Abstract
Background: We previously reported results of the phase 2, multicenter PINNACLE study, which confirmed the substantial single-agent activity of bortezomib in patients with relapsed or refractory mantle cell lymphoma (MCL).
Materials and methods: We report updated time-to-event data, in all patients and by response to treatment, after extended follow-up (median 26.4 months).
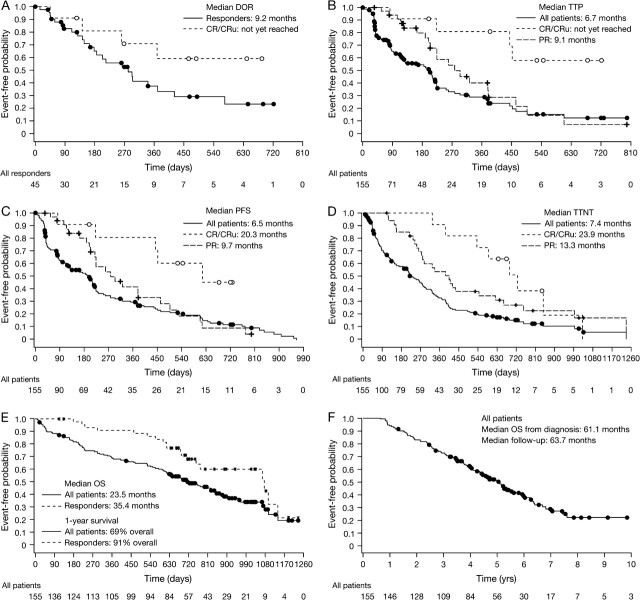
Results: Median time to progression (TTP) was 6.7 months. Median time to next therapy (TTNT) was 7.4 months. Median overall survival (OS) was 23.5 months. In responding patients, median TTP was 12.4 months, median duration of response (DOR) was 9.2 months, median TTNT was 14.3 months, and median OS was 35.4 months. Patients achieving complete response had heterogeneous disease characteristics; among these patients, median TTP and DOR were not reached, and median OS was 36.0 months. One-year survival rate was 69% overall and 91% in responding patients. Median OS from diagnosis was 61.1 months, after median follow-up of 63.7 months. Activity was seen in patients with refractory disease and patients relapsing following high-intensity treatment. Toxicity was generally manageable.
Conclusions: Single-agent bortezomib is associated with lengthy responses and notable survival in patients with relapsed or refractory MCL, with considerable TTP and TTNT in responding patients, suggesting substantial clinical benefit.
Keywords: bortezomib, mantle cell lymphoma, refractory, relapsed, response, survival
introduction
Mantle cell lymphoma (MCL), an aggressive subtype of non-Hodgkin's lymphoma, is characterized by cyclin D1 overexpression resulting from the t(11;14)(q13;q32) translocation [1, 2]. MCL patients generally have a poor prognosis [1, 2]; median overall survival (OS) is typically 3–4 years from diagnosis [3, 4]. Standard front-line treatment has not been established [1, 4]. High-dose therapy plus stem-cell transplant (HDT-SCT) appears beneficial for progression-free survival (PFS) or event-free survival compared with standard chemotherapy [5, 6]. Dose-intense regimens such as hyperfractionated cyclophosphamide, with vincristine, doxorubicin, and dexamethasone (hyper-CVAD), alternating with high-dose methotrexate and cytarabine, in combination with rituximab, are frequently employed [1, 4]. Although high-intensity treatments have demonstrated notable OS [7, 8], median PFS has nevertheless been reported as only 3 years [7]. Despite high response rates to front-line regimens, patients relapse [1, 4], often demonstrating acquired chemotherapy resistance, and typically achieving short duration of response (DOR) to conventional chemotherapy, illustrating the need for novel therapies for relapsed/refractory MCL.
