Abstract
Background. Mostly anecdotal reports describe a high prevalence of chronic kidney disease in northwestern Nicaragua, predominantly among younger men, resulting in substantial morbidity and mortality. The true prevalence, nature and aetiology of kidney disease in this region remain unknown.
Methods. We performed a population-based prevalence study in Quezalguaque, Nicaragua to assess the frequency of estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2, and compared the prevalence of reduced eGFR in Quezalguaque with the USA using the NHANES 1999–2006 data. We also conducted an embedded case–control study in a subset of participants to assess kidney disease risk factors.
Results. From 1882 eligible households, 771 individuals from 300 households participated in the prevalence study, 98 (13%) of whom had reduced eGFR. Reduced eGFR was more common among older participants, men and participants living at lower altitudes. Among 18–29-year-old participants, 2.6% had reduced eGFR, and among 30–41-year-old participants, 7.4% had reduced eGFR; this compares with 0.2% and 0.8%, respectively, in NHANES. No individuals in these age groups were diabetic. Among cases, only 27% had dipstick proteinuria of 1+ or greater, compared with 7% of controls. Haematuria did not significantly differ between cases and controls (24% versus 18%). In age- and sex-adjusted models, hypertension and residence at lower altitude were independently associated with reduced eGFR, while occupational history was not associated with reduced eGFR.
Conclusions. Kidney disease appears common in residents of Quezalguaque, Nicaragua, particularly in younger men, with features most consistent with tubulointerstitial disease. Further research is needed to elucidate the causes of kidney disease in this region.
Keywords: chronic kidney disease, epidemiology, Latin America, Nicaragua, prevalence
Introduction
For more than two decades, multiple reports have described a high prevalence of chronic kidney disease (CKD) in northwestern Nicaragua and coastal El Salvador, predominantly among younger men [1–6]. Many of these reports have methodological concerns, and a few have reached the peer-reviewed literature. Putative risk factors described in these studies include agricultural work [1,4–7], pesticide exposure [1,5,6], living at lower altitude [8], alcoholism in general [4–6,9] and, more specifically, consumption of commercially produced bulk rum known as ‘Guaro Lija’ [4,5]. In addition, local physicians posit that substantial volume depletion occurring in agricultural workers in the cotton and sugar cane industries may be an important contributing factor to the progression of the kidney disease (personal communications by D.W. with Drs Felix Zelaya, Mauricio Jarquin and Erwin Reyes, June 2009). Importantly, traditional risk factors for CKD, such as diabetes, hypertension and cardiovascular disease, are not implicated as causes of CKD in this region [1,5,6,9,10].
A recent study by Torres and colleagues explored the prevalence of reduced glomerular filtration rate (GFR) in five communities in the department of León, Nicaragua, describing a high prevalence of elevated serum creatinine (≥1.3 mg/dL) with minimal proteinuria in villages where mining and subsistence farming (26% of men, age 20–60 years) and banana/sugar cane farming (22% of men, age 20–60 years) are the primary industry [11]. In order to further define the prevalence of and explore risk factors for kidney disease in this region, we carried out a population-based prevalence study in the town of Quezalguaque, Nicaragua, building on methodology used in the Torres study. To contextualize disease rates, we compared the prevalence of decreased GFR in Quezalguaque with the USA based on the National Health and Nutrition Examination Survey (NHANES) 1999–2006 data. We also conducted an embedded case–control study in a subset of participants to assess potential risk factors for decreased GFR.
Materials and methods
Study overview and population
Quezalguaque is a small, rural municipality located in the department of León on the Pacific coast of Nicaragua, comprised of 22 communities with an adult population of 5749. In July 2008, we conducted a population-based prevalence study in Quezalguaque with an embedded case–control study. Study design and data collection materials were consistent with a concurrent study being conducted in the department of León by the Universidad Nacional Autónoma de Nicaragua-León (UNAN-León) [11]. The Brookline–Quezalguaque Sister City Committee (Brookline, MA) provided resources and organizational support, and the Institutional Review Boards of Boston University and the UNAN-León approved all study activities.
Prevalence study
Individuals were enrolled from all 22 communities within Quezalguaque. A random sample of households in each community was selected from the 2007 Quezalguaque census; the number of households sampled in each community was proportional to the population of that community relative to the entire municipality. All adult members of each sampled household residing in the household during the study period were eligible for the prevalence study. Households were approached twice to account for absence in the first attempted encounter. If some but not all family members were present, the family was approached once more at a time of their recommendation. Research assistants explained the study to each individual and obtained oral informed consent prior to screening and data collection. Age, sex, height, weight and serum creatinine level were recorded for all participants in the prevalence study. The location of each household was determined using a portable GPS device.
The Stat Sensor, a hand-held, battery-operated device (Nova Biomedical, Waltham, MA, USA), was used to determine serum creatinine and estimated GFR (eGFR). As the Stat Sensor has not been calibrated to an IDMS-standardized creatinine assay, eGFR for individuals was calculated using the original four-variable MDRD Study equation (eGFR = 186 × creatinine− 1.154 × age− 0.203 × 0.742 if female) [12]. Individuals with eGFR <60 mL/min/1.73 m2 were designated ‘cases’ and included in the case–control portion of the study.
