Abstract
Background
Antibiotic resistance is increasing worldwide, being of special concern in low- and middle-income countries. The aim of this study was to determine the antimicrobial susceptibility and mechanisms of resistance in 205 enterotoxigenic Escherichia coli (ETEC) isolates from two cohort studies in children <24 months in Lima, Peru.
Methods
ETEC were identified by an in-house multiplex real-time PCR. Susceptibility to 13 antimicrobial agents was tested by disk diffusion; mechanisms of resistance were evaluated by PCR.
Results
ETEC isolates were resistant to ampicillin (64%), cotrimoxazole (52%), tetracycline (37%); 39% of the isolates were multidrug-resistant. Heat-stable toxin producing (ETEC-st) (48%) and heat-labile toxin producing ETEC (ETEC-lt) (40%) had higher rates of multidrug resistance than isolates producing both toxins (ETEC-lt-st) (21%), p<0.05. Only 10% of isolates were resistant to nalidixic acid and none to ciprofloxacin or cefotaxime. Ampicillin and sulfamethoxazole resistance were most often associated with blaTEM (69%) and sul2 genes (68%), respectively. Tetracycline resistance was associated with tet(A) (49%) and tet(B) (39%) genes. Azithromycin inhibitory diameters were ≤15 mm in 36% of isolates, with 5% of those presenting the mph(A) gene.
Conclusions
ETEC from Peruvian children are often resistant to older, inexpensive antibiotics, while remaining susceptible to ciprofloxacin, cephalosporins and furazolidone. Fluoroquinolones and azithromycin remain the drugs of choice for ETEC infections in Peru. However, further development of resistance should be closely monitored.
Keywords: Antibiotic resistance, Children, Diarrhoea, Enterotoxigenic Escherichia coli, Peru
Introduction
Among children under 5 years of age, there are approximately 2.5 billion cases of diarrhoea per year reported worldwide,1 resulting in 700 000–800 000 deaths per year globally, principally in low- and middle-income countries.2,3 In Peru, childhood diarrhoea is the third leading cause of death in children under 5,4 with its highest incidence in periurban and rural areas.
Rotavirus and diarrhoeagenic Escherichia coli (DEC) account for approximately 200 000 and 120 000 deaths per year, respectively, accounting collectively for 40% of all deaths due to childhood diarrhoea.2 Among DEC pathotypes, enterotoxigenic E. coli (ETEC) accounts for more than 40 000 deaths each year in children from developing countries, in addition to causing significant morbidity in adult travellers from industrialised countries to the developing world.5–7
Diarrhoea due to ETEC occurs between 8 and 72 hours after initial infection, usually following the ingestion of contaminated food or water.6 The severity of disease varies from a mild illness to severe disease and potentially to death. Typical symptoms include nausea, vomiting and resulting dehydration. Leukocytes and blood are usually not found in faeces.8 Oral rehydration therapy or, in severe cases, intravenous fluid replacement is the cornerstone of treatment. Antibiotic treatment plays a role in specific cases, such as persistent diarrhoea, nutritional deficiencies, or the presence of other pathology.9,10 Patients with persistent diarrhoea and abdominal pain due to ETEC may benefit from antibiotic therapy, with a more rapid resolution of symptoms.9 Patients with nutritional deficiencies or medical comorbidities may similarly have more severe presentations and require the use of antimicrobial agents.10 Unfortunately, the rapid emergence of antibiotic-resistant strains limits their usefulness.7,11,12 The dissemination of antimicrobial resistance genes among bacteria is an increasingly serious problem throughout the world.13–15
Peru is a developing country with an expanding tourism sector. Diarrhoea caused by contaminated food or water is very common in Peru and is a potentially serious threat for travellers visiting historical sites, including Lima. Thus, circulating strains in a particular country that primarily affect children and contaminate local water and food sources (as well as the hands of the food handlers) may determine the type of ETEC infecting travellers.6
Given the widespread geographical reach of ETEC, studies in different regions are mandatory to evaluate local variations in its virulence profile.16,17 Until a licensed ETEC vaccine is available, the profile of ETEC antimicrobial susceptibility must also be known in order to avoid inadequate therapy.6 Unfortunately, resistance to older, inexpensive antimicrobial agents, such as ampicillin or cotrimoxazole, is widespread among ETEC isolates in most low-income countries.17,18 In upper-middle-income countries such as Peru, the scenario is more complex, due to the relatively easy access to most modern antimicrobial agents, the availability of antibiotics without a prescription, and both the overprescription and misuse of antimicrobial agents.19
The aims of this study were to determine the antibiotic susceptibility of ETEC isolates from two cohort studies in Peruvian children, establishing the molecular mechanisms of resistance in these strains.
Materials and methods
Study population
The specimens analysed in this study were obtained as part of two previous studies.21,22 The first study (Cohort 1) was a prospective, passive surveillance cohort diarrhoea study of children 2 to 24 months of age, conducted in low socioeconomic communities in the southern districts of Lima, Peru, between September 2006 and December 2007 (1034 children younger than 1 year of age) and from January to July 2008 (529 children, 1–2 years of age, from the initial cohort were followed during this period).20 The second study (Cohort 2) was a community-based randomised double-blind placebo controlled trial comparing supplementation with bovine lactoferrin versus placebo conducted in low socioeconomic communities in the northern districts of Lima, Peru, between January 2008 and May 2011, in which 555 weaned children were enrolled at 12–18 months of age and followed for 6 months with daily home visits for data and sample collection and supplement administration.21 In this study, there were no differences in the frequency of ETEC isolation in both groups. Therefore, all strains were included in the current study. Both cohort studies were approved by the Institutional Review Board of the Universidad Peruana Cayetano Heredia, in Lima, Peru, and other participating institutions.
