Abstract
Purpose
Interactive multimedia’s potential to improve biobank informed consent has yet to be investigated. The aim of this study was to test the separate effectiveness of interactivity and multimedia at improving participant understanding and confidence of understanding of informed consent, compared to a standard, face-to-face (F2F) biobank consent process.
Methods
A 2 (F2F versus multimedia) × 2 (standard versus enhanced interactivity) experimental design was used with 200 patients randomly assigned to receive informed consent. All patients received the same information provided in the Biobank’s 9-page consent document.
Results
Interactivity (F(1,196)=7.56, p=0.007, partial η2=0.037) and Media (F(1,196)=4.27, p=0.04, partial η2=0.021) independently improved participants’ understanding of the Biobank consent. Interactivity (F(1,196) = 6.793, p = 0.01, partial η2=0.033), but not Media (F(1,196) = 0.455, n.s.), resulted in increased participant confidence in their understanding of the Biobank’s consent. Patients took more time to complete the multimedia (M=18.2 min.) than the F2F (M=12.6 min.) conditions.
Conclusion
This study demonstrated that interactivity and multimedia each can be effective at promoting individuals’ understanding and confidence in understanding of a biobank consent, albeit with additional time investment. Researchers should not assume that multimedia is inherently interactive, but rather separate the two constructs when studying electronic consent.
Keywords: interactive, multimedia, consent, biobanking, randomized
INTRODUCTION
Biobanks collecting genetic material for research report considerable interest in using online portals and technologies such as computerized kiosks and tablet computers (tablets) to obtain informed consent.1,2 Informed consent promotes deliberate, voluntary participation in research and is important in biobanking given the potency of genomic information, the open-ended nature of biobank-supported research, and constraints on privacy and confidentiality.3 However, traditional face-to-face (F2F) consent processes pose challenges for biobanks.
Of the many types of biobanks, one subset is designed to support human health research using human biological samples and associated data.4 Obtaining informed consent for many of these biobanks is resource intensive and costly, requiring trained consenters to staff multiple recruitment points in clinical and/or community settings.5 Information in biobank consent materials also presents unique challenges. Individuals are typically asked to consent to future research studies that can be described only in the most general terms. Many are faced with information that requires a challenging level of genetic and genomic literacy.6 Studies have found that individuals misunderstand issues such as the risks of biobanking to privacy and confidentiality, ownership of samples, and the disclosure of individual results from genomic research.7 Thus, both the efficiency and effectiveness of informed consent at promoting adequate understanding of biobank participation are in need of improvement.
Electronic Delivery of Informed Consent
A potential alternative to more traditional methods of obtaining consent is to automate the process and deliver information via a website or stand-alone computer program. Improvements in electronic delivery have made possible a shift in the consent format from text to multimedia, including the addition of graphics, audio narration, and video. Electronic technologies can shift F2F delivery of consent information to methods that provide patients more control over the pace of delivery and more engagement with research concepts through interactivity and other proactive instructional strategies.
Electronic delivery of study information also offers the potential to reach more participants using less staff time. Tablets, for example, can become portable, paperless means of presenting consent information and documenting consent. Online availability provides broader access and allows participants to review consent information at their own pace, possibly even in their homes. Touchscreen technology can be used to capture participant signatures. Electronic tools can also support digital document storage and efficient retrieval, as well as ongoing education about a biobank’s activities, the (non)disclosure of research results, and recontact with participants.8
Multimedia
Research has yet to demonstrate that electronic consent (e-consent) processes communicate study information more effectively than traditional F2F methods. Efforts to improve participants’ understanding of information presented in the consent process for research through electronic media, primarily multimedia, have proved challenging.9 Some interventions have been successful at improving research participant understanding,10,11 while others have not.8 Most studies have compared multimedia to F2F methods without considering the theoretical underpinnings of the delivery strategies or carefully controlling variables across conditions. Mahnke et al.12 posited that the inconsistency in multimedia study results may be due to a lack of rigorous development of multimedia and alternative materials.
The current study applied theories of human perception and cognition to multimedia instruction13 in designing a multimedia informed consent presentation. According to dual coding theory,14 people process information through two simultaneous pathways, verbal (words and symbols) and spatial (pictures and movement). Well-designed instruction capitalizes on multimedia, the ability to combine visual and auditory information,13,15 and strategically presents information through both modalities to enhance the efficiency and effectiveness of instruction.13,16-18
In addition, cognitive load theory (CLT) suggests that multimedia can be designed to enhance learning by maintaining an optimal level of cognitive load, the amount of information in use by working memory.19-21 By selecting content and designing presentation of instruction to optimize load, learning engages but does not overwhelm the learner.20,22,23 To optimize cognitive load, designers focus on both the content and the delivery (e.g., how much information is presented and how it is organized) to improve participant learning.
Interactivity
Whereas studies of multimedia informed consent for research have had inconsistent results,9 use of interactive (e.g., test-feedback) strategies to engage participants in the consent process have generally shown improved understanding of informed consent information.24,25 Because definitions of interactivity differ across domains (computer science, communication studies, psychology), an empirically verifiable definition of “interactivity” is difficult to identify and even harder to apply to informed consent.26-30 Defined in the most general terms common among theorists, similar to Yacci’s31 “loop,” interactivity consists of three parts: 1) a prompt, 2) a response from the participant, and 3) feedback to the response.
