Abstract
Monocytes fundamentally contribute to immune surveillance and the inflammatory response in immunoinflammatory diseases like atherosclerosis. Recruitment of these cells to the site of injury requires their trafficking across the blood vessel wall. A series of events, including capture, rolling, slow rolling, arrest, adhesion strengthening, and lateral locomotion, precede monocyte transmigration. Recent investigations have revealed new aspects of this cascade. This article revisits some conventional paradigms and selectively highlights new findings, including novel insights into monocyte differentiation and recently identified functional mediators, signalling pathways, and new structural aspects of monocyte extravasation. The emerging roles of endothelial junctional molecules like vascular endothelial-cadherin and the junctional adhesion molecule family, adhesion molecules such as intercellular adhesion molecule-1, molecules localized to the lateral border recycling compartment like cluster of differentiation 99, platelet/endothelial cell adhesion molecule-1, and poliovirus receptor (CD155), as well as other cell surface molecules such as cluster of differentiation 146 and ephrins in transendothelial migration are discussed.
Keywords: Monocyte migration, Monocyte subsets, Extravasation
1. Migration of monocytes into tissue
Monocytes play a pivotal role in tissue homeostasis, protective immunity, and both promotion and resolution of inflammation.1,2 They and their offspring, macrophages and dendritic cells, are essential for the innate and adaptive immune responses to pathogens. Monocytes exert many of their functions outside the vascular compartment; thus, trafficking and migration are required. Recruitment of blood monocytes to the site of injury or infection and their diapedesis through the endothelium (also called extravasation) are crucial events in early inflammation, followed by monocyte differentiation and subsequent downstream events of the inflammatory response.3,4
In atherosclerosis, monocytes show a profound inflammatory response that involves hypercholesterolemia-associated5 trafficking to inflamed arteries, mainly dependent on the chemokines CCL2 and CCL5, extravasation, subendothelial accumulation, and differentiation into macrophages. Eventually, these cells give rise to pathogenic lipid-laden foam cells.1,6,7
A tightly regulated, multistep process, consisting of a series of interactions between the immune and the endothelial cells, precedes the actual transmigration step. For monocytes, this process is essentially believed to follow the ‘cascade’ paradigm originally established for neutrophils, but some molecules are of specific importance in monocyte recruitment5 and will be particularly highlighted. A summary of the most important molecules and events in monocyte extravasation is provided in Table 1 and Figure 1.
Table 1.
Important molecules involved in monocyte trafficking across the vessel wall
| Class | Common name | Immunological name | Gene name (mouse) | Function | Main ligands | Expressed on | Number of clinical trials | Of which open | References |
|---|---|---|---|---|---|---|---|---|---|
| Integrins | LFA-1 | CD11a, CD18 | Itgal, Itgb2 | Patrolling, locomotion, arrest | ICAM-1, -2 | Monocytes | 39 | 8 | 8–10 |
| Mac-1 | CD11b, CD18 | Itgam, Itgb2 | Arrest, locomotion, transmigration, activation | Multiple | Monocytes | 575 | 180 | 10 | |
| VLA-4 | CD 49d, CD29 | Itga4, Itgb1 | Adhesion, transmigration | VCAM-1 | Monocytes | 9 | 4 | 10 | |
| Immunoglobulin superfamily | ICAM-1 | CD54 | Icam1 | Adhesion, transmigration | LFA-1 | EC | 119 | 25 | 10 |
| ICAM-2 | CD102 | Icam2 | Adhesion, transmigration | LFA-1 | EC | 81 | 13 | ||
| MCAM | CD146 | Mcam | Transmigration | EC | 38 | 16 | 11 | ||
| JAM-A | F11r | Transmigration | LFA-1, JAM-A | EC | 2 | 1 | 9 | ||
| JAM-B | |||||||||
| JAM-B | CD322 | Jam2 | Transmigration | VLA-4 | EC | 202 | 2 | 12 | |
| JAM-C | |||||||||
| Mac-1 | |||||||||
| JAM-C | Jam3 | Reverse transmigration | CAR | EC | 164 | 20 | 12,13 | ||
| JAML | Amica1 | Arrest, transmigration | VLA-4 | Monocytes | 116 | 19 | 14 | ||
| VCAM-1 | CD106C | Vcam1 | Arrest | PECAM-1 | EC | 93 | 20 | 10 | |
| PECAM-1 | D31 | Pecam1 | Transmigration | EC, monocytes | 7 | 2 | 15 | ||
| Poliovirus receptor | CD155C | Pvr | Transmigration | CD96, DNAM-1, TIGIT PILR |
EC, monocytes | 11 | 5 | 16 | |
| MIC2 | D99 | Cd99 | Transmigration | EC, monocytes | 115 | 49 | 17 | ||
| ICD99L2 | Cd99l2 | Transmigration | ? | EC, monocytes | 7 | 0 | 18 | ||
| Selectins | E- selectin | CD62E | Sele | Rolling | ESL-1 PSGL-1 CD44 |
EC | 78 | 21 | 19 |
| P- selectin | CD62P | Selp | Rolling, arrest | PSGL-1 | EC | 72 | 22 | 20 | |
| L-selectin | CD62L | Sell | Rolling, signalling | PSGL1, CD34 | Monocytes | 44 | 11 | 21 | |
| Chemokine receptors | CCR1 | CD191 | Ccr1 | Arrest | CCL5 | Monocytes | 249 | 71 | 22 |
| CCR5 | CD195 | Ccr5 | Spreading, arrest, transmigration | CCL5 | Monocytes | 226 | 83 | 23 | |
| CCR2 | CD192 | Ccr2 | Adhesion, transmgration | CCL2, -7, -12 | Monocytes | 277 | 97 | 24 | |
| Other | VE- cadherin | CD144 | Cdh5 | Transmigration | EC | 7 | 3 | 25 | |
| Eph A1, -2 | Epha1 | Transmigration | Ephrin-A ligands | Monocytes | 8 | 5 | 26 | ||
| Eph B1–4 | Ephb1 | Transmigration | Ephrin-B ligands | Monocytes | 17 | 5 | 26 |
Figure 1.
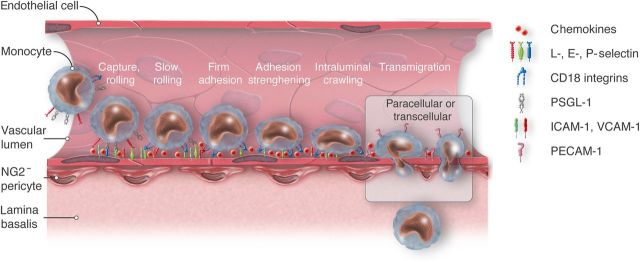
Schematic view of the monocyte adhesion cascade. ICAM-1, intercellular adhesion molecule-1; VCAM-1, vascular cell adhesion molecule-1; PECAM-1, platelet endothelial cell adhesion molecule; PSGL-1, P-selectin glycoprotein ligand-1; CD18 integrins, cluster of differentiation 18, beta subunit of integrins LFA-1 and Mac-1. A multistep cascade of capture, rolling, slow rolling, firm adhesion, adhesion strenghtening, and intraluminal crawling precedes the transendothelial migration of monocytes. Two modes of transmigration, a paracellular and a transcellular, can be distinguished. Activated endothelial cells express adhesion molecules and chemokines that interact with monocytic ligands. Such interactions essentially mediate the various steps of the adhesion cascade. This article focuses on how monocytes negotiate the endothelial monolayer.