Strains
A total of 120 ETEC strains from children with diarrhoea (defined as three or more liquid or semiliquid stools in 24 hours or a single bloody semiliquid stool in 24 hours) and 85 ETEC strains from control children without diarrhoea 1 week before and 1 week after the stool collection sample were obtained from both cohorts. Briefly, stool samples were evaluated for common enteric pathogens (Shigella, Salmonella, Vibrio, Campylobacter, Giardia lamblia, Cryptosporidium and rotavirus) by conventional methods.23 Five lactose-positive colonies were isolated from MacConkey plates and tested by a multiplex PCR with specific DNA primers to detect virulence factors associated with enteropathogenic E. coli (EPEC), enteroinvasive E. coli (EIEC), enteroaggregative E. coli (EAEC), diffusely adherent E. coli (DAEC), Shiga toxin-producing E. coli (STEC) and ETEC, as described previously, using a validated five-colony pool analysis method.24,25 Subsequently, individual colonies from positive ETEC samples were analysed using separate PCR assays for the LT (heat-labile toxin) and ST (heat-stabile toxin) genes.24 Only one ST gene-positive and/or LT gene-positive colony per sample was then selected for further analysis. The ETEC strains were stored at -70°C in skim milk broth until use. In all cases, the isolates were re-identified prior to use amplifying the uidA gene as previously described.26
Antimicrobial susceptibility testing
Antimicrobial susceptibility was established by disk diffusion, according to Clinical and Laboratory Standards Institute (CLSI) guidelines.27 The antibiotics analysed were: ampicillin (Amp; 10-μg), cotrimoxazole (Sxt; 23.75/1.25-μg), tetracycline (Tet; 30-μg), nitrofurantoin (Nit; 300-μg), nalidixic acid (Nal; 30-μg), chloramphenicol (Chl; 30-μg), ciprofloxacin (Cip; 5-μg), gentamicin (Gm; 10-μg), cefotaxime (Ctx; 30-μg), amoxicillin-clavulanic acid (Amc; 30-μg), ceftazidime (Caz; 30-μg), and azithromycin (Azm; 15-μg). As no specific furazolidone resistant breakpoints are available, those of Nit were examined. The antibiotics tested were selected considering both their usefulness in the treatment of diarrhoea or their relevance at epidemiological level. The E. coli strain ATCC 25922 was used as quality control. Multidrug resistance (MDR) was defined as resistance to three or more classes of antimicrobial agents. Additionally, the presence of extended-spectrum β-lactamases (ESBLs) was established by the double disk synergy test as described elsewhere.28
Molecular mechanisms of antimicrobial resistance
Genes encoding common resistance mechanisms to β-lactams (blaTEM-like, blaCARB-like, blaSHV-like, blaOXA1-like, blaOXA2-like and blaOXA5-like), to Tet (tet(A) and tet(B) genes), Chl (cmlA, floR and cat genes), Sxt (dfrA1, dfrA5, dfrA6, dfrA7, dfrA8, dfrA12, dfrA13, dfrA14, dfrA15, dfrA15b, dfrA16, dfrA16b, and dfrA17 genes for trimethoprim and sul1, sul2, and sul3 genes for sulfamethoxazole), and Azm (mph(A), erm(A) and erm(B) genes) were studied by conventional PCR using previously described primers and conditions (Table 1) in those isolates presenting resistance to the corresponding antibiotic. As no clinical breakpoints have been established for Azm, the genes encoding resistance to this antimicrobial agent were sought in those isolates with an Azm inhibitory diameter zone of ≤15 mm.11 In all cases, the amplified products were visualized in 1.5% agarose gels containing 5% of SYBR® Safe Gel Stain (Invitrogen, Eugene, OR, USA). To determine the specific dfr genes, the amplified products were analysed by restriction fragment length polymorphism (RFLP) analysis as previously described.29
Table 1.
Primers and conditions for identification of molecular mechanisms of antibiotic resistance on enterotoxigenic Escherichia coli (ETEC) strains
| Antibiotic | Gene | Primer sequence |
Size of product (bp) | Annealing temperature (°C) | Reference | |
|---|---|---|---|---|---|---|
| Forward (5′–3′) | Reverse (5′–3′) | |||||
| β-Lactams | blaTEM-like | ATTCTTGAAGACGAAAGGGC | ACGCTCAGTGGAACGAAAAC | 1150 | 60 | 35 |
| blaSHV-like | ATGCGTTATATTCGCCTGTG | TTAGCGTTGCCAGTGCTCG | 841 | 55 | 61 | |
| blaCARB-like | AATGGCAATCAGCGCTTC | GGGGCTTGATGCTCACT | 586 | 56 | 58 | |
| blaOXA1-like | ACCAGATTCAACTTTCAA | TCTTGGCTTTTATGCTTG | 598 | 55 | 59 | |
| blaOXA2-like | CGATAGTTGTGGCAGACGAA | CCACTCAACCCATCCTACCC | 550 | 55 | 64 | |
| blaOXA5-like | TATATTCCAGCATCAACATT | ATGATGCCCTCACTTGCCAT | 605 | 55 | 61 | |
| Chloramphenicol | cmlA | TGTCATTTACGGCATACTCG | ATCAGGCATCCCATTCCCAT | 435 | 55 | 35 |
| floR | CACGTTGAGCCTCTATAT | ATGCAGAAGTAGAACGCG | 868 | 55 | 35 | |
| cat | GGTGAGCTGGTGATATGG | GGGATTGGCTGAGACGA | 209 | 48 | 62 | |
| Tetracycline | tet(A) | GTAATTCTGAGCACTGTCGC | CTGCCTGGACAACATTGCTT | 950 | 62 | 35 |
| tet(B) | CTCAGTATTCCAAGCCTTTG | CTAAGCACTTGTCTCCTGTT | 435 | 57 | 35 | |
| Trimethoprim | dfr1A, dfrA5, dfrA15, dfrA15b, dfrA16, dfrA16b | GTGAAACTATCACTAATGG | TTAACCCTTTTGCCAGATTT | 474 | 55 | 29 |
| dfrA6, dfrA14 | GAGCAGCTICTITTIAAAGC | TTAGCCCTTTIICCAATTTT | 393 | 60 | 29 | |
| dfrA7, dfrA17 | TTGAAAATTTCATTGATT | TTAGCCTTTTTTCCAAATCT | 474 | 55 | 29 | |
| dfrA8 | GAGCTTCCGGGTGTTCGTGAC | CTTCCATGCCATTCTGCTCGTAGT | 247 | 43 | 63 | |
| dfrA12, dfrA13 | GGTGGCGCAGAAGATTTTTCGC | TGGGAAGAAGGCGTCACCCTC | 319 | 60 | 29 | |
| Sulfamethoxazole | sul1 | TGGTGACGGTGTTCGGCATTC | GCGAGGGTTTCCGAGAAGGTG | 789 | 63 | 35 |
| sul2 | CGGCATCGTCAACATAACC | GTGTGCGGATGAAGTCAG | 722 | 50 | 35 | |
| sul3 | CATTCTAGAAAACAGTCGTAGTTCG | CATCTGCAGCTAACCTAGGGCTTTGGA | 990 | 51 | 35 | |
| Azithromycin | mph(A) | GTGAGGAGGAGCTTCGCGAG | TGCCGCAGGACTCGGAGGTC | 403 | 60 | 60 |
| erm(A) | TCTAAAAAGCATGTAAAAGAAA | CGATACTTTTTGTAGTCCTTC | 533 | 52 | 60 | |
| erm(B) | GAAAAAGTACTCAACCAAATA | AGTAACGGTACTTAAATT | 639 | 45 | 60 | |
In all cases, E. coli positive controls from bacterial collection of the Centre de Recerca en Salut Internacional de Barcelona (Barcelona, Spain) were used.