In a standard F2F consent session, participants may be prompted by hearing information they do not understand to ask a question (response) and receive feedback from the researcher. Because this is unstructured and inconsistent in F2F encounters, we consider this a lower level of interactivity. However, interactivity can also be structured and made consistent through strategically placed multiple-choice questions (i.e., prompts), which require a response from the participant and feedback to correct misconceptions or reinforce correct responses. This would qualify as higher-level interactivity, because such questions could be specifically targeted to improve understanding of difficult concepts. Although F2F discussions between participants and researchers can be highly interactive in the broadest sense of the term, standard consent processes do not ensure that a patient-driven dialogue occurs. For this study, we have included a simplified interactivity construct which can be controlled and improve our empirical understanding of interactivity in the consent process.
The interactive, multimedia presentation reported on here is based on these distinct but interconnected concepts of multimedia and interactivity. Other ways of designing electronic and interactive multimedia consent9 are possible. Because producing interactive, multimedia materials can be time consuming and expensive, researchers need good theory and evidence-based principles to maximize their investment. The current study was designed to test the hypotheses that multimedia and interactivity each would improve understanding and confidence of understanding of a biobank’s informed consent.
MATERIALS AND METHODS
Overview
A prospective randomized study was integrated into the ongoing recruitment process for a comprehensive biobank (Biobank) at University of Iowa Hospitals and Clinics (UIHC) in Iowa City, IA. The UI Biobank collects saliva samples (with the option to collect blood and tissue) directly from patients for storage and future research. Informed consent is obtained using a 9-page consent document containing segments on HIPAA, GINA, and informational elements required by the federal code of regulations on informed consent.32 The consent document addresses all 15 topics that experts suggest need to be addressed in biobank consent documents.3 The document is presented to eligible individuals as part of an opt-in, broad consent process, developed with the institution’s IRB from data showing a statewide preference for an opt-in, active consent model.33
The Biobank and IRB permitted the researchers to enroll patients into the Biobank after randomization into one of four consenting conditions. Because the study involved experimental procedures for the consent process, participants’ comprehension of the information was assessed, and participants were automatically provided additional instruction for any missed questions prior to enrollment in the Biobank. To avoid confusing individuals with two consent processes, contaminating data, a waiver of consent/authorization of consent was obtained for this minimal-risk study.
Any patient approached by the Biobank in 2013 was considered eligible for the study. This included patients in dermatology, rheumatology/immunology, pulmonology, and family medicine clinics, and those recruited by the Biobank through posters, fliers, ResearchMatch.org, and announcements distributed on the medical campus and in universitywide email blasts and newsletters. To maintain consistency with Biobank procedures, research associates (RAs) were trained by the Biobank supervisor to conduct F2F consent and collect specimens. Training included specific points to emphasize during the consent process. In addition, researchers for the current study trained the RAs on this study’s protocol and how to use the interactive and multimedia components. Mock consent sessions were practiced until RAs reported a moderate level of comfort with the study protocol.
Study Conditions
This study tested the effectiveness of adding multimedia and enhancing interactivity on participants’ understanding of and confidence in their understanding of biobank consent information using a 2 (F2F versus multimedia) × 2 (standard versus enhanced interactivity) experimental design. Participants were randomly assigned to receive informed consent using one of four processes:
F2F Standard Interactivity – paper consent document with standard researcher-participant discussion (Control)
F2F Enhanced Interactivity – paper consent document, researcher-participant discussion, and 13 targeted, interactive questions
Multimedia Standard Interactivity – electronic consent document text, graphics, and verbatim narration of text with participants able to ask questions at any time
Multimedia Enhanced Interactivity – multimedia consent procedure as above and 13 targeted, interactive questions
All four conditions received the same information in the Biobank’s 9-page paper consent document.
F2F conditions
The F2F Standard Interactivity condition was the standard informed consent process used by the Biobank. RAs reviewed the informed consent document with participants and answered any participant questions. In the F2F Enhanced Interactivity condition, the informed consent document was reviewed as in the F2F Standard Interactivity condition, with the addition of 13 stoppage points where the RAs asked participants scripted, multiple-choice questions about critical elements of the consent, such as the purpose of the Biobank, sample types, and the risks and benefits of participation. After each question, RAs provided scripted feedback based on the participants’ response, reinforcing correct answers and clarifying misconceptions. RAs were trained for consistency in their communication style and feedback for both F2F conditions.
Multimedia conditions
For the multimedia conditions, a PowerPoint-type module was developed. CLT principles for effective multimedia learning were used to guide the chunking of text and selection of supporting graphics. The words from the paper consent document were divided (“chunked”) across slides, with graphics added to some slides to reinforce specific concepts, yielding a 93-slide presentation. All text was narrated verbatim. Hyperlinks were not used, and participants were not allowed to skip pages.
Tablets were used to present the Flash-based multimedia module. Participants could adjust the volume or mute the audio and could move forward or backward in the slideshow. The slideshow was incorporated into a delivery system developed by a commercial company (Patient Education Institute, Inc.) with experience in patient education software using Flash-based web technologies.
In the Multimedia (MM) Standard Interactivity condition, participants were presented the slideshow, including the consent text, graphics, and narration of the text. The MM Enhanced Interactivity condition included all slides from the MM Standard Interactivity condition, with the addition of 13 interactive questions also asked in the F2F Enhanced Interactivity condition. On answering the interactive questions, participants received automated feedback based on their responses, reinforcing correct answers and clarifying misconceptions.