Initially, inflammatory cytokines such as tumour necrosis factor-α or IL-1β, originating from tissue macrophages, transiently activate the endothelium. They induce the rapid expression of adhesion molecules, most importantly the adhesion molecules E- and P-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1), and the presentation of tethered chemokines on the luminal surface of the endothelium.27–29 Selectins interact with O-glycosylated carbohydrate ligands displayed on P-selectin glycoprotein ligand-1 (PSGL-1) and expressed on all monocytes. This interaction allows monocytes to roll on the endothelium and negotiate the high shear stress exerted by the blood flow in the vasculature. PSGL-1 is expressed at a significantly higher level by inflammatory Ly6Chi monocytes than by resident Ly6Clow monocytes (see classification given below and Table 2), which likely facilitates their adhesion to atherosclerotic lesions.20 Atherosclerosis is characterized by an up-regulation of endothelial adhesion molecules like E- and P-selectin and VCAM-1 in lesion-prone areas.40 Deficiency in P- and E-selectin as well as in ICAM-1 was shown to significantly reduce atherosclerotic lesion size in apoE−/− mice individually.41 In inflammatory conditions, monocyte rolling has also been shown to strongly depend on monocyte-expressed very late antigen-4 (VLA-4 α4β1 integrin)5,42 and CD44.6,43 VCAM-1 is an endothelial adhesion molecule that binds to VLA-4 and mediates slow rolling on cytokine-activated endothelium, thus facilitating the transition between rolling and firm arrest.44–46 Monocyte firm adhesion to the endothelium is coordinated by C-C and C-X-C chemokines such as CCL2 and IL-8.47–49 Monocytes can also use VLA-4 for firm adhesion.49 Blockage of VLA-4 in atherosclerosis-prone mice was shown to inhibit monocyte adhesion in carotid arteries.50 A recent study has found that the hormone resistin facilitates monocyte–endothelial cell adhesion by up-regulation of ICAM-1 and VCAM-1 on endothelial cells.51 Monocyte arrest is followed by a directional chemotactic and mechanotactic52 step, in which monocytes spread, polarize, and subsequently locomote laterally to find preferred sites of extravasation.53 This intraluminal crawling depends on the leucocyte integrins lymphocyte function-associated antigen-1 (LFA-1) and macrophage-1-antigen (Mac-1) as well as the endothelial ligands ICAM-1 and ICAM-2, as blocking of these adhesion molecules was shown to disable crawling and the subsequent transmigration. This step involves probing of the apical surface with membrane protrusions called lamellipodia.53,54 Note that this crawling is different from patrolling, which is strictly intravascular and does not lead to extravasation in steady state,32 as outlined below. Finally, to exit the vessel, monocytes must negotiate the endothelium, the lamina basalis and the embedded pericytes (Figure 1). Transmigration seems to be the crucial event in the monocyte adhesion cascade, as it is the only step that is hardly ever reversed.55
Table 2.
Nomenclature and features of blood monocytes
| % monocytes | Subset | Surface markers | Function | References | |
|---|---|---|---|---|---|
| Mouse | 50 | LY6Chi, inflammatory | LY6Chi CCR2+ Gr1+ | Pro-inflammatory, pathogen defence, phagocytosis | 30,31 |
| 50 | LY6Clow, patrolling | Ly6Clow CX3CR1+ Gr1−LFA1hi | Patrolling, promotion of tissue repair | 32–34 | |
| Human | 80–90 | Classical | CD14++CD16− CD62L+CCR2++ | Phagocytosis | 35 |
| 2–11 | Intermediate | CD14++CD16+ CCR2+CX3CR1++CCR5+ | Pro-inflammatory | 35–38 | |
| 10–20 | Non-classical patrolling | CD14+(CD14 dim) CD16++ CX3CR1++ | Local surveillance of tissue, antiviral | 35,39 |
+ = approximately 10-fold increase in surface expression compared with isotype control; ++ = approximately 100-fold increase in surface expression compared with isotype control.
2. Models and methods employed to elucidate monocyte transmigration
The recent past has seen major progress in determining the molecular pathways associated with monocyte transendothelial migration. Blocking antibodies that arrest monocytes at different stages of diapedesis have generated valuable insights into the spatial and temporal regulation of this complex process. Additionally, function-blocking antibodies directed against signalling molecules and receptors have been used to identify different players involved in migration to the site of inflammation and extravasation.56,57 Among others, blocking antibodies against platelet/endothelial cell adhesion molecule-1 (PECAM-1)58 and cluster of differentiation 99 (CD99)17 were used in vitro and subsequently validated in vivo, as discussed below. Inhibiting antibodies also constitute a potent therapeutic approach to treating inflammatory conditions. In addition to impeding transmigration with antibodies, tissue-specific conditional knockout and inducible and cell type specific gene knockout and knockin animals have proved to be powerful tools for uncovering the intricacies of transmigration in vivo. More recently, microscopy-based approaches for live cell visualization have led to an expanded definition of the leucocyte adhesion cascade42,59,60 and have greatly enhanced the understanding of dynamic changes during transmigration. Among others, intravital real-time imaging,61 multiphoton and confocal laser scanning microscopy,62 time-lapse video microscopy,63 and electron micoscropy64 were employed to meticulously study the extravasation process in vitro and in vivo.
3. Monocyte heterogeneity and homeostasis
The understanding of monocyte ontogeny and differentiation has changed significantly during the past few years. Monocytes and their descendants constitute a highly complex and dynamic cellular system. Blood monocytes account for 4 and 10% of the circulating leucocytes in mice and humans, respectively.3 This review will highlight some key aspects of the recent findings, but does not claim to be comprehensive. References [2,3] and [45] offer deeper insights into functions and the development of monocytes.
Monocytes are grouped into subsets on the basis of their location, phenotype and function, as well as by characteristic chemokine receptor expression and the presence of specific surface molecules1,4,65 (Table 2). They arise from haematopoietic stem cells in the bone marrow via a linage-negative (LIN−), CD117+, CD135+ clonotypic monocyte and dendritic cell precursor (MDP) founder cell, in a macrophage colony-stimulating factor receptor (MCSF-R, CD115)-dependent manner.3 Emerging data suggest the existence of a developmentally committed clonogenic, monocyte-, and macrophage-specific progenitor downstream from MDP.66 The largest human monocyte subpopulation expresses high levels of the LPS co-receptor CD14 (CD14++). These monocytes are called classical monocytes, express CCR2, and produce IL-10 in response to LPS. CD14+, CD16 (low-affinity IgG receptor)++ non-classical monocytes lack CCR2, but express high levels of CX3CR1. A small intermediate group with high CD14 and moderate CD16 expression67 (CD14++CD16− intermediate subset in Table 2) has recently been reported to not merely be a transitory state as previously assumed, but also to possess unique features and differ from traditional subsets inter alia with respect to inflammatory cytokine secretion.68
The exact correspondence between mouse and human monocyte subsets is subject of debate. There is evidence that suggests that Ly6Clow monocytes may be equated to the CD14+CD16++ non-classical human monocytes, based on gene expression profiles.69,70 Similarly, the Ly6Chi subset seems to correspond to the human CD14++CD16− classical subgroup. Although data comparisons and lineage tracing studies between the potentially matching subsets show overall correspondence, significant differences, both, in subset-specific gene expression and functional behaviour remain, thus forbidding direct translation between murine and human subpopulations. Reference [69] offers a comprehensive review on the correlation of monocyte subsets within a spectrum of species. Some have proposed that Ly6Chi cells can give rise to a Ly6ClowCX3CR1+ subset, but this is highly controversial.4,45,71,72
Ly6Clow mouse monocytes and CD14dim human monocytes constitutively crawl on the luminal side of the non-inflamed endothelium (‘patrolling monocytes’). They are thought to scavenge microparticles and debris from the endothelial surface in a Toll-like receptor 7 (TLR7)-dependent manner.32,33 The differentiation and survival of such patrolling monocytes critically depends on the nuclear receptor Nur77 (Nr4a1).73 Interaction of the integrin LFA1 with its endothelial ligands ICAM-1 and -2 mediates patrolling of Ly6Clow monocytes on the endothelium.33 In resting tissue, <1% of all patrolling monocytes cross the endothelium.32 Interestingly, in the event of tissue damage, Ly6Clow monocytes rapidly extravasate and transiently produce inflammatory cytokines, thus initiating a very early innate response.3,32 The role of monocyte subsets in vascular remodelling is unknown. As little is known about molecules, pathways, and signals specifically involved in transmigration of patrolling monocytes, the emphasis of this review lies on extravasation of Ly6Chi monocytes in an inflammatory setting.
In pathogen-challenged mice, numbers of circulating LY6Chi monocytes, often referred to as inflammatory monocytes, are drastically increased and selectively populate sites of inflammation,30 reviewed in Shi and Pamer45. Their recruitment from the bone marrow occurs preferentially via interaction of CCL2 and CCL7 with the chemokine receptor CCR2.74 Swirski et al.75 reported a 14-fold increase of the Ly6Chi subset in the setting of late atherosclerosis. At the focus of inflammation, monocytes extravasate and may differentiate into macrophages and dendritic cells. The early dogma that all tissue-resident macrophages derive from monocytes,4,76 however, needs to be reformulated. Studies have recently provided evidence that in some locations, monocytes do not substantially contribute to the tissue macrophages at steady state.2,77,78 Compelling lineage analyses have shown the existence of macrophages that derive from embryonic pre-haematopoietic precursors, persist to adulthood, and maintain themselves by self-renewal,78–80 thoroughly independent of monocyte replenishment. In fact, recent studies suggest that recruited endogenous monocytes do extravasate, but may not imperatively differentiate into macrophages or dendritic cells. Instead, these monocytes serve as surveillance and effector cells and migrate to lymph nodes.80 Blood monocyte contribution to the tissue macrophage compartment seems to be largely limited to inflammatory settings. However, there is strong variation between tissues: while there is evidence that the pool of resident macrophages of the CNS, the microglia, originates from primitive myeloid precursors,78,81 and the pool of intestinal macrophages crucially requires CCR2-dependent influx of Ly6Chi monocytes.82 Other tissues show a mixture of both early seeding with self-renewal and monocyte-derived recruitment. An interesting recent study proposes a third model of macrophage provenance, providing evidence that lung alveolar macrophages perinatally derive from fetal monocytes, followed by self-maintenance throughout life.83
In macrophages, two main activation states represent the extremes of a spectrum of possible functional polarizations. In these two well-described conditions, macrophages exert opposing functions. M1 macrophages have been described to play a pro-inflammatory role,84 whereas a wound healing role has been attributed to M2 macrophages.85,86 Emerging evidence suggests that the macrophage activation status depends on several variables, such as location, cytokines, and other microenvironmental cues.87–89 For example, immediately after cardiac ischaemia, the damaged myocardium recruits Ly6Chi monocytes that give rise to an abundance of inflammatory M1 macrophages.90 References [34,91] and [92] suggest that in the following reparative phase, the predominant macrophage phenotype in the ischaemic tissue changes towards an alternatively activated state, clearly expressing M2-macrophage-associated genes.
