Abstract
Parathyroid hormone (PTH) suppresses the expression of a bone formation inhibitor sclerostin (Sost) in osteocytes by inducing nuclear accumulation of histone deacetylases (HDACs) to inhibit the myocyte enhancer factor 2 (MEF2)-dependent Sost bone enhancer. Previous studies revealed that lipoprotein receptor–related protein 6 (LRP6) mediates the intracellular signaling activation and the anabolic bone effect of PTH. Here, we investigated whether LRP6 mediates the inhibitory effect of PTH on Sost using an osteoblast-specific LRP6-deficient (LRP6-KO) mouse model. An increased level of Sost mRNA expression was detected in femur tissue from LRP6-KO mice, compared to wild-type littermates. The number of osteocytes expressing sclerostin was also increased in bone tissue of LRP6-KO mice, indicating a negative regulatory role of LRP6 on Sost/sclerostin. In wild-type mice, intermittent PTH treatment significantly suppressed Sost mRNA expression in bone and the number of sclerostin+ osteocytes, while the effect of PTH was much less significant in LRP6-KO mice. Additionally, PTH-induced down-regulation of MEF2C and 2D, as well as HDAC changes in osteocytes, were abrogated in LRP6-KO mice. These data indicate that LRP6 is required for PTH suppression of Sost expression.
Keywords: PTH, LRP6, Sost, osteocyte, MEF2, HDAC, sclerostin
Introduction
Parathyroid hormone (PTH), an 84 amino acid peptide secreted by parathyroid glands, controls calcium homeostasis and stimulates bone remodeling by binding to PTH/PTH-related peptide receptor (PTH1R).1-3 Intermittent increases of PTH in the circulation, as achieved by daily injections, lead to bone gain. Owing to this property, PTH is the only current US Food and Drug Administration (FDA)-approved bone anabolic agent for osteoporosis. The PTH bone anabolic effect has been mainly attributed to the ability of the hormone to stimulate a rapid increase in osteoblasts and bone formation.4-6 At the molecular level, PTH binds to PTH1R, a class II G protein–coupled receptor that activates several signaling pathways, including the Gsα-linked cAMP-dependent protein kinase A (PKA) and the Gq/11-linked phosphatidyl inositol-specific phospholipase C (PLC)-protein kinase C signaling pathways.7-9 The specific roles of these distinct PTH1R signaling pathways in bone have been examined in vivo.10-13
Our previous work suggested that PTH also orchestrates signaling of local factors, including, but not limited to, TGF-β, Wnt, BMP, and IGF-1,14-18 in modulation of bone modeling. In particular, PTH stimulates formation of a complex of PTH1R and lipoprotein receptor-related protein 6 (LRP6) in osteoblastic lineage cells, resulting in the activation of both β-catenin-Tcf/Lef signaling 16, 19, 20 and cAMP–PKA signaling.21, 22 Importantly, LRP6 is not only essential for the survival and differentiation of osteoblastic lineage cells during skeletal growth 69 and bone remodeling, 23 but it is also required for PTH-associated anabolic effects in mice.23
At the cellular level, osteoblasts have been regarded as the main target cells of the PTH anabolic effect, but recent increasing evidence identifies osteocytes as critical effectors of PTH action.24-29 Osteocytes, the most abundant cells in bone, comprising more than 90% of cells within the matrix or on bone surfaces, are the master signal sensors, integrators, and transducers of the skeleton.30, 31 Osteocytes are highly connected with cells on the bone surface and within the mineralized bone matrix, which allows the transport of proteins secreted by osteocytes to other cells via the osteocytic lacunar–canalicular system. One of the molecules secreted by osteocytes is sclerostin, the product of the gene Sost.32-34 Sclerostin binds to LRP5 and LPR6 and prevents activation of Wnt signaling, as well as antagonizes the actions of BMPs, 35-37 which are critical for osteoblastogenesis and bone formation. As LRP6 is an essential element in PTH–PTH1R signaling pathway, sclerostin was also found to antagonize PTH action in osteoblasts by binding to LRP6.22 Loss of sclerostin in humans causes the high bone mass disorders Van Buchem's disease and sclerosteosis,38, 39 and in mice high bone mass.40, 41 Conversely, mice overexpressing Sost exhibit low bone mass.26, 32 Neutralizing antibodies against sclerostin restore bone mineral density.42-44 Thus, sclerostin, secreted by osteocytes, is an important negative regulator of bone mass through the inhibition of osteoblastic bone formation.
The expression of Sost in osteocytes is negatively regulated by PTH. Both continuous and intermittent PTH administration suppresses Sost mRNA and sclerostin protein expression in cells from mice,34, 45, 46 rats,47, 48 and humans.49, 50 PTH exerts its repressive effect by inhibiting myocyte enhancer factor 2 (MEF2) transcription factors, which bind to a distant downstream enhancer that is required for Sost expression in adult bone.46, 51-53 Vertebrates express four MEF2 proteins, MEF2A, B, C, and D. It has been demonstrated that MEF2A, C, and D, but not MEF2B, are expressed in adult bone.46 Moreover, mice lacking Mef2c in osteocytes or lacking the Mef2c downstream enhancer region display reduced sclerostin levels and high bone mass.51, 54 The activity of MEF2s is controlled by a variety of signaling pathways. Specifically, histone deacetylases (HDACs), a family of enzymes capable of deacetylating lysine residues in a wide variety of cellular proteins, including histones,55 are important regulators of the transcription activity of MEF2 genes. Baertschi et al recently showed that class I HDAC1, 2, and 3 are required for constitutive Sost expression, whereas PTH-induced Sost suppression was associated with specific, rapid nuclear accumulation of class II HDAC5 and co-localization with MEF2 proteins.56 Moreover, mice lacking Hdac5 show increased sclerostin levels in osteocytes, low bone density, and reduced bone formation.57 In agreement with an important role of MEF2C and HDAC5 in control of Sost expression, Mef2c and Hdac5 were identified as two of 20 loci affecting bone mineral density in a meta-analysis of five genome-wide association studies of femoral neck and lumbar spine bone mineral density.58 Therefore, HDAC–MEF2–Sost pathway is important in the regulation of bone metabolism.
PTH-regulated Sost suppression has been shown to be initiated by the cAMP signaling pathway downstream of the PTH1R,45, 59 a pathway in which LRP6 is an essential component in osteoblastic-lineage cells.16, 19-23 We have reported that LRP6 in osteoblasts is required for osteoblastic differentiation during bone remodeling and for the anabolic effects of PTH by using an osteoblast-specific LRP6-deficient mouse model.23 Using the same mouse model, we now show that the expression levels of Sost mRNA, sclerostin protein, HDAC2–4, and transcription factors MEF2C and 2D are significantly upregulated in osteocytes of LRP6-KO mice compared to wild-type mice. More importantly, the effects of intermittent PTH treatment on Sost repression and MEF2 protein downregulation in osteocytes of femurs are blunted in LRP6-KO mice. Our results suggest that LRP6 is essential for the inhibitory effect of PTH on Sost/sclerostin expression in osteocytes.