Statistical analysis
Results were analysed using EpiInfo version 3.4.3 (Centers for Disease Control and Prevention, Atlanta, GA, USA). The χ2 test or Fisher's exact test was used for comparisons between groups, as appropriate. Differences were considered statistically significant when p<0.05.
Results
A high proportion of ETEC isolates were resistant to Amp (64%), Sxt (52%), and Tet (37%). Only 10% of all ETEC isolates were resistant to Nal, while resistance rates for the other antibiotics evaluated were ≤10%. No isolate was resistant to Cip or Ctx. Thirty-nine percent (79/205) of the isolates were multidrug-resistant. ETEC-st isolates were more resistant than ETEC-lt-st to Amp (74% vs 47%, p<0.01), Sxt (62% vs 33%, p<0.01) and Tet (34% vs 16%, p<0.001), whereas ETEC-lt-st isolates presented higher resistance to Nal than ETEC-lt isolates (21% vs 7%, p<0.05) (Table 2). ETEC-st and ETEC-lt isolates (48% and 40%, respectively) presented with high rates of multidrug resistance than ETEC-lt-st (21%), p<0.05.
Table 2.
Antibiotic resistance rates of enterotoxigenic Escherichia coli (ETEC) strains isolated from Peruvian children
| No. (%) of ETEC strains |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Antibiotic | Cohort 1 |
Cohort 2 |
Toxin type |
All strains | ||||||
| Diarrhoea | Control | Total | Diarrhoea | Control | Total | LT | ST | LT-ST | ||
| (n=57) | (n=26) | (n=83) | (n=63) | (n=59) | (n=122) | (n=99) | (n=63) | (n=43) | (n=205) | |
| Amp | 42 (74) | 17 (65) | 59 (71)a | 36 (57) | 36 (61) | 72 (59)a | 73 (74) | 38 (60)c | 20 (47)c | 131 (64) |
| Sxt | 35 (61) | 16 (62) | 51 (61) | 29 (46) | 27 (46) | 56 (46) | 61 (62) | 32 (51)c | 14 (33)c | 107 (52) |
| Tet | 22 (39) | 12 (46) | 34 (41) | 20 (32) | 22 (37) | 42 (34) | 34 (34) | 35 (56)c | 7 (16)c | 76 (37) |
| Amc | 11 (19) | 4 (15) | 15 (18)b | 1 (2) | 2 (3) | 3 (2)b | 12 (12) | 3 (5) | 3 (7) | 18 (9) |
| Nit | 8 (14) | 3 (12) | 11 (13) | 4 (6) | 2 (3) | 6 (5) | 5 (5) | 12 (19) | 0 | 17 (8) |
| Nal | 4 (7) | 3 (12) | 7 (8) | 7 (11) | 7 (12) | 14 (11) | 7 (7)a | 5 (8) | 9 (21)a | 21 (10) |
| Chl | 2 (4) | 3 (12) | 5 (6) | 5 (8) | 4 (7) | 9 (7) | 11 (11) | 2 (3) | 1 (2) | 14 (7) |
| Caz | 2 (4) | 0 | 2 (2) | 0 | 1 (2) | 1 (1) | 2 (2) | 0 | 1 (2) | 3 (1) |
| Gm | 0 | 0 | 0 | 0 | 1 (2) | 1 (1) | 1 (1) | 0 | 0 | 1 (0) |
| Multiresistant | 22 (39) | 12 (46) | 34 (41) | 24 (38) | 21 (36) | 45 (37) | 40 (40)a | 30 (48)a | 9 (21)a | 79 (39) |
Amc: amoxicillin-clavulanic acid; Amp: ampicillin; Caz: ceftazidime; Chl: chloramphenicol; Gm: gentamicin; LT: heat-labile toxin; Nal: nalidixic acid; Nit: nitrofurantoin; ST: heat-stabile toxin; Sxt: cotrimoxazole; Tet: tetracycline. No isolate was resistant to ciprofloxacin or cefotaxime.
a p<0.05.
b p<0.001.
c p<0.01.
The rates of antibiotic resistance of Cohort 1 (n=83) were significantly more resistant to Sxt (61% vs 46%, p<0.05) and to Amc (18% vs 2%, p<0.001) when compared with those of Cohort 2 (n=122). Cohort 1 also had higher resistance rates than Cohort 2 for most commonly-used antibiotics such as Amp (71% vs 59%), Tet (41% vs 34%) and Nit (13% vs 5%) (Table 2). ETEC isolates from Cohort 1 tended to be more multiresistant than Cohort 2 (41% vs 37%), however this difference was not statistically significant. Although no evidence of ESBLs was observed, 1% of strains (three isolates) were resistant to Caz. Resistance to three or more antibiotics was more commonly detected in ETEC strains from Cohort 1 than those of Cohort 2 (47% vs. 32%, respectively, p<0.05). The most common multi-drug resistance phenotype was AmpR SxtR TetR present, with or without resistance to other antibiotics (57 out 205 strains, 27.8%) (Table 3).
Table 3.
Antibiotic resistance patterns for the enterotoxigenic Escherichia coli (ETEC) strains isolated from Peruvian children
| Resistant ETEC isolates, n (%) |
||||||
|---|---|---|---|---|---|---|
| Pattern | MDR resistance patterns | Cohort 1 | Cohort 2 | Diarrhoea | Control | All strains |
| (n=83) | (n=122) | (n=120) | (n=85) | (n=205) | ||
| I | AmpR CazR AmcR SxtR TetR NitR | 0 | 1 (1) | 0 | 1 (1) | 1 (0.5) |
| II | AmpR AmcR SxtR TetR NitR | 3 (4) | 0 | 3 (3) | 0 | 3 (1.5) |
| III | AmpR NalR GmR SxtR TetR | 0 | 1 (1) | 0 | 1 (1) | 1 (0.5) |
| IV | AmpR SxtR TetR NitR | 5 (6) | 2 (2) | 6 (5) | 1 (1) | 7 (3.4) |
| V | AmpR NalR SxtR TetR | 1 (1) | 4 (3) | 1 (1) | 4 (5) | 5 (2.4) |
| VI | AmpR AmcR SxtR TetR | 3 (4) | 1 (1) | 1 (1) | 3 (4) | 4 (2.0) |
| VII | AmpR SxtR TetR ChlR | 0 | 3 (2) | 2 (2) | 1 (1) | 3 (1.5) |
| VIII | NalR SxtR TetR ChlR | 2 (2) | 0 | 0 | 2 (2) | 2 (1.0) |
| IX | AmpR CazR AmcR SxtR | 1 (1) | 0 | 1 (1) | 0 | 1 (0.5) |
| X | AmpR AmcR TetR ChlR | 1 (1) | 0 | 0 | 1 (1) | 1 (0.5) |
| XI | AmpR SxtR TetR | 14 (17) | 19 (16) | 18 (15) | 15 (18) | 33 (16.1) |
| XII | AmpR NalR SxtR | 2 (2) | 4 (3) | 5 (4) | 1 (1) | 6 (2.9) |
| XIII | AmpR AmcR SxtR | 5 (6) | 0 | 4 (3) | 1 (1) | 5 (2.4) |
| XIV | AmcR TetR ChlR | 1 (1) | 2 (2) | 3 (3) | 0 | 3 (1.5) |
| XV | AmpR SxtR NitR | 0 | 2 (2) | 2 (2) | 0 | 2 (1.0) |
| XVI | AmpR AmcR ChlR | 1 (1) | 0 | 1 (1) | 0 | 1 (0.5) |
| NA | Resistance to three or more antibiotics (MDR) | 39 (47)a | 39 (32)a | 47 (39) | 31 (36) | 78 (38) |
| NA | Resistance to two antibiotics | 21 (25) | 25 (20) | 28 (23) | 18 (21) | 46 (22.4) |
| NA | Resistance to only one antibiotic | 6 (7) | 22 (18) | 14 (12) | 14 (16) | 28 (13.7) |
| NA | Pan-susceptible | 17 (20) | 36 (30) | 31 (26) | 22 (26) | 53 (25.9) |
Amc: amoxicillin-clavulanic acid; Amp: ampicillin; Caz: ceftazidime; Chl: chloramphenicol; Gm: gentamicin; MDR: multidrug resistance; NA: not applicable; Nal: nalidixic acid; Nit: nitrofurantoin; Sxt: cotrimoxazole; Tet: tetracycline.
