Abstract
Background
Adults ≥ 65 years dually enrolled in Medicare and Medicaid (Duals) are an at-risk group in healthcare, however outcomes of women with gynecologic cancers in this population are unknown.
Methods
This is a population-based cohort study of North Carolina state cancer registry cases of uterine, ovarian, cervical, and vulvar/vaginal cancers (2003-09), with linked enrollment in Medicare and state Medicaid. Outcomes of all-cause mortality and stage of diagnosis were analyzed as a function of enrollment status using multivariate analysis and survival curves.
Results
Of 4,522 women ≥ 65, (3,702 (82%) Medicare and 820 (18%) were Dually enrolled), there were 2286 (51%) uterine, 1587 (35%) ovarian, 302 (7%) cervix, and 347 (8%) vulvar/vaginal cancers. Dual enrollees had increased all-cause mortality overall (aHR 1.34, 95%CI 1.19–1.49), and within each cancer site: uterine aHR 1.22 (95%CI 1.02-1.47); ovarian aHR 1.25 (95%CI 1.05-1.49); cervical aHR 1.34 (95%CI 0.96–1.87); and vulvar/vaginal aHR 1.93 (95%CI 1.36–2.72). Increased odds of advanced stage disease at diagnosis among Dual enrollees was only present in uterine cancer (aOR 1.38, 95%CI 1.06–1.79). Stratified survival curves demonstrate the strongest disparities amongst women with early stage uterine and early stage vulvar/vaginal cancers.
Conclusions
Women ≥ 65 dually enrolled in Medicare and Medicaid have an overall 34% increase in all-cause mortality after diagnosis with a gynecologic cancer compared to the non-dual Medicare population. Women with early stage uterine and vulvar/vaginal cancers have the most disparate outcomes. As these malignancies are generally curable they have the most potential for benefit from targeted interventions.
Keywords: Female Genital Neoplasms, Medicare, Medicaid, Aged, Outcomes Research
Introduction
By 2030, 70% of cancer patients in the United States will be over the age of 65 1. There is a growing emphasis on the outcomes of this population across the spectrum of cancers, including gynecologic malignancies (uterine, ovarian, cervical, and vulvar/vaginal) 2-5. There are over 736,000 women with gynecologic cancer over the age of 60, representing 1.5% of all cancers in this age group6. The elderly gynecologic cancer population has been shown to have poorer outcomes compared to younger cohorts due to more aggressive tumor types, increased comorbid conditions and deviance from guideline adherent care delivery7-12. Within the elderly population however, there have been limited studies addressing factors of disparity.
Despite Medicare's broad coverage of US citizens over 65 years of age, insurance coverage is not uniform among this group13. To secure insurance coverage beyond the inpatient services covered by Medicare Part A, older adults must enroll and pay premiums for Medicare Part B, including annual deductibles and co-payment fees 14, or be enrolled in Medicare Advantage, a health maintenance organization (HMO) with associated fees. Supplemental private insurance may be purchased in addition to or in place of Part B for those with the financial means. Older adults with individual annual incomes at or near the federal poverty line can qualify for supplemental Medicaid to cover the cost of Medicare Part B 15; these individuals are generally called “dual”-eligible or dually–enrolled (Duals) due to their coverage by both Medicare and Medicaid programs. In North Carolina the individual income eligibility for complete dual coverage is 100% of the federal poverty level (≤ $10,400 in 2008)16.
With its strict income criteria, dual enrollment is an individual marker of low socioeconomic status (SES), which in turn, can be a primary driver of health-related disparities17. These patients have been identified as an at-risk group in cancer care, with evidence suggesting that disparities are greatest among those cancer types most amenable to intervention18. In gynecologic oncology, race and SES have been identified as drivers of outcomes in ovarian, uterine, and cervical cancer. Black women have been shown to be less likely to have surgical treatment and receive guideline-adherent care in each disease site19-24. In military25 and clinical trial26 settings however, where socioeconomic barriers to care are minimized and treatment is standardized, mortality differences by race are not seen. Low SES is also consistently associated with poor care delivery and worse outcomes23,27. In cervix cancer, SES based disparities have actually been increasing over time28 and Fedewa et al reported that patients without private health insurance had worse uterine cancer survival than insured counterparts29. Studies addressing insurance-based disparities in gynecologic oncology within the elderly population, however, are limited. In addition, population-based studies involving more than one gynecologic cancer site are rare, therefore we know little about how SES disparities compare across tumor sites.
Our objective was to analyze the outcomes of stage at diagnosis and mortality of dually-enrolled women ≥ 65, diagnosed with gynecologic cancers in North Carolina, compared to Medicare recipients.
Methods
Data Source and Study Population
This study was approved by the North Carolina Institutional Review Board (# 13-2863). The North Carolina Central Cancer Registry (NCCCR) was used to identify all women in North Carolina diagnosed with a primary gynecologic cancer from 2003–2009. Women with benign or in-situ histology (including low malignant potential tumors), or who were diagnosed at death or autopsy were excluded using NCCCR flags and ICD-O-3 codes (See eTable 1).
The North Carolina Integrated Cancer Information and Surveillance System (ICISS) links identified cancer cases from the NCCCR with administrative data from Medicare, Medicaid and beneficiaries in privately insured health plans across the state 30. We restricted the sample to women ≥ 65 years old with linked enrollment in Medicare and Medicaid. Medicare enrollees with a primary eligibility reason of disability were excluded.