Bortezomib (VELCADE®; Millennium: The Takeda Oncology Company, and Johnson & Johnson Pharmaceutical Research & Development, L.L.C.) is approved in the United States for the treatment of patients with MCL following at least one prior therapy, and patients with multiple myeloma. We previously demonstrated substantial activity with single-agent bortezomib in patients with relapsed or refractory MCL in an initial analysis of the phase 2 PINNACLE study [9], the largest prospective study to date in this population. Response rate was 33%, including 8% complete response (CR)/unconfirmed CR (CRu), as defined in materials and methods; median DOR was 9.2 months, and median time to progression (TTP) was 6.2 months, after median follow-up of 13.4 months [9]. Our findings confirmed the activity seen in smaller phase 1 and 2 studies [10–15] and resulted in the approval of bortezomib in this setting. In our previous report, 12 patients remained on treatment and 16 and 72 were in short-term follow-up for disease progression and long-term follow-up for survival, respectively [9]. All patients have now completed treatment; here we report updated data after extended follow-up.
materials and methods
The PINNACLE study design has been reported [9]. Study objectives were to evaluate response rate [CR/CRu + partial response (PR)], DOR, TTP, and OS. Eligible patients were ≥18 years old and had measurable/assessable, pathologically confirmed MCL, including cyclin D1 overexpression and/or evidence of the t(11;14) translocation, with documented relapse or progression after one or two lines of antineoplastic therapy (including exposure to an anthracycline or mitoxantrone, and rituximab). In total, 155 patients received bortezomib 1.3 mg/m2, days 1, 4, 8, 11, every 21 days, for up to 17 cycles or four cycles beyond initial reporting of CR/CRu, or until discontinuation for progressive disease (PD), unacceptable toxicity, or patient/investigator decision. Efficacy was assessed every 6 weeks (two cycles) for 18 weeks (six cycles), then every 12 weeks until PD or alternative antineoplastic therapy. Disease response and progression were determined by International Workshop Response Criteria [16], using independent radiology review. Patients discontinuing for reasons other than PD continued in short-term follow-up every 6 weeks until week 18, then every 12 weeks until PD or alternative therapy. All patients underwent long-term follow-up every 3 months. Safety was assessed throughout by investigators and through laboratory evaluations. Adverse events (AEs) were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 3.0 [17]. The study was conducted in accordance with International Conference on Harmonization Good Clinical Practice guidelines and the Declaration of Helsinki. The study was approved by the responsible institutional review board for each investigative site; all patients provided written informed consent.
At this analysis, additional scans were assessed and existing scans reassessed by independent radiology review. Updated response rate and DOR analyses were conducted in 141 response-assessable patients and in patient subgroups with refractory disease (no response or response with TTP <6 months after last prior therapy; n = 58, including 51 response-assessable patients) and with prior high-intensity therapy (therapies containing high-dose cytarabine or ifosfamide/carboplatin/etoposide; n = 58, including 52 response-assessable patients). Updated analyses of TTP, PFS, time to next therapy (TTNT; time from first bortezomib dose to start of next therapy), and OS were conducted for all patients, by response, and in the above subgroups, using Kaplan–Meier methods. A post hoc analysis of OS from diagnosis was conducted using Kaplan–Meier methods.
results
patient characteristics and disposition
Baseline characteristics have been reported; median age was 65 years, and 46% of patients had received two or more prior lines of therapy [9]. At the time of enrollment, 44% of patients had an International Prognostic Index score ≥3, 36% had lactate dehydrogenase levels above the upper limit of normal, and 77% had stage IV MCL [9]. After a median follow-up of 26.4 months, 55 patients (35%) remained in follow-up (four in short-term and 51 in long-term follow-up), 93 (60%) had died, two (1%) had withdrawn consent, and five (3%) were lost to follow-up. Patients received a median of four treatment cycles (range 1–21) overall; responding patients received a median of eight cycles (range 2–21).
efficacy
Response rate at this updated analysis was 32% [95% confidence interval (CI) 24%, 40%], including 8% CR/CRu (95% CI 4%, 14%). Median time to first response was 1.4 months; response rate after two cycles was 18% (95% CI 12%, 25%), with 2% CR/CRu (95% CI 0%, 6%), and after four cycles was 27% (95% CI 20%, 35%), with 4% CR/CRu (95% CI 1%, 8%). Patients who achieved CR/CRu were aged 52–79 years and had heterogeneous disease characteristics at baseline, including some patients with bulky disease (5 of 11 had lesions >5 cm on the long axis), Table 1.
Table 1.
