Abstract
Re-examination of Dermacentor taiwanensis Sugimoto, 1935 specimens in the United States National Tick Collection revealed that two morphologically distinct Dermacentor species were identified under this name. One of them corresponds to Sugimoto’s description of D. taiwanensis, while another species is identical to Schulze’s Dermacentor bellulus (Schulze, 1935). The latter species has not been considered valid by recent workers. D. bellulus is reinstated here as a valid species and all its stages are redescribed. The adults of D. taiwanensis are also redescribed, and its immature stages are described for the first time. Males and females of D. bellulus can be distinguished from those of D. taiwanensis by the shape of the conscutum and scutum, color pattern, genital structures, size of the palpi and cornua, and the spurs of coxa I. Nymphs of D. bellulus can be distinguished from those of D. taiwanensis by the shape of the scutum, basis capituli, and the hypostomal dentition. Larvae of D. bellulus can be differentiated from those of D. taiwanensis by the shape of the basis capituli, and the degree of development of the auriculae and spur on palpal segment III ventrally. D. bellulus has been recorded from China, Japan, Nepal, Taiwan, and Vietnam; adults have been collected from wild boars, bears, panda, dog, and human; the immature stages are known from rodents, hares, ferret-badger, and bamboo-partridge. D. taiwanensis is found in China, Taiwan, and Vietnam; adults have been collected from wild boars; the immature stages are known from rodents, hares, mustelids, and domestic dog.
Keywords: Dermacentor bellulus, Dermacentor taiwanensis, description
Ticks of the genus Dermacentor Koch, 1844 that occur chiefly in the Oriental zoogeographic region comprise a compact and apparently natural group, designated subgenus Indocentor Schulze, 1933. Currently, six Indocentor species are generally recognized: D. atrosignatus Neumann, 1906, D. auratus Supino, 1897, D. compactus Neumann, 1901, D. confragus (Schulze, 1933), D. steini (Schulze, 1933), and D. taiwanensis Sugimoto, 1935. The systematics of subgenus Indocentor was largely developed by Schulze (1933, 1935) and revised in a series of papers by Wassef and Hoogstraal (1983; 1984a,b; 1986a,b). Nevertheless, Indocentor species remain little studied and poorly known, possibly because all active life history stages parasitize wild animals, mostly mammals, in the primary forests of South and Southeast Asia.
During re-examination of supposed D. taiwanensis specimens in the United States National Tick Collection (USNTC), two morphologically distinct Dermacentor species were found. Both had been identified as D. taiwanensis by previous workers. Comparison of these specimens with other described Dermacentor taxa revealed that they are identical to two species described from Taiwan, namely D. taiwanensis and D. bellulus (Schulze, 1935). The latter name has not been recognized as valid in all recent lists (Guglielmone et al. 2010, 2014) and was synonymized with D. atrosignatus (Sugimoto 1937, Estrada-Peña 1991), D. auratus (Teng and Jiang 1991), or D. taiwanensis (Camicas et al. 1998, Sun and Xu 2013) or considered incertae cedis in Guglielmone and Nava (2014). The presence of a number of distinctive diagnostic characters in the male, female, nymph, and larva persuaded us to reinstate D. bellulus as a valid species and redescribe all its parasitic stages. For comparative purposes, we also redescribe the male and female of true D. taiwanensis and describe its nymph and larva for the first time.
Materials and Methods
The material examined is summarized in Tables 1 and 2. Field-collected and laboratory-reared ticks were available for study. The specimens that were examined are deposited in the USNTC (Georgia Southern University, Statesboro), Institute of Zoology of the Chinese Academy of Sciences (IZAS; Beijing, China), Zoological Museum of Moscow State University (ZMMO; Moscow, Russia), and Zoological Institute of the Russian Academy of Sciences (ZIAC; St. Petersburg, Russia).
Table 1.
Dermacentor bellulus, material examined
| No. of ticksa |
Host | Locality; altitude (m) | Date | Collectorb | Accession no.c | |||
|---|---|---|---|---|---|---|---|---|
| ♂ | ♀ | N | L | |||||
| China | ||||||||
| 4 | Sus scrofa | Fujian, Nanping County | 1.VI.1963 | D-048-05 | ||||
| 1 | Ursus sp. | Fujian, Shaowu County | XI.1963 | DW | D-048-01 | |||
| 1 | 1 | Ailuropoda melanoleuca | Sichuan, Tianquan County | 24.V.1986 | WQ | D-047-11 | ||
| 1 | Ursus thibetanus | Xizang, Medog County, Gutang; 2400 | 4.X.1982 | D 300 | ||||
| Japan | ||||||||
| 1 | Sus scrofa | Fukui, Shizuhara | 12.XII.1955 | JA | 00714209 | |||
| 2 | Kagoshima, Yuwan-dake | VIII.1963 | VT | 00714173 | ||||
| 2 | Sus scrofa | Kagoshima, Uken | 19.III.1967 | 00714408 | ||||
| 4 | 2 | Sus scrofa | Kagoshima, Uken | 13.III.1967 | 00714992 | |||
| 1 | Sus scrofa | Kagoshima, Uken | 19.III.1967 | 00714613 | ||||
| 4d | 2d | 2;5d,e | 7;5d | Vegetationf | Kanagawa, Kuribana & Kyoto, Mineyamaf | 10.IV.1969 | YS | 00714414 |
| 1 | Sus scrofa | Kyoto, Kyoto | 00714289 | |||||
| 1 | Apodemus speciosus | Kyoto, Ohara | 1.V.1952 | 00714535 | ||||
| Nepal | ||||||||
| 1 | Vegetation | Nuwakot, Syahru; 1829 | 6.VI.1970 | RMM | 00714845 | |||
| 1 | Vegetation | Rasuwa, Syaurbensi; 1448 | 2.VI.1970 | RMM | 00714917 | |||
| Taiwan | ||||||||
| 7 | 47 | Bandicota indica | Taipei, San-chih, Ch’e-ch’eng; 376 | 8.III.1973 | FJS | 00714185 | ||
| 2d | 2e | Bandicota indica | Taipei, San-chih, Ch’e-ch’eng; 376 | 7.XII.1973 | FJS | 00714509 | ||
| 1 | Mus caroli | Taipei, San-chih, Ch’e-ch’eng; 376 | 7.III.1973 | FJS | 00714423 | |||
| 2d | 4d | 1e | Niviventer coninga | Taipei, San-chih, Ch’e-ch’eng; 376 | 10.IV.1973 | FJS | 00714798 | |
| 1d | 1e | Niviventer coninga | Taipei, San-chih, Ch’e-ch’eng; 376 | 10.IV.1973 | FJS | 00714800 | ||
| 1d | Niviventer coninga | Taipei, San-chih, Ch’e-ch’eng; 376 | 10.IV.1973 org10.III.1974 | FJS | 00714609 | |||
| 1d | 1e | Niviventer coninga | Taipei, San-chih, Ch’e-ch’eng; 376 | 10.IV.1973 or10.III.1974 | FJS | 00714531 | ||
| 1e | Niviventer coninga | Taipei, San-chih, Ch’e-ch’eng; 376 | 10.IV.1973 or10.III.1974 | FJS | 00714204 | |||
| 2 | Rattus losea | Taipei, San-chih, Ch’e-ch’eng; 376 | 7.III.1973 | FJS | 00714085 | |||
| 1 | Rattus losea | Taipei, San-chih, Ch’e-ch’eng; 376 | 8.III.1973 | FJS | 00714571 | |||
| 2d | 1 e | Rattus losea | Taipei, San-chih, Ch’e-ch’eng; 376 | 9.IV.1974 | FJS | 00714135 | ||
| 1 | Rattus losea | Taipei, San-chih, Ch’e-ch’eng; 376 | 10.IV.1973 or10.III.1974 | FJS | 00714938 | |||
| 1e | Rattus losea | Taipei, San-chih, Ch’e-ch’eng; 376 | 24.IV.1973 or10.III.1974 | FJS | 00714586 | |||
| 1e | Rattus losea | Taipei, San-chih, Ch’e-ch’eng; 376 | 24.IV.1973 or10.III.1974 | FJS | 00714840 | |||
| 2 | Rattus losea | Taipei, San-chih, Ch’e-ch’eng; 376 | 24.IV.1973 or10.III.1974 | FJS | 00714153 | |||
| 1e | Rattus losea | Taipei, San-chih, Ch’e-ch’eng; 376 | 24.IV.1973 or10.III.1974 | FJS | 00714556 | |||
| 1d | Bandicota indica | Taipei, San-chih, Ch’e-ch’eng; 475 | 4.IX.1974 | FJS | 00714987 | |||
| 1e | Rattus losea or Niviventer coninga | Taipei, San-chih, Ch’e-ch’eng or Shu-hsing Li, Hsing-fu-liao; 332 | 24 or 25.IV.1973 | FJS | 00714804 | |||
| 2 | Melogale moschata | Taipei, Ta-t’un Shan, Pai-liu-chia | 26.III.1973 | FJS | 00714354 | |||
| 7 | 5;3e | Lepus sinensis | Taipei, Ta-t’un Shan, Pai-liu-chia; 594-891 | 9.XI.1973 | FJS | 00714074 | ||
| 2 | Bandicota indica | Taipei, Ta-t’un Shan, Pai-liu-chia; 693 | 2.V.1973 | FJS | 00714932 | |||
| 1e | Bandicota indica | Taipei, Ta-t’un Shan, Pai-liu-chia; 693 | 2.V.1973 | FJS | 00714700 | |||
| 1e | Bandicota indica | Taipei, Ta-t’un Shan, Pai-liu-chia; 693 | 2.V.1973 | FJS | 00714997 | |||
| 1 | Bambusicola thoracica | Taipei, Ta-t’un Shan, Pai-liu-chia or San-chih, Ch’e-ch’eng; 376 | 28.III.1973 | FJS | 00714582 | |||
| 3 | 2 | Sus scrofa | Taipei, Tan-shui, Pai-liu-chia-ting | 5.II.1975 | FJS | 00714171 | ||
| 1 | Lepus sinensis | Taipei, Tan-shui, Pai-liu-chia-ting; 500 | 15.II.1973 | FJS | 00714576 | |||
| 2 | Lepus sinensis | Taipei, Tan-shui, Pai-liu-chia-ting; 500 | 21.II.1973 | FJS | 00714640 | |||
| 8 | Sus scrofa | Taipei, Wu-lai | 25.II.1959 | REK | 00714335 | |||
| 2 | 2 | Sus scrofa | Taipei, Yang-ming-shan | 17.XI.1961 | REK | 00714355 | ||
| 1 | 1 | Sus scrofa | Taipei, Yang-ming-shan | 17.XI.1961 | REK | 00714077 | ||
| 1 | Sus scrofa | Taito | 18.X.1936 | 00714962 | ||||
| 16 | 3 | Sus scrofa | Taitung, Pei-yuan; 198 | 10 or 18.XI.1971 | CIC | 00714904 | ||
| 2 | 3 | Sus scrofa | Taitung, Pei-yuan; 300 | 11 or 14.II.1971 | CIC | 00714425 | ||
| 2 | 1 | Sus scrofa | Taitung, Pei-yuan; 300 | 14.II.1971 | CIC | 00714659 | ||
| 11 | Sus scrofa | Taitung, Pei-yuan, Shih-nan; 198 | 10.XII.1970 | CIC | 00714604 | |||
| 10 | 3 | Sus scrofa | Taitung, Pei-yuan, Shih-nan; 198 | 10.I.1972 or 10.I.1973 | FJS | 00714747 | ||
| 4 | 1 | Sus scrofa | Taitung, Pei-yuan, Shih-nan; 198 | 10.I.1973 | FJS | 00714735 | ||
| 3 | Sus scrofa | Taitung, Pei-yuan or Tung-ho | 5 or 11.II.1971 | CIC | 00714168 | |||
| 1 | Sus scrofa | Taitung, Tung-ho | 11.I.1972 | CIC | 00714768 | |||
| 2 | 1 | Sus scrofa | Taitung, Tung-ho, Pei-yuan | 15.XI.1965 | SYL | 00714291 | ||
| 1 | 1 | Sus scrofa | Taitung, Tung-ho, Pei-yuan | 4.V.1970 | JCL | 00714066 | ||
| 18 | 1 | Sus scrofa | Taitung, Tung-ho or Pei-yuan; 300 | 5.II. or 12.IX.1970 | CIC | 00714839 | ||
| 1 | Sus scrofa | Tosei | 1935 | S | 00714922 | |||
| 9 | No data | 10.III.1974 | FJS | 00714847 | ||||
| Vietnam | ||||||||
| 3 | Rattus tanezumi | Da Nang, Da Nang; 10 | 1.V.1966 | FJS | 00860989 | |||
| 5 | 3 | Rattus norvegicus | Da Nang, Da Nang | 11.VIII.1969 | RHG | 00860986 | ||
| 6 | Rattus sp. | Da Nang, Mt. Sontra; 594 | 7.IX.1967 | PFDVP | 00714147 | |||
| 1 | Rattus tanezumi | Da Nang, Mt. Sontra; 164 | 20.IV.1966 | FJS | 00714969 | |||
| 2 | Rattus sp. | Da Nang, Mt. Sontra; 446 | 12.VIII.1967 | PFDVP | 00860991 | |||
| 1 | 3 | Rattus sp. | Da Nang, Mt. Sontra; 594 | 7.IX.1967 | PFDVP | 00860997 | ||
| 2 | Rattus rattus | Da Nang, Mt. Sontra; 396 | 11.VIII.1967 | GSJ | 00860990 | |||
| 1 | 1 | Rattus rattus | Da Nang, Mt. Sontra; 425 | 4.X.1967 | GSJ | 00860988 | ||
| 1 | Rattus rattus | Da Nang, Mt. Sontra; 97 | IV.1969 | PFDVP | 00860992 | |||
| 2 | Rattus sp. | Da Nang, Mt. Sontra; 10 | 8.VIII.1967 | PFDVP | 00860998 | |||
| 21 | 1 | Rats | Da Nang, Sontra | V-VI.1989 | VS | Tdi | ||
| 1 | Tupaia glis | Da Nang, Sontra | 4.VI.1989 | Tdi | ||||
| 1 | Rattus rattus | Da Nang, Sontra | 9.VI.1989 | Tdi | ||||
| 127 | Rats | Da Nang, Sontra | V-VI.1989 | VS | Tdi | |||
| 50 | Rodents | Da Nang, Sontra | VII-VIII.1989 | VS | Tdi | |||
| 2 | 2 | Rattus rattus | Da Nang, Sontra, 629 | 19.VII.1989 | Tdi | |||
| 1 | Clothing | Dak Lak, Krong Kmar, Chu Yang Sin National Park; 950 | V.2014 | AVA | ZIN | |||
| 4 | Rattus rattus | Dong Nai, Ma Da | 17.I.1994 | SS | Tdi | |||
| 6 | Bandicota bengalensis | Dong Nai, Ma Da | 4.III.1991 | VS | Tdi | |||
| 1 | Niviventer sp. | Lam Dong, Da Lat | 7.XII.1995 | GK | Tdi | |||
| 1 | Niviventer sp. | Lam Dong, Da Lat | 6.XII.1995 | Tdi | ||||
| 7 | Rodents | Lam Dong, Da Lat | XII.1995 | GK, VS | Tdi | |||
| 2 | Niviventer sp. | Lam Dong, Da Lat, Cong-Troi pass | 4.XII.1995 | Tdi | ||||
| 2 | Niviventer sp. | Lam Dong, Da Lat, Cong-Troi pass | 7.XII.1995 | VS | Tdi | |||
| 1 | Niviventer sp. | Lam Dong, Da Lat, Cong-Troi pass | 2.XII.1995 | GK | Tdi | |||
| 1 | clothing | Lam Dong, Loc Bao, 35 km NW of Loc Bao Town; 650 | IV.2012 | AVA | ZIN | |||
| 1 | N. confucianus | Lao Cai, Hoang Lien Son, Chapa | NMT, VVB | Tdi | ||||
| 1 | 1 | Lao Cai, Van Ban | 8.IV.2005 | K | Tdi-1965 | |||
| 1 | Human | Quang Binh, ?Tuyen-Hoa | 1.V.1999 | GVK | Tdi-4710 | |||
| 2 | Rattus rattus | Quang Tri, Mt. Thon Ke Tri; 10 | 26.III.1969 | PFDVP | 00860985 | |||
| 112 | 36 | 93 | 301 | Total | ||||
a L, larvae; N, nymphs.
