Abstract
Background
Appalachian residents have a higher overall cancer burden than the rest of the United States because of the unique features of the region. Treatment delays vary widely within Appalachia, with colorectal cancer patients undergoing median treatment delays of 5 days in Kentucky compared to 9 days for patients in Pennsylvania, Ohio, and North Carolina combined.
Objective
This study identified the source of this disparity in treatment delay using statistical decomposition techniques.
Methodology
This study used linked 2006 to 2008 cancer registry and Medicare claims data for the Appalachian counties of Kentucky, Pennsylvania, Ohio, and North Carolina to estimate a 2-part model of treatment delay. An Oaxaca Decomposition of the 2-part model revealed the contribution of the individual determinants to the disparity in delay between Kentucky counties and the remaining 3 states.
Results
The Oaxaca Decomposition revealed that the higher percentage of patients treated at for-profit facilities in Kentucky proved the key contributor to the observed disparity. In Kentucky, 22.3% patients began their treatment at a for-profit facility compared to 1.4% in the remaining states. Patients initiating treatment at for-profit facilities explained 79% of the observed difference in immediate treatment (<2 days after diagnosis) and 72% of Kentucky’s advantage in log days to treatment.
Conclusions
The unique role of for-profit facilities led to reduced treatment delay for colorectal cancer patients in Kentucky. However, it remains unknown whether for-profit-hospitals’ more rapid treatment converts to better health outcomes for colorectal cancer patients.
Keywords: Appalachia, colorectal cancer, decomposition analysis, disparities
Treatment delay remains an important topic for patients, academics, and the cancer advocacy communities. Cancer patients frequently view timeliness of care as an important quality indicator for cancer treatment.1 Similarly, recent academic work showing longer colorectal cancer (CRC) treatment delays at Veterans Affairs (VA) hospitals2 has led to a rash of negative headlines for the VA and questions about whether the treatment delays led to veterans’ deaths. Finally, advocacy by the American Cancer Society frequently indicates that access to timely, quality care is a key barrier to winning the war on cancer.3
The Institute of Medicine has shown that minority, poor, and other medically underserved communities are the least likely to survive a cancer diagnosis.4 In this paper, we focus on cancer treatment disparities within one medically underserved community, Appalachia. Appalachia served as the focal point for the launch of the “War on Poverty” in the 1960s, but in 2014, much of Appalachia still faces a combination of both high morbidity and high poverty. Appalachian residents have a higher overall cancer burden than the rest of the United States, with a colorectal cancer incidence of 66.7 per 100,000 persons compared to 59.7 for the rest of the country.5 Colorectal cancer incidence varies widely across the region from a high of 71.0 in northern Appalachia to a low of 59.2 in southern Appalachia. Higher levels of poverty and geographic isolation contribute to Appalachia’s overall elevated morbidity compared to the nation, but little is known about the determinants of disparities within Appalachia.6,7
Previous work has suggested that the inadequate availability of cancer care resources is a potential contributor to Appalachian cancer disparities.8 Only a limited supply of health care resources is available to treat Appalachia’s elevated cancer burden. As of 2004, the Centers for Medicare and Medicaid Services (CMS) classified 297 of 410 counties (72%) in Appalachia as health professional shortage areas,9 and the closest available National Cancer Institute (NCI) comprehensive cancer centers (of Birmingham, Alabama; Winston-Salem, North Carolina; and Pittsburgh, Pennsylvania) are found at the outer edges of Appalachia. Moreover, research on this population is hampered by the fact that Appalachia is not included in the Surveillance, Epidemiology, and End Results Programs (SEER) data, with the exception of Kentucky and Georgia.
Perspectives on treatment delay vary widely. From the patient and advocacy perspective, timely care is viewed as better care. The time after a cancer diagnosis represents extreme stress for both patients and families facing frequently uncoordinated care. This concern has led to multiple experiments using patient navigators to facilitate and reduce delays in the system.10 From a clinical perspective, it remains unclear whether treatment delay affects survivability. Recent reviews are mixed with some indicating prompt care reduces mortality while others find no link between treatment delay and colorectal cancer mortality.11,12
This paper decomposes the determinants of treatment delays in Appalachian Kentucky compared to the Appalachian counties of 3 other states (North Carolina, Ohio, and Pennsylvania), utilizing a unique database created by linking state cancer registries and Medicare claims data. We make 2 new contributions to the literature. First, we find that treatment delay in these Appalachian counties compares favorably with other multi-state estimates. Our estimates indicate that colorectal cancer patients face a median treatment delay of 5 days in Kentucky and 9 days for patients in Pennsylvania, Ohio, and North Carolina. Second, we use the Oaxaca Decomposition to explain these disparities within Appalachia. Our 2-part model estimates the determinants of (1) whether the patient receives immediate treatment (< 2 days from diagnosis) and (2) the number of days between diagnosis and treatment if ≥ 2 days. The Oaxaca Decomposition of the 2-part model results reveals that observed differences in covariates can explain the entire disparity in treatment delay. The key determinant driving the observed disparity in treatment delay is the higher percentage of patients treated at for-profit facilities in Kentucky.