Materials and methods
Mice and treatment
Lrp6f/f mice were obtained from Van Andel Research Institute. 60, 61 Transgenic mice expressing the Cre recombinase under the control of a 3.9-kb fragment of the human osteocalcin promoter (OC-Cre) were obtained from T. Clemens (Baltimore, MD).62 The generation of homozygous deletion Cre+/−;Lrp6f/f mice (LRP6-KO hereafter) and control Cre−/−;Lrp6f/f (WT hereafter) were described previously.23 Mice were maintained on a mixed background of C57Bl/6J, 129, and FVB/N. All animals were maintained in the Animal Facility of the Johns Hopkins University School of Medicine. The experimental protocol was reviewed and approved by the Institutional Animal Care and Use Committee of the Johns Hopkins University, Baltimore, MD. Genomic DNA extraction and genotyping of the animals were prepared as described previously.23 Primers for Cre recombinase and the loxP sites were used for PCR. For PTH treatment, two month-old male WT mice and KO mice were randomized into four groups: WT-vehicle, WT-PTH, KO-vehicle, and KO-PTH. Six mice of each treatment group were used. Mice were subcutaneously injected with either human PTH1–34 or vehicle (1 mM acetic acid in phosphate buffered saline (PBS)) at a dosage of 80 μg/kg daily, five days per week, for 4 weeks. Human PTH1–34 was purchased from Bachem Bioscience Inc. (King of Prussia, PA).
Immunohistochemical and immunofluorescence analysis of the bone tissue sections
Left femurs of mice were decalcified in 10% EDTA (pH 7.4, 5 N NaOH) for 14 days. Samples were then embedded in paraffin wax and 4 μm longitudinal sections were cut on a microtome (HM325, Thermo Scientific). To standardize staining, consecutive longitudinal sections from each femur sample were stained for a single batch of immunohistochemical and immunofluorescence staining, and the staining using the same antibody was repeated three times per sample. A total of six mice per treatment group were used. Bone sections were processed for antigen retrieval by digestion in 0.05% trypsin for 15 min at 37 °C and then blocked with PBS containing 5% bovine serum albumin (BSA) for 1 hr, and then incubated with antibodies against rabbit sclerostin (diluted 1:50, Abcam, MA), rabbit LRP6 (diluted 1:100, Abcam, MA), rabbit MEF2C (diluted 1:100, Abcam, MA), rabbit HDAC2 (diluted 1:250, Abcam, MA), rabbit HDAC3 (diluted 1:250, Abcam, MA) overnight at 4 °C. An HRP- or AP-streptavidin detection system (Dako) was subsequently used to detect the immunoactivity followed by counterstaining with hematoxylin (Sigma). Sections incubated with polyclonal rabbit IgG (R&D Systems, AB-105-C) under the same concentrations and conditions as negative control. Double immunofluorescence staining was performed as described previously.16 After blocking in 0.5% horse serum, sections were incubated first with antibodies, followed by incubation with FITC- or Cy3-conjugated secondary antibodies (Jackson ImmunoResearch). Nuclei were counterstained with DAPI (Sigma). The sections were observed under a fluorescence microscope (BX51, Olympus). To quantify the osteocyte numbers that were positive for the detected signaling, five random high power fields per tissue section were selected, and averages per section were taken as the final measures. The percentage of osteocytes that stained positive for each antibody, out of the total osteocytes, was then calculated.
Quantitative real-time PCR
Femora were harvested and both ends and surrounding soft tissues of the bone were removed. The remaining bones were flushed to remove bone marrow cells and digested with a protease solution (2 mg/ml collagenase A and 2.5 mg/ml trypsin in PBS) for 20 min to remove the osteoblasts and osteo-progenitors on bone surface. Total RNA for quantitative real-time (qRT)-PCR was extracted from the bone tissue using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. RNA purity was tested by measuring the absorbance at 260 and 280 nm. For qRT-PCR, cDNA was prepared with random primers using the SuperScript FirstStrand Synthesis System (Invitrogen) and analyzed with SYBR GreenMaster Mix (Qiagen, Valencia, CA) in the thermal cycler with two sets of primers specific for each targeted gene. Relative expression was calculated for each gene by 2-ΔΔCT method, with GAPDH for normalization. Primers used for qRT-PCR are listed in Supplememtary Table 1.
Statistical analysis
All data were presented as mean ± SEM. All statistical tests were two-sided. A P-value less that 0.05 was considered significant. Comparability of two groups of data was assessed using a Students t-test.
Results
Osteocytes with LRP6 deficiency secrete high levels of sclerostin
We previously generated mice lacking LRP6 specifically in mature osteoblasts (LRP6-KO) by crossing mice expressing the Cre recombinase driven by the human osteocalcin promoter (OC-cre) with mice expressing loxP-flanked Lrp6 (Lrp6f/f). We have confirmed that Lrp6 was deleted specifically in skeletal tissue in LRP6-KO mice.23 We examined whether LRP6 expression is also affected in osteocytes— terminal differentiated cells derived from osteoblasts—in the mice. Immunohistochemical analysis revealed that LRP6 expression was detected in most (approximately 75%) of the osteocytes in cortical bone of femora in wild-type mice, but only in a small portion (about 23%) of osteocytes in femurs of the LRP6-KO mice (Fig. 1A and 1B). Similarly, Lrp6 mRNA level was decreased approximately 77% in bone tissue measured by qRT-PCR (Fig.1C). Therefore, LRP6 protein is also deleted in most of the osteocytes in LRP6 KO mice. As LRP5 and LRP6 are highly homologous proteins that transduce the same canonical Wnt signaling, we also examined LRP5 expression in osteocytes in LRP6-KO mice. As shown in Fig. 1D and 1E, more LRP5+ osteocytes were detected in cortical bone of femora in LRP6-KO mice compared to WT mice, indicating a compensatory role of LRP5 in regulating the activities of osteocytes. We then examined whether the levels of Sost and sclerostin expression in osteocytes were affected by Lrp6 deletion. The number of sclerostin+ osteocytes was significantly increased in cortical bone of femora in LRP6-KO mice, compared with WT mice, by immunohistochemical analysis (Fig.1F and G). Consistently, in real-time PCR assays elevated Sost mRNA level was observed in femur tissue of LRP6-KO mice compared with WT mice (Fig.1H).
Figure 1.
