Abstract
Aims
Patients with left ventricular systolic dysfunction frequently show abnormal coronary vascular function, even in the absence of overt coronary artery disease. Moreover, the severity of vascular dysfunction might be related to the aetiology of cardiomyopathy.
We sought to determine the incremental value of assessing coronary vascular dysfunction among patients with ischaemic (ICM) and non-ischaemic (NICM) cardiomyopathy at risk for adverse cardiovascular outcomes.
Methods and results
Coronary flow reserve (CFR, stress/rest myocardial blood flow) was quantified in 510 consecutive patients with rest left ventricular ejection fraction (LVEF) ≤45% referred for rest/stress myocardial perfusion PET imaging. The primary end point was a composite of major adverse cardiovascular events (MACE) including cardiac death, heart failure hospitalization, late revascularization, and aborted sudden cardiac death.
Median follow-up was 8.2 months. Cox proportional hazards model was used to adjust for clinical variables. The annualized MACE rate was 26.3%. Patients in the lowest two tertiles of CFR (CFR ≤ 1.65) experienced higher MACE rates than those in the highest tertile (32.6 vs. 15.5% per year, respectively, P = 0.004), irrespective of aetiology of cardiomyopathy.
Conclusion
Impaired coronary vascular function, as assessed by reduced CFR by PET imaging, is common in patients with both ischaemic and non-ischaemic cardiomyopathy and is associated with MACE.
Keywords: coronary flow reserve, cardiomyopathy, positron emission tomography, myocardial blood flow, coronary vascular dysfunction, clinical outcomes
Introduction
Accurate characterization of the aetiology of left ventricular systolic dysfunction and risk stratification are important goals in the management of patients with heart failure (HF). Clinically, patients are classified as having ischaemic or non-ischaemic left ventricular systolic dysfunction based on a history of myocardial infarction and/or objective evidence of coronary artery disease (CAD) on angiography. However, the severity of left ventricular dysfunction and remodelling is often out of proportion to the severity of angiographic CAD. This is compounded by the fact that many patients without angiographic CAD present with typical angina, not infrequently, show regional wall motion and/or myocardial perfusion abnormalities on non-invasive imaging studies. This suggests that structural and/or functional abnormalities downstream from the epicardial coronary arteries may contribute to symptoms, cardiac function, and prognosis. Abnormal coronary flow reserve, an integrated measure of the haemodynamic effects of epicardial coronary atherosclerosis and microvascular dysfunction/remodelling, is frequently found in patients with cardiomyopathy even in the presence of angiographically normal coronary arteries1–4 and is associated with increased risk of adverse ventricular remodelling independent of clinical severity of HF.5,6
In this study, we tested the hypothesis that the severity of global coronary vascular dysfunction may differ based on aetiology of cardiomyopathy and is associated with clinical outcomes independently of traditional clinical and imaging risk markers. We used positron emission tomography (PET) to quantify coronary blood flow and flow reserve in a large cohort of patients with ischaemic and non-ischaemic cardiomyopathy.
Methods
Study population
Consecutive patients referred to the Brigham and Women's Hospital between January 2006 and June 2010 for rest/stress myocardial perfusion PET were included if they had a resting left ventricular ejection fraction (LVEF) ≤45% by two-dimensional transthoracic echocardiogram within 90 days of the index PET scan. The study was approved by the Partners Healthcare Institutional Review Board and conducted in accordance with institutional guidelines.
PET imaging
Myocardial perfusion PET imaging was performed on a whole-body PET-CT scanner (Discovery RX or STE LightSpeed 64, GE Healthcare, Milwaukee, WI, USA) after an overnight fast. Patients were instructed to refrain from caffeine- and methylxanthine-containing substances for 24 h prior to their scans. Myocardial blood flow (MBF) was measured during rest and peak stress with rubidium-82 as a perfusion tracer, and adenosine, regadenoson, or dipyridamole as the stress agent, as described previously.7 Heart rate, blood pressure, and 12-lead ECG were recorded at baseline and every minute during and after pharmacological stress.
Image analysis and interpretation
Myocardial perfusion
Semi-quantitative 17-segment visual interpretation of the summed myocardial perfusion images was performed using a standard 5-point scoring system (0–4), as described previously.7 Summed rest and stress scores were calculated as the sum of individual segmental scores, and their difference was recorded as summed difference score. Summed rest, stress, and difference scores were converted into percentages of total myocardium by dividing each score by the maximum possible score (i.e. 68).
Quantitative myocardial blood flow and flow reserve
Absolute MBF (mL/g/min) was computed from dynamic rest and stress imaging series using previously validated commercially available software (Corridor4DM; Ann Arbor, MI).8 Briefly, factor analysis was used to generate blood pool and tissue time-activity curves. Global rest and peak stress MBFs were calculated using a two-compartment tracer-kinetic model, as described previously. Per-patient global CFR was calculated as the ratio of absolute MBF at stress over rest. Four operators performed quantification of MBF without knowledge of aetiology; the intraclass correlation coefficient for CFR among these four readers was 0.94 [95% confidence interval (CI), 0.88–0.98].
Definition of the aetiology of cardiomyopathy
The aetiology of cardiomyopathy was adjudicated by two cardiologists blinded to the coronary flow reserve data. Blinded adjudication used historical data, results of non-invasive imaging tests and coronary angiography, and physicians' notes to classify patients into two groups. Consistent with Felker9 criteria, patients were defined as having non-ischaemic cardiomyopathy if there was no prior history of coronary revascularization and/or myocardial infarction and they had either a normal myocardial perfusion PET study (summed stress score <3), or they had a coronary angiogram without evidence of obstructive coronary artery stenosis in more than one vessel. If subjects had evidence of multivessel obstructive coronary artery stenosis or significant ischaemia or scar on PET (summed stress score ≥3), they were characterized as ischaemic cardiomyopathy.
Assessment of outcomes
The primary outcome was the first occurrence of a major adverse cardiovascular event (MACE)—a composite end point that included cardiac death, HF hospitalization, aborted sudden cardiac death (SCD), and late revascularization. Patients who died of non-cardiac causes were censored at the time of death. MACE was adjudicated by manual chart review by three cardiologists. Differences between reviewers were resolved by consensus. Detailed definitions used for adjudication of major adverse events are provided in Supplementary data online, Methods.