All women in the cohort were required to be continuously enrolled in Medicare Part A. Insurance groupings of exclusive Medicare and dual coverage (Medicare and Medicaid) were constructed. Due to lags in data administration, the Medicaid enrollment file extended to 2008; in order to enable an additional year study period, Medicaid dual-eligibility as reported by monthly indicator variables in the Medicare enrollment file (2009) was used to identify the dual population for this year of the study, as is commonly defined 31,32.
Outcome Variables and Covariates
The primary outcomes assessed were stage at diagnosis and all-cause mortality, both reported by the NCCCR. Stage at diagnosis is reported in the summary staging variable systematically reported by state and national cancer registries and was consistent throughout the study period. Stage, as an outcome, was defined in a binary fashion: early stage (local) and advanced stage (regional and distant). For the analysis of stage as an outcome, the missing/unknown category was excluded. Mortality was updated annually by the registry and, at the time of study analysis, was available through 2011. For the analysis of mortality as an outcome, all stage categories were included.
Age, race/ethnicity, geography, stage, cancer site, first or subsequent cancer diagnosis (‘diagnosis order’), and comorbidity were all included as covariates. Age at diagnosis, race, and diagnosis order data were reported from the NCCCR. All cases were linked to the American Community Survey for census-tract level population data30. Rural/urban classification from the United States Department of Agriculture was dichotomized at the county level into Metro vs. Non-metro based on Rural/Urban Continuum codes from 2013. Cancer site was defined by International Classification of Disease (ICD-O-3) morphology codes (See eTable 1). Comorbidity was assessed using the Charlson Comorbidity Index33, from diagnosis codes present in Medicare Part A claims for the 6 months prior to cancer diagnosis.
Statistical Analysis
Univariate and bivariate analysis of insurance groups, covariates, and the outcomes of stage at diagnosis and mortality were performed. Student's t-test and chi square statistic were used to assess the relationships between independent variables and outcome variables as appropriate. We completed multivariate survival analyses using Cox proportional hazard models to generate adjusted hazard ratios (HR) for time to death (mortality). Proportional hazard assumptions were tested using time interactions for each independent variable. Multivariate logistic regression was used generate odds ratios (OR) of likelihood of early versus late stage diagnosis. Unadjusted Kaplan-Meier survival plots were generated, with stratification for selected variables. Statistical significance was set at p<0.05. Analysis was performed using SAS v9.3 (Cary, NC).
Results
Descriptive
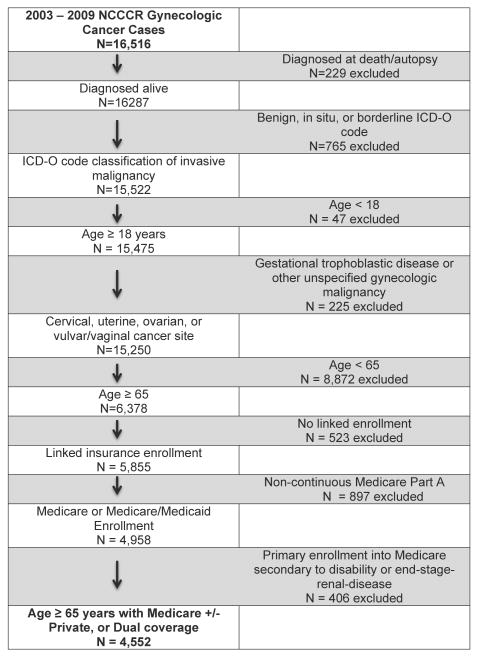
A total of 16,516 unique cases of gynecologic cancers during 2003-2009 were identified from the North Carolina Central Cancer Registry. After applying tumor-level and demographic exclusions, there were 6,378 cases of uterine, ovarian, cervical, and vulvar/vaginal cancers. The majority (N=5,855, 92%) were successfully linked to enrollment files. After payer-level exclusions were applied, the final cohort was comprised of 4,522 women enrolled in Medicare only (N=3,702, 82%) or Medicare + Medicaid (“Dual”, N=820, 18%) enrollment (Figure 1).
Figure 1. Study Population.
The cohort mean age was 76 years, with 83% White Non-Hispanic, and 64% residing in metropolitan areas. Nearly half (N=1870, 41%) of the cohort had early-stage (local) disease at presentation. The frequency of cancer sites was consistent with national trends with uterine cancer being the most common (51%) and vulvar/vaginal the least (8%) common. There were differences in all baseline characteristics among the insurance groups. Mean age was higher in the Medicare cohort (78 years), and a larger percentage of Black women were in the Dual coverage (35%) group, than in Medicare (10%). Dual enrollees were more likely to live in non-Metro areas compared the other groups and to have greater Charlson comorbidity. With regard to cancer site, dual enrollees also had a higher prevalence of HPV related (cervical and vulvar/vaginal) cancers (Table 1).