a p<0.05
The distribution of the growth inhibitory zones to Azm varied between Cohort 1 and Cohort 2, with inhibitory zone diameters less than 10 mm in 5% and 2%; 11–15 mm in 46% and 24%; 16–20 mm in 18% and 68%; and more than 21 mm in 31% and 6%, respectively.
Among all ETEC isolates, antibiotic resistance was related mainly to the presence of blaTEM-like β-lactamases (91/131, 69.5%) for Amp; dfrA15 gene (14/107, 13.1%) for trimethoprim, sul2 gene (73/107, 68.2%) for sulfamethoxazole, tet(A) gene (37/76, 49%) for Tet, and cat gene (10/14, 71%) for Chl resistance (Table 4). Seventy-three out of 205 isolates (36%) had Azm inhibitory diameter zones of less than 15 mm, and 5% (4/73) of those were related to the presence of mph(A).
Table 4.
Mechanisms of resistance of enterotoxigenic Escherichia coli (ETEC) isolates from Peruvian children
| Antimicrobial | Mechanism of resistance | No. of isolates with indicated mechanism of resistance/total no. of resistant isolates (%) |
||||
|---|---|---|---|---|---|---|
| Cohort 1 | Cohort 2 | Diarrhoea | Control | All strains | ||
| Ampicillina | blaTEM-like | 31/59 (53)b | 60/72 (83)b | 53/78 (68) | 38/53 (72) | 91/131 (69) |
| blaOXA1-like | 3/59 (5) | 8/72 (11) | 7/78 (9) | 4/53 (8) | 11/131 (8) | |
| blaCARB-like | 0/59 (0) | 2/72 (3) | 1/78 (1) | 1/53 (2) | 2/131 (2) | |
| Non determined | 25/59 (42)c | 6/72 (8)c | 20/78 (26) | 11/53 (21) | 31/131 (24) | |
| Chloramphenicol | cat | 3/5 (60) | 7/9 (78) | 6/7 (86) | 4/7 (57) | 10/14 (71) |
| floR | 1/5 (20) | 1/9 (11) | 1/7 (14) | 1/7 (14) | 2/14 (14) | |
| Non determined | 1/5 (20) | 1/9 (11) | 0/7 (0) | 2/7 (29) | 2/14 (14) | |
| Tetracyclined | tet(A) | 15/34 (44) | 22/42 (52) | 21/42 (50) | 16/34 (47) | 37/76 (49) |
| tetB | 8/34 (24)e | 22/42 (52)e | 17/42 (40) | 13/34 (38) | 30/76 (39) | |
| Non determined | 11/34 (32)c | 0/52 (0)c | 5/42 (12) | 6/34 (18) | 11/76 (14) | |
| Sulfamethoxazolef | sul2 | 30/51 (59)g | 43/56 (77)g | 44/64 (69) | 29/43 (67) | 73/107 (68) |
| sul1 | 13/51 (25) | 13/56 (23) | 14/64 (22) | 12/43 (28) | 26/107 (24) | |
| sul3 | 1/51 (2) | 0/56 (0) | 1/64 (2) | 0/43 (0) | 1/107 (1) | |
| Non determined | 9/51 (18) | 5/56 (9) | 7/64 (11) | 7/43 (16) | 14/107 (13) | |
| Trimethoprimh | dfrA1 | 4/51 (8) | 7/56 (13) | 8/64 (13) | 3/43 (7) | 11/107 (10) |
| dfrA15 | 8/51 (16) | 6/56 (11) | 8/64 (13) | 6/43 (14) | 14/107 (13) | |
| dfrA7 | 2/51 (4) | 5/56 (9) | 4/64 (6) | 3/43 (7) | 7/107 (7) | |
| dfrA5 | 0/51 (0) | 2/56 (4) | 2/64 (3) | 2/43 (5) | 4/107 (4) | |
| dfrA14 | 0/51 (0) | 3/56 (5) | 2/64 (3) | 1/43 (2) | 3/107 (3) | |
| dfrA17 | 0/51 (0) | 2/56 (4) | 0/64 (0) | 2/43 (5) | 2/107 (2) | |
| Non determined | 38/51 (75)c | 31/56 (55)c | 41/64 (64) | 27/43 (63) | 68/107 (64) | |
| Azithromycin | mph(A) | 1/42 (2) | 3/31 (10) | 2/29 (7) | 2/44 (5) | 4/73 (5) |
| Non determined | 41/42 (98) | 28/31 (90) | 27/29 (93) | 42/44 (95) | 69/73 (95) | |
a Two isolates from Cohort 2 presented blaTEM-like and blaOXA1-like concomitantly, also both blaCARB-like were found concomitantly with blaTEM-like.
b p<0.001.
c p=0.0001.
d Two isolates from Cohort 2 possessed both the tet(A) and tet(B) genes.
e p<0.05.
f Seven isolates (2 from Cohort 1, and 5 from Cohort 2) presented the sul1 and sul2 genes concomitantly.
g p=0.061.
h the dfrA7 gene was found concomitantly with dfrA1 gene (1 case) and dfrA15 gene (1 case).