Participants were provided a paper copy of the consent document to look at during the consent process. In keeping with the Biobank’s consent practices, participants were encouraged to ask RAs questions at any time during the consent process. RAs were not blinded to the conditions, nor could they be blinded to the interactive questions. However, the assessment questions (i.e., knowledge, satisfaction, demographics) were provided on tablets for all conditions. Thus, RAs were not privy to the assessment questions or participants’ performance on those questions (outcome measures).
Formative evaluation
Various stakeholders participated in a formative evaluation of the multimedia presentation (evaluating chunking and graphics) and the interactive components (questions and feedback) for the enhanced interactivity conditions. Ethics consultants with expertise in biobanking, two biobank directors and an IRB Chair participated in a first-round evaluation by completing an heuristic evaluation form.34 Next, five community members participated in two iterative rounds of evaluation, completing the MM Enhanced Interactivity module using think-aloud protocols35 and a debriefing with study investigators. The research team used stakeholder feedback, wherever feasible and appropriate, to improve the presentation. One notable change as a result of stakeholder feedback was to add a preliminary statement that participants would be asked questions and that the questions were for their benefit in the enhanced interactive conditions. Some graphics were modified or replaced and interactive questions refined based on stakeholder feedback.
Outcome Measures
To assess understanding of the information presented in the informed consent, a two-part assessment was developed modeled on Joffe et al.’s36 design for cancer clinical trials and Ormond et al.’s7 adaptation for biobanking consent. Participants in all conditions completed the same assessment on a tablet using an online survey format. RAs were present during the assessment to answer participant questions or address technical problems.
The Understanding of Consent contained 27 true/false statements covering the eight essential components of informed consent as specified in the federal regulations32 (see Table 1). For each item, participants could agree, disagree, or declare they were unsure about the statement. Following Ormond et al.7 and Joffe et al.,36 participants received 100 points for each correct response, 50 points for unsure responses, and zero points for incorrect responses. Points were averaged (total points/27) to yield an Understanding Consent score between 0 and 100 for each participant.
Table 1.
Understanding of Consent Items
| A1. The University of Iowa (UI) Biobank collects and stores blood, tissue, and saliva samples for research. |
| A2. Your samples and health information could be used in many different kinds of research studies. |
| A3. The UI Biobank will not allow genetic research on your samples and health information. |
| A4. Researchers who use the UI Biobank hope to find new ways to understand, detect, and treat health problems. |
| A5. Only University of Iowa researchers will be given access to your samples and health information. |
| A6. The UI Biobank may ask you to donate blood through a blood draw. |
| A7. Samples in the UI Biobank will be destroyed after five years. |
| A8. The UI Biobank may contact you up to two times a year for additional information. |
| A9. You may be contacted to provide additional blood or saliva samples. |
| A10. You cannot continue participating in the UI Biobank unless you agree to being recontacted. |
| A11. Your sample will be given a code to help protect your confidentiality and privacy. |
| A12. The Genetic Information Nondiscrimination Act (GINA) makes it illegal for health insurance companies, health plans, and large employers to discriminate against you because of your genetic information. |
| A13. The UI Biobank guarantees that your privacy and confidentiality will never be violated. |
| A14. There is no physical risk to you of providing a saliva sample for the UI Biobank. |
| A15. There is no risk that you will be upset or discriminated against if you participate in the UI Biobank. |
| A16. You will not directly benefit from participating in the UI Biobank. |
| A17. The hope is that other people may benefit from the research that will be supported by the UI Biobank. |
| A18. You should expect to receive individual research results from the UI Biobank. |
| A19. If researchers using your sample discover something important about your health, you will be contacted to see if you want to learn more. |
| A20. You will be paid for being in the UI Biobank. |
| A21. There are no plans to compensate you for any products or patents resulting from UI Biobank’s research. |
| A22. There will be no cost to you or your insurance company if you participate in the UI Biobank. |
| A23. The consent form for the UI Biobank lists the name of the person (or persons) whom you should contact if you have any questions or concerns. |
| A24. Participation in the UI Biobank is voluntary. |
| A25. The UI Biobank promises you better treatment at the University of Iowa Healthcare. |
| A26. To stop participating in the UI Biobank, there is a phone number you can call. |
| A27. By signing the consent form, you agree to include your samples and health information in the UI Biobank. |
Note: Disagree is correct response for bolded items.
Confidence in Understanding consisted of 23 questions in which participants rated their confidence from 1 (“I Didn’t Understand This at All”) to 5 (“I Understood This Very Well”). The Confidence in Understanding items were summed, averaged, normalized in accordance with Joffe’s36 equation [(Average Confidence – 1) × 25] to create a Confidence score.
In addition, Participant Consent Time was calculated as the elapsed time in seconds from the beginning of the consent to the end of the consent, before participants began the assessment instruments. For all conditions, this included time that RAs answered any participants’ questions.
Data Management and Analysis
To test the hypotheses that multimedia and enhanced interactivity would improve participant understanding and confidence, the outcome measures (Understanding of Consent, Confidence in Understanding, and Consent Time) were analyzed using 2 × 2 factorial MANOVAs with Media (F2F vs. MM) and Interactivity (Standard vs. Enhanced) as independent variables, in SPSS version 21. Responses to open-ended, qualitative questions probing participants’ likes, dislikes, and suggestions were reviewed and coded by two independent coders. Coders developed a code book and met several times with a study PI to refine the code book and coding categories.
