Abstract
Aims
Recent studies have suggested that esophageal submucosal gland (ESMG) ducts harbor progenitor cells that may contribute to esophageal metaplasia. Our objective was to determine if histologic differences exist between the ESMGs of individuals with and without esophageal adenocarcinoma (EAC).
Methods and Results
We performed histologic assessment of 343 unique ESMGs from 30 control patients, 24 patients with treatment-naïve high-grade columnar dysplasia (HGD) or EAC, and 23 non-EAC esophagectomy cases. A gastrointestinal pathologist assessed H&E stained ESMG images using a scoring system that assigns individual ESMG acini to five histologic types (mucous, serous, oncocytic, dilated, or ductal metaplastic). In our model, ductal metaplastic acini were more common in patients with HGD/EAC with 12.7% and 3.5% in controls (p=0.006). We also identified greater proportions of acini with dilation (21.9%, p<0.001) and, to a lesser extent, ductal metaplasia (4.3%, p=0.001) in non-EAC esophagectomy cases versus controls. Ductal metaplasia tended to occur in areas of mucosal ulceration or tumor.
Conclusions
We found a clear association between ductal metaplastic ESMG acini and HGD/EAC. Non-EAC cases had dilated acini and some ductal dilation. Because ESMGs and ducts harbor putative progenitor cells, these associations could have significance for the understanding the pathogenesis of EAC.
Keywords: Esophagus, esophageal neoplasm, Barrett’s Esophagus, ductal metaplasia, esophageal submucosal gland
Introduction
The overall five-year survival of esophageal adenocarcinoma (EAC) is a dismal 17%1, 2, and incidence of EAC is increasing. Clinical guidelines promoting screening and surveillance for Barrett’s esophagus (BE) represent an effort to improve long-term outcomes3. However, in part because the vast majority of patients who present with EAC have never been previously diagnosed with BE4, 5, these guidelines have not resulted in an appreciable decrease in EAC2. In addition, many resected EACs do not demonstrate a histologic background of BE, suggesting an alternative pre-neoplastic process6–9.
The origin of esophageal progenitor cells remains an area of debate10–14. One of the most intriguing theories focuses on esophageal submucosal glands (ESMGs), which occupy a protected position within the esophageal submucosa and connect to the mucosal layer via ducts. The role of ESMGs as a potential stem cell niche remains incompletely understood in part due to limitations of animal models. Mouse studies have demonstrated progenitor populations in the basal layer of squamous epithelium15, and at the squamo-columnar junction12, 14. However, mouse esophagus does not contain ESMGs16, reducing the ability of the mouse to adequately model human esophageal biology. As a result, direct studies of human ESMGs are necessary in order to further our understanding of ESMGs in human esophageal disease.
ESMGs are a defining histologic feature of human esophagus17,18, 19. Autopsy studies have been used to evaluate ESMGs in normal esophagus while esophagectomy studies have focused on ESMGs in diseased esophagus20, 21. Each ESMG is comprised of clusters of cells known as acini. Each acinus is surrounded by basement membrane and a myoepithelial cell layer. Human ESMG acini contain variable numbers of mucous cells, serous cells, and oncocytes22–24. Mucous-producing cells demonstrate abundant clear to pale mucinous cytoplasm with small, basally-oriented nuclei and represent the predominant acinar cell type in ESMGs. Serous cells have abundant pale pink cytoplasm and produce neutral and sialated mucins as well as lysozyme and pepsinogen24. Oncocytes are characterized by abundant bright pink cytoplasm consisting of densely packed mitochondria22.
In vivo, small ducts draining each acinus coalesce to form ducts of progressively greater diameter until a main duct is formed. Main gland ducts traverse the muscularis mucosa and subepithelial connective tissue of the mucosa, allowing ESMGs to release their products into the esophageal lumen19. Duct cells within and near ESMGs appear cuboidal. As the lumen of the esophagus is approached, main duct cells transition to a squamous appearance.
Early investigations into ESMG ducts demonstrated continuity between ductal epithelium and neosquamous epithelium after squamous esophageal injury25. Later reports described continuity between gland duct epithelium and a biphenotypic multilayered esophageal epithelium with both squamous and columnar features26. Recently, ESMG ducts have been found in direct connection with both columnar BE as well as squamous islands occurring in a background of BE27. However, the histologic changes occurring in the actual ESMG distal to the duct represent an area in need of further scientific investigation.
In our own practice, we noted ESMGs in esophagectomy specimens had a heterogeneous appearance. ESMGs from patients with EAC often lacked the classic appearance of predominantly mucinous acini. In order to quantify our observations, we systematically characterized the appearance of the ESMGs in patients with and without EAC or high grade dysplasia (HGD) in a blinded fashion. We used these data to test the hypothesis that the proportions of the various acinar types in ESMGs from patients with HGD/EAC differ from those found in ESMGs from patients without HGD/EAC.
Materials and Methods
Cases and Controls
Prior approval for this study was obtained from Duke University’s Institutional Review Board. Using an existing database of patients who had undergone esophagectomy or esophagogastrectomy; cases from three years were selected for clinical and pathologic review. Cases (n=24) were included if the pathologic diagnosis was HGD/EAC. Because of well-characterized histologic changes occurring in patients after radiation therapy, patients who had received any thoracic cavity radiation therapy were excluded. Similarly, patients with neoadjuvant chemotherapy were excluded. Finally, cases were excluded if pathologic review of all available archival paraffin blocks failed to disclose ≥ 1 complete ESMG. All tumor sections were reviewed for confirmation of pathologic diagnosis.
For controls, we selected autopsy cases; further discussion of this choice of control is provided in Supporting Information. Autopsy files were sequentially reviewed. Controls (n=30) were included if the patient was ≥ 18 without clinical history or histologic finding of HGD/EAC. Standard histologic sections taken during autopsy at Duke include the anatomic gastroesophageal junction and the mid-tubular esophagus. All tubular esophagus sections and gastroesophageal junction sections containing squamous epithelium were reviewed. Controls were excluded if no ESMGs were identified in sections or if autolysis prevented detailed examination of ESMGs. One control sample was obtained from a surgical patient without BE or EAC who underwent esophagectomy for stricture. This group comprised our initial control group.
After the initial analysis of HGD/EAC cases and controls, an additional new comparison group (n=23) was formed from the esophagectomy cohort. The goal was to evaluate a separate group of esophagectomy cases in order to assess a continuum of esophageal disease that included esophagitis and/or BE without HGD or EAC. We queried our 2000–2010 esopahgectomy database for a group of cases without adenocarcinoma and without a history of neoadjuvant therapy. Diagnosis of achalasia and fistula were also excluded due to suspected anatomic distortion. From this group, 33 non-EAC esophagectomy cases were identified and tissue blocks were evaluated for presence of ESMGs. In ten cases, there were insufficient ESMGs; the remaining 23 non-EAC controls were available for scoring and analysis.
Clinical data collected for all esophagectomy cases included demographics, treatment history, and BE. Limited clinical data were available for autopsy cases and included basic demographics.