The presence of antibiotic resistance mechanisms was also analysed by source (Cohort 1 vs Cohort 2). All studied molecular mechanisms of resistance present in ETEC from Cohort 2 were also present in the strains from Cohort 1, except for dfrA5, dfrA14 and dfrA17 genes conferring trimethoprim resistance and blaCARB-like β-lactamase conferring Amp resistance (Table 4). The blaTEM-like genes (83% vs 53%, p<0.001) and tet(B) gene (52% vs 24%, p<0.05) were more frequently detected in Cohort 2-strains than in Cohort 1-strains, respectively (p<0.05). Additionally, in other cases, as account for sul2, a clear trend toward higher presence in Cohort 2 was also observed, but under significance breakpoint. The concomitant presence of multiple resistance mechanisms of resistance affecting the same antibacterial family was detected in a subset of cases, most often from Cohort 2 (Table 4).
No blaSHV-like, blaOXA2-like and blaOXA5-like genes were detected. Similarly, we did not detect any isolates with cmlA, dfrA6, dfrA8, dfrA12, dfrA13, dfrA15b, dfrA16, or dfrA16b genes.
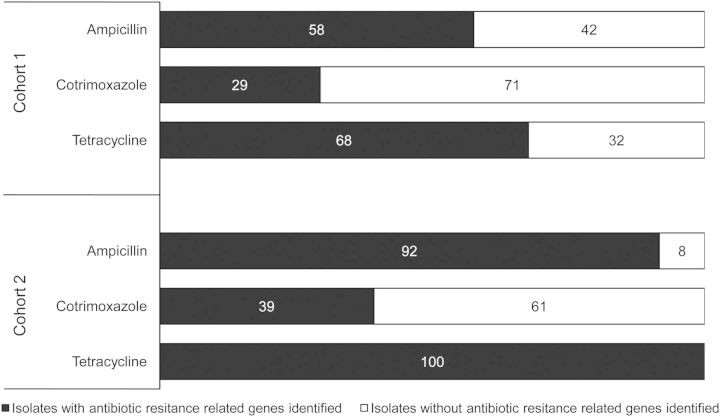
Significant proportions of antibiotic-resistant strains did not exhibit any of the resistance genes tested. These isolates predominated in Cohort 1, in which a significant number of isolates did not have identifiable mechanisms of resistance to trimethoprim (p<0.05), tetracycline, or β-lactam agents (p=0.0001 in both cases). In Cohort 1, only 58% of Amp-resistant strains had at least one of the β-lactamase genes identified, while 68% of Tet-resistant strains had at least one specific mechanism of resistance found (Table 4). A small proportion of Sxt-resistant isolates possessed at least one associated resistance mechanisms (29%) (Figure 1). In Cohort 2, the majority of the Amp-resistant isolates had at least one β-lactamase gene detected (92%), while all Tet-resistant isolates had the tet(A) and/or tet(B) genes. Only 39% of Sxt-resistant isolates in Cohort 2 possessed any of the Sxt-resistance genes under evaluation.
Figure 1.
Presence of antimicrobial resistance-related genes. Percentages of resistant ETEC isolates presenting at least one of the analysed mechanisms of antibiotic resistance for each included antibiotic family. For Sxt it is considered the concomitant presence of at least one gene related to sulfamethoxazole resistance and at least another for trimethoprim resistance. In black, presence of antibiotic resistance genes. In white, absence of antibiotic resistance genes.
Discussion
Antibiotics are often used in patients with severe enteritis, dysentery and persistent diarrhoea.30 However, in paediatric diarrhoea, the risks and benefits of antibiotic use are not fully defined because of the multiple bacterial and viral agents implicated in childhood infections and the inability to distinguish between pathogens on clinical grounds. In addition, conventional laboratory diagnostics are limited in their ability to detect many common enteropathogens, particularly ETEC and other DEC. As such, it has been difficult to study the effect of antimicrobials in children with specific pathogens such as ETEC. This often results in widespread empiric use of antimicrobial agents which drive towards antibiotic resistance. Antimicrobial resistance in Peru is currently a great challenge, related in part to the high rates of antibiotic use as well as inadequate dosing and inappropriate antibiotic selection for a given infection.31 This situation may be extrapolated to most other low and middle income countries, each one with its particularities.
Antibiotic resistance was widespread among analysed ETEC strains, mainly to common antibiotics such as Amp (64%) and Sxt (52%). This finding is similar to descriptions of children in different countries, such as Egypt (63% and 52%), Mexico (73% and 65%) and Mozambique (46% and 61%) or to that described in international travellers (63% in both cases).7,18,32,33 These antimicrobial agents had been largely used for treatment of diarrhoea in children for years,34 and despite the aforementioned general high resistance rates, they remained in common use in many countries due to the lack of available alternatives. EUCAST criteria (http://www.eucast.org/clinical_breakpoints/), which are based on clinical parameters and epidemiological cut-off values, generally use lower breakpoints to classify Enterobacteriaceae as resistant, especially for β-lactam antibiotics and fluoroquinolones. However, the application of EUCAST criteria does not alter the quantified level of antibiotic resistance described in terms of zone inhibition. Qualitatively, only a few isolates in our study would be re-classified as resistant to a given antimicrobial agent, mainly due to the fact that relatively few microorganisms classified as susceptible in our study had borderline-to-intermediate levels of susceptibility.
This is a worrisome finding because ETEC isolates, and especially ETEC-st, are among the diarrhoeagenic pathogens most associated with child mortality worldwide.2,5 In the present study and in accordance with that described by different authors,35,36 the most commonly detected β-lactamases encoding genes were the blaTEM-like genes, sometimes co-existing with other β-lactamases such as blaOXA1-like or blaCARB-like. Meanwhile different dfrA and sul genes were identified.
Based on our findings, these high levels of resistance should preclude the empiric use of Amp or Sxt if ETEC is suspected or confirmed. Different alternatives may be considered, including fluoroquinolones, cephalosporins, macrolides or furazolidone.