Cardiac death
Vital status of all patients was ascertained by integrating data from the Social Security Death Index (SSDI), the National Death Index (NDI), and Partners Healthcare Research Patient Data Repository (RPDR), as described previously.
HF hospitalization
For hospitalization events to be classified as due to HF, the event had to meet all of the following criteria: (i) the patient was admitted to the hospital for a primary diagnosis of HF, (ii) the patient presented with new or worsening symptoms due to HF on presentation, (iii) the patient had objective evidence of new or worsening HF, and (iv) the patient received initiation or intensification of treatment specifically directed at HF.
Aborted SCD
Implantable cardioverter defibrillator (ICD) therapies within 12 months after the index PET scan were categorized by reviewing all stored ICD internal electrograms of suspected VT/VF episodes to verify their ventricular origin. The episodes were identified in terms of cycle lengths recorded by the ICD and were also manually checked by examining the printout electrograms. Aborted SCD was defined by delivery of an appropriate ICD shock or anti-tachycardia pacing for ventricular tachycardia or fibrillation. Inappropriate ICD therapy was defined as a discharge triggered by sinus tachycardia, supraventricular tachycardia, atrial fibrillation, or device malfunction.
Late revascularization
All revascularization procedures ≥90 days from the index PET scan were adjudicated and included as MACE.
Statistical analysis
Statistical significance was assessed using the Wilcoxon, Fisher exact, and χ2 tests for continuous, dichotomous, and categorical variables, respectively. Two-sided values of P < 0.05 were considered significant. All statistical analyses were performed with SAS 9.3 (SAS Institute Inc., Cary, NC, USA).
Survival modelling
Survival analysis was conducted using Kaplan–Meier analysis. Because some patients experienced multiple events, for each analysis follow-up was censored at the first relevant event. Nested Cox proportional hazards models were generated to evaluate the incremental prognostic value of coronary flow reserve beyond clinical covariates and imaging variables. Because of high collinearity between end-diastolic and end-systolic volume indices, only the former was included in models as it resulted in greater improvement in model fit. Incremental prognostic value was evaluated by the global χ2 statistic, c-index, and net reclassification improvement.10–12
Results
Patient characteristics
A total of 510 patients met inclusion criteria and were followed for a median of 8.2 months (IQR 2.7–18.7 months). Complete follow-up was obtained for 94% of patients. Baseline characteristics are provided in Table 1. Median age was 67.3 years, with men comprising 73.7% of the study population. Ischaemia was the predominant cause of cardiomyopathy, accounting for 382 patients (74.9%). Baseline cardiovascular risk factors such as hypertension, dyslipidaemia, and diabetes mellitus were higher among patients with ICM compared with NICM. Patients with ICM had lower mean LVEF (32.1 vs. 34.7%, P < 0.01) and higher scar burden (26.0 vs. 2.1% myocardium, P < 0.0001).
Table 1.
Patient characteristics
| Variable | HF aetiology (n = 510) |
P-value | |
|---|---|---|---|
| Ischaemic | Non-ischaemic | ||
| n = 382 (74.9) | n = 128 (25.1) | ||
| Demographics | |||
| Age (years) | 70.2 (61.5–78.2) | 65.4 (55.4–75) | 0.0003 |
| Male | 306 (80.1) | 70 (54.7) | <0.0001 |
| BMI | 26.6 (23.7–30.8) | 27.4 (22.3–32.3) | 0.6 |
| CV risk factors | |||
| Hypertension | 337 (88.2) | 104 (81.3) | 0.05 |
| Dyslipidaemia | 308 (80.6) | 60 (46.9) | <0.0001 |
| Diabetes mellitus | 181 (47.4) | 36 (28.1) | 0.0001 |
| Prior history | |||
| Family history | 101 (26.4) | 21 (16.4) | 0.02 |
| Coronary artery disease | 345 (90.3) | 30 (23.4) | <0.0001 |
| Prior myocardial infarction | 186 (48.7) | 11 (8.6) | <0.0001 |
| Prior revascularization | 259 (67.8) | 0 (0.0) | <0.0001 |
| Medications | |||
| ACE-I/ARB | 197 (51.6) | 69 (53.9) | 0.65 |
| Beta blocker | 307 (80.4) | 83 (64.8) | <0.001 |
| Diuretic | 221 (57.9) | 62 (48.4) | 0.07 |
| Reason for PET study | |||
| Chest pain | 109 (28.5) | 43 (33.6) | 0.31 |
| Dyspnoea | 139 (36.4) | 58 (45.3) | 0.08 |
| Pre-op evaluation | 65 (17.0) | 10 (7.8) | 0.01 |
| Laboratory data | |||
| BNP | 611 (217–1238) | 709 (287–1748) | 0.34 |
| GFR (MDRD) | 57.5 (38.4–77.1) | 56.5 (33.1–80.9) | 0.91 |
| Imaging findings | |||
| LVEF % | 33 (25–39) | 36 (29–42) | 0.004 |
| ESVI | 59.8 (44–83.6) | 50.9 (38.7–72.6) | 0.007 |
| Rest MBF | 0.77 (0.62–1.02) | 0.87 (0.69–1.15) | 0.006 |
| Peak MBF | 1.07 (0.83–1.45) | 1.49 (1.11–1.92) | <0.0001 |
| Global CFR | 1.33 (1.07–1.71) | 1.67 (1.36–2.08) | <0.0001 |
| SSS (% myocardium) | 25 (13.2–36.8) | 0 (0–2.9) | <0.0001 |
BMI, body mass index; ACE-I, angiotensin-converting enzyme-inhibitor; ARB, angiotensin receptor blocker; BNP, B-type natriuretic peptide; GFR, glomerular filtration rate; ESVI, end-systolic volume index; SSS, summed stress score.