RESULTS
Sample
Two hundred individuals participated in the study, 50 per condition. Participants had a mean age of 47 (range 18-86), were more likely to be female (76%) and predominantly Caucasian (96%). Participants were well educated, with 79% reporting at least a college degree or higher. Close to one of every four (24%) reported a total household income of $100,000 or more.
Understanding of the Biobank Consent
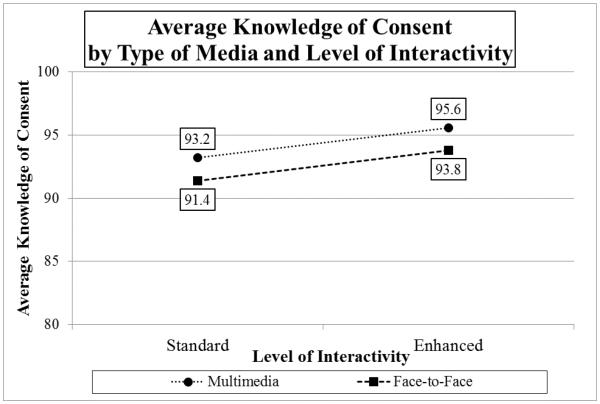
Testing the hypothesis that multimedia and enhanced interactivity would improve participant understanding (Media × Interactivity MANOVA on Understanding Consent scores), both main effects for Interactivity (F(1,196)=7.56, p=0.007, partial η2=0.037) and Media (F(1,196)=4.27, p=0.04, partial η2=0.021) were statistically significant, although the interaction between Interactivity and Media was not (F(1,196)=0.004, n.s.). Based on main effects, individuals in the enhanced interactivity conditions (collapsed across type of media) demonstrated better understanding (M=94.7) than those in the standard (M=92.3) conditions. Similarly, individuals in both standard and enhanced MM conditions (collapsed across level of interactivity) demonstrated better understanding (M=94.4) than those in the F2F (M=92.6) conditions (See Graph 1). For the total sample (N=200), the average Understanding of Consent score was 93.5.
Graph 1.
Average Knowledge of Consent by Type of Media and Level of Interactivity
Although average understanding was high, differences between groups on certain critical items were of note (see Table 2). For example, fewer than half of the participants in the F2F Standard Interactivity (48%) and just over half of those in the F2F Enhanced Interactivity (58%) conditions, compared to most of the MM participants (Standard Interactivity 82%, Enhanced Interactivity 86%), correctly understood that the Biobank allowed access to samples and health information by outside researchers (not affiliated with the University of Iowa). Almost half of the F2F Standard interactivity participants (42%) misunderstood that the Biobank could not guarantee that their privacy and confidentiality would never be violated, compared to 18% of the MM Standard Interactivity, 20% of the MM Enhanced Interactivity, and 24% of the F2F Enhanced Interactivity participants.
Table 2.
Item Response Rates On Understanding of Consent By Study Condition
| Item | F2F Standard Interactivity | F2F Enhanced Interactivity | MM Standard Interactivity | MM Enhanced Interactivity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disagree | Unsure | Agree | Disagree | Unsure | Agree | Disagree | Unsure | Agree | Disagree | Unsure | Agree | |
| Count | Count | Count | Count | Count | Count | Count | Count | Count | Count | Count | Count | |
| A1. | 0.0% | 0.0% | 100.0% | 2.0% | 0.0% | 98.0% | 0.0% | 0.0% | 100.0% | 0.0% | 0.0% | 100.0% |
| A2. | 0.0% | 0.0% | 100.0% | 0.0% | 0.0% | 100.0% | 0.0% | 0.0% | 100.0% | 0.0% | 0.0% | 100.0% |
| A3. | 82.0% | 0.0% | 18.0% | 94.0% | 2.0% | 4.0% | 82.0% | 6.0% | 12.0% | 94.0% | 4.0% | 2.0% |
| A4. | 0.0% | 0.0% | 100.0% | 0.0% | 2.0% | 98.0% | 0.0% | 0.0% | 100.0% | 0.0% | 0.0% | 100.0% |