b AVA, A.V. Abramov; CIC, C.I. Cheng; DW, D. Wang; FJS, F.J. Santana; GSJ, G.S. Jones; GVK, G.V. Kuznetsov; JA, J. Akiyama; JCL, J.C. Lien; K, Kalyakin; NMT, Nguyen Minh Tam; PFDVP, P.F.D. Van Peenen; REK, R.E. Kuntz; RHG, R.H. Grothaus; RMM, R.M. Mitchell; S, Sugimoto; SS, S. Shilova; SYL, S.Y. Lin; VS, V. Suntzov; VT, V. Tipton; VVB, V.V. Bobrov; WQ, W. Qui; YS, Y. Saito.
c USNMENT number is given for USNTC collection unless specified: D—IZAS; Tdi—ZMMO; ZIN—ZIAC.
d Reared specimen.
e Molting skin.
f Place where parent larvae were collected.
g Here and below “or” in the columns Host, Locality, and Date indicates that the data on the label and in the Harry Hoogstraal Tick Collection catalogue for this collection do not agree; data presented as follows: label data or Harry Hoogstraal Tick Collection catalogue data.
Table 2.
Dermacentor taiwanensis, material examined
| No. of ticksa |
Host | Locality; altitude (m) | Date | Collectorb | Accession no.c | |||
|---|---|---|---|---|---|---|---|---|
| ♂ | ♀ | N | L | |||||
| China | ||||||||
| 1 | Hainan, Jianfengling | IV.1980 | D-047-14 | |||||
| Taiwan | ||||||||
| 1d | 1d | Bandicota indica | Taipei, Hou Shan ore Shu-hsing Li, Hsing-fu-liao; 323 | 30.VI.1972 | FJS | 00714971 | ||
| 1d | 1f | Bandicota indica | Taipei, San-chih, Ch’e-ch’eng; 376 | 11.V.1973 | FJS | 00714584 | ||
| 1d | Bandicota indica | Taipei, San-chih, Ch’e-ch’eng; 376 | 11.V.1973 | FJS | 00714094 | |||
| 1f | Bandicota indica | Taipei, San-chih, Ch’e-ch’eng; 376 | 11.V.1973 | FJS | 00714083 | |||
| 1f | Bandicota indica | Taipei, San-chih, Ch’e-ch’eng; 376 | 11.V.1973 | FJS | 00714402 | |||
| 1f | Bandicota indica | Taipei, San-chih, Ch’e-ch’eng; 376 | 11.V.1973 | FJS | 00714633 | |||
| 1f | Bandicota indica | Taipei, San-chih, Ch’e-ch’eng; 376 | 11.V.1973 | FJS | 00714734 | |||
| 1f | Bandicota indica | Taipei, San-chih, Ch’e-ch’eng; 376 | 11.V.1973 | FJS | 00714961 | |||
| 6 | Bandicota indica | Taipei, San-chih, Ch’e-ch’eng; 475 | 7.VIII.1974 | FJS | 00714293 | |||
| 1d | 1f | Bandicota indica | Taipei, San-chih, Ch’e-ch’eng; 475 | 4.IX.1974 | FJS | 00714264 | ||
| 1d | 1f | Bandicota indica | Taipei, Shu-hsing Li, Hsing-fu-liao; 323 | 7.VII.1972 | FJS | 00714318 | ||
| 1 | Bandicota indica | Taipei, Shu-hsing Li, Hsing-fu-liao; 320 | 10 or 20.IX.1972 | FJS | 00714480 | |||
| 1 | Bandicota indica | Taipei, Shu-hsing Li, Hsing-fu-Liao , Tan-Shui; 320 | 10.VII.1972 | FJS | 00714176 | |||
| 14 | Bandicota indica | Taipei, Shu-hsing Li, Hsing-fu-liao, Tan-shui; 320 | 30.VI.1972 | FJS | 00714976 | |||
| 3 | 1 | Sus scrofa | Taipei, Tan-shui, Pai-liu-chia | 5.II.1975 | FJS | 00714110 | ||
| 1d | 1f | Callosciurus erythraeus | Taipei, Ta-t’un Shan, Pia-liu-chia; 594-891 | 30.VIII.1973 | FJS | 00714548 | ||
| 4d | 1;11f | Lepus sinensis | Taipei, Ta-t’un Shan, Pai-liu-chia; 594-891 | 23.VII.1973 | FJS | 00714903 | ||
| 2d | 1;2f | Lepus sinensis | Taipei, Ta-t’un Shan, Pai-liu-chia; 594-891 | 26.VII.1973 | FJS | 00714376 | ||
| 1 | Melogale moschata | Taipei, Ta-t’un Shan, Pai-liu-chia; 594-891 | 9.VIII.1973 | FJS | 00714813 | |||
| 1 | Melogale moschata | Taipei, Ta-t’un Shan, Pai-liu-chia; 594-891 | 30.VIII.1973 | FJS | 00714925 | |||
| 1d | 1f | Melogale moschata | Taipei, Ta-t'un Shan, Pia-liu-chia; 596-891 | 6.IX.1973 | FJS | 00714466 | ||
| 9f | Mustela sibirica | Taipei, Ta-t’un Shan, Pia-liu-chia; 594-891 | 24.VII.1973 | FJS | 00714433 | |||
| 1f | Bandicota indica | Taipei, Ta-t’un Shan, Pai-liu-chia; 693 | 2.V.1973 | FJS | 00714564 | |||
| 1 | Melogale moschata | Taipei, Ta-t’un Shan, Pai-liu-chia; 693 | 11.V. or 19.VII.1973 | FJS | 00714099 | |||
| 1 | Melogale moschata | Taipei, Ta-t’un Shan, Pai-liu-chia; 693 | 11.V. or 19.VII.1973 | FJS | 00714399 | |||
| 10 | Sus scrofa | Taipei, Wu-lai | 25.II.1959 | REK | 00714486 | |||
| 1 | 1 | Sus scrofa | Taipei, Yang-ming-shan | 17.XI.1961 | REK | 00714352 | ||
| 2 | 2 | Sus scrofa | Taipei, Yang-ming-shan | 17.XI.1961 | REK | 00714213 | ||
| 1 | Sus scrofa | Taito | 18.X.1936 | 00714490 | ||||
| 5 | 5 | Sus scrofa | Taitung, Pei-yuan; 198 | 10 or 18.XI.1971 | CIC | 00714302 | ||
| 6 | Sus scrofa | Taitung, Pei-yuan; 300 | 14.II.1971 | CIC | 00714776 | |||
| 1 | Sus scrofa | Taitung, Pei-yuan; 300 | 11 or 14.II.1971 | CIC | 00714835 | |||
| 1 | 1 | Sus scrofa | Taitung, Pei-yuan, Shih-nan | 10.XII.1970 | CIC | Tdi-5079 | ||
| 2 | Sus scrofa | Taitung, Pei-yuan, Shih-nan; 198 | 10.XII.1970 | CIC | 00714501 | |||
| 5 | 2 | Sus scrofa | Taitung, Pei-yuan, Shih-nan; 198 | 10.I.1972 or 10.I.1973 | FJS | 00714182 | ||
| 1 | Sus scrofa | Taitung, Pei-yuan or Tung-ho; 300 | 4 or 12.IX.1970 | CIC | 00714597 | |||
| 1 | Sus scrofa | Taitung, Tung-ho | 11.I.1972 | CIC | 00714488 | |||
| 7 | Sus scrofa | Taitung, Tung-ho or Pei-yuan; 300 | 5.II. or 12.IX.1970 | CIC | 00714026 | |||
| 1 | Sus scrofa | Taitung, Tung-ho, Pei-yuan | 15.XI.1965 | SYL | 00714186 | |||
| Vietnam | ||||||||
| 3 | Vegetation | Bac Thai | 29.VI.1985 | Tdi | ||||
| 23 | 1 | Callosciurus erythraeus | Bac Thai | 29.VI.1985 | Tdi | |||
| 1 | Tamiops mcclellandii | Bac Thai | 27.VI.1985 | GVK | Tdi | |||
| 1 | Domestic dog | Bac Thai | 27.VI.-2.VII.1985 | Tdi | ||||
| 1 | Vegetation | Hanoi, Khoshon-Bin’, Mai-Choo | 31.X.-4.XI.1990 | EPN | ZIN 5721 | |||
| 1 | Rattus sp. | Vinh Phuc, Tam Dao | V.1997 | NLO | Tdi | |||
| 51 | 23 | 80 | 12 | Total | ||||
a L, larvae; N, nymphs.
b CIC, C.I. Cheng; EPN, E.P. Narchuk; FJS, F.J. Santana; GVK, G.V. Kolonin; HH, H. Hoogstraal; L, Lowery; M, Muschinske; NLO, N.L. Orlov; REK, R.E. Kuntz; SYL, S.Y. Lin; VS, V. Suntzov.
c USNMENT number is given for USNTC collections unless specified: D—IZAS; Tdi—ZMMO; ZIN—ZIAC.
d Reared specimen.
e Here and below “or” in the columns Host, Locality, and Date indicates that the data on the label and in the Harry Hoogstraal Tick Collection catalogue for this collection do not agree; data presented as follows: label data or Harry Hoogstraal Tick Collection catalogue data.
f Molting skin.
The immature stages were mounted on glass slides and examined under a light microscope (Olympus BX41, Olympus Corporation, Tokyo, Japan). All stages were also studied by means of a stereoscopic microscope (Olympus SZX16, Olympus Corporation, Tokyo, Japan) and a scanning electron microscope (JOEL JSM6610LV, JOEL Ltd., Tokyo, Japan). Measurements for the male and female are given in millimeters and those for the various features of the nymph and larva in micrometers. The measurements are arranged as follows: minimum–maximum (mean ± SD, n = number of specimens measured). The nomenclature of larval setae is that of Clifford and Anastos (1960). Host nomenclature is that of Wilson and Reeder (2005). Illustrations presented in figures 1 and 8 have been drawn by D.A. Apanaskevich and colored by M.A. Apanaskevich.