4. Two modes of monocyte transmigration across the vascular endothelium
The thin monolayer of endothelial cells (cell thickness 0.1 in the periphery to 1 µm over the nucleus) constitutes the primary physical barrier between blood and tissue. Endothelial cells have no true tight junctions. Adherens junctions between the cells and integrin and cadherin anchors to the basement membrane (BM) form a network that tightly regulates vascular homeostasis and restrains leucocyte transendothelial migration.93 In addition to the predominant paracellular route that leads monocytes through the junctions between endothelial cells and requires junctional remodelling, a different route directly through fusing vesicles in the endothelial cell cytoplasm constitutes an established mode of monocyte transmigration62,94–96 (Figure 1). Under most circumstances, monocytes choose transcellular migration in only 10–30% of events.96,97 Strong activating stimuli are thought to increase overall occurrence of transcellular transendothelial migration in monocytes.96,97 Live cell visualization in vitro62 and live imaging through confocal intravital microscopy95 have greatly advanced our understanding in this field. However, it remains difficult to accurately quantify paracellular vs. transcellular migration, because light microscopy cannot clearly determine whether a cell is migrating through a junction or merely very close to it.98
5. VE-cadherin as a gatekeeper of transendothelial migration
The control of endothelial junctions constitutes a central event in determining the time and location of leucocyte extravasation. For paracellular transmigration, signals generated by adherent monocytes dissociate the adherens junctions that create cell contact integrity at endothelial cell–cell borders.25,99 One essential stabilizing component of adherens junctions is the membrane glycoprotein vascular endothelial-cadherin (VE-cadherin), linked to the actin cytoskeleton through α-catenin. Drugs disrupting this interaction drastically increase vascular permeability.100 Recently, the Vestweber group elegantly showed the VE-cadherin complex to be of dominant importance to transendothelial migration by replacing VE-cadherin with a VE-cadherin–α-catenin fusion protein, thus strongly inhibiting leucocyte extravasation.101 The endothelial-specific vascular endothelial tyrosine phosphatase associates with VE-cadherin and regulates its function by dephosphorylation. Monocyte adhesion to activated endothelial cells triggers the disruption of this association and has been shown to be critical for paracellular monocyte diapedesis.102 Non-receptor protein tyrosine kinases Src- and Pyk2-dependent phosphorylation of VE-cadherin dissociates its binding to α-catenin, thus removing it from the junction.103 Alterations in endothelial junctional structure during transmigration of monocytes were visualized in real-time in vitro,104 and ICAM-1 signalling was shown to affect VE-cadherin rearrangement to accommodate monocyte transmigration.105 Monocyte transendothelial migration may augment subsequent transmigratory activity by decreasing VE-cadherin expression.106
6. JAM family
The junctional adhesion molecule (JAM) family consists of six immunoglobulin-like proteins, JAM-A, JAM-B, JAM-C, JAM-4, ESAM, and CAR.12 They are expressed on endothelial cells and can be expressed on leucocytes. JAMs and JAM multimers mainly interact with each other and with integrin counter-receptors, for instance JAM-A binds LFA-19 and JAM-C interacts with Mac-1.107 JAM-C is specifically required to prevent reverse transmigration of monocytes back into the vascular lumen and provides regulated and polarized monocyte transmigration.13 In this study, anti-JAM-C antibodies significantly reduced monocytes in inflamed tissue, but increased the number of not transmigrated monocytes with a reverse-transmigratory phenotype. These monocytes show abluminal to luminal migration after primary extravasation, due to impaired JAM-C activity.13 Recently, a study revealed JAM-A to be of significant importance for arterial monocyte recruitment in the context of diet-induced atherosclerosis. Here, it was shown that the same number of monocytes adhered to JAM-A-deficient endothelial monolayers as to JAM-A-bearing endothelial cells, but the number of monocytes that transmigrated beneath the monolayer was markedly reduced in the JAM-A-deficient model.108 JAM-like protein (JAM-L) is closely related to the JAM family and is restricted to leucocytes, mainly monocytes and granulocytes. Under inflammatory conditions, monocytic JAM-L expression is up-regulated, and JAM-L and its endothelial counter-receptor CAR play a critical role in mediating monocyte migration across the endothelium in vitro. In this process, VLA-4 facilitates JAM-L-receptor binding.14,109
7. Other junctional molecules
The type 1 membrane glycoprotein PECAM-1 has long been known to be crucial for the diapedesis of monocytes, as monoclonal antibodies to PECAM-1 block monocyte transmigration by 70–90% in some models.110 Such antibodies have been demonstrated to attenuate inflammation in several in vivo models.58,111–113 PECAM-1 deficiency in ApoE double-KO mice (ApoE−/−/PECAM-1−/−) was found to reduce atherosclerotic plaque burden in some areas of the aorta.114–116
Recently, poliovirus receptor (PVR, CD155), located among others at the endothelial borders and on monocytes,117 was identified as a regulator of monocyte extravasation that acts downstream of PECAM-1. Antibody blockage of certain transmigration steps is reversible by thorough washing. The group showed that resumed extravasation of isolated adherent monocytes released from anti-PECAM-1 blockage could still be arrested by anti-PVR or antibodies against its monocyte ligand DNAX accessory molecule-1 (DNAM-1) in vitro. The reverse (arresting diapedesis by anti-PECAM-1 after previous blockage with anti-PVR) was not possible, suggesting that monocytes have already passed the PECAM-1-dependent step when secondly arrested by anti-PVR.118
Antibodies directed against two other type 1 membrane proteins, CD99 and CD99L2,18 impair monocyte influx into the site of inflammation to an equal, if not greater degree in vitro56 as well as in vivo,17 compared with anti-PECAM-1. However, while anti-PECAM-1 and anti-CD155 antibodies arrest adherent monocytes on the apical surface of the endothelial cells, anti-CD99 and anti-CD99L2 arrest monocytes partway through the endothelial junction.56 This indicates that CD99 and CD99L2 both act downstream of CD155 and PECAM-1.
PECAM-1, CD155, CD99, and CD99L2 all participate in the extravasation process but not in capture or adhesion to the blood vessel wall, as revealed by intravital microscopy. PECAM-1, CD99, and CD99L2 are enriched at endothelial cell junctions and expressed diffusely on the monocyte surface, and facilitate transmigration by homophilic binding between the molecules on the two cell types, respectively.14,118
8. The lateral border recycling compartment
Mamdouh and Muller recently discovered119 in in vitro studies that approximately one-third of PECAM-1 and considerable amounts of CD99, CD155,118 JAM-A, and other molecules involved in transendothelial migration, but not in VE-cadherin, reside in an subjunctional, intracellular endothelial membrane reticulum, forming a ‘transmigration complex’ that is actively transported to the site of diapedesis by kinesin-microtubule motors and surrounds the transmigrating cell120 (Figure 2). This membrane reticulum has been named the lateral border recycling compartment (LBRC).121 The 50 nm vesicle-like structures are connected with each other and the endothelial cell border. In resting endothelial cells, there is an incessant flux of membrane between the junction and the LBRC. Homophilic interaction between PECAM on endothelial cells and PECAM on monocytes, however, triggers extensive and rapid targeted recycling of PECAM-bearing membrane to the site of transmigration.119 Thus, the migrating cell is supplied with functional molecules required for it to be able to transmigrate. Inhibition of targeted recycling, for instance by depolymerization of microtubules or disruption of PECAM–PECAM interaction, blocks monocyte extravasation by over 85%,121 arresting adherent monocytes above the cell junctions, unable to initiate transmigration. These findings suggest a critical role for the LBRC and targeted recycling in transendothelial migration. The accumulation of adhesion molecules around the migrating cell is observed not only in paracellular, but the LBRC is also recruited to mediate transcellular migration and seems to fulfil nearly identical rules in the two crossing routes.120 However, suitable markers to study the LBRC in vivo remain to be identified;121 thus, the role of the LBRC in vivo is unknown.