Methods
Defining the Sample
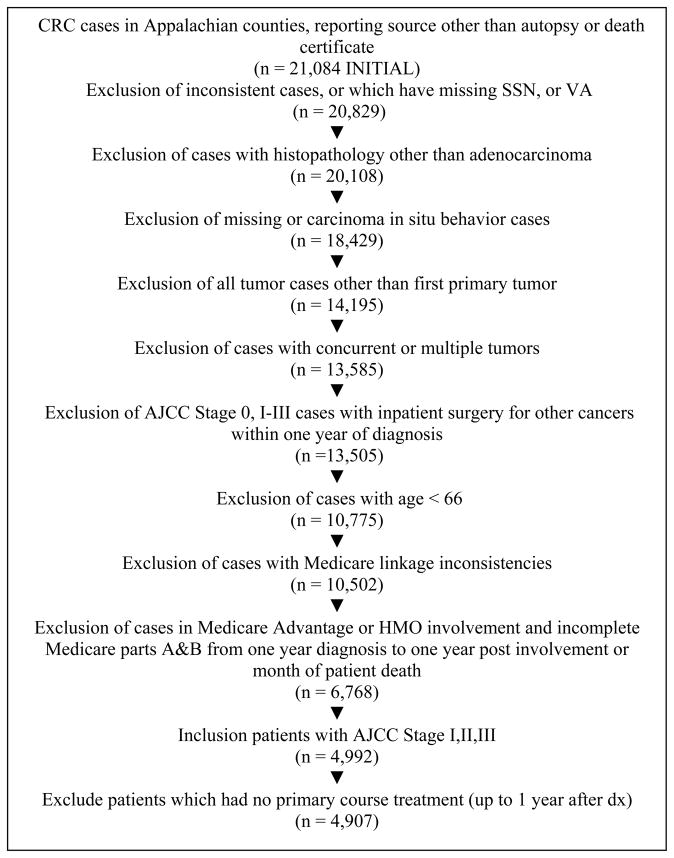
Cases were collected from 4 state cancer registries (Kentucky, Ohio, Pennsylvania, and North Carolina) for CRC patients diagnosed during a 3-year period 2006–2008 in 167 Appalachian counties as defined by the Appalachian Regional Commission (ARC).. Inclusion and exclusion criteria are detailed in Figure 1. The initial sample consisted of 21,084 cases reported in Appalachian counties.
Figure 1.
Study Participants and Exclusion Criteria for Colorectal Cancer Patients Living in Appalachian Counties in 4 States 2006–2008
Only cases with adenocarcinoma malignant behavior, with a first primary non-concurrent tumor, no inpatient surgery for other cancers, and with a patient age greater than 65 were included the sample. These criteria produced a provisional total of 10,502 cases. Furthermore, cases had to be FFS (fee for service) Medicare enrolled in parts A&B during 1 year prior and 1 year after the diagnosis or until patient’s death, have American Joint Committee on Cancer (AJCC) stage I-III, and have treatment (as defined in the section below) recorded during the year after diagnosis. The final sample consisted of 4,907 cases.
Definition of the Measures Used
Chemotherapy, radiation, and oncologic resection treatment dates were identified from the Medicare claims stream for each patient (available on request from the authors), starting from 2 weeks before to 1 year after the confirmed diagnosis date recorded in the Registry. The date of Central Cancer Registry (CCR) primary site surgery was initially assigned as the earliest treatment date. If no CCR surgery date was present, the earliest resection date, if found in the Medicare claims stream, was chosen as the earliest treatment date. The earliest treatment date was shifted back if evidence of chemotherapy or radiation treatment was found using the Medicare claims stream or CCR treatment date.
We selected treatment facility associated with initiation by selecting either CCR reporting facility and/or claims-specific facilities associated within treatment initiation. We used the CCR class of case variable, which identified whether reporting facility was either the diagnosis facility, treatment facility, or both. In 4,639 (94.5%) of the cases, reporting facility, claim-based facility, and class of cases were in agreement with regard to treatment facility. In 240 (4.9%) of the cases, claim-based facility was used to indicate treatment facility, as reporting facility was only diagnosis facility. In 28 (0.6%) of the cases, assignment was subject to uncertainty as the claim-based facility was missing and the reporting facility was listed as a diagnosis facility only. In these cases, we decided to treat the reporting facility as a replacement for treatment facility. In addition, we identified the performing provider associated with the most extensive surgery during the year after diagnosis using a study-specific algorithm (available upon request).
Predictors of time until treatment included the following variables extracted from the CCR: patient age at diagnosis, gender, race/Hispanic combination variable derived from the corresponding CCR fields, state of residence, metropolitan/non-metropolitan county, collaborative stage tumor size, AJCC-derived stage grouping, cancer grade, and histology type. Metro/non-metro location was a county-level variable based on patient address at time of diagnosis and the 2003 USDA rural-urban continuum codes.13 A claims-based Adult Comorbidity Evaluation-27 (ACE-27) measure was selected as a measure of comorbidity burden based on 26 different comorbidities with 4 levels of severity: none, mild, moderate, and severe.14 Treatment indicators were also calculated combining information from the Medicare claims stream and CCR fields to flag oncological surgery, chemotherapy, and radiation treatment within 30 days of CRC diagnosis. Additionally, for each case, type of cancer was subdivided into colon cancer (ICD-O-3 C180–C189) and rectal cancer (C199 rectoid-sigmoid, C209 rectum).