Fig. 1.
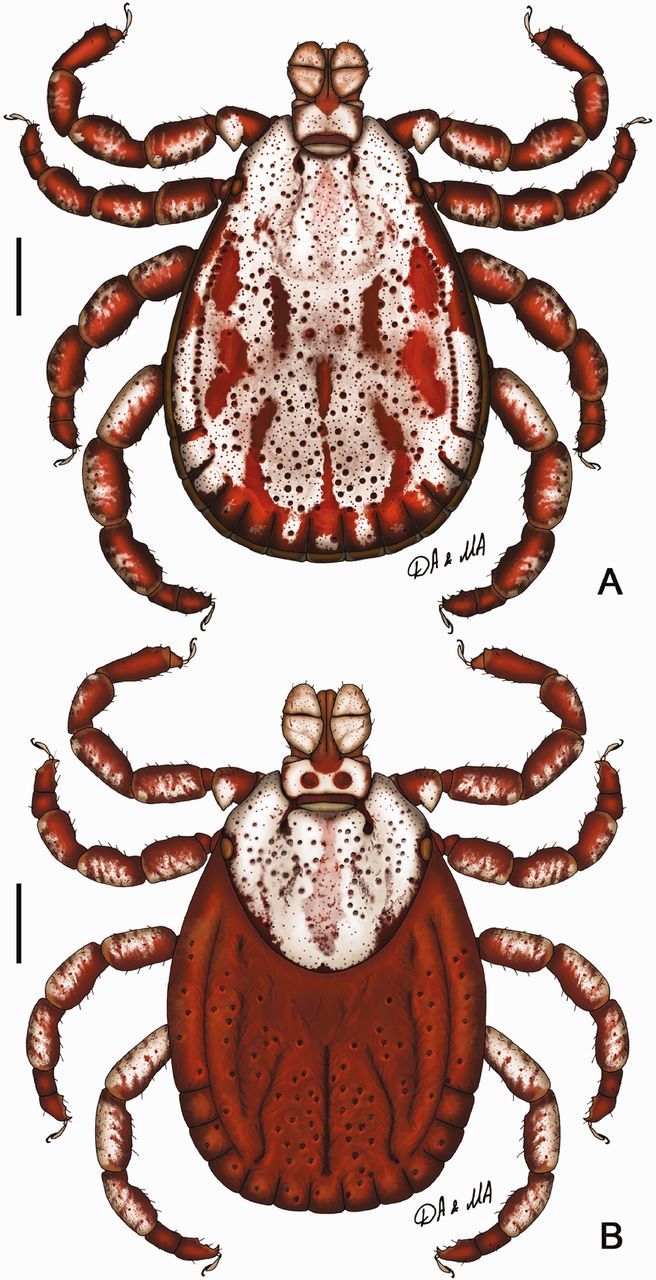
Dermacentor bellulus, dorsally. (A) Male (Taiwan, Taitung, Pei-yuan, Shih-nan, USNMENT 00714604). (B) Female (Taiwan, Taitung, Tung-ho, Pei-yuan, USNMENT 00714066). Scale bar = 1 mm.
Fig. 8.
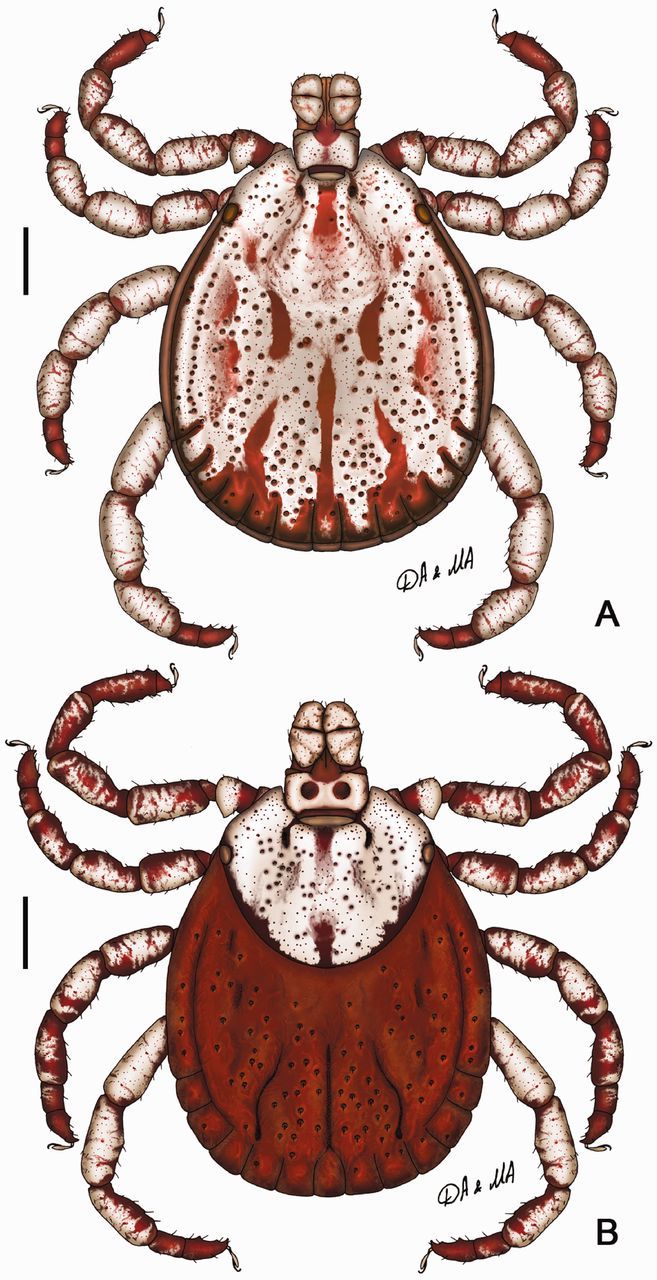
Dermacentor taiwanensis, dorsally. (A) Male (Taiwan, Taitung, Pei-yuan, USNMENT 00714776). (B) Female (Taiwan, Taitung, Pei-yuan, Shih-nan, USNMENT 00714182). Scale bar = 1 mm.
Dermacentor bellulus (Schulze, 1935)(Figs. 1–6)
Male
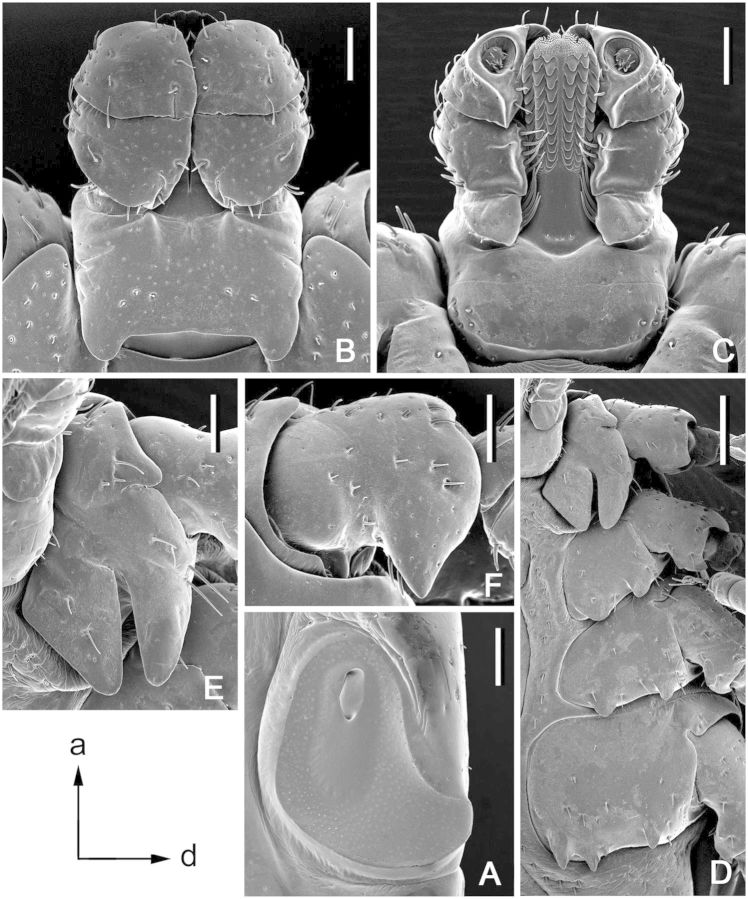
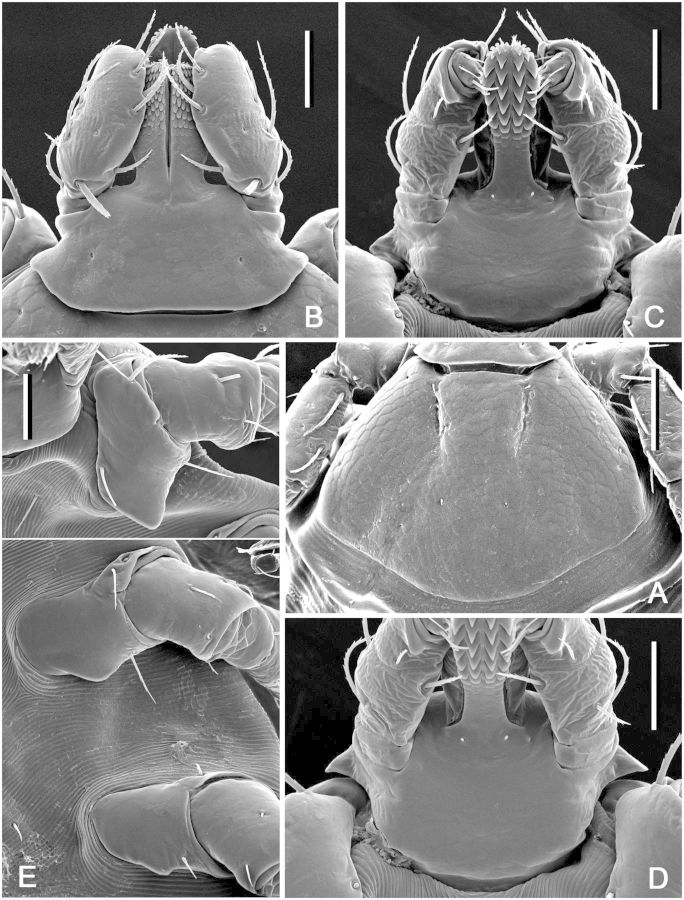
(redescription) (Figs. 1A, 2, and 3). Conscutum (Figs. 1A and 2): broadly oval with moderately convex lateral margins, widest at posterior third of conscutal length; distance from scapular apices to posterior margin of conscutum 3.69–6.12 (4.99 ± 0.54; n = 112), maximum width 2.69–4.50 (3.59 ± 0.41; n = 112), ratio length to width 1.31–1.51 (1.39 ± 0.04; n = 112). Coloration as illustrated: pale ivory-colored ornamentation moderate to extensive, light- to dark-brown background forms several patches, often with indistinct hazy margins; a pair of narrow cervical patches extending from cervical pits to posterior margin of pseudoscutum; a pair of small patches lateral to cervical patches; broad and hazy central patch on pseudoscutum; posteromedian margin of pseudoscutum indicated by narrow hazy strip; lateral field with two brown patches: anterior broad patch extending medially to lateral grooves from eyes to midlength and posterior narrow patch (often poorly defined or rarely indistinct) along most of lateral margins of conscutum; two pairs of oval patches medial to lateral groove; a pair of central patches; narrow stripes in posteromedian and paramedian regions; first and second festoons mostly ivory colored, third and fourth festoons mostly brown, paramedian and median festoons with large ivory spots; all punctations from whitish- to dark-brown. In lighter or darker colored specimens (Fig. 2A–D) some patches may become indistinct or oppositely fused together. Central field posterior to pseudoscutum often flat, imparting a concave appearance to conscutum; conscutum smooth. Cervical grooves shallow; a pair of central slight depressions, and a second posterior pair of slight depressions that correspond to paramedian grooves; lateral grooves distinct, extending from just posterior to eyes to first festoons and aligned with large punctations; 11 distinct festoons, median festoon usually as broad as paramedian festoons. Very large and deep punctations moderately dense, distributed over entire conscutum but denser laterally and posteriorly; large punctations on pseudoscutum rather sparse; fine punctations dense, evenly distributed. Eyes oval, very slightly convex, at anterior one-fifth of conscutal length. Setae relatively short and inconspicuous. Spiracular plates (Fig. 3A): suboval; dorsal prolongation moderately long and broad, with narrow, unperforated widening anteriorly; perforations very small and fairly numerous. Sclerotized plaques on festoons ventrally inornate or with small ivory-colored spot. Gnathosoma (Figs. 1A, 3B and C): length from palpal apices to cornual apices dorsally 0.92–1.46 (1.15 ± 0.10; n = 112), width of basis capituli 0.60–0.97 (0.79 ± 0.07; n = 113), ratio length to width 1.32–1.57 (1.46 ± 0.05; n = 112). Basis capituli (Figs. 1A, 3B and C): dorsally subrectangular; posterior margin nearly straight or slightly concave; length 0.38–0.60 (0.49 ± 0.05; n = 112), ratio width to length 1.47–2.00 (1.61 ± 0.08; n = 112); cornua broad, short, total length of basis capituli, including cornua, 3.67–8.60 (5.01 ± 0.80; n = 112) cornual length; dorsally with extensive whitish enameling. Basis capituli ventrally subrectangular; posterior margin convex. Palpi (Figs. 1A, 3B and C): short, broad; length dorsally (segments I–III) 0.54–0.87 (0.67 ± 0.06; n = 113), width 0.32–0.51 (0.42 ± 0.04; n = 113), ratio length to width 1.41–1.96 (1.60 ± 0.09; n = 113), length of segments in descending order: 2, 3, 1, 4; segment I well developed ventrally; segment II narrower at base and thereafter widening, without clear denticle at posterior margin dorsally; segment III subrectangular with broadly rounded apex; segments II and III with extensive whitish enameling on dorsal surfaces. Hypostome (Fig. 3C): club-shaped; dental formula 3/3. Legs (Fig. 1A): of medium length, moderately robust; with extensive whitish enameling mostly on dorsal and lateral aspects of leg segments. Coxae (Fig. 3D and E): coxa I with relatively long, triangular, closely spaced internal and external spurs, internal spur broadly triangular with narrowly rounded apex, external spur narrowly triangular with broadly to narrowly rounded apex, internal and external spurs nearly equal in length, both spurs of coxa I generally directed posteriorly; coxae II and III each with moderate triangular external and internal spurs, external spur with narrowly rounded or tapering apex, internal spur with broadly or narrowly rounded apex; coxa IV with moderate triangular external spur with tapering apex and with several moderate triangular internal spurs, each with tapering apex; coxa IV enlarged, ratio length to width 0.81–1.10 (0.99 ± 0.05; n = 111); coxae inornate or, especially coxae I, with small spots of whitish enameling. Trochanter I (Figs. 1A and 3F) with moderate, broadly triangular dorsal spur with tapering apex. Genu and Tibia (Fig. 1A) with two rows of very short projections ventrally. Genu IV length 0.75–1.29 (1.04 ± 0.12; n = 101), width 0.37–0.66 (0.51 ± 0.07; n = 101), ratio length to width 1.85–2.25 (2.02 ± 0.07; n = 101).
