Abstract
Purpose
Preclinical modeling in non-small cell lung cancer (NSCLC) showed that stimulation with hepatocyte growth factor (HGF), the ligand for MET, could reverse the cytostatic and cytotoxic effects of the epidermal-growth factor receptor (EGFR) inhibitor erlotinib in erlotinib-sensitive cell lines. Inhibitors of AKT signaling mitigated this HGF-mediated resistance, partially restoring erlotinib activity. We conducted a phase II trial of erlotinib plus MK2206, a highly selective inhibitor of AKT, in NSCLC patients.
Experimental Design
Eligible patients must have progressed following prior benefit from erlotinib, defined as response or stable disease > 12 weeks. Treatment consisted of erlotinib 150 mg po QD + MK-2206 45 mg po QOD on a 28 day cycle. Primary endpoints were RECIST response rate > 30% (stratum 1: EGFR mutant) and disease control rate (DCR) > 20% at 12 weeks (stratum 2: EGFR wild type).
Results
Eighty patients were enrolled, 45 and 35 in stratum 1 and 2, respectively. Most common attributable adverse events (all grade 3) were rash, diarrhea, fatigue, and mucositis. Response and DCR were respectively 9% and 40% in stratum 1; 3% and 47% in stratum 2. Median progression free survival was 4.4 months in stratum 1 and 4.6 months in stratum 2.
Conclusions
Combination MK2206 and erlotinib met its primary endpoint in erlotinib-pretreated patients with EGFR wild type NSCLC. While activity was seen in EGFR mutated NSCLC, this did not exceed a priori estimates. AKT pathway inhibition merits further clinical evaluation in EGFR wild type NSCLC.
INTRODUCTION
Non-small cell lung cancer (NSCLC) is the most common cause of cancer-related death in the United States. Most patients present with advanced stage disease at the time of initial diagnosis and are therefore incurable, accounting for the high mortality rate. In the past, patients with metastatic NSCLC were often treated with platinum-based chemotherapy which had previously been shown to improve survival and quality of life.(1)
More recently, activating mutations in the epidermal growth factor receptor’s (EGFR) tyrosine kinase domain – seen in approximately 10–15% of lung adenocarcinomas in the U.S. - have been associated with remarkable responses to EGFR tyrosine kinase inhibitors such as erlotinib.(2) Unfortunately and inevitably, these oncogene-addicted tumors subsequently develop resistance to EGFR TKIs due to various mechanisms including emergence of resistance mutations (such as T790M in about 50% of cases) and increased signal transduction through complementary pathways. In the latter case, up-regulation of AKT activity through alternative kinase activation (such as Met), may account for a substantial proportion of the resistant population.(3) The addition of an AKT inhibitor to erlotinib in patients who initially responded to erlotinib but have acquired resistance may be of significant clinical benefit, provided it can be safely administered.
MK-2206 is a potent allosteric inhibitor of AKT with anti-proliferative activity alone and in combination with other agents in human cancer cell lines including breast, ovarian, lung, and prostate cancer. (4–6) Additionally, MK-2206 has been shown to have synergistic antitumor activity when combined with erlotinib, docetaxel, and carboplatin in vivo in various human tumor xenograft models. In vitro investigations in NSCLC cell lines showed that in some erlotinib-sensitive cell lines (whether EGFR mutated or not) stimulation with hepatocyte growth factor (HGF), the ligand for MET, reverses the cytotoxic and cytostatic effects of erlotinib treatment. [7] AKT inhibition with MK-2206 overcame HGF-mediated resistance to erlotinib, partially restoring erlotinib activity. Additionally, significantly elevated HGF plasma levels were observed in patients who progressed on erlotinib therapy, suggesting that peripheral plasma concentrations may be an indicator of -or a contributing factor to - erlotinib resistance in patients with WT-EGFR.