Selected facility characteristics were linked to each case using the Provider of Service file available from the CMS, including total number of beds in the facility, type of control (ie, for-profit, government-owned, non-profit), and radiology services offered. The Facility Commission of Cancer status (COC) variable was created by searching the COC website Facility locator from 2011.15 Surgical provider specialty was identified from Internet sources.16,17 Medicare Physician Identification and Eligibility Registry (MPIER) and the National Provider Identification File, both from the CMS, were used in this study.
We derived 2 additional volumes of care variables from 2008 Medicare claims associated with facilities and providers in our sample. We examined all claims with procedure codes for colorectal cancer resection procedures (colectomies, procto-sigmoidectomies, protectomies, colostomies/ileostomies) associated with ICD-9 colorectal cancer diagnosis codes (153,154,154.8) and facility or provider of interest. We then summed all non-duplicate unique claims associated with facility or provider and categorized them into quartiles.
Finally, an approximate road distance variable between patient residence at diagnosis and treatment facility at initiation was created by estimating distances between Zip code centroids by querying Google maps for each case.
Due to the large fraction of patients who had treatment 2 days or earlier from confirmed diagnosis, time to treatment was modeled assuming that 2 processes were at work. The first process determined whether the patient received treatment within 2 days, considered as “immediate treatment”; the second process determined time to treatment conditional on the treatment not being immediate. This led to implementing a 2-part model18 in 2 steps. In the first step, the determinants of immediate treatment were evaluated by fitting a Linear Probability Model (LPM) with robust standard errors. In the second step, the determinants of delayed treatment were determined by fitting an ordinary least squares (OLS) model on the logarithm of time (in days).
To identify the main contributors to the treatment time disparity between Kentucky and other Appalachian states, we applied an Oaxaca Decomposition in each of these 2 steps,19 a technique developed to identify determinants of gender differences in labor markets which has also been extended to explain health care disparities.20 We implemented a 2-way decomposition, which separates the difference in expected means between both groups into 2 components separately viewed as “difference in characteristics” and “difference in coefficients.” Formula 1 illustrates the decomposition in matrix notation:
Where β̂KY, β̂OTH are coefficient estimates vectors corresponding to each group, X̄KY, X̄OTH are the sample average of covariates vectors, and ȲKY, ȲOTH are the expected values. The first term in the decomposition represents the weighted sum of difference in averages, where the weights are KY coefficients, and the second term represents the weighted sum of difference in coefficients, where the weights are the covariate averages from the non-KY group. These 2 terms and their components can be statistically tested. The Stata version 12.1 Oaxaca package was used to perform the decomposition (StataCorp LP, College Station, Texas).
Results
Table 1 shows the descriptive statistics for the sample. More patients (44.2%) in Kentucky received immediate treatment (0–2 days from diagnosis) when compared to CRC patients in NC, OH, and PA combined (37.7%). Similarly, more Kentucky patients (84.7%) were receiving treatment in the first month (compared to 76.2% among patients in NC, OH, and PA combined. Furthermore, patients in Kentucky faced a median treatment delay of 5 days compared to 9 days in the other 3 states.
Table 1.
Days to Treatment After Diagnosis for Colorectal Cancer Patients - Sample Descriptive Statistics
| Kentucky (%) | NC+OH+PA (%) | All 4 States (%) | |
|---|---|---|---|
| Number of Cases | 561 | 4,346 | 4,907 |
| Median Days From Diagnosis to Treatment | 5.0 | 9.0 | 8.0 |
| Days From Diagnosis to Treatment | |||
| 0–2 Days | 44.2 | 37.7 | 38.5 |
| 3–29 Days | 40.5 | 38.5 | 38.7 |
| 30–59 Days | 12.5 | 18.3 | 17.7 |
| 60+ Days | 2.9 | 5.4 | 5.1 |
| Age | |||
| 66–73 | 41.0 | 29.2 | 30.5 |
| 74–80 | 30.5 | 31.3 | 31.2 |
| 81+ | 28.5 | 39.6 | 38.3 |
| Gender | |||
| Female | 45.3 | 54.9 | 53.8 |
| Male | 54.7 | 45.1 | 46.2 |
| Race | |||
| White, Non-Hispanic | 98.9 | 97.0 | 97.2 |
| Black, Non-Hispanic | 0.5 | 2.1 | 1.9 |