| A5. | 48.0% | 12.0% | 40.0% | 58.0% | 8.0% | 34.0% | 82.0% | 4.0% | 14.0% | 86.0% | 2.0% | 12.0% |
| A6. | 0.0% | 2.0% | 98.0% | 2.0% | 0.0% | 98.0% | 2.0% | 2.0% | 96.0% | 4.0% | 0.0% | 96.0% |
| A7. | 76.0% | 20.0% | 4.0% | 92.0% | 8.0% | 0.0% | 82.0% | 12.0% | 6.0% | 96.0% | 4.0% | 0.0% |
| A8. | 0.0% | 10.0% | 90.0% | 4.0% | 8.0% | 88.0% | 2.0% | 14.0% | 84.0% | 0.0% | 12.0% | 88.0% |
| A9. | 2.0% | 0.0% | 98.0% | 2.0% | 0.0% | 98.0% | 0.0% | 2.0% | 98.0% | 2.0% | 0.0% | 98.0% |
| A10. | 60.0% | 14.0% | 26.0% | 80.0% | 0.0% | 20.0% | 56.0% | 16.0% | 28.0% | 88.0% | 8.0% | 4.0% |
| A11. | 0.0% | 0.0% | 100.0% | 0.0% | 0.0% | 100.0% | 0.0% | 0.0% | 100.0% | 0.0% | 0.0% | 100.0% |
| A12. | 0.0% | 0.0% | 100.0% | 0.0% | 0.0% | 100.0% | 2.0% | 0.0% | 98.0% | 4.0% | 0.0% | 96.0% |
| A13. | 56.0% | 2.0% | 42.0% | 74.0% | 2.0% | 24.0% | 74.0% | 8.0% | 18.0% | 78.0% | 2.0% | 20.0% |
| A14. | 2.0% | 0.0% | 98.0% | 10.0% | 0.0% | 90.0% | 8.0% | 2.0% | 90.0% | 2.0% | 2.0% | 96.0% |
| A15. | 66.0% | 6.0% | 28.0% | 78.0% | 2.0% | 20.0% | 70.0% | 8.0% | 22.0% | 68.0% | 10.0% | 22.0% |
| A16.. | 2.0% | 2.0% | 96.0% | 6.0% | 0.0% | 94.0% | 8.0% | 2.0% | 90.0% | 2.0% | 2.0% | 96.0% |
| A17. | 0.0% | 0.0% | 100.0% | 0.0% | 0.0% | 100.0% | 0.0% | 0.0% | 100.0% | 0.0% | 2.0% | 98.0% |
| A18. | 92.0% | 4.0% | 4.0% | 90.0% | 2.0% | 8.0% | 94.0% | 2.0% | 4.0% | 96.0% | 4.0% | 0.0% |
| A19. | 0.0% | 4.0% | 96.0% | 2.0% | 0.0% | 98.0% | 4.0% | 4.0% | 92.0% | 2.0% | 4.0% | 94.0% |
| A20. | 92.0% | 2.0% | 6.0% | 98.0% | 0.0% | 2.0% | 100.0% | 0.0% | 0.0% | 100.0% | 0.0% | 0.0% |
| A21. | 0.0% | 0.0% | 100.0% | 0.0% | 2.0% | 98.0% | 0.0% | 0.0% | 100.0% | 2.0% | 2.0% | 96.0% |
| A22. | 0.0% | 4.0% | 96.0% | 2.0% | 2.0% | 96.0% | 0.0% | 0.0% | 100.0% | 0.0% | 2.0% | 98.0% |
| A23. | 2.0% | 6.0% | 92.0% | 2.0% | 0.0% | 98.0% | 0.0% | 2.0% | 98.0% | 0.0% | 4.0% | 96.0% |
| A24. | 0.0% | 0.0% | 100.0% | 2.0% | 0.0% | 98.0% | 0.0% | 0.0% | 100.0% | 0.0% | 0.0% | 100.0% |
| A25. | 88.0% | 8.0% | 4.0% | 100.0% | 0.0% | 0.0% | 94.0% | 0.0% | 6.0% | 96.0% | 2.0% | 2.0% |
| A26. | 2.0% | 2.0% | 96.0% | 0.0% | 6.0% | 94.0% | 2.0% | 0.0% | 98.0% | 2.0% | 2.0% | 96.0% |
| A27 | 0.0% | 0.0% | 100.0% | 0.0% | 0.0% | 100.0% | 4.0% | 0.0% | 96.0% | 2.0% | 0.0% | 98.0% |
Participant Confidence in Understanding
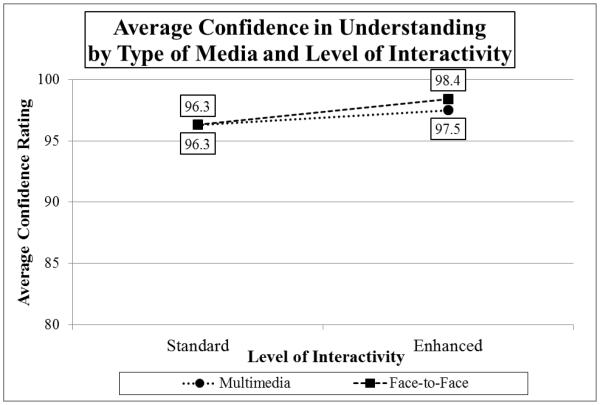
Testing the hypotheses that MM and enhanced interactivity would improve participants’ confidence in their understanding of the Biobank consent (Media × Interactivity MANOVA on Confidence scores), the main effect for level of interactivity was statistically significant (F(1,196)=6.793, p=0.01, partial η2=0.033), although Media (F(1,196)=0.455, n.s.) and the interaction between Interactivity and Media (F(1,196)=0.502, n.s.) were not. In other words, individuals who received enhanced interactivity (13 multiple-choice questions) reported higher confidence in their understanding of the Biobank consent (M=97.9), compared to individuals in the standard interactivity conditions (M=96.3) (see Graph 2). For the total sample (N=200), the average Confidence score was 97.1.
Graph 2.
