Abstract
Ductal carcinoma in situ (DCIS) is a non-invasive breast cancer that comprises approximately 20% of new breast cancer diagnoses. DCIS is predominantly detected by screening mammography prior to the development of any clinical symptoms. Prognosis following a DCIS diagnosis is excellent, due to both the availability of effective treatments and the frequently benign nature of the disease. However, a DCIS diagnosis and its treatment have psychological and physical impacts that often lead to adverse changes in health-related behaviors, including changes in physical activity, body weight, alcohol intake, and smoking, which may represent a greater threat to the woman's overall health than the DCIS itself. Depending on age at diagnosis, women diagnosed with DCIS are 3-13 times more likely to die from non-breast cancer related causes, such as cardiovascular disease, than from breast cancer. Thus, the maintenance and improvement of healthy behaviors that influence a variety of outcomes after diagnosis may warrant increased attention during DCIS management. This may also represent an important opportunity to promote the adoption of healthy behaviors, given that DCIS carries the psychological impact of a cancer diagnosis but also a favorable prognosis. Particular focus is needed to address these issues in vulnerable patient subgroups with pre-existing higher rates of unhealthy behaviors and demonstrated health disparities.
Introduction
Breast cancer mortality has declined steadily in the US over the past 20 years due to advances in population screening and the development of new treatments.1 However, overdiagnosis has emerged as an unintended consequence and an important harm associated with mammography screening for breast cancer.2 Various lines of evidence suggest that anywhere between 5-40% of early stage screen-detected breast cancers would never have emerged clinically if they had not been detected through screening.3 Concerns about overdiagnosis are heightened for ductal carcinoma in situ (DCIS), the earliest stage of breast cancer. Two decades of increased screening, diagnosis, and treatment of DCIS has not led to a substantial reduction in invasive cancer incidence, suggesting that a large proportion of DCIS cases would not progress if left undetected.4 DCIS is a non-obligate precursor of invasive breast cancer and is detected predominantly by screening mammography among women who have no clinical symptoms.5 It is a heterogeneous diagnosis, with variations in histopathological features, radiological characteristics, natural history, and molecular marker expression.6 Large increases in the incidence of DCIS began in the early 1980s (Figure 1), concurrent with the widespread adoption of screening mammography.7 Every year over 60,000 new DCIS cases are diagnosed,8 representing 18% of new breast cancer diagnoses overall,9 with projections that by 2016 there will be over 1 million women living with a DCIS diagnosis in the US.10 While the prevalence of DCIS continues to increase, the prognosis is excellent. A recent analysis demonstrated that only 3.2% of women diagnosed with DCIS die from breast cancer over 20 years of follow-up.11 As such, DCIS patients are a growing cancer survivor population at risk for competing causes of death. Modification of health-related behaviors may promote breast-cancer specific survivorship as well as protect against competing causes of death. Many health-related behaviors are associated with reduced risk of breast cancer recurrence in addition to decreased risk of a wide array of other common health outcomes (e.g., cardiovascular disease). Importantly, a DCIS diagnosis appears to have a comparable psychological impact to that of a localized invasive breast cancer diagnosis, which may include increased patient motivation to adopt health-improving strategies.12 Thus, DCIS management may offer a unique opportunity for health care providers to assist a large number of patients in adopting healthy behaviors that improve both breast cancer and non-breast-cancer outcomes.
Figure 1.

Annual age-adjusted incidence of ductal carcinoma in situ. Data from the Surveillance, Epidemiology, and End Results (SEER) Program.115
Health Outcomes after a DCIS Diagnosis
Analyses of data from the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) program have demonstrated that women diagnosed with DCIS are more likely to die from other causes compared to their risk of dying from breast cancer. As with the general population, cardiovascular disease is the leading cause of mortality among women with DCIS. Ernster et al. found that among women diagnosed with DCIS from 1978-1989, 11% of deaths were due to breast cancer while 32% were due to cardiovascular disease (CVD).13 In a more recent analysis of cases diagnosed between 1978 and 2010, the cumulative risk of cause-specific death at 20 years of follow-up was 3.2% for death due to breast cancer compared to 13.2% for death due to cardiovascular disease and 23.2% for death due to other causes.11 Notably, the relative frequency of other cause death compared to breast cancer death increased with age at DCIS diagnosis (Figure 2). Women diagnosed with DCIS between the ages of 40-49 were approximately 3 times more likely to die from other causes than from breast cancer; women diagnosed at ages 60-69 were nearly 13 times more likely to die from other causes than breast cancer. This phenomenon occurs because the risk of CVD and other cause mortality increases more rapidly with age compared to the risk of breast cancer mortality after a DCIS diagnosis.
Figure 2.
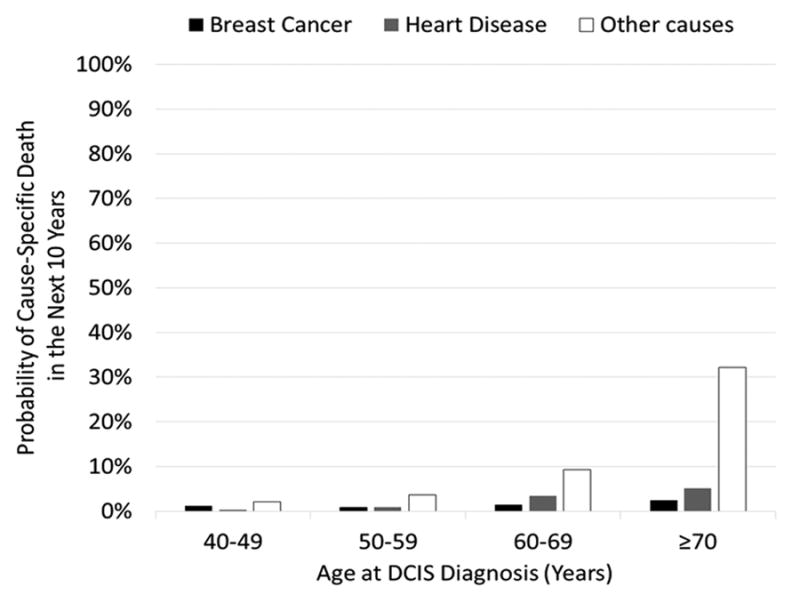
Ten-year cumulative incidence of death due to breast cancer, heart disease, and other causes following a DCIS diagnosis. Data from the Surveillance, Epidemiology, and End Results (SEER) Program.11
Health-related Quality of Life and the Psychological Impact of a DCIS Diagnosis
Despite having an excellent prognosis, women with DCIS experience substantial reductions in health-related quality of life, comparable to women with localized invasive breast cancer.14 These generally are more prominent among the mental, rather than physical, domains of health-related quality of life. In the Nurses' Health Study, the impact of a DCIS diagnosis was examined using the SF-36 health-related quality of life survey.15 Clinically significant declines in social functioning and mental health were observed within the six months following diagnosis. Small declines in the domains of role limitations due to physical problems and vitality were also observed in the first four years after a diagnosis. In a separate cohort of women with DCIS, notable declines were observed in general health, vitality, and mental health at 9 and 18 months after diagnosis, compared to pre-diagnosis scores.16 In comparison to population controls, patients with DCIS had lower emotional functioning, general health, vitality, sexual interest, poorer mental health, and higher rates of depression.17
These decrements in mental health-related quality of life likely stem from fear of breast cancer recurrence and mortality. Importantly, levels of fear appear similar in women with DCIS and women with more advanced diagnoses. In a study assessing factors related to fear of recurrence, 29% of DCIS patients reported moderate to high fear, which was not significantly different from patients diagnosed with stage IIA breast cancer.18 Fear of recurrence is known to affect psychological state and quality of life, through its association with increased anxiety, depression, and functional impairments.19 In a separate study, it was found that risk perceptions among women with DCIS were overestimated and did not change over an 18 month follow-up period from diagnosis, and about 10% of women experienced substantial anxiety related to these misperceptions.16 Furthermore, using the Hospital Anxiety and Depression Scale, Kennedy et al. found that about 50% of women with DCIS experienced problematic anxiety at diagnosis, which persisted through 9 months of follow up, with 33.3% of women continuing to experience high levels of anxiety.20 Finally, Rakovitch et al. found that 56% of DCIS patients exhibited any level of anxiety, which was similar to anxiety rates among women with early invasive breast cancer.12