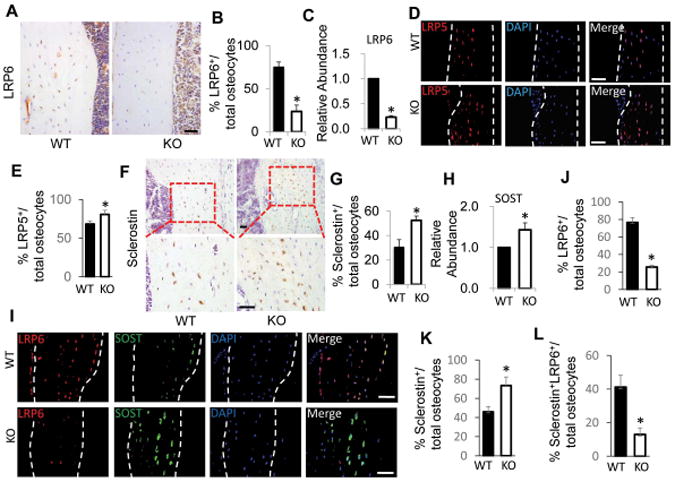
Osteocytes in femur tissue of Oc-Cre–mediated conditional LRP6-deficient mice had increased Sost/sclerostin expression. (A and B) Immunohistochemical analysis of LRP6 expression in cortical bone of femur from 3 month-old male Oc-Cre;Lrp6f/f (KO) and Cre−/−;Lrp6f/f (WT) mice. Representative images of immunohistochemical staining with an antibody to LRP6 (brown), A. Scale bars: 50μm. Quantification of LRP6+ osteocytes out of total osteocytes, B. (C) qRT-PCR analysis of Lrp6 expression in cortical bone extracts from 3-month-old male WT and KO mice. (D and E) Immunofluorescence staining of LRP5 in cortical bone of femur from 3 month-old male WT and KO mice. Representative images, D, and quantification of LRP5-positive osteocytes out of total osteocytes, E, are shown. Scale bars: 50μm. (F and G) Immunohistochemical analysis of sclerostin expression in cortical bone of femur from 3 month-old male WT and KO mice. Representative images of immunohistochemical staining with an antibody to sclerostin (brown) in low power (F, upper panels) and high power (F, bottom panels). Scale bars: 50μm. Quantification of sclerostin+ osteocytes out of total osteocytes, G. (H) qRT-PCR analysis of Sost expression in cortical bone extracts from 3-month-old male WT and KO mice. (I–L) Double-immunofluorescence staining of LRP6 and sclerostin in cortical bone of femur from 3 month-old male WT and KO mice. Representative images, I, and quantification of LRP6+, J, sclerostin+, K, and LRP6+sclerostin+, L, osteocytes out of total osteocytes were shown. Scale bars: 50μm. For all the experiments, a total of three femur sections from each mouse, and six mice per treatment group were analyzed. *P < 0.01 vs. WT mice.
To further examine if the association of the increased sclerostin expression with LRP6 deficiency in ostyocytes, a double-immunofluorescence staining of the femoral tissue from the WT and LRP6-KO mice was performed. Consistent with Fig. 1A and 1F, LRP6 expression was detected in most of the osteocytes in cortical bone of femora in WT mice, but in many fewer osteocytes in LRP6-KO mice (Fig. 1I and J). The number of the sclerostin+ osteocytes was significantly increased in cortical bone of femora from LRP6-KO mice, compared with WT mice (Fig. 1I and K). Most importantly, double-stained LRP6+ sclerostin+ osteocytes were almost undetectable in LRP6-KO mice (Fig. 1I and L). Thus, the upregulation of sclerostin in osteocytes is directly associated with LRP6 deficiency at a single cell level in bone tissue.
LRP6 deficiency abolishes the suppressive effect of PTH on sclerostin expression in osteocytes
To determine whether PTH-repressed Sost expression in osteocytes is also affected by LRP6 deficiency, we examined the expression changes of Sost and sclerostin in osteocytes in femur tissue from WT mice and LRP6-KO mice after daily injection of either PTH1–34 or vehicle for 4 weeks. PTH administration in WT mice reduced the number of sclerostin+ osteocytes (about 40%) relative to vehicle treatment (Fig. 2A and B). In LRP6-KO mice, reduced number of sclerostin+ osteocytes was still observed with PTH treatment versus vehicle treatment, but the reduction is much less significant (about 13% reduction) (Fig. 2A and B). Moreover, the numbers of sclerostin+ osteocytes in KO mice were dramatically increased compared with those in WT mice in either vehicle- or PTH-treatment groups. In a quite similar fashion, the reduction of Sost mRNA level in femur tissue was dramatic (about 45%) in WT mice when compared PTH treatment with vehicle treatment, whereas this reduction was much less (about 12%) in KO mice (Fig. 2C). Thus, the suppressive effect of PTH on Sost/sclerostin in osteocytes was alleviated by LRP6 deficiency.
Figure 2.
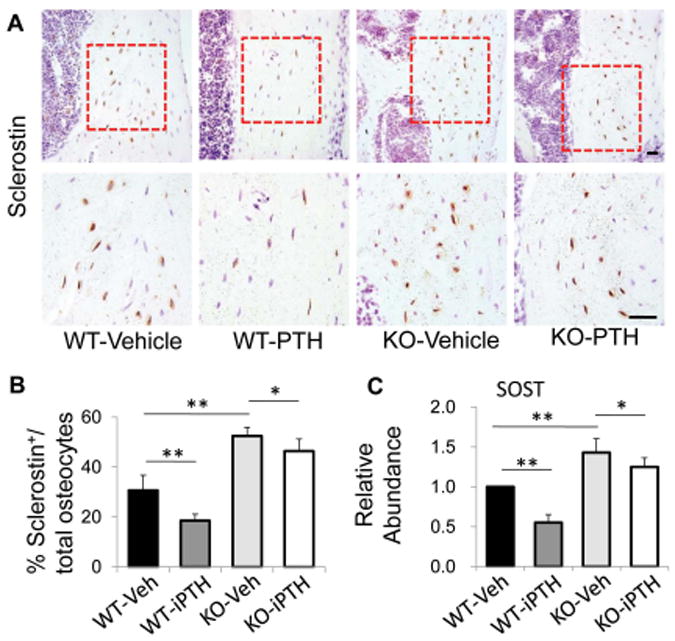
PTH treatment failed to inhibit sclerostin/Sost expression in osteocyte in LRP6-deficient mice. (A) Representative images of immunohistochemical staining with an antibody to sclerostin (brown) in 3-month-old male WT and KO mice treated with vehicle or PTH1–34 daily at 80 mg/kg bw, 5 days a week for 4 weeks. Scale bars: 50 μm. (B) Quantification of sclerostin+ osteocytes out of total osteocytes. (C) qRT-PCR analysis of Sost expression in cortical bone extracts from 3-month-old male WT and KO mice treated with vehicle or PTH. For all the experiments, a total of three femur sections from each mouse, and six mice per treatment group were analyzed. *P < 0.05, **P < 0.01.
LRP6 deficiency abolishes the inhibitory effect of PTH on MEF2 expression in osteocytes
As transcription factor MEF2s have been reported to mediate the effect of PTH on Sost repression,46, 51-53 we examined the expression of MEF2s in osteocytes in femur tissue from WT mice and LRP6 KO mice after PTH treatment. In vehicle-treated mice, elevated mRNA levels of MEF2C and 2D but decreased levels of MEF2A and 2B were observed in femur tissue of LRP6-KO mice relative to WT mice (Fig. 3A–D, compare third and first bars). PTH treatment induced a decrease in the levels of MEF2A, 2C, and 2D in femur tissue of WT mice (Fig. 3A–D, compare second and first bars), but the reductions in all three genes caused by PTH were abolished in LRP6-KO mice (Fig. 3A–D, compare fourth and third bars). Consistently, immunohistochemical analysis revealed that the number of MEF2C-positive osteocytes was increased in KO mice compared to WT mice with vehicle treatment (Fig. 4A–B, compare third and first panels). Unlike in WT mice, PTH treatment failed to inhibit MEF2C expression in osteocytes in KO mice (Fig. 4A–B, compare fourth and third panels). Therefore, LRP6 is required for PTH suppression of MEF protein expression in osteocytes.
Figure 3.