Table 1. Cohort Characteristics of Women ≥ 65 with Gynecologic Cancers in North Carolina: Overall and by Insurance Type (2003 – 2009).
| Characteristics | Total N=4,522 |
Medicare N=3702 (82%) |
Dual N=820 (18%) |
p-value | |
|---|---|---|---|---|---|
| Age | Mean (S.D.) | 75.5 (8) | 75.0 (7) | 77.9 (8) | <.001b |
|
| |||||
| Race/Ethnicity | White | 3775 (83%) | 3276 (88%) | 499 (61%) | |
| Black | 664 (15%) | 377 (10%) | 287 (35%) | ||
| Hispanic/Other | 71(2%) | - | - | ||
| Unknown | - | - | - | <.001c | |
|
| |||||
| Geographic | Metro | 2912 (64%) | 2449 (66%) | 463 (56%) | |
| Density | Non-Metro | 1609 (36%) | 1252 (34%) | 357 (44%) | |
| Unknown | - | - | - | <.001 c | |
|
| |||||
| Stage | Local | 1870 (41%) | 1580 (43%) | 290 (35%) | |
| Regional | 978 (22%) | 775 (21%) | 203 (25%) | ||
| Distant | 1381 (31%) | 1151 (31%) | 230 (28%) | ||
| Unknown | 293 (6%) | 196 (5%) | 97 (12%) | <.001 c | |
|
| |||||
| Cancer Site | Uterus | 2286 (51%) | 1925 (52%) | 361 (44%) | |
| Ovary | 1587 (35%) | 1341 (36%) | 246 (30%) | ||
| Cervix | 302 (7%) | 194 (5%) | 108 (13%) | ||
| Vulvar/Vaginal | 347 (8%) | 242 (7%) | 105 (13%) | <.001 c | |
|
| |||||
| Diagnosis Order | First and Only | 3434 (76%) | 2789 (75%) | 645 (79%) | |
| Higher Order | 1088 (24%) | 913 (25%) | 175 (21%) | 0.04 c | |
|
| |||||
| Charlson Comorbidity | 0 | 2281 (50%) | 1956 (53%) | 325 (40%) | |
| ≥ 1 | 736 (16%) | 507 (14%) | 229 (28%) | ||
| Missing | 1505 (33%) | 1239 (33%) | 266 (32%) | <.001c | |
Cells with values <10 were suppressed
one way analysis of variance
chi-square test
Within each cancer site, the mean age at diagnoses for uterine, ovarian, and cervical cancers was 75 – 76 years, and for vulvar/vaginal was 79 years. Eighty-seven percent of patients with uterine cancer, 22% of ovarian, 72% of cervical, and 86% of vulvar/vaginal cases were diagnosed in early stages (eTable 2). All-cause mortality during the study period was 31%, 69%, 54%, and 46% for uterine, ovarian, cervical, and vulvar/vaginal respectively.
Insurance Type and Stage at Diagnosis
Using logistic regression adjusting for all covariates including cancer site, there was no increased likelihood of advanced stage diagnosis by insurance type (HR 1.13 95% CI 0.91 – 1.36). Black race (HR 1.42, 95% CI: 1.15 – 1.76) and cancer site, especially ovary (HR 20.8 95%CI 16.9 – 25.6), were the primary factors driving stage at diagnosis (eTable 3). When stratified by cancer site, there was no impact of insurance type on stage at the time of diagnosis with the exception of uterine cancer, where dual enrollment was associated with an OR of 1.38 (95% CI 1.06 – 1.79) with advanced stage diagnosis (Table 2).
Table 2. Odds of Late Stage of Diagnosis at Presentation by Insurance Type – Stratified by Cancer Site1.
| Gynecologic Cancer Site | Insurance Type | N | Adjusted Odds Ratio | 95% CI | p-value2 |
|---|---|---|---|---|---|
| Uterus | Medicare | 1857 | 1.0 | -- | referent |
| Dual | 329 | 1.38 | 1.06 – 1.79 | 0.02 | |
|
| |||||
| Ovary | Medicare | 1235 | 1.0 | -- | referent |
| Dual | 201 | 0.85 | 0.49 – 1.50 | 0.58 | |
|
| |||||
| Cervix | Medicare | 182 | 1.0 | -- | referent |
| Dual | 94 | 0.69 | 0.39 – 1.21 | 0.19 | |
|
| |||||
| Vulva/Vagina | Medicare | 231 | 1.0 | -- | referent |
| Dual | 99 | 0.97 | 0.57 – 1.63 | 0.89 | |
Logistic regression with indicator variables and Wald confidence intervals, adjusted for age, race, geographic density, diagnosis order, and comorbidity
Chi-square p value for beta coefficient from logistic regression model
Insurance Enrollment and Mortality
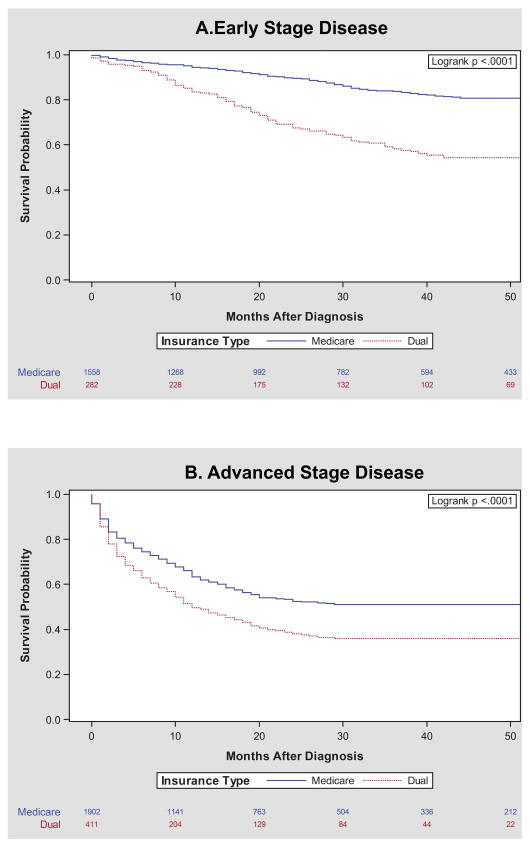
The probability of overall survival varied significantly by insurance type. For women diagnosed with both early and late stage disease, Dual enrollees had a higher mortality rate compared to Medicare enrollees (Figure 2). Stratified by race and stage, the relative impact of insurance type on mortality was most prominent among White women. Lower survival probability among duals was also present among Black women in both early and advanced stage categories, but the disparity was not as pronounced (eFigure 1).