Cip remains appropriate as empiric therapy in adults, and its safety and utility in children with enteric infections has been previously shown.37,38 However, the 10% of strains resistant to Nal observed in this study, and the 68% and 28% of resistance to Nal and Cip, respectively, detected in commensal E. coli strains isolated from Peruvian children, highlight the importance of ongoing surveillance.39 Given the additive effect of fluoroquinolone resistance mechanisms, the presence of Nal-resistance is a risk factor for the development of complete fluoroquinolone resistance and subsequent treatment failures.40,41 Of additional concern is the spread of different transferable mechanisms of quinolone resistance,13,42 which may facilitate the development of fluoroquinolone resistance in absence of previous detected Nal resistance.41
Our results show that cephalosporins remain highly active against ETEC isolates. Only three isolates showed resistance to Caz and none to Ctx. Although no ESBL production was detected by double disk diffusion tests in our study, high rates of ESBLs (in excess of 75%) have been described among Enterobacteriaceae, including pathogenic E. coli, in other clinical syndromes in Peru.43 In the present study, in the absence of ESBLs, Caz resistance may be secondary to other mechanisms such as overexpression of efflux pumps, or, more probably, to the presence of plasmid-encoded AmpC. The ability of AmpC to hydrolyse cephalosporins is not uniform across the class and may result with resistance to some specific cephalosporins, with others retaining activity. An analysis of the effect of plasmid-encoded AmpC showed that 33% and 50% of Enterobacteriaceae isolates carrying AmpC were Ctx-susceptible and -intermediate respectively, while only 16% of them were susceptible or intermediate to Caz.44
The situation of Azm is also worrisome, with a high number of isolates with a halo diameter lower than 15 mm. However, our analysis of the most prevalent transferable mechanisms of resistance showed the presence of only four isolates carrying the mph(A) gene, the most extensively described worldwide among Enterobacteriaceae.45,46 This may be partially explained by the problems related to the use of Azm disks, as previously described.47 The presence of a number of isolates in which no halo was observed confirms the presence of Azm-resistance in the ETEC isolates circulating in the study area, which may be related to target alterations, other transferable mechanisms of resistance (http://faculty.washington.edu/marilynr/), or the overexpression of efflux pumps.48
Low resistance levels to Nit were observed. Despite concerns about the carcinogenic potential of nitrofurans, furazolidone is considered acceptable for the treatment of paediatric diarrhoea in Peru and other Latin American countries.49,50 These data, together with the low frequency of selection of furazolidone-resistant mutants,51 show its utility in clinical usage.
In most low and middle income countries, enteric co-infections may be the rule rather than the exception. Moreover, asymptomatic carriage of diarrhoeagenic pathogens, as described in the present study, is common.52 With the high sensitivity of current molecular diagnostic techniques,53 co-infections (or co-colonization) may be more frequently identified. In certain cases, quantitative measurement of pathogen DNA in stools may clarify the specific aetiology of a particular case of diarrhoea.54 The high rate of asymptomatic carriage in developing countries highlights the need to include samples from apparent healthy people in epidemiological studies,55 as well as the need for a greater understanding of the role of the general microbiome of symptomatic and asymptomatic hosts alike.
Overall, the study showed a wide variety of transferable antibiotic-resistance mechanisms among isolates. The ease with which ETEC may acquire antibiotic resistance mechanisms highlights the need for stricter control of antimicrobial agents in order to maximize the benefits of antibiotic therapies when needed.
Both cohorts showed differences in the rates of antimicrobial resistance. Interestingly, we were unable to detect specific antibiotic resistance mechanisms in some of the strains analysed. In the case of Sxt, the overall high number of isolates from both cohorts in which no detectable dfrA gene was found, suggests that the most frequent mechanisms for Sxt resistance in the region of Lima remain undetected. In the case of Tet and Amp, the resistant isolates in which no mechanisms of resistance were detected were limited to Cohort 1. This may reflect differences in the nature of the antibiotic pressure which is exerted over microorganisms on both studied areas. Interestingly, high levels of resistance to rifaximin mediated by the overexpression of efflux pumps have been described in the same area as Cohort 1.56 Overexpression of efflux pumps may also be involved in the resistance to Amp and other antibiotics and may be induced by different bacterial stressors, including the presence of specific environmental toxics.57
A limitation of the study is that molecular mechanisms of antibiotic resistance were only studied in antibiotic-resistant or intermediate isolates but not in susceptible isolates; these could hypothetically act as a hidden antibiotic resistance reservoir while remaining unexpressed.
In conclusion, ETEC isolates in periurban Lima showed high rates of resistance to the most available and inexpensive antibiotics while remaining broadly susceptible to cephalosporins, furazolidone and fluoroquinolones. An effort to control the use of antimicrobial agents is needed in order to preserve their utility when necessary. Ongoing surveillance of antimicrobial resistance is also needed to detect the presence of isolates exhibiting resistance to first-line therapies.
Acknowledgments
Authors' disclaimers: The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, the U.S. Government, nor that of the National Institutes of Health and other funding institutions.
MB, RM and RCM are employees of the U.S. Government. This work was prepared as part of their official duties. Title 17 U.S.C. §105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.’ Title 17 U.S.C. §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person's official duties.
Authors' contributions: AMM and FPR contributed equally to this work. RCM, CFL, JR and TJO conceived the study; AMM, FPR, MJP, MR, CG, MB, RM, LH, EC-W, AIG develop all the laboratory procedures. AMM, FPR, TJO and JR analyse the data. AMM, FPR, TJO and HR drafted the manuscript. MJP, CG, TJO and JR critically revised the manuscript for intellectual content. All authors read and approve the final manuscript. TJO and JR are guarantors of the paper.
Funding: This work was partially supported by: Agencia Española de Cooperación Internacional para el Desarrollo (AECID), Spain, Programa de Cooperación Interuniversitaria e Investigación Científica con Iberoamérica [D/019499/08, D/024648/09, D/030509/10, and A1/035720/11] (JR and TJO); National Institute of Child Health and Human Development, USA [Public Health Service award R01-HD051716] (EC-W. and TJO); by the Spanish Network for the Research in Infectious Diseases [REIPI RD12/0015] (JR); and by Generalitat de Catalunya, Departament d'Universitats, Recerca i Societat de la Informació [2014 SGR 26] (JR); AMM attended a 3-month training through the CRESIB-UPCH agreement, training was partially funded by Consejo Nacional de Ciencia, Tecnología e Innovación Tecnológica (CONCYTEC, FONDECYT) in Peru. JR has a fellowship from the program I3, of the ISCIII [grant number CES11/012]. MJP has a postdoctoral fellowship from CONCYTEC/FONDECYT.
Competing interests: None declared.
Ethical approval: Not required.