| Other, Non-Hispanic | 0.2 | 0.3 | 0.3 |
| Hispanic | 0.4 | 0.4 | 0.4 |
| State | |||
| Kentucky | 100.0 | 0.0 | 11.4 |
| North Carolina | 0.0 | 16.5 | 14.6 |
| Ohio | 0.0 | 23.2 | 20.5 |
| Pennsylvania | 0.0 | 60.4 | 53.5 |
| Tumor Size | |||
| < 0.5 cm | 0.5 | 1.1 | 1.0 |
| 0.5 – < 2 cm | 8.0 | 9.0 | 8.8 |
| 2 – 4 cm | 31.0 | 28.9 | 29.1 |
| 4 + cm | 44.2 | 49.2 | 48.6 |
| Not Available | 16.2 | 11.9 | 12.4 |
| Stage | |||
| Stage I | 33.7 | 31.3 | 31.5 |
| Stage II | 32.3 | 37.2 | 36.6 |
| Stage III | 34.0 | 31.6 | 31.9 |
| Grade | |||
| Well differentiated | 6.1 | 9.8 | 9.4 |
| Moderately differentiated | 67.9 | 67.6 | 67.7 |
| Poorly/undifferentiated | 18.0 | 17.7 | 17.7 |
| Not determined/not applicable | 8.0 | 4.8 | 5.2 |
| Histology | |||
| Adenocarcinoma from polyp | 20.1 | 15.5 | 16.1 |
| Adenocarcinoma, NOS | 70.4 | 73.4 | 73.1 |
| Mucinous adenocarcinoma | 8.9 | 10.2 | 10.0 |
| Signet ring carcinoma | 0.5 | 0.9 | 0.9 |
| ACE Severity | |||
| None | 13.0 | 12.1 | 12.2 |
| Mild | 40.5 | 39.9 | 40.0 |
| Moderate | 16.0 | 16.8 | 16.8 |
| Severe | 30.5 | 31.1 | 31.0 |
| ONC surgery within 30 days of treatment initiation | |||
| No | 5.7 | 6.5 | 6.4 |
| Yes | 94.3 | 93.5 | 93.6 |
| Chemotherapy within 30 days of treatment initiation | |||
| No | 89.1 | 92.0 | 91.7 |
| Yes | 10.9 | 8.0 | 8.3 |
| Radiation within 30 days of treatment initiation | |||
| No | 93.6 | 93.1 | 93.2 |
| Yes | 6.4 | 6.9 | 6.8 |
| Type of Cancer | |||
| Colon Cancer | 76.6 | 78.0 | 77.9 |
| Rectal Cancer | 23.4 | 22.0 | 22.1 |
| Facility bed size | |||
| < 50 | 5.3 | 3.5 | 3.7 |
| 50–100 | 12.5 | 6.2 | 6.9 |
| 100–200 | 27.3 | 19.3 | 20.2 |
| 200–500 | 54.4 | 42.4 | 43.8 |
| 500+ | 0.4 | 27.6 | 24.5 |
| COC status of facility where treatment initiated | |||
| Community Cancer Program | 29.6 | 11.5 | 13.6 |
| Comprehensive Community Cancer Program | 20.7 | 35.4 | 33.7 |
| NCI Designated Comprehensive | 0.0 | 4.6 | 4.1 |
| Network Cancer Program | 0.0 | 2.2 | 2.0 |
| No designation | 45.6 | 31.7 | 33.3 |
| Teaching Hospital Cancer Program | 3.7 | 13.8 | 12.7 |
| Facility Ownership Status | |||
| For profit | 22.3 | 1.4 | 3.8 |
| Government | 8.0 | 6.5 | 6.6 |
| Not-for profit | 69.5 | 91.4 | 88.9 |
| Metropolitan Status | |||
| Metro | 14.3 | 60.1 | 54.9 |
| Non-metro | 85.7 | 39.9 | 45.1 |
| Resection volume at facility where treatment initiated | |||
| Quartile 1 | 37.3 | 22.5 | 24.2 |
| Quartile 2 | 28.2 | 24.7 | 25.1 |
| Quartile 3 | 16.4 | 26.2 | 25.1 |
| Quartile 4 | 18.0 | 25.3 | 24.4 |
| Radiation treatment offered at facility where treatment initiated | |||
| No | 19.3 | 23.2 | 22.7 |
| Yes | 80.6 | 76.0 | 76.6 |
| Surgery provider resection volume | |||
| Quartile 1 | 18.7 | 18.1 | 18.1 |
| Quartile 2 | 30.7 | 29.8 | 29.9 |
| Quartile 3 | 18.4 | 22.6 | 22.1 |
| Quartile 4 | 25.1 | 22.0 | 22.4 |
| Surgery provider specialty | |||
| Colorectal Surgery | 10.2 | 15.6 | 15.0 |
| Other | 82.5 | 76.8 | 77.4 |
| Year of diagnosis | |||
| 2006 | 34.6 | 37.4 | 37.0 |
| 2007 | 35.7 | 33.4 | 33.6 |
| 2008 | 29.8 | 29.2 | 29.3 |
| Class of case | |||
| DX elsewhere, TX @ REP facility | 15.5 | 12.7 | 13.1 |
| Dx and Tx @ REP facility | 83.2 | 82.1 | 82.3 |
| Other | 1.2 | 5.1 | 4.7 |
| Distance quartile | |||
| Quartile 1 | 14.1 | 26.3 | 24.9 |
| Quartile 2 | 12.3 | 26.5 | 24.9 |
| Quartile 3 | 25.1 | 24.8 | 24.8 |
| Quartile 4 | 48.3 | 21.7 | 24.7 |
Table 2 presents the coefficients from the 2-part model. The first 2 columns list the coefficients from the linear probability model predicting whether the patient received treatment within the first 2 days of diagnosis for (1) Kentucky and (2) the comparison states. In this model, the outcome variable is coded as Y=1 if treatment was received within 0–2 days and Y=0 if delay was greater than 2 days. Positive coefficients indicate that the patient was more likely to receive timely treatment within the first 2 days of diagnosis. The statistically significant coefficients illustrate who receives immediate treatment after diagnosis. Patients starting treatment within 2 days of diagnosis were more likely to have larger tumors, have a lower comorbidity burden, receive treatment at for-profit facilities, have a lower volume surgeon, and receive treatment at their diagnosis facility. Although many of the coefficient estimates appear different across the 2 models, only rural status and the facility’s bed size had differing effects across the 2 groups of states (P = .05). These differences will be discussed with the decomposition results.
Table 2.