Autopsy and esophagectomy specimens had been fixed in 10% neutral buffered formalin and processed into paraffin blocks. Appropriate blocks were selected for preparation of research sections. Sections representing anatomic esophagus were identified; the anatomic esophagus was defined by either the presence of squamous epithelium or the presence of ESMGs, even if the overlying epithelium was columnar17. Five micrometer sections were cut from the blocks and used for standard hematoxylin and eosin (H&E) staining or immunohistochemical staining as described below.
ESMG Image Capture
A pathologist reviewed all slides to confirmation pathologic diagnosis, presence of autolysis, and presence of evaluable ESMGs using an Olympus BX-46 microscope. Subsequently, H&E-stained slides from selected paraffin blocks were examined using an Olympus IX71 microscope. An Olympus DP2-BSW digital camera and software was used to capture photomicrographs (Olympus Corporation, Tokyo, Japan). All ESMGs in case and control blocks were identified, and digital images were captured. Prior to scoring, each image was processed to obscure overlying epithelium. Blinded to clinical diagnosis, esophageal cancer status, and overlying esophageal epithelial type, a subspecialty fellowship-trained gastrointestinal pathologist evaluated isolated images of 343 ESMGs in the initial cases and controls.
Histologic Scoring System Developed
For quantification of acinar types, two investigators (KG, SM) devised an ESMG scoring schema (Table 1). The approach was based on the assumption that each ESMG acinus could be assigned to one of three types: mucinous (purely mucinous cells, no discernible lumen), serous (acinus containing any number of serous cells) or oncocytic (acinus containing any number of oncocytic cells). However, we noted the variable presence of a discernible lumen within some mucinous acini. Because normal ESMG are predominantly comprised of acini with tightly-clustered mucin-producing cells, the presence of a prominent discernible lumen within mucinous-cell lined acini was assigned a “dilated” category. We observed that while many dilated mucinous acini were lined by cells with a clear mucinous phenotype, some dilated acini appeared to be lined by cuboidal cells without histologic evidence of mucin production that appeared neither oncocytic nor serous. We assigned a fifth category of putative “ductal metaplastic” based on these histologic features. More detailed description of acinar types is provided in Supporting Information.
Table 1. ESMG scoring system for inflammation and acinar type.
The left column provides categories to score overall inflammation within ESMG. The second column scores different acinar types (as defined in column three) with by counting acinar types present in an ESMG. Each acinus within an ESMG should only be assigned one category. The bottom cell of this column should represent the total tally of the five different acinar types and should equal all acini within an ESMG. The far right column allows calculation of percentages of each acinar type per ESMG.
| Scoring of Each ESMG | Types of Acini (score below) |
Definitions of Acinar Types |
% Each Acinar Type of Total # Acinar Units |
|---|---|---|---|
| Overall inflammation (score below) | # Mucinous | Pale, plump, glandular cells | |
| 0 = none | # Serous | Small dense granules and peripherally located nucleus | |
| 1 = mild | # Oncocytic | Eosinophilic (mitochrondria-rich) with central nucleus | |
| 2 = moderate | # Dilated Mucinous | Pale mucinous cells with dilated lumen | |
| 3 = marked | # Ductular Metaplasia | Ductal-type epithelium with dilated lumen | |
| Total # of Acinar Units | Calculated as sum of five different acinar types | Should add to 100% |
In addition to assessment of acinar types, inflammation was subjectively graded for each ESMG unit. Inflammation was considered significant and scored if it was present around distal acini within the ESMG. Periductal inflammation was not considered significant28. Inflammation grading used a typical pathologic scale of 0=none, 1=mild, 2=moderate, 3=marked.
Scoring was performed manually. The total number of acini per ESMG was counted. Acini were classified as one of the five types. Proportions were then calculated based upon total counts. The initial scoring analysis of cases and controls was performed on digital images processed to obscure overlying epithelium. Subsequently, the additional non-EAC esophagectomy cases were scored and HGD/EAC cases and the non-EAC esophagectomy cases were then evaluated to determine the type of epithelium overlying each ESMG.
Data Analysis
Study data were collected and managed using REDCap electronic data capture tools hosted at Duke University29. Statistical Analysis was performed using SAS software, Version 9.3 of the SAS System for Windows, Copyright © 2002–2010 SAS Institute Inc. See supporting information for REDCap and SAS details as well as more detailed description of statistical analysis. The initial analysis compared HGD/EAC cases to controls. In brief, data were structured at the ESMG level per patient. Each patient possessed a variable number of ESMGs. For each ESMG, the number of acini was counted for each of five acinar types: mucinous, serous, oncocytic, dilated and ductal metaplastic. Proportions of acinar types within each ESMG were calculated. A general estimating equation (GEE) model that accounted for differences due to cases versus controls, acinar types, and the interaction of cases/controls and acinar types was used to calculate the predicted proportions of acini. Predicted proportions and interaction p-values are reported instead of odds ratios to allow the differences in acinar rates to be quantified. This type of model was selected for the analysis because it is designed to account for possible correlation caused by observing multiple glands and multiple acinar types per patient.30 A description of this model (and justification for its selection over a multinomial model) are provided in supplemental material). Later, the same model was used to examine non-EAC esopahgectomy cases versus HGD/EAC cases versus non-EAC controls.
Analysis of the association between proportion of ESMGs with different inflammation scores by group (HGD/EAC, non-EAC cases and controls) was performed using Chi-squared or Fisher’s exact test, as appropriate.
Immunohistochemistry
Cytokeratin 7 (CK7) faintly and peripherally stains normal ESMG mucous cells but stains ductal cells in a dense cytoplasmic pattern (Supplementary Figure 1). Esophageal squamous epithelium does not stain for CK726, 31. To verify the ductal metaplasia, we assessed CK7 in ESMGs and ducts (Dako Antibody M7018). Detailed methods are reported in Supporting Information. Pepsin retrieval was performed, protein blocking was performed, and then the primary antibody was applied and incubated for 1 hour at room temperature. Antibody detection was performed with diaminobenzidine (Dako, Carpinteria, CA) and counterstaining was performed with hematoxylin solution and bluing agent.
Results
Patient Characteristics
The initial cohort included 54 patients: 24 cases and 30 controls. The cases had undergone esophagectomy or esophagogastrectomy for a diagnosis of HGD (n=7) or EAC (n=17). Of the 24 EAC/HGD cases, 21 (87.5%) had histologic evidence of BE. As per exclusion criteria, none of the 24 patients with HGD/EAC had previously received chemotherapy or thoracic radiation. All control patients were without HGD/EAC; no control patients had BE. After the initial analysis, an additional non-EAC esophagectomy comparison group was added consisting of 23 diseased esophagus (without adenocarcinoma) but affected by other esophageal conditions. Diagnoses in this group included esophagitis (n=7), esophageal squamous cell cancer (ESCC) (n=10, 2 with BE), paraesophageal hernia (n=2), leiomyoma (n=2, 1 with BE), liposarcoma (n=1).