Average Confidence in Understanding by Type of Media and Level of Interactivity
Participant Consent Time
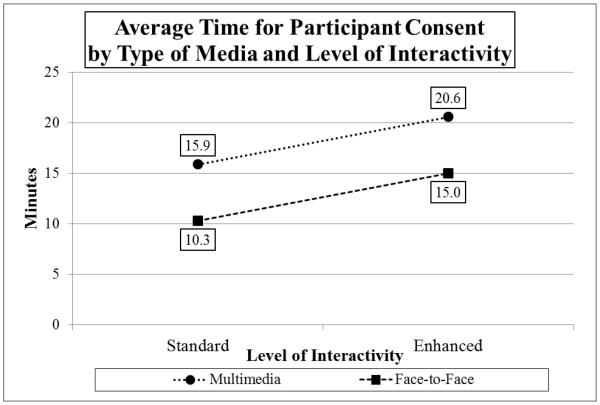
The MANOVA (Media × Interactivity) on Consent Time revealed that both main effects for Media (F(1,195)=42.15, p<0.001) and Interactivity (F(1,195)=29.94, p,0.001) were statistically significant, although the interaction of Interactivity and Media was not (F(1,196)<0.001, n.s.). Collapsing across Interactivity, participants in the MM conditions took more time to complete the consent (M=18.2 min.) than those in the F2F conditions (M=12.6 min.). Collapsing across Media, participants receiving enhanced interactivity took more time to complete the consent (M=17.8 min.) compared to those in the standard conditions (M=13.1 min.). Participants in the MM Enhanced Interactivity condition took twice the time (M=20.6 min.) to complete the consent compared to those in the F2F Standard Interactivity condition (M=10.3 min.) (See Graph 3). The average elapsed time for the total sample (N=196) was 15.4 minutes. Times for four participants were excluded because of missing data.
Graph 3.
Average Time for Participant Consent by Type of Media and Level of Interactivity.
Attitudinal Data
Using three open-ended questions, all study participants were asked what they liked, disliked, and suggested to improve the Biobank’s informed consent process. A total of 93 participants (46.5%) responded to at least one of these questions. Participants liked elements of all four study conditions. Likable elements of the MM conditions included the tablet and the interactive interface, the touchscreen and the “simple and direct” interface, the recorded verbal narration of the consent text, and the graphics. Likable elements of the F2F conditions included being “well explained,” “quick,” and “easy,” interactive by virtue of the presence of a consenter, and the perceived professionalism, “humor,” and “friendliness” of the consenter.
The length of the consent process was disliked by some participants in all four conditions, as well as the perceived “redundancy” of some of the content in the consent document. Length of consent was a more prominent theme among participants in the MM conditions, compared to the F2F conditions. However, participants in the MM Enhanced Interactivity, which took the most time, did not criticize the duration of the consent process more than those in the MM Standard Interactivity group.
Suggestions for improving the Biobank consent process included shortening the presentation, bulleting key information, and adding a short video to the MM presentation. Participants also suggested making the informed consent document available online and providing it prior to patients’ appointments.
Decision to Enroll
Of the 200 participants, four (2%) chose not to enroll in the Biobank after completing the informed consent. Two were in the MM Enhanced Interactivity condition, one was in the MM Standard Interactivity condition, and one was in the F2F Standard Interactivity condition.
DISCUSSION
Results from this study suggest that interactivity and multimedia are at least as good as, and perhaps better than, a biobank’s standard F2F consent process at promoting understanding of the information presented in its informed consent document. Thus, incorporating interactivity and/or multimedia into a biobank’s consent process is one option for improving the process. Converting a biobank’s informed consent document to multimedia delivery by chunking text into slides, adding relevant graphics, and narrating the text benefited consent participants. Enhancing interactivity by adding multiple choice questions with feedback improved participant understanding of and confidence in the informed consent information for both MM and F2F conditions. Comparing understanding of the F2F Standard Interactivity group to the MM Enhanced Interactivity group results in a large effect size (Cohen’s d=0.67), suggesting that further study of this combination is warranted. Given the individual effects of multimedia and interactivity, however, researchers should not assume that multimedia is inherently interactive, but rather should separate the two constructs when studying electronic consent.
Results suggest that interactivity, which has not been treated as a separate construct in past multimedia research, deserves more attention. Adding multiple choice questions with feedback offered a relatively easy and straightforward means of improving understanding, even in a F2F consent process. This means that enhancing interactivity can be incorporated into different delivery approaches, as this study illustrates. Other forms of interactivity, such as repetition and elaboration of information on incorrect answers to questions37 or extended discussions with researchers based on question responses,38 should be studied as potential ways to improve interactivity in multimedia informed consent.
Biobanks will need to consider the tradeoff of improved participant understanding to increased participant time. Although participants in the MM conditions understood the material better, the gains were small and the MM conditions took more time for participants to complete than the F2F conditions. MM participants likely listened to all the slide narration, similar to reading the complete informed consent document, whereas RAs in the F2F conditions summarized the main points.
The qualitative feedback from study participants suggests that interactive multimedia is well tolerated, but that the length of a biobank consent process and redundancy may be problematic from a participant standpoint, regardless of how the information is presented. Participant feedback suggests that biobanks should consider providing staff-assisted consent options in addition to electronic processes for people who prefer a “human touch” and/or a paper document. Some individuals may prefer to listen to the audio narration in a multimedia slideshow, while others may prefer to read the text without narration. Preferences for interactivity may also vary. Research is also warranted into the feasibility and benefits of providing the consent document online ahead of any formal consent process in the clinic environment. Multimedia consenting may be one of several options (i.e., in the form of a “menu-type” approach) offered to accommodate a diverse population with different levels of technology access, physical and perceptual ability, literacy, and other capabilities.
Biobanks should also consider that interactive multimedia consenting tools require a number of developmental steps. Formative evaluation and community consultation12 provide key insights into stakeholder needs and preferences with respect to the usability of e-consent. Evaluation processes used in the current study resulted in improvements to the materials, which may have improved participant usage and satisfaction.