Figure 2.
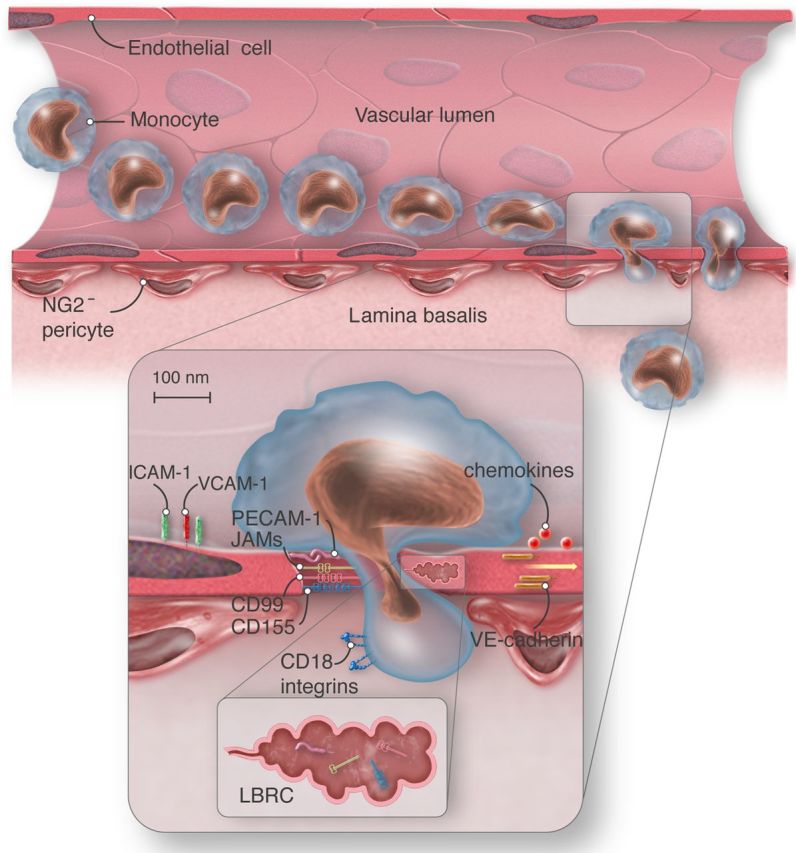
Schematic view of paracellular monocyte transmigration. ICAM-1, intercellular adhesion molecule-1; PECAM-1, platelet endothelial cell adhesion molecule; CD99, cluster of differentiation 99; CD155, cluster of differentiation 155; VE-cadherin, vascular endothelial-cadherin; CD18 integrins, cluster of differentiation 18, beta subunit of integrins LFA-1 and Mac-1; JAMs, junctional adhesion molecules; LBRC, lateral border recycling compartment; NG2−, neuron-glial-2 negative. Vascular endothelial cell, red; monocyte, light blue; pericytes, red-brown. Monocyte extravasation into the site of inflammation requires transendothelial migration and penetration of the lamina basalis NG2− pericytes. Monocytes transmigrate at sites of low matrix protein density. Monocyte engagement of endothelial cell surface molecules activates targeted recycling of the LBCR and thus enlarges the transmigration gap. The LBRC (brown) contains PECAM-1, CD99, CD155, and JAM-A, but not VE-cadherin, and supplies the transmigrating monocyte with additional functional molecules. VE-cadherin stabilizes junctional integrity in steady-state and transiently abandons site of transmigration under inflammatory conditions.
9. Transmigratory cups and endothelial calcium signalling
To initiate cytoskeletal reorganization and facilitate transmigration, VCAM-1, ICAM-1, and E-selectin form clusters, employing their leucocyte integrin counter-receptors.122,123 The clusters, also called docking structures, initiate activation of Src, Rac-1, and RhoA and cause an increase of cytosolic-free calcium in endothelial cells. The resulting activation of Ca2+/calmodulin-dependent myosin light chain kinase (MLCK) and phosphorylation of VE-cadherin are crucial to release the associated catenins, loosen the junctions, and allow diapedesis.124,125 Studies in monocytes, lymphocytes, and neutrophils revealed these clusters to frequently be located on upright endothelial membrane processes and organized to so-called transmigratory cups by tetraspanins.123 These structures seem to be generated as a response to endothelial cell binding to LFA-1 and VLA-4 and to partially envelop the leucocytes during extravasation. Here, VCAM-1 and ICAM-1 also interact directly with the ezrin/radixin/moesin complex, which serves as a cytoskeletal linker and a signal transducer in cytoskeletal remodelling.122 The transmigratory cups play a guiding role in paracellular, as well as in transcellular, extravasation. Under certain in vitro conditions, 96% of transmigrating monocytes were observed to be associated with such ICAM-1-enriched projections.96
10. Metalloproteinases
Broad spectrum inhibition of metalloproteinase activity almost doubles the duration of the diapedesis step in monocytes. This delayed transmigration is accompanied by significant elevation of monocyte surface integrin Mac-1.63,126
11. Cluster of differentiation 146 (MCAM)
Cluster of differentiation 146 (MCAM, CD146) is a member of the immunoglobulin superfamily and is, among others, strongly expressed on blood vessel endothelium. The inflammatory cytokine TNF increases CD146 expression and triggers metalloproteinase-dependent release of soluble CD146. By the use of blocking antibodies and small-interfering RNA (siRNA), both soluble and tethered CD146 were reported to be crucial in monocyte trafficking across the endothelium. However, the mechanism by which this important role is exerted and a potential monocytic counter-receptor for CD146 remain to be discovered.11
12. LIM domain binding 2
A recent genetic study provided compelling evidence for the involvement of the transcriptional regulator LIM domain binding 2 (LDB2) in the extravasation of monocytes in atherosclerosis.127 The authors found that this co-transcription factor, which is present in mouse and human atherosclerotic lesions, regulates many genes associated with monocyte transendothelial migration in atherosclerosis. LDB2 deficiency leads to increased monocyte migration in vitro, responsible for significantly larger lesion size in ldb2−/−ldlr−/−apoB100/100 mice. In younger, atherosclerosis-free ldb2−/− mice, 40 genes were differentially expressed, as assessed by transcriptional profiling.127 Among them, a considerable up-regulation of the adhesion molecule VCAM-1 became apparent in ldb2−/− monocytes as well as the arterial wall.
13. Ephrins and netrin-1
The receptor protein tyrosin kinase family of eph receptors and their ligands ephrins was recently implicated in monocyte activation and migration into inflamed tissue and the development of atherosclerosis. Monocytes among others express EphA1 and 2 and EphB1–4 receptors.26 EphrinB1 and its cognate receptor, Eph receptor B2,128 as well as EphrinA1 and EphA4,129 are expressed on arterial and some venous endothelial cells and in the atherosclerotic plaque. It was demonstrated that ephrin-A1-induced EphA4 forward signalling in endothelial cells increases monocyte adhesion129 and ephrinA1-dependent activation of the EphA2 receptor influences the expression of VCAM-1.130 Monocytes were activated by interaction with EphrinB2.131 Through EphB4 forward signalling and ephrin B2 reverse signalling, Ephrin B2–Eph B interaction was shown to influence monocyte adhesion and transendothelial migration.132 Netrin-1 is crucial in retaining monocytes and macrophages, preventing them from leaving atherosclerotic lesions.133
14. Transcriptional changes in monocytes driven by transmigration
It has been established that the gene expression profile of trafficking monocytes is altered dramatically between constitutive and inflammatory conditions.134 Adhesion of monocytes to endothelial cells triggers regulation of extravasation-specific genes and initiates changes towards a more differentiated phenotype.135 Following monocyte diapedesis across activated endothelial cells, 489 genes have been shown to be up- and 203 to be down-regulated in monocytes. Among the highly up-regulated genes were MCP-1 (CCL2) and -3 (CCL7), both chemokines that attract monocytes, suggesting that monocytes may recruit other monocytes to the site of diapedesis.135 Other up-regulated genes identified are mainly involved in the immune response and inhibition of apoptosis.136 Taken together, these findings indicate that transmigration promotes further recruitment of monocytes and inhibits apoptosis, but additional stimuli are necessary for differentiation into macrophage or dendritic cell phenotypes.