Women in the Wisconsin In Situ Cohort (WISC) study were 57% more likely to use antidepressants after their DCIS diagnosis than they were one year prior to diagnosis.21 This is consistent with data from invasive breast cancer populations, which have found depression rates as high as 46% among survivors.22 Depression among women with breast cancer is correlated with a lower quality of life in physical, emotional, and social dimensions.23 Additionally, preliminary data from WISC suggest that DCIS cases experience declines in mental health that worsen over time since their diagnosis. Similar patterns were observed across all treatment groups and were strongest among women diagnosed at a younger age (<50 years). These declines contrast the typical pattern of increasing mental health measures with aging in the general population. There was little variation in physical quality of life scores according to time since diagnosis, and overall DCIS cases appeared to report similar physical quality of life as controls from the general population of the same age.24
Health-related Behaviors after a DCIS Diagnosis
There is evidence that the decline in mental health-related quality of life following a DCIS diagnosis is accompanied by adverse changes in health-related behaviors. The WISC study was designed to study changes in health-related behaviors and their impacts on health outcomes among women diagnosed with in situ breast cancer.21, 25 Approximately 1900 DCIS cases diagnosed during 1997-2006 were recruited from the mandatory statewide cancer registry shortly after their diagnosis. A baseline interview was conducted, which asked patients to recall a number of health-related behaviors prior to diagnosis, report current health-related behaviors, and provide information on topics including treatments received, socio-demographics, and quality of life measures. Participation in the study was approximately 78%. Successive interviews or mailed surveys were conducted in 2 year increments during up to 15 years of follow-up, with greater than 72% participation at each round.
Body weight is a convenient measure that reflects the combined effects of health behaviors such as patient diet and physical activity, which are more challenging to measure in large population-based studies. In the WISC study, a DCIS diagnosis was associated with a 2.2 kg weight gain (beyond that associated with aging) that persisted throughout 10 years of follow-up.21 Similar findings were reported in the Health, Eating, Activity, and Lifestyle (HEAL) study that observed weight gain among women diagnosed with DCIS as well as those diagnosed with invasive breast cancer.26 Weight gain after diagnosis is likely impacted by a multiple factors related to diagnosis and its treatment. There is evidence that women tend to decrease physical activity levels after a DCIS diagnosis. In the HEAL study, women with DCIS decreased their total physical activity levels by about 4% per week and their vigorous physical activity by 33% per week in the year following diagnosis.27 A separate study found that at baseline 67.4% of women with DCIS reported infrequent vigorous physical activity, with only half of the study population performing any type of exercise more than two times per week.28
Side effects stemming from DCIS treatment are another possible contributor to weight gain following diagnosis. Surgical treatment of breast cancer, particularly mastectomy, can lead to range of motion deficits and lymphedema.29 Gho et al. found the most pronounced weight gain in women who underwent a mastectomy compared to other surgical treatments.30 Among women with DCIS, those who were treated with mastectomy were more likely to report a decrease in physical activity levels (HR 2.4, 95% CI 1.3-4.4) compared to women treated with a lumpectomy or excisional biopsy.28 Among women with early stage breast cancer treated with breast conserving surgery and radiation, the two most common side effects during radiotherapy treatment were fatigue and difficulty sleeping, with up to 67% of women rating their fatigue as a least moderately distressing.31 Fatigue has been demonstrated to be a barrier to physical activity among breast cancer survivors,32 and the fatigue caused by DCIS treatments is a possible contributor to the decrease in physical activity and weight gain exhibited in this population as well. Finally, use of tamoxifen therapy for breast cancer has been associated with weight gain, though the evidence remains inconsistent.33 In the WISC cohort, weight gain was highest among women who received tamoxifen, yet substantial weight gain was also observed in women who had not.21
Emotional and mental health changes may also contribute to weight gain in the DCIS population. As indicated earlier, antidepressant use and depression are prevalent among women with a DCIS diagnosis. It is difficult to disentangle the relationship of antidepressant use with depression among DCIS survivors, as antidepressants are also used in the breast cancer survivor population to manage hot flashes and menopausal symptoms.34 However, both depression and use of antidepressants are associated with weight gain,35 thus linking the mental health changes and medication use among women with DCIS to their tendency to gain weight, and further contributing to their risk of developing other chronic conditions. The HEAL study found that women who had high anxiety levels at the time of DCIS diagnosis were more likely to experience a decrease in physical activity levels (HR 2.1, 95% CI 1.1-4.1).28
In contrast to the adverse changes in physical activity and body weight, women with DCIS tend to make positive changes in smoking cessation after diagnosis. In the WISC cohort, 14.5% of women were smokers one year prior to DCIS diagnosis. Among these smokers, 38% reported quitting smoking after diagnosis.21 Similarly, in the invasive breast cancer population, Skeie et al. found that 33.4% of baseline smokers had quit smoking after diagnosis.36 Another study found that mean tobacco consumption decreased by 1.1 grams per day at up to 6 years after a breast cancer diagnosis and that the percentage of women who did not smoke increased by 7%.37 These positive changes may in part be due to physician's focus on smoking cessation. In a recent analysis of risk factors in cancer survivors, including breast cancer survivors, it was found that 87% of current smokers reported discussing smoking cessation with their physicians. In this same cohort, overweight and inactivity were more prevalent risk factors than smoking, however, only 61% and 68% of survivors reported discussions of diet and exercise, respectively, with their physician.38 Overall, these data suggest that health behavior change interventions can be successful in the DCIS population when appropriate attention is given to them by patients and providers.
Evidence is sparse regarding changes in other health-related behaviors after DCIS, including alcohol consumption and diet. In the only known study addressing change in these variables in the DCIS population specifically, no statistically significant differences were noted before and after diagnosis.21 There is additional data on change in alcohol use and diet after an invasive breast cancer diagnosis, though mainly from European studies. Among women in a Danish cohort with grades I-III breast cancer, alcohol consumption increased by 0.6 grams/day after diagnosis.37 In the Norwegian Women and Cancer cohort study, Skeie et al. found that after a breast cancer diagnosis, women significantly increased their fruit and vegetable consumption, compared to women who remained cancer-free.36
The Importance of Health-Related Behaviors after a DCIS Diagnosis
The association between several health-related behaviors and breast cancer risk has long been recognized.39, 40 More recently, evidence has emerged to demonstrate that these lifestyle factors are also associated with disease progression after a breast cancer diagnosis.41-43 Weight gain of ≥ 2 kg after diagnosis has been associated with a 50% increased risk of recurrence.41 Physical activity has been shown to decrease risk of recurrence, with women participating in 9-14.9 metabolic equivalent-hours (MET-hrs) per week reducing their recurrence risk by about 50%, compared to women participating in <3 MET-hrs per week.44 Drinking ≥ 6 grams/day of alcohol has been associated with an increased risk of recurrence of 35%, compared to no drinking.43 The evidence specific to DCIS remains sparse. An early study indicated that women in the upper decile of BMI were twice as likely to have a recurrence as those in the lower four deciles.45 This was not replicated in a recent analysis of data in the WISC cohort, though risk of a second breast cancer diagnosis after DCIS was elevated with increasing alcohol consumption.25
Given that women diagnosed with DCIS are more likely to die from causes other than breast cancer, it is important to consider the impact of health-related behaviors on other health outcomes in this population (Table 1). There are long established associations between physical inactivity and weight gain, smoking, and other health-related behaviors with a variety of health outcomes in the general population.46-49
Table 1.