Comparison with NHANES
We compared results from the prevalence study with CKD prevalence estimates in the USA using the NHANES. The NHANES is a cross-sectional, multistage, stratified, clustered probability sample of the civilian, non-institutionalized US population. The NHANES population encompassed 19 781 participants from the 1999–2006 surveys who were 18 years and older and were not missing serum creatinine measurements. In the NHANES, serum creatinine was measured using a kinetic rate Jaffe method and re-calibrated to standardized creatinine measurements obtained at the Cleveland Clinic Research Laboratory (Cleveland, OH, USA) [13]. GFR was estimated using the four-variable MDRD Study equation re-expressed for standardized values [14]. Stata version 10.0 (StataCorp, College Station, TX, USA) was used for analyses to incorporate the NHANES sampling weights to obtain unbiased prevalence estimates from the complex NHANES sampling design. Standard errors for all estimates were obtained using the Taylor series (linearization) method following the NHANES recommended procedures [15].
Case–control study
Selection of Quezalguaque residents for the prevalence and case–control studies is summarized in Figure 1. Based on the Quezalguaque census, occupants 18 years old and older in each household in Quezalguaque were randomly ordered. Upon obtaining consent from each participating household, occupants with and without reduced eGFR were identified from households participating in the prevalence study. Using the randomly ordered list, we sought to enrol one control per household. If the selected individual declined or could not be located, the next person on the list for that household became the control, and so on. If the randomly selected person to be a control for that household was identified as ‘case’, we did not select an additional control for the household. Men were oversampled in the control selection by a pre-specified 2:1 ratio, reflecting previously reported higher prevalence of CKD in Nicaraguan men. A detailed questionnaire was administered to all controls and cases, and included questions about literacy, education and work history, exposure to pesticides and other chemicals, alcohol and cigarette use, and personal and family medical histories. Blood pressure was assessed in both cases and controls with the participant seated after a 5-min rest, using either a standard sphygmomanometer and stethoscope or an automated blood pressure cuff. Additional blood samples were obtained from all participants in the case–control study, stored and refrigerated at the local health centre, and transported to Nova Biomedical for analysis. Glucose was assayed on the Stat Strip Xpress glucometer from Nova Biomedical, and lead was assayed on LeadCare II analyser from ESA Biosciences (Chelmsford, MA, USA). Urinalysis was performed on-site using Siemens Multistick 10 SG reagent strips.
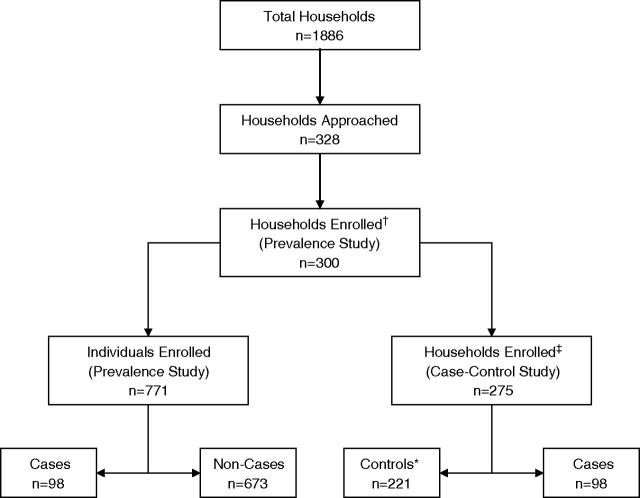
Fig. 1.
Derivation of the participant populations for the cohort and case–control studies. Thirteen households (dagger) were unavailable on two attempts, and 15 declined evaluation. Twenty-five (double dagger) declined further evaluation. In 54 of 272 households (asterisk), the randomly selected control was a case, and no further occupant was selected as a control.
Study outcomes
For both the prevalence and case–control studies, the outcome of interest was CKD stage 3 or worse, defined by eGFR <60 mL/min/1.73 m2 on a single measurement. For case–control participants, proteinuria and haematuria were dichotomized at 1+ or greater on urine dipstick analysis.
Statistical analyses
The prevalence of CKD was determined and compared across different subgroups using t-tests and chi-square analyses as appropriate. In the case–control analyses, in addition to the variables available in the prevalence study, other variables of interest included diabetes (random serum glucose ≥200 mg/dL or self-report of diabetes), hypertension (blood pressure ≥140/90 mmHg or self-report of high blood pressure), type of work (agricultural, and specifically cotton and sugar cane), literacy and schooling, exposure to pesticides (self-report), water source (well, piped or other), and medication use (NSAIDS, antibiotics or antihypertensive agents). In a sensitivity analysis, we used a glucose level of ≥126 mg/dL to define diabetes. t-tests and chi-square analyses were performed, as appropriate, and Mantel–Haenzsel odds ratios were calculated, controlling for sex and age. Analyses were performed with SAS version 9.2 (Cary, NC, USA). All testing was two-sided.
Results
Prevalence study
Of the 1882 eligible households in the municipality of Quezalguaque, 771 individuals from 300 households participated in the prevalence study (Figure 1). The majority of the study population was female (61%) with a median age of 35 years (Table 1). Eighty percent of the population lived in areas <500 m above sea level. Among participants, 33% had a body mass index (BMI) between 25 and 30 kg/m2 (overweight), and 24% were above 30 kg/m2 (obese) (Table 1).
Table 1.