Myocardial blood flow and coronary flow reserve
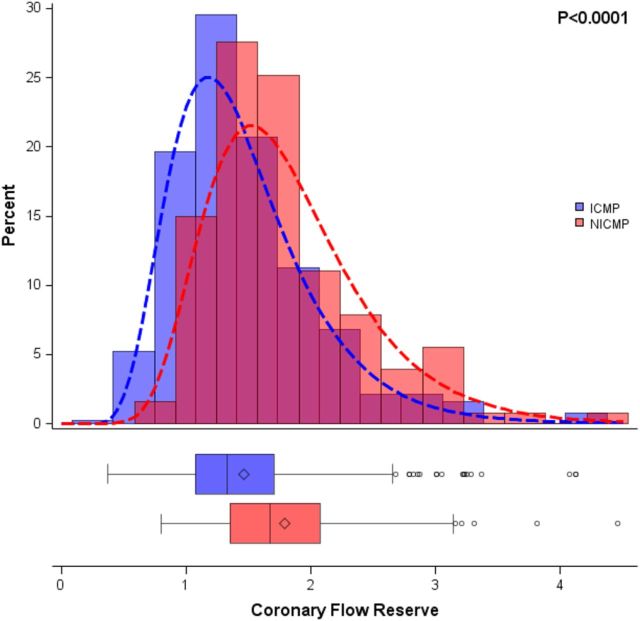
Patients with ICM had a trend for lower MBF at rest compared with NICM (0.87 ± 0.47 vs. 0.95 ± 0.38 mL/min/g, respectively, P = 0.08) and significantly lower blood flow during peak stress (1.20 ± 0.56 vs. 1.65 ± 0.77 mL/min/g, respectively, P < 0.0001). Mean CFR was significantly lower among patients with ICM compared with those with NICM (1.46 ± 0.59 vs. 1.82 ± 0.73, respectively, P < 0.0001, Figure 1). Overall, 417 patients (81.8%) had abnormal CFR (<2.0). Abnormal CFR was more prevalent in patients with ICM (85.1%) than in those with NICM (71.9%) (P = 0.001), but occurred in the majority of patients in each group. We found that in multivariable models containing age, gender, hypertension, dyslipidaemia, tobacco use, diabetes, family history of CAD, prior CAD, prior PCI, prior CABG, and medications (beta blockers, ACE inhibitors/ARBs, and diuretics), ischaemic HF aetiology remained associated with lower stress MBF (β = −0.29; P = 0.002) and CFR (β = −0.28; P = 0.003) but not resting MBF (P = 0.45).
Figure 1.
Distribution of CFR by HF aetiology, showing that patients with non-ischaemic cardiomyopathy (NICM, red) have significantly higher CFR than those with ischaemic cardiomyopathy (ICM, blue). Significance was evaluated with a t-test assuming an underlying log-normal distribution.
Relationship between coronary flow reserve abnormalities and symptoms
CFR was abnormal (<2.0) in 77.5% of the 152 patients with chest pain. Among patients with chest pain, CFR was quantitatively lower in patients with ICM than those with NICM (1.54 ± 0.63 vs. 1.89 ± 0.68, respectively; P = 0.003). Among those with dyspnoea, CFR was abnormal in 84.3% of patients. Among patients with dyspnoea, CFR was lower in those with ICM compared with those with NICM (1.40 ± 0.54 vs. 1.74 ± 0.59, respectively; P = 0.0001).
Patient outcomes
Overall, 135 out of 510 (26.5%) met the primary composite outcome of MACE (Table 2). Compared with those without MACE, those who experienced MACE were slightly older, had higher prevalence of prior CAD and revascularization, had more severe ventricular dysfunction, and higher incidence of severely impaired CFR (see Supplementary data online, Table S1).
Table 2.
Primary outcomes stratified by CFR
| CFR tertiles | |||||
|---|---|---|---|---|---|
| Outcome | All patients (n = 510), n (%) | Low (n = 170), n (%) | Medium (n = 170), n (%) | High (n = 170), n (%) | P-value |
| MACE | 135 (26.5) | 53 (31.2) | 53 (31.2) | 29 (17.1) | 0.002 |
| Cardiac death | 76 (14.9) | 32 (18.8) | 30 (17.6) | 14 (8.2) | 0.01 |
| HF hospitalization | 72 (14.1) | 27 (15.9) | 27 (15.9) | 18 (10.6) | 0.26 |
| Aborted SCD | 18 (3.5) | 3 (1.8) | 11 (6.5) | 4 (2.4) | 0.05 |
| Late revascularization | 13 (2.5) | 6 (3.5) | 6 (3.5) | 1 (0.6) | 0.12 |
| Outcome | All patients (n = 510) | CFR ≤ 1.65 (n = 340) | CFR > 1.65 (n = 170) | P-value |
|---|---|---|---|---|
| MACE | 135 (26.5) | 106 (31.2) | 29 (17.1) | 0.0006 |
| Cardiac death | 76 (14.9) | 62 (18.2) | 14 (8.2) | 0.002 |
| HF hospitalization | 72 (14.1) | 54 (15.9) | 18 (10.6) | 0.14 |
| Aborted SCD | 18 (3.5) | 14 (4.1) | 4 (2.4) | 0.45 |
| Late revascularization | 13 (2.5) | 12 (3.5) | 1 (0.6) | 0.07 |
CFR=coronary flow reserve, HF= heart failure, MACE=major adverse cardiovascular events, SCD=sudden cardiac death.
Analysis by CFR tertiles revealed similar annualized rates of MACE in the low and medium tertiles subgroups (35.3 vs. 30.3%, P = 0.43), compared with the high CFR tertile (CFR > 1.65) cohort (35.3 and 30.3% vs. 15.5%, P = 0.0004 and P = 0.004 for low vs. high and medium vs. high, respectively). Thus, for all subsequent analyses, patients in low and medium CFR tertiles (CFR ≤ 1.65) were grouped together and compared with patients in the highest CFR tertile group (Table 2). Overall, patients with CFR ≤ 1.65 experienced higher annualized rates of MACE, cardiac death, HF hospitalization, and late revascularization (Figure 2).
Figure 2.