Examples of associations between health-related behaviors and various health outcomes.
| Behavior | Association with Breast Cancer Recurrence | Association with CVD Incidence | Association with Diabetes Incidence | Association with Incidence of Other Cancers | Association with All-Cause Mortality |
|---|---|---|---|---|---|
| Lack of Physical Activity | ↑50%86 | ↑30-150%50, 55 | ↑55 -110%47, 50 | ↑20-55%52-54 | ↑50 -135%50, 87 |
| Obesity | ↑130-145%45, 88 | ↑50-220%89, 90 | ↑450-700%57, 91 | ↑25-155%39, 58 | ↑50-250%61, 92 |
| Smoking | ↑20%93 | ↑200-400%94 | ↑40%64 | ↑20-4000%63, 95, 96 | ↑20-240%97, 98 |
| Heavy Alcohol Consumption | ↑35-160%25, 43 | ↑140%99 | ↑65%100 | ↑20-150%40, 101, 102 | ↑60-120%103, 104 |
Compared to those who engage in leisure time physical activity, increased sedentary behavior is associated with increased risk of diabetes,50 hypertension,51 certain site-specific cancers,52-54 and CVD.55 Sedentary behavior and decreased physical activity have also been found to contribute to elevated risks of all-cause, CVD, and cancer mortality.56 Importantly, physical inactivity and sedentary behavior can lead to weight gain. Excess weight is associated with increased risk of multiple chronic diseases, including diabetes, hypertension, osteoarthritis, coronary heart disease, and cancer.46, 57, 58 Though modest, a 2.2kg change in body weight, as seen in the WISC cohort, contributes to all of the aforementioned chronic conditions. Weight gains of <5 kg have been associated with increases in women's risk of developing hypertension, diabetes, and coronary heart disease.59,60,61 Importantly, weight gain is also associated with increased risk of mortality from these conditions, as well as increased risk of all-cause mortality.61, 62
Interestingly, smoking cessation appears to be the most successful behavior change after DCIS diagnosis, despite the association of smoking with breast cancer risk or recurrence remaining unclear, 63 compared to the well-established associations of physical inactivity and BMI with breast cancer outcomes. Nevertheless, many women continue smoking after their DCIS diagnosis (e.g., 62% of those smoking prior to diagnosis in the WISC study continued to smoke).21 Given the well-known impact of smoking on several chronic conditions, this remains concerning. Compared to those who never smoked, current smokers are about 1.5 times more likely to develop diabetes,64 and 2-4 times more likely to develop coronary heart disease.65 Smoking is also a strong risk factor for countless other cancers.66
Vulnerable Populations
Data from the WISC study indicate that SES and living situation are key factors in determining the likelihood of undergoing adverse health behavior changes after a DCIS diagnosis.67 Women with a college degree and those living with a partner were about 30% less likely to gain >10% body weight after a DCIS diagnosis compared to high school graduates and those not living with a partner. Higher educational attainment, higher annual income, and living with a partner were also associated with lower likelihood of consistent sedentary behavior after a DCIS diagnosis. Similar factors affected alcohol use and smoking status after DCIS diagnosis. For example, women with a college degree were 2.4 times more likely to quit smoking after their DCIS diagnosis than women who had not attended college. A separate study demonstrated that women with medium or low SES were more likely to report increased anxiety and depression after a DCIS diagnosis than those with high SES.68 Furthermore, fear of recurrence after initial DCIS diagnosis was diminished in those patients with increased social support.69 While we are unaware of studies examining SES in relation to breast cancer mortality after a DCIS diagnosis, there is extensive literature demonstrating poorer outcomes among low SES women following an invasive breast cancer diagnosis.70, 71 These studies have generally found that SES disparities in breast cancer and all-cause mortality persist even after adjusting for screening utilization, cancer characteristics, and treatment factors; this suggests that other health equity factors, including patient health behaviors, are likely additional factors contributing to variations in outcome according to SES. Prior research suggests that potentially modifiable behavioral factors such as smoking, alcohol consumption, dietary patterns, and physical activity may correlate with socioeconomic differences in mortality.72 Interventions specifically targeted to individual health behaviors among women from vulnerable populations may be effective in improving outcomes and reducing health inequalities related to breast cancer.
An Opportunity for Intervention
Importantly, education and physician emphasis on health promoting behaviors are key components of capitalizing on the teachable moment that occurs after a cancer diagnosis.73, 74 A DCIS diagnosis may represent a particularly unique opportunity to intervene, given that it carries the psychological impact of a cancer diagnosis but an excellent prognosis. The success in smoking cessation following a DCIS diagnosis suggests that women with DCIS are receptive to positive lifestyle changes. The time following DCIS diagnosis may represent a key window for clinicians to educate patients on the benefits of physical activity and weight loss and introduce positive lifestyle behaviors. In many cases, this is currently a missed opportunity. Exercise, the most frequently discussed lifestyle behavior by physicians to DCIS patients, is only discussed with 53% of women with a DCIS diagnosis and weight was only discussed with 43% of DCIS patients.75 A wide array of interventions have been shown to be efficacious in improving health-related behaviors (Table 2). Interventions aimed at improvement of physical activity levels and weight management have been implemented successfully among women with an invasive breast cancer diagnosis,76, 77 as well as in mixed stage populations that included DCIS patients.78 Among breast cancer survivors, supervised exercise as well as home-based exercise and telephone coaching have been successful in increasing physical activity levels, with increases seen up to 271 minutes/week.79-81 Weight loss interventions, utilizing in person or telephone coaching have also been successful in this population with weight loss of up to 12.5 kg achieved.82-84 These studies demonstrate the potential for health behavior modification following a DCIS diagnosis. However, access to these successful interventions remains limited. The majority of these interventions require training and resources that are not be readily available to clinical care providers. Further studies are needed to evaluate effectiveness in community practice, cost-effectiveness, and dissemination strategies that require limited resources and training to make such interventions readily accessible to patients and providers.
Table 2.