Two-part Model Estimates of Days From Diagnosis to Treatment Initiation for Colorectal Cancer Patients
| Treatment Within 2 days of Diagnosis | Log of Days to Treatment if > 2 days | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kentucky | PA, OH, NC | Kentucky | PA, OH, NC | |||||||||||
| Coef. | Std. Err. | Coef. | Std. Err. | Coef. | Std. Err. | Coef. | Std. Err. | |||||||
| Female | −0.042 | 0.0459 | 0.007 | 0.0154 | −0.123 | 0.1033 | −0.053 | 0.0366 | ||||||
| Ages 66–73 | −0.031 | 0.0541 | 0.013 | 0.0197 | −0.086 | 0.1212 | 0.064 | 0.0462 | ||||||
| Ages 81+ | −0.032 | 0.0567 | 0.031 | 0.0181 | * | −0.103 | 0.1254 | −0.080 | 0.0434 | * | ||||
| White | 0.209 | 0.2113 | 0.097 | 0.0458 | ** | 0.271 | 0.4040 | 0.116 | 0.1038 | |||||
| Rural | −0.080 | 0.0905 | 0.032 | 0.0178 | * | ++ | 0.215 | 0.2004 | 0.010 | 0.0435 | ||||
| Year of Diagnosis = 2006 | −0.035 | 0.0556 | −0.039 | 0.0186 | ** | 0.038 | 0.1262 | −0.081 | 0.0449 | * | ||||
| Year of Diagnosis = 2007 | −0.046 | 0.0554 | −0.032 | 0.0190 | * | −0.243 | 0.1225 | ** | −0.032 | 0.0456 | ||||
| Tumor size: < 2 cm | 0.029 | 0.0849 | 0.039 | 0.0269 | 0.019 | 0.1849 | 0.335 | 0.0650 | *** | |||||
| Tumor size: 2 cm – 4 cm | −0.086 | 0.0508 | * | −0.062 | 0.0170 | *** | 0.191 | 0.1122 | * | 0.211 | 0.0398 | *** | + | |
| Tumor Grade = Well Differentiated | 0.109 | 0.1120 | 0.114 | 0.0306 | *** | −0.095 | 0.2831 | 0.091 | 0.0775 | |||||
| Tumor Grade = Moderately Differentiated | −0.066 | 0.0579 | −0.003 | 0.0199 | 0.081 | 0.1323 | 0.119 | 0.0473 | ** | |||||
| ACE Severity = Low and None | −0.224 | 0.0752 | *** | −0.156 | 0.0260 | *** | 0.045 | 0.1576 | 0.183 | 0.0602 | *** | |||
| ACE Severity = Mild | −0.110 | 0.0549 | ** | −0.118 | 0.0181 | *** | 0.001 | 0.1285 | 0.164 | 0.0442 | *** | |||
| ACE Severity = Moderate | −0.014 | 0.0671 | −0.082 | 0.0224 | *** | 0.021 | 0.1578 | 0.079 | 0.0550 | |||||
| Facility bed size: < 50 | −0.001 | 0.1667 | 0.119 | 0.0510 | ** | −0.508 | 0.3491 | 0.321 | 0.1316 | ** | ++ | |||
| Facility bed size: 50 – 99 | 0.105 | 0.1200 | 0.063 | 0.0407 | −0.225 | 0.2643 | 0.269 | 0.0998 | *** | + | ||||
| Facility bed size: 100 – 199 | 0.165 | 0.0850 | * | 0.005 | 0.0249 | ++ | −0.503 | 0.2011 | ** | 0.111 | 0.0598 | * | +++ | |
| COC Status = None | 0.040 | 0.0612 | −0.019 | 0.0207 | 0.109 | 0.1334 | −0.113 | 0.0488 | ** | |||||
| Public Facility | −0.190 | 0.1112 | * | −0.009 | 0.0786 | 1.053 | 0.2335 | *** | 0.015 | 0.2060 | +++ | |||
| Not-for-Profit Facility | −0.187 | 0.0747 | ** | −0.090 | 0.0722 | 0.679 | 0.1862 | *** | 0.040 | 0.1889 | ++ | |||
| Facility surgery vol. = Quartile 1 | −0.030 | 0.1032 | 0.025 | 0.0317 | 0.031 | 0.2254 | −0.194 | 0.0746 | *** | |||||
| Facility surgery vol. = Quartile 2 | 0.059 | 0.0988 | 0.122 | 0.0242 | *** | 0.337 | 0.2225 | −0.152 | 0.0588 | *** | +++ | |||
| Facility surgery vol. = Quartile 3 | −0.118 | 0.1055 | 0.032 | 0.0216 | 0.349 | 0.2230 | 0.026 | 0.0501 | ||||||
| Radiation treatment available at facility | 0.076 | 0.0955 | −0.025 | 0.0214 | 0.250 | 0.2199 | 0.017 | 0.0531 | ||||||
| Surgeon volume quartiles 1–3 | 0.028 | 0.0611 | 0.086 | 0.0187 | *** | 0.027 | 0.1285 | −0.118 | 0.0428 | *** | ||||
| Tumor Stage I | −0.019 | 0.0643 | −0.029 | 0.0212 | 0.266 | 0.1373 | * | 0.144 | 0.0497 | *** | ||||
| Tumor Stage II | 0.020 | 0.0533 | −0.027 | 0.0180 | 0.190 | 0.1187 | 0.020 | 0.0428 | ||||||
| Chemotherapy within 30 days of treatment start | −0.098 | 0.0926 | −0.037 | 0.0429 | 0.203 | 0.2026 | −0.146 | 0.0971 | ++ | |||||
| Radiation within 30 days of treatment start | −0.186 | 0.1300 | −0.230 | 0.0488 | *** | 0.080 | 0.2592 | 0.399 | 0.1048 | *** | ||||
| Distance = Quartile 1 | 0.001 | 0.0804 | −0.033 | 0.0249 | 0.305 | 0.1866 | −0.186 | 0.0600 | *** | ++ | ||||
| Distance = Quartile 2 | 0.040 | 0.0832 | −0.039 | 0.0246 | 0.212 | 0.1927 | −0.199 | 0.0587 | *** | ++ | ||||
| Distance = Quartile 3 | 0.027 | 0.0653 | −0.014 | 0.0239 | −0.058 | 0.1560 | −0.138 | 0.0571 | ** | |||||
| Diagnosis and Treatment at same Facility | 0.286 | 0.0640 | *** | 0.214 | 0.0211 | *** | −0.323 | 0.1249 | *** | −0.294 | 0.0456 | *** | ||
| Constant | 0.188 | 0.2689 | 0.197 | 0.0969 | ** | 1.698 | 0.5838 | *** | 3.025 | 0.2418 | *** | ++ | ||
P < .1,
P < .05,
P < .01
Significant difference between coefficients, P = .10