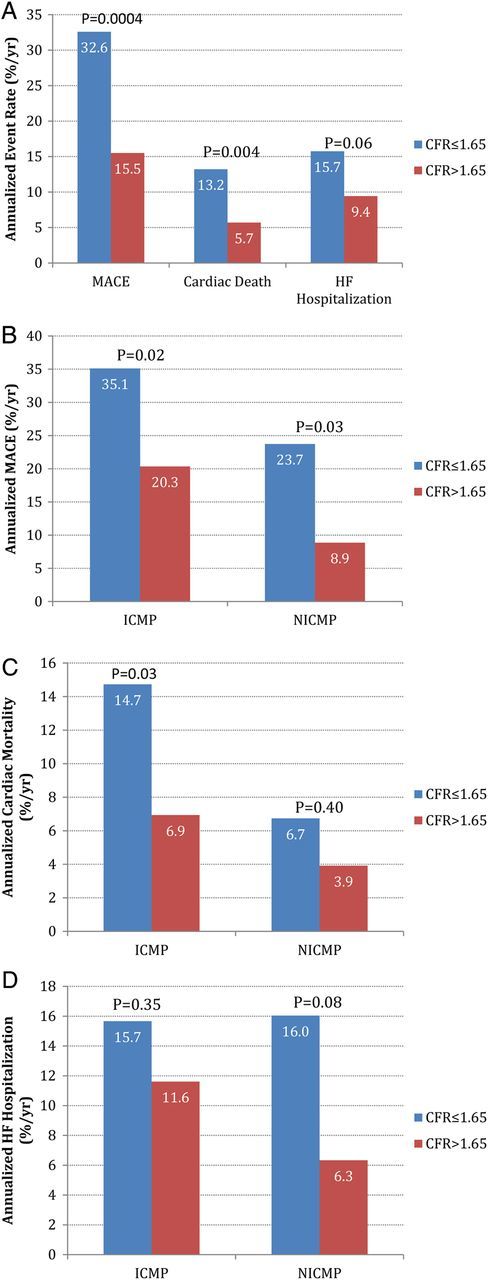
Annualized event rates stratified by CFR above (red) and below (blue) the median. (A) Annualized event rates for MACEs, cardiac death, and HF hospitalization; (B) annualized MACE rate in ICM and NICM; (C) annualized cardiac death in ICM and NICM; (D) annualized HF hospitalization rate in ICM and NICM.
HF aetiology-based analysis revealed that patients with ICM experienced higher rates of MACE, cardiac death, and late revascularization, compared with those with NICM (Table 3).
Table 3.
Primary outcomes stratified by aetiology of cardiomyopathy
| Outcome | All patients (n = 510) | ICM (n = 382) | NICM (n = 128) | P-value |
|---|---|---|---|---|
| MACE | 135 (26.5) | 111 (29.1) | 24 (18.8) | 0.03 |
| Cardiac death | 76 (14.9) | 66 (17.3) | 10 (7.8) | 0.009 |
| HF hospitalization | 72 (14.1) | 55 (14.4) | 17 (13.3) | 0.88 |
| Aborted SCD | 18 (3.5) | 16 (4.2) | 2 (1.6) | 0.27 |
| Late revascularization | 13 (2.5) | 13 (3.4) | 0 (0) | 0.05 |
| Outcome | All ICM (n = 382) | CFR ≤ 1.65 (n = 280) | CFR > 1.65 (n = 102) | P-value |
| MACE | 111 (29.1) | 89 (31.8) | 22 (21.6) | 0.06 |
| Cardiac death | 66 (17.3) | 56 (20) | 10 (9.8) | 0.02 |
| HF hospitalization | 55 (14.4) | 42 (15) | 13 (12.7) | 0.63 |
| Aborted SCD | 16 (4.2) | 12 (4.3) | 4 (3.9) | 1 |
| Late revascularization | 13 (3.4) | 12 (4.3) | 1 (1.0) | 0.2 |
| Outcome | All NICM (n = 128) | CFR ≤ 1.65 (n = 60) | CFR > 1.65 (n = 68) | P-value |
| MACE | 24 (18.8) | 17 (28.3) | 7 (10.3) | 0.01 |
| Cardiac death | 10 (7.8) | 6 (10) | 4 (5.9) | 0.51 |
| HF hospitalization | 17 (13.3) | 12 (20) | 5 (7.4) | 0.04 |
| Aborted SCD | 2 (1.6) | 2 (3.3) | 0 (0) | 0.22 |
| Late revascularization | 24 (18.8) | 17 (28.3) | 7 (10.3) | 0.01 |
CFR=coronary flow reserve, HF= heart failure, ICM=ischemic cardiomyopathy, MACE=major adverse cardiovascular events, NICM=non-ischemic cardiomyopathy, SCD=sudden cardiac death.
Unadjusted and adjusted event-free survival
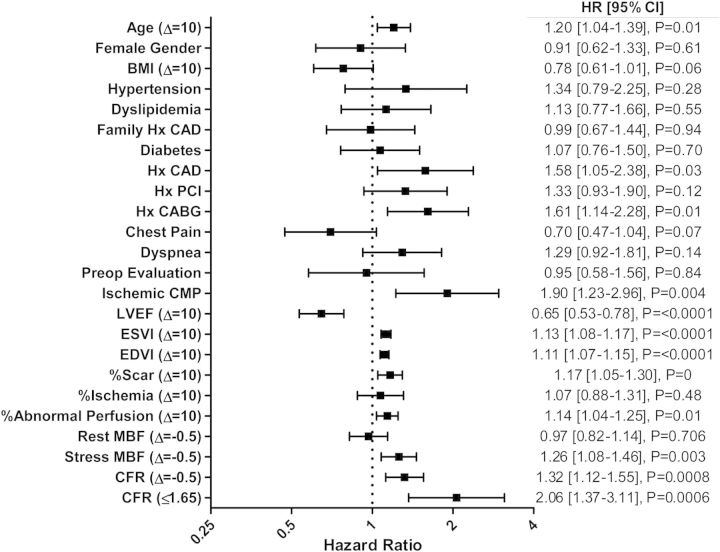
Variables included in the Cox proportional hazards model and results of univariate and multivariate event-free survival analysis are reported in Tables 4 and 5. In univariate analysis, age, history of CAD or CABG, ICM, ventricular function, end-systolic and end-diastolic volumes, combined amount of myocardial scar and ischaemia (abnormal perfusion), as well as CFR were all significantly associated with MACE (Figure 3). Unadjusted Kaplan–Meier analyses revealed an event-free survival rate of 52.4 and 78.3% for patients with CFR ≤ 1.65 and CFR > 1.65, respectively (P < 0.0001; Figure 4); and a 2-year event-free survival rate of 58.3 and 68.7% for patients with ICM and NICM, respectively (P = 0.0006; see Supplementary data online, Figure S1).