Baseline characteristics of patients achieving CR/CRu to bortezomib by independent radiology review
| Patient | Age (years) | Time since diagnosis (years) | Disease stage | IPI | Prior therapies | Refractory disease | Largest tumor mass (cm) |
| 1 | 52 | 4.6 | III | 1 | CHOP; rituximab | Yes | 11.0 × 7.7 |
| 2 | 73 | 2.3 | IV | 2 | R-EPOCH | No | 7.3 × 6.7 |
| 3 | 68 | 4.1 | IV | 2 | R-CHOP; hyper-CVAD + ASCT | No | 6.8 × 1.9 |
| 4 | 63 | 5.1 | IV | 2 | Hyper-CVAD + ASCT; R-CVP | Yes | 5.9 × 4.4 |
| 5 | 72 | 2.7 | IV | 3 | R-CHOP; 90Y-ibritumomab tiuxetan | Yes | 5.5 × 3.0 |
| 6 | 58 | 5.2 | IV | 3 | R-hyper-CVAD + ASCT; 90Y-ibritumomab tiuxetan | No | 4.7 × 2.6 |
| 7 | 58 | 1.0 | III | 1 | R-hyper-CVAD | No | 3.9 × 2.8 |
| 8 | 78 | 2.0 | IV | 3 | R-EPOCH; CNOP | No | 3.0 × 2.3 |
| 9 | 79 | 3.0 | IV | 3 | CVP; rituximab; R-pentostatin + mitoxantrone | No | 3.0 × 2.7 |
| 10 | 74 | 0.9 | III | 2 | R-CHOP + oblimersen | No | 2.8 × 2.5 |
| 11 | 62 | 4.3 | IV | 2 | R-CHOP; hyper-CVAD + ASCT | No | 1.5 × 1.1 |
CR, complete response; CRu, unconfirmed complete response; IPI, International Prognostic Index; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; ASCT, autologous stem cell transplant; R-CVP, rituximab, cyclophosphamide, vincristine, prednisone; CNOP, cyclophosphamide, mitoxantrone, vincristine, prednisone; R-EPOCH, rituximab, etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin; hyper-CVAD, hyperfractionated cyclophosphamide, with vincristine, doxorubicin, and dexamethasone.
Median DOR was 9.2 months (95% CI 5.9, 13.8) in all responders (Figure 1A). It was not reached (95% CI 8.7, not estimable) in patients achieving CR/CRu and was 6.7 months (95% CI 4.9, 9.7) in patients achieving PR. There was no correlation between DOR and time to response. Table 2 shows medians for TTP, PFS, TTNT, and OS for all patients and by response.
Figure 1.
Kaplan–Meier distribution curves of time-to-event data in patients with relapsed or refractory mantle cell lymphoma receiving bortezomib. (A) Duration of response in all responders and in patients achieving complete response/unconfirmed complete response. (B) Time to progression, (C) progression-free survival, (D) time to next therapy, and (E) overall survival (OS) for all patients and by response after a median follow-up of 26.4 months. (F) OS for all patients from diagnosis, after a median follow-up of 63.7 months.
Table 2.
Median TTP, PFS, TTNT, and OS in months (95% confidence interval) in all patients and by response
| All patients (n = 155) | Responders (n = 45)a | CR/CRu (n = 11)a | PR (n = 34)a | SD (n = 52)a | PD (n = 34)a | |
| TTP | 6.7 (4.0, 7.3) | 12.4 (7.4, 16.3) | NE (14.6, NE) | 9.1 (7.4, 12.5) | 6.9 (4.2, 7.2) | 1.2 (1.2, 1.3) |
| PFS | 6.5 (4.0, 7.2) | 12.4 (7.4, 17.3) | 20.3 (14.6, NE) | 9.7 (7.2, 15.2) | 7.2 (6.4, 11.6) | 1.2 (1.2, 1.3) |
| TTNT | 7.4 (5.6, 9.3) | 14.3 (11.1, 22.6) | 23.9 (17.6, 33.9) | 13.3 (9.0, 20.5) | 7.0 (4.4, 8.7) | 2.3 (1.9, 2.9) |
| OS | 23.5 (20.3, 27.9) | 35.4 (24.9, 37.5) | 36.0 (NE, NE) | 35.1 (23.4, 37.5) | 27.8 (21.3, NE) | 13.7 (6.7, 22.3) |
Of the 141 response-evaluable patients, 10 had no postbaseline assessment.