Figure 2. Mortality after Gynecologic Cancer Diagnosis of Women ≥ 65 by Insurance Enrollment in North Carolina (2003 – 2009) stratified by Stage at Diagnosis.
Kaplan Meier survival curves stratified by insurance enrollment into Medicare and Dual during the 2003 – 2009 study period. Mortality is measured from month of diagnosis until death or censoring. Stage is dichotomized into Early (local) and Advanced (regional and distant) disease.
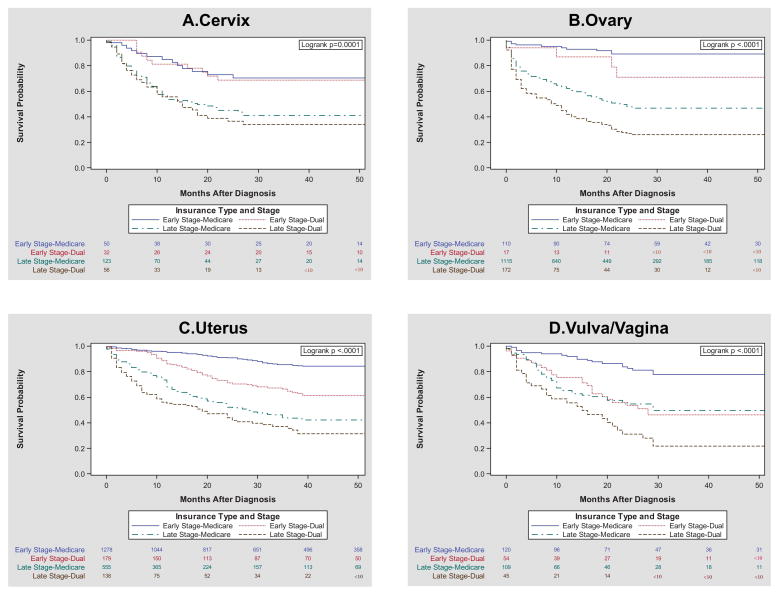
In multivariate modeling controlling for age, race/ethnicity, geographic density, stage at diagnosis, diagnosis order, gynecologic cancer site, and comorbidity, Dual enrollees had a hazard ratio associated with mortality of 1.34 (95%CI 1.19 – 1.49) compared to Medicare enrollees (Table 3, full model eTable4). When stratified by cancer site, the results were similar with dual enrollment associated with an increased HR for mortality for three of the four cancer sites, but most notably in vulvar/vaginal cancer (HR 1.93, 95% CI 1.36 – 2.72) (Table 3). In the smallest group, cervical cancer, the result was not statistically significant (HR 1.34, 95% CI 0.96 – 1.87) although the magnitude and direction of effect was similar. Stratified survival curves suggest the largest disparities by insurance type to be among women with advanced stage ovarian cancer (Figure 3B), early stage uterine cancer (Figure 3C), and early stage vulvar cancers (Figure 3D).
Table 3. Mortality After Gynecologic Cancer Diagnosis – Overall and Stratified by Cancer Sitea.
| Gynecologic Cancer Siteb | Insurance Type | N | aHRc | 95% CI | p-value |
|---|---|---|---|---|---|
| All Sitesc N=4,552 |
Medicare | 3702 | 1.0 | -- | Referent |
| Dual | 820 | 1.34 | 1.19 – 1.49 | <.001 | |
|
| |||||
| Uterus N=2285 |
Medicare | 1924 | 1.0 | -- | Referent |
| Dual | 361 | 1.22 | 1.02 – 1.47 | 0.03 | |
|
| |||||
| Ovary N=1587 |
Medicare | 1341 | 1.0 | -- | referent |
| Dual | 246 | 1.25 | 1.05 – 1.49 | 0.01 | |
|
| |||||
| Cervix N=302 |
Medicare | 194 | 1.0 | -- | referent |
| Dual | 108 | 1.34 | 0.96 – 1.87 | 0.08 | |
|
| |||||
| Vulva/Vagina N=347 |
Medicare | 242 | 1.0 | -- | referent |
| Dual | 105 | 1.93 | 1.36 – 2.72 | <.002 | |
Cox proportional hazard models with covariate values and Wald confidence limits shown.
Total of number of deaths per site as follows: All (1,998) Uterus (704), Ovary (961), Cervix (164), and Vulva/Vagina (159).
Adjusted Hazard ratios presented are adjusted for age, race, geographic density, stage, other cancer diagnosis, and comorbidity in Cox proportional models. Overall cohort is also adjusted for cancer site.