Table 4.
Variables in the Cox proportional hazards model
| Model 1 |
Model 2 |
Model 3 |
Model 4 |
Model 5 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | P | HR | P | HR | P | HR | P | HR | P | |
| Age (Δ = 10 years) | 1.17 (1–1.36) | 0.04 | 1.16 (0.99–1.35) | 0.07 | 1.22 (1.04–1.43) | 0.01 | 1.2 (1.02–1.41) | 0.03 | 1.17 (0.99–1.37) | 0.06 |
| Female gender | 1.07 (0.71–1.61) | 0.75 | 1.22 (0.8–1.85) | 0.36 | 1.44 (0.93–2.22) | 0.1 | 1.48 (0.96–2.29) | 0.08 | 1.44 (0.93–2.22) | 0.1 |
| Hypertension | 1.16 (0.67–2) | 0.59 | 1.25 (0.72–2.15) | 0.43 | 1.17 (0.68–2.02) | 0.57 | 1.16 (0.67–2) | 0.59 | 1.12 (0.65–1.94) | 0.69 |
| Dyslipidaemia | 0.92 (0.59–1.43) | 0.71 | 0.96 (0.62–1.49) | 0.87 | 0.9 (0.58–1.4) | 0.64 | 0.87 (0.56–1.36) | 0.54 | 0.88 (0.57–1.37) | 0.58 |
| Family hx CAD | 0.83 (0.56–1.25) | 0.38 | 0.76 (0.51–1.15) | 0.19 | 0.77 (0.51–1.16) | 0.21 | 0.74 (0.49–1.12) | 0.15 | 0.73 (0.49–1.1) | 0.13 |
| DM | 0.91 (0.63–1.3) | 0.6 | 0.94 (0.65–1.35) | 0.74 | 1.02 (0.71–1.48) | 0.9 | 1 (0.69–1.44) | 0.98 | 0.98 (0.67–1.42) | 0.9 |
| Hx of CAD | 1.28 (0.74–2.21) | 0.37 | 1.3 (0.75–2.26) | 0.35 | 1.26 (0.73–2.18) | 0.41 | 1.01 (0.56–1.85) | 0.97 | 0.98 (0.54–1.78) | 0.94 |
| Hx of PCI | 1.3 (0.87–1.93) | 0.2 | 1.32 (0.89–1.98) | 0.17 | 1.46 (0.97–2.18) | 0.07 | 1.34 (0.89–2.03) | 0.16 | 1.35 (0.9–2.04) | 0.15 |
| Hx of CABG | 1.42 (0.95–2.13) | 0.09 | 1.39 (0.93–2.08) | 0.11 | 1.34 (0.9–2.01) | 0.15 | 1.24 (0.82–1.87) | 0.31 | 1.23 (0.81–1.85) | 0.33 |
| Early revascularization | 0.78 (0.45–1.34) | 0.37 | 0.81 (0.47–1.41) | 0.46 | 0.9 (0.52–1.55) | 0.69 | 0.79 (0.45–1.39) | 0.42 | 0.73 (0.41–1.29) | 0.28 |
| Rest LVEF (Δ = 10%) | 0.64 (0.52–0.78) | <0.0001 | 0.83 (0.65–1.07) | 0.15 | 0.85 (0.66–1.1) | 0.21 | 0.84 (0.65–1.08) | 0.18 | ||
| Rest LVEDVI (Δ = 10 mL/m2) | 1.11 (1.05–1.17) | 0.0001 | 1.11 (1.05–1.17) | 0.0001 | 1.1 (1.04–1.16) | 0.0006 | ||||
| Ischaemic aetiology | 1.73 (0.92–3.23) | 0.09 | 1.63 (0.87–3.07) | 0.13 | ||||||
| CFR ≤ 1.65 | 1.78 (1.16–2.74) | 0.01 | ||||||||
CABG, coronary artery bypass grafting; DM, diabetes mellitus; IDI, integrated discrimination improvement; LVEDVI, left ventricular end-diastolic volume index; NRI, net reclassification index; PCI, percutaneous coronary intervention.
Table 5.
Multivariable modelling
| Model 1 |
Model 2 |
Model 3 |
Model 4 |
Model 5 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | P | Estimate | P | Estimate | P | Estimate | P | Estimate | P | |
| Global χ2 | 17.9 | ref | 37.1 | <0.0001 | 50.5 | 0.0002 | 53.5 | 0.08 | 61 | 0.006 |
| AIC | 1448 | ref | 1430.8 | <0.0001 | 1419.4 | 0.0007 | 1418.4 | 0.32 | 1412.9 | 0.02 |
| C-index | 0.613 (0.565 to 0.661) | ref | 0.659 (0.608 to 0.709) | 0.02 | 0.681 (0.635 to 0.727) | 0.19 | 0.696 (0.652 to 0.74) | <0.05 | 0.706 (0.663 to 0.749) | 0.38 |
| Calibration χ2 | 10 | 0.35 | 11.7 | 0.23 | 17.3 | 0.04 | 4.1 | 0.9 | 3.3 | 0.95 |
| IDI | ref | ref | 0.042 (0.032 to 0.053) | <0.05 | 0.022 (0.010 to 0.034) | <0.05 | 0.006 (0.002 to 0.011) | <0.05 | 0.018 (0.010 to 0.025) | <0.05 |
| NRI | ref | ref | 0.436 (0.196 to 0.686) | <0.05 | 0.322 (0.089 to 0.565) | <0.05 | 0.152 (−0.085 to 0.397) | NS | 0.381 (0.144 to 0.590) | <0.05 |
AIC, Akaike's information criterion.
Figure 3.