TTP, time to progression; PFS, progression-free survival; TTNT, time to next therapy; OS, overall survival; CR, complete response; CRu, unconfirmed complete response; PR, partial response; SD, stable disease; PD, progressive disease; NE, not estimable.
Among the 11 patients achieving CR/CRu, four had progressed by independent radiology review (one of these patients had not received subsequent antineoplastic therapy), and eight had received subsequent antineoplastic therapy (five of these patients had not progressed by independent radiology review). Subsequent therapies received by the three patients who had progressed by independent radiology review were fludarabine plus rituximab; bortezomib plus fludarabine, mitoxantrone, and dexamethasone; and etoposide, methylprednisolone, cytarabine, cisplatin. Of the five patients who had not progressed by independent radiology review, two had rituximab added to bortezomib, one received radiation therapy following progression by investigator assessment, one received methotrexate for a nonmalignant condition (seronegative symmetrical synovitis), and one received rituximab despite not having progressed by either independent radiology review or investigator assessment. Among five patients with lesions >5 cm on the long axis, two had progressed by independent radiology review; the others included one patient with progression by investigator assessment, the patient treated for synovitis, and the patient treated despite not having progressed.
Figure 1B–E shows distribution curves for TTP, PFS, TTNT, and OS. The 1-year survival rate was 69% overall and 91% in responders. Median OS from diagnosis was 61.1 months (95% CI 52.1, 70.4), after median follow-up of 63.7 months; the distribution curve is shown in Figure 1F. In all patients, median TTP following their last prior therapy was 12.0 months (95% CI 11.1, 15.0); median TTP following last prior therapy was 16.0 months (95% CI 12.0, 20.1) among patients who responded to bortezomib, and 17.0 months (95% CI 10.0, 21.0) among patients who achieved CR/CRu with bortezomib.
Bortezomib demonstrated activity in patients with refractory MCL and patients with prior high-intensity therapy. Response rates, DOR, TTP, PFS, TTNT, and OS among these populations are summarized in Table 3.
Table 3.
Response rate, DOR, TTP, PFS, TTNT, and OS in patients with refractory MCL and patients with prior high-intensity therapya
| Refractory MCL, n = 58 | Prior high-intensity therapy, n = 58 | |
| Response rate (95% CI) | n = 51 | n = 52 |
| CR + PR | 29% (17%, 44%) | 25% (14%, 39%) |
| CR/CRu | 6% (1%, 16%) | 10% (3%, 21%) |
| Median DOR, months (95% CI) | ||
| All responders | 5.9 (4.7, NE) | Not reached (6.1, NE) |
| Patients achieving CR/CRu | Not reached (4.7, NE) | NE |
| Patients achieving PR | 5.9 (4.9, 9.6) | NE |
| Median TTP, months (95% CI) | 3.9 (1.7, 7.3) | 4.2 (4.0, 7.4) |
| Median PFS, months (95% CI) | 4.1 (1.7, 6.9) | 4.5 (2.8, 7.4) |
| Median TTNT, months (95% CI) | 4.6 (2.9, 8.0) | 6.2 (3.4, 9.7) |
| Median OS, months (95% CI) | 17.3 (7.7, 27.2) | 20.3 (12.0, 28.8) |
Note that patients with prior high-intensity therapy may also be included with patients refractory to their prior therapy.
DOR, duration of response; TTP, time to progression; PFS, progression-free survival; TTNT, time to next therapy; OS, overall survival; MCL, mantle cell lymphoma; CI, confidence interval; CR, complete response; PR, partial response; CRu, unconfirmed complete response; NE, not estimable.
safety
The safety profile of bortezomib in this analysis was similar to that previously reported [9]. The most common grade 3 or higher non-hematologic AE was peripheral neuropathy, seen in 20 (13%) patients. Median time to onset of peripheral neuropathy of any grade was four cycles (∼12 weeks); time to onset of grade 3 or higher peripheral neuropathy ranged from 4 to 30 weeks. Data on reversibility of this AE were not collected. Lymphopenia was seen in 104 (67%) patients, including 52 (34%) with grade ≥3 lymphopenia [absolute lymphocyte count (ALC) <500 cells/mm3]. By the end-of-treatment visit, typically 30 days after last dose of bortezomib, ALC had recovered to normal in 38 of 104 (37%) patients and had improved by at least one NCI CTCAE grade in a further 36 (35%) patients. Among patients with grade ≥3 lymphopenia, nine (17%) had recovered to normal and a further 29 (58%) had improved by at least one NCI CTCAE grade by their end-of-treatment visit.