As expected, the cohort of EAC/HGD cases tended to be male (87.5%). In comparison, gender in the control group and non-EAC esophagectomy group were more balanced (53.3% and 52.2% male respectively). Median age of the HGD/EAC cases was 66 years (range 38- over age 80). This was similar to controls with a median age of 64 years (range 26-over age 90). The non-EAC esophagectomy comparison group had a mean age of 61 years (range 27–77).
ESMG Histology for cases and controls
An average of 1.2 esophageal blocks containing ESMGs was identified for review from both cases and controls (range 1–2 in cases and range 1–4 in controls). Histology of all 343 ESMGs identified was reviewed. The data analyzed were complex with variable numbers of ESMGs per patient: ESMG counts ranged from 1 to 24 with a total of 147 ESMGs for cases (mean 6.1) and 196 ESMGs for controls (mean 6.5). The number of acini within each ESMG ranged from 1 to 320. As expected, the most common type of acinus within the ESMGs was the mucinous acinus, comprised solely of mucous-producing cells clustered tightly without a discernible lumen (Figure 1). In addition to mucinous acini, oncocytic acini (containing oncocytic cells as in Figure 2) and serous acini (containing serous cells) were variably present. We also identified a dilated mucinous (with a central lumen) type of acinus, and found histologic evidence suggesting ductal metaplasia within some ESMGs (defined in Table 1 and presented in Figure 3). Dense and diffuse cytoplasmic positivity for CK7 confirmed ductal phenotype. Mucinous acini express CK7; it is generally peripheral and faint. Representative CK7 staining is presented in Figure 3 and in Supplemental Figure 1.
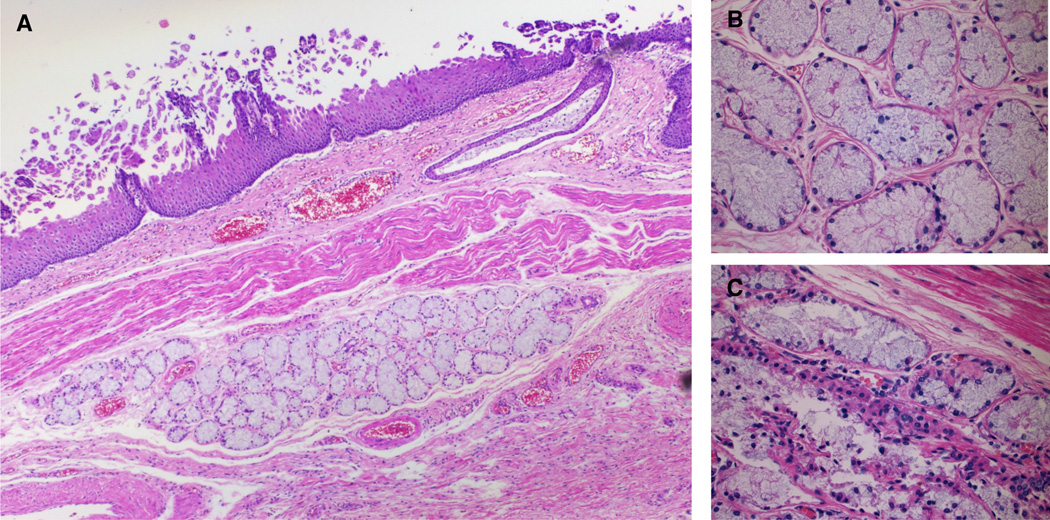
Figure 1.
Normal-appearing ESMG from a control with no history of esophageal cancer: A. A 40× magnification image with squamous epithelium is present, and an esophageal duct is seen reaching in the direction of the ESMG to the lumen of the esophagus. This ESMG demonstrates predominantly non-dilated mucinous acini and there is no evidence of inflammation in this ESMG. B. 200× magnification demonstrates normal appearing mucinous acini without discernible lumens. C. Serous cell with light pink cytoplasm in a different ESMG from the same autopsy case (arrow).
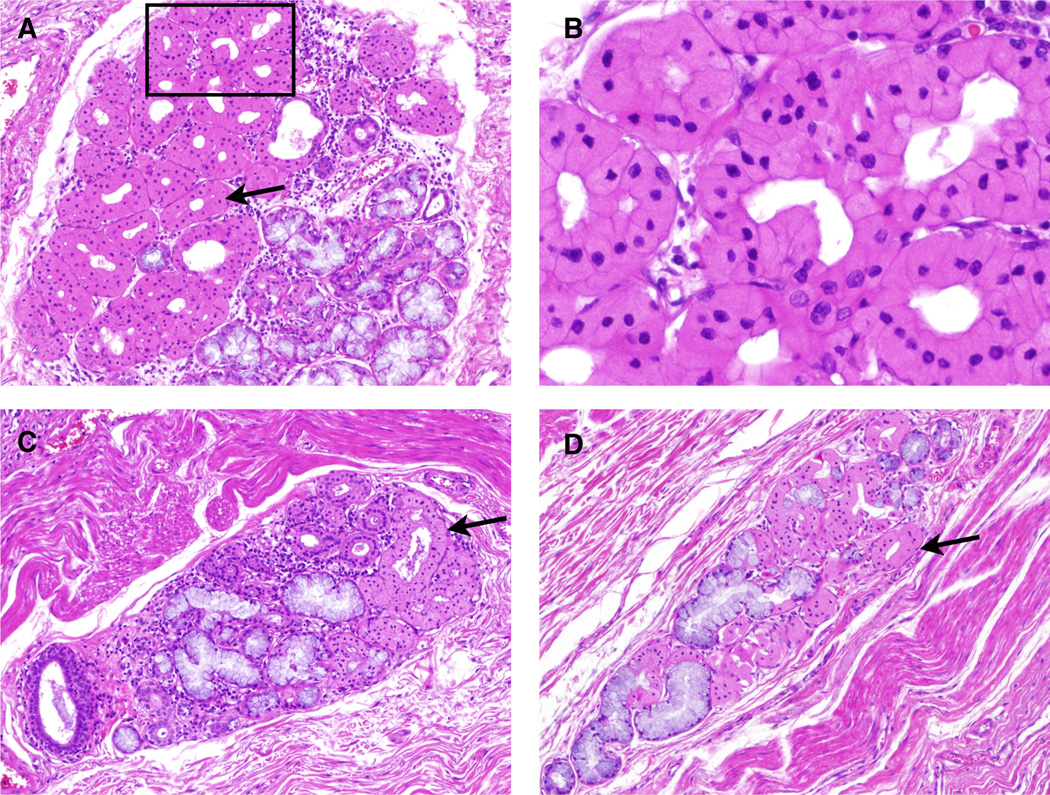
Figure 2.
ESMGs containing oncocytic acini from controls: Oncocytes appear densely eosinophilic with a centrally located nucleus. A. 100× view of an ESMG containing 70% oncocytic acini, present on the left (arrows). Mucinous acini, characterized by their pale mucosa are present right side of this ESMG. B. 200× view of oncocytes from Panel A. C. 40× view of ESMG that contains 25% oncocytic acini. D. 40× view of ESMG containing 62% oncocytic acini.