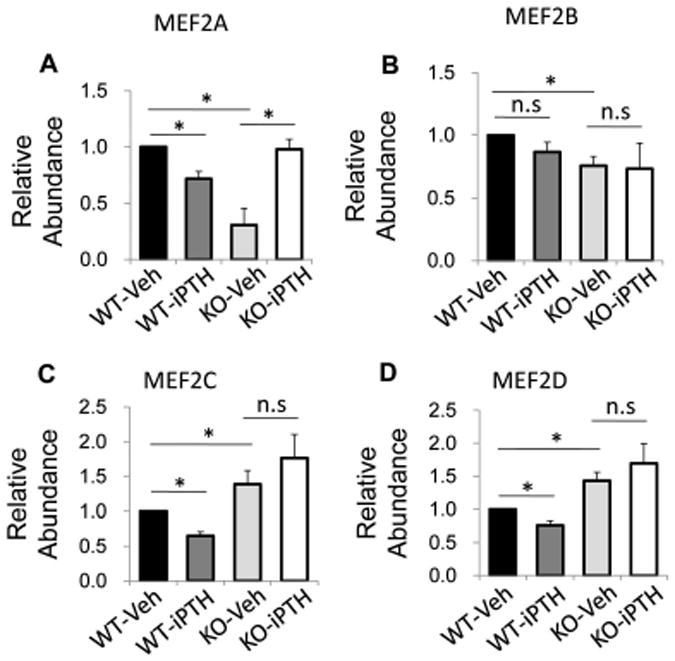
PTH treatment failed to inhibit MEF2s mRNA expression in bone of LRP6-deficient mice. QRT-PCR analysis of Mef2a (A), Mef2b (B), Mef2c (C), and Mef2d (D) gene expression in cortical bone extracts from 3-month-old male WT and KO mice treated with vehicle or PTH daily at 80 mg/kg bw, 5 days a week for 4 weeks. Six mice per treatment group were analyzed. *P < 0.01.
Figure 4.
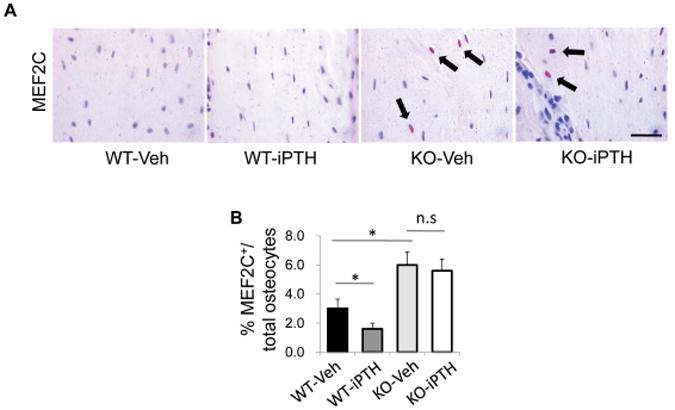
PTH treatment failed to inhibit MEF2s protein expression in osteocytes of LRP6 deficient mice. (A) Representative images of immunohistochemical staining with an antibody to MEF2C (pink) in 3-month-old male WT and KO mice treated with vehicle or PTH1–34 daily at 80mg/kg bw, 5 days a week for 4 weeks. Scale bars: 50μm. (B) Quantification of MEF2C-positive osteocytes out of total osteocytes. A total of three femur sections from each mouse, and six mice per treatment group were analyzed. *P < 0.01.
LRP6 regulates the expression of HDACs in osteocytes
It is known that HDACs interact with MEF2 transcription factors, and the MEF2–HDAC axis plays an important role in regulating development, differentiation, and tissue homeostasis.63 Two recent reports demonstrated that HDACs regulate MEF2C-driven sclerostin expression in osteocytes.56, 57
We examined the expression levels of HDACs in osteocytes in femur tissue from wild-type mice and LRP6-KO mice after PTH1–34 or vehicle treatment. Upregulated mRNA levels of Hdac2, 3, and 4, but not Hdac1 and 5, were observed in femur tissue from LRP6-KO mice compared with WT mice (Fig 5A–E, compare third and first bars). Surprisingly, PTH treatment significantly decreased mRNA levels of Hdac2, 3, and 4 in WT mice (Fig 5B–D, compare second and first bars), while the effect of PTH treatment was not significant in LRP6-KO mice (Fig 5B–D, compare fourth and third bars). We also examined the in situ expression of HDAC2 and 3 in osteocytes of femur tissue by immunohistochemical analysis. Similar to the results of qRT-PCR, the numbers of HDAC2+ and HDAC3+ osteocytes in femur tissue of the LRP6-KO mice were significantly increased compared to WT mice in both vehicle- and PTH-treatment groups (Fig. 6A and B). The results are consistent with the conclusion that LRP6 is an important regulator of HDACs in osteocytes.
Figure 5.
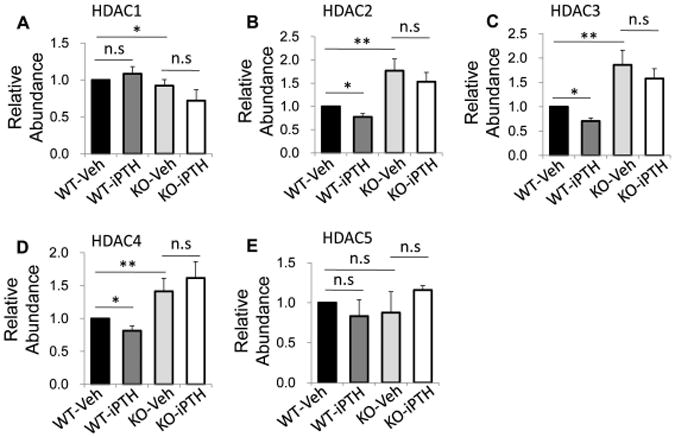
Inhibitory effect of PTH on Hdac mRNA expression in bone is abolished by LRP6 deficiency. Quantitative RT-PCR analysis of Hdac1 (A), Hdac2 (B), Hdac3 (C), Hdac4 (D), Hdac5 (E) mRNA levels in cortical bone extracts from 3-month-old male WT and KO mice treated with vehicle or PTH daily at 80 mg/kg bw, 5 days a week for 4 weeks. Six mice per treatment group were analyzed. *P < 0.05, **P < 0.01.
Figure 6.
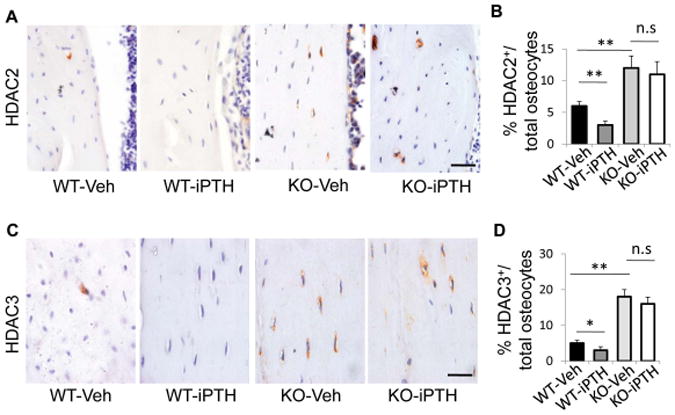
Inhibitory effect of PTH on HDAC 2 and 3 protein expression in osteocytes is abolished by LRP6 deficiency. (A) Representative images of immunohistochemical staining with an antibody to HDAC2 (brown) in 3-month-old male WT and KO mice treated with vehicle or PTH1–34 daily at 80 mg/kg bw, 5 days a week for 4 weeks. Scale bars: 50μm. (B) Quantification of HDAC2+ osteocytes out of total osteocytes; n = 6; *P < 0.05, **P < 0.01. (C) Representative images of immunohistochemical staining with an antibody to HDAC3 (brown) in 3-month-old male WT and KO mice treated with vehicle or PTH1–34 daily at 80 mg/kg bw, 5 days a week for 4 weeks. Scale bars: 50 μm. (D) Quantification of HDAC3+ osteocytes out of total osteocytes. A total of three femur sections from each mouse, and six mice per treatment group were analyzed. *P < 0.01.