Twelve patients (8%) died within 28 days of their last dose of bortezomib, including four (3%) whose cause of death was considered related to bortezomib (three due to non-neutropenic sepsis, one due to respiratory failure) [9].
discussion
These updated results from the PINNACLE study after extended follow-up confirm the substantial activity of single-agent bortezomib in patients with relapsed or refractory MCL. Bortezomib was associated with lengthy responses (median DOR of 9.2 months) and a notable median OS of ∼2 years, with substantial TTP and TTNT in responding patients, indicating clinical benefit. These data are of particular note given that almost half the patients entered the study following second relapse; clinical experience at the time of this study suggested survival of only 1–2 years following first relapse. Furthermore, analysis of OS from diagnosis demonstrated a median OS of >5 years, similar to that reported recently for low–intermediate risk MCL patients [18]. This appears longer than historically reported data [3, 4] and more consistent with studies of HDT-SCT in first remission [19] and front-line dose-intense therapy [20, 21]. Patient selection may also have contributed to the long median OS. In addition, our findings confirm that bortezomib is active in refractory disease and in patients relapsing following high-intensity treatment. The latter is important given the frequent front-line use of high-intensity therapies. Our analysis of patients who achieved CR/CRu showed that responses were seen in patients with heterogeneous baseline demographic and disease characteristics, including patients with bulky disease.
The safety profile in this updated analysis was similar to that previously reported, with bortezomib-associated toxic effects proving manageable. Although data on the reversibility of peripheral neuropathy were not collected, studies of bortezomib in myeloma have shown peripheral neuropathy to be reversible in the majority of patients [22–24]. In the present study, bortezomib-associated lymphopenia was reversible in the majority of patients within 30 days of treatment discontinuation, with approximately one-third recovering to a normal ALC and a further one-third demonstrating an improvement of at least one NCI CTCAE grade within this time period.
Immunohistochemical analyses for potential biomarkers of bortezomib activity are ongoing using PINNACLE data, with preliminary results suggesting Ki67, nuclear factor kappa-B–p65, and PSMA5 levels correlate with TTP [25]. Another analysis suggested p27 and p65 correlate with PFS with bortezomib [26]. These analyses may help identify surrogate markers of response to bortezomib. In other ongoing studies, bortezomib is being investigated in relapsed/refractory and previously untreated MCL in combination with standard agents and regimens including high-dose cytarabine [27], rituximab, and dexamethasone [28], rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) [29, 30], R-hyper-CVAD [31], and rituximab, etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin [32], with promising early results. Due to the heterogeneous natural history of MCL and the consequent difficulty in comparing results between studies, randomized trials involving bortezomib alone or in combination may be warranted to confirm the benefit of this agent; a phase 3 study is currently comparing R-CHOP to bortezomib, rituximab, cyclophosphamide, doxorubicin, prednison (VcR-CAP) in front-line MCL.
In conclusion, our updated results confirm that bortezomib is highly active in patients with relapsed or refractory MCL and has a manageable toxicity profile. Bortezomib is associated with lengthy responses and notable survival, with substantial TTP and TTNT in responding patients, especially those achieving CR/CRu. These findings indicate that bortezomib provides clinical benefit and represents a valuable treatment option for patients with relapsed/refractory MCL, including those who have relapsed following high-intensity therapy.