A phase I trial of erlotinib + MK2206 had previously been reported, showing that the combination was feasible and tolerable. (8) Both QOD and QW dosing schedules of MK-2206 were evaluated in that trial. MK-2206 at 45 mg QOD and erlotinib at 150 mg daily appeared to be reasonably well-tolerated and was the dose-schedule selected for this current study.
PATIENTS AND METHODS
Eligibility Criteria
Institutional review boards at each study center approved the trial, and all patients provided written informed consent. Eligible patients were required to have histologically or cytologically confirmed NSCLC of any histologic subtype and progressive disease following prior benefit (response or stable disease) to EGFR-TKI therapy (erlotinib) administered either as a single agent or in combination with other agents for at least 12 weeks prior to progression. Patients may have received intervening systemic therapy after initial erlotinib progression. Patients must also have documentation of radiographic progression within the preceding three months prior to study entry. Any number of prior chemotherapy regimens was allowed. A Karnofsky Performance Status of at least 60% was required. Patients must have acceptable hepatic, renal, and bone marrow function and were required to provide signed written informed consent document. Patients with asymptomatic controlled or treated (e.g., with radiation and/or surgery) brain metastases were eligible as long as corticosteroids given expressly for brain metastases have been stopped for at least 14 days. Patients with history of allergic reactions attributed to compounds of similar chemical or biologic composition to MK-2206 or erlotinib were excluded
Study Design and Treatment Plan
This was a stratified phase II trial of MK-2206 plus erlotinib in previously erlotinib-treated metastatic NSCLC patients. Patients received erlotinib at 150 mg orally once daily plus MK-2206 at 200 mg orally every week with a cycle length of 28 days. Patients were stratified into two groups: STRATUM 1 - those whose tumors have EGFR activating mutations (in exons 19 and 21); and STRATUM 2 - those whose tumors are EGFR wild-type.
Patients developing a rash no worse than grade 2 were managed at the discretion of the treating physician. Grade 3 or higher rash required a dose reduction. Patients with grade 3 or worse diarrhea occurring despite the optimal use of loperamide required a dose reduction. Patients developing grade 2 keratitis required a dose interruption until resolution or amelioration of findings to ≤ grade 1 and then could be retreated at the discretion of the physician with a dose reduction. For grade 2 medically concerning non-hematological toxicity (e.g., prolonged cardiac, pulmonary, or neurotoxicity), treatment was held until resolution to ≤ grade 1 and will be reinstituted with a dose reduction. For other forms of toxicities of grade 3 or higher (with the exception of alopecia), treatment was held until toxicity resolved to grade 1 or less; treatment was then be resumed with a dose reduction. No dose re-escalations were permitted.
Statistical Considerations
The primary objective for this phase II trial was to determine the efficacy of MK-2206 and erlotinib in combination in two different patient strata: those with EGFR-mutated tumors and those with EGFR wild type tumors. Secondary objectives included progression-free survival and safety. Enrollment to stratum 1 was done in two stages. In the first stage, 21 patients in stratum 1 were to be treated. If one or fewer exhibited a RECIST objective response, enrollment to this stratum was to be closed. If 2 or more patients had an objective response, the study was to continue enrollment to the final sample size of 41 subjects. If 5 or more of the 41 patients responded, the trial was to be regarded as indicating adequate activity in tumors with EGFR-mutations, providing other factors, such as toxicity and time to progression, also appear favorable. The probability of indicating activity by this criterion was no more than 0.05 if the underlying response rate was 5%, and it was at least 0.90 if the underlying response rate was 20%.
Enrollment to stratum 2 was also done in two stages. In the first stage, 21 patients in stratum 2 were to be treated. If one or fewer exhibit disease control (DC) at 12 weeks, enrollment to this stratum was to be closed. If 2 or more patients had DC at 12 weeks, the study was to continue enrollment to the final sample size of 41 subjects. If 5 or more of the 41 patients had DC at 12 weeks, the trial was to be regarded as indicating adequate activity in tumors with wild type EGFR, providing other factors, such as toxicity and time to progression, also appear favorable. Similar to stratum 1, the probability of indicating activity by this criterion was no more than 0.05 if the underlying DC rate was 5%, and it was at least 0.90 if the underlying rate was 20%.