Significant difference between coefficients, P = .05
Significant difference between coefficients, P = .01
The second group of coefficients in Table 2 shows that Kentucky differs fundamentally from the other 3 states for its determinants predicting the log of days until treatment, conditional upon a treatment delay greater than 2 days. In contrast to the results of the first step of the 2-part model in columns 1 and 2, the positive coefficients in the second 2 columns indicate longer treatment delays. Unlike the determinants of immediate treatment (<2 days), no standard profile emerges across the 2 groups of states, with significant differences between the coefficients for a third of the determinants.
Table 3 provides a summary of the Oaxaca Decomposition results (the full set of coefficients are available upon request). The estimates from the first step of the 2-part model indicate that a patient receiving treatment in Kentucky would have a predicted probability of 40.8% to receive treatment within 2 days of diagnosis. In contrast, patients receiving treatment in Pennsylvania, Ohio, or North Carolina can expect a 35.2% chance of treatment within 2 days. Of the 5.6 percentage point gap, observed differences in the covariates can explain more than the entire difference (11 percentage points) between Kentucky and the other states. With few coefficients significantly different across the 2 groups, the portion of the gap between Kentucky and the other states due to the coefficients is not significantly different from zero.
Table 3.
Oaxaca Decomposition – Two-part Model Estimates of Days From Diagnosis to Treatment Initiation
| Treatment Within 2 days of Diagnosis | Coef. | Std. Err. | |
|---|---|---|---|
| Kentucky - Predicted probability of treatment within 2 days | 0.408 | 0.024 | *** |
| PA, OH, NC - Predicted probability of treatment within 2 days | 0.352 | 0.013 | *** |
| Net Difference | 0.056 | 0.027 | ** |
| Difference due to observed characteristics | 0.106 | 0.044 | ** |
| Difference due to coefficients | −0.050 | 0.044 |
| Log of Days to Treatment if > 2 days | Coef. | Std. Err. | |
|---|---|---|---|
| Kentucky - Predicted log of days until treatment | 2.763 | 0.056 | *** |
| PA, OH, NC - Predicted log of days until treatment | 2.981 | 0.029 | *** |
| Net Difference | −0.218 | 0.063 | *** |
| Difference due to observed characteristics | −0.300 | 0.096 | *** |
| Difference due to coefficients | 0.082 | 0.110 |
P < .1,
P < .05,
P < .01
The key determinant driving the observed differences is a higher percentage of patients treated at for-profit facilities in Kentucky. In Kentucky, 22.3% of patients began their treatment at a for-profit facility compared to 1.4% in the remaining states. With the higher likelihood of immediate treatment (< 2 days) at for-profit facilities, more patients treated at for-profit facilities explain 79% of the observed difference (4.4 of 5.6 percentage points). No other observed difference in determinants is significant at the P = .10 level.
Table 3 also indicates that most of the gap in the log of days to treatment can be explained by differences in the covariates. In Kentucky, the models predict that CRC patients who do not receive immediate treatment (< 2 days) can expect a delay of 2.76 log days (15.8 days). Similarly, patients in the other 3 states can expect delays of 3.0 log days (19.7 days). Of the 0.24 log day difference between Kentucky and the other states, the covariates can explain more than the entire difference (0.30 log days). As before, facility ownership is the only significant difference in the observed determinants. Specifically, the difference in for-profit and not-for-profit ownership covers 72% of the difference in log of days until treatment.
Unlike the first step of the 2-part model, many coefficients differ between Kentucky and the other states for the determinants of log of days to treatment. The facility size, facility ownership, facility surgery volume, and distance to the treatment facility variables all have a different effect in Kentucky than they do in the other states (P = .05). However, the decomposition in Table 3 indicates that these differences in coefficients cancel out and make no significant contribution to the net difference in log days to treatment.