funding
Millennium Pharmaceuticals, Inc.; Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Acknowledgments
The authors would like to thank Steve Hill and Rosemary Washbrook for assistance in drafting the manuscript. Steve Hill is a medical writer and Rosemary Washbrook is a medical editor with Gardiner-Caldwell London. The PINNACLE Study Group: Said Baidas, MD, Georgetown University Medical Center, Washington, DC; Nancy Bartlett, MD, Washington University School of Medicine, St Louis, MO; Robert Belt, MD, Kansas City Cancer Center, Kansas City, KC; Jesus Berdeja, MD, Loma Linda University Medical Center, Loma Linda, CA; SB, MD, University of Rochester, James P Wilmot Cancer Center, Rochester, NY; Myron Czuczman, MD, Roswell Park Cancer Institute, Buffalo, NY; SdV, MD, UCLA School of Medicine, Los Angeles, CA; BD, MD PhD, H. Lee Moffitt Cancer Center & Research Institute, Tampa, FL; Martin Dreyling, MD, University Clinic of Munich–Grosshadern, Munich, Germany; EE, MD, Oregon Health and Science University, Portland, OR; RIF, MD, University of Rochester, James P. Wilmot Cancer Center, Rochester, NY; Andres Forero, MD, University of Alabama at Birmingham, Birmingham, AL; AG, MD, Hackensack University Medical Center, Hackensack, NJ; Stephanie Gregory, MD, Rush Presbyterian St. Lukes Medical Center, Chicago, IL; Michael Grossbard, MD, St Luke's Roosevelt Hospital, NY; John Hainsworth, MD, Sarah Cannon Cancer Center, Nashville, TN; Sandra Horning, MD, Stanford Cancer Center, Stanford, CA; David Hurd, MD, Wake Forest University Health Sciences, Winston-Salem, NC; David Irwin, MD, Alta Bates Comprehensive Cancer Center, Berkeley, CA; BK, MD, University Hospital Madison, Madison, WI; Lawrence Kaplan, MD, University of California San Francisco, San Francisco, CA; Alan Keller, MD, Cancer Care Associates, Tulsa, OK; AK, MD, City of Hope National Medical Center, Duarte, CA; JL, MD, Weill Medical College of Cornell University/New York Presbyterian Hospital, NY; John Lister, MD, Western Pennsylvania Hospital, Pittsburgh, PA; SL, MD, Emory University Winship Cancer Institute, Atlanta, GA; James W. Lynch, MD FACP, Florida VA Hospital, Gainesville, FL; Romeo Mandanas, MD, Cancer Care Associates, Oklahoma City, OK; David Morgan, MD, Vanderbilt University Medical Center, Nashville, TN; Martin Oken, MD, North Memorial Hospital Hubert Humphrey Cancer Center, Robbinsdale, MN; MR, MD, Indiana University Medical Center, Indianapolis, IN; Jorge Romaguera, MD, University of Texas MD Anderson Cancer Center, Houston, TX; Simon Rule, MD, Plymouth Hospitals NHS Trust, Derriford Hospital, Plymouth, UK; Mansoor Saleh, MD, Georgia Cancer Specialists, Tucker, GA; Edward Stadtmauer, MD, University of Pennsylvania Cancer Center, Philadelphia, PA; James Wooldridge, MD, University of Iowa Cancer Center, Iowa. Disclosures: AG, SHB, BSK, SdV, EE, AK, JPL, SL, OAO, HS, ALB, and RIF have declared a potential conflict of interest. AG, SHB, BSK, EE, JPL, SL, OAO, and RIF have received honoraria from Millennium Pharmaceuticals, Inc., and AK has received honoraria from Celgene and Genentech; SHB, BSK, SdV, AK, JPL, SL, and RIF disclose consultancy with Millennium Pharmaceuticals, Inc., AK and RIF with Celgene and Genentech, and JPL with Johnson & Johnson. OAO discloses research funding from Millennium Pharmaceuticals, Inc. and AK from Biogen Idec. Two of the authors (HS and ALB) are employed by and had an ownership interest in Millennium Pharmaceuticals, Inc, whose product [VELCADE (bortezomib)] was studied in the present work. AK discloses an ownership interest in Celgene. BD and MJR have no financial interests to declare.