Population characteristics stratified by eGFR level
| Variable | eGFR <60 n = 98 (13%) | eGFR ≥60 n = 673 (87%) | Total n = 771 | P-value |
| Men, women | 20.1% (60), 8.0% (38) | 79.9% (238), 92.0% (435) | 39% (298), 61% (473) | <0.001 |
| eGFR | 38.7 ± 15.8 | 101.0 ± 25.5 | 93.1 ± 32.1 | |
| Age (years) | 55.0 ± 16.7 | 36.1 ± 14.4 | 38.5 ± 16.0 | <0.001 |
| Age group | ||||
| 18–30 years | 7.1% (7) | 40.3% (271) | 36.1% (278) | <0.001 |
| 31–41 years | 15.3% (15) | 28.2% (190) | 26.6% (205) | |
| 42–56 years | 27.6% (27) | 20.7% (139) | 21.5% (166) | |
| 57+years | 50.0% (49) | 10.9% (73) | 15.8% (122) | |
| BMI (kg/m2) | 26.2 ± 5.0 | 26.8 ± 5.6 | 26.8 ± 5.5 | 0.33 |
| BMI | ||||
| <20 kg/m2 | 7.3% (7) | 7.5% (50) | 7.4% (57) | 0.36 |
| 20–24.9 kg/m2 | 41.7% (40) | 34.0% (228) | 35.0% (268) | |
| 25–29.9 kg/m2 | 26.0% (25) | 34.5% (231) | 33.4% (256) | |
| 30+kg/m2 | 25.0% (24) | 24.0% (161) | 24.2% (185) | |
| Elevation | ||||
| <500 m | 88.7% (86) | 79.3% (532) | 80.5% (618) | 0.04 |
| ≥500 m | 11.3% (11) | 20.7% (139) | 19.5% (150) | |
eGFR values are truncated to 150 mL/min/1.73 m2.
BMI, body mass index.
Of these 771 participants, 98 (13%) had eGFR <60 mL/min/1.73 m2. There was a higher prevalence of low eGFR among older participants, men and participants living at lower altitudes (Table 1). Although older adults were more likely to have lower eGFR, there were a substantial number of individuals among the youngest participants with eGFR <60 mL/min/1.73 m2 (2.5% of the 278 participants between 18 and 30 years; Table 2). Only 16 people had diabetes; the youngest of whom was 44 years old.
Table 2.
Comparison of frequency of CKD stages in Quezalguaque with the USA using the NHANES data
| Age group | GFR category | Total |
Men |
Women |
|||
| Quezalguaque (n = 771) | US | Quezalguaque (n = 298) | US | Quezalguaque (n = 473) | US | ||
| Total | GFR 60+ | 87.3 (673) | 93.1 | 80.0 (238) | 94.4 | 92.0 (435) | 91.8 |
| Stage 3 (GFR 30–59) | 8.7 (67) | 6.5 | 11.1 (33) | 5.1 | 7.2 (34) | 7.7 | |
| Stage 4 and 5 (GFR <30) | 4.0 (31) | 0.5 | 9.1 (27) | 0.5 | 0.9 (4) | 0.5 | |
| 18–<30 | GFR 60+ | 97.5 (271) | 99.8 | 97.0 (95) | 99.7 | 97.8 (176) | 99.9 |
| Stage 3 (GFR 30–59) | 2.2 (6) | 0.1 | 2.0 (2) | 0.2 | 2.2 (4) | 0.1 | |
| Stage 4 and 5 (GFR <30) | 0.4 (1) | 0.1 | 1.0 (1) | 0.1 | 0 (0) | <0.1 | |
| 30–<42 | GFR 60+ | 92.7 (190) | 99.2 | 86.6 (71) | 99.2 | 96.8 (119) | 99.2 |
| Stage 3 (GFR 30–59) | 5.4 (11) | 0.7 | 8.5 (7) | 0.7 | 3.3 (4) | 0.7 | |
| Stage 4 and 5 (GFR <30) | 2.0 (4) | 0.1 | 4.9 (4) | 0.1 | 0 (0) | 0.1 | |
| 42–<57 | GFR 60+ | 83.7 (139) | 96.5 | 72.3 (47) | 97.3 | 91.1 (92) | 95.7 |
| Stage 3 (GFR 30–59) | 10.2 (17) | 3.3 | 15.4 (10) | 2.5 | 6.9 (7) | 4.1 | |
| Stage 4 and 5 (GFR <30) | 6.0 (10) | 0.2 | 12.3 (8) | 0.2 | 2.0 (2) | 0.1 | |
| 57+ | GFR 60+ | 59.8 (73) | 77.8 | 47.2 (25) | 81.0 | 69.6 (48) | 75.2 |
| Stage 3 (GFR 30–59) | 27.1 (33) | 20.7 | 26.4 (14) | 17.5 | 27.5 (19) | 23.3 | |
| Stage 4 and 5 (GFR <30) | 13.1 (16) | 1.5 | 26.4 (14) | 1.5 | 2.9 (2) | 1.6 | |
All data are in percentage, with the overall number of individuals in each group in parentheses for Quezalguaque only.
GFR, estimated glomerular filtration rate in mL/min/1.73 m2.
Comparison with the NHANES 1999–2006
Table 2 and Figure 2 compare the distribution of eGFR among age and sex groups in Quezalguaque with the same groups in the USA. Age groups reflect quartiles generated from the case–control population. The overall prevalence of eGFR <60 mL/min/1.73 m2 is 1.8 times greater in Quezalguaque than in the USA, with higher rates apparent at all age groups. Unlike in the USA, where CKD rates are similar among men and women, the prevalence of CKD in Quezalguaque is 2.6 times greater among men than among women. The greatest discrepancy between Quezalguaque and the US rates is seen in men ages 30–41, with rates 16 times higher in Quezalguaque. Similarly, for women, the ratio of CKD prevalence in Quezalguaque to that in the USA is highest in the youngest age group (Table 2). In a sensitivity analysis, using the CKD–EPI equation, there was substantial reclassification of individuals initially categorized with eGFR between 30 and 59 mL/min/1.73 m2, particularly among younger participants; however, the degree of reclassification was similar in magnitude to that seen in the US population. There was only a minimal change in eGFR for individuals with levels consistent with stage 4 CKD (data not shown).