Discussion
This study found that colorectal cancer patients in the Appalachian counties of Kentucky face a median treatment delay of 5 days compared to 9 days in the Appalachian counties of North Carolina, Ohio, and Pennsylvania. Statistical techniques developed to examine wage disparities reveal that this treatment disparity can be explained by observable factors. Of these observable covariates, treatment at for-profit facilities proved particularly important for the reduced treatment delay in Kentucky. The Oaxaca Decomposition showed treatment in for-profit facilities explains 79% of Kentucky’s advantage in immediate treatment and 72% of Kentucky’s advantage in log days to treatment. This finding that for-profit facilities drive Kentucky’s reduced treatment delay faces a very important limitation. It remains unclear whether the more rapid treatment in Kentucky leads to better outcomes.
Benchmarks and standards for treatment delay for colorectal cancers in the US are limited. The median 8-day delay faced by Appalachian patients compares favorably with other multi-state estimates. One study using SEER data found a median treatment delay of 13 days for colon cancer patients and 16 days for rectal cancer.21 This literature shows that patients receiving treatment at the same facility consistently face shorter delays. Multi-state estimates from the National Cancer Database find a median delay from diagnosis to treatment of 12 days at the same hospital and 21 days if referred to a different facility.22 In this study, we find patients treated in the same facility are more likely to receive immediate treatment within 2 days and shorter delays if not receiving immediate treatment.
It is unclear whether prompt treatment leads to better survivability. A recent review of 40 studies found that the majority did not report a significant link between therapeutic delay and survival.23 The review found several studies with a significant association between longer delays and better survival. Furthermore, some evidence suggests delay may affect colon and rectal cancers differently. In a recent study, treatment delay was negatively associated with long-term rectal cancer survival, while the association was insignificant in case of colon cancer. However, excluding therapeutic delay (the interval from the onset of symptoms until the initiation of treatment), the negative association for rectal cancer disappeared.24,25 Finally, a recent US population-based study also reported that CRC treatment delays of up to 120 days did not appear to elevate the risk of death.26
Our key finding is that patients in Kentucky face a 5-day delay compared to 9 days in the other 3 states. While the delay is only half as long in Kentucky, a 4-day shorter delay is unlikely to improve survivability in these Appalachian counties. First, a 5-day treatment delay or even 9 days is better than the median delay observed in other multi-state studies. Second, other authors have argued that a reasonable delay allows more detailed preoperative evaluation and tumor staging. 27 In that study, a median treatment delay of 23 days was too short for the tumor to advance in stage for most cases. Hence, the authors advanced the argument that triaging or prioritizing cases proved more important than overall treatment delay.
Finally, more for-profit hospitals explained the shorter treatment delays in Kentucky. Recent research has found for-profit hospitals adopting newer, costly treatments for breast cancers.28 This finding is consistent with previous research showing for-profit facilities respond to changes in demand and technology faster than other hospitals. However, the literature finds little difference between not-for-profit and for-profit hospital behavior, especially when compared to hospitals operated by public/government entities.29 Our findings reveal important avenues for further research. First, treatment delays in these Appalachian counties compare favorably with other multi-state estimates. Second, while the statistical decomposition technique we used was first developed to examine wage disparities, it is equally valuable in revealing the source of frequently observed health disparities. Contextual or community factors are as important in understanding health disparities as individual factors. We found that for-profit facilities explained treatment disparities within these Appalachian states, but as mentioned previously, the literature is mixed on whether more rapid treatment converts to improved cancer survival. Future research will be needed to disentangle the relationships between facility ownership, facility quality, treatment delay, and cancer survival.
Acknowledgments
Funding: This work was supported by a grant from the National Cancer Institute (1 R01 CA140335-01A1).
Footnotes
Disclosures: The authors have no financial relationships relevant to this article to disclose and no conflicts of interest to disclose.
References
- 1.Hess LM, Pohl G. Perspectives of Quality Care in Cancer Treatment: A Review of the Literature. Am Health Drug Benefits. 2013;6(6):321–329. [PMC free article] [PubMed] [Google Scholar]
- 2.Merkow RP, Bilimoria KY, Sherman KL, et al. Efficiency of Colorectal Cancer Care Among Veterans: Analysis of Treatment Wait Times at Veterans Affairs Medical Centers. J Oncol Pract. 2013;9(4):e154–e163. doi: 10.1200/JOP.2012.000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Cancer Society. [Accessed February 13, 2015];Expanding Access to Care. 2008 Apr; Available at: http://action.acscan.org/site/DocServer/Policy_-_A2C_CAN_one_pager_w_TQs_and_4As_FINAL.pdf?docID=6822.
- 4.Hewitt M, Greenfield S, Stovall E, editors. Committee on Cancer Survivorship: Improving Care and Quality of Life, Institute of Medicine and National Research Council. Washington, DC: The National Academies Press; 2006. From Cancer Patient to Cancer Survivor: Lost in Transition. [Google Scholar]
- 5.Wingo PA, Tucker TC, Jamison PM, et al. Cancer in Applalachia, 2001–2003. Cancer. 2007;112(1):181–192. doi: 10.1002/cncr.23132. [DOI] [PubMed] [Google Scholar]
- 6.Yao N, Matthews SA, Hillemeir MM, et al. Radiation Therapy Resources and Guideline-Concordant Radiotherapy for Early-Stage Breast Cancer Patients in an Underserved Region. Health Serv Res. 2013;48(4):1433–1449. doi: 10.1111/1475-6773.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lengerich EJ, Tucker TC, Powell RK, et al. Cancer incidence in Kentucky, Pennsylvania, and West Virginia: disparities in Appalachia. J Rural Health. 2005;21(1):39–47. doi: 10.1111/j.1748-0361.2005.tb00060.x. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong LR, Thompson T, Hall HI, Coughlin SS, Steele B, Rogers JD. Colorectal carcinoma mortality among Appalachian men and women, 1969–1999. Cancer. 2004;101(12):2851–2858. doi: 10.1002/cncr.20667. [DOI] [PubMed] [Google Scholar]
- 9.Appalachian Regional Commission. [Accessed February 13, 2015];An Analysis of Disparities in Health Status and Access to Health Care in the Appalachian Region. 2004 Available at: http://www.etsu.edu/health/index_files/halversonreport.pdf.