References
- 1.UNICEF/WHO. Diarrhoea: Why children are still dying and what can be done. Geneva: World Health Organization; 2009 [Google Scholar]
- 2.Lanata CF, Fischer-Walker CL, Olascoaga AC, et al. Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PLoS One 2013;8:Le72788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 2012;379:2151–61. [DOI] [PubMed] [Google Scholar]
- 4.Huicho L, Trelles M, Gonzales F. National and sub-national under-five mortality profiles in Peru: a basis for informed policy decisions. BMC Public Health 2006;6:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 2013;382:209–22. [DOI] [PubMed] [Google Scholar]
- 6.Qadri F, Svennerholm AM, Faruque AS, Sack RB. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin Microbiol Rev 2005;18:465–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruiz J, Mensa L, O'Callaghan C, et al. In vitro antimicrobial activity of rifaximin against enteropathogens causing traveler's diarrhea. Diagn Microbiol Infect Dis 2007;59:473–5. [DOI] [PubMed] [Google Scholar]
- 8.Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat Rev Microbiol 2004;2:123–40. [DOI] [PubMed] [Google Scholar]
- 9.Becker SL, Vogt J, Knopp S, et al. Persistent digestive disorders in the tropics: causative infectious pathogens and reference diagnostic tests. BMC Infect Dis 2013;13:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Ryan GM, Ashkenazi-Hoffnung L, O'Ryan-Soriano MA, Ashkenazi S. Management of acute infectious diarrhea for children living in resource-limited settings. Expert Rev Anti Infect Ther. 2014;12:621–32. [DOI] [PubMed] [Google Scholar]
- 11.Ochoa TJ, Ruiz J, Molina M, et al. High frequency of antimicrobial drug resistance of diarrheagenic Escherichia coli in infants in Peru. Am J Trop Med Hyg 2009;81:296–301. [PMC free article] [PubMed] [Google Scholar]
- 12.Wennerås C, Erling V. Prevalence of enterotoxigenic Escherichia coli-associated diarrhoea and carrier state in the developing world. J Health Pop Nutr 2004;22:370–82. [PubMed] [Google Scholar]
- 13.Pons MJ, Mosquito S, Gomes C, et al. Analysis of quinolone-resistance in commensal and diarrheagenic Escherichia coli isolates from infants in Lima, Peru. Trans R Soc Trop Med Hyg 2014;108:22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pons MJ, Vubil D, Guiral E, et al. 2015. Characterisation of extended spectrum β-Lactamases among Klebsiella pneumoniae isolates causing bacteraemia and urinary tract infection in Mozambique. J Global Antimicrob Resist 2015;3:19–25 [DOI] [PubMed] [Google Scholar]
- 15.Wellington EM, Boxall AB, Cross P, et al. The role of the natural environment in the emergence of antibiotic resistance in gram-negative bacteria. Lancet Infect Dis 2013;13:155–65. [DOI] [PubMed] [Google Scholar]
- 16.Rivera FP, Ochoa TJ, Maves RC, et al. Genotypic and phenotypic characterization of enterotoxigenic Escherichia coli strains isolated from Peruvian children. J Clin Microbiol 2010;48:3198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rivera FP, Medina AM, Aldasoro E, et al. Genotypic characterization of enterotoxigenic Escherichia coli strains causing traveler's diarrhea. J Clin Microbiol 2013;51:633–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandomando IM, Macete EV, Ruiz J, et al. Etiology of diarrhea in children younger than 5 years of age admitted in a rural hospital of southern Mozambique. Am J Trop Med Hyg 2007;76:522–7. [PubMed] [Google Scholar]
- 19.Okeke IN. Diarrheagenic Escherichia coli in sub-Saharan Africa: status, uncertainties and necessities. J Infect Dev Ctries 2009;3:817–42. [DOI] [PubMed] [Google Scholar]
- 20.Ecker L, Ochoa TJ, Vargas M, et al. Factors affecting caregivers' use of antibiotics available without a prescription in Peru. Pediatrics 2013;131:1771–9. [DOI] [PubMed] [Google Scholar]
- 21.Ochoa TJ, Chea-Woo E, Baiocchi N, et al. Randomized double-blind controlled trial of bovine lactoferrin for prevention of diarrhea in children. J Pediatr 2013;162:349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ochoa TJ, Ecker L, Barletta F, et al. Age-related susceptibility to infection with diarrheagenic Escherichia coli among infants from Periurban areas in Lima, Peru. Clin Infect Dis 2009;49:1694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murray PR, Baron EJ, Jorgensen JH, et al. Manual of Clinical Microbiology. 9th ed Washington, DC, USA: ASM Press; 2007. [Google Scholar]
- 24.Guion CE, Ochoa TJ, Walker CM, et al. Detection of diarrheagenic Escherichia coli by use of melting-curve analysis and real-time multiplex PCR. J Clin Microbiol 2008;46:1752–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barletta F, Ochoa TJ, Ecker L, Gil AI, et al. Validation of a five-colony pool analysis using Multiplex Real Time PCR for detection of diarrheagenic Escherichia coli. J Clin Microbiol 2009;47:1915–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walk ST, Alm EW, Gordon DM, et al. Cryptic lineages of the genus Escherichia. Appl Environ Microbiol 2009;75:6534–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.CLSI. Performance standards for antimicrobial susceptibility testing. Twenty-first Informational Supplement M100-S21.2011. Wayne, PA, USA: Clinical and Laboratory Standards Institute; 2011. [Google Scholar]
- 28.Drieux L, Brossier F, Sougakoff W, Jarlier V. Phenotypic detection of extended-spectrum β-lactamase production in Enterobacteriaceae: review and bench guide. Clin Microbiol Infect 2008;14(Suppl 1):90–103. [DOI] [PubMed] [Google Scholar]
- 29.Navia MM, Ruiz J, Sanchez-Cespedes J, Vila J. Detection of dihydrofolate reductase genes by PCR and RFLP. Diagn Microbiol Infect Dis 2003;46:295–8. [DOI] [PubMed] [Google Scholar]
- 30.Pickering LK. Antimicrobial resistance among enteric pathogens. Semin Pediatr Infect Dis 2004;15:71–7. [DOI] [PubMed] [Google Scholar]
- 31.Ecker L, Olarte L, Vilchez G, et al. Physicians' responsibility for antibiotic use in infants from periurban Lima, Peru. Rev Panam Salud Publica 2011;30:574–9. [PubMed] [Google Scholar]
- 32.Estrada-García T, Cerna JF, Paheco-Gil L, et al. Drug-resistant diarrheogenic Escherichia coli, Mexico. Emerg Infect Dis 2005;11:1306–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaheen HI, Khalil SB, Rao MR, et al. Phenotypic profiles of enterotoxigenic Escherichia coli associated with early childhood diarrhea in rural Egypt. J Clin Microbiol 2004;42:5588–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diniz-Santos DR, Silva LR, Silva N. Antibiotics for the empirical treatment of acute infectious diarrhea in children. Braz J Infect Dis 2006;10:217–27. [DOI] [PubMed] [Google Scholar]