Limitations
A limitation of the current study is that it was conducted at a single, Midwestern, nonurban institution with a primarily Caucasian and relatively well educated sample. Because of small group sizes, comparison of racial/ethnic differences or differences in recruitment settings was not possible. Further study of these findings with a more diverse population in different regions of the country and different recruitment settings is warranted. In addition, the lack of blinding of RAs to study conditions may have affected results. It is possible that RA exposure to the interactive questions in the F2F Enhanced Interactivity condition may have influenced their handling of the Standard F2F condition, accounting in part for the relatively high mean understanding score for participants in this condition. Researchers may want to consider employing two sets of RAs in similar future research, one set devoted to multimedia and another to F2F conditions, to minimize possible contamination.
Finally, this study produced little data on whether electronic consenting might affect participation in biobanks, though there was no indication that an electronic consent would impact participation. In addition, biobanks need to have more information about how electronic consent processes affect staff time and patient relationships.
Conclusion
Biobanks are diverse entities that may benefit in multiple ways from electronic interfacing with research participants. Issues of time and costs for developing electronic materials, computer access, user preference and integration with electronic patient records will need to be addressed in efforts to harness the benefits of electronic informed consent. From an ethical and legal standpoint, electronic consent processes need to be at least as good as standard F2F consent processes at promoting informed, deliberate choices about research participation. Results from this study showing improvement in understanding and confidence of understanding of a biobank informed consent document indicate the potential for improving the biobank consent process using interactive multimedia.
Acknowledgements
The authors would like to thank Joyce Craig, Abe Klein, Jamie L’Heureux, Jenni Rigdon, and Laura Shinkunas, as well as Jeff Murray, University of Iowa Biobank director, for their assistance and support in this study. Excellent feedback from Andrew Bertolatus, Betsy Chrischilles, Lauris Kaldjian and the journal’s anonymous reviewers was also incorporated into the paper.
Source of Funds Supporting the Work:
This work was supported by a grant from the National Human Genome Institute at the National Institutes of Health (R21HG006293).
Footnotes
DISCLOSURE
The authors declare no conflict of interest.
REFERENCES
- 1.Simon CM, Klein DW, Schartz HA. Traditional and electronic informed consent for biobanking: A survey of U.S. biobanks. Biopreserv Biobank. doi: 10.1089/bio.2014.0045. In press. [DOI] [PubMed] [Google Scholar]
- 2.Thiel DB, Platt J, Platt T, et al. Testing an online, dynamic consent portal for large population biobank research. Public Health Genomics. 2014 Oct 30; doi: 10.1159/000366128. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beskow LM, Dombeck CB, Thompson CP, Watson-Ormond JK, Weinfurt KP. Informed consent for biobanking: consensus-based guidelines for adequate comprehension. Genet Med. 2014 Aug 21; doi: 10.1038/gim.2014.102. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simeon-Dubach D, Watson P. Biobanking 3.0: evidence based and customer focused biobanking. Clin Biochem. 2014;47:300–308. doi: 10.1016/j.clinbiochem.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed FE. Biobanking perspective on challenges in sample handling, collection, processing, storage, analysis and retrieval for genomics, transcriptomics and proteomics data. Anal Methods. 2011;3:1029–1038. [Google Scholar]
- 6.Kaphingst KA, Facio FM, Cheng MR, et al. Effects of informed consent for individual genome sequencing on relevant knowledge. Clin Genet. 2012;82:408–415. doi: 10.1111/j.1399-0004.2012.01909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ormond KE, Cirino AL, Helenowski IB, Chisholm RL, Wolf WA. Assessing the understanding of biobank participants. Am J Med Genet A. 2009;149A:188–198. doi: 10.1002/ajmg.a.32635. [DOI] [PubMed] [Google Scholar]
- 8.Chalil Madathil K, Koikkara R, Obeid J, et al. An investigation of the efficacy of electronic consenting interfaces of research permissions management system in a hospital setting. Int J Med Inform. 2013;82:854–863. doi: 10.1016/j.ijmedinf.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishimura A, Carey J, Erwin PJ, Tilburt JC, Murad MH, McCormick JB. Improving understanding in the research informed consent process: a systematic review of 54 interventions tested in randomized control trials. BMC Med Ethics. 2013;14:28. doi: 10.1186/1472-6939-14-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karunaratne AS, Korenman SG, Thomas SL, Myles PS, Komesaroff PA. Improving communication when seeking informed consent: a randomised controlled study of a computer-based method for providing information to prospective clinical trial participants. Med J Aust. 2010;192:388–392. doi: 10.5694/j.1326-5377.2010.tb03561.x. [DOI] [PubMed] [Google Scholar]