Univariate predictors of MACE hazard ratios (HRs) are presented for a 1-unit increase except for age (increase of 10 years), BMI (increase of 10 units), LVEF (decrease of 10%), ESVI and EDVI (increase of 10 cc), extent of myocardial ischaemia and scar combined and each separately (increase of 10%), MBF (decrease of 0.5 mL/kg/min), and CFR (decrease of 0.5). Abnormal perfusion refers to combined scar and ischaemia. BMI, body mass index; CABG, coronary artery bypass grafting; CMP, cardiomyopathy; ESVI, end-systolic volume index; EDVI, end-diastolic volume index; PCI, percutaneous coronary intervention.
Figure 4.
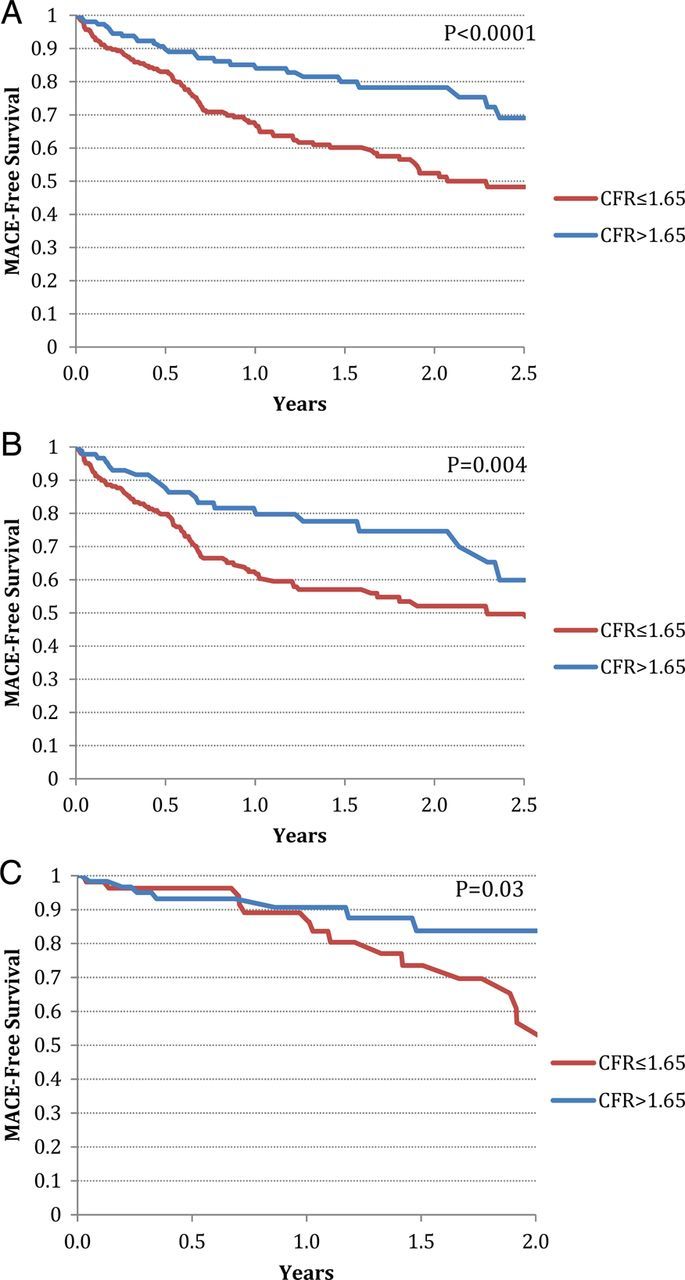
Kaplan–Meier event-free survival stratified by CFR above (blue) and below (red) the median for (A) all patients, (B) ICM only, and (C) NICM only.
Next, a series of multivariable models were constructed to assess the incremental prognostic value of CFR for MACE after adjustment for clinical covariates, LVEF, left ventricular end-diastolic volume index, and HF aetiology (Tables 4 and 5). Because HF aetiology and myocardial scar burden showed significant collinearity, only the former was included in the models. Addition of CFR was associated with a significant increase in global χ2, indicating improved model fit. Compared with CFR ≤ 1.65, the adjusted hazard ratio for MACE for patients with CFR > 1.65 was 0.62 (95% CI, 0.39–0.85; P < 0.04). Of note, an interaction term between CFR and HF aetiology was non-informative, suggesting that the effect of CFR on MACE was consistent regardless of aetiology of HF. Furthermore, use of beta blockers, ACE inhibitors/ARBs, and diuretics was not related to MACE and did not modify the effect of CFR on MACE. Direct adjusted survival plots demonstrated that patients with CFR ≥ 1.65 had a significantly better event-free survival compared with those with CFR ≤ 1.65 (Figure 5). Finally, when CFR was corrected by the rate pressure product to account for resting cardiac work, similar results were obtained.
Figure 5.
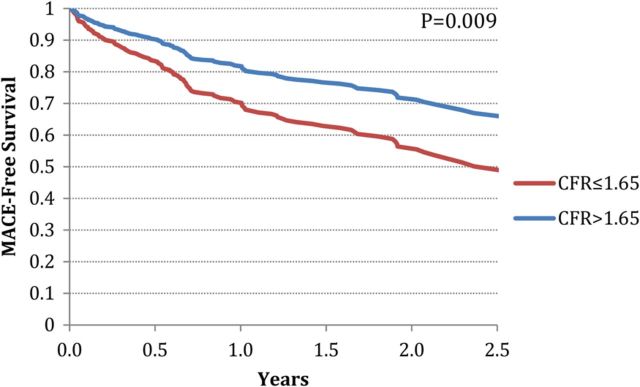
Direct adjusted MACE-free survival after adjustment for age, gender, hypertension, dyslipidaemia, diabetes mellitus, family history of coronary artery disease, history of coronary revascularization, early revascularization, left ventricular ejection fraction, end-diastolic volume index, and HF aetiology, showing a significant association between CFR and MACE-free survival.
Risk reclassification
The addition of CFR category to a pre-CFR risk model that included clinical covariates, LVEF, LVEDVI, and HF aetiology (model 5, Tables 4 and 5) resulted in a net correct reclassification of 57.2% of events and a net incorrect reclassification of 19.1% of non-events. The continuous net reclassification improvement was 0.381 (95% CI, 0.144–0.590).