Figure 3. Mortality after Gynecologic Cancer Diagnosis of Women ≥ 65 by Insurance Enrollment in North Carolina (2003 – 2009): Differences By Cancer Site.
Kaplan Meier survival curves with insurance stratification, grouped by ICD-O-3 tumor site codes in the North Carolina Central Cancer Registry, further stratified by early and advanced disease groups. Data does not include Hispanic, Other, or Missing cases.
Sensitivity Analyses
Because of the potential selection bias in requiring continuous enrollment in Medicare Part A, we repeated all analyses without this criterion. To address different exposure definitions, sensitivity analyses were performed excluding 2009 and also models were performed using the Medicaid eligibility (state-buy in) variable. Depending on year, actual enrollment in the Medicaid beneficiary file represents between 60-80% of ‘dually-eligible’ women for that year as defined by the state buy-in indicator in Medicare. The results from these sensitivity analyses were similar in magnitude and precision to our final results.
Discussion
This is the first study comparing population-level outcomes of elderly women across the spectrum of gynecologic cancers. In the state of North Carolina, older women diagnosed with gynecologic cancers who are dually enrolled in Medicare and Medicaid experienced 34% higher all-cause mortality rates than those enrolled in Medicare alone, even when accounting for comorbidity and other demographic variables. When addressing health care disparities, it is crucial to identify the clinical subgroups that are most at risk and most amenable to intervention. Early stage vulvar cancer, early stage uterine cancer and advanced stage ovarian cancer groups drove the overall mortality differences seen in this population.
The Theory of Fundamental Causes of health inequalities is born out of socio-behavioral research, and holds that the relative impact of SES-related mortality on disease outcomes is dependent upon the overall treatability of the specific disease 18. For example, in our cohort, there was greater disparity seen among women with early stage disease compared to advanced stage disease. Advanced stage gynecologic cancer patients have less effective treatment options and high mortality. This clinical reality minimizes the contribution of SES-driven insurance type to outcomes. It is the diseases that are most amenable to treatment that have the highest potential for income-based disparity.
We found that early stage vulvar cancer, early stage uterine cancer and advanced stage ovarian cancer groups demonstrated the largest gap in survival between Medicare and Dual populations. Based on the Fundamental Cause theory, these data supports a strategy of targeting early stage uterine and vulvar/vaginal cancers for interventions to improve gynecologic cancer outcomes. There have been several studies focused solely on disparities in ovarian cancer outcomes by race and SES, uniformly reporting worse survival in vulnerable groups27,34-37. Ovarian cancer, however, is a highly fatal illness, with 70% of women diagnosed in advanced stages and a 10-year survival of less than 25%38. In contrast, early stage uterine and vulvar cancers are curable diseases with 5-year survival rates of 80% and 71%, respectively 6. Early diagnosis is feasible and appropriate treatment result in high cure rates. It is this precise clinical environment where interventions that are resource-blind, such as community education of early symptomatology and universal access to specialty surgery, can make the biggest differences in decreasing mortality and eliminating SES-driven disparities.
With regard to uterine cancer, it is the only disease site in our cohort where Dually enrolled women present at later stages compared to the Medicare population (HR 1.38, 95%CI 1.06 – 1.79). Stage is the main driver of cure rates for this disease. Postmenopausal bleeding is a hallmark symptom of uterine cancer, and when acted upon promptly, often results in timely diagnosis of an early stage disease. Public health campaigns to raise awareness of the link between postmenopausal bleeding and uterine cancer, is the kind resource-blind intervention that can make a significant impact on mortality in this vulnerable population.
Among women with vulvar/vaginal cancers, we found the strongest association of Dual enrollment with mortality (HR 1.93 (95%CI: 1.36 – 2.72). This finding highlights an especially vulnerable group of women, and calls into question what potential care processes or demographic factors make it particularly difficult for older poor women to survive a vulvar cancer diagnosis. Adequate care of vulvar cancer requires timely diagnosis by tissue biopsy, appropriate surgical evaluation including lymph node biopsy, possible receipt of adjuvant radiation therapy, and close follow up for recurrence. Any or all of these process points may be mediators for the increased mortality among dual enrollees and important points of potential intervention39. A potential common mediator may be the level of interaction with specialized gynecologic oncology surgical care 35,40,41.
We saw no differences in outcomes among the cervical cancer cohort. We postulate that the lack of SES-insurance based disparities seen in this group is because of the commonalities of the environment and behaviors that leads to persistent HPV infection likely mitigate the benefits of the higher SES Medicare population.
Interestingly, stratification by race uncovered differences in the strength of the association of Dual enrollment and mortality. Although dually enrolled Black women experienced higher mortality rates than Medicare enrolled Black women, the magnitude of the difference was smaller than among Whites (eFigure 2). There are residual racial drivers of treatment outcomes, independent of SES. Black women with ovarian cancer are less likely to receive guideline adherent care, independent of insurance type and income level 27. In cases of equal access to cancer care, Black race is associated with poorer patient-physician communication 42, and more provider distrust 43. Specific to this study, North Carolina as a state ranks at the bottom (51st of 51) on measures of race/ethnicity equity in health care according to the 2014 Commonwealth Fund report 44. Our findings of differential effect size among races may be state-specific, in that Black women in states of gaping health equity may not benefit from the same health resources as Whites.