Discussion
In this study we used a genetic approach in mice to define the role of LRP6 in PTH-induced Sost suppression in osteocytes. We provide evidence that LRP6 is a negative regulator of MEF2 activity and Sost expression in osteocytes. More importantly, we demonstrate that LRP6 expression is required for PTH-induced Sost suppression. Previous studies revealed that LRP6 is a key element in PTH–PTH1R-stimulated β-catenin-Tcf/Lef 16, 19, 20 and cAMP-PKA signaling pathways21, 22 in osteoblastic lineage cells, and therefore essential for the PTH bone anabolic effect.23 The present finding that LRP6 mediates the PTH effect on the MEF2–Sost pathway adds an additional dimension to the current understanding of LRP6-mediated PTH activity on bone (Fig. 7).
Figure 7.
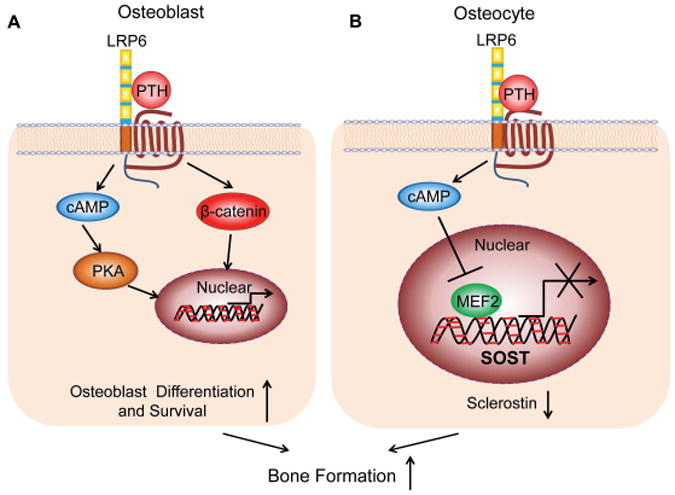
Schematic model of the involvement of LRP6 in the PTH-stimulated anabolic bone effect. PTH-stimulated complex formation of PTH–PTH1R–LRP6 results in the activation of cAMP-PKA and β-catenin-Tcf/Lef signaling pathways in osteoblastic lineage cells. In earlier stages of osteoblastic lineage cells, LRP6-mediated activation of both pathways by PTH promotes the survival and differentiation of osteoblasts, leading to increased osteoblastic bone formation (A). In osteocytes, LRP6-mediated activation of cAMP signaling pathway by PTH inhibits MEF2 transcription factor–stimulated activity of Sost transcription and reduces sclerostin production, leading to increased osteoblastic bone formation (B).
It is known that LRP6 deficiency disrupted the differentiation and survival of osteoblastic lineage cells, resulting in impaired bone formation during bone growth and bone remodeling in adults.23, 64 The current finding that LRP6 deficiency results in increased expression of Sost/sclerostin in osteocytes may be one of the major mechanisms for LRP6 deficiency-caused low bone mass. In this study, we used osteocalcin promoter-driven Cre to delete Lrp6 specifically in mature osteoblasts. In addition to the deficiency of LRP6 in bone surface osteoblasts,23 we found that most of the osteocytes (approximately 69%) in bone matrix also lost LRP6 expression. We found that sclerostin+ osteocytes were almost doubled in LRP6-KO mice, compared to WT mice, and almost all osteocytes that had strong sclerostin expression were LRP6-deficient in cortical bone tissue of the mice. The results strongly suggest that LRP6 in osteocytes is a strong native inhibitor of sclerostin production. It will be interesting to examine in future studies if serum sclerostin levels are increased in the LRP6-deficient mice and whether sclerostin antibody administration in LRP6-deficient mice will restore osteoblast activities and bone formation. One of the reasons for the negative regulation of LRP6 on Sost expression is that LRP6 is required for PTH-stimulated cAMP signaling, which directly suppresses Sost expression in osteocytes.46 Indeed, our finding that intermittent PTH treatment-induced Sost suppression was significantly alleviated by LRP6 deficiency supports this concept. We note that Sost expression suppressed by PTH was not completely abolished in LRP6-KO mice. The most reasonable explanation is that Lrp6 was not completely deleted in all osteocytes in the OC-Cre mouse model. Future studies in an osteocyte-specific Lrp6 deletion mouse model are needed to define if LRP6 in osteocytes is essential to mediate the PTH effect on Sost suppression.
We cannot exclude the possibility that LRP6 also mediates the suppressive effect of other stimuli/factors on Sost expression. The fact that more LRP5+ osteocytes in cortical bone of femora in LRP6-KO mice suggests that increased LRP5 may serve as a compensatory mechanism in regulating the activities of osteocytes. However, it is known that LRP6, but not LRP5, is an essential mediator for PTH-elicited bone anabolic effect during bone remodeling.23, 64, 65 Therefore, it is most likely that the increased LRP5 in osteocytes in LRP6-KO mice has no effect on transducing PTH-induced SOST suppression but instead participates in regulating other activities of osteocytes. LRP6 has been proposed to be a central organizer for the extracellular antagonist network for the regulating signaling pathways of different local growth factors.17 It has been reported that TGF-β166 and prostaglandin E2 (PGE2),67 local factors released in bone microenvironment, regulate Sost transcription in bone. It will be interesting to determine if LRP6 is involved in the effects of these factors on Sost expression.