Examples of effective interventions to improve health-related behaviors.
| Behavior | Strategy to Improve | Effectiveness |
|---|---|---|
| Lack of Physical Activity | Supervised aerobic exercise intervention | ↑45.4 -129 minutes/week79, 105 |
| Home based exercise program with weekly telephone counseling | ↑28.8 -271 minutes/week80, 81 | |
|
| ||
| Obesity | Telephone based diet and physical activity goal coaching | ↓3.1 - 12.5 kg82, 83 |
| In person diet, behavioral weight loss, and physical activity coaching | ↓3.3 -6.3 kg84, 106 | |
|
| ||
| Smoking | Internet and printed counseling intervention | ↓12.5 -15.6% smoking rate107, 108 |
| Clinician administered cognitive behavioral therapy and medication | ↓21 -47%109, 110 | |
|
| ||
| Heavy Alcohol | Peer or clinician led brief motivational intervention | ↓2.33 -3.32 drinks/week111, 112 |
| Internet/text message counseling intervention | ↓0.31 drinks/day113, ↓3.9 drinks/week114 | |
Evidence from the WISC cohort to date suggests that women with lower SES are particularly susceptible to adverse health behavior changes after a DCIS diagnosis. Importantly, there is also ample data indicating that socioeconomic disparities impact breast cancer survival. Thus, interventions may have the greatest impact if targeted at women belonging to these vulnerable populations. There is also evidence that women with low social support, including those not living with a partner, are at elevated risk of adverse health behavior change and thus may particularly benefit from an intervention. The potential benefit of targeting interventions to other groups is less clear. Further research is needed to identify patient sub-groups most likely to benefit from targeted interventions, and inform effective strategies in cancer survivorship.
Conclusion
Health outcomes for women with DCIS may be improved by adopting a more global outlook on threats to long term health. This will require a shift in the typical focus of patients, oncologists, and primary care providers during the management of DCIS. However, recognition of the low frequency of breast cancer mortality among women with DCIS and the comparatively higher rates of other causes of death should help to facilitate this shift. As early as the initial treatment decision, women may be counselled on the potential negative impacts of cancer therapy and encouraged to initiate lifestyle changes that will improve long-term health outcomes. The increased motivation of patients and heightened attention of medical providers during the time of a DCIS diagnosis may offer a strong opportunity to initiate healthy behaviors that will improve a wide range of health outcomes. Increased attention to health behaviors among women from lower socioeconomic backgrounds and other vulnerable populations may also provide a means to reduce observed disparities in health outcomes. DCIS has one of the highest cancer-specific survival rates of all cancers; as such, it may serve as an ideal setting for a targeted approach to positive lifestyle changes. Success in this framework could then provide justification for a similar approach with other types of cancer as well as other life-threatening disease as survival rates improve.
Acknowledgments
All authors contributed to the drafting and editing of this manuscript.
Funding: This work was supported in part by grants from the National Institutes of Health (P20GM103644, U54CA163303, R01CA067264, and P30CA014520).
Footnotes
Conflict of interest statement: The authors declare that there are no conflicts of interest.
References
- 1.Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, Mandelblatt JS, Yakovlev AY, Habbema JD, Feuer EJ. Effect of screening and adjuvant therapy on mortality from breast cancer. The New England journal of medicine. 2005;353:1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 2.The benefits and harms of breast cancer screening: An independent review. Lancet. 2012;380:1778–1786. doi: 10.1016/S0140-6736(12)61611-0. [DOI] [PubMed] [Google Scholar]
- 3.Etzioni R, Gulati R, Mallinger L, Mandelblatt J. Influence of study features and methods on overdiagnosis estimates in breast and prostate cancer screening. Ann Intern Med. 2013;158:831–838. doi: 10.7326/0003-4819-158-11-201306040-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esserman L, Shieh Y, Thompson I. Rethinking screening for breast cancer and prostate cancer. JAMA. 2009;302:1685–1692. doi: 10.1001/jama.2009.1498. [DOI] [PubMed] [Google Scholar]
- 5.Lee RJ, Vallow LA, McLaughlin SA, Tzou KS, Hines SL, Peterson JL. Ductal carcinoma in situ of the breast. International journal of surgical oncology. 2012;2012:123549. doi: 10.1155/2012/123549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mokbel K, Cutuli B. Heterogeneity of ductal carcinoma in situ and its effects on management. The lancet oncology. 2006;7:756–765. doi: 10.1016/S1470-2045(06)70861-0. [DOI] [PubMed] [Google Scholar]
- 7.Ernster VL, Barclay J, Kerlikowske K, Grady D, Henderson C. Incidence of and treatment for ductal carcinoma in situ of the breast. JAMA. 1996;275:913–918. [PubMed] [Google Scholar]
- 8.American Cancer Society. Cancer facts & figures 2009-2010. 2009 [Google Scholar]
- 9.Surveillance Epidemiology and End Results (SEER) Program. Seer*stat database: Incidence - seer 9 regs research data, nov 2009 sub (1973-2007) <katrina/rita population adjustment> - linked to county attributes - total u S., 1969-2007 counties, national cancer institute, dccps, surveillance research program, cancer statistics branch released april 2010, based on the november 2009 submission ( www.Seer.Cancer.Gov)
- 10.Sprague BL, Trentham-Dietz A. Prevalence of breast carcinoma in situ in the united states. JAMA. 2009;302:846–848. doi: 10.1001/jama.2009.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berkman A, B FC, Ades PA, Dickey S, Higgins ST, Trentham-Dietz A, Sprague BL, Lakoski SG. Racial differences in breast cancer, cardiovascular disease, and all-cause mortality among women with ductal carcinoma in situ of the breast. Breast cancer research and treatment. 2014;148:407–413. doi: 10.1007/s10549-014-3168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rakovitch E, Franssen E, Kim J, Ackerman I, Pignol JP, Paszat L, Pritchard KI, Ho C, Redelmeier DA. A comparison of risk perception and psychological morbidity in women with ductal carcinoma in situ and early invasive breast cancer. Breast Cancer Res Treat. 2003;77:285–293. doi: 10.1023/a:1021853302033. [DOI] [PubMed] [Google Scholar]
- 13.Ernster VL, Barclay J, Kerlikowske K, Wilkie H, Ballard-Barbash R. Mortality among women with ductal carcinoma in situ of the breast in the population-based surveillance, epidemiology and end results program. Arch Intern Med. 2000;160:953–958. doi: 10.1001/archinte.160.7.953. [DOI] [PubMed] [Google Scholar]
- 14.Ganz PA. Quality-of-life issues in patients with ductal carcinoma in situ. J Natl Cancer Inst Monogr. 2010;2010:218–222. doi: 10.1093/jncimonographs/lgq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nekhlyudov L, Kroenke CH, Jung I, Holmes MD, Colditz GA. Prospective changes in quality of life after ductal carcinoma-in-situ: Results from the nurses' health study. J Clin Oncol. 2006;24:2822–2827. doi: 10.1200/JCO.2005.04.6219. [DOI] [PubMed] [Google Scholar]