Fig. 2.
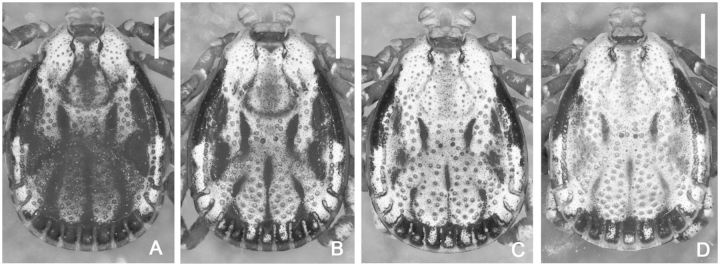
(A–D) Dermacentor bellulus, variations in the coloration of male conscuta. (Taiwan, Taitung, Pei-yuan, Shih-nan, USNMENT 00714604). Scale bar = 1 mm.
Fig. 3.
Dermacentor bellulus, male (Taiwan, Taitung, Tung-ho, Pei-yuan, USNMENT 00714291). (A) Spiracular plate. Scale bar = 0.2 mm. Arrows show orientation of spiracular plate (a—anterior; d—dorsal). (B) Gnathosoma dorsally. Scale bar = 0.2 mm. (C) Gnathosoma ventrally. Scale bar = 0.2 mm. (D) Coxae. Scale bar = 0.5 mm. (E) Coxa I. Scale bar = 0.2 mm. (F) Trochanter I. Scale bar = 0.2 mm.
Female
(redescription) (Figs. 1B and 4). Idiosoma (Fig. 1B): broadly oval, widest just anterior to mid-length. Scutum (Fig. 1B): length 1.87–2.69 (2.37 ± 0.21; n = 35), width 2.16–2.85 (2.51 ± 0.19; n = 35), ratio length to width 0.85–1.01 (0.94 ± 0.04; n = 35), margins diverge posteriorly in anterior third of total length, thereafter gradually converging to broadly rounded posterior margin, posterolateral projections indistinct. Coloration: ornamentation very extensive, major portion of scutal surface covered with whitish enameling; brown colored patches arranged as follows: two pairs of small patches in cervical pits, a pair of narrow patches in cervical fields, large and broad central patch extending from anterior margin to posterior margin of scutum, hazy with speckles of small, darker patches, a pair of narrow patches bordering posterior margin of scutum extending from just anterior to eyes, widening at posterior margin of cervical grooves and connecting at posterior apex of scutum. Cervical grooves distinct, moderately deep. Surface of scutum smooth; very large and deep punctations moderately dense to fairly sparse and situated mostly in cervical grooves and anterior part of central field; fine punctations dense, evenly distributed over scutum. Eyes oval, very slightly convex, positioned at second third of scutal length. Setae relatively sparse and short. Alloscutum (Fig. 1B): as illustrated; 11 festoons. Setae of alloscutum relatively short (ca. 0.061), moderately dense. Genital aperture (Fig. 4A and B): at level of coxae II, moderately narrow and U-shaped, semi-oval sclerites bordering genital aperture laterally distinct; preatrial fold conspicuously bulging. Spiracular plates (Fig. 4C): suboval; dorsal prolongation moderately long and broad, broadly rounded to its apex, with broad unperforated widening anteriorly; perforations very small and fairly numerous. Gnathosoma (Figs. 1B, 4D and E): length from palpal apices to posterior margin of basis capituli dorsally 1.12–1.52 (1.34 ± 0.10; n = 34), width of basis capituli 0.86–1.17 (1.03 ± 0.08; n = 34), ratio length to width 1.18–1.40 (1.30 ± 0.05; n = 34). Basis capituli (Figs. 1B, 4D and E): dorsally subrectangular; posterior margin nearly straight or slightly concave, length 0.42–0.59 (0.50 ± 0.04; n = 34), ratio width to length 1.79–2.32 (2.08 ± 0.11; n = 34); cornua broad, short, total length of basis capituli, including cornua, 5.90–11.00 (8.53 ± 1.32; n = 34) cornual length; dorsally extensively ornate with whitish enameling. Porose areas moderate, circular, deeply sunken with clearly circumscribed borders, separated by space less than their width. Basis capituli ventrally subrectangular, with convex posterior margin. Palpi (Figs. 1B, 4D and E): short and broad; length dorsally (segments I–III) 0.70–0.96 (0.85 ± 0.06; n = 35), width 0.36–0.54 (0.46 ± 0.05; n = 35), ratio length to width 1.67–2.10 (1.86 ± 0.10; n = 35), length of segments in descending order: 2, 3, 1, 4; segment I well developed ventrally; segment II narrower at base and thereafter parallel-sided, without clear denticle at posterior margin dorsally; segment III broad, subrectangular with broadly rounded apex; segments II and III with extensive whitish enameling on dorsal surfaces. Hypostome (Fig. 4E): club-shaped; dental formula 3/3. Legs (Fig. 1B): of medium length, slender; extensively ornate with whitish enameling mostly on dorsal and lateral aspects of leg segments. Coxae (Fig. 4F and G): coxa I with relatively long, triangular, closely spaced internal and external spurs, internal spur broadly triangular with narrowly rounded apex, external spur narrowly triangular with broadly to narrowly rounded apex, internal and external spurs nearly equal in length, both spurs of coxa I generally directed posteriorly; coxae II and III each with moderate triangular external and internal spurs, external spur with narrowly rounded or tapering apex, internal spur with broadly rounded apex on coxa II and narrowly to broadly rounded apex on coxa III; coxa IV with moderate triangular subequal external and internal spurs, each with narrowly rounded to tapering apex; coxae inornate or, especially coxae I, with small spots of ivory enameling. Trochanter I (Figs. 1B and 4H) with moderate, broadly triangular dorsal spur with tapering apex. Genu IV length 0.81–1.16 (1.02 ± 0.08; n = 33), width 0.37–0.56 (0.46 ± 0.05; n = 33), ratio length to width 2.06–2.43 (2.22 ± 0.08; n = 33).
Fig. 4.
Dermacentor bellulus, female (Taiwan, Taitung, Tung-ho, Pei-yuan, USNMENT 00714291). (A) Genital aperture, ventral view. Scale bar = 0.1 mm. (B) Genital aperture, ventrolateral view. Scale bar = 0.1 mm. (C) Spiracular plate. Scale bar = 0.2 mm. Arrows show orientation of spiracular plate (a—anterior; d—dorsal). (D) Gnathosoma dorsally. Scale bar = 0.2 mm. (E) Gnathosoma ventrally. Scale bar = 0.2 mm. (F) Coxae. Scale bar = 0.5 mm. (G) Coxa I. Scale bar = 0.2 mm. (H) Trochanter I. Scale bar = 0.2 mm.