The molecular mechanisms controlling Sost transcription are only beginning to be unraveled. Both recent in vitro and in vivo studies suggest the importance of the MEF2–HDAC axis in regulating Sost expression in osteocytes. Specifically, MEF2A, C, and D are robustly expressed in osteocytes and control Sost expression in a synergistic manner.46 HDAC5 interacts with MEF2 at the Sost enhancer and directly inhibits MEF2-driven sclerostin expression in osteocytes, 57 as well as in UMR106 osteosarcoma cells.56
We found that osteocytes in LRP6-KO mice have increased levels of MEF2C and D, consistent with LRP6 being a negative regulator of the MEF2–Sost pathway. Our data also show, consistent with previous reports, that PTH treatment significantly reduced the levels of MEF2C and D in osteocytes in wild-type mice, and that this effect of PTH was completely abolished in LRP6-KO mice. These data suggest that LRP6 mediates the suppressive effect of PTH on the MEF2C–Sost pathway. We noted that unlike MEF2C and 2D, the level of MEF2A was dramatically down-regulated after Lrp6 deletion. It is unclear whether this effect of LRP6 is associated with Sost regulation. Future studies, by performing ChIP assay using specific MEF2A antibody and primers flanking the MEF2 binding site of Sost, are needed to clarify the effect of LRP6. A recent study reported that HDAC1–3 are required for constitutive Sost expression, whereas HDAC5 rapidly translocates into nucleus in response to PTH treatment and contributes to PTH-induced Sost suppression.56 Unexpectedly, we found that the levels of HDAC2-4 were up-regulated by LRP6 deficiency and down-regulated by PTH, but the level of HDAC5 was not affected by either LRP6 deficiency or PTH treatment. One explanation is that although the expression of HDAC5 is unchanged, translocation into nucleus may be relatively higher after PTH treatment. Our data suggest that LRP6 primarily regulates the expression HDAC2–4 in osteocytes. It is not clear whether this effect of LRP6 is associated with its inhibitory effect on Sost expression. It is possible that the change of HDAC2–4 in osteocytes by PTH treatment or Lrp6 deletion is associated with the transcriptional regulation of genes other than Sost. Obri et al.68 reported that PTH induces RANKL (Tnfsf11) expression by triggering the protein degradation of HDAC4, which releases MEF2C and transactivates the Tnfsf11 promoter. Further biochemical studies will help determine whether LRP6 plays a role in the PTH-regulated MEF2–HDAC–RANKL pathway, and whether LRP6 is involved in HDAC5-associated regulation of Sost expression in osteocytes.
Supplementary Material
Acknowledgments
This work was supported the National Institutes of Health DK083350 (to M.W.). We thank Bart O. Williams (Van Andel Research Institute) for providing the Lrp6f/f mice and Thomas L. Clemens (Johns Hopkins University School of Medicine) for providing the Oc-cre mice.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
References
- 1.Juppner H, bou-Samra AB, Freeman M, Kong XF, Schipani E, Richards J, Kolakowski LF, Jr, Hock J, Potts JT, Jr, Kronenberg HM. A G protein-linked receptor for parathyroid hormone and parathyroid hormone-related peptide. Science. 1991;254(5034):1024–1026. doi: 10.1126/science.1658941. [DOI] [PubMed] [Google Scholar]
- 2.bou-Samra AB, Juppner H, Force T, Freeman MW, Kong XF, Schipani E, Urena P, Richards J, Bonventre JV, Potts JT., Jr Expression cloning of a common receptor for parathyroid hormone and parathyroid hormone-related peptide from rat osteoblast-like cells: a single receptor stimulates intracellular accumulation of both cAMP and inositol trisphosphates and increases intracellular free calcium. Proc Natl Acad Sci U S A. 1992;89(7):2732–2736. doi: 10.1073/pnas.89.7.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray TM, Rao LG, Divieti P, Bringhurst FR. Parathyroid hormone secretion and action: evidence for discrete receptors for the carboxyl-terminal region and related biological actions of carboxyl- terminal ligands. Endocr Rev. 2005;26(1):78–113. doi: 10.1210/er.2003-0024. [DOI] [PubMed] [Google Scholar]
- 4.Jilka RL. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007;40(6):1434–1446. doi: 10.1016/j.bone.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim SW, Pajevic PD, Selig M, Barry KJ, Yang JY, Shin CS, Baek WY, Kim JE, Kronenberg HM. Intermittent parathyroid hormone administration converts quiescent lining cells to active osteoblasts. J Bone Miner Res. 2012;27(10):2075–2084. doi: 10.1002/jbmr.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Potts JT, Gardella TJ. Progress, paradox, and potential: parathyroid hormone research over five decades. Ann N Y Acad Sci. 2007;1117:196–208. doi: 10.1196/annals.1402.088. [DOI] [PubMed] [Google Scholar]
- 7.Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3(9):639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 8.McCudden CR, Hains MD, Kimple RJ, Siderovski DP, Willard FS. G-protein signaling: back to the future. Cell Mol Life Sci. 2005;62(5):551–577. doi: 10.1007/s00018-004-4462-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin L, Raggatt LJ, Partridge NC. Parathyroid hormone: a double-edged sword for bone metabolism. Trends Endocrinol Metab. 2004;15(2):60–65. doi: 10.1016/j.tem.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 10.rmamento-Villareal R, Ziambaras K, bbasi-Jarhomi SH, Dimarogonas A, Halstead L, Fausto A, Avioli LV, Civitelli R. An intact N terminus is required for the anabolic action of parathyroid hormone on adult female rats. J Bone Miner Res. 1997;12(3):384–392. doi: 10.1359/jbmr.1997.12.3.384. [DOI] [PubMed] [Google Scholar]
- 11.Siddappa R, Martens A, Doorn J, Leusink A, Olivo C, Licht R, van RL, Gaspar C, Fodde R, Janssen F, van BC, de BJ. cAMP/PKA pathway activation in human mesenchymal stem cells in vitro results in robust bone formation in vivo. Proc Natl Acad Sci U S A. 2008;105(20):7281–7286. doi: 10.1073/pnas.0711190105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tintut Y, Parhami F, Bostrom K, Jackson SM, Demer LL. cAMP stimulates osteoblast-like differentiation of calcifying vascular cells. Potential signaling pathway for vascular calcification. J Biol Chem. 1998;273(13):7547–7553. doi: 10.1074/jbc.273.13.7547. [DOI] [PubMed] [Google Scholar]