- 35.Sáenz Y, Ruiz J, Zarazaga M, et al. Effect of efflux pump inhibitor Phe-Arg-β-naphthylamide on the MIC values of quinolones, tetracycline and chloramphenicol in Escherichia coli isolates of different origin. J Antimicrob Chemother 2002;53:544–5. [DOI] [PubMed] [Google Scholar]
- 36.Salverda ML, De Visser JA, Barlow M. Natural evolution of TEM-1 β-lactamase: experimental reconstruction and clinical relevance. FEMS Microbiol Rev 2010;34:1015–36. [DOI] [PubMed] [Google Scholar]
- 37.Murray TS, Baltimore RS. Pediatric uses of fluoroquinolone antibiotics. Pediatr Ann 2007;36:336–42. [DOI] [PubMed] [Google Scholar]
- 38.Principi N, Esposito S. Appropriate use of fluoroquinolones in children. Int J Antimicrob Agents 2015;45:341–6. [DOI] [PubMed] [Google Scholar]
- 39.Mosquito S, Ruiz J, Pons MJ, et al. Molecular mechanisms of antibiotic resistance in diarrhoeagenic Escherichia coli isolated from children. Int J Antimicrob Agents 2012;40:544–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruiz J. Mechanisms of resistance to quinolones: Target alterations, decreased accumulation and DNA gyrase protection. J Antimicrob Chemother 2003;51:1109–17. [DOI] [PubMed] [Google Scholar]
- 41.Ruiz J, Pons MJ, Gomes C. Transferable mechanisms of quinolone resistance. Int J Antimicrob Agents 2012;40:196–203. [DOI] [PubMed] [Google Scholar]
- 42.Riveros M, Riccobbono E, Durand D, et al. Plasmid-mediated quinolone-resistance genes in enteroaggregative Escherichia coli (EAEC) from infants in Lima, Perú. Int J Antimicrob Agents 2012;39:540–2. [DOI] [PubMed] [Google Scholar]
- 43.Garcia C, Horna G, Linares E, et al. Antimicrobial drug resistance in Peru. Emerg Infect Dis 2012;18:520–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kohner PC, Robberts FJ, Cockerill FR, 3rd, Patel R. Cephalosporin MIC distribution of extended-spectrum-β-lactamase- and pAmpC-producing Escherichia coli and Klebsiella species. J Clin Microbiol 2009;47:2419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Howie RL, Folster JP, Bowen A, et al. Reduced azithromycin susceptibility in Shigella sonnei, United States. Microb Drug Resist 2010;16:245–8. [DOI] [PubMed] [Google Scholar]
- 46.Wu G, Day MJ, Mafura MT, et al. Comparative analysis of ESBL-positive Escherichia coli isolates from animals and humans from the UK, The Netherlands and Germany. PLoS One 2013;8:e75392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jain SK, Gupta A, Glanz B, et al. Antimicrobial-resistant Shigella sonnei: limited antimicrobial treatment options for children and challenges of interpreting in vitro azithromycin susceptibility. Pediatr Infect Dis J 2005;24:494–7. [DOI] [PubMed] [Google Scholar]
- 48.Gomes C, Pons MJ, Magallon-Tejada A, et al. In vitro development and analysis of Escherichia coli and Shigella boydii azithromycin-resistant mutants. Microb Drug Resist 2013;19:88–93. [DOI] [PubMed] [Google Scholar]
- 49.Tratamiento de neumonía, shigellosis e ITU. Boletin AIS-COIME 2006;29:9–10. [Google Scholar]
- 50.Ecker L, Ochoa TJ, Vargas M, et al. Preferencias de uso de antibióticos en niños menores de 5 años por médicos de centros de atención primaria en zonas peri-urbanas de Lima, Perú. Rev Peru Med Exp Salud Publica. 2013;30:181–9. [PubMed] [Google Scholar]
- 51.Martinez-Puchol S, Gomes C, Pons MJ, et al. Development and analysis of furazolidone-resistant diarrhoeagenic Escherichia coli mutants. APMIS 2015DOI:10.1111/apm.12401. DOI:10.1111/apm.12401. [DOI] [PubMed] [Google Scholar]
- 52.Nhampossa T, Mandomando I, Acacio S, et al. Diarrheal disease in rural Mozambique: burden, risk factors and etiology of diarrheal disease among children aged 0–59 months seeking care at health facilities. PLoS One 2015;10:e0119824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frickmann H, Schwarz NG, Rakotozandrindrainy R, et al. PCR for enteric pathogens in high-prevalence settings. What does a positive signal tell us? Infect Dis (Lond) 2015;47:491–8. [DOI] [PubMed] [Google Scholar]
- 54.Barletta F, Ochoa TJ, Mercado E, et al. Quantitative Real-Time polymerase chain reaction for enteropathogenic Escherichia coli: a tool for investigation of asymptomatic versus symptomatic infections. Clin Infect Dis 2011;53:1223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dubourg G, Fenollar F. Epidemiologic studies need asymptomatic controls. Clin Microbiol Infect 2015;21:e51–2. [DOI] [PubMed] [Google Scholar]
- 56.Gomes C, Ruiz L, Pons MJ, et al. Relevant role of efflux pumps in the high levels of rifaximin resistance in Escherichia coli clinical isolates. Trans R Soc Trop Med Hyg 2013;107:545–9. [DOI] [PubMed] [Google Scholar]
- 57.Ramos JL, Duque E, Gallegos MT, et al. Mechanisms of solvent tolerance in gram-negative bacteria. Annu Rev Microbiol 2002;56:743–8. [DOI] [PubMed] [Google Scholar]
- 58.Arlet G, Philippon A. Construction by polymerase chain reaction and use of intragenic DNA probes for three main types of transferable β-lactamases (TEM, SHV, CARB). FEMS Microbiol Lett 1991;66:19–25. [DOI] [PubMed] [Google Scholar]
- 59.Gallardo F, Ruiz J, Marco F, et al. Increase in incidence of resistance to ampicillin, chloramphenicol and trimethoprim in clinical isolates of Salmonella serotype Typhimurium with investigation of molecular epidemiology and mechanisms of resistance. J Med Microbiol 1999;48:367–74. [DOI] [PubMed] [Google Scholar]
- 60.Phuc Nguyen MC, Woerther P, Bouvet M, et al. Escherichia coli as reservoir for macrolide resistance genes. Emerg Infect Dis 2009;15:1648–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Salazar de Vegas EZ, Nieves B, Ruiz M, et al. Molecular epidemiology and characterization of resistance mechanisms to various antimicrobial agents in Acinetobacter baumannii isolated in Mérida, Venezuela. Med Sci Monit 2007;13:BR89–94. [PubMed] [Google Scholar]
- 62.Sunde M, Norström M. The prevalence of, associations between and conjugal transfer of antibiotic resistance genes in Escherichia coli isolated from Norwegian meat and meat products. J Antimicrob Chemother 2006;58:741–7. [DOI] [PubMed] [Google Scholar]
- 63.Toro CS, Farfán M, Contreras I, et al. Genetic analysis of antibiotic-resistance determinants in multidrug-resistant Shigella strains isolated from Chilean children. Epidemiol Infect 2005;133:81–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vila J, Navia M, Ruiz J, Casals C. Cloning and nucleotide sequence analysis of a gene encoding an OXA-derived β-lactamase in Acinetobacter baumannii. Antimicrob Agents Chemother 1997;41:2757–9. [DOI] [PMC free article] [PubMed] [Google Scholar]