Fig. 2.
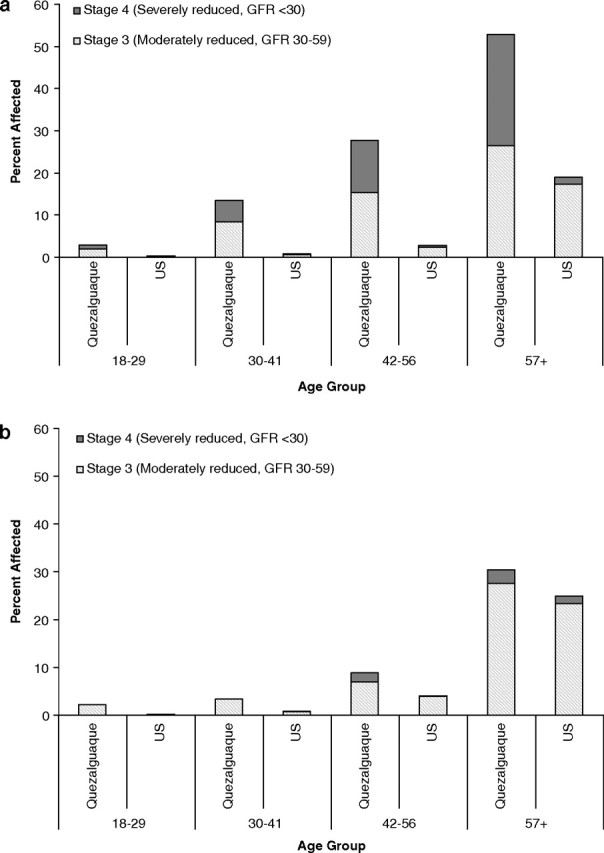
Prevalence of kidney disease in Quezalguaque compared with the USA using the NHANES 1999–2006 data: men (a) and women (b).
Case–control study
A total of 221 participants in the prevalence study were designated as controls and enrolled into the case–control portion of the study (Figure 1). Of the 98 cases, 27% had urine protein 1+ or higher, compared with 7% of controls (P < 0.001); 24% had 1+ or higher haematuria, compared with 18% of controls (P = 0.24) (Table 3).
Table 3.
Risk factors for eGFR <60 mL/min/1.73 m2 from the case–control study
| Cases n = 98 (30.7%) | Controls n = 221 (69.3%) | Odds ratio (unadjusted) | Odds ratio (age- and sex-adjusted) | |
| Demographics | ||||
| Age, per 5 years | 55.0 ± 16.7 | 36.5 ± 13.8 | 1.45 (1.32, 1.59) | – |
| Age group <30 | 7 (7.1%) | 78 (35.3%) | Reference | – |
| 30–41 | 15 (15.3%) | 72 (32.6%) | 2.32 (0.90, 6.02) | – |
| 42–56 | 27 (27.6%) | 51 (23.1%) | 5.90 (2.39, 14.7) | – |
| 57+ | 49 (50.0%) | 20 (9.1%) | 27.3 (10.8, 69.3) | – |
| Men | 60 (61.2%) | 101 (45.7%) | 1.88 (1.16, 3.05) | – |
| Elevation ≥500 m | 11 (11.3%) | 52 (23.6%) | 0.39 (0.19, 0.82) | 0.39 (0.18, 0.85) |
| Residence near field | 50 (53.2%) | 121 (55.5%) | 0.91 (0.56, 1.48) | 0.92 (0.52, 1.63) |
| Water source Well | 40 (42.1%) | 77 (35.2%) | Reference | Reference |
| Piped | 51 (53.7%) | 139 (63.5%) | 0.71 (0.43, 1.16) | 0.69 (0.38, 1.23) |
| Other | 4 (4.2%) | 3 (1.4%) | 2.57 (0.55, 12.0) | 0.93 (0.13, 6.71) |
| Medical history | ||||
| Diabetes | 8 (8.2%) | 8 (3.6%) | 2.37 (0.86, 6.50) | 0.64 (0.21, 1.99) |
| Hypertension composite | 65 (68.4%) | 99 (45.2%) | 2.63 (1.58, 4.36) | 2.30 (1.25, 4.23) |
| History of hypertension | 34 (36.2%) | 51 (26.4%) | 1.58 (0.93, 2.68) | 1.52 (0.80, 2.89) |
| Systolic BP, per 5 mmHg | 135.4 ± 18.2 | 126.3 ± 21.3 | 1.11 (1.05, 1.18) | 1.05 (0.98, 1.13) |
| Diastolic BP, per 5 mmHg | 85.7 ± 13.2 | 78.5 ± 14.1 | 1.20 (1.10, 1.32) | 1.17 (1.05, 1.30) |
| NSAID use | 43 (45.7%) | 93 (43.1%) | 1.12 (0.69, 1.82) | 1.42 (0.79, 2.56) |
| Antibiotic use | 16 (17.0%) | 33 (15.3%) | 1.14 (0.59, 2.19) | 1.18 (0.55, 2.53) |
| Work history | ||||
| Agricultural work | 81 (86.2%) | 164 (74.9%) | 2.09 (1.08, 4.05) | 1.00 (0.44, 2.27) |
| Duration 0 year | 13 (13.8%) | 55 (25.1%) | Reference | Reference |
| <1 year | 13 (13.8%) | 40 (18.3%) | 1.38 (0.58, 3.28) | 0.52 (0.18, 1.49) |
| 1–5 years | 8 (8.5%) | 30 (13.7%) | 1.13 (0.42, 3.03) | 1.13 (0.34, 3.69) |
| 6–20 years | 24 (25.5%) | 51 (23.3%) | 1.99 (0.92, 4.32) | 1.41 (0.55, 3.62) |
| 20+ years | 36 (38.3%) | 43 (19.6%) | 3.54 (1.67, 7.49) | 1.18 (0.44, 3.15) |
| Any pesticide exposure | 75 (83.3%) | 141 (67.1%) | 2.45 (1.31, 4.57) | 1.85 (0.84, 4.07) |
| Mix or apply pesticides | 47 (50%) | 78 (35.9%) | 1.78 (1.09, 2.91) | 1.32 (0.66, 2.64) |
| Cotton work | 77 (83.7%) | 126 (57.5%) | 3.79 (2.05, 7.01) | 1.33 (0.64, 2.79) |
| Sugar cane work | 32 (36.0%) | 61 (28.4%) | 1.42 (0.84, 2.40) | 1.52 (0.77, 2.99) |
| Heavy exertion <1 h | 50 (53.2%) | 88 (40.2%) | Reference | Reference |
| 1–2 h | 15 (16.0%) | 48 (21.9%) | 0.55 (0.28, 1.08) | 0.54 (0.24, 1.19) |