References
- 1.Williams ME, Densmore JJ. Biology and therapy of mantle cell lymphoma. Curr Opin Oncol. 2005;17:425–431. doi: 10.1097/01.cco.0000174039.69656.2b. [DOI] [PubMed] [Google Scholar]
- 2.Bertoni F, Rinaldi A, Zucca E, et al. Update on the molecular biology of mantle cell lymphoma. Hematol Oncol. 2006;24:22–27. doi: 10.1002/hon.767. [DOI] [PubMed] [Google Scholar]
- 3.Lenz G, Dreyling M, Hiddemann W. Mantle cell lymphoma: established therapeutic options and future directions. Ann Hematol. 2004;83:71–77. doi: 10.1007/s00277-003-0774-2. [DOI] [PubMed] [Google Scholar]
- 4.Witzig TE. Current treatment approaches for mantle-cell lymphoma. J Clin Oncol. 2005;23:6409–6414. doi: 10.1200/JCO.2005.55.017. [DOI] [PubMed] [Google Scholar]
- 5.Dreyling M, Lenz G, Hoster E, et al. Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma: results of a prospective randomized trial of the European MCL Network. Blood. 2005;105:2677–2684. doi: 10.1182/blood-2004-10-3883. [DOI] [PubMed] [Google Scholar]
- 6.Lefrere F, Delmer A, Suzan F, et al. Sequential chemotherapy by CHOP and DHAP regimens followed by high-dose therapy with stem cell transplantation induces a high rate of complete response and improves event-free survival in mantle cell lymphoma: a prospective study. Leukemia. 2002;16:587–593. doi: 10.1038/sj.leu.2402406. [DOI] [PubMed] [Google Scholar]
- 7.Vigouroux S, Gaillard F, Moreau P, et al. High-dose therapy with autologous stem cell transplantation in first response in mantle cell lymphoma. Haematologica. 2005;90:1580–1582. [PubMed] [Google Scholar]
- 8.Khouri IF, Romaguera J, Kantarjian H, et al. Hyper-CVAD and high-dose methotrexate/cytarabine followed by stem-cell transplantation: an active regimen for aggressive mantle-cell lymphoma. J Clin Oncol. 1998;16:3803–3809. doi: 10.1200/JCO.1998.16.12.3803. [DOI] [PubMed] [Google Scholar]
- 9.Fisher RI, Bernstein SH, Kahl BS, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2006;24:4867–4874. doi: 10.1200/JCO.2006.07.9665. [DOI] [PubMed] [Google Scholar]
- 10.Belch A, Kouroukis CT, Crump M, et al. A phase II study of bortezomib in mantle cell lymphoma: the National Cancer Institute of Canada Clinical Trials Group trial IND.150. Ann Oncol. 2007;18:116–121. doi: 10.1093/annonc/mdl316. [DOI] [PubMed] [Google Scholar]
- 11.Goy A, Younes A, McLaughlin P, et al. Phase II study of proteasome inhibitor bortezomib in relapsed or refractory B-cell non-Hodgkin's lymphoma. J Clin Oncol. 2005;23:667–675. doi: 10.1200/JCO.2005.03.108. [DOI] [PubMed] [Google Scholar]
- 12.O'Connor OA, Wright J, Moskowitz C, et al. Targeting the proteasome pathway with bortezomib in patients with mantle cell (MCL) and follicular lymphoma (FL) produces prolonged progression free survival among responding patients: results of a multicenter phase II experience. Ann Oncol. 2005;16(Suppl 5):v66. [Google Scholar]
- 13.Orlowski RZ, Stinchcombe TE, Mitchell BS, et al. Phase I trial of the proteasome inhibitor PS-341 in patients with refractory hematologic malignancies. J Clin Oncol. 2002;20:4420–4427. doi: 10.1200/JCO.2002.01.133. [DOI] [PubMed] [Google Scholar]
- 14.Strauss SJ, Maharaj L, Hoare S, et al. Bortezomib therapy in patients with relapsed or refractory lymphoma: potential correlation of in vitro sensitivity and tumor necrosis factor alpha response with clinical activity. J Clin Oncol. 2006;24:2105–2112. doi: 10.1200/JCO.2005.04.6789. [DOI] [PubMed] [Google Scholar]
- 15.O'Connor OA, Wright J, Moskowitz C, et al. Phase II clinical experience with the novel proteasome inhibitor bortezomib in patients with indolent non-Hodgkin's lymphoma and mantle cell lymphoma. J Clin Oncol. 2005;23:676–684. doi: 10.1200/JCO.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 16.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244–1253. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 17.National Cancer Institute. National Cancer Institute Cancer Therapy Evaluation Program Common Terminology Criteria for Adverse Events. Accessed 1 May 2008 http://ctep.cancer.gov/forms/CTCAEv3.pdf. [Google Scholar]