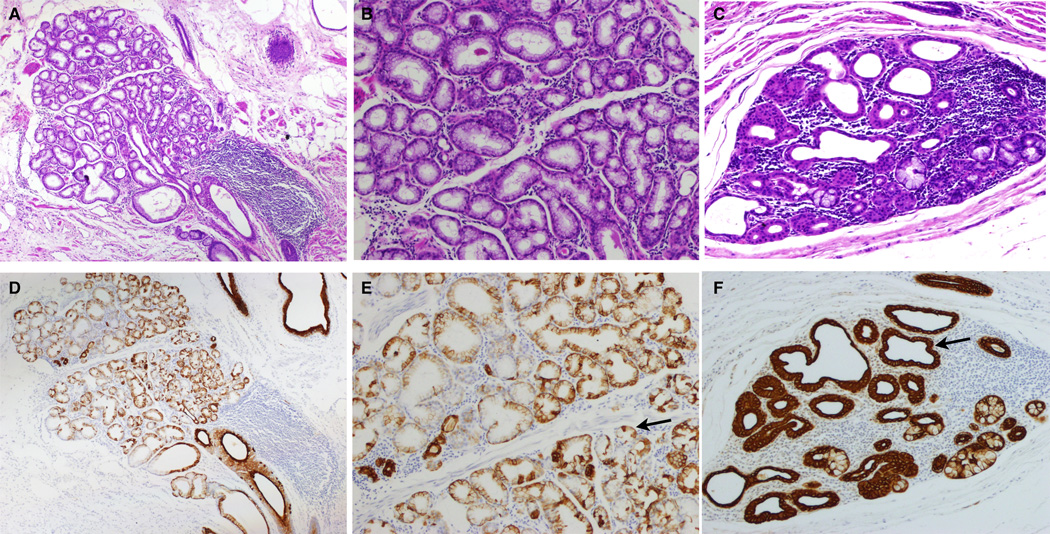
Figure 3.
Dilated acinar phenotype (A, B, D, E) and Ductal metaplasia (C and F). A. 40× view of H& E of ESMG from a patient with BE with high-grade dysplasia; 88% of the acini were scored as dilated. B. 100× view of same ESMG with H&E illustrating dilated mucinous glands. C. 100× view of ESMG from an EAC patient with 84% ductal metaplastic acini and prominent inflammation. D. 40× view of CK7 staining illustrating contrast between dilated mucinous glands and more densly staining ducts of ESMG shown in panel A. E. 100× view of same ESMG illustrating dilated mucinous glands with CK7 staining; the arrow in panel E indicates normal CK7 pattern in dilated mucinous acini.. F. 100× view of CK7 staining of ductal metaplasia and inflammation within the ESMGs shown in Panel C; the arrow in panel F indicates ductal pattern of prominent cytoplasmic CK7 staining. Both patients had BE with high-grade dysplasia and EAC and both were treatment naive (no radiation or chemotherapy) prior to esophagectomy.
Proportions of dilated acini, acini with ductal metaplasia, and oncocytic acini within ESMG varied significantly between the HGD/EAC cases and controls. While there was no substantial difference in the proportion of serous acini between controls and cases (10.8% and 7.9%, p=0.37), the proportion of dilated mucinous acini in HGD/EAC cases was almost twice as high as in controls (11.6% to 5.8%, p=0.05) and the proportion of acini with ductular metaplasia was 3.6 times as high in HGD/EAC cases as in controls (12.7% to 3.5%, p=0.006) The proportion of oncocytic acini was lower in HGD/EAC patients when compared to controls (1.0% to 7.9%, p=0.005). Predicted proportions of acinar types for for HGD/EAC cases and controls are presented in Table 2 along with results for an additional group of non-EAC esopahgectomy cases that was added to the analysis in order to consider the relationship of ductal metaplasia to mucosal inflammation. The non-EAC esophagectomy group is described in more detail later in the results section.
Table 2. Predicted proportions of acinar types for HGD/EAC cases, controls, and the non-EAC esophagectomy group.
Proportions of acinar types were predicted using a general estimating equation model that controlled for the repeated measurements of the variable number of ESMGs per subject. Predicted proportions of four acinar types are presented for the HGD/EAC cases, the autopsy controls and the non-EAC esophagectomy group. P-values are provided for the comparisons between groups by acinar type.
| HGD/EAC Cases |
Controls | Non-EAC | P-Values | |||
|---|---|---|---|---|---|---|
| EAC vs. Controls |
Non-EAC vs. Controls |
Non-EAC vs. EAC |
||||
| Dilated | 11.6% | 5.8% | 21.9% | 0.05 | <0.001 | <0.001 |
| Ductular Metaplasia | 12.7% | 3.5% | 4.3% | 0.006 | 0.001 | 0.19 |
| Oncocytic | 1.0% | 6.7% | 2.6% | 0.005 | 0.19 | 0.01 |
| Serous | 7.9% | 10.8% | 0.9% | reference | reference | reference |
The grade of inflammation within ESMGs correlated with proportions of acinar types. Inflammation scores of ESMG spanned the range of 0 (none) to 3 (marked) although marked inflammation was only present in 9 ESMGs in the cases (6.1%) and 1 ESMG in the controls (0.5%). Examples of ESMG inflammation grades are presented in Figure 4. Overall, 14 cases (58.3%) had at least one ESMG with grade 2 (moderate) or grade 3 (marked) inflammation compared to eight controls (26.7%). Mean proportions of ESMGs by inflammation grade are presented in Table 3 for cases, controls, and non-EAC esophagectomy cases. ESMGs with any amount of inflammation (ESMG inflammation grade higher than zero) were associated with higher proportions of all four non-mucinous acinar types (dilated, ductal metaplasia, oncocytic, and serous). The correlation between non-mucinous acinar types was stronger with higher grades of inflammation as for grade 2 versus grade 0 (p-value = 0.03). For all grades of ESMG inflammation, proportions of dilated acini and acini with ductal metaplasia were smaller for controls than for HGD/EAC cases and the proportions of oncocytic and serous acini were larger for controls than HGD/EAC cases. Including grade of ESMG inflammation in the model heightened overall differences between HGD/EAC cases and controls, data not shown.