| 3+ h | 29 (30.9%) | 83 (37.9%) | 0.62 (0.36, 1.06) | 0.59 (0.30, 1.13) |
| Social history | ||||
| Smoking status Never | 48 (51.6%) | 141 (66.8%) | Reference | Reference |
| Former | 23 (24.7%) | 35 (16.6%) | 1.93 (1.04, 3.59) | 0.60 (0.26, 1.42) |
| Current | 22 (23.7%) | 35 (16.6%) | 1.85 (0.99, 3.45) | 1.50 (0.66, 3.38) |
| Current/former alcohol | 61 (64.9%) | 108 (49.8%) | 1.87 (1.31, 3.08) | 1.97 (0.86, 4.48) |
| Current/former Lija | 28 (38.9%) | 33 (22.2%) | 2.24 (1.21, 4.12) | 1.12 (0.51, 2.45) |
| Literate | 66 (73.3%) | 187 (90.3%) | 0.29 (0.15, 0.57) | 0.59 (0.27, 1.27) |
| Attended school | 71 (75.5%) | 191 (87.6%) | 0.44 (0.24, 0.81) | 1.23 (0.58, 2.63) |
| Laboratory testing | ||||
| eGFR (mL/min/1.73 m2) | 38.7 ± 15.8 | 100.7 ± 30.5 | ||
| Proteinuria None | 49 (52.1%) | 177 (81.2%) | Reference | Reference |
| Trace | 19 (20.2%) | 33 (15.1%) | 2.08 (1.09, 3.97) | 2.28 (1.06, 4.89) |
| 1+ | 6 (6.4%) | 3 (1.4%) | 7.22 (1.74, 29.9) | 4.59 (0.93, 22.6) |
| ≥2+ | 20 (21.3%) | 5 (2.3%) | 14.5 (5.16, 40.4) | 14.0 (4.23, 46.2) |
| Haematuria None | 55 (59.1%) | 155 (70.8%) | Reference | Reference |
| Trace | 16 (17.2%) | 29 (13.2%) | 1.56 (0.79, 3.08) | 1.19 (0.52, 2.70) |
| 1+ | 4 (4.3%) | 3 (1.4%) | 3.76 (0.82, 17.3) | 2.88 (0.44, 18.7) |
| ≥2+ | 18 (19.4%) | 32 (14.6%) | 1.59 (0.82, 3.05) | 1.56 (0.73, 3.37) |
| Lead (μg/dL) <3 | 81 (93.1%) | 197 (92.2%) | Reference | Reference |
| 3–5 | 5 (5.8%) | 11 (5.2%) | 1.11 (0.37, 3.28) | 0.64 (0.17, 2.40) |
| >5 | 1 (1.2%) | 4 (1.9%) | 0.61 (0.07, 5.53) | 1.22 (0.13, 11.8) |
All values are mean ± standard deviation or n (%). Diabetes was defined by either personal history or random serum glucose ≥200 mg/dL. Hypertension composite was defined by personal history of hypertension, systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg. Odds ratios for blood pressure are per 5 mmHg rise. Exertional work was defined by the answer regarding how many hours an individual would sweat while at work. Lija consumption was missing for 111 entries, equally distributed among cases and controls.
A higher percentage of cases (61%) than controls (50%) were men (P = 0.05 in univariate and P = 0.06 in analyses adjusted for age) and lived at lower altitudes (89% vs. 79%; P = 0.03). Cases were older than controls (55 ± 17 years vs. 37 ± 14 years; P < 0.001). Cases were also less literate than controls (73% vs. 90%; P < 0.001) and less likely to have attended school (76% vs. 88%; P = 0.009). Other significant univariate associations included working in cotton industry and Lija consumption (Table 3). After adjusting for age and sex, only hypertension and residence at an altitude <500 m were associated with CKD. Agricultural work and Lija consumption, both putative risk factors for CKD in other reports, were not significantly associated in adjusted analyses. Given the relatively small size of our study, it is worth noting that pesticide exposure (OR = 1.85, 95% CI = 0.84–4.07) and current or former alcohol consumption (OR = 1.97, 95% CI = 0.86–4.48) were positively, albeit not significantly, associated with CKD.