15. Migration beyond the endothelium
After successfully overcoming the endothelium, monocytes are confronted with the vascular BM and encounter the pericytes that discontinuously wrap around the microvasculature. The non-cellular matrix of the BM constitutes a tight mesh-like network, formed mainly by collagen IV and laminins, interconnected by glycoproteins. In 2009, the Nourshargh group provided evidence that monocytes preferentially negotiate the BM at permissive regions of low matrix protein density by changing their shape and squeezing through small pre-existing gaps,137 reviewed in Nourshargh et al.138 Pericytes and the BM may have an active role in the innate immune response. Neuron-glial-2 negative (NG2−) pericytes line postcapillary venules (Figure 2), whereas NG2+ pericytes reside in arterioles and capillaries. Monocytes first encounter NG2− pericytes and crawl along them. In response to inflammatory stimuli, NG2+ pericytes secrete chemoattractants and express ICAM-1, thus attracting the transmigrated cells.139 Discoidin domain receptor 1a (DDR1a), a surface receptor that has only been detected on leucocytes once they reach the extravascular space, seems to be involved in monocyte migration beyond the endothelium. Studies show that DDR1a interaction with collagen leads to shape changes that enable monocytes to travel through the tight collagen network of the BM.140
16. Concluding remarks
The abundance of molecules and pathways implicated in the migration of monocytes out of the blood stream (summarized in Table 1) is of clear translational potential. Thousands of clinical trials have been conducted on the molecules listed (see Table 1, right two columns). A humanized mAb against the alpha4 subunit of VLA-4, natalizumab, is FDA-approved for the treatment of multiple sclerosis and Crohn's disease.141 In 2014, the integrin α4β7 mAb vedolizumab was introduced to the market as an inhibitor of inflammatory cell influx selectively into gut mucosal tissue in the setting of ulcerative colitis and Crohn's disease.142,143 However, none of these treatments specifically inhibits the transmigration of monocytes; for example, α4β1 is also expressed on activated lymphocytes, endothelial cells, and other cells, and α4β7 is also expressed by activated lymphocytes. In addition to drugs, imaging agents and diagnostic biomarkers are also under development.
Funding
T.G. is supported by a Boehringer Ingelheim Fonds MD fellowship. K.L. is supported by NIH HL 115232, HL 055798, HL 118765, HL 112276, HL 088093, HL 126543, HL 121697, and DK 091222.
Acknowledgments
Conflict of interest: none declared.
References
- 1.Yona S, Jung S. Monocytes: subsets, origins, fates and functions. Curr Opin Hematol 2010;17:53–59. [DOI] [PubMed] [Google Scholar]
- 2.Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol 2014;14:392–404. [DOI] [PubMed] [Google Scholar]
- 3.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol 2009;27:669–692. [DOI] [PubMed] [Google Scholar]
- 4.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science 2010;327:656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mestas J, Ley K. Monocyte-endothelial cell interactions in the development of atherosclerosis. Trends Cardiovasc Med 2008;18:228–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carman CV. Teasing out monocyte trafficking mechanisms. Blood 2008;112:929–930. [DOI] [PubMed] [Google Scholar]
- 7.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol 2010;7:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sumagin R, Prizant H, Lomakina E, Waugh RE, Sarelius IH. LFA-1 and Mac-1 define characteristically different intralumenal crawling and emigration patterns for monocytes and neutrophils in situ. J Immunol 2010;185:7057–7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ostermann G, Weber KS, Zernecke A, Schroder A, Weber C. JAM-1 is a ligand of the beta(2) integrin LFA-1 involved in transendothelial migration of leukocytes. Nat Immunol 2002;3:151–158. [DOI] [PubMed] [Google Scholar]
- 10.Meerschaert J, Furie MB. The adhesion molecules used by monocytes for migration across endothelium include CD11a/CD18, CD11b/CD18, and VLA-4 on monocytes and ICAM-1, VCAM-1, and other ligands on endothelium. J Immunol 1995;154:4099–4112. [PubMed] [Google Scholar]
- 11.Bardin N, Blot-Chabaud M, Despoix N, Kebir A, Harhouri K, Arsanto JP, Espinosa L, Perrin P, Robert S, Vely F, Sabatier F, Le Bivic A, Kaplanski G, Sampol J, Dignat-George F. CD146 and its soluble form regulate monocyte transendothelial migration. Arterioscler Thromb Vasc Biol 2009;29:746–753. [DOI] [PubMed] [Google Scholar]
- 12.Bazzoni G. The JAM family of junctional adhesion molecules. Curr Opin Cell Biol 2003;15:525–530. [DOI] [PubMed] [Google Scholar]
- 13.Bradfield PF, Scheiermann C, Nourshargh S, Ody C, Luscinskas FW, Rainger GE, Nash GB, Miljkovic-Licina M, Aurrand-Lions M, Imhof BA. JAM-C regulates unidirectional monocyte transendothelial migration in inflammation. Blood 2007;110:2545–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luissint AC, Lutz PG, Calderwood DA, Couraud PO, Bourdoulous S. JAM-L-mediated leukocyte adhesion to endothelial cells is regulated in cis by alpha4beta1 integrin activation. J Cell Biol 2008;183:1159–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newman PJ, Newman DK. Signal transduction pathways mediated by PECAM-1: new roles for an old molecule in platelet and vascular cell biology. Arterioscler Thromb Vasc Biol 2003;23:953–964. [DOI] [PubMed] [Google Scholar]
- 16.Bernhardt G. TACTILE becomes tangible: CD96 discloses its inhibitory peculiarities. Nat Immunol 2014;15:406–408. [DOI] [PubMed] [Google Scholar]
- 17.Dufour EM, Deroche A, Bae Y, Muller WA. CD99 is essential for leukocyte diapedesis in vivo. Cell Commun Adhes 2008;15:351–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schenkel AR, Dufour EM, Chew TW, Sorg E, Muller WA. The murine CD99-related molecule CD99-like 2 (CD99L2) is an adhesion molecule involved in the inflammatory response. Cell Commun Adhes 2007;14:227–237. [DOI] [PubMed] [Google Scholar]
- 19.Zarbock A, Ley K, McEver RP, Hidalgo A. Leukocyte ligands for endothelial selectins: specialized glycoconjugates that mediate rolling and signaling under flow. Blood 2011;118:6743–6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.An G, Wang H, Tang R, Yago T, McDaniel JM, McGee S, Huo Y, Xia L. P-selectin glycoprotein ligand-1 is highly expressed on Ly-6Chi monocytes and a major determinant for Ly-6Chi monocyte recruitment to sites of atherosclerosis in mice. Circulation 2008;117:3227–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosen SD. Ligands for L-selectin: homing, inflammation, and beyond. Annu Rev Immunol 2004;22:129–156. [DOI] [PubMed] [Google Scholar]
- 22.Weber C, Weber KSC, Klier C, Gu S, Wank R, Horuk R, Nelson PJ. Specialized roles of the chemokine receptors CCR1 and CCR5 in the recruitment of monocytes and TH1-like/CD45RO+T cells. Blood 2001;97:1144–1146. [DOI] [PubMed] [Google Scholar]
- 23.Kamei M, Carman CV. New observations on the trafficking and diapedesis of monocytes. Curr Opin Hematol 2010;17:43–52. [DOI] [PubMed] [Google Scholar]
- 24.Kuziel WA, Morgan SJ, Dawson TC, Griffin S, Smithies O, Ley K, Maeda N. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc Natl Acad Sci 1997;94:12053–12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giannotta M, Trani M, Dejana E. VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev Cell 2013;26:441–454. [DOI] [PubMed] [Google Scholar]
- 26.Sakamoto A, Sugamoto Y, Tokunaga Y, Yoshimuta T, Hayashi K, Konno T, Kawashiri M, Takeda Y, Yamagishi M. Expression profiling of the Ephrin (EFN) and Eph receptor (EPH) family of genes in atherosclerosis-related human cells. J Int Med Res 2011;39:522–527. [DOI] [PubMed] [Google Scholar]
- 27.Leon B, Ardavin C. Monocyte migration to inflamed skin and lymph nodes is differentially controlled by L-selectin and PSGL-1. Blood 2008;111:3126–3130. [DOI] [PubMed] [Google Scholar]
- 28.Imhof BA, Aurrand-Lions M. Adhesion mechanisms regulating the migration of monocytes. Nat Rev Immunol 2004;4:432–444. [DOI] [PubMed] [Google Scholar]
- 29.Min JK, Kim YM, Kim SW, Kwon MC, Kong YY, Hwang IK, Won MH, Rho J, Kwon YG. TNF-related activation-induced cytokine enhances leukocyte adhesiveness: induction of ICAM-1 and VCAM-1 via TNF receptor-associated factor and protein kinase C-dependent NF- B activation in endothelial cells. J Immunol 2005;175:531–540. [DOI] [PubMed] [Google Scholar]