- 10.Freund KM, Battaglia TA, Calhoun E, et al. Impact of Patient Navigation on Timely Cancer Care: The Patient Navigation Research Program. J Natl Cancer I. 2014;106(6):dju115. doi: 10.1093/jnci/dju115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramos M, Esteva M, Cabeza E, Campillo C, Llobera J, Aguiló A. Relationship of diagnostic and therapeutic delay with survival in colorectal cancer: a review. Eur J Cancer. 2007;43(17):2467–2478. doi: 10.1016/j.ejca.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 12.Langenbach MR, Schmidt J, Neumann J, Zirngibl H. Delay in treatment of colorectal cancer: multifactorial problem. World J Surg. 2003;27(3):304–308. doi: 10.1007/s00268-002-6678-9. [DOI] [PubMed] [Google Scholar]
- 13.USDA. [Accessed February 13, 2015];Rural-Urban Continuum Codes. 2000 Available at: http://www.ers.usda.gov/topics/rural-economy-population/rural-classifications.aspx.
- 14.Fleming ST, Sabatino SA, Kimmick G, et al. for the Patterns of Care Study Group. Developing a claim-based version of the ACE-27 comorbidity index: a comparison with medical record review. Med Care. 2011;49:752–760. doi: 10.1097/MLR.0b013e318215d7dd. [DOI] [PubMed] [Google Scholar]
- 15. [Accessed February 13, 2015]; http://www.facs.org/cancerprogram/index.html.
- 16. [Accessed February 13, 2015]; www.healthgrades.com/
- 17. [Accessed February 13, 2015]; http://npi.ecare.com/
- 18.Mihaylova B, Briggs A, O’Hagan A, Thompson SG. Review of statistical methods for analysing healthcare resources and costs. Health Econ. 2011;20(8):897–916. doi: 10.1002/hec.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oaxaca RL. Male-Female Wage Differentials in Urban Labor Markets. Int Econ Rev. 1973;14(3):693–709. [Google Scholar]
- 20.Zuvekas SH, Taliaferro GS. Pathways to Access: Health Insurance, the Health Care Delivery System and Racial and Ethnic Disparities, 1996–1999. Health Affair. 2003;22(2):139–153. doi: 10.1377/hlthaff.22.2.139. [DOI] [PubMed] [Google Scholar]
- 21.Pruitt SL, Harzke AJ, Davidson NO, Schootman M. Do diagnostic and treatment delays for colorectal cancer increase risk of death? Cancer Cause Control. 2013;24:961–977. doi: 10.1007/s10552-013-0172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bilimoria KY, Clifford YK, Tomlinson JS, et al. Wait Times for Cancer Surger in the United States: Trends and Predictors of Delays. Ann Surg. 2011;252(4):779–785. doi: 10.1097/SLA.0b013e318211cc0f. [DOI] [PubMed] [Google Scholar]
- 23.Ramos M, Esteva M, Cabeza E, Campillo C, Llobera J, Aguiló A. Relationship of diagnostic and therapeutic delay with survival in colorectal cancer: a review. Eur J Cancer. 2007;43(17):2467–2478. doi: 10.1016/j.ejca.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 24.Langenbach MR, Schmidt J, Neumann J, Zirngibl H. Delay in treatment of colorectal cancer: multifactorial problem. World J Surg. 2003;27(3):304–308. doi: 10.1007/s00268-002-6678-9. [DOI] [PubMed] [Google Scholar]
- 25.Iversen LH, Antonsen S, Laurberg S, Lautrup MD. Therapeutic delay reduces survival of rectal cancer but not of colonic cancer. Brit J Surg. 2009;96(10):1183–1189. doi: 10.1002/bjs.6700. [DOI] [PubMed] [Google Scholar]
- 26.Pruitt SL, Harzke AJ, Davidson NO, Schootman M. Do diagnostic and treatment delays for colorectal cancer increase risk of death? Cancer Cause Control. 2013;24(5):961–977. doi: 10.1007/s10552-013-0172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amri R, Bordeianou LG, Sylla P, Berger DL. Treatment Delay in Surgically-Treated Colon Cancer: Does It Affect Outcomes. Ann Surg Oncol. 2014;21:3909–3916. doi: 10.1245/s10434-014-3800-9. [DOI] [PubMed] [Google Scholar]
- 28.Sen S, Soulos PR, Herrin J, et al. For-profit hospital ownership status and use of brachytherapy after breast-conserving surgery. Surgery. 2014;155(5):776–788. doi: 10.1016/j.surg.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Folland S, Goodman AC, Stano M. Economics of Health and Healthcare. 7. Upper Saddle River, NJ: Prentice Hall; 2013. [Google Scholar]