Historically, cotton was the major crop in this region; accordingly, cotton work was assessed in a subgroup of individuals with no sugar cane work experience to rule out masking of a possible interaction between age and agricultural work as an explanation for the association between cotton work and kidney disease. In this subgroup analysis, a higher percentage of cases than controls had a history of cotton work (75.4% vs. 51.3%; P = 0.002; data not shown); however, there was no significant association after adjusting for age and sex. Defining diabetes by glucose ≥126 mg/dL or personal history, we found a statistically significant difference between the groups, with 32% of cases and 18% of controls (P = 0.004) classified with diabetes; this was not significant in age- and sex-adjusted models. Overall, lead levels were low, and there was no association between blood lead and kidney disease status.
Discussion
In the current study, performed in the municipality of Quezalguaque, Nicaragua in response to anecdotal reports of a high prevalence of kidney disease in agricultural regions in northwestern Nicaragua, we found an overall prevalence of decreased eGFR (<60 mL/min/1.73 m2) of 12.7%, a rate that is substantially higher than the 7.0% prevalence observed in the US NHANES data and reflects disease occurring at a younger age. Our data are consistent with another recent report from this region, which also noted a high prevalence of reduced eGFR [11]. Although present in all age groups, the difference in kidney disease prevalence between Quezalguaque and the USA is most striking in younger men, with rates approximately nine times higher in Quezalguaque. Given the absence of high-grade proteinuria in affected individuals, this finding suggests that there are different aetiologies responsible for the development of kidney disease in Nicaragua versus the USA.
The results of our case–control study confirm that the epidemiology of kidney disease in Nicaragua is different than that seen in the USA and other developed nations. Although Quezalguaque residents with diabetes and hypertension did have a higher prevalence of decreased eGFR and proteinuria, these factors accounted for only a small portion of prevalent CKD in Quezalguaque, with many cases of hypertension potentially resulting from rather than causing kidney disease. Critically, the vast majority of cases, particularly those occurring in younger adults, did not have evidence of significant proteinuria, making diabetes, hypertension and glomerulonephritides unlikely the causes of kidney disease in this region. The association between lower elevation and increased prevalence of decreased GFR has been described elsewhere [1]. It is unclear whether this association represents a direct environmental effect (e.g. water quality) or is a marker for other activities that might be associated with CKD (e.g. occupation). Some investigators in Nicaragua and other regions along the west coast of Latin America have hypothesized that volume depletion in the setting of intensive agricultural work and extremely high ambient temperatures may predispose residents to CKD [16]. In theory, kidney effects of volume depletion could be exacerbated by frequent use of non-steroidal anti-inflammatory drugs and nephrotoxic antibiotics, and slightly lower temperatures (as seen at higher altitudes) protective against volume depletion. However, in the current study, perhaps due to recall and indication bias, we obtained contradictory results about volume depletion. The development of a more comprehensive assessment for volume depletion could be helpful in determining its association with CKD in Nicaragua.
In recent years, increasing worldwide attention has been paid to the increase in the prevalence and the public health approach in diagnosing and treating CKD [17–20]. In the industrialized world, much of the rising prevalence reflects an increase in traditional kidney disease risk factors, such as diabetes and hypertension, as well as an ageing and increasingly overweight population [21]. In many developing nations, the rising prevalence of CKD may be attributable to similar processes [22], but the aetiology of CKD in other regions remains uncertain [23]. In some regions, increased prevalence appears localized, and in the case of Balkan endemic nephropathy, this geographic pattern helped define a distinct aetiology of kidney disease [24].
In Nicaragua, given the absence of both traditional CKD causes and proteinuria in individuals with reduced GFR, tubulointerstitial disease is the most likely aetiology of kidney disease. Unfortunately, it is very difficult to discriminate among the various causes of tubulointerstitial kidney disease. However, lessons may be drawn from other regions with high rates of tubulointerstitial kidney disease. For example, in Balkan endemic nephropathy, it is hypothesized that seeds from plants of the genus Aristolochia contaminate local grain sources and, when consumed, predispose to the indolent development of a chronic interstitial nephritis and progressive interstitial fibrosis. Sri Lanka has also recently observed a very high rate of kidney failure which, similar to that seen in the Balkans and in Latin America, manifests with minimal proteinuria and a male predominance [25]. Several other regional CKD epidemics previously have been described; these include likely ingestion of food contaminated with cadmium and mercury in Japan [26,27], and contamination of food with ochratoxin in Tunisia [28]. Based on the male predominance and tubulointerstitial manifestation of the disease, the current study in Quezalguaque suggests that an exposure, perhaps occupational or environmental, may be contributing to CKD; unfortunately, no causal agents have been identified.
Our study has several strengths, including a population-based sampling strategy, potentially allowing for greater generalizability than prior studies that were conducted in specific sub-populations. Additionally, we coordinated our efforts with the local health centre, giving us greater credibility and enabling a very high response rate, with >90% of households approached participating in the study. Our study also has several limitations. First, women were over-represented in the study population, likely reflecting that many men either worked late hours or had left the region to find employment. Accordingly, we oversampled men in the case–control study in an attempt to include a sufficient number for analysis. Second, urinalysis was performed only in participants in the case–control study. As such, we were only able to evaluate the prevalence of reduced eGFR and cannot draw conclusions about earlier stages of CKD. However, even in individuals with reduced eGFR, there was a relatively low prevalence of dipstick proteinuria. In future studies, quantitative analysis of urine proteins will be considered. Third, the creatinine assay used to calculate the eGFR was not calibrated to standardized assays. Although differences in calibration and specific estimating equations can have substantial effects on prevalence estimates [29,30], variation associated with assays and equations has larger effects at lower levels of creatinine, and at eGFR levels <60 mL/min/1.73 m2 and, particularly, at levels <45 mL/min/1.73 m2; bias introduced by measurement and estimation inaccuracies is likely small, making the high prevalence of late-stage CKD in Quezalguaque, particularly stage 4, likely robust to these effects. Additionally, classification of both kidney function and albuminuria was based on a single measure. While this is suboptimal, individuals assessed were in their usual state of health, likely lessening misclassification. Fourth, we did not ascertain how many participants already knew or suspected they had CKD; this may have affected their answers regarding current and past exposures. Fifth, the racial distribution of western Nicaragua, comprised predominantly of a mestizo population (mixed Amerindian and white), does not have an equivalent within the NHANES, potentially limiting comparisons between populations. Importantly, this also raises the possibility that different genetic factors may contribute to the pathogenesis of CKD in this region. Sixth, we lack blood pressure data on the larger cohort and specific medication use for participants in the case–control study. While the use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may have affected serum creatinine levels and proteinuria, these medications are only intermittently available in Nicaragua [31], and their use is not commonly adjusted for and in generation of CKD prevalence estimates in studies like the NHANES.