- 16.Partridge A, Adloff K, Blood E, Dees EC, Kaelin C, Golshan M, Ligibel J, de Moor JS, Weeks J, Emmons K, Winer E. Risk perceptions and psychosocial outcomes of women with ductal carcinoma in situ: Longitudinal results from a cohort study. J Natl Cancer Inst. 2008;100:243–251. doi: 10.1093/jnci/djn010. [DOI] [PubMed] [Google Scholar]
- 17.Claus EB, Petruzella S, Carter D, Kasl S. Quality of life for women diagnosed with breast carcinoma in situ. J Clin Oncol. 2006;24:4875–4881. doi: 10.1200/JCO.2005.05.2290. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Perez M, Schootman M, Aft RL, Gillanders WE, Jeffe DB. Correlates of fear of cancer recurrence in women with ductal carcinoma in situ and early invasive breast cancer. Breast Cancer Res Treat. 2011;130:165–173. doi: 10.1007/s10549-011-1551-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simard S, Savard J, Ivers H. Fear of cancer recurrence: Specific profiles and nature of intrusive thoughts. Journal of cancer survivorship : research and practice. 2010;4:361–371. doi: 10.1007/s11764-010-0136-8. [DOI] [PubMed] [Google Scholar]
- 20.Kennedy F, Harcourt D, Rumsey N, White P. The psychosocial impact of ductal carcinoma in situ (dcis): A longitudinal prospective study. Breast. 2010;19:382–387. doi: 10.1016/j.breast.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 21.Sprague BL, Trentham-Dietz A, Nichols HB, Hampton JM, Newcomb PA. Change in lifestyle behaviors and medication use after a diagnosis of ductal carcinoma in situ. Breast Cancer Res Treat. 2010;124:487–495. doi: 10.1007/s10549-010-0869-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004:57–71. doi: 10.1093/jncimonographs/lgh014. [DOI] [PubMed] [Google Scholar]
- 23.Reich M, Lesur A, Perdrizet-Chevallier C. Depression, quality of life and breast cancer: A review of the literature. Breast Cancer Res Treat. 2008;110:9–17. doi: 10.1007/s10549-007-9706-5. [DOI] [PubMed] [Google Scholar]
- 24.Hart V, Lakoski SG, Hampton J, Trentham-Dietz A, Sprague BL. Trends in health related quality of life following diagnosis of ductal carcinoma in situ. In preparation. American Society of Preventive Oncology. 2014 Mar; doi: 10.1200/JCO.2015.62.7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McLaughlin VH, Trentham-Dietz A, Hampton JM, Newcomb PA, Sprague BL. Lifestyle factors and the risk of a second breast cancer after ductal carcinoma in situ. Cancer Epidemiol Biomarkers Prev. 2014;23:450–460. doi: 10.1158/1055-9965.EPI-13-0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Irwin ML, McTiernan A, Baumgartner RN, Baumgartner KB, Bernstein L, Gilliland FD, Ballard-Barbash R. Changes in body fat and weight after a breast cancer diagnosis: Influence of demographic, prognostic, and lifestyle factors. J Clin Oncol. 2005;23:774–782. doi: 10.1200/JCO.2005.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Irwin ML, Crumley D, McTiernan A, Bernstein L, Baumgartner R, Gilliland FD, Kriska A, Ballard-Barbash R. Physical activity levels before and after a diagnosis of breast carcinoma: The health, eating, activity, and lifestyle (heal) study. Cancer. 2003;97:1746–1757. doi: 10.1002/cncr.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ligibel JA, Partridge A, Giobbie-Hurder A, Golshan M, Emmons K, Winer EP. Physical activity behaviors in women with newly diagnosed ductal carcinoma-in-situ. Ann Surg Oncol. 2009;16:106–112. doi: 10.1245/s10434-008-0174-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayes SC, Johansson K, Stout NL, Prosnitz R, Armer JM, Gabram S, Schmitz KH. Upper-body morbidity after breast cancer: Incidence and evidence for evaluation, prevention, and management within a prospective surveillance model of care. Cancer. 2012;118:2237–2249. doi: 10.1002/cncr.27467. [DOI] [PubMed] [Google Scholar]
- 30.Gho SA, Steele JR, Jones SC, Munro BJ. Self-reported side effects of breast cancer treatment: A cross-sectional study of incidence, associations, and the influence of exercise. Cancer Causes Control. 2013;24:517–528. doi: 10.1007/s10552-012-0142-4. [DOI] [PubMed] [Google Scholar]
- 31.Sundaresan P, Sullivan L, Pendlebury S, Kirby A, Rodger A, Joseph D, Campbell I, Dhillon HM, Stockler MR. Patients' perceptions of health-related quality of life during and after adjuvant radiotherapy for t1n0m0 breast cancer. Clinical oncology. 2014 doi: 10.1016/j.clon.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Ventura EE, Ganz PA, Bower JE, Abascal L, Petersen L, Stanton AL, Crespi CM. Barriers to physical activity and healthy eating in young breast cancer survivors: Modifiable risk factors and associations with body mass index. Breast Cancer Res Treat. 2013;142:423–433. doi: 10.1007/s10549-013-2749-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sestak I, Harvie M, Howell A, Forbes JF, Dowsett M, Cuzick J. Weight change associated with anastrozole and tamoxifen treatment in postmenopausal women with or at high risk of developing breast cancer. Breast Cancer Res Treat. 2012;134:727–734. doi: 10.1007/s10549-012-2085-6. [DOI] [PubMed] [Google Scholar]
- 34.Bordeleau L, Pritchard K, Goodwin P, Loprinzi C. Therapeutic options for the management of hot flashes in breast cancer survivors: An evidence-based review. Clinical therapeutics. 2007;29:230–241. doi: 10.1016/j.clinthera.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Grundy A, Cotterchio M, Kirsh VA, Kreiger N. Associations between anxiety, depression, antidepressant medication, obesity and weight gain among canadian women. PloS one. 2014;9:e99780. doi: 10.1371/journal.pone.0099780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skeie G, Hjartaker A, Braaten T, Lund E. Dietary change among breast and colorectal cancer survivors and cancer-free women in the norwegian women and cancer cohort study. Cancer Causes Control. 2009;20:1955–1966. doi: 10.1007/s10552-009-9390-3. [DOI] [PubMed] [Google Scholar]
- 37.Bidstrup PE, Dalton SO, Christensen J, Tjonneland A, Larsen SB, Karlsen R, Brewster A, Bondy M, Johansen C. Changes in body mass index and alcohol and tobacco consumption among breast cancer survivors and cancer-free women: A prospective study in the danish diet, cancer and health cohort. Acta oncologica. 2013;52:327–335. doi: 10.3109/0284186X.2012.746466. [DOI] [PubMed] [Google Scholar]
- 38.Weaver KE, Foraker RE, Alfano CM, Rowland JH, Arora NK, Bellizzi KM, Hamilton AS, Oakley-Girvan I, Keel G, Aziz NM. Cardiovascular risk factors among long-term survivors of breast, prostate, colorectal, and gynecologic cancers: A gap in survivorship care? Journal of cancer survivorship : research and practice. 2013;7:253–261. doi: 10.1007/s11764-013-0267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Catsburg C, Kirsh VA, Soskolne CL, Kreiger N, Bruce E, Ho T, Leatherdale ST, Rohan TE. Associations between anthropometric characteristics, physical activity, and breast cancer risk in a canadian cohort. Breast Cancer Res Treat. 2014;145:545–552. doi: 10.1007/s10549-014-2973-z. [DOI] [PubMed] [Google Scholar]