Our study has several limitations, many of which are consistent with registry-linked claims data45. The NCCCR is a gold certified registry under the North American Association of Cancer Registries, meeting rigorous standards of review. The research team has extensive experience using claims data and is familiar with the types of bias and misclassification which can be present46-52. First, we were unable to measure more sensitive and specific person-level indicators of SES as a covariate. Dual enrollment operates as a proxy in this study as it is driven by Medicaid income limit restrictions exclusively, and we excluded people who qualified at enrollment for Medicare based on disability. Second, we are limited to a single state (North Carolina), so our results may not be generalizable nationwide. Third, we are also limited by a claims-based definition of comorbidity, using the well-validated Charlson comorbidity assessment, which may underestimate comorbidities and does not measure frailty or functional status in an elderly population. Fourth, there are tiers of supplemental Medicaid coverage based on income limits that go up to 250% of the federal poverty line. Our analysis was completed grouping any level of supplemental Medicaid into the dual enrollment group and may mask differences of populations within each tier of coverage. Finally, due to the nature of our data linkage based on insurance enrollment, we do not have data on older (>65 years) cancer patients who were never enrolled in Medicaid or Medicare.
By using actual enrollment files for both payers, we have increased accuracy of in identification of Medicaid status compared to cancer registry reporting alone 53. We have also included a measure of comorbidity which was omitted in prior population based studies on ovarian and uterine cancer outcomes.27 The gynecologic cancer spectrum may be ideal for studying the disparate effects of social factors on cancer care. Each of the cancer sites – ovarian, uterine, cervical, and vulvar/vaginal – has unique and well described social and biological risk factors that define the population, yet all are seen and treated by a relatively small number of gynecologic oncologists within a similar care framework.
In an environment of limited health care resources, changing laws regarding insurance coverage, and increasing calls to mitigate cancer care disparities, this data is crucial in highlighting target populations most in need, and most likely to benefit from intervention. With a narrower focus on these populations, the possibilities for effective, tailored interventions increase.
Our study is the first to our knowledge to address the outcomes across the spectrum of gynecologic cancer among this population of women ≥ 65 years who are Dual enrollees. We found that disparities in outcomes exist among the most curable subgroups: early stage uterine cancer and early stage vulvar cancer. As gynecologic oncologists frequently provide diagnosis, surgical management, and adjuvant therapy for these cancers, there is great potential for intervention during multiple points of the cancer care process, within the scope of a single practice.
Supplementary Material
Acknowledgments
Funding: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number R25CA116339.
Footnotes
The authors have no financial disclosures.
References
- 1.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009 Jun 10;27(17):2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 2.Wright JD, Lewin SN, Barrena Medel NI, et al. Morbidity and mortality of surgery for endometrial cancer in the oldest old. Am J Obstet Gynecol. 2011 Jul;205(1):66, e61–68. doi: 10.1016/j.ajog.2011.02.067. [DOI] [PubMed] [Google Scholar]
- 3.Sharma C, Deutsch I, Horowitz DP, et al. Patterns of care and treatment outcomes for elderly women with cervical cancer. Cancer. 2012 Jul 15;118(14):3618–3626. doi: 10.1002/cncr.26589. [DOI] [PubMed] [Google Scholar]
- 4.Wright JD, Lewin SN, Deutsch I, et al. Defining the limits of radical cytoreductive surgery for ovarian cancer. Gynecologic oncology. 2011 Dec;123(3):467–473. doi: 10.1016/j.ygyno.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 5.Garg G, Yee C, Schwartz K, Mutch DG, Morris RT, Powell MA. Patterns of care, predictors, and outcomes of chemotherapy in elderly women with early-stage uterine carcinosarcoma: A population-based analysis. Gynecol Oncol. 2014 Feb 19; doi: 10.1016/j.ygyno.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howlander N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2012. National Cancer Institute. 2015 http://seer.cancer.gov/csr/1975_2012/
- 7.Truong PT, Kader HA, Lacy B, et al. The effects of age and comorbidity on treatment and outcomes in women with endometrial cancer. Am J Clin Oncol. 2005 Apr;28(2):157–164. doi: 10.1097/01.coc.0000143049.05090.12. [DOI] [PubMed] [Google Scholar]
- 8.Wright JD, Lewin SN, Barrena Medel NI, et al. Endometrial cancer in the oldest old: Tumor characteristics, patterns of care, and outcome. Gynecol Oncol. 2011 Jul;122(1):69–74. doi: 10.1016/j.ygyno.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed A, Zamba G, DeGeest K, Lynch CF. The impact of surgery on survival of elderly women with endometrial cancer in the SEER program from 1992-2002. Gynecol Oncol. 2008 Oct;111(1):35–40. doi: 10.1016/j.ygyno.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 10.Mahdi H, Lockhart D, Maurer KA. Impact of age on 30-day mortality and morbidity in patients undergoing surgery for endometrial cancer. Gynecol Oncol. 2015 Apr;137(1):106–111. doi: 10.1016/j.ygyno.2015.01.543. [DOI] [PubMed] [Google Scholar]
- 11.Suh DH, Kang S, Lim MC, et al. Management of the elderly patient with gynecologic cancer: report of the 2011 workshop in geriatric gynecologic oncology. Int J Gynecol Cancer. 2012 Jan;22(1):161–169. doi: 10.1097/IGC.0b013e318234f8d5. [DOI] [PubMed] [Google Scholar]
- 12.Sabatier R, Calderon B, Jr, Lambaudie E, et al. Prognostic Factors for Ovarian Epithelial Cancer in the Elderly: A Case-Control Study. Int J Gynecol Cancer. 2015 Mar 12; doi: 10.1097/IGC.0000000000000418. [DOI] [PubMed] [Google Scholar]
- 13.Mold JW, Fryer GE, Thomas CH. Who are the uninsured elderly in the United States. J Am Geriatr Soc. 2004 Apr;52(4):601–606. doi: 10.1111/j.1532-5415.2004.52169.x. [DOI] [PubMed] [Google Scholar]
- 14.Services DoHaH. The Official U.S Government Site for Medicare. [Accessed April 30, 2014];2014 http://www.medicare.gov.