In summary, we have demonstrated an elevated prevalence of decreased GFR in Quezalguaque, Nicaragua, described that this condition is non-proteinuric and not associated with diabetes, and identified potential risk factors that warrant further investigation. Given the predilection of CKD in this region for workers of a productive age as well as the limited availability of medications and kidney replacement therapies, this illness has led to severe personal, economic, and social effects for individuals suffering from the disease, their families and their communities. Future research will require sufficient resources to support larger and more methodologically advanced and rigorous studies if the underlying causes of the increase in CKD in this area are to be discovered.
Acknowledgments
We would like to acknowledge the assistance of Drs Cecelia Torres and Aurora Aragon of UNAN-Leon who assisted us with the study approval and provided us with the survey instrument that we adapted for use in this study. This study could not have occurred without the efforts of the individuals who performed the screenings in Quezalguaque (Ann Marie Borchelt, Eddy Calderon, Heidi Chase, Fabiola Mendoza, Luisa Silva, Yorlenys Urbina, Tom Van MD and Monica Zigman); the physicians, nurses and technicians who provided logistic support and advice in Nicaragua (Mayra Arias RN, Benjamin Barreto MD, Miriam Cruz, Luzmila Escoto, Francisco Iglesias, Gilberto Moreno MD, Mario Navarette MD and Vera Orozco MD); the advice and assistance of Bijan Roshan MD; the support of the Sister City Committee in Brookline, MA, USA; Nova Biomedical, which donated serum creatinine assays and instruments; ESA Biosciences in Chelmsford, MA, USA, which greatly assisted in lead analyses; the support of the East Boston Neighborhood Health Center; the Clinical and Translational Research Institute (CTSI) at Tufts University; and, most importantly, the residents of Quezalguaque who welcomed us into their community.
Conflict of interest statement. None declared.
References
- 1.Garcia-Trabanino R, Dominguez J, Jansa J, et al. Proteinuria e insuficiencia renal crónica en la costa de El Salvador: detección con métodos de bajo costo y factores asociados [Proteinuria and chronic renal failure in the coast of El Salvador: detection with low cost methods and associated factors] Nefrologia. 2005;25:31–38. [PubMed] [Google Scholar]
- 2.García-Trabanino R, Aguilar R, Reyes S, et al. Nefropatía terminal en pacientes de un hospital de referencia en El Salvador [Terminal nephropathology in patients in a reference hospital in El Salvador]. Revista Panamericana de Salud Pública. Pan Am J Public Health. 2002;12:202–206. doi: 10.1590/s1020-49892002000900009. [DOI] [PubMed] [Google Scholar]
- 3.Cuadra S, Jakobsson K, Hogstedt C, et al. Chronic kidney disease: assessment of current knowledge and feasibility for regional research collaboration in Central America. SALTRA Technical Series on Work & Health 2, Heredia, Costa Rica. 2006. http://www.saltra.info/index.php?module=Pagesetter&tid=11.
- 4.Alonso Medrano A, Perea W. Insuficiencia Renal Crónica (IRC) en trabajadores de caña de azúcar, Chinandega, Nicaragua: Febrero-Marzo 2002. In: Cuadra SN, Jakobsson K, Hogstedt C, Wesseling C. Chronic kidney disease: assessment of current knowledge and feasibility for regional research collaboration in Central America. Heredia, Costa Rica: SALTRA, IRET-UNA. 2006:11–42. [Google Scholar]
- 5.Callejas Callejas L, Alonso Medrano CD, Mendoza Canales B. Insuficiencia renal crónica (IRC) en trabajadores de caña de azúcar, El Viejo, Chinandega, Nicaragua. In: Cuadra SN, Jakobsson K, Hogstedt C, Wesseling C. Chronic kidney disease: Assessment of current knowledge and feasibility for regional research collaboration in Central America. Heredia, Costa Rica: SALTRA, IRET-UNA. 2006:11–42. [Google Scholar]
- 6.Domínguez J, Montoya Pérez C, Jansá JM. Análisis de prevalencia y determinantes de la insuficiencia renal crónica de la costa del Océano Pacífico: Sur fr México, Guatemala, El Salvador y Honduras. In: Cuadra SN, Jakobsson K, Hogstedt C, Wesseling C. Chronic kidney disease: Assessment of current knowledge and feasibility for regional research collaboration in Central America. Heredia, Costa Rica: SALTRA, IRET-UNA. 2006:11–42. [Google Scholar]
- 7.Marín Ruiz J, Berroterán J. Insuficiencia renal crónica: Cuadro clínico y situación epidemiológica en Nicaragua. Managua, Nicaragua: Ministerio de Salud Nicaragua. 2002 [Google Scholar]
- 8.Castillo M. Factores de riesgo asociados a insuficiencia renal crónica en pacientes ingresados al departamento de medicina interna. UNAN LEON. 2001 Unpublished data. [Google Scholar]
- 9.Rivas FAZ, Iglesias MJ, Orozco AM. Insuficiencia renal crónica en Nicaragua [Chronic kidney disease in Nicaragua]. Comité Nacional de Productores de Azúcar (CNPA) http://www.cnpa.com.ni/irc.pdf 2 December 2009, date last accessed. [Google Scholar]
- 10.Durón R, Sierra F, Osorio JR, et al. Característica de los pacientes en el programa de diálisis peritoneal del Hospital Escuela. Tegucigalpa. Revista Médica Hondurena. 2003;66:123–128. [Google Scholar]
- 11.Torres C, Aragon A, González M, et al. Decreased kidney function of unknown cause in Nicaragua: a community-based survey. Am J Kidney Dis. 2010;55:485–496. doi: 10.1053/j.ajkd.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 13.Selvin E, Manzi J, Stevens LA, et al. Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988–1994, 1999–2004. Am J Kidney Dis. 2007;50:918–926. doi: 10.1053/j.ajkd.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 15.National Center for Health Statistics, Centers for Disease Control and Prevention The National Health and Nutrition Examination Survey (NHANES): analytic and reporting guidelines. Available at: http://www.cdc.gov/nchs/nhanes/nhanes2003-2004/analytical_guidelines.htm 2006 3 May 2010, date last accessed.