Nymph
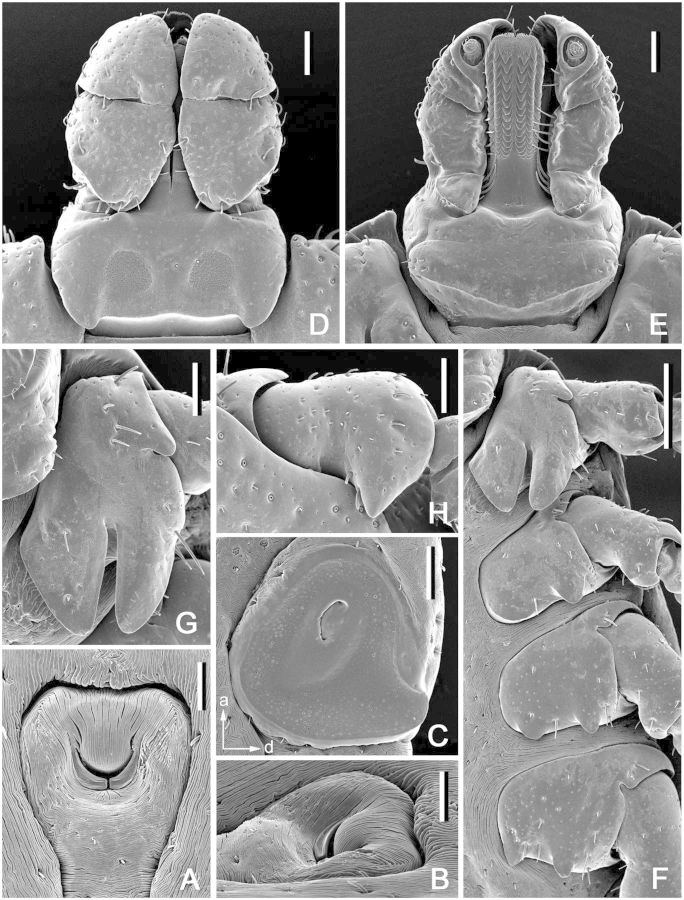
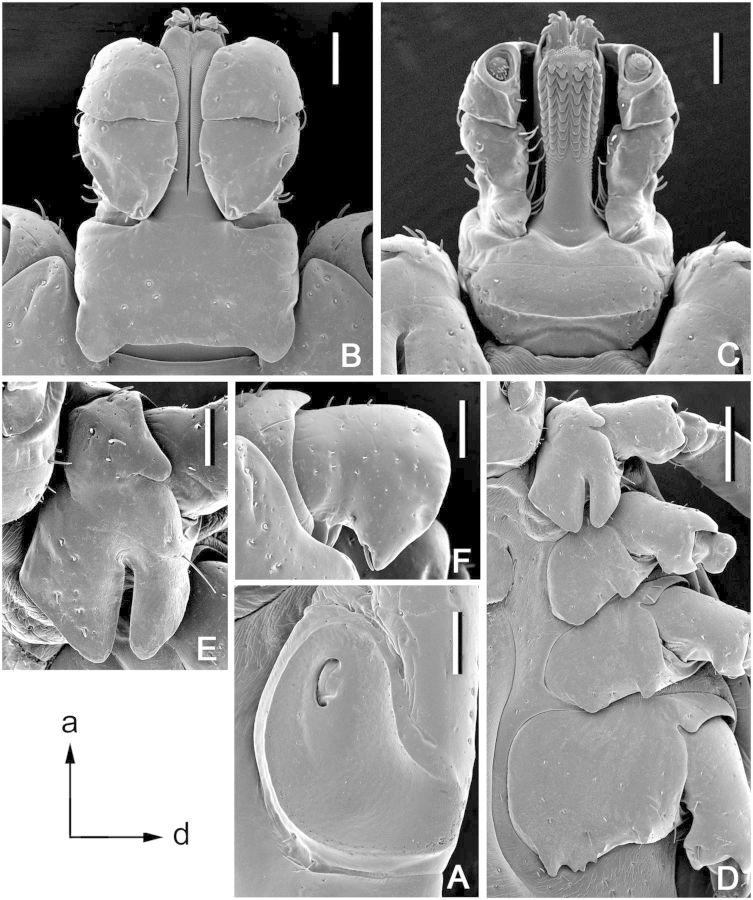
(redescription) (Fig. 5). Idiosoma: suboval, widest at level of posterior margin of coxae IV. Scutum (Fig. 5A): length 670–730 (703 ± 25; n = 5), width 610–690 (659 ± 30; n = 5), ratio length to width 1.06–1.10 (1.07 ± 0.02; n = 5); pentagonal, anterolateral margins slightly diverging, subparallel; posterolateral margins converging to moderately narrow rounded apex, posterolateral depressions and posterolateral angles slight; cervical grooves distinct, shallow. Setae approximately 12–18 (14; n = 5) pairs, length of setae in posterior portion of central field 30–36 (33 ± 2; n = 5). Eyes suboval, slightly bulging, located on lateral margins of scutum at posterior third of its length; length of scutum portion posterior to eyes 210–230 (220 ± 7; n = 5), ratio of scutum length to length of its portion posterior to eyes 3.04–3.48 (3.22 ± 0.18; n = 5). Number of pairs of dorsal setae on alloscutum 47–54 (50; n = 4); length of setae in anterolateral portion of alloscutum 47–54 (51 ± 3; n = 5); setae in central rows length 39–46 (41 ± 3; n = 5); setae with denticles (Fig. 5B). Fovea with two or three (in one case, four) openings. Two to four pairs of setae at the level of coxae II ventrally. Spiracular plates (Fig. 5C): irregularly oval, moderate to large, greatest diameter in antero-posterior plane, longitudinal diameter of spiracular plate considerably larger than longitudinal diameter of sclerite around anal valves; submarginal row of perforations complete. Gnathosoma (Fig. 5D and E): length from palpal apices to posterior dorsal margin of basis capituli 340–395 (368 ± 23; n = 5), width at apices of dorsolateral projections 402–460 (438 ± 24; n = 5); ratio length to width 0.80–0.90 (0.84 ± 0.04; n = 5). Basis capituli (Fig. 5D and E): dorsally subtriangular, with very long and sharply pointed lateral projections; lacking auriculae ventrally; with two pairs of ventral setae and one pair of posthypostomal setae. Palpi (Fig. 5D and E): elongate, length (segments II-III) 252–292 (278 ± 16; n = 5), maximum width 60–67 (64 ± 3, n = 5), ratio length to width 4.18–4.55 (4.33 ± 0.14; n = 5); segment I well developed ventrally, cylindrical, clear suture between segments II and III, segment II the longest, narrow proximally and gradually expanding distally, segment III broadly rounded at apex; segment I with one ventral seta, segment II with four dorsal and three ventral setae, segment III with five dorsal and two ventral setae. Hypostome (Fig. 5E): length from apex to level of posthypostomal setae 202–232 (220 ± 12; n = 5), width at narrowest portion 60–77 (68 ± 7; n = 2), ratio length to width 3.00–3.44 (3.26 ± 0.19; n = 5); club-shaped; dental formula: distal 1 row on either side (rarely on both sides) of larger denticles 3/3, proximal approximately 7–8 rows 2/2; 3/3 portion of hypostome comprises approximately 1/10 of its total length (ratio avg. 10.08). Coxae (Fig. 5F): coxa I with relatively long internal and external spurs; internal spur slightly shorter than external; both spurs triangular with bluntly tapering apices; external spur on coxae II–IV relatively large, broadly triangular with bluntly tapering apices; size of external spurs slightly decreasing from coxa II to coxa IV; coxa II without distinct internal spur; spur on coxa IV usually not protruding beyond posterior coxal margin; coxal “pores” (openings of dermal glands or sensilla usually situated close to mid- margin of coxae) present only on coxae I (rarely asymmetrically present on other coxae). Genu IV: length 255–285 (269 ± 15; n = 5), width 95–107 (102 ± 7; n = 3), ratio length to width 2.65–2.74 (2.70 ± 0.04; n = 3).
Fig. 5.
Dermacentor bellulus, nymph (Taiwan, Taipei, Ta-t’un Shan, Pai-liu-chia, USNMENT 00714074). (A) Scutum. Scale bar = 200 μm. (B) Setae of alloscutum. Scale bar = 10 μm. (C) Spiracular plate. Scale bar = 50 μm. Arrows show orientation of spiracular plate (a—anterior; d—dorsal). (D) Gnathosoma dorsally. Scale bar = 100 μm. (E) Gnathosoma ventrally. Scale bar = 100 μm. (F) Coxae. Scale bar = 100 μm.
Larva
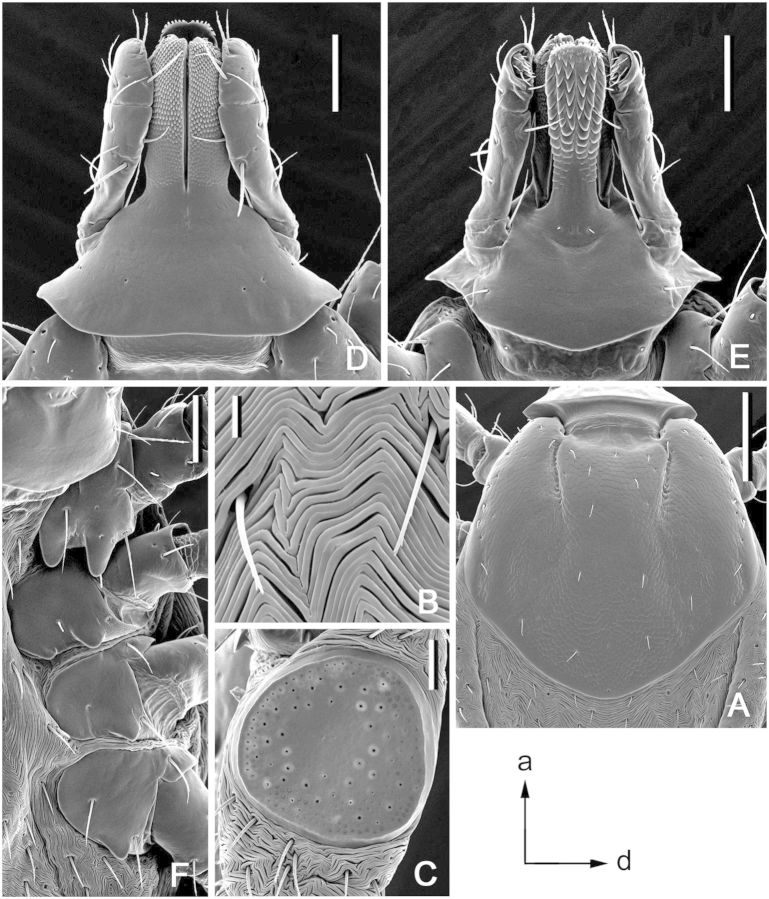
(Fig. 6). Idiosoma: suboval, widest at level of coxae III. Scutum (Fig. 6A): length 320–345 (332 ± 7; n = 15), width 435–480 (455 ± 13; n = 15), ratio length to width 0.71–0.77 (0.73 ± 0.01; n = 15); pentagonal, posterior margin broadly rounded, posterolateral marginal depressions slight; cervical grooves as faint, shallow depressions. Eyes suboval, slightly bulging, located on lateral margins of scutum in posterior third of scutum length; length of scutum portion posterior to eyes 95–115 (106 ± 6; n = 15), ratio of scutum length to length of its portion posterior to eyes 2.95–3.42 (3.14 ± 0.14; n = 15). Setae 3 pairs, Sc2 19–22 (20 ± 1; n = 5), Sc3 16–19 (17 ± 1; n = 5). Dorsal setae of alloscutum 10 pairs; 2 pairs of central dorsals, Cd1 17–20 (19 ± 1; n = 10), Cd2 19–21 (20 ± 1; n = 5); 8 pairs of marginal dorsals, Md1 21–26 (23 ± 2; n = 6), Md8 17–21 (18 ± 1; n = 10). Ventral setae 14 pairs plus 1 pair on anal valves; 3 pairs of sternals, St1 35–40 (37 ± 1; n = 11); 2 pairs of preanals, Pa1 26–32 (29 ± 2; n = 9), Pa2 25–29 (27 ± 2; n = 8); 4 pairs of premarginals, Pm1 28–37 (31 ± 2; n = 10); 5 pairs of marginal ventrals, Mv1 18–24 (21 ± 2; n = 13), Mv5 17–21 (20 ± 1; n = 14). Gnathosoma (Fig. 6B–D): length from palpal apices to posterior dorsal margin of basis capituli 167–195 (180 ± 8; n = 15), width at apices of dorsolateral projections 200–222 (213 ± 7; n = 15); ratio length to width 0.80–0.90 (0.84 ± 0.03; n = 15). Basis capituli (Fig. 6B–D): dorsally subtriangular, with long and tapering lateral projections; ventrally rectangular; anterior angle of basis capituli slightly obtuse; auriculae as short, broad, and blunt projections. Posthypostomal setae 1 pair. Palpi (Fig. 6B–D): elongate, length (segments II-III) 122–135 (130 ± 4; n = 15), width 50–55 (52 ± 1, n = 15), ratio length to width 2.38–2.65 (2.49 ± 0.07; n = 15); segment I well developed ventrally, cylindrical, faint suture between segments II and III, segment III with relatively large denticle ventrally; segment I without setae, segment II with four dorsal and two ventral setae, segment III with five dorsal and one ventral setae. Hypostome (Fig. 6C): length from apex to level of posthypostomal setae 92–105 (98 ± 3; n = 14), minimum width 26–30 (28 ± 1; n = 14), ratio length to width 3.36–3.64 (3.49 ± 0.08; n = 14); club-shaped, dental formula 2/2 throughout length, ca. 6 larger denticles in median files; denticulated portion comprises approximately half of hypostomal length. Coxae (Fig. 6E): coxa I with large triangular spur with tapering apex, coxae II and III each with relatively large triangular spurs. Genu I: length 150–170 (161 ± 6; n = 15), width 67–77 (74 ± 3; n = 13), ratio length to width 2.03–2.28 (2.18 ± 0.08; n = 13).
Fig. 6.
Dermacentor bellulus, larva (Taiwan, Taipei, San-chih, Ch’e-Ch’eng, USNMENT 00714185). (A) Scutum. Scale bar = 100 μm. (B) Gnathosoma dorsally. Scale bar = 50 μm. (C) Gnathosoma ventrally. Scale bar = 50 μm. (D) Gnathosoma anteroventrally. Scale bar = 50 μm. (E) Coxae. Scale bar = 50 μm.
HOLOTYPE
Male, from dog, Kosempo (23° 42′ N, 121° 3′ E), Nantou County, Taiwan, collected by Sauter. Schulze (1935) indicated that this specimen is in the Entomological Institute Berlin-Dahlem (Germany). All noninsect specimens from the Senckenberg Deutsches Entomologisches Intitut (Senckenberg German Entomological Institute, Müncheberg) were transferred to the Museum für Naturkunde (Berlin) but unfortunately the type specimen of D. bellulus was not among them (Jason Dunlop, personal communication) . We were unable to confirm the existence of the holotype. Based on the original description and illustrations, it is clear that Schulze (1935) had additional specimens of D. bellulus (paratypes) but their whereabouts are also unknown.
The male of D. bellulus was described and illustrated by Schulze (1935). Later, a male of this species was described and illustrated in Sugimoto (1937) as D. atrosignatus. Keegan and Toshioka (1957) referred to this taxon as Dermacentor sp., as did Yamaguti et al. (1971). Wassef and Hoogstraal (1986a) illustrated the male as D. taiwanensis but provided a description that was based on specimens of both D. taiwanensis and D. bellulus. Apparently, the first and only description of the female of D. bellulus is that of Sugimoto (1937), again as D. atrosignatus. Unfortunately, however, key features are not illustrated and it is therefore difficult to ascertain the identity of Sugimoto’s female specimen. The nymph and larva of D. bellulus were described by Kitaoka and Suzuki (1981) as D. taiwanensis and later reproduced in Teng and Jiang (1991), also as D. taiwanensis.
Synonyms
Indocentor bellulus Schulze, 1935.
Schulze (1935) originally described D. bellulus in the genus Indocentor. Luh and Woo (1950) moved this species to the genus Dermacentor.