Study Conduct
The two-stage design was applied independently to the two strata. EGFR mutational status was not initially required at the start of the study; however, this was required to be ascertained within 6 weeks of enrolment so that patients can be allocated to their appropriate stratum for subsequent analysis. To avoid interrupting accrual while endpoints were being evaluated during the first stage, over-enrollment of up to 5 extra patients on the first stages were allowed. If a second favorable outcome occurred among these additional patients, the stratum was allowed continue accrual past the first stage (type I error rate was maintained at 0.050). In the event that one stratum closed while the other was accruing, knowledge of EGFR mutation status was then required to be established prior to registration. This was allowed to occur at the first stage, or at the end of the study.
By March 2013, the criterion for regarding the regimen promising in the stratum 2 was exceeded by a wide margin, with 13 of 32 subjects then enrolled in the stratum exhibiting DC at 12 weeks, and 7 with DC at 24 weeks. The lower end of an exact 95% confidence interval for the probability of DC at 12 weeks was 24%, which was above the 20% rate that was regarded as promising when establishing the two-stage design. The 2.5th percentile of the posterior distribution using a flat prior was 26%, also well above the target. At this point, permission to close the trial was granted by the trial sponsor NCI-CTEP. By the time the trial officially closed in April 2013, 36 patients had been enrolled in this stratum.
RESULTS
Patient Demographics
Patient characteristics are summarized in Table 1. A total of 80 patients were enrolled in the trial. Forty five patients were enrolled in stratum 1 while 35 patients were in stratum 2. Median age was 64 years, while the majority of patients were female. Approximately 80% of patients in both strata had adenocarcinoma histology. Sixty three patients (79%) had prior cytotoxic chemotherapy. Erlotinib was the immediate prior therapy for 55% and 60% of patients, respectively. The median number of prior drug therapies for stratum 1 was 2 (range 1–8) and for stratum 2 the median was 3 (range 1–8). EGFR mutational status was as follows: del 19 (27 patients), L858R (11 patients), and Exon 21/other (7 patients).
Table 1.
Patient Characteristics
| Variable | Stratum | |
|---|---|---|
| EGFR mutant | EGFR wild type | |
| Number of patients | 45 | 35 |
| Age, median in years (range) | 64 (44–86) | 63 (40–83) |
| Male sex, n (%) | 14 (31%) | 15 (43%) |
| Race, n (%) | ||
| Asian | 21 (47%) | 6 (17%) |
| Caucasian | 20 (44%) | 27 (77%) |
| Black | 1 (2%) | 1 (3%) |
| Other | 3 (7%) | 1 (3%) |
| Karnofsky Performance Status, n (%) | ||
| 90–100% | 25 (54%) | 28 (82%) |
| 70–80% | 20 (44%) | 7 (20%) |
| Histology | ||
| Adenocarcinoma | 36 (80%) | 28 (80%) |
| Squamous cell | 2 (4%) | 3 (9%) |
| Mixed tumor or NOS | 7 (16%) | 4 (11%) |
| Erlotinib as immediate prior therapy, n (%) | 25 (56%) | 21 (60%) |
Safety and Treatment Delivery
The combination of MK-2206 and erlotinib appeared to be feasible and tolerable (Table 2). In stratum 1, the median number of cycles was 3 (range 1–21) while in stratum 2, the median number of cycles was likewise 3 (range 1–23). Dose delays, principally due to toxicity, were similar between strata. Of the 230 total number of cycles delivered in stratum 1, 30 (13%) required dose delay. Overall, 31 patients (39%) required dose modification of either or both drugs at some point during the course of the trial. Of the 203 total number of cycles delivered in stratum 2, 26 (13%) required a dose delay. Of the 80 subjects treated, 52 subjects experienced least one AE of grade 3 or worse. In 41 of these subjects, at least one grade 3 AE was at least possibly attributed to MK-2206 or to erlotinib, and in 3 subjects, the attributable AE was worse that grade 3. Table 2 enumerates the number of subjects with any grade 3 or higher adverse event. The most common attributable adverse events (all grade 3) were rash (n=12), diarrhea (n=11), fatigue (n=8), and mucositis (n=5). There was only 1 grade 4 attributable event (lung infection). There was 1 treatment-related death (pneumonia). The primary reason for study discontinuation was disease progression in 56 patients (70%).