- 16.Delgado Cortez O. Heat stress assessment among workers in a Nicaraguan sugarcane farm. Global Health Action. 2009;2:2069. doi: 10.3402/gha.v2i0.2069. DOI: 10.3402/gha.v2i0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L, Zhang P, Wang F, et al. Prevalence and factors associated with CKD: a population study from Beijing. Am J Kidney Dis. 2008;51:373–384. doi: 10.1053/j.ajkd.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Jafar TH. The growing burden of chronic kidney disease in Pakistan. N Engl J Med. 2006;354:995–997. doi: 10.1056/NEJMp058319. [DOI] [PubMed] [Google Scholar]
- 19.Stevens LA, Coresh J, Levey AS. CKD in the elderly—old questions and new challenges: World Kidney Day 2008. Am J Kidney Dis. 2008;51:353–357. doi: 10.1053/j.ajkd.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Schoolwerth AC, Burrows NR, et al. Comprehensive public health strategies for preventing the development, progression, and complications of CKD: report of an expert panel convened by the Centers for Disease Control and Prevention. Am J Kidney Dis. 2009;53:522–535. doi: 10.1053/j.ajkd.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 21.Elsayed EF, Sarnak M, Tighiouart H, et al. Waist-to-hip ratio, body mass index and subsequent kidney disease and death. Am J Kidney Dis. 2008;52:29–38. doi: 10.1053/j.ajkd.2008.02.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gutierrez-Padilla JA, Mendoza-Garcia M, Plascencia-Perez S, et al. Screening for CKD and cardiovascular disease risk factors using mobile clinics in Jalisco, Mexico. Am J Kidney Dis. 2010;55:474–484. doi: 10.1053/j.ajkd.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 23.Wanigasuriya KP, Peiris-John RJ, Wickremasinghe R, et al. Chronic renal failure in North Central Province of Sri Lanka: an environmentally induced disease. Trans R Soc Trop Med Hyg. 2007;101:1013–1017. doi: 10.1016/j.trstmh.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Bamias G, Boletis J. Balkan nephropathy: evolution of our knowledge. Am J Kidney Dis. 2008;52:606–616. doi: 10.1053/j.ajkd.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization Sri Lanka . Chronic kidney disease of unknown aetiology (CKDu): a new threat to health. www.searo.who.int/LinkFiles/News_Letters_CKDu.pdf 3 May 2010, date last accessed. [Google Scholar]
- 26.Iesato K, Wakashin M, Wakashin Y, et al. Renal tubular dysfunction in Minamata disease. Detection of renal tubular antigen and beta-2-microglobin in the urine. Ann Intern Med. 1977;86:731–737. doi: 10.7326/0003-4819-86-6-731. [DOI] [PubMed] [Google Scholar]
- 27.Jarup L. Cadmium overload and toxicity. Nephrol Dial Transplant. 2002;17:35–39. doi: 10.1093/ndt/17.suppl_2.35. [DOI] [PubMed] [Google Scholar]
- 28.Abid S, Hassen W, Achour A, et al. Ochratoxin A and human chronic nephropathy in Tunisia: is the situation endemic? Hum Exp Toxicol. 2003;22:77–84. doi: 10.1191/0960327103ht328oa. [DOI] [PubMed] [Google Scholar]
- 29.Stevens LA, Manzi J, Levey AS, et al. Impact of creatinine calibration on performance of GFR estimating equations in a pooled individual patient database. Am J Kidney Dis. 2007;50:21–35. doi: 10.1053/j.ajkd.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Coresh J, Eknoyan G, Levey AS. Estimating the prevalence of low glomerular filtration rate requires attention to the creatinine assay calibration. J Am Soc Nephrol. 2002;13:2811–2812. doi: 10.1097/01.asn.0000037420.89149.c9. author reply 2812–2816. [DOI] [PubMed] [Google Scholar]
- 31.Needs assessment: options to improve immediate and long-term care for people suffering from chronic renal insufficiency, October 2009. The Office of the Compliance Advisor/Ombudsman of the World Bank Group. http://www.cao-ombudsman.org/cases/document-links/links-82.aspx. 27 May 2010, date last accessed.