- 15.Services NCDoHaH. A Consumer's Guide to Medicare Savings Programs Within North Carolina Medicaid. http://www.ncdhhs.gov.
- 16.Services DoHaH. 2008 HHS Poverty Guidelines. Federal Registrar. 2008;73(15):3971–3972. [Google Scholar]
- 17.Phelan JC, Link BG, Tehranifar P. Social conditions as fundamental causes of health inequalities: theory, evidence, and policy implications. J Health Soc Behav. 2010;51(Suppl):S28–40. doi: 10.1177/0022146510383498. [DOI] [PubMed] [Google Scholar]
- 18.Tehranifar P, Neugut AI, Phelan JC, et al. Medical advances and racial/ethnic disparities in cancer survival. Cancer Epidemiol Biomarkers Prev. 2009 Oct;18(10):2701–2708. doi: 10.1158/1055-9965.EPI-09-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collins Y, Holcomb K, Chapman-Davis E, Khabele D, Farley JH. Gynecologic cancer disparities: a report from the Health Disparities Taskforce of the Society of Gynecologic Oncology. Gynecol Oncol. 2014 May;133(2):353–361. doi: 10.1016/j.ygyno.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill HA, Eley JW, Harlan LC, Greenberg RS, Barrett RJ, 2nd, Chen VW. Racial differences in endometrial cancer survival: the black/white cancer survival study. Obstet Gynecol. 1996 Dec;88(6):919–926. doi: 10.1016/s0029-7844(96)00341-9. [DOI] [PubMed] [Google Scholar]
- 21.Randall TC, Armstrong K. Differences in treatment and outcome between African-American and white women with endometrial cancer. J Clin Oncol. 2003 Nov 15;21(22):4200–4206. doi: 10.1200/JCO.2003.01.218. [DOI] [PubMed] [Google Scholar]
- 22.Bristow RE, Zahurak ML, Ibeanu OA. Racial disparities in ovarian cancer surgical care: a population-based analysis. Gynecol Oncol. 2011 May 1;121(2):364–368. doi: 10.1016/j.ygyno.2010.12.347. [DOI] [PubMed] [Google Scholar]
- 23.Madison T, Schottenfeld D, James SA, Schwartz AG, Gruber SB. Endometrial cancer: socioeconomic status and racial/ethnic differences in stage at diagnosis, treatment, and survival. Am J Public Health. 2004 Dec;94(12):2104–2111. doi: 10.2105/ajph.94.12.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.del Carmen MG, Montz FJ, Bristow RE, Bovicelli A, Cornelison T, Trimble E. Ethnic differences in patterns of care of stage 1A(1) and stage 1A(2) cervical cancer: a SEER database study. Gynecol Oncol. 1999 Oct;75(1):113–117. doi: 10.1006/gyno.1999.5543. [DOI] [PubMed] [Google Scholar]
- 25.Farley JH, Hines JF, Taylor RR, et al. Equal care ensures equal survival for African-American women with cervical carcinoma. Cancer. 2001 Feb 15;91(4):869–873. [PubMed] [Google Scholar]
- 26.Winter WE, 3rd, Maxwell GL, Tian C, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007 Aug 20;25(24):3621–3627. doi: 10.1200/JCO.2006.10.2517. [DOI] [PubMed] [Google Scholar]
- 27.Bristow RE, Powell MA, Al-Hammadi N, et al. Disparities in ovarian cancer care quality and survival according to race and socioeconomic status. J Natl Cancer Inst. 2013 Jun 5;105(11):823–832. doi: 10.1093/jnci/djt065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simard EP, Naishadham D, Saslow D, Jemal A. Age-specific trends in black-white disparities in cervical cancer incidence in the United States: 1975-2009. Gynecol Oncol. 2012 Dec;127(3):611–615. doi: 10.1016/j.ygyno.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 29.Fedewa SA, Lerro C, Chase D, Ward EM. Insurance status and racial differences in uterine cancer survival: a study of patients in the National Cancer Database. Gynecol Oncol. 2011 Jul;122(1):63–68. doi: 10.1016/j.ygyno.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Center UoNCLCC. ICISS Data Resources. [Accessed January 1st, 2013]; iciss.unc.edu/data.php.