- 40.Scoccianti C, Lauby-Secretan B, Bello PY, Chajes V, Romieu I. Female breast cancer and alcohol consumption: A review of the literature. American journal of preventive medicine. 2014;46:S16–25. doi: 10.1016/j.amepre.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 41.Kroenke CH, Chen WY, Rosner B, Holmes MD. Weight, weight gain, and survival after breast cancer diagnosis. J Clin Oncol. 2005;23:1370–1378. doi: 10.1200/JCO.2005.01.079. [DOI] [PubMed] [Google Scholar]
- 42.Bertram LA, Stefanick ML, Saquib N, Natarajan L, Patterson RE, Bardwell W, Flatt SW, Newman VA, Rock CL, Thomson CA, Pierce JP. Physical activity, additional breast cancer events, and mortality among early-stage breast cancer survivors: Findings from the whel study. Cancer Causes Control. 2011;22:427–435. doi: 10.1007/s10552-010-9714-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kwan ML, Kushi LH, Weltzien E, Tam EK, Castillo A, Sweeney C, Caan BJ. Alcohol consumption and breast cancer recurrence and survival among women with early-stage breast cancer: The life after cancer epidemiology study. J Clin Oncol. 2010;28:4410–4416. doi: 10.1200/JCO.2010.29.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. Jama. 2005;293:2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 45.Habel LA, Daling JR, Newcomb PA, Self SG, Porter PL, Stanford JL, Seidel K, Weiss NS. Risk of recurrence after ductal carcinoma in situ of the breast. Cancer Epidemiol Biomarkers Prev. 1998;7:689–696. [PubMed] [Google Scholar]
- 46.Wilson PW, D'Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: The framingham experience. Arch Intern Med. 2002;162:1867–1872. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 47.Hamilton MT, Hamilton DG, Zderic TW. Sedentary behavior as a mediator of type 2 diabetes. Medicine and sport science. 2014;60:11–26. doi: 10.1159/000357332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Folsom AR, Caspersen CJ, Taylor HL, Jacobs DR, Jr, Luepker RV, Gomez-Marin O, Gillum RF, Blackburn H. Leisure time physical activity and its relationship to coronary risk factors in a population-based sample. The minnesota heart survey. Am J Epidemiol. 1985;121:570–579. doi: 10.1093/oxfordjournals.aje.a114035. [DOI] [PubMed] [Google Scholar]
- 49.Villablanca AC, McDonald JM, Rutledge JC. Smoking and cardiovascular disease. Clinics in chest medicine. 2000;21:159–172. doi: 10.1016/s0272-5231(05)70015-0. [DOI] [PubMed] [Google Scholar]
- 50.Wilmot EG, Edwardson CL, Achana FA, Davies MJ, Gorely T, Gray LJ, Khunti K, Yates T, Biddle SJ. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: Systematic review and meta-analysis. Diabetologia. 2012;55:2895–2905. doi: 10.1007/s00125-012-2677-z. [DOI] [PubMed] [Google Scholar]
- 51.Huai P, Xun H, Reilly KH, Wang Y, Ma W, Xi B. Physical activity and risk of hypertension: A meta-analysis of prospective cohort studies. Hypertension. 2013;62:1021–1026. doi: 10.1161/HYPERTENSIONAHA.113.01965. [DOI] [PubMed] [Google Scholar]
- 52.Gierach GL, Chang SC, Brinton LA, Lacey JV, Jr, Hollenbeck AR, Schatzkin A, Leitzmann MF. Physical activity, sedentary behavior, and endometrial cancer risk in the nih-aarp diet and health study. International journal of cancer Journal international du cancer. 2009;124:2139–2147. doi: 10.1002/ijc.24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patel AV, Rodriguez C, Pavluck AL, Thun MJ, Calle EE. Recreational physical activity and sedentary behavior in relation to ovarian cancer risk in a large cohort of us women. Am J Epidemiol. 2006;163:709–716. doi: 10.1093/aje/kwj098. [DOI] [PubMed] [Google Scholar]
- 54.Howard RA, Freedman DM, Park Y, Hollenbeck A, Schatzkin A, Leitzmann MF. Physical activity, sedentary behavior, and the risk of colon and rectal cancer in the nih-aarp diet and health study. Cancer Causes Control. 2008;19:939–953. doi: 10.1007/s10552-008-9159-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chomistek AK, Manson JE, Stefanick ML, Lu B, Sands-Lincoln M, Going SB, Garcia L, Allison MA, Sims ST, LaMonte MJ, Johnson KC, Eaton CB. Relationship of sedentary behavior and physical activity to incident cardiovascular disease: Results from the women's health initiative. Journal of the American College of Cardiology. 2013;61:2346–2354. doi: 10.1016/j.jacc.2013.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim Y, Wilkens LR, Park SY, Goodman MT, Monroe KR, Kolonel LN. Association between various sedentary behaviours and all-cause, cardiovascular disease and cancer mortality: The multiethnic cohort study. Int J Epidemiol. 2013;42:1040–1056. doi: 10.1093/ije/dyt108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122:481–486. doi: 10.7326/0003-4819-122-7-199504010-00001. [DOI] [PubMed] [Google Scholar]
- 58.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 59.Huang Z, Willett WC, Manson JE, Rosner B, Stampfer MJ, Speizer FE, Colditz GA. Body weight, weight change, and risk for hypertension in women. Ann Intern Med. 1998;128:81–88. doi: 10.7326/0003-4819-128-2-199801150-00001. [DOI] [PubMed] [Google Scholar]
- 60.Resnick HE, Valsania P, Halter JB, Lin X. Relation of weight gain and weight loss on subsequent diabetes risk in overweight adults. Journal of epidemiology and community health. 2000;54:596–602. doi: 10.1136/jech.54.8.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Manson JE, Willett WC, Stampfer MJ, Colditz GA, Hunter DJ, Hankinson SE, Hennekens CH, Speizer FE. Body weight and mortality among women. The New England journal of medicine. 1995;333:677–685. doi: 10.1056/NEJM199509143331101. [DOI] [PubMed] [Google Scholar]
- 62.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028–2037. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 63.Gaudet MM, Gapstur SM, Sun J, Diver WR, Hannan LM, Thun MJ. Active smoking and breast cancer risk: Original cohort data and meta-analysis. J Natl Cancer Inst. 2013;105:515–525. doi: 10.1093/jnci/djt023. [DOI] [PubMed] [Google Scholar]
- 64.Yeh HC, Duncan BB, Schmidt MI, Wang NY, Brancati FL. Smoking, smoking cessation, and risk for type 2 diabetes mellitus: A cohort study. Ann Intern Med. 2010;152:10–17. doi: 10.7326/0003-4819-152-1-201001050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smoking & tobacco use. 2012 [Google Scholar]
- 66.Sasco AJ, Secretan MB, Straif K. Tobacco smoking and cancer: A brief review of recent epidemiological evidence. Lung cancer. 2004;45(Suppl 2):S3–9. doi: 10.1016/j.lungcan.2004.07.998. [DOI] [PubMed] [Google Scholar]
- 67.Khadanga SHV, Ba Y, Higgins ST, Ades PA, Trentham-Dietz A, Sprague BL, Lakoski SG. Living situation and socioeconomic factors are associated with change in health behavior after a diagnosis of ductal carcinoma in situ. 2014 submitted. [Google Scholar]