- 13.Guo J, Liu M, Yang D, Bouxsein ML, Thomas CC, Schipani E, Bringhurst FR, Kronenberg HM. Phospholipase C signaling via the parathyroid hormone (PTH)/PTH-related peptide receptor is essential for normal bone responses to PTH. Endocrinology. 2010;151(8):3502–3513. doi: 10.1210/en.2009-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang Y, Wu X, Lei W, Pang L, Wan C, Shi Z, Zhao L, Nagy TR, Peng X, Hu J, Feng X, Van HW, Wan M, Cao X. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med. 2009;15(7):757–765. doi: 10.1038/nm.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu X, Pang L, Lei W, Lu W, Li J, Li Z, Frassica FJ, Chen X, Wan M, Cao X. Inhibition of Sca-1-positive skeletal stem cell recruitment by alendronate blunts the anabolic effects of parathyroid hormone on bone remodeling. Cell Stem Cell. 2010;7(5):571–580. doi: 10.1016/j.stem.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wan M, Yang C, Li J, Wu X, Yuan H, Ma H, He X, Nie S, Chang C, Cao X. Parathyroid hormone signaling through low-density lipoprotein-related protein 6. Genes Dev. 2008;22(21):2968–2979. doi: 10.1101/gad.1702708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu B, Zhao X, Yang C, Crane J, Xian L, Lu W, Wan M, Cao X. Parathyroid hormone induces differentiation of mesenchymal stromal/stem cells by enhancing bone morphogenetic protein signaling. J Bone Miner Res. 2012;27(9):2001–2014. doi: 10.1002/jbmr.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xian L, Wu X, Pang L, Lou M, Rosen CJ, Qiu T, Crane J, Frassica F, Zhang L, Rodriguez JP, Xiaofeng J, Shoshana Y, Shouhong X, Argiris E, Mei W, Xu C. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat Med. 2012;18(7):1095–1101. doi: 10.1038/nm.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Revollo L, Kading J, Jeong SY, Li J, Salazar V, Mbalaviele G, Civitelli R. N-cadherin Restrains PTH Activation of Lrp6/beta-catenin Signaling and Osteoanabolic Action. J Bone Miner Res. 2014 doi: 10.1002/jbmr.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo J, Liu M, Yang D, Bouxsein ML, Saito H, Galvin RJ, Kuhstoss SA, Thomas CC, Schipani E, Baron R, Bringhurst FR, Kronenberg HM. Suppression of Wnt signaling by Dkk1 attenuates PTH-mediated stromal cell response and new bone formation. Cell Metab. 2010;11(2):161–171. doi: 10.1016/j.cmet.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wan M, Li J, Herbst K, Zhang J, Yu B, Wu X, Qiu T, Lei W, Lindvall C, Williams BO, Ma H, Zhang F, Cao X. LRP6 mediates cAMP generation by G protein-coupled receptors through regulating the membrane targeting of Galpha(s) Sci Signal. 2011;4(164):ra15. doi: 10.1126/scisignal.2001464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi C, Li J, Wang W, Cao W, Cao X, Wan M. Antagonists of LRP6 regulate PTH-induced cAMP generation. Ann N Y Acad Sci. 2011;1237:39–46. doi: 10.1111/j.1749-6632.2011.06226.x. [DOI] [PubMed] [Google Scholar]
- 23.Li C, Xing Q, Yu B, Xie H, Wang W, Shi C, Crane JL, Cao X, Wan M. Disruption of LRP6 in osteoblasts blunts the bone anabolic activity of PTH. J Bone Miner Res. 2013;28(10):2094–2108. doi: 10.1002/jbmr.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fermor B, Skerry TM. PTH/PTHrP receptor expression on osteoblasts and osteocytes but not resorbing bone surfaces in growing rats. J Bone Miner Res. 1995;10(12):1935–1943. doi: 10.1002/jbmr.5650101213. [DOI] [PubMed] [Google Scholar]
- 25.O'Brien CA, Plotkin LI, Galli C, Goellner JJ, Gortazar AR, Allen MR, Robling AG, Bouxsein M, Schipani E, Turner CH, Jilka RL, Weinstein RS, Manolagas SC, Bellido T. Control of bone mass and remodeling by PTH receptor signaling in osteocytes. PLoS One. 2008;3(8):e2942. doi: 10.1371/journal.pone.0002942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rhee Y, Allen MR, Condon K, Lezcano V, Ronda AC, Galli C, Olivos N, Passeri G, O'Brien CA, Bivi N, Plotkin LI, Bellido T. PTH receptor signaling in osteocytes governs periosteal bone formation and intracortical remodeling. J Bone Miner Res. 2011;26(5):1035–1046. doi: 10.1002/jbmr.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powell WF, Jr, Barry KJ, Tulum I, Kobayashi T, Harris SE, Bringhurst FR, Pajevic PD. Targeted ablation of the PTH/PTHrP receptor in osteocytes impairs bone structure and homeostatic calcemic responses. J Endocrinol. 2011;209(1):21–32. doi: 10.1530/JOE-10-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bellido T, Saini V, Pajevic PD. Effects of PTH on osteocyte function. Bone. 2013;54(2):250–257. doi: 10.1016/j.bone.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Compton JT, Lee FY. A Review of Osteocyte Function and the Emerging Importance of Sclerostin. J Bone Joint Surg Am. 2014;96(19):1659–1668. doi: 10.2106/JBJS.M.01096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aarden EM, Burger EH, Nijweide PJ. Function of osteocytes in bone. J Cell Biochem. 1994;55(3):287–299. doi: 10.1002/jcb.240550304. [DOI] [PubMed] [Google Scholar]
- 31.Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26(2):229–238. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR, Staehling-Hampton K, Appleby M, Brunkow ME, Latham JA. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003;22(23):6267–6276. doi: 10.1093/emboj/cdg599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Bezooijen RL, Roelen BA, Visser A, van dWP, de WE, Karperien M, Hamersma H, Papapoulos SE, ten DP, Lowik CW. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med. 2004;199(6):805–814. doi: 10.1084/jem.20031454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bellido T, Ali AA, Gubrij I, Plotkin LI, Fu Q, O'Brien CA, Manolagas SC, Jilka RL. Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology. 2005;146(11):4577–4583. doi: 10.1210/en.2005-0239. [DOI] [PubMed] [Google Scholar]
- 35.Semenov M, Tamai K, He X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem. 2005;280(29):26770–26775. doi: 10.1074/jbc.M504308200. [DOI] [PubMed] [Google Scholar]
- 36.Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, Harris SE, Wu D. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005;280(20):19883–19887. doi: 10.1074/jbc.M413274200. [DOI] [PubMed] [Google Scholar]
- 37.Choi HY, Dieckmann M, Herz J, Niemeier A. Lrp4, a novel receptor for Dickkopf 1 and sclerostin, is expressed by osteoblasts and regulates bone growth and turnover in vivo. PLoS One. 2009;4(11):e7930. doi: 10.1371/journal.pone.0007930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brunkow ME, Gardner JC, Van NJ, Paeper BW, Kovacevich BR, Proll S, Skonier JE, Zhao L, Sabo PJ, Fu Y, Alisch RS, Gillett L, Colbert T, Tacconi P, Galas D, Hamersma H, Beighton P, Mulligan J. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet. 2001;68(3):577–589. doi: 10.1086/318811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balemans W, Ebeling M, Patel N, Van HE, Olson P, Dioszegi M, Lacza C, Wuyts W, Van Den EJ, Willems P, Paes-Alves AF, Hill S, Bueno M, Ramos FJ, Tacconi P, Dikkers FG, Stratakis C, Lindpaintner K, Vickery B, Foernzler D, Van HW. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST) Hum Mol Genet. 2001;10(5):537–543. doi: 10.1093/hmg/10.5.537. [DOI] [PubMed] [Google Scholar]
- 40.Loots GG, Kneissel M, Keller H, Baptist M, Chang J, Collette NM, Ovcharenko D, Plajzer-Frick I, Rubin EM. Genomic deletion of a long-range bone enhancer misregulates sclerostin in Van Buchem disease. Genome Res. 2005;15(7):928–935. doi: 10.1101/gr.3437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, D'Agostin D, Kurahara C, Gao Y, Cao J, Gong J, Asuncion F, Barrero M, Warmington K, Dwyer D, Stolina M, Morony S, Sarosi I, Kostenuik PJ, Lacey DL, Simonet WS, Ke HZ, Paszty C. Targeted Deletion of the Sclerostin Gene in Mice Results in Increased Bone Formation and Bone Strength*. J Bone Miner Res. 2008 doi: 10.1359/jbmr.080216. [DOI] [PubMed] [Google Scholar]