Hosts
The host data for D. bellulus are summarized in Table 1. Most adults have been collected from wild boar, Sus scrofa L. A few adults are from giant panda, Ailuropoda melanoleuca (David), Asian black bear, Ursus thibetanus Cuvier, and unidentified bear, Ursus sp. A single male was taken from a human and a single female was collected off clothing. The type specimens were collected from domestic dog (Schulze, 1935). Both nymphs and larvae were collected from Chinese hare, Lepus sinensis Gray, greater bandicoot rat, Bandicota indica (Bechstein), Losea rat, Rattus losea (Swinhoe), brown rat, Rattus norvegicus (Berkenhout), roof rat, Rattus rattus (L.), rats, Rattus sp., and unidentified “rats.” Additionally, nymphs have been found on large Japanese field mouse, Apodemus speciosus (Temminck), Confucian niviventer, Niviventer confucianus (Milne-Edwards), spiny Taiwan niviventer, Niviventer coninga (Swinhoe), niviventer, Niviventer sp., Oriental house rat, Rattus tanezumi Temminck, common treeshrew, Tupaia glis (Diard) and clothing, while larvae have been recorded from lesser bandicoot rat, Bandicota bengalensis (Gray), Ryukyu mouse, Mus caroli Bonhote, unidentified “rodents,” Chinese ferret-badger, Melogale moschata (Gray), and Chinese bamboo-partridge Bambusicola thoracicus (Temminck).
Adults apparently can be collected throughout the year. In material that we have studied, adult D. bellulus were collected during all months except July and September, with the maximum number of collections made in February. Nymphs of D. bellulus were collected during all months except February, with the maximum number of collections made in April, while larvae were collected during all months except January, with the maximum number of collections in March.
Distribution
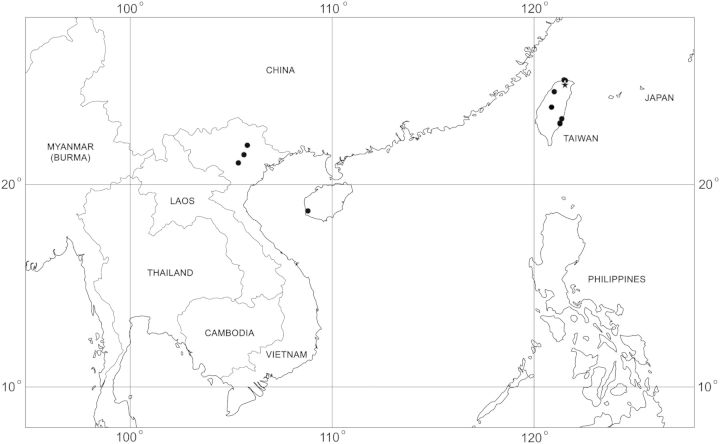
(Fig. 7). Distribution data for D. bellulus are summarized in Table 1. The known distribution of this species is confined to China (Fujian, Sichuan, and Tibet Provinces), Japan (Fukui, Kagoshima, Kanagawa, and Kyoto Prefectures), Nepal (Nuwakot and Rasuwa Districts), Taiwan (Taipei and Taitung Counties), and Vietnam (Da Nang, Dak Lak, Dong Nai, Lam Dong, Lao Cai, Quang Binh, and Quang Tri Provinces). This is the only Indocentor species occurring in both the Oriental and Palearctic Zoogeographic Regions.
Fig. 7.
Dermacentor bellulus, map of geographical distribution. Star shows type locality, filled circles show confirmed localities.
Etymology
The specific name is derived from the Latin “bellulus,” meaning “beautiful,” and apparently refers to the extensive bright ivory ornamentation of the male conscutum.
Dermacentor taiwanensis Sugimoto, 1935 (Figs. 8–12)
Male
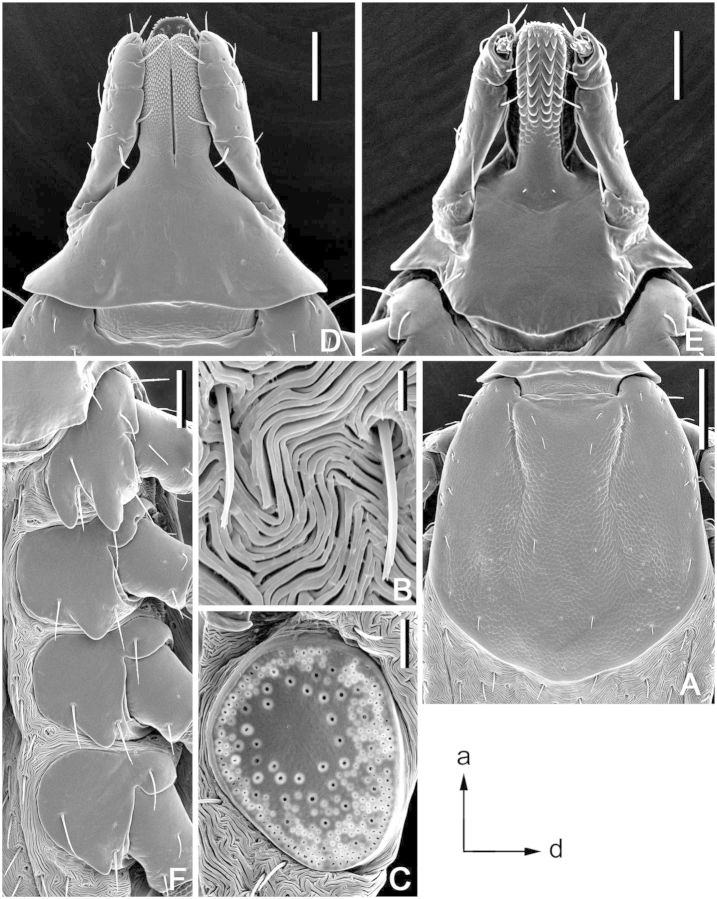
(redescription) (Figs. 8A and 9). Conscutum (Fig. 8A): broadly oval with distinctly convex lateral margins, widest approximately at mid-length; distance from scapular apices to posterior margin of conscutum 2.87–5.94 (4.99 ± 0.68; n = 55), maximum width 2.31–4.81 (3.89 ± 0.54; n = 55), ratio length to width 1.22–1.37 (1.28 ± 0.03; n = 55). Coloration as illustrated: pale ivory-colored ornamentation very extensive, light- to dark-brown background forms several patches, often with indistinct hazy margins; a pair of narrow cervical patches extending from cervical pits to posterior margin of pseudoscutum; a pair of small patches lateral to cervical patches; broad, well-defined anterior and hazy posterior brownish central patch on pseudoscutum; posteromedian margin of pseudoscutum indicated by narrow hazy strip; lateral field with one narrow brown patch extending along most of lateral margins of conscutum from eyes to first festoon; two pairs of oval patches medial to lateral grooves; a pair of central patches; narrow stripes on posteromedian and paramedian regions; first, second, and paramedian festoons mostly ivory colored, third and fourth festoons mostly brown, median festoon with large ivory spot; all punctations light- to dark-brown. Central field posterior to pseudoscutum often is flat, imparting a concave appearance to conscutum; surface of conscutum smooth. Cervical grooves shallow; a pair of central depressions, and a second posterior pair that correspond to paramedian grooves; lateral grooves superficial, but distinct because they align with large punctations; 11 distinct festoons, median festoon conspicuously narrower than paramedian. Very large and deep punctations moderately dense, distributed over entire conscutum but denser laterally and posteriorly; large punctations on pseudoscutum fairly sparse; fine punctations dense, evenly distributed. Eyes oval, very slightly convex, at anterior one-fifth of conscutal length. Setae relatively short and inconspicuous. Spiracular plates (Fig. 9A): suboval; dorsal prolongation moderately long and broad, with broad, unperforated widening anteriorly; perforations very small and fairly numerous. Sclerotized plaques on festoons ventrally inornate or with small ivory-colored spot. Gnathosoma (Figs. 8A, 9B and C): length from palpal apices to cornual apices dorsally 0.70–1.41 (1.21 ± 0.14; n = 54), width of basis capituli 0.49–1.00 (0.85 ± 0.10; n = 54), ratio length to width 1.27–1.53 (1.42 ± 0.05; n = 54). Basis capituli (Figs. 8A, 9B and C): dorsally subrectangular; posterior margin nearly straight or slightly concave; length 0.30–0.60 (0.52 ± 0.06; n = 54), ratio width to length 1.51–1.84 (1.65 ± 0.07; n = 54); cornua broad, short, total length of basis capituli, including cornua, 4.67–9.33 (6.62 ± 1.00; n = 54) cornual length; dorsally with extensive whitish enameling. Basis capituli ventrally subrectangular; posterior margin convex. Palpi (Figs. 8A, 9B and C): short, broad; length dorsally (segments I–III) 0.41–0.83 (0.70 ± 0.08; n = 54), width 0.22–0.48 (0.39 ± 0.05; n = 54), ratio length to width 1.58–2.16 (1.80 ± 0.12; n = 54), length of segments in descending order: 2, 3, 1, 4; segment I well developed ventrally; segment II narrower at base and thereafter widening, without clear denticle at posterior margin dorsally; segment III subrectangular with broadly rounded apex; segments II and III with extensive whitish enameling on dorsal surfaces. Hypostome (Fig. 9C): club-shaped; dental formula 3/3. Legs (Fig. 8A): of medium length, moderately robust; with extensive whitish enameling mostly on dorsal and lateral aspects of leg segments. Coxae (Fig. 9D and E): coxa I with relatively long, triangular, closely spaced internal and external spurs, internal spur broadly triangular with narrowly rounded apex, external spur narrowly triangular with broadly to narrowly rounded apex, internal and external spurs nearly equal in length, both spurs of coxa I generally directed posteriorly; coxae II and III each with moderate triangular external and internal spurs, external spur with narrowly rounded or tapering apex, internal spur with broadly or narrowly rounded apex; coxa IV with moderate triangular external spur with tapering apex and with several moderate triangular internal spurs, each with tapering apex; coxa IV enlarged, ratio length to width 0.78–1.08 (0.95 ± 0.08; n = 55). Trochanter I (Figs. 8A and 9F) with moderately long, broadly triangular dorsal spur with tapering apex. Genu and Tibia (Fig. 8A) with two rows of very short projections ventrally. Genu IV length 0.57–1.38 (1.12 ± 0.17; n = 53), width 0.25–0.70 (0.53 ± 0.10; n = 53), ratio length to width 1.86–2.35 (2.13 ± 0.12; n = 53).
Fig. 9.
Dermacentor taiwanensis, male (Taiwan, Taitung, Pei-yuan, USNMENT 00714776). (A) Spiracular plate. Scale bar = 0.2 mm. Arrows show orientation of spiracular plate (a—anterior; d—dorsal). (B) Gnathosoma dorsally. Scale bar = 0.2 mm. (C) Gnathosoma ventrally. Scale bar = 0.2 mm. (D) Coxae. Scale bar = 0.5 mm. (E) Coxa I. Scale bar = 0.2 mm. (F) Trochanter I. Scale bar = 0.2 mm.
Female
(redescription) (Figs. 8B and 10). Idiosoma (Fig. 8B): broadly oval, widest near mid-length. Scutum (Fig. 8B): length 1.95–2.75 (2.41 ± 0.19; n = 23), width 2.30–3.25 (2.88 ± 0.23; n = 23), ratio length to width 0.76–0.90 (0.84 ± 0.03; n = 23), margins diverge posteriorly in anterior third of total length, thereafter gradually converging to broadly rounded posterior margin, slight posterolateral angular projections may be present. Coloration: ornamentation very extensive, major portion of scutal surface covered with whitish enameling; brown colored patches arranged as follows: two pairs of small patches in cervical pits, a pair of narrow patches in cervical fields, narrow central patch brown anteriorly and posteriorly, hazy toward center and disappearing at center of scutum, a pair of narrow patches bordering posterior margin of scutum extending from just anterior to eyes, widening at posterior margin of cervical grooves, and connecting at posterior apex of scutum. Cervical grooves distinct, moderately deep. Surface of scutum smooth; very large and deep punctations sparse and situated mostly in cervical grooves and anterior part of central field; fine punctations dense, evenly distributed over scutum. Eyes oval, very slightly convex, positioned at second third of scutal length. Setae relatively sparse and short. Alloscutum (Fig. 8B): as illustrated; 11 festoons. Setae of alloscutum relatively short (ca. 0.076), moderately dense. Genital aperture (Fig. 10A and B): at level of coxae II, broadly V-shaped, sclerites bordering genital aperture laterally small and indistinct; preatrial fold slightly convex. Spiracular plates (Fig. 10C): suboval; dorsal prolongation moderately long and broad, broadly rounded to its apex, with broad unperforated widening anteriorly; perforations very small and fairly numerous. Gnathosoma (Figs. 8B, 10D and E): length from palpal apices to posterior margin of basis capituli dorsally 1.22–1.58 (1.43 ± 0.09; n = 23), width of basis capituli 0.90–1.20 (1.05 ± 0.07; n = 23), ratio length to width 1.28–1.45 (1.36 ± 0.05; n = 23). Basis capituli (Figs. 8B, 10D and E): dorsally subrectangular; posterior margin nearly straight or slightly concave, length 0.46–0.62 (0.54 ± 0.04; n = 23), ratio width to length 1.79–2.35 (1.93 ± 0.12; n = 23); cornua broad, short, total length of basis capituli, including cornua, 7.00–14.50 (9.07 ± 1.52; n = 23) cornual length; dorsally extensively ornate with whitish enameling. Porose areas moderate, circular, deeply sunken with clearly circumscribed borders, separated by space nearly equal to their width. Basis capituli ventrally subrectangular; with convex posterior margin. Palpi (Figs. 8B, 10D and E): short and broad; length dorsally (segments I–III) 0.77–0.96 (0.88 ± 0.05; n = 23), width 0.35–0.50 (0.44 ± 0.04; n = 23), ratio length to width 1.82–2.25 (2.02 ± 0.13; n = 23), length of segments in descending order: 2, 3, 1, 4; segment I well developed ventrally; segment II narrower at base and thereafter parallel-sided, without clear denticle at posterior margin dorsally; segment III broad, subrectangular with broadly rounded apex; segments II and III with extensive whitish enameling on dorsal surfaces. Hypostome (Fig. 10E): club-shaped; dental formula 3/3. Legs (Fig. 8B): of medium length, slender; extensively ornate with whitish enameling mostly on dorsal and lateral aspects of leg segments. Coxae (Fig. 10F and G): coxa I with relatively long, triangular, closely spaced internal and external spurs, internal spur broadly triangular with narrowly rounded apex, external spur narrowly triangular with broadly to narrowly rounded apex, internal and external spurs nearly equal in length, both spurs of coxa I generally directed posteriorly; coxae II and III each with moderate triangular external and internal spurs, external spur with narrowly rounded or tapering apex, internal spur with broadly rounded apex on coxa II and narrowly to broadly rounded apex on coxa III; coxa IV with moderate triangular subequal external and internal spurs, with narrowly rounded to tapering apex; coxae inornate or, especially coxae I, with small spots of ivory enameling. Trochanter I (Figs. 8B and 10H) with moderate, broad, triangular dorsal spur with tapering apex. Genu IV length 0.96–1.30 (1.16 ± 0.08; n = 19), width 0.42–0.57 (0.49 ± 0.04; n = 19), ratio length to width 2.22–2.45 (2.34 ± 0.07; n = 19).