- 68.de Moor JS, Partridge AH, Winer EP, Ligibel J, Emmons KM. The role of socioeconomic status in adjustment after ductal carcinoma in situ. Cancer. 2010;116:1218–1225. doi: 10.1002/cncr.24832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu Y, Perez M, Schootman M, Aft RL, Gillanders WE, Ellis MJ, Jeffe DB. A longitudinal study of factors associated with perceived risk of recurrence in women with ductal carcinoma in situ and early-stage invasive breast cancer. Breast cancer research and treatment. 2010;124:835–844. doi: 10.1007/s10549-010-0912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sprague BL, Trentham-Dietz A, Gangnon RE, Ramchandani R, Hampton JM, Robert SA, Remington PL, Newcomb PA. Socioeconomic status and survival after an invasive breast cancer diagnosis. Cancer. 2011;117:1542–1551. doi: 10.1002/cncr.25589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Byers TE, Wolf HJ, Bauer KR, Bolick-Aldrich S, Chen VW, Finch JL, Fulton JP, Schymura MJ, Shen T, Van Heest S, Yin X Patterns of Care Study G. The impact of socioeconomic status on survival after cancer in the united states : Findings from the national program of cancer registries patterns of care study. Cancer. 2008;113:582–591. doi: 10.1002/cncr.23567. [DOI] [PubMed] [Google Scholar]
- 72.Stringhini S, Sabia S, Shipley M, Brunner E, Nabi H, Kivimaki M, Singh-Manoux A. Association of socioeconomic position with health behaviors and mortality. JAMA. 2010;303:1159–1166. doi: 10.1001/jama.2010.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gritz ER, Fingeret MC, Vidrine DJ, Lazev AB, Mehta NV, Reece GP. Successes and failures of the teachable moment: Smoking cessation in cancer patients. Cancer. 2006;106:17–27. doi: 10.1002/cncr.21598. [DOI] [PubMed] [Google Scholar]
- 74.Demark-Wahnefried W, Aziz NM, Rowland JH, Pinto BM. Riding the crest of the teachable moment: Promoting long-term health after the diagnosis of cancer. J Clin Oncol. 2005;23:5814–5830. doi: 10.1200/JCO.2005.01.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lopez ME, Kaplan CP, Napoles AM, Livaudais JC, Hwang ES, Stewart SL, Bloom J, Karliner L. Ductal carcinoma in situ (dcis): Posttreatment follow-up care among latina and non-latina white women. Journal of cancer survivorship : research and practice. 2013;7:219–226. doi: 10.1007/s11764-012-0262-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Campbell KL, Van Patten CL, Neil SE, Kirkham AA, Gotay CC, Gelmon KA, McKenzie DC. Feasibility of a lifestyle intervention on body weight and serum biomarkers in breast cancer survivors with overweight and obesity. Journal of the Academy of Nutrition and Dietetics. 2012;112:559–567. doi: 10.1016/j.jada.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 77.Kim SH, Shin MS, Lee HS, Lee ES, Ro JS, Kang HS, Kim SW, Lee WH, Kim HS, Kim CJ, Kim J, Yun YH. Randomized pilot test of a simultaneous stage-matched exercise and diet intervention for breast cancer survivors. Oncology nursing forum. 2011;38:E97–106. doi: 10.1188/11.ONF.E97-E106. [DOI] [PubMed] [Google Scholar]
- 78.Reeves MM, Terranova CO, Eakin EG, Demark-Wahnefried W. Weight loss intervention trials in women with breast cancer: A systematic review. Obes Rev. 2014;15:749–768. doi: 10.1111/obr.12190. [DOI] [PubMed] [Google Scholar]
- 79.Rogers LQ, Courneya KS, Anton PM, Hopkins-Price P, Verhulst S, Vicari SK, Robbs RS, Mocharnuk R, McAuley E. Effects of the beat cancer physical activity behavior change intervention on physical activity, aerobic fitness, and quality of life in breast cancer survivors: A multicenter randomized controlled trial. Breast Cancer Res Treat. 2014 doi: 10.1007/s10549-014-3216-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Spector D, Deal AM, Amos KD, Yang H, Battaglini CL. A pilot study of a home-based motivational exercise program for african american breast cancer survivors: Clinical and quality-of-life outcomes. Integrative cancer therapies. 2014;13:121–132. doi: 10.1177/1534735413503546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pinto BM, Papandonatos GD, Goldstein MG. A randomized trial to promote physical activity among breast cancer patients. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2013;32:616–626. doi: 10.1037/a0029886. [DOI] [PubMed] [Google Scholar]
- 82.Goodwin PJ, Segal RJ, Vallis M, Ligibel JA, Pond GR, Robidoux A, Blackburn GL, Findlay B, Gralow JR, Mukherjee S, Levine M, Pritchard KI. Randomized trial of a telephone-based weight loss intervention in postmenopausal women with breast cancer receiving letrozole: The lisa trial. J Clin Oncol. 2014;32:2231–2239. doi: 10.1200/JCO.2013.53.1517. [DOI] [PubMed] [Google Scholar]
- 83.Befort CA, Klemp JR, Austin HL, Perri MG, Schmitz KH, Sullivan DK, Fabian CJ. Outcomes of a weight loss intervention among rural breast cancer survivors. Breast Cancer Res Treat. 2012;132:631–639. doi: 10.1007/s10549-011-1922-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Travier N, Fonseca-Nunes A, Javierre C, Guillamo E, Arribas L, Peiro I, Buckland G, Moreno F, Urruticoechea A, Oviedo GR, Roca A, Hurtos L, Ortega V, Munoz M, Garrigos L, Cirauqui B, Del Barco S, Arcusa A, Segui MA, Borras JM, Gonzalez CA, Agudo A. Effect of a diet and physical activity intervention on body weight and nutritional patterns in overweight and obese breast cancer survivors. Medical oncology. 2014;31:783. doi: 10.1007/s12032-013-0783-5. [DOI] [PubMed] [Google Scholar]
- 85.Shariff-Marco S, Yang J, John EM, Sangaramoorthy M, Hertz A, Koo J, Nelson DO, Schupp CW, Shema SJ, Cockburn M, Satariano WA, Yen IH, Ponce NA, Winkleby M, Keegan TH, Gomez SL. Impact of neighborhood and individual socioeconomic status on survival after breast cancer varies by race/ethnicity: The neighborhood and breast cancer study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2014;23:793–811. doi: 10.1158/1055-9965.EPI-13-0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schmidt ME, Chang-Claude J, Vrieling A, Seibold P, Heinz J, Obi N, Flesch-Janys D, Steindorf K. Association of pre-diagnosis physical activity with recurrence and mortality among women with breast cancer. International journal of cancer Journal international du cancer. 2013;133:1431–1440. doi: 10.1002/ijc.28130. [DOI] [PubMed] [Google Scholar]
- 87.Borch KB, Braaten T, Lund E, Weiderpass E. Physical activity and mortality among norwegian women - the norwegian women and cancer study. Clinical epidemiology. 2011;3:229–235. doi: 10.2147/CLEP.S22681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kamineni A, Anderson ML, White E, Taplin SH, Porter P, Ballard-Barbash R, Malone K, Buist DS. Body mass index, tumor characteristics, and prognosis following diagnosis of early-stage breast cancer in a mammographically screened population. Cancer Causes Control. 2013;24:305–312. doi: 10.1007/s10552-012-0115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Harris TB, Ballard-Barbasch R, Madans J, Makuc DM, Feldman JJ. Overweight, weight loss, and risk of coronary heart disease in older women. The nhanes i epidemiologic follow-up study. Am J Epidemiol. 1993;137:1318–1327. doi: 10.1093/oxfordjournals.aje.a116641. [DOI] [PubMed] [Google Scholar]
- 90.Manson JE, Colditz GA, Stampfer MJ, Willett WC, Rosner B, Monson RR, Speizer FE, Hennekens CH. A prospective study of obesity and risk of coronary heart disease in women. The New England journal of medicine. 1990;322:882–889. doi: 10.1056/NEJM199003293221303. [DOI] [PubMed] [Google Scholar]
- 91.Dotevall A, Johansson S, Wilhelmsen L, Rosengren A. Increased levels of triglycerides, bmi and blood pressure and low physical activity increase the risk of diabetes in swedish women. A prospective 18-year follow-up of the beda study. Diabetic medicine : a journal of the British Diabetic Association. 2004;21:615–622. doi: 10.1111/j.1464-5491.2004.01189.x. [DOI] [PubMed] [Google Scholar]