- 30.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 2003;19:71–82. [DOI] [PubMed] [Google Scholar]
- 31.Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol 2008;26:421–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 2007;317:666–670. [DOI] [PubMed] [Google Scholar]
- 33.Carlin LM, Stamatiades EG, Auffray C, Hanna RN, Glover L, Vizcay-Barrena G, Hedrick CC, Cook HT, Diebold S, Geissmann F. Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell 2013;153:362–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 2007;204:3037–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong KL, Tai JJ, Wong WC, Han H, Sem X, Yeap WH, Kourilsky P, Wong SC. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood 2011;118:e16–e31. [DOI] [PubMed] [Google Scholar]
- 36.Merino A, Buendia P, Martin-Malo A, Aljama P, Ramirez R, Carracedo J. Senescent CD14+CD16+ monocytes exhibit proinflammatory and proatherosclerotic activity. J Immunol 2011;186:1809–1815. [DOI] [PubMed] [Google Scholar]
- 37.Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol 2007;81:584–592. [DOI] [PubMed] [Google Scholar]
- 38.Yang J, Zhang L, Yu C, Yang X-F, Wang H. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark Res 2014;2:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, Puel A, Biswas SK, Moshous D, Picard C, Jais JP, D'Cruz D, Casanova JL, Trouillet C, Geissmann F. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity 2010;33:375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakashima Y, Raines EW, Plump AS, Breslow JL, Ross R. Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the ApoE-deficient mouse. Arterioscler Thromb Vasc Biol 1998;18:842–851. [DOI] [PubMed] [Google Scholar]
- 41.Collins RG, Velji R, Guevara NV, Hicks MJ, Chan L, Beaudet AL. P-selectin or intercellular adhesion molecule (ICAM)-1 deficiency substantially protects against atherosclerosis in apolipoprotein E-deficient mice. J Exp Med 2000;191:189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 2007;7:678–689. [DOI] [PubMed] [Google Scholar]
- 43.Xu H, Manivannan A, Crane I, Dawson R, Liversidge J. Critical but divergent roles for CD62L and CD44 in directing blood monocyte trafficking in vivo during inflammation. Blood 2008;112:1166–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zarbock A, Abram CL, Hundt M, Altman A, Lowell CA, Ley K. PSGL-1 engagement by E-selectin signals through Src kinase Fgr and ITAM adapters DAP12 and FcR gamma to induce slow leukocyte rolling. J Exp Med 2008;205:2339–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol 2011;11:762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmitz B, Vischer P, Brand E, Schmidt-Petersen K, Korb-Pap A, Guske K, Nedele J, Schelleckes M, Hillen J, Rotrige A, Simmet T, Paul M, Cambien F, Brand SM. Increased monocyte adhesion by endothelial expression of VCAM-1 missense variation in vitro. Atherosclerosis 2013;230:185–190. [DOI] [PubMed] [Google Scholar]
- 47.Gerszten RE, Garcia-Zepeda EA, Lim YC, Yoshida M, Ding HA, Gimbrone MA, Jr, Luster AD, Luscinskas FW, Rosenzweig A. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature 1999;398:718–723. [DOI] [PubMed] [Google Scholar]
- 48.Luscinskas FW, Gerszten RE, Garcia-Zepeda EA, Lim YC, Yoshida M, Ding HA, Gimbrone MA, Jr, Luster AD, Rosenzweig A. C-C and C-X-C chemokines trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Ann N Y Acad Sci 2000;902:288–293. [DOI] [PubMed] [Google Scholar]
- 49.Huo Y, Weber C, Forlow SB, Sperandio M, Thatte J, Mack M, Jung S, Littman DR, Ley K. The chemokine KC, but not monocyte chemoattractant protein-1, triggers monocyte arrest on early atherosclerotic endothelium. J Clin Invest 2001;108:1307–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huo Y, Hafezi-Moghadam A, Ley K. Role of vascular cell adhesion molecule-1 and fibronectin connecting segment-1 in monocyte rolling and adhesion on early atherosclerotic lesions. Circ Res 2000;87:153–159. [DOI] [PubMed] [Google Scholar]
- 51.Hsu WY, Chao YW, Tsai YL, Lien CC, Chang CF, Deng MC, Ho LT, Kwok CF, Juan CC. Resistin induces monocyte-endothelial cell adhesion by increasing ICAM-1 and VCAM-1 expression in endothelial cells via p38MAPK-dependent pathway. J Cell Physiol 2011;226:2181–2188. [DOI] [PubMed] [Google Scholar]
- 52.Phillipson M, Heit B, Parsons SA, Petri B, Mullaly SC, Colarusso P, Gower RM, Neely G, Simon SI, Kubes P. Vav1 is essential for mechanotactic crawling and migration of neutrophils out of the inflamed microvasculature. J Immunol 2009;182:6870–6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schenkel AR, Mamdouh Z, Muller WA. Locomotion of monocytes on endothelium is a critical step during extravasation. Nat Immunol 2004;5:393–400. [DOI] [PubMed] [Google Scholar]
- 54.Wong CH, Heit B, Kubes P. Molecular regulators of leucocyte chemotaxis during inflammation. Cardiovasc Res 2010;86:183–191. [DOI] [PubMed] [Google Scholar]
- 55.Muller W. PECAM: regulating the start of diapedesis. In: Ley K, ed. Adhesion molecules: function and inhibition. Birkhäuser, Basel, 2007; p. 201–220. [Google Scholar]
- 56.Schenkel AR, Mamdouh Z, Chen X, Liebman RM, Muller WA. CD99 plays a major role in the migration of monocytes through endothelial junctions. Nat Immunol 2002;3:143–150. [DOI] [PubMed] [Google Scholar]
- 57.Petri B, Bixel MG. Molecular events during leukocyte diapedesis. FEBS J 2006;273:4399–4407. [DOI] [PubMed] [Google Scholar]
- 58.Bogen S, Pak J, Garifallou M, Deng X, Muller WA. Monoclonal antibody to murine PECAM-1 (CD31) blocks acute inflammation in vivo. J Exp Med 1994;179:1059–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chavakis E, Choi EY, Chavakis T. Novel aspects in the regulation of the leukocyte adhesion cascade. Thromb Haemost 2009;102:191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muller WA. Getting leukocytes to the site of inflammation. Vet Pathol 2013;50:7–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zarbock A, Ley K. New insights into leukocyte recruitment by intravital microscopy. In: Dustin M, McGavern D, eds. Visualizing immunity. Springer Berlin Heidelberg, 2009; p. 129–152. [DOI] [PubMed] [Google Scholar]
- 62.Hashimoto K, Kataoka N, Nakamura E, Hagihara K, Okamoto T, Kanouchi H, Mohri S, Tsujioka K, Kajiya F. Live-cell visualization of the trans-cellular mode of monocyte transmigration across the vascular endothelium, and its relationship with endothelial PECAM-1. J Physiol Sci 2012;62:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsubota Y, Frey JM, Tai PW, Welikson RE, Raines EW. Monocyte ADAM17 promotes diapedesis during transendothelial migration: identification of steps and substrates targeted by metalloproteinases. J Immunol 2013;190:4236–4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rautou PE, Leroyer AS, Ramkhelawon B, Devue C, Duflaut D, Vion AC, Nalbone G, Castier Y, Leseche G, Lehoux S, Tedgui A, Boulanger CM. Microparticles from human atherosclerotic plaques promote endothelial ICAM-1-dependent monocyte adhesion and transendothelial migration. Circ Res 2011;108:335–343. [DOI] [PubMed] [Google Scholar]
- 65.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol 2005;5:953–964. [DOI] [PubMed] [Google Scholar]
- 66.Hettinger J, Richards DM, Hansson J, Barra MM, Joschko AC, Krijgsveld J, Feuerer M. Origin of monocytes and macrophages in a committed progenitor. Nat Immunol 2013;14:821–830. [DOI] [PubMed] [Google Scholar]
- 67.Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ, Scherberich J, Schmitz J, Shortman K, Sozzani S, Strobl H, Zembala M, Austyn JM, Lutz MB. Nomenclature of monocytes and dendritic cells in blood. Blood 2010;116:e74–e80. [DOI] [PubMed] [Google Scholar]
- 68.Ziegler-Heitbrock L, Hofer TP. Toward a refined definition of monocyte subsets. Front Immunol 2013;4:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ziegler-Heitbrock L. Monocyte subsets in man and other species. Cell Immunol 2014;289:135–139. [DOI] [PubMed] [Google Scholar]