Fig. 10.
Dermacentor taiwanensis, female (Taiwan, Taitung, Pei-yuan, Shih-nan, USNMENT 00714182). (A) Genital aperture, ventral view. Scale bar = 0.1 mm. (B) Genital aperture, ventrolateral view. Scale bar = 0.1 mm. (C) Spiracular plate. Scale bar = 0.2 mm. Arrows show orientation of spiracular plate (a—anterior; d—dorsal). (D) Gnathosoma dorsally. Scale bar = 0.2 mm. (E) Gnathosoma ventrally. Scale bar = 0.2 mm. (F) Coxae. Scale bar = 0.5 mm. (G) Coxa I. Scale bar = 0.2 mm. (H) Trochanter I. Scale bar = 0.2 mm.
Nymph
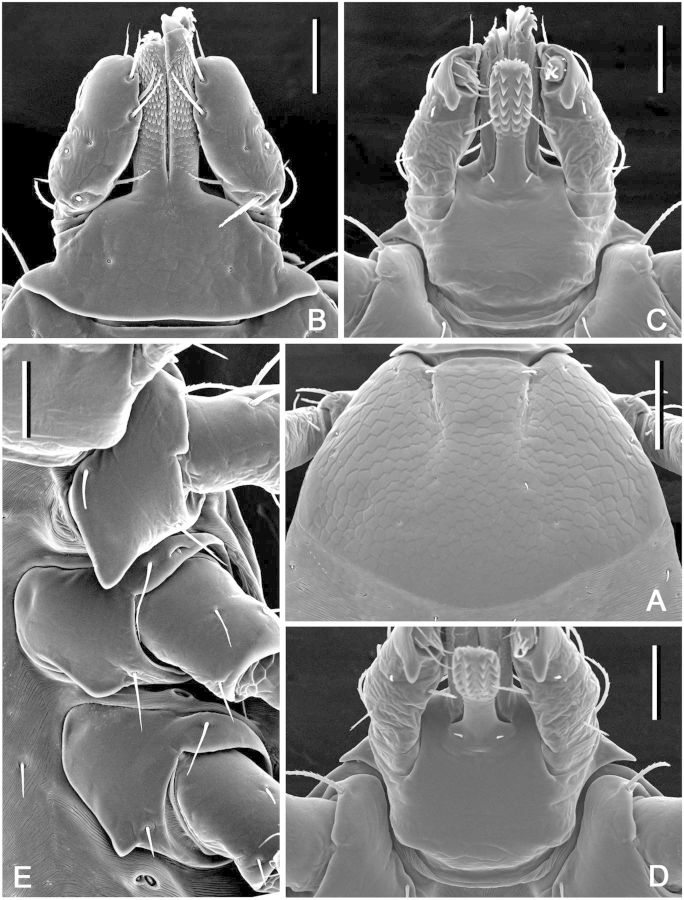
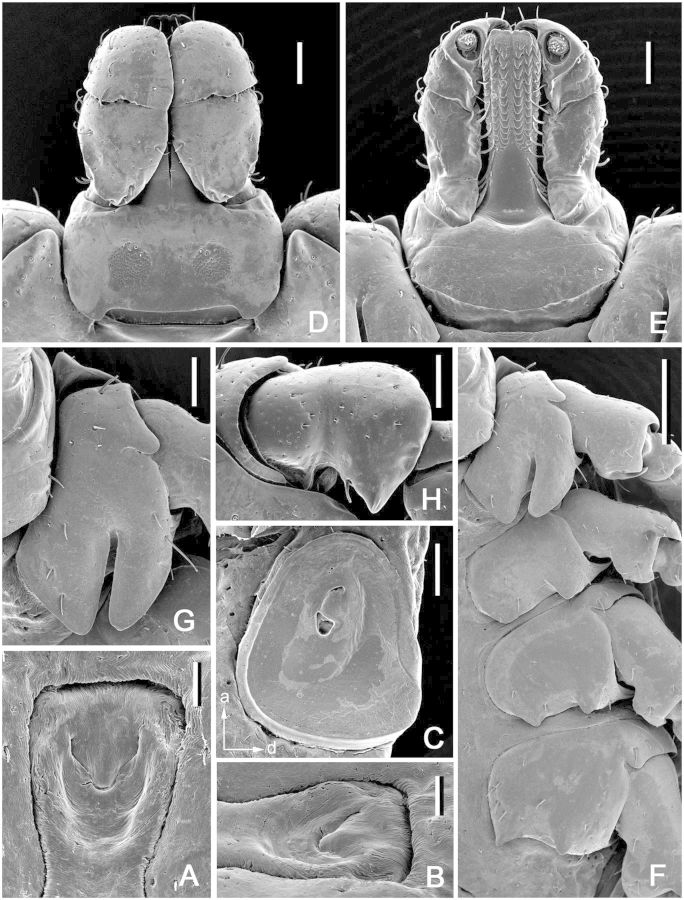
(description) (Fig. 11). Idiosoma: suboval, widest at level of posterior margin of coxae IV. Scutum (Fig. 11A): length 640–645 (642; n = 2), width 650 (n = 2), ratio length to width 0.98–0.99 (0.99; n = 2); pentagonal, anterolateral margins distinctly diverging, posterolateral margins converging to moderately narrow rounded apex, posterolateral depressions and posterolateral angles very slight; cervical grooves distinct, shallow. Setae approximately 12–14 (13; n = 2) pairs, length of setae in posterior portion of central field 29–32 (31; n = 2). Eyes suboval, slightly bulging, located on lateral margins of scutum slightly posterior its midlength; length of scutum portion posterior to eyes 250–270 (260; n = 2), ratio of scutum length to length of its portion posterior to eyes 2.39–2.56 (2.47; n = 2). Number of pairs of dorsal setae on alloscutum 43–44 (n = 2); length of setae in anterolateral portion of alloscutum 35–40 (37; n = 2); setae in central rows length 31–32 (32; n = 2); setae with denticles (Fig. 11B). Fovea with two or three (one to four) openings. Two pairs of setae at the level of coxae II ventrally. Spiracular plates (Fig. 11C): irregularly subcircular, relatively large, greatest diameter often in antero-posterior plane, longitudinal diameter of spiracular plate considerably larger than longitudinal diameter of sclerite around anal valves; submarginal row of perforations complete. Gnathosoma (Fig. 11D and E): length from palpal apices to posterior dorsal margin of basis capituli 335–340 (337; n = 2), width at apices of dorsolateral projections 382–385 (384; n = 2); ratio length to width 0.87–0.89 (0.88; n = 2). Basis capituli (Fig. 11D and E): dorsally subhexagonal, with long and sharply pointed lateral projections; lacking auriculae ventrally; with two pairs of ventral setae and one pair of posthypostomal setae. Palpi (Fig. 11D and E): elongate, length (segments II-III) 240–245 (242; n = 2), maximum width 61–62 (62, n = 2), ratio length to width 3.92 (n = 2); segment I well developed ventrally, cylindrical, clear suture between segments II and III, segment II longest, narrow proximally and gradually expanding distally, segment III broadly rounded at apex; segment I with one ventral seta, segment II with four dorsal and three ventral setae, segment III with five dorsal and two ventral setae. Hypostome (Fig. 11E): length from apex to level of posthypostomal setae 222–230 (226; n = 2), width at narrowest portion 80 (n = 2), ratio length to width 2.78–2.87 (2.83; n = 2); club-shaped; dental formula of approximately 5 rows of larger denticles distally 3/3, formula of approximately 3–4 rows proximally 2/2; 3/3 portion of hypostome comprises slightly more than 1/2 of its total length (ratio avg. 2.5). Coxae (Fig. 11F): coxa I with relatively long internal and external spurs; internal spur slightly shorter than external; both spurs triangular with bluntly tapering apices; external spur on coxae II–IV relatively large, triangular with bluntly tapering apices; size of external spurs slightly decreasing from coxa II to coxa IV; coxa II with short triangular internal spur; spur on coxa IV either not protruding or slightly protruding beyond posterior coxal margin; coxal “pores” (openings of dermal glands or sensilla usually situated close to mid-lateral margin of coxae) present only on coxae I. Genu IV: length 245–255 (250; n = 2), width 100 (n = 2), ratio length to width 2.45–2.55 (2.50; n = 2).
Fig. 11.
Dermacentor taiwanensis, nymph (Taiwan, Taipei, San-chih, Ch’e-ch’eng, USNMENT 00714094). (A) Scutum. Scale bar = 200 μm. (B) Setae of alloscutum. Scale bar = 10 μm. (C) Spiracular plate. Scale bar = 50 μm. Arrows show orientation of spiracular plate (a—anterior; d—dorsal). (D) Gnathosoma dorsally. Scale bar = 100 μm. (E) Gnathosoma ventrally. Scale bar = 100 μm. (F) Coxae. Scale bar = 100 μm.
Larva
(Fig. 12). Idiosoma: suboval, widest at level of coxae III. Scutum (Fig. 12A): length 330 (n = 1), width 440–450 (445; n = 2), ratio length to width 0.73 (n = 1); pentagonal, posterior margin broadly rounded, posterolateral marginal depressions slight; cervical grooves as faint, shallow depressions. Eyes suboval, slightly bulging, located on lateral margins of scutum at posterior third of scutum length; length of scutum portion posterior to eyes 90–100 (95; n = 2), ratio of scutum length to length of its portion posterior to eyes 3.30 (n = 1). Setae 3 pairs, Sc2 25 (n = 1), Sc3 20 (n = 2). Dorsal setae of alloscutum 10 pairs; 2 pairs of central dorsals, Cd1 17 (n = 1), Cd2 18 (n = 1); 8 pairs of marginal dorsals, Md1 23 (n = 1), Md8 20 (n = 1). Ventral setae 14 pairs plus 1 pair on anal valves; 3 pairs of sternals, St1 36 (n = 1); 2 pairs of preanals, Pa1 25 (n = 1), Pa2 25 (n = 1); 4 pairs of premarginals, Pm1 33 (n = 1); 5 pairs of marginal ventrals, Mv1 30 (n = 1), Mv5 24 (n = 1). Gnathosoma (Fig. 12B–D): length from palpal apices to posterior dorsal margin of basis capituli 190 (n = 1), width at apices of dorsolateral projections 177–202 (190; n = 2); ratio length to width 0.94 (n = 1). Basis capituli (Fig. 12B–D): dorsally subhexagonal, with relatively short and tapering lateral projections; ventrally rectangular; anterior angle of basis capituli either right or slightly obtuse; auriculae indistinct. Posthypostomal setae 1 pair. Palpi (Fig. 12B–D): elongate, length (segments II-III) 112–122 (117; n = 2), width 47–54 (51; n = 2), ratio length to width 2.28–2.37 (2.32; n = 2); segment I well developed ventrally, cylindrical, faint suture between segments II and III, segment III with relatively small denticle ventrally; segment I without setae, segment II with four dorsal and two ventral setae, segment III with five dorsal and one ventral setae. Hypostome (Fig. 12C): length from apex to the level of posthypostomal setae 100–105 (102; n = 2), minimal width 27–30 (29; n = 2), ratio length to width 3.50–3.64 (3.57; n = 2); club-shaped, dental formula 2/2 throughout length, ca. 5 or 6 larger denticles in median files; denticulated portion comprises approximately half of hypostomal length. Coxae (Fig. 12E): coxa I with large triangular spur with tapering apex, coxae II and III each with moderate triangular spurs. Genu I: length 165–177 (171; n = 2), width 80 (n = 1), ratio length to width 2.22 (n = 1).