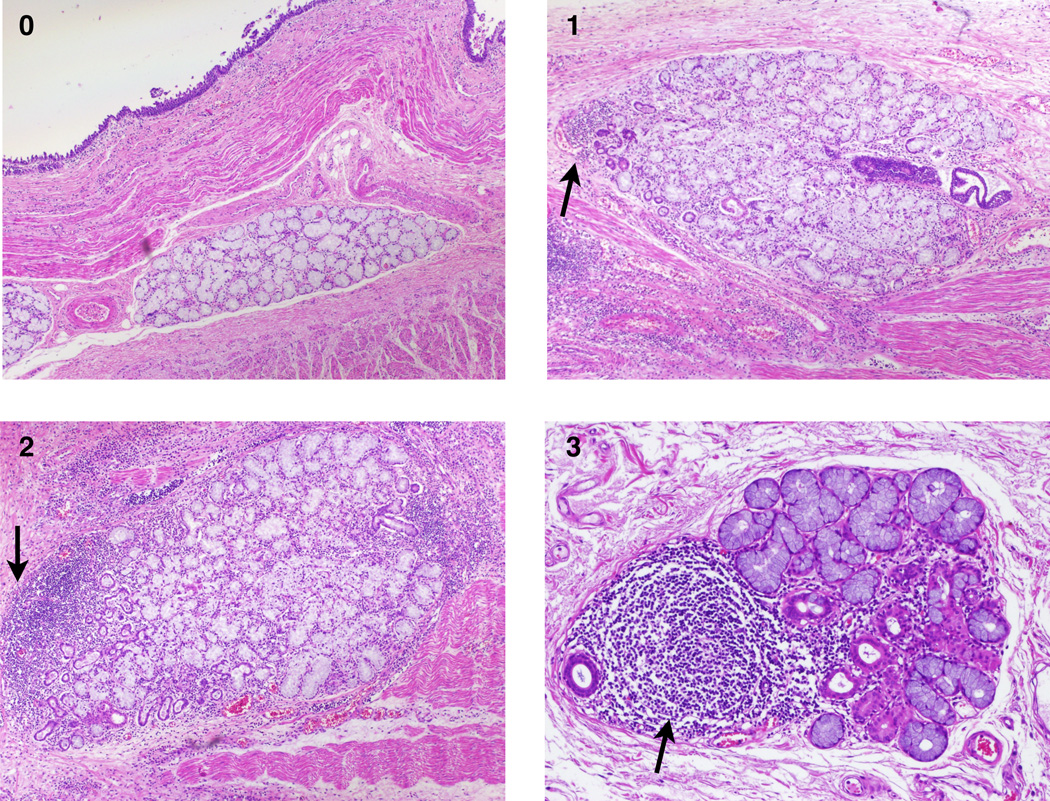
Figure 4.
Examples of different grades (0–3) of ESMG inflammation: Numbers on the images represent the ESMG inflammation score. Arrows indicate inflammatory infiltrate within ESMGs. As shown in panel 3, lymphoid follicle formation is designated grade 3 (arrow).
Table 3. Proportion of ESMGs with Inflammation Grades 0–3 for Cases (N=147 ESMGs) and Controls (N=195 ESMGs) and non-EAC (N=695 ESMGs).
The proportion of ESMGs with each category of inflammation Grades 0–3 as scored (where 0=none, 1=mild, 2=moderate, 3=marked) was calculated for both cases (EAC and non-EAC) and controls. Chi-squared or Fisher’s Exact Tests, as appropriate, were used to compare the proportions between Non-EAC cases, EAC cases, and controls.
| Grade | HGD/EAC Cases | Controls | Non-EAC Esophagectomy |
P-Values |
|---|---|---|---|---|
| 0 | 33 (22.5%) | 59 (30.3%) | 434 (62.5%) | <0.001 |
| 1 | 84 (57.1%) | 125 (64.1%) | 206 (29.6%) | <0.001 |
| 2 | 21 (14.3%) | 10 (5.1%) | 46 (6.6%) | 0.002 |
| 3 | 9 (6.1%) | 1 (0.5%) | 9 (1.3%) | <0.001 |
Increased patient age was also associated with increased proportion of non-mucinous acinar types (dilated ductal, oncocytic and serous), (p=0.006) in HGD/EAC cases versus controls. When age was added to a model that included cases versus controls, acinar types, and the interaction of these two factors, there continued to be more dilated acini and ductal metaplasia in the HGD/EAC cases versus the controls, and there were fewer oncocytic and serous acini in HGD/EAC cases versus controls. Furthermore, as age increased, the size of the differences between these proportions also increased, data not shown. When the model was used to evaluate the association between presence or absence of BE in the HGD/EAC cases and acinar types within ESMGs, presence of BE in the cases did not exert much effect (p=0.88).
An additional group of esophagectomy patients was added to the study following the initial analysis. This new group was added in order to evaluate ESMGs associated with non-EAC esophageal diseases. The new non-EAC esophagectomy group was heterogenous, including cases of esophagectomy performed for ulcers and intractable inflammation. In these cases, pathologists were responsible for carefully excluding cancer. As a result, these non-EAC esophagectomy cases tended to have many blocks of esophageal tissue available for clinical evaluation. Compared to the cases and controls (with mean 1.2 blocks per case with ESMGs), the non-EAC esophagectomy group had an average of 5.1 blocks per case with ESMGs (range 1–26). The total glands evaluated for the non-EAC esophagectomy cases was 698 (mean 30.3 per case, range 1–178).
Non-EAC esophagectomy cases had the highest proportion of dilated mucinous acini (21.9%) compared to EAC cases (11.6%, p-value <0.001) and controls (5.8%, p-value <0.001). While non-EAC esophagectomy cases also had more ductal metaplasia (4.3%) than was found in the control group (3.5%, p-value 0.001), there was not a significant difference from HGD/EAC group (12.7%, p-value 0.19); these results are presented in Table 2. Supplemental Table 1 provides unadjusted counts for all three groups: HGD/EAC cases, non-EAC esophagectomy, and controls.
An analysis of the overlying epithelium was also performed for the HGD/EAC cases as well as the new non-EAC esophagectomy cases. As per the initial study-design, the overlying epithelium in the control group was entirely squamous. Table 4 provides data on frequency of various types of overlying epithelium found over ESMGs in the esophagectomy cases with HGD/EAC and the non-EAC esophagectomy cases. Mean unadjusted proportions of ductal metaplasia for ESMGs with different types of overlying epithelia is reported in Table 4. Overall, ESMGs underlying EAC, ulcer, or SCC frequently contained ductal metaplasia. Very acute injury such as perforation with peritonitis was not associated with increased proportions of ductal metaplasia in ESMGs. BE was much less commonly found overlying ESMGs than was squamous epithelium. Interestingly, in the HGD/EAC cases, there were many instances of squamous islands identified overlying the ESMGs.
Table 4. Types of esophageal epithelium directly over ESMGs and amount of ductal metaplasia associated with epithelial types.
Type of epithelium overlying each ESMG was assessed after the initial blinded scoring. In HGD/EAC cases, there were several instances where a squamous island was associated with ESMG. Those ESMGs had low-levels of ductal metaplasia. In contrast, HGD/EAC as well as SCC and ulcer were associated with ESMGs in the submucosa. Overlying epithelium could not be determined for a small number of ESMGs in the HGD/EAC group.
| EAC (143 ESMGs) | Non-EAC (698 ESMGs) | ||||
|---|---|---|---|---|---|
| # ESMGs |
Mean proportion ductal metaplasia in ESMGs |
# ESMGs | Mean proportion ductal metaplasia in ESMGs |
||
| Squamous epithelium | 74 | 15.6% | Squamous epithelium | 551 | 10.8% |
| Squamous island | 17 | 5.4% | |||
| BE | 28 | 11.8% | BE | 8 | 26.9% |
| Columnar | 11 | 20.9% | |||
| EAC | 13 | 37.3% | SCC | 58 | 28.3% |
| Gastroesophageal Junction | 36 | 8.5% | |||
| Ulcer | 11 | 54.4% | |||
| Peritonitis | 34 | 5.0% | |||
Discussion
Our results provide the first evidence that proportions of ESMG acinar types differ between HGD/EAC patients and autopsy controls, and specifically that increased ductal metaplasia in ESMGs is seen in patients with HGD/EAC. There is a collection of studies demonstrating continuity between ESMG ducts and squamous islands, multilayered (biphenotypic) esophageal epithelium, and/or BE itself26,27. However our study is one of the first to examine the ESMG themselves32, and our study provides a scoring schema for quantitative ESMG assessment that will be useful as these investigations continue.