- 18.Hoster E, Dreyling M, Klapper W, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111:558–565. doi: 10.1182/blood-2007-06-095331. [DOI] [PubMed] [Google Scholar]
- 19.Vandenberghe E, Ruiz de EC, Loberiza FR, et al. Outcome of autologous transplantation for mantle cell lymphoma: a study by the European Blood and Bone Marrow Transplant and Autologous Blood and Marrow Transplant Registries. Br J Haematol. 2003;120:793–800. doi: 10.1046/j.1365-2141.2003.04140.x. [DOI] [PubMed] [Google Scholar]
- 20.Fayad L, Thomas D, Romaguera J. Update of the M.D. Anderson Cancer Center experience with hyper-CVAD and rituximab for the treatment of mantle cell and Burkitt-type lymphomas. Clin Lymphoma Myeloma. 2007;8(Suppl 2):S57–S62. doi: 10.3816/clm.2007.s.034. [DOI] [PubMed] [Google Scholar]
- 21.Evens AM, Winter JN, Hou N, et al. A phase II clinical trial of intensive chemotherapy followed by consolidative stem cell transplant: long-term follow-up in newly diagnosed mantle cell lymphoma. Br J Haematol. 2008;140:385–393. doi: 10.1111/j.1365-2141.2007.06908.x. [DOI] [PubMed] [Google Scholar]
- 22.Richardson PG, Briemberg H, Jagannath S, et al. Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. J Clin Oncol. 2006;24:3113–3120. doi: 10.1200/JCO.2005.04.7779. [DOI] [PubMed] [Google Scholar]
- 23.San Miguel JF, Richardson P, Sonneveld P, et al. Frequency, characteristics, and reversibility of peripheral neuropathy (PN) in the APEX trial. Blood. 2005;106:111a. [Google Scholar]
- 24.San Miguel JF, Schlag R, Khuageva N, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:964–966. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 25.Goy A, Bernstein SH, McDonald A, et al. Immunohistochemical analyses for potential biomarkers of bortezomib activity in mantle cell lymphoma from the PINNACLE phase 2 trial. Blood. 2007;110:758a–759a. doi: 10.3109/10428194.2010.483302. [DOI] [PubMed] [Google Scholar]
- 26.Gerecitano J, Gounder S, Feldstein J, et al. Pre-treatment p27 and Bcl-6 staining levels correlate with response to bortezomib in non-Hodgkin lymphoma: results from a tissue microarray analysis. Blood. 2007;110:389a. [Google Scholar]
- 27.Weigert O, Weidmann E, Mueck R, et al. High dose cytarabine salvage regimen combined with bortezomib is feasible and highly effective in relapsed mantle cell lymphoma. Blood. 2006;108:693a–694a. [Google Scholar]
- 28.Drach J, Kaufmann H, Pichelmayer O, et al. Bortezomib, rituximab, and dexamethason (BORID) induces high response rates and durable complete remissions in patients with relapsed/refractory mantle cell lymphoma. Haematologica. 2007;92:435. [Google Scholar]
- 29.Leonard JP, Furman RR, Cheung Y-K, et al. Phase I/II trial of bortezomib + CHOP-rituximab in diffuse large B cell (DLBCL) and mantle cell lymphoma (MCL): phase I results. Blood. 2005;106:147a. [Google Scholar]
- 30.Mounier N, Ribrag V, Haioun C, et al. Efficacy and toxicity of two schedules of R-CHOP plus bortezomib in front-line B lymphoma patients: a randomized phase II trial from the Groupe d'Etude des Lymphomes de l'Adulte (GELA) J Clin Oncol. 2007;25:8010. [Google Scholar]
- 31.Kahl BS, Peterson C, Blank J, et al. A feasibility study of VcR-CVAD with maintenance rituximab for untreated mantle cell lymphoma. J Clin Oncol. 2007;25:456s. [Google Scholar]
- 32.Wiestner A, Dunleavy K, Rizzatti EG, et al. Potent single agent activity and tumor selectivity of bortezomib in mantle cell lymphoma: first impressions from a randomized phase II study of EPOCH-rituximab-bortezomib in untreated mantle cell lymphoma. Blood. 2005;106:266b. [Google Scholar]