- 70.Ingersoll MA, Spanbroek R, Lottaz C, Gautier EL, Frankenberger M, Hoffmann R, Lang R, Haniffa M, Collin M, Tacke F, Habenicht AJ, Ziegler-Heitbrock L, Randolph GJ. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood 2010;115:e10–e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, Jung S. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 2013;38:79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Varol C, Landsman L, Fogg DK, Greenshtein L, Gildor B, Margalit R, Kalchenko V, Geissmann F, Jung S. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med 2007;204:171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hanna RN, Carlin LM, Hubbeling HG, Nackiewicz D, Green AM, Punt JA, Geissmann F, Hedrick CC. The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C- monocytes. Nat Immunol 2011;12:778–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest 2007;117:902–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest 2007;117:195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van Furth R, Cohn ZA. The origin and kinetics of mononuclear phagocytes. J Exp Med 1968;128:415–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, Greter M, Mortha A, Boyer SW, Forsberg EC, Tanaka M, van Rooijen N, Garcia-Sastre A, Stanley ER, Ginhoux F, Frenette PS, Merad M. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 2013;38:792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010;330:841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 2012;336:86–90. [DOI] [PubMed] [Google Scholar]
- 80.Jakubzick C, Gautier EL, Gibbings SL, Sojka DK, Schlitzer A, Johnson TE, Ivanov S, Duan Q, Bala S, Condon T, van Rooijen N, Grainger JR, Belkaid Y, Ma'ayan A, Riches DW, Yokoyama WM, Ginhoux F, Henson PM, Randolph GJ. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity 2013;39:599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci 2014;15:300–312. [DOI] [PubMed] [Google Scholar]
- 82.Bain CC, Bravo-Blas A, Scott CL, Gomez Perdiguero E, Geissmann F, Henri S, Malissen B, Osborne LC, Artis D, Mowat AM. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol 2014;15:929–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guilliams M, De Kleer I, Henri S, Post S, Vanhoutte L, De Prijck S, Deswarte K, Malissen B, Hammad H, Lambrecht BN. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp Med 2013;210:1977–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gordon S. Alternative activation of macrophages. Nat Rev Immunol 2003;3:23–35. [DOI] [PubMed] [Google Scholar]
- 85.Mills C. M1 and M2 macrophages: oracles of health and disease. Crit Rev Immunol 2012;32:463–488. [DOI] [PubMed] [Google Scholar]
- 86.Mills CD, Ley K. M1 and M2 macrophages: the chicken and the egg of immunity. J Innate Immun 2014;6:716–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol 2014;5:514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000prime Rep 2014;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Davis MJ, Tsang TM, Qiu Y, Dayrit JK, Freij JB, Huffnagle GB, Olszewski MA. Macrophage M1/M2 polarization dynamically adapts to changes in cytokine microenvironments in Cryptococcus neoformans infection. mBio 2013;4:e00264–e00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Frantz S, Nahrendorf M. Cardiac macrophages and their role in ischaemic heart disease. Cardiovasc Res 2014;102:240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Troidl C, Möllmann H, Nef H, Masseli F, Voss S, Szardien S, Willmer M, Rolf A, Rixe J, Troidl K, Kostin S, Hamm C, Elsässer A. Classically and alternatively activated macrophages contribute to tissue remodelling after myocardial infarction. J Cell Mol Med 2009;13:3485–3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hilgendorf I, Gerhardt LM, Tan TC, Winter C, Holderried TA, Chousterman BG, Iwamoto Y, Liao R, Zirlik A, Scherer-Crosbie M, Hedrick CC, Libby P, Nahrendorf M, Weissleder R, Swirski FK. Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ Res 2014;114:1611–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dejana E, Orsenigo F, Molendini C, Baluk P, McDonald DM. Organization and signaling of endothelial cell-to-cell junctions in various regions of the blood and lymphatic vascular trees. Cell Tissue Res 2009;335:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ferreira AM, McNeil CJ, Stallaert KM, Rogers KA, Sandig M. Interleukin-1beta reduces transcellular monocyte diapedesis and compromises endothelial adherens junction integrity. Microcirculation 2005;12:563–579. [DOI] [PubMed] [Google Scholar]
- 95.Woodfin A, Voisin MB, Beyrau M, Colom B, Caille D, Diapouli FM, Nash GB, Chavakis T, Albelda SM, Rainger GE, Meda P, Imhof BA, Nourshargh S. The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat Immunol 2011;12:761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Carman CV, Springer TA. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J Cell Biol 2004;167:377–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Carman CV, Sage PT, Sciuto TE, de la Fuente MA, Geha RS, Ochs HD, Dvorak HF, Dvorak AM, Springer TA. Transcellular diapedesis is initiated by invasive podosomes. Immunity 2007;26:784–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vestweber D. Novel insights into leukocyte extravasation. Curr Opin Hematol 2012;19:212–217. [DOI] [PubMed] [Google Scholar]
- 99.Vestweber D. Relevance of endothelial junctions in leukocyte extravasation and vascular permeability. Ann N Y Acad Sci 2012;1257:184–192. [DOI] [PubMed] [Google Scholar]
- 100.Vandenbroucke St Amant E, Tauseef M, Vogel SM, Gao XP, Mehta D, Komarova YA, Malik AB. PKCalpha activation of p120-catenin serine 879 phospho-switch disassembles VE-cadherin junctions and disrupts vascular integrity. Circ Res 2012;111:739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schulte D, Kuppers V, Dartsch N, Broermann A, Li H, Zarbock A, Kamenyeva O, Kiefer F, Khandoga A, Massberg S, Vestweber D. Stabilizing the VE-cadherin-catenin complex blocks leukocyte extravasation and vascular permeability. EMBO J 2011;30:4157–4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Broermann A, Winderlich M, Block H, Frye M, Rossaint J, Zarbock A, Cagna G, Linnepe R, Schulte D, Nottebaum AF, Vestweber D. Dissociation of VE-PTP from VE-cadherin is required for leukocyte extravasation and for VEGF-induced vascular permeability in vivo. J Exp Med 2011;208:2393–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Allingham MJ, van Buul JD, Burridge K. ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration. J Immunol 2007;179:4053–4064. [DOI] [PubMed] [Google Scholar]
- 104.Shaw SK, Bamba PS, Perkins BN, Luscinskas FW. Real-time imaging of vascular endothelial-cadherin during leukocyte transmigration across endothelium. J Immunol 2001;167:2323–2330. [DOI] [PubMed] [Google Scholar]
- 105.Sumagin R, Sarelius IH. Intercellular adhesion molecule-1 enrichment near tricellular endothelial junctions is preferentially associated with leukocyte transmigration and signals for reorganization of these junctions to accommodate leukocyte passage. J Immunol 2010;184:5242–5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hashimoto K, Kataoka N, Nakamura E, Hagihara K, Hatano M, Okamoto T, Kanouchi H, Minatogawa Y, Mohri S, Tsujioka K, Kajiya F. Monocyte trans-endothelial migration augments subsequent transmigratory activity with increased PECAM-1 and decreased VE-cadherin at endothelial junctions. Int J Cardiol 2011;149:232–239. [DOI] [PubMed] [Google Scholar]
- 107.Santoso S, Sachs UJH, Kroll H, Linder M, Ruf A, Preissner KT, Chavakis T. The junctional adhesion molecule 3 (jam-3) on human platelets is a counterreceptor for the leukocyte integrin Mac-1. J Exp Med 2002;196:679–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schmitt MM, Megens RT, Zernecke A, Bidzhekov K, van den Akker NM, Rademakers T, van Zandvoort MA, Hackeng TM, Koenen RR, Weber C. Endothelial junctional adhesion molecule-a guides monocytes into flow-dependent predilection sites of atherosclerosis. Circulation 2014;129:66–76. [DOI] [PubMed] [Google Scholar]
- 109.Guo YL, Bai R, Chen CX, Liu DQ, Liu Y, Zhang CY, Zen K. Role of junctional adhesion molecule-like protein in mediating monocyte transendothelial migration. Arterioscler Thromb Vasc Biol 2009;29:75–83. [DOI] [PubMed] [Google Scholar]
- 110.Muller WA, Weigl SA, Deng X, Phillips DM. PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med 1993;178:449–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schenkel AR, Chew TW, Muller WA. Platelet endothelial cell adhesion molecule deficiency or blockade significantly reduces leukocyte emigration in a majority of mouse strains. J Immunol 2004;173:6403–6408. [DOI] [PubMed] [Google Scholar]