- 42.Costa AG, Bilezikian JP, Lewiecki EM. Update on romosozumab : a humanized monoclonal antibody to sclerostin. Expert Opin Biol Ther. 2014;14(5):697–707. doi: 10.1517/14712598.2014.895808. [DOI] [PubMed] [Google Scholar]
- 43.Lewiecki EM. Sclerostin monoclonal antibody therapy with AMG 785: a potential treatment for osteoporosis. Expert Opin Biol Ther. 2011;11(1):117–127. doi: 10.1517/14712598.2011.540565. [DOI] [PubMed] [Google Scholar]
- 44.Lewiecki EM. Sclerostin: a novel target for intervention in the treatment of osteoporosis. Discov Med. 2011;12(65):263–273. [PubMed] [Google Scholar]
- 45.Keller H, Kneissel M. SOST is a target gene for PTH in bone. Bone. 2005;37(2):148–158. doi: 10.1016/j.bone.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 46.Leupin O, Kramer I, Collette NM, Loots GG, Natt F, Kneissel M, Keller H. Control of the SOST bone enhancer by PTH using MEF2 transcription factors. J Bone Miner Res. 2007;22(12):1957–1967. doi: 10.1359/jbmr.070804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gooi JH, Pompolo S, Karsdal MA, Kulkarni NH, Kalajzic I, McAhren SH, Han B, Onyia JE, Ho PW, Gillespie MT, Walsh NC, Chia LY, Quinn JM, Martin TJ, Sims NA. Calcitonin impairs the anabolic effect of PTH in young rats and stimulates expression of sclerostin by osteocytes. Bone. 2010;46(6):1486–1497. doi: 10.1016/j.bone.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 48.Silvestrini G, Ballanti P, Leopizzi M, Sebastiani M, Berni S, Di VM, Bonucci E. Effects of intermittent parathyroid hormone (PTH) administration on SOST mRNA and protein in rat bone. J Mol Histol. 2007;38(4):261–269. doi: 10.1007/s10735-007-9096-3. [DOI] [PubMed] [Google Scholar]
- 49.Mirza FS, Padhi ID, Raisz LG, Lorenzo JA. Serum sclerostin levels negatively correlate with parathyroid hormone levels and free estrogen index in postmenopausal women. J Clin Endocrinol Metab. 2010;95(4):1991–1997. doi: 10.1210/jc.2009-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drake MT, Srinivasan B, Modder UI, Peterson JM, McCready LK, Riggs BL, Dwyer D, Stolina M, Kostenuik P, Khosla S. Effects of parathyroid hormone treatment on circulating sclerostin levels in postmenopausal women. J Clin Endocrinol Metab. 2010;95(11):5056–5062. doi: 10.1210/jc.2010-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kramer I, Baertschi S, Halleux C, Keller H, Kneissel M. Mef2c deletion in osteocytes results in increased bone mass. J Bone Miner Res. 2012;27(2):360–373. doi: 10.1002/jbmr.1492. [DOI] [PubMed] [Google Scholar]
- 52.Jia HB, Ma JX, Ma XL, Yu JT, Feng R, Xu LY, Wang J, Xing D, Zhu SW, Wang Y. Estrogen alone or in combination with parathyroid hormone can decrease vertebral MEF2 and sclerostin expression and increase vertebral bone mass in ovariectomized rats. Osteoporos Int. 2014;25(12):2743–2754. doi: 10.1007/s00198-014-2818-y. [DOI] [PubMed] [Google Scholar]
- 53.Bonnet N, Conway SJ, Ferrari SL. Regulation of beta catenin signaling and parathyroid hormone anabolic effects in bone by the matricellular protein periostin. Proc Natl Acad Sci U S A. 2012;109(37):15048–15053. doi: 10.1073/pnas.1203085109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Collette NM, Genetos DC, Economides AN, Xie L, Shahnazari M, Yao W, Lane NE, Harland RM, Loots GG. Targeted deletion of Sost distal enhancer increases bone formation and bone mass. Proc Natl Acad Sci U S A. 2012;109(35):14092–14097. doi: 10.1073/pnas.1207188109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325(5942):834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 56.Baertschi S, Baur N, Lueders-Lefevre V, Voshol J, Keller H. Class I and IIa histone deacetylases have opposite effects on sclerostin gene regulation. J Biol Chem. 2014;289(36):24995–25009. doi: 10.1074/jbc.M114.564997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wein MN, Spatz J, Nishimori S, Doench J, Root D, Babij P, Nagano K, Baron R, Brooks D, Bouxsein M, Pajevic PD, Kronenberg HM. HDAC5 controls MEF2C-driven sclerostin expression in osteocytes. J Bone Miner Res. 2014 doi: 10.1002/jbmr.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rivadeneira F, Styrkarsdottir U, Estrada K, Halldorsson BV, Hsu YH, Richards JB, Zillikens MC, et al. Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat Genet. 2009;41(11):1199–1206. doi: 10.1038/ng.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kramer I, Keller H, Leupin O, Kneissel M. Does osteocytic SOST suppression mediate PTH bone anabolism? Trends Endocrinol Metab. 2010;21(4):237–244. doi: 10.1016/j.tem.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 60.Zylstra CR, Wan C, VanKoevering KK, Sanders AK, Lindvall C, Clemens TL, Williams BO. Gene targeting approaches in mice: assessing the roles of LRP5 and LRP6 in osteoblasts. J Musculoskelet Neuronal Interact. 2008;8(4):291–293. [PubMed] [Google Scholar]
- 61.Joeng KS, Schumacher CA, Zylstra-Diegel CR, Long F, Williams BO. Lrp5 and Lrp6 redundantly control skeletal development in the mouse embryo. Dev Biol. 2011;359(2):222–229. doi: 10.1016/j.ydbio.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang M, Xuan S, Bouxsein ML, von SD, Akeno N, Faugere MC, Malluche H, Zhao G, Rosen CJ, Efstratiadis A, Clemens TL. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem. 2002;277(46):44005–44012. doi: 10.1074/jbc.M208265200. [DOI] [PubMed] [Google Scholar]
- 63.Potthoff MJ, Olson EN. MEF2: a central regulator of diverse developmental programs. Development. 2007;134(23):4131–4140. doi: 10.1242/dev.008367. [DOI] [PubMed] [Google Scholar]
- 64.Sawakami K, Robling AG, Ai M, Pitner ND, Liu D, Warden SJ, Li J, Maye P, Rowe DW, Duncan RL, Warman ML, Turner CH. The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J Biol Chem. 2006;281(33):23698–23711. doi: 10.1074/jbc.M601000200. [DOI] [PubMed] [Google Scholar]
- 65.Iwaniec UT, Wronski TJ, Liu J, Rivera MF, Arzaga RR, Hansen G, Brommage R. PTH stimulates bone formation in mice deficient in Lrp5. J Bone Miner Res. 2007;22(3):394–402. doi: 10.1359/jbmr.061118. [DOI] [PubMed] [Google Scholar]
- 66.Loots GG, Keller H, Leupin O, Murugesh D, Collette NM, Genetos DC. TGF-beta regulates sclerostin expression via the ECR5 enhancer. Bone. 2012;50(3):663–669. doi: 10.1016/j.bone.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Genetos DC, Yellowley CE, Loots GG. Prostaglandin E2 signals through PTGER2 to regulate sclerostin expression. PLoS One. 2011;6(3):e17772. doi: 10.1371/journal.pone.0017772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Obri A, Makinistoglu MP, Zhang H, Karsenty G. HDAC4 integrates PTH and sympathetic signaling in osteoblasts. J Cell Biol. 2014;205(6):771–780. doi: 10.1083/jcb.201403138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li C, Williams BO, Cao X, Wan M. LRP6 in mesenchymal stem cells is required for bone formation during bone growth and bone remodeling. Bone Res. 2014;2:43–54. doi: 10.1038/boneres.2014.6. 14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