In some patients with BE, ESMG ducts and overlying metaplastic epithelia have been described as sharing a common mutation in the tumor suppressor gene p16, suggesting an association between the underlying ESMG ducts and overlying BE31. In addition, when mitochondrial mutations in the cytochrome C oxidase gene were used to map the clonal origins of neoplastic esophageal epithelial cells, a common mitochondrial gene mutation was also found in both the submucosa and the surface epithelium33. This suggests that investigations of esophageal neoplasia should include not only the ESMG ducts, but the ESMGs themselves.
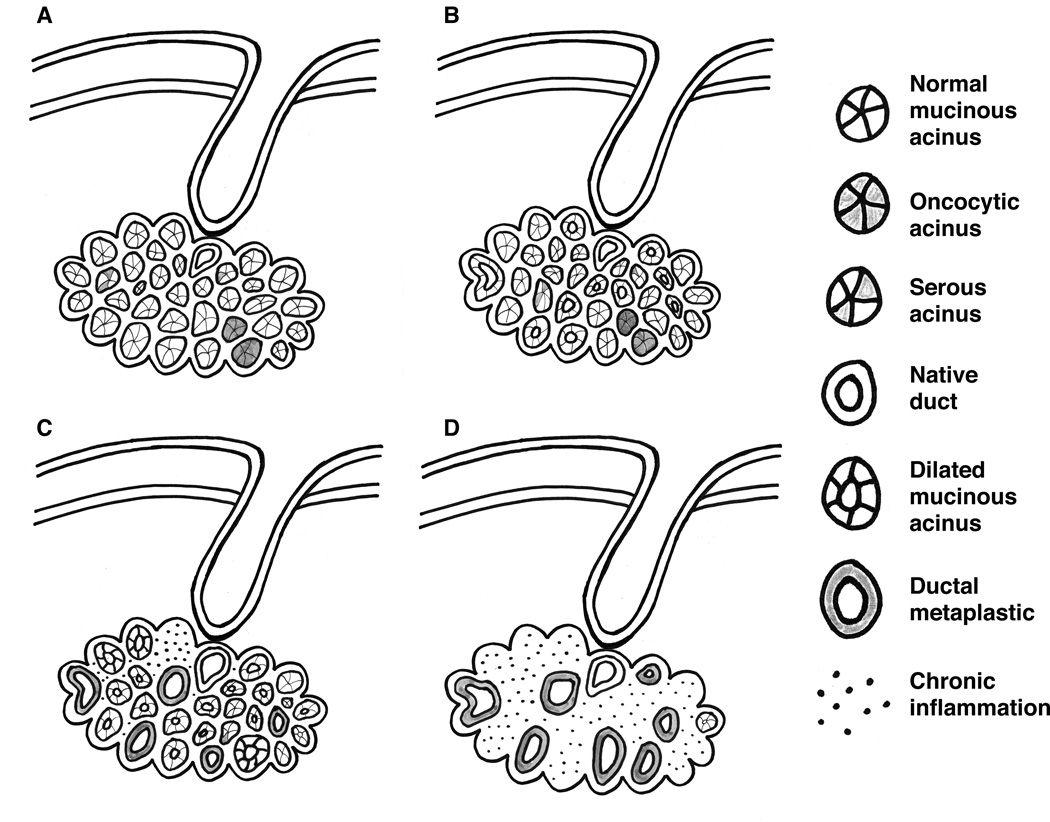
Our results support the concept that ESMGs themselves may undergo metaplasia, particularly in the setting of ESMG inflammation. To illustrate this concept, we propose a possible schema of changes in ESMGs (i.e., loss of acinar type variability, dilation of mucinous acini, ductal metaplasia of mucinous acini) that may be associated with changes in the overlying mucosa occurring during the evolution of HGD/EAC in Figure 5.
Figure 5.
Proposed progression of ESMG from normal, development of ductal dilation and then ductal metaplasia. A. Normal ESMG B. ESMG with ductal dilation and early ductal metaplasia C. Ductal metaplasia
A recent paper by Braxton et al described ESMGs with a process previously reported in minor salivary glands called “necrotizing sialometaplasia.”32 This was defined as replacement of normal ESMG acini with duct-like structures. This group also reported a different process where normal ESMGs are replaced with “oncocytic glandular metaplasia.” Using an esophagectomy database divided into those with and without BE, their group reported an association of both abnormal ESMG phenotypes with BE; 22% of non-BE-controls had metaplasia compared to 56% of BE cases.
Our study supports the novel concept that abnormal ESMG phenotypes are associated with abnormal esophageal epithelial phenotypes. Both the Braxton study and our analysis note the ductal metaplastic change associated with EAC. However, there are several important differences between our two studies. In this current investigation, we focused on ESMGs associated with HGD/EAC versus ESMGs associated with relatively normal esophagus controls. We specifically excluded patients with radiation and/or chemotherapy in an effort to eliminate the possible effects of these therapies on ESMG phenotype. Rather than assigning ESMG phenotypic changes into broad categories such as necrotizing sialometaplasia and oncocytic glandular metaplasia, we developed a quantitative schema for assessing individual acini within ESMGs.
Our additional analysis of non-EAC esophagectomy cases establishes that ESMG ductal metaplasia is also present in the setting of active esophageal inflammation (such as esophageal ulceration). In other organs, duct-like progenitors have been reported34, 35. We hypothesize that in ESMGs, the ductal metaplasia associated with inflammation may persist in some instances, and a signaling climate may develop to support and foster the development of EAC. Interestingly, ductal metaplasia was very rarely identified in ESMGs underlying squamous islands in patients in this group. Model systems are needed to prospectively study the association we report between ductal metaplasia in ESMGs and esophageal ulcer.
This study was not designed to assess the association between ESMGs and esophageal squamous cell carcinoma. However, in the few cases of esophageal squamous cell cancer evaluated as part of the non-EAC esopahgectomy cohort, we were surprised to find some ductal metaplasia. We noted a few cases of squamoid metaplasia in the ducts of patients with esophageal squamous cell carcinoma, and we propose further research in this area to more fully evaluate this finding.
The ductal metaplasia we identified in ESMGs is consistent with previous studies that suggest association of expanded ductular compartment with inflammation and neoplasia in other organs such as pancreas36,37. A more detailed discussion of supporting studies is provided in Supporting Information. The association between ductal metaplasia in ESMGs and EAC is further supported by case reports of EAC developing within an ESMG38. Our data present strong evidence to support future research in this area.