- 112.Dasgupta B, Chew T, deRoche A, Muller WA. Blocking platelet/endothelial cell adhesion molecule 1 (PECAM) inhibits disease progression and prevents joint erosion in established collagen antibody-induced arthritis. Exp Mol Pathol 2010;88:210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Reinke EK, Lee J, Zozulya A, Karman J, Muller WA, Sandor M, Fabry Z. Short-term sPECAM-Fc treatment ameliorates EAE while chronic use hastens onset of symptoms. J Neuroimmunol 2007;186:86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Goel R, Schrank BR, Arora S, Boylan B, Fleming B, Miura H, Newman PJ, Molthen RC, Newman DK. Site-specific effects of PECAM-1 on atherosclerosis in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol 2008;28:1996–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stevens HY, Melchior B, Bell KS, Yun S, Yeh JC, Frangos JA. PECAM-1 is a critical mediator of atherosclerosis. Dis Models Mech 2008;1:175–181; discussion 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Harry BL, Sanders JM, Feaver RE, Lansey M, Deem TL, Zarbock A, Bruce AC, Pryor AW, Gelfand BD, Blackman BR, Schwartz MA, Ley K. Endothelial cell PECAM-1 promotes atherosclerotic lesions in areas of disturbed flow in ApoE-deficient mice. Arterioscler Thromb Vasc Biol 2008;28:2003–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Reymond N, Imbert AM, Devilard E, Fabre S, Chabannon C, Xerri L, Farnarier C, Cantoni C, Bottino C, Moretta A, Dubreuil P, Lopez M. DNAM-1 and PVR regulate monocyte migration through endothelial junctions. J Exp Med 2004;199:1331–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sullivan DP, Seidman MA, Muller WA. Poliovirus receptor (CD155) regulates a step in transendothelial migration between PECAM and CD99. Am J Pathol 2013;182:1031–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mamdouh Z, Chen X, Pierini LM, Maxfield FR, Muller WA. Targeted recycling of PECAM from endothelial surface-connected compartments during diapedesis. Nature 2003;421:748–753. [DOI] [PubMed] [Google Scholar]
- 120.Mamdouh Z, Mikhailov A, Muller WA. Transcellular migration of leukocytes is mediated by the endothelial lateral border recycling compartment. J Exp Med 2009;206:2795–2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mamdouh Z, Kreitzer GE, Muller WA. Leukocyte transmigration requires kinesin-mediated microtubule-dependent membrane trafficking from the lateral border recycling compartment. J Exp Med 2008;205:951–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Barreiro O, Yanez-Mo M, Serrador JM, Montoya MC, Vicente-Manzanares M, Tejedor R, Furthmayr H, Sanchez-Madrid F. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J Cell Biol 2002;157:1233–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Carman CV, Jun CD, Salas A, Springer TA. Endothelial cells proactively form microvilli-like membrane projections upon intercellular adhesion molecule 1 engagement of leukocyte LFA-1. J Immunol 2003;171:6135–6144. [DOI] [PubMed] [Google Scholar]
- 124.Sullivan D, Muller W. Neutrophil and monocyte recruitment by PECAM, CD99, and other molecules via the LBRC. Semin Immunopathol 2014;36:193–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wittchen ES, van Buul JD, Burridge K, Worthylake RA. Trading spaces: Rap, Rac, and Rho as architects of transendothelial migration. Curr Opin Hematol 2005;12:14–21. [DOI] [PubMed] [Google Scholar]
- 126.Zen K, Guo YL, Li LM, Bian Z, Zhang CY, Liu Y. Cleavage of the CD11b extracellular domain by the leukocyte serprocidins is critical for neutrophil detachment during chemotaxis. Blood 2011;117:4885–4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shang MM, Talukdar HA, Hofmann JJ, Niaudet C, Asl HF, Jain RK, Rossignoli A, Cedergren C, Silveira A, Gigante B, Leander K, de Faire U, Hamsten A, Ruusalepp A, Melander O, Ivert T, Michoel T, Schadt EE, Betsholtz C, Skogsberg J, Bjorkegren JL. Lim domain binding 2: a key driver of transendothelial migration of leukocytes and atherosclerosis. Arterioscler Thromb Vasc Biol 2014;34:2068–2077. [DOI] [PubMed] [Google Scholar]
- 128.Korff T, Braun J, Pfaff D, Augustin HG, Hecker M. Role of ephrinB2 expression in endothelial cells during arteriogenesis: impact on smooth muscle cell migration and monocyte recruitment. Blood 2008;112:73–81. [DOI] [PubMed] [Google Scholar]
- 129.Jellinghaus S, Poitz DM, Ende G, Augstein A, Weinert S, Stutz B, Braun-Dullaeus RC, Pasquale EB, Strasser RH. Ephrin-A1/EphA4-mediated adhesion of monocytes to endothelial cells. Biochim Biophys Acta 2013;1833:2201–2211. [DOI] [PubMed] [Google Scholar]
- 130.Funk SD, Yurdagul A, Jr, Albert P, Traylor JG, Jr, Jin L, Chen J, Orr AW. EphA2 activation promotes the endothelial cell inflammatory response: a potential role in atherosclerosis. Arterioscler Thromb Vasc Biol 2012;32:686–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Braun J, Hoffmann SC, Feldner A, Ludwig T, Henning R, Hecker M, Korff T. Endothelial cell ephrinB2-dependent activation of monocytes in arteriosclerosis. Arterioscler Thromb Vasc Biol 2011;31:297–305. [DOI] [PubMed] [Google Scholar]
- 132.Pfaff D, Heroult M, Riedel M, Reiss Y, Kirmse R, Ludwig T, Korff T, Hecker M, Augustin HG. Involvement of endothelial ephrin-B2 in adhesion and transmigration of EphB-receptor-expressing monocytes. J Cell Sci 2008;121:3842–3850. [DOI] [PubMed] [Google Scholar]
- 133.van Gils JM, Derby MC, Fernandes LR, Ramkhelawon B, Ray TD, Rayner KJ, Parathath S, Distel E, Feig JL, Alvarez-Leite JI, Rayner AJ, McDonald TO, O'Brien KD, Stuart LM, Fisher EA, Lacy-Hulbert A, Moore KJ. The neuroimmune guidance cue netrin-1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques. Nat Immunol 2012;13:136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Srivastava M, Jung S, Wilhelm J, Fink L, Buhling F, Welte T, Bohle RM, Seeger W, Lohmeyer J, Maus UA. The inflammatory versus constitutive trafficking of mononuclear phagocytes into the alveolar space of mice is associated with drastic changes in their gene expression profiles. J Immunol 2005;175:1884–1893. [DOI] [PubMed] [Google Scholar]
- 135.Thomas-Ecker S, Lindecke A, Hatzmann W, Kaltschmidt C, Zanker KS, Dittmar T. Alteration in the gene expression pattern of primary monocytes after adhesion to endothelial cells. Proc Natl Acad Sci U S A 2007;104:5539–5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Williams MR, Sakurai Y, Zughaier SM, Eskin SG, McIntire LV. Transmigration across activated endothelium induces transcriptional changes, inhibits apoptosis, and decreases antimicrobial protein expression in human monocytes. J Leukoc Biol 2009;86:1331–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Voisin MB, Woodfin A, Nourshargh S. Monocytes and neutrophils exhibit both distinct and common mechanisms in penetrating the vascular basement membrane in vivo. Arterioscler Thromb Vasc Biol 2009;29:1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nourshargh S, Hordijk PL, Sixt M. Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat Rev Mol Cell Biol 2010;11:366–378. [DOI] [PubMed] [Google Scholar]
- 139.Stark K, Eckart A, Haidari S, Tirniceriu A, Lorenz M, von Bruhl ML, Gartner F, Khandoga AG, Legate KR, Pless R, Hepper I, Lauber K, Walzog B, Massberg S. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and ‘instruct’ them with pattern-recognition and motility programs. Nat Immunol 2013;14:41–51. [DOI] [PubMed] [Google Scholar]
- 140.Kamohara H, Yamashiro S, Galligan C, Yoshimura T. Discoidin domain receptor 1 isoform-a (DDR1alpha) promotes migration of leukocytes in three-dimensional collagen lattices. FASEB J 2001;15:2724–2726. [DOI] [PubMed] [Google Scholar]
- 141.Engelhardt B, Kappos L. Natalizumab: targeting alpha4-integrins in multiple sclerosis. Neurodegener Dis 2008;5:16–22. [DOI] [PubMed] [Google Scholar]
- 142.Wyant T, Leach T, Sankoh S, Wang Y, Paolino J, Pasetti MF, Feagan BG, Parikh A. Vedolizumab affects antibody responses to immunisation selectively in the gastrointestinal tract: randomised controlled trial results. Gut 2015;64:77–83. [DOI] [PubMed] [Google Scholar]
- 143.Rietdijk ST, D'Haens GR. Vedolizumab for the treatment of ulcerative colitis. Exp Rev Clin Pharmacol 2014;7:423–430. [DOI] [PubMed] [Google Scholar]