- 92.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr Body-mass index and mortality in a prospective cohort of u.S. Adults. The New England journal of medicine. 1999;341:1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 93.Pierce JP, Patterson RE, Senger CM, Flatt SW, Caan BJ, Natarajan L, Nechuta SJ, Poole EM, Shu XO, Chen WY. Lifetime cigarette smoking and breast cancer prognosis in the after breast cancer pooling project. J Natl Cancer Inst. 2014;106:djt359. doi: 10.1093/jnci/djt359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lakier JB. Smoking and cardiovascular disease. The American journal of medicine. 1992;93:8S–12S. doi: 10.1016/0002-9343(92)90620-q. [DOI] [PubMed] [Google Scholar]
- 95.Nyante SJ, Gierach GL, Dallal CM, Freedman ND, Park Y, Danforth KN, Hollenbeck AR, Brinton LA. Cigarette smoking and postmenopausal breast cancer risk in a prospective cohort. British journal of cancer. 2014;110:2339–2347. doi: 10.1038/bjc.2014.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stellman SD, Takezaki T, Wang L, Chen Y, Citron ML, Djordjevic MV, Harlap S, Muscat JE, Neugut AI, Wynder EL, Ogawa H, Tajima K, Aoki K. Smoking and lung cancer risk in american and japanese men: An international case-control study. Cancer Epidemiol Biomarkers Prev. 2001;10:1193–1199. [PubMed] [Google Scholar]
- 97.Shaw BA, Agahi N. Smoking and physical inactivity patterns during midlife as predictors of all-cause mortality and disability: A 39-year prospective study. European journal of ageing. 2014;11:195–204. doi: 10.1007/s10433-013-0298-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gellert C, Schottker B, Brenner H. Smoking and all-cause mortality in older people: Systematic review and meta-analysis. Arch Intern Med. 2012;172:837–844. doi: 10.1001/archinternmed.2012.1397. [DOI] [PubMed] [Google Scholar]
- 99.Hvidtfeldt UA, Frederiksen ME, Thygesen LC, Kamper-Jorgensen M, Becker U, Gronbaek M. Incidence of cardiovascular and cerebrovascular disease in danish men and women with a prolonged heavy alcohol intake. Alcoholism, clinical and experimental research. 2008;32:1920–1924. doi: 10.1111/j.1530-0277.2008.00776.x. [DOI] [PubMed] [Google Scholar]
- 100.Cullmann M, Hilding A, Ostenson CG. Alcohol consumption and risk of pre-diabetes and type 2 diabetes development in a swedish population. Diabetic medicine : a journal of the British Diabetic Association. 2012;29:441–452. doi: 10.1111/j.1464-5491.2011.03450.x. [DOI] [PubMed] [Google Scholar]
- 101.Turati F, Garavello W, Tramacere I, Pelucchi C, Galeone C, Bagnardi V, Corrao G, Islami F, Fedirko V, Boffetta P, La Vecchia C, Negri E. A meta-analysis of alcohol drinking and oral and pharyngeal cancers: Results from subgroup analyses. Alcohol and alcoholism. 2013;48:107–118. doi: 10.1093/alcalc/ags100. [DOI] [PubMed] [Google Scholar]
- 102.Tramacere I, Negri E, Pelucchi C, Bagnardi V, Rota M, Scotti L, Islami F, Corrao G, La Vecchia C, Boffetta P. A meta-analysis on alcohol drinking and gastric cancer risk. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2012;23:28–36. doi: 10.1093/annonc/mdr135. [DOI] [PubMed] [Google Scholar]
- 103.Rostron B. Alcohol consumption and mortality risks in the USA. Alcohol and alcoholism. 2012;47:334–339. doi: 10.1093/alcalc/agr171. [DOI] [PubMed] [Google Scholar]
- 104.Klatsky AL, Armstrong MA, Friedman GD. Alcohol and mortality. Ann Intern Med. 1992;117:646–654. doi: 10.7326/0003-4819-117-8-646. [DOI] [PubMed] [Google Scholar]
- 105.Irwin ML, Cadmus L, Alvarez-Reeves M, O'Neil M, Mierzejewski E, Latka R, Yu H, Dipietro L, Jones B, Knobf MT, Chung GG, Mayne ST. Recruiting and retaining breast cancer survivors into a randomized controlled exercise trial: The yale exercise and survivorship study. Cancer. 2008;112:2593–2606. doi: 10.1002/cncr.23446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Harris MN, Swift DL, Myers VH, Earnest CP, Johannsen NM, Champagne CM, Parker BD, Levy E, Cash KC, Church TS. Cancer survival through lifestyle change (castle): A pilot study of weight loss. International journal of behavioral medicine. 2013;20:403–412. doi: 10.1007/s12529-012-9234-5. [DOI] [PubMed] [Google Scholar]
- 107.Emmons KM, Puleo E, Sprunck-Harrild K, Ford J, Ostroff JS, Hodgson D, Greenberg M, Diller L, de Moor J, Tyc V. Partnership for health-2, a web-based versus print smoking cessation intervention for childhood and young adult cancer survivors: Randomized comparative effectiveness study. Journal of medical Internet research. 2013;15:e218. doi: 10.2196/jmir.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.van der Aalst CM, de Koning HJ, van den Bergh KA, Willemsen MC, van Klaveren RJ. The effectiveness of a computer-tailored smoking cessation intervention for participants in lung cancer screening: A randomised controlled trial. Lung cancer. 2012;76:204–210. doi: 10.1016/j.lungcan.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 109.Duffy SA, Ronis DL, Valenstein M, Lambert MT, Fowler KE, Gregory L, Bishop C, Myers LL, Blow FC, Terrell JE. A tailored smoking, alcohol, and depression intervention for head and neck cancer patients. Cancer Epidemiol Biomarkers Prev. 2006;15:2203–2208. doi: 10.1158/1055-9965.EPI-05-0880. [DOI] [PubMed] [Google Scholar]
- 110.Griebel B, Wewers ME, Baker CA. The effectiveness of a nurse-managed minimal smoking-cessation intervention among hospitalized patients with cancer. Oncology nursing forum. 1998;25:897–902. [PubMed] [Google Scholar]
- 111.Mastroleo NR, Oakley WC, Eaton EM, Borsari B. Response of heavy-drinking voluntary and mandated college students to a peer-led brief motivational intervention addressing alcohol use. Journal of substance abuse treatment. 2014;47:321–328. doi: 10.1016/j.jsat.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Crawford MJ, Sanatinia R, Barrett B, Byford S, Dean M, Green J, Jones R, Leurent B, Lingford-Hughes A, Sweeting M, Touquet R, Tyrer P, Ward H. The clinical effectiveness and cost-effectiveness of brief intervention for excessive alcohol consumption among people attending sexual health clinics: A randomised controlled trial (shear) Health technology assessment. 2014;18:1–48. doi: 10.3310/hta18300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Suffoletto B, Kristan J, Callaway C, Kim KH, Chung T, Monti PM, Clark DB. A text message alcohol intervention for young adult emergency department patients: A randomized clinical trial. Annals of emergency medicine. 2014;64:664–672 e664. doi: 10.1016/j.annemergmed.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schulz DN, Candel MJ, Kremers SP, Reinwand DA, Jander A, de Vries H. Effects of a web-based tailored intervention to reduce alcohol consumption in adults: Randomized controlled trial. Journal of medical Internet research. 2013;15:e206. doi: 10.2196/jmir.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Seer*stat database: Incidence - seer 9 regs research data, nov 2013 sub (1973-2011). released April 2014, based on the November 2013 submission