Fig. 12.
Dermacentor taiwanensis, larva (Taiwan, Taipei, San-chih, Ch’e-ch’eng, USNMENT 00714293). (A) Scutum. Scale bar = 100 μm. (B) Gnathosoma dorsally. Scale bar = 50 μm. (C) Gnathosoma ventrally. Scale bar = 50 μm. (D) Gnathosoma anteroventrally. Scale bar = 50 μm. (E) Coxae. Scale bar = 50 μm.
HOLOTYPE
Male, from Sus sp., Aoshan, Xindian (24° 57′ N, 121° 32′ E), New Taipei City, Taiwan, collected by Nishiyama; depository unknown. We were unable to determine the current location of the Sugimoto collection and verify the existence of the holotype.
The male of Dermacentor taiwanensis was described and illustrated by Sugimoto (1935, 1937). Arthur (1960), Teng (1978), and Teng and Jiang (1991) reproduced original illustrations from Sugimoto. Wassef and Hoogstraal (1986a) illustrated the female of true D. taiwanensis but presented a description that was based on both D. taiwanensis and D. bellulus specimens.
Hosts
The host data for D. taiwanensis are summarized in Table 2. Adults have been collected only from wild boar, Sus scrofa L. Both nymphs and larvae were collected from greater bandicoot rat, Bandicota indica (Bechstein), Pallas's squirrel, Callosciurus erythraeus (Pallas), Chinese hare, Lepus sinensis Gray, and Chinese ferret-badger, Melogale moschata (Gray). Additionally, nymphs were collected from Himalayan striped squirrel, Tamiops mcclellandii (Horsfield), rat, Ruttus sp., Siberian weasel, Mustela sibirica Pallas, and domestic dog.
Adults apparently can be collected throughout the year. In material that we have studied, adult D. taiwanensis were collected during all months except March, May, July, and August, with the maximum number of collections made in February and November. Nymphs of D. taiwanensis were collected from May to September, with most collections made in May and July, while few larvae were collected from June to September.
Distribution
(Fig. 13). Distribution data for D. taiwanensis are summarized in Table 2. The known distribution of this species includes China (Hainan Province), Taiwan (New Taipei, Taipei, and Taitung Counties), and Vietnam (former Bac Thai, Hanoi, and Vinh Phuc Provinces).
Fig. 13.
Dermacentor taiwanensis, map of geographical distribution. Star shows type locality, filled circles show confirmed localities.
Mariana et al. (2005, 2008) and Sun and Xu (2013) recorded D. taiwanensis from Malaysia and continental China, respectively. We cannot confirm the identity of this species in these regions and consider such records doubtful and in need of confirmation.
Etymology
The specific name is apparently derived from Taiwan, an island and country in East Asia, where the holotype was collected.
Related Species
Morphologically, all parasitic stages of D. bellulus are quite distinct from those of D. taiwanensis.
Males of D. bellulus can be distinguished from those of D. taiwanensis by the following suite of characters: narrower conscutum with less convex lateral sides (broader conscutum with more convex lateral margins in D. taiwanensis); coloration pattern of lateral field: two brown patches, broad anterior and small, often indistinct posterior (one narrow brown line along entire lateral field in D. taiwanensis); longer cornua: total length of basis capituli, including cornua, avg. 5.01 cornual length (shorter cornua: total length of basis capituli, including cornua, avg. 6.62 cornual length in D. taiwanensis); shorter and broader palpi: ratio length to width avg. 1.60 (longer and narrower palpi in D. taiwanensis: ratio length to width avg. 1.80); longer spurs on coxae I (shorter in D. taiwanensis). Females of D. bellulus can be distinguished from those of D. taiwanensis by the shape of the genital structures: narrowly U-shaped genital aperture, highly bulging preatrial fold, and clearly distinct lateral sclerites in D. bellulus, but broadly V-shaped genital aperture, slightly bulging preatrial fold, and indistinct sclerites in D. taiwanensis. Additionally, females of D. bellulus can be distinguished from those of D. taiwanensis by the following suite of characters: narrower scutum: ratio length to width avg. 0.94 (broader scutum: ratio length to width avg. 0.84 in D. taiwanensis); longer cornua: total length of basis capituli, including cornua, 8.53 cornual length (shorter cornua: total length of basis capituli, including cornua, 9.07 cornual length in D. taiwanensis); broader and shorter palpi: ratio length to width avg. 1.86 (longer and narrower palpi in D. taiwanensis: ratio length to width avg. 2.02); longer spurs on coxae I (shorter in D. taiwanensis). Nymphs of D. bellulus can be distinguished from those of D. taiwanensis by the following suite of characters: lateral margins of scutum slightly divergent and subparallel (distinctly divergent in D. taiwanensis), eyes situated roughly at 2/3 of scutal length (slightly posterior to midlength in D. taiwanensis), basis capituli subtriangular with long lateral projections (subhexagonal with shorter lateral projections in D. taiwanensis), only ca. 1 row of hypostomal denticles with 3/3 formula, all other rows are 2/2 (5 rows, i.e., more than half of hypostome’s length is 3/3 in D. taiwanensis), and lacking internal spur on coxae II (small spur in D. taiwanensis). Larvae of D. bellulus can be distinguished from those of D. taiwanensis by the following suite of characters: subtriangular basis capituli with longer and sharper lateral projections dorsally (subhexagonal basis capituli with shorter and more obtuse lateral projections dorsally in D. taiwanensis), more pronounced auriculae (indistinct in D. taiwanensis), larger denticle on palpal segment III ventrally (smaller in D. taiwanensis).
Acknowledgments
We express our sincere thanks to Jun Chen and Wei Duan (Institute of Zoology, Chinese Academy of Sciences, Beijing, China), Natalia A. Filippova and Alexei V. Abramov (Zoological Institute, Russian Academy of Sciences, St. Petersburg, Russia), and Olga V. Voltzit (Zoological Museum, Moscow State University, Moscow, Russia) for making their specimens available for examination and for their assistance throughout this study. We are in debt to Jason Dunlop and Anja Friederichs (Museum für Naturkunde, Berlin, Germany) for verifying the presence of type specimens of various Oriental Dermacentor species in their collections and making them available for examination. We also thank Richard G. Robbins (Armed Forces Pest Management Board, Office of the Assistant Secretary of Defense for Energy, Installations and Environment, Washington, DC) for his scrupulous editing and reviewing of our manuscript. Dmitry Apanaskevich’s part of this project was supported by Grant R15AI096317 from the National Institute of Allergy and Infectious Diseases.
References Cited
- Arthur D. R. 1960. Ticks. A monograph of the Ixodoidea. Part V. On the genera Dermacentor, Anocentor, Cosmiomma, Boophilus and Margaropus. Cambridge at the University Press, Cambridge, United Kingdom. [Google Scholar]
- Camicas J. L., Hervy J. P., Adam F., Morel P. C. 1998. The ticks of the world (Acarida, Ixodida). Nomenclature, described stages, hosts, distribution. Orstom éditions, Paris, France. [Google Scholar]
- Clifford C. M., Anastos G. 1960. The use of chaetotaxy in the identification of larval ticks (Acarina: Ixodidae). J. Parasitol. 46: 567–578. [PubMed] [Google Scholar]
- Estrada-Peña A. 1991. Check-list of the species of ticks (Ixodoidea). Part I. Genera Haemaphysalis, Anocentor, Cosmiomma and Dermacentor. Documents de Travail de l’Institut Royal des Sciences Naturelles de Belgique 63, Koninklijk Belgisch Instituut voor Natuurwetenschappen, Brussels. [Google Scholar]
- Guglielmone A. A., Nava S. 2014. Names for Ixodidae (Acari: Ixodoidea): valid, synonyms, incertae sedis, nomina dubia, nomina nuda, lapsus, incorrect and suppressed names – with notes on confusion and misidentifications. Zootaxa 3767: 1–256. [DOI] [PubMed] [Google Scholar]
- Guglielmone A. A., Robbins R. G., Apanaskevich D. A., Petney T. N., Estrada-Peña A., Horak I. G., Shao R., Barker S. C. 2010. The Argasidae, Ixodidae and Nuttalliellidae (Acari: Ixodida) of the world: A list of valid species names. Zootaxa 2528: 1–28. [Google Scholar]
- Guglielmone A. A., Robbins R. G., Apanaskevich D. A., Petney T. N., Estrada-Peña A., Horak I. G. 2014. The hard ticks of the world (Acari: Ixodida: Ixodidae). Springer, Dordrecht, Heidelberg, New York, London. [Google Scholar]
- Keegan H. L., Toshioka S. 1957. Ixodid ticks of Japan, Korea and the Ryukyu Islands. 406th Medical General Laboratory, Camp Zama, Japan. [Google Scholar]
- Kitaoka S., Suzuki H. 1981. Dermacentor taiwanensis Sugimoto, 1935 (Acarina: Ixodidae): The immature stages and notes on hosts and distribution in Japan. Trop. Med. 23: 205–211. [Google Scholar]
- Luh P. L., Woo W. C. 1950. A list of Chinese Ticks. Chin. J. Entomol. 1: 195–222. [Google Scholar]
- Mariana A., Zuraidawati Z., Ho T. M., Mohd Kulaimi B., Saleh I., Shukor M. N., Shahrul-Anuar M. S. 2005. A survey of ectoparasites in Gunung Stong Forest Reserve, Kelantan, Malaysia. Southeast Asian J. Trop. Med. Pub. Health 36: 1125–1131. [PubMed] [Google Scholar]
- Mariana A., Zuraidawati Z., Ho T. M., Mohd Kulaimi B., Saleh I., Shukor M. N., Shahrul-Anuar M. S. 2008. Ticks (Ixodidae) and other ectoparasites in Ulu Muda Forest Reserve, Kedah, Malaysia. Southeast Asian J. Trop. Med. Pub. Health 39: 496–506. [PubMed] [Google Scholar]
- Schulze P. 1933. Die Arten der Zeckengattung Dermacentor s.l. aus Europa, Asien und Neu-Guinea. Zeitschrift für Parasitenkunde 6: 416–431. [Google Scholar]
- Schulze P. 1935. Zur zeckenfauna Formosas. Zoologischer Anzeiger 112: 233–237. [Google Scholar]
- Sugimoto M. 1935. On the Ixodoidea of Formosa. Taiwan Hakubutsu Gakkai Kaiho 3: 1–7. [Google Scholar]
- Sugimoto M. 1937. Notes on the ticks in the Formosan mountain reservation for aborigines. J. Cent. Soc. Vet. Med. 50:303–340. [Google Scholar]
- Sun Y., Xu R. 2013. The genus Dermacentor and the subgenus Indocentor (Acari: Ixodidae) from China. Oriental Insects 47: 155–168. [Google Scholar]
- Teng K. F. 1978. Economic insect fauna of China. Fasc. 15. Acarina: Ixodoidea. Science Press, Beijing, China. [Google Scholar]
- Teng K. F., Jiang Z. J. 1991. Economic insect fauna of China. Fasc 39. Acari: Ixodidae. Science Press, Bejing, China. [Google Scholar]
- Wassef H. Y., Hoogstraal H. 1983. Dermacentor (Indocentor) compactus (Acari: Ixodoidea: Ixodidae): Identity of male and female. J. Med. Entomol. 20: 648–652. [DOI] [PubMed] [Google Scholar]
- Wassef H. Y., Hoogstraal H. 1984a. Dermacentor (Indocentor) auratus (Acari: Ixodoidea: Ixodidae): Identity of male and female. J. Med. Entomol. 21: 169–173. [DOI] [PubMed] [Google Scholar]
- Wassef H. Y., Hoogstraal H. 1984b. Dermacentor (Indocentor) atrosignatus (Acari: Ixodoidea: Ixodidae): Identity of male and female. J. Med. Entomol. 21: 586–591. [DOI] [PubMed] [Google Scholar]
- Wassef H. Y., Hoogstraal H. 1986a. Dermacentor (Indocentor) taiwanensis (Acari: Ixodoidea: Ixodidae): Identity of male and female. J. Med. Entomol. 23: 173–177. [DOI] [PubMed] [Google Scholar]
- Wassef H. Y., Hoogstraal H. 1986b. Dermacentor (Indocentor) steini (Acari: Ixodoidea: Ixodidae): Identity of male and female. J. Med. Entomol. 23: 532–537. [DOI] [PubMed] [Google Scholar]
- Wilson D. E., Reeder D. M. 2005. Mammal species of the world: a taxonomic and geographic reference, 3rd ed The Johns Hopkins University Press, Baltimore, MD. [Google Scholar]
- Yamaguti N., Tipton V. J., Keegan H. L., Toshioka S. 1971. Ticks of Japan, Korea, and the Ryukyu Islands, vol. 15, pp. 1–226. Brigham Young University Science Bulletin, Biological Series, Provo, Utah. [Google Scholar]