Discussion
This study of coronary flow reserve in cardiomyopathy reveals that coronary vascular dysfunction is highly prevalent among patients with cardiomyopathy, even among those classified clinically as NICM although coronary flow abnormalities are quantitatively more severe among those with ICM. Second, even among those with chest pain or dyspnoea, CFR was lower in those with ICM compared with those with NICM. Third, CFR ≤ 1.65 was associated with a two-fold higher annualized frequency of MACE in patients with both ICM and NICM, compared with those with a CFR > 1.65. Interestingly, the higher frequency of annualized MACE among those with severely reduced CFR vs. those with relatively preserved CFR was dominated by cardiac death in ICM and by HF admissions in those with NICM. In multivariable modelling adjusting for clinical risk, ejection fraction, left ventricular end-diastolic volume index, and aetiology of cardiomyopathy, an impaired CFR was independently associated with adverse cardiovascular events.
Few studies have examined abnormalities in coronary vascular function and its association with clinical outcomes in patients with LV systolic dysfunction. These studies were small and included only patients with NICM. Neglia et al.1 evaluated 67 patients and showed a 3.5-fold increase in the risk of death and progression of HF in the setting of impaired CFR as measured by PET. Two other studies using a less well-validated method for assessing coronary function (echocardiography) also showed that a reduced CFR associated with cardiovascular events.3,13 Our findings extend the observations of these studies in three important ways: by characterizing the differences in coronary flow abnormalities in a significantly larger cohort that included patients with both ICM and NICM, by describing the interrelationship between the abnormalities in coronary function and patients' symptoms, and by describing the prognostic significance of impaired CFR in patients with ICM and NICM.
Although multiple factors contribute to abnormal CFR among patients with CAD and ischaemic cardiomyopathy, such as severity and distribution of obstructive CAD, the extent of diffuse distal atherosclerosis, and microvascular dysfunction,14–16 the mechanisms underlying the reduced CFR in patients with NICM are not well understood.17,18 Some of the proposed mechanisms for alterations in coronary blood flow in patients with cardiomyopathies include endothelial and autonomic nervous system dysfunction, vasostructural changes such as macro- and microvascular obstruction, changes in myocardial capillary density, and vascular remodelling, as well as extravascular compressive forces.17,19,20
From a clinical viewpoint, these data suggest that assessment of CFR may provide another element to include in risk stratification for patients with HF. While it is not clear whether and how this might direct therapy, it would seem plausible that patients with abnormal CFR and exertional limitation may potentially benefit from vasodilators already used in some patients with HF, such as nitrates, which have been suggested to improve exercise tolerance in NICM as well as in ICM, or hydralazine, which has improved multiple HF outcomes.21,22 In patients with ICM, impaired CFR in the peri-infarct or remote myocardium may increase the risk of sudden death from fatal arrhythmias triggered by ischaemia in the location of scar or in remote myocardium.23
Our study has several limitations. First, this was a single-centre observational study in a large academic medical centre and, thus, is subject to bias. In the present cohort, there is a high rate of cardiac death, but this is quite comparable to other patient populations in epidemiologic studies of patients with left ventricular systolic dysfunction and/or congestive HF.24–26 Despite accounting for several important clinical covariates, it is likely that additional confounding bias persists. However, an advantage of this type of analysis is that it represents a real-world population, and thus, the results should be generalizable. Second, coronary angiography was not available in all patients. In this regard, it is possible that reliance on the absence of stress perfusion defects may misclassify a small number of patients (e.g. with multivessel CAD and globally depressed CFR). However, in general, the absence of PET perfusion defects is associated with lack of significant CAD,27 and future prospective studies in this space may utilize CT or invasive angiography to confirm these findings. Finally, stress MBF was not associated with outcome in our study, suggesting that CFR as an integrated physiologic measure of flow reserve is a uniquely prognostic PET parameter.
Conclusion
In the present study, we show that impaired coronary vascular function is common in patients with ischaemic and non-ischaemic cardiomyopathy. It may contribute to chest pain and dyspnoea on exertion, thus limiting exercise in patients with and without significant CAD. Furthermore, the presence of coronary vascular dysfunction is associated with MACEs, including cardiovascular death and HF hospitalization. Future work should be focused on investigating whether and how quantitative measurements of CFR can direct therapy in patients with HF.
Supplementary material
Supplementary data are available at European Heart Journal – Cardiovascular Imaging online.
Funding
The study was funded in part by grants from the National Institutes of Health (K23HL092299). M.D.M. was supported by a NIH training grant to the Brigham and Women's Hospital, Noninvasive Cardiovascular Imaging Program (HL-094301-03). M.F.D.C. received research grant support from Gilead. S.D. received research grant support from Astellas.
Conflict of interest: None declared.