The retrospective nature of our study presents inherent limitations. Previous research demonstrated heterogeneous distribution of ESMGs within the human tubular esophagus39. The esophagus could not be fully mapped and evaluated for ESMGs as only selected blocks were available for review, however the average number of slides for cases and controls did not differ. The work presented here justifies future prospective studies in autopsy and esophagectomy cases for more complete mapping of ESMGs including relationship to BE segments and distribution of ESMGs with phenotypic changes within the esophagus.
Identifying ductal metaplasia in ESMGs may have important clinical implications. Dysplastic BE may be treated with surgery, endoscopic mucosal resection, or ablation40,41.However, of those who respond to radiofrequency ablation (RFA), in two years post-ablation, BE subsequently re-emerges in 33%42. It is possible that persistent abnormalities in the ESMG compartment could explain failure to maintain normal squamous epithelium following ablative mucosal therapies.
We did not identify an independent association between BE and ductal metaplasia in ESMGs. However, our study was not designed to evaluate this association. Because our cohort of cases was comprised of advanced disease (in which short segments of BE could be replaced by cancer), it is not possible to determine if ductal metaplasia in ESMGs precedes development of HGD/EAC. To further evaluate the presence of ductal metaplasia in BE, additional studies are required. Endoscopic mucosal resection cases may provide further opportunity for such research.
Endoscopic mucosal resection (EMR) and endoscopic mucosal dissection (EMD) remove both the esophageal mucosa and the esophageal submucosa. As EMR/EMD become more common, pathologists will have the opportunity to evaluate more ESMGs. We have provided a basic framework for scoring ESMG inflammation and quantifying acinar types within the ESMGs. In this study, scoring was performed with non-automated (manual) counting of each acinar type. In future, it may be beneficial to develop automated scoring based upon image analysis software. There is an opportunity and a need for prospective validation of the dilated and ductal metaplastic acinar phenotypes in both BE and EAC.
Supplementary Material
Acknowledgements
Funding provided by NIH NIDDK K08-DK098528 (KG) and the Duke Endowment (AMD).
IRB Approval was obtained for this work through two protocols: Esopahgectomy Database Protocol # 39682 (initial approval 07/31/2012) and Immunohistochemistry #19479 (initial approval/exemption 09/02/2009).
The authors gratefully acknowledge the Duke University Department of Pathology Research Histology and Immunohistochemistry Laboratory for assistance preparing histology sections and performing hematoxylin and eosin staining. We also thank Susan J. Henning for reviewing the manuscript and her ongoing mentorship (KG).
Footnotes
Conflicts of Interest Statement: None of the authors have conflicts of interest to report.
Author contributions:
Katherine S. Garman, Leandi Kruger, Marzena Swiderska-Syn and Shannon McCall performed the research. Katherine S. Garman, Anna Mae Diehl and Shannon McCall designed the research study. Anna Mae Diehl and Marzena Swiderska-Syn contributed essential reagents and tools. Katherine Garman, Samantha Thomas and Barry Moser analyzed the data. Katherine Garman and Shannon McCall wrote the paper with input from Barry Moser, Leandi Kruger, and Anna Mae Diehl and all authors reviewed the manuscript.
List of Online Supporting Information:
1. Details on Methods including: Selection of Controls, Gland Scoring, Data Integrity and Analysis Software, Statistical Analysis, Immunohistochemistry
2. Results of Inflammation Assessment in ESMGs
3. Discussion of Ductal Metaplasia in other organs
4. Supplemental Table 1 - Ductal Metaplasia in HGD/EAC Cases, Controls and non-EAC Esophagectomy Cases
5. Supplemental Figure 1 – CK7 in ESMG from autopsy control, in ESMG from a patient with EAC and in EAC.
References
- 1.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97:142–146. doi: 10.1093/jnci/dji024. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, et al. Seer cancer statistics review. Bethesda, MD: National Cancer Institute; 2011. based upon November 2010 SEER data submission. [Google Scholar]
- 3.Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ. American gastroenterological association medical position statement on the management of barrett's esophagus. Gastroenterology. 2011;140:1084–1091. doi: 10.1053/j.gastro.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 4.Dulai GS, Guha S, Kahn KL, Gornbein J, Weinstein WM. Preoperative prevalence of barrett's esophagus in esophageal adenocarcinoma: A systematic review. Gastroenterology. 2002;122:26–33. doi: 10.1053/gast.2002.30297. [DOI] [PubMed] [Google Scholar]
- 5.Hvid-Jensen F, Pedersen L, Drewes AM, Sorensen HT, Funch-Jensen P. Incidence of adenocarcinoma among patients with barrett's esophagus. N Engl J Med. 2011;365:1375–1383. doi: 10.1056/NEJMoa1103042. [DOI] [PubMed] [Google Scholar]
- 6.Liu S, Dai JY, Yao L, et al. Esophageal adenocarcinoma and its rare association with barrett's esophagus in henan, china. PLoS One. 2014;9:e110348. doi: 10.1371/journal.pone.0110348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamilton SR, Smith RR, Cameron JL. Prevalence and characteristics of barrett esophagus in patients with adenocarcinoma of the esophagus or esophagogastric junction. Hum Pathol. 1988;19:942–948. doi: 10.1016/s0046-8177(88)80010-8. [DOI] [PubMed] [Google Scholar]
- 8.Hoff SJ, Sawyers JL, Blanke CD, Choy H, Stewart JR. Prognosis of adenocarcinoma arising in barrett's esophagus. Ann Thorac Surg. 1998;65:176–180. doi: 10.1016/s0003-4975(97)01178-8. discussion 180-171. [DOI] [PubMed] [Google Scholar]
- 9.Sabel MS, Pastore K, Toon H, Smith JL. Adenocarcinoma of the esophagus with and without barrett mucosa. Arch Surg. 2000;135:831–835. doi: 10.1001/archsurg.135.7.831. discussion 836. [DOI] [PubMed] [Google Scholar]