- 11.Rowbotham MC, Astin J, Greene K, Cummings SR. Interactive informed consent: randomized comparison with paper consents. PLoS One. 2013;8:e58603. doi: 10.1371/journal.pone.0058603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahnke AN, Plasek JM, Hoffman DG, et al. A rural community's involvement in the design and usability testing of a computer-based informed consent process for the Personalized Medicine Research Project. Am J Med Genet A. 2014;164A:129–140. doi: 10.1002/ajmg.a.36220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayer RE. Multimedia learning. 2nd Cambridge University Press; Cambridge; New York: 2009. [Google Scholar]
- 14.Low R, Sweller J. The modality principle in multimedia learning. In: Mayer RE, editor. The Cambridge handbook of multimedia learning. Cambridge University Press; New York, NY: 2005. pp. 147–158. [Google Scholar]
- 15.Mayer R. Introduction to multimedia learning. In: Mayer RE, editor. The Cambridge handbook of multimedia learning. Cambridge University Press; New York, NY: 2005. pp. 1–16. [Google Scholar]
- 16.Mayer RE. Cognitive theory and the design of multimedia instruction: an example of the two-way street between cognition and instruction. New Directions for Teaching & Learning. 2002;2002:55–71. [Google Scholar]
- 17.Mousavi SY, Low R, Sweller J. Reducing cognitive load by mixing auditory and visual presentation modes. J Educ Psychol. 1995;87:319–334. [Google Scholar]
- 18.Sadoski M, Paivio A. Imagery and text: a dual coding theory of reading and writing. Lawrence Erlbaum Associates; Mahwah, NJ: 2001. [Google Scholar]
- 19.Schnotz W, Kürschner C. A reconsideration of cognitive load theory. Educ Psychol Rev. 2007;19:469–508. [Google Scholar]
- 20.Sweller J, Van Merrienboer JJ, Paas FG. Cognitive architecture and instructional design. Educ Psychol Rev. 1998;10:251–296. [Google Scholar]
- 21.Van Merrienboer JJ, Sweller J. Cognitive load theory and complex learning: recent developments and future directions. Educ Psychol Rev. 2005;17:147–177. [Google Scholar]
- 22.Chandler P, Sweller J. Cognitive load theory and the format of instruction. Cognition and Instruction. 1991;8:293–332. [Google Scholar]
- 23.Mayer RE, Moreno R. Nine ways to reduce cognitive load in multimedia learning. Educ Psychol. 2003;38:43–52. [Google Scholar]
- 24.Flory J, Emanuel E. Interventions to improve research participants' understanding in informed consent for research: a systematic review. JAMA. 2004;292:1593–1601. doi: 10.1001/jama.292.13.1593. [DOI] [PubMed] [Google Scholar]
- 25.Schenker Y, Fernandez A, Sudore R, Schillinger D. Interventions to improve patient comprehension in informed consent for medical and surgical procedures: a systematic review. Med Decis Making. 2011;31:151–173. doi: 10.1177/0272989X10364247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Downes EJ, McMillan SJ. Defining interactivity: A qualitative identification of key dimensions. New Media and Society. 2000;2:157–179. [Google Scholar]
- 27.Heeter C. Interactivity in the context of designed experiences. Journal of Interactive Advertising. 2000;1:3–14. [Google Scholar]
- 28.Jensen JF. The concept of interactivity--revisited: four new typologies for a new media landscape. Proceedings of the 1st International Conference on Designing Interactive User Experiences for TV and Video.2008. [Google Scholar]
- 29.Kiousis S. Interactivity: a concept explication. New Media and Society. 2002;4:355–383. [Google Scholar]
- 30.Koolstra CM, Bos MJ. The development of an instrument to determine different levels of interactivity. International Communication Gazette. 2009;71:373–391. [Google Scholar]
- 31.Yacci M. Interactivity demystified: A structural definition for distance education and intelligent CBT. Educational Technology. 2000;40:5–16. [Google Scholar]
- 32.General requirements for informed consent (45CFR46.116). 45 C.F.R. Sect. 46.116. 2009 Available from: http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.html. Accessed November 20, 2014.
- 33.Simon CM, L'Heureux J, Murray JC, et al. Active choice but not too active: public perspectives on biobank consent models. Genet Med. 2011;13:821–831. doi: 10.1097/GIM.0b013e31821d2f88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alessi SM, Trollip SR. 3rd Allyn and Bacon; Boston: 2001. Multimedia for learning: methods and development. [Google Scholar]
- 35.Krug S. Usability test script: Advanced Common Sense website. 2012 Available at: http://sensible.com/downloads-dmmt.html. Accessed July 18, 2012.
- 36.Joffe S, Cook EF, Cleary PD, Clark JW, Weeks JC. Quality of informed consent: A new measure of understanding among research subjects. J Natl Cancer Inst. 2001;93:139–147. doi: 10.1093/jnci/93.2.139. [DOI] [PubMed] [Google Scholar]
- 37.Wirshing DA, Sergi MJ, Mintz J. A videotape intervention to enhance the informed consent process for medical and psychiatric treatment research. Am J Psychiatry. 2005;162:186–188. doi: 10.1176/appi.ajp.162.1.186. [DOI] [PubMed] [Google Scholar]
- 38.Coletti AS, Heagerty P, Sheon AR, et al. Randomized, controlled evaluation of a prototype informed consent process for HIV vaccine efficacy trials. J Acquir Immune Defic Syndr. 2003;32:161–169. doi: 10.1097/00126334-200302010-00008. [DOI] [PubMed] [Google Scholar]
- 39.L'Heureux J, Murray JC, Newbury E, Shinkunas L, Simon CM. Public perspectives on biospecimen procurement: what biorepositories should consider. Biopreserv Biobank. 2013;11:137–143. doi: 10.1089/bio.2013.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tait AR, Voepel-Lewis T, Malviya S. Do they understand? (part I): parental consent for children participating in clinical anesthesia and surgery research. Anesthesiology. 2003;98:603–608. doi: 10.1097/00000542-200303000-00005. [DOI] [PubMed] [Google Scholar]