- 31.Koroukian SM, Dahman B, Copeland G, Bradley CJ. The utility of the state buy-in variable in the Medicare denominator file to identify dually eligible Medicare-Medicaid beneficiaries: a validation study. Health Serv Res. 2010 Feb;45(1):265–282. doi: 10.1111/j.1475-6773.2009.01051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O' Donnell B, Schneider K, Roozeboom M. Options for Determining Which Medicare Beneficiaries are Dually Eligible: Technical Guidance. 2012 [Google Scholar]
- 33.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 34.Goff BA, Matthews BJ, Larson EH, et al. Predictors of comprehensive surgical treatment in patients with ovarian cancer. Cancer. 2007 May 15;109(10):2031–2042. doi: 10.1002/cncr.22604. [DOI] [PubMed] [Google Scholar]
- 35.Bristow RE, Palis BE, Chi DS, Cliby WA. The National Cancer Database report on advanced-stage epithelial ovarian cancer: impact of hospital surgical case volume on overall survival and surgical treatment paradigm. Gynecol Oncol. 2010 Sep;118(3):262–267. doi: 10.1016/j.ygyno.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 36.Chan JK, Urban R, Cheung MK, et al. Ovarian cancer in younger vs older women: a population-based analysis. Br J Cancer. 2006 Nov 20;95(10):1314–1320. doi: 10.1038/sj.bjc.6603457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan JK, Cheung MK, Husain A, et al. Patterns and progress in ovarian cancer over 14 years. Obstet Gynecol. 2006 Sep;108(3 Pt 1):521–528. doi: 10.1097/01.AOG.0000231680.58221.a7. [DOI] [PubMed] [Google Scholar]
- 38.Baldwin LA, Huang B, Miller RW, et al. Ten-year relative survival for epithelial ovarian cancer. Obstet Gynecol. 2012 Sep;120(3):612–618. doi: 10.1097/AOG.0b013e318264f794. [DOI] [PubMed] [Google Scholar]
- 39.Rauh-Hain JA, Clemmer J, Clark RM, et al. Management and outcomes for elderly women with vulvar cancer over time. BJOG. 2014 May;121(6):719–727. doi: 10.1111/1471-0528.12580. discussion 727. [DOI] [PubMed] [Google Scholar]
- 40.Earle CC, Schrag D, Neville BA, et al. Effect of surgeon specialty on processes of care and outcomes for ovarian cancer patients. J Natl Cancer Inst. 2006 Feb 1;98(3):172–180. doi: 10.1093/jnci/djj019. [DOI] [PubMed] [Google Scholar]
- 41.Chan JK, Sherman AE, Kapp DS, et al. Influence of gynecologic oncologists on the survival of patients with endometrial cancer. J Clin Oncol. 2011 Mar 1;29(7):832–838. doi: 10.1200/JCO.2010.31.2124. [DOI] [PubMed] [Google Scholar]
- 42.Gordon HS, Street RL, Jr, Sharf BF, Souchek J. Racial differences in doctors' information-giving and patients' participation. Cancer. 2006 Sep 15;107(6):1313–1320. doi: 10.1002/cncr.22122. [DOI] [PubMed] [Google Scholar]
- 43.Gordon HS, Street RL, Jr, Sharf BF, Kelly PA, Souchek J. Racial differences in trust and lung cancer patients' perceptions of physician communication. J Clin Oncol. 2006 Feb 20;24(6):904–909. doi: 10.1200/JCO.2005.03.1955. [DOI] [PubMed] [Google Scholar]
- 44.Radley DC, McCarthy D, Lippa JA, Hayes SC, Schoen C. Aiming Higher: Results from a Scorecard on State Health System Performance, 2014. The Commonwealth Fund. 2014 May; 2014. [Google Scholar]
- 45.Hershman DL, Wright JD. Comparative effectiveness research in oncology methodology: observational data. J Clin Oncol. 2012 Dec 1;30(34):4215–4222. doi: 10.1200/JCO.2012.41.6701. [DOI] [PubMed] [Google Scholar]
- 46.Glynn RJ, Schneeweiss S, Sturmer T. Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol. 2006 Mar;98(3):253–259. doi: 10.1111/j.1742-7843.2006.pto_293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hernan MA, Alonso A, Logan R, et al. Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidemiology. 2008 Nov;19(6):766–779. doi: 10.1097/EDE.0b013e3181875e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meyer AM, Wheeler SB, Weinberger M, Chen RC, Carpenter WR. An overview of methods for comparative effectiveness research. Semin Radiat Oncol. 2014 Jan;24(1):5–13. doi: 10.1016/j.semradonc.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 49.Sturmer T, Rothman KJ, Avorn J, Glynn RJ. Treatment effects in the presence of unmeasured confounding: dealing with observations in the tails of the propensity score distribution--a simulation study. Am J Epidemiol. 2010 Oct 1;172(7):843–854. doi: 10.1093/aje/kwq198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walker AM. Confounding by indication. Epidemiology. 1996 Jul;7(4):335–336. [PubMed] [Google Scholar]
- 51.Newhouse JP, McClellan M. Econometrics in outcomes research: the use of instrumental variables. Annu Rev Public Health. 1998;19:17–34. doi: 10.1146/annurev.publhealth.19.1.17. [DOI] [PubMed] [Google Scholar]
- 52.Brookhart MA, Rassen JA, Schneeweiss S. Instrumental variable methods in comparative safety and effectiveness research. Pharmacoepidemiol Drug Saf. 2010 Jun;19(6):537–554. doi: 10.1002/pds.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chan JK, Gomez SL, O'Malley CD, Perkins CI, Clarke CA. Validity of cancer registry medicaid status against enrollment files: implications for population-based studies of cancer outcomes. Med Care. 2006 Oct;44(10):952–955. doi: 10.1097/01.mlr.0000220830.46929.43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.