- 10.Doupe DP, Alcolea MP, Roshan A, et al. A single progenitor population switches behavior to maintain and repair esophageal epithelium. Science. 2012;337:1091–1093. doi: 10.1126/science.1218835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan Q, Nicholson AM, Barr H, et al. Identification of lineage-uncommitted, longlived, label-retaining cells in healthy human esophagus and stomach, and in metaplastic esophagus. Gastroenterology. 2013;144:761–770. doi: 10.1053/j.gastro.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 12.Quante M, Bhagat G, Abrams JA, et al. Bile acid and inflammation activate gastric cardia stem cells in a mouse model of barrett-like metaplasia. Cancer Cell. 2012;21:36–51. doi: 10.1016/j.ccr.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seery JP, Watt FM. Asymmetric stem-cell divisions define the architecture of human oesophageal epithelium. Curr Biol. 2000;10:1447–1450. doi: 10.1016/s0960-9822(00)00803-4. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Ouyang H, Yamamoto Y, et al. Residual embryonic cells as precursors of a barrett's-like metaplasia. Cell. 2011;145:1023–1035. doi: 10.1016/j.cell.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalabis J, Oyama K, Okawa T, et al. A subpopulation of mouse esophageal basal cells has properties of stem cells with the capacity for self-renewal and lineage specification. J Clin Invest. 2008;118:3860–3869. doi: 10.1172/JCI35012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garman KS, Orlando RC, Chen XL. Experimental models for barrett's esophagus and esophageal adenocarcinoma. Am J Physiol Gastrointest Liver Physiol. 2012 doi: 10.1152/ajpgi.00509.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi L, Der R, Ma Y, Peters J, Demeester T, Chandrasoma P. Gland ducts and multilayered epithelium in mucosal biopsies from gastroesophageal-junction region are useful in characterizing esophageal location. Dis Esophagus. 2005;18:87–92. doi: 10.1111/j.1442-2050.2005.00456.x. [DOI] [PubMed] [Google Scholar]
- 18.Chandrasoma PT, Der R, Ma Y, Dalton P, Taira M. Histology of the gastroesophageal junction: An autopsy study. Am J Surg Pathol. 2000;24:402–409. doi: 10.1097/00000478-200003000-00009. [DOI] [PubMed] [Google Scholar]
- 19.DeNardi FG, Riddell RH. The normal esophagus. Am J Surg Pathol. 1991;15:296–309. doi: 10.1097/00000478-199103000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Medeiros LJ, Doos WG, Balogh K. Esophageal intramural pseudodiverticulosis: A report of two cases with analysis of similar, less extensive changes in "normal" autopsy esophagi. Hum Pathol. 1988;19:928–931. doi: 10.1016/s0046-8177(88)80008-x. [DOI] [PubMed] [Google Scholar]
- 21.Lorinc E, Oberg S. Submucosal glands in the columnar-lined oesophagus: Evidence of an association with metaplasia and neosquamous epithelium. Histopathology. 2012;61:53–58. doi: 10.1111/j.1365-2559.2012.04180.x. [DOI] [PubMed] [Google Scholar]
- 22.Al Yassin TM, Toner PG. Fine structure of squamous epitheilum and submucosal glands of human oesophagus. J Anat. 1977;123:705–721. [PMC free article] [PubMed] [Google Scholar]
- 23.Long JD, Orlando RC. Esophageal submucosal glands: Structure and function. Am J Gastroenterol. 1999;94:2818–2824. doi: 10.1111/j.1572-0241.1999.1422_b.x. [DOI] [PubMed] [Google Scholar]
- 24.Hopwood D, Coghill G, Sanders DS. Human oesophageal submucosal glands. Their detection mucin, enzyme and secretory protein content. Histochemistry. 1986;86:107–112. doi: 10.1007/BF00492353. [DOI] [PubMed] [Google Scholar]
- 25.Biddlestone LR, Barham CP, Wilkinson SP, Barr H, Shepherd NA. The histopathology of treated barrett's esophagus: Squamous reepithelialization after acid suppression and laser and photodynamic therapy. Am J Surg Pathol. 1998;22:239–245. doi: 10.1097/00000478-199802000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Glickman JN, Chen YY, Wang HH, Antonioli DA, Odze RD. Phenotypic characteristics of a distinctive multilayered epithelium suggests that it is a precursor in the development of barrett's esophagus. Am J Surg Pathol. 2001;25:569–578. doi: 10.1097/00000478-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Coad RA, Woodman AC, Warner PJ, Barr H, Wright NA, Shepherd NA. On the histogenesis of barrett's oesophagus and its associated squamous islands: A threedimensional study of their morphological relationship with native oesophageal gland ducts. J Pathol. 2005;206:388–394. doi: 10.1002/path.1804. [DOI] [PubMed] [Google Scholar]
- 28.McClave SA, Boyce HW, Jr, Gottfried MR. Early diagnosis of columnar-lined esophagus: A new endoscopic diagnostic criterion. Gastrointest Endosc. 1987;33:413–416. doi: 10.1016/s0016-5107(87)71676-9. [DOI] [PubMed] [Google Scholar]
- 29.Harris PATR, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (redcap) - a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lipsitz SR, Kim K, Zhao L. Analysis of repeated categorical data using generalized estimating equations. Statistics in medicine. 1994;13:1149–1163. doi: 10.1002/sim.4780131106. [DOI] [PubMed] [Google Scholar]
- 31.Leedham SJ, Preston SL, McDonald SA, et al. Individual crypt genetic heterogeneity and the origin of metaplastic glandular epithelium in human barrett's oesophagus. Gut. 2008;57:1041–1048. doi: 10.1136/gut.2007.143339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braxton DR, Nickleach DC, Liu Y, Farris AB., 3rd Necrotizing sialometaplasia-like change of the esophageal submucosal glands is associated with barrett's esophagus. Virchows Arch. 2014;465:135–143. doi: 10.1007/s00428-014-1590-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicholson AM, Graham TA, Simpson A, et al. Barrett's metaplasia glands are clonal, contain multiple stem cells and share a common squamous progenitor. Gut. 2012;61:1380–1389. doi: 10.1136/gutjnl-2011-301174. [DOI] [PubMed] [Google Scholar]
- 34.Huch M. Regenerative biology: The versatile and plastic liver. Nature. 2015;517:155–156. doi: 10.1038/517155a. [DOI] [PubMed] [Google Scholar]
- 35.Tarlow BD, Pelz C, Naugler WE, et al. Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes. Cell stem cell. 2014;15:605–618. doi: 10.1016/j.stem.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strobel O, Rosow DE, Rakhlin EY, et al. Pancreatic duct glands are distinct ductal compartments that react to chronic injury and mediate shh-induced metaplasia. Gastroenterology. 2010;138:1166–1177. doi: 10.1053/j.gastro.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi G, DiRenzo D, Qu C, Barney D, Miley D, Konieczny SF. Maintenance of acinar cell organization is critical to preventing kras-induced acinar-ductal metaplasia. Oncogene. 2013;32:1950–1958. doi: 10.1038/onc.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang SW, Chang CS, Wang J, Yeh HZ. Early adenocarcinoma originating in submucosal gland of thoracic esophagus presenting as submucosal tumor. Endoscopy. 2008;40(Suppl 2):E237–E238. doi: 10.1055/s-2008-1077680. [DOI] [PubMed] [Google Scholar]
- 39.Goetsch E. The structure of the mammalian oesophagus. The American Journal of Anatomy. 1910;10:1–40. [Google Scholar]
- 40.Chadwick G, Groene O, Markar SR, Hoare J, Cromwell D, Hanna GB. Systematic review comparing radiofrequency ablation and complete endoscopic resection in treating dysplastic barrett's esophagus: A critical assessment of histologic outcomes and adverse events. Gastrointest Endosc. 2014;79:718–731. e713. doi: 10.1016/j.gie.2013.11.030. [DOI] [PubMed] [Google Scholar]
- 41.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in barrett's esophagus with dysplasia. N Engl J Med. 2009;360:2277–2288. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 42.Gupta M, Iyer PG, Lutzke L, et al. Recurrence of esophageal intestinal metaplasia after endoscopic mucosal resection and radiofrequency ablation of barrett's esophagus: Results from a us multicenter consortium. Gastroenterology. 2013;145:79–86. e71. doi: 10.1053/j.gastro.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.