References
- 1.Neglia D, Michelassi C, Trivieri MG, Sambuceti G, Giorgetti A, Pratali L, et al. Prognostic role of myocardial blood flow impairment in idiopathic left ventricular dysfunction. Circulation 2002;105:186–93. [DOI] [PubMed] [Google Scholar]
- 2.Rigo F, Gherardi S, Galderisi M, Pratali L, Cortigiani L, Sicari R, et al. The prognostic impact of coronary flow-reserve assessed by Doppler echocardiography in non-ischaemic dilated cardiomyopathy. Eur Heart J 2006;27:1319–23. [DOI] [PubMed] [Google Scholar]
- 3.Rigo F, Gherardi S, Galderisi M, Sicari R, Picano E. The independent prognostic value of contractile and coronary flow reserve determined by dipyridamole stress echocardiography in patients with idiopathic dilated cardiomyopathy. Am J Cardiol 2007;99:1154–8. [DOI] [PubMed] [Google Scholar]
- 4.Rigo F, Ciampi Q, Ossena G, Grolla E, Picano E, Sicari R. Prognostic value of left and right coronary flow reserve assessment in nonischemic dilated cardiomyopathy by transthoracic Doppler echocardiography. J Card Fail 2011;17:39–46. [DOI] [PubMed] [Google Scholar]
- 5.Neglia D, Parodi O, Gallopin M, Sambuceti G, Giorgetti A, Pratali L, et al. Myocardial blood flow response to pacing tachycardia and to dipyridamole infusion in patients with dilated cardiomyopathy without overt heart failure. A quantitative assessment by positron emission tomography. Circulation 1995;92:796–804. [DOI] [PubMed] [Google Scholar]
- 6.Morales MA, Neglia D, L'Abbate A. Reduction of myocardial blood flow reserve in idiopathic dilated cardiomyopathy without overt heart failure and its relation with functional indices: an echo-Doppler and positron emission tomography study. J Cardiovasc Med (Hagerstown) 2008;9:778–82. [DOI] [PubMed] [Google Scholar]
- 7.Murthy VL, Naya M, Foster CR, Hayner J, Gaber M, Di Carli G, et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation 2011;124:2215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El Fakhri G, Kardan A, Sitek A, Dorbala S, Abi-Hatem N, Lahoud Y, et al. Reproducibility and accuracy of quantitative myocardial blood flow assessment with (82)Rb PET: comparison with (13)N-ammonia PET. J Nucl Med 2009;50:1062–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felker GM. A standardized definition of ischemic cardiomyopathy for use in clinical research. J Am Coll Card 2002;39:210–8. [DOI] [PubMed] [Google Scholar]
- 10.Hosmer DW, Hosmer T, Le Cessie S, Lemeshow S. A comparison of goodness-of-fit tests for the logistic regression model. Stat Med 1997;16:965–80. [DOI] [PubMed] [Google Scholar]
- 11.Pencina MJ, D'Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med 2004;23:2109–23. [DOI] [PubMed] [Google Scholar]
- 12.Antolini L, Boracchi P, Biganzoli E. A time-dependent discrimination index for survival data. Stat Med 2005;24:3927–44. [DOI] [PubMed] [Google Scholar]
- 13.Anantharam B, Janardhanan R, Hayat S, Hickman M, Chahal M, Basseti P, et al. Coronary flow reserve assessed by myocardial contrast echocardiography predicts mortality in patients with heart failure. Eur J Echocardiogr 2011;12:69–75. [DOI] [PubMed] [Google Scholar]
- 14.Gould KL, Nakagawa Y, Nakagawa K, Sdringola S, Hess MJ, Haynie M, et al. Frequency and clinical implications of fluid dynamically significant diffuse coronary artery disease manifest as graded, longitudinal, base-to-apex myocardial perfusion abnormalities by noninvasive positron emission tomography. Circulation 2000;101:1931–9. [DOI] [PubMed] [Google Scholar]
- 15.Uren NG, Melin JA, De Bruyne B, Wijns W, Baudhuin T, Camici PG. Relation between myocardial blood flow and the severity of coronary-artery stenosis. N Engl J Med 1994;330:1782–8. [DOI] [PubMed] [Google Scholar]
- 16.Di Carli MF, Asgarzadie F, Schelbert HR, Brunken RC, Laks H, Phelps ME, et al. Quantitative relation between myocardial viability and improvement in heart failure symptoms after revascularization in patients with ischemic cardiomyopathy. Circulation 1995;92:3436–44. [DOI] [PubMed] [Google Scholar]
- 17.Herrmann J, Kaski JC, Lerman A. Coronary microvascular dysfunction in the clinical setting: from mystery to reality. Eur Heart J 2012;33:2771–2782b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson NP, Gould KL. Integrating noninvasive absolute flow, coronary flow reserve, and ischemic thresholds into a comprehensive map of physiological severity. JACC Cardiovasc Imaging 2012;5:430–40. [DOI] [PubMed] [Google Scholar]
- 19.Tsagalou EP, Anastasiou-Nana M, Agapitos E, Gika A, Drakos SG, Terrovitis JV, et al. Depressed coronary flow reserve is associated with decreased myocardial capillary density in patients with heart failure due to idiopathic dilated cardiomyopathy. J Am Coll Cardiol 2008;52:1391–8. [DOI] [PubMed] [Google Scholar]
- 20.Camici PG, Olivotto I, Rimoldi OE. The coronary circulation and blood flow in left ventricular hypertrophy. J Mol Cell Cardiol 2012;52:857–64. [DOI] [PubMed] [Google Scholar]
- 21.Cole RT, Kalogeropoulos AP, Georgiopoulou VV, Gheorghiade M, Quyyumi A, Yancy C, et al. Hydralazine and isosorbide dinitrate in heart failure: historical perspective, mechanisms, and future directions. Circulation 2011;123:2414–22. [DOI] [PubMed] [Google Scholar]
- 22.Gupta D, Georgiopoulou VV, Kalogeropoulos AP, Marti CN, Yancy CW, Gheorghiade M, et al. Nitrate therapy for heart failure: benefits and strategies to overcome tolerance. JACC Heart Fail 2013;1:183–91. [DOI] [PubMed] [Google Scholar]
- 23.Rijnierse MT, de Haan S, Harms HJ, Robbers LF, Wu L, Danad I, et al. Impaired hyperemic myocardial blood flow is associated with inducibility of ventricular arrhythmia in ischemic cardiomyopathy. Circ Cardiovasc Imaging 2014;7:20–30. [DOI] [PubMed] [Google Scholar]
- 24.Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka M, Ho K, et al. Long-term trends in incidence of and survival with heart failure. N Engl J Med 2002;347:1397–402. [DOI] [PubMed] [Google Scholar]
- 25.Solomon SD, Anavekar N, Skali H, McMurray JJV, Swedberg K, Yusuf S, et al. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation 2005;112:3738–44. [DOI] [PubMed] [Google Scholar]
- 26.Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol 2011;8:30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaarsma C, Leiner T, Bekkers SC, Crijns HJ, Wildberger JE, Nagel E, et al. Diagnostic performance of noninvasive myocardial perfusion imaging using single-photon emission computer tomography, cardiac magnetic resonance, and positron emission tomography imaging for the detection of obstruction coronary artery disease: a meta-analysis. J Am Coll Cardiol 2012;59:1719–28. [DOI] [PubMed] [Google Scholar]