Table 2.
Subjects with any grade 3 or higher adverse event by MEDDRA code, at least possibly attributable to MK-2206
| Number of subjects with attributable grade 3+ adverse event | |||
|---|---|---|---|
| MEDDRA Code | EGFR mutant | EGFR wild type | Total |
| Anemia | 0 | 1 | 1 |
| Diarrhea | 5 | 5 | 10 |
| Mucositis | 4 | 1 | 5 |
| Nausea | 1 | 2 | 3 |
| Vomiting | 0 | 1 | 1 |
| Fatigue | 5 | 3 | 8 |
| Skin infection | 0 | 1 | 1 |
| Lung infection | 2 | 0 | 2 |
| Urinary tract infection | 1 | 1 | 2 |
| Creatinine increased | 0 | 1 | 1 |
| Lymphocyte count decreased | 6 | 3 | 11 |
| Anorexia | 0 | 1 | 1 |
| Dehydration | 3 | 0 | 3 |
| hyperglycemia | 3 | 0 | 3 |
| Hypokalemia | 2 | 0 | 2 |
| Hyponatremia | 0 | 2 | 2 |
| Hypophophatemia | 1 | 0 | 1 |
| Myalgia | 1 | 0 | 1 |
| Neoplasm | 0 | 1 | 1 |
| Dyspnea | 1 | 1 | 2 |
| Dry skin | 0 | 3 | 3 |
| Erythema multiforme | 1 | 0 | 1 |
| Skin peeling (feet) | 0 | 1* | 1* |
| Pruritus | 0 | 1 | 1 |
| Rash | 9 | 3 | 12 |
| Hypertension | 1 | 0 | 1 |
Efficacy
There were no complete responders in either stratum. In the EGFR mutant stratum (N=45), four patients (9%) had a partial response while 14 patients had stable disease for an overall DCR at 12 weeks of 40%. For this stratum, median PFS was 4.4 months (95% CI 2.7,6.6). In the EGFR wild type stratum (N=35), there was only 1 patient with unconfirmed partial response and 14 patients with stable disease. Thus, DCR at 12 weeks was seen in 15 patients (43%). Two patients in the wild type stratum quit therapy at 12 weeks despite having stable disease. If we count these as failures, the rate of 13 SD out of 35 (37%) implies 21.5% as the lower bound of a 95% confidence interval, still above the 20% target. In this stratum, median progression-free survival was 4.6 months (95% CI 2.9, 8.5). Efficacy results are summarized in Table 3 while Kaplan Meier curves for PFS are illustrated in Figure 1. Images from computed tomography chest scans from a female patient in Stratum 1 who experienced a partial response to therapy are shown in Figure 2.
Table 3.
Response rate, disease control rate (DCR), and progression free survival according to stratum
| Stratum | Response Rate, N (%) | DCR at 12 weeks, N (%) | Median Progression-Free Survival, Months (95% CI) |
|---|---|---|---|
|
| |||
| 1: EGFR mutant (N=45) | 4 (9%) | 18 (40%) | 4.4 (2.7,6.6) |
| Erlotinib in last regimen (n=25) | 2 (8%) | 8 (32%) | 3.1 (2.7, 13.9) |
| No erlotinib in last regimen (n=20) | 2 (10%) | 10 (50%) | 5.3 (2.6, 11.3) |
|
| |||
| 2: EGFR wild type (N=35) | 1 (3%) | 15 (43%) | 4.6 (2.9, 8.5) |
| Erlotinib in last regimen (21) | 0 (0%) | 10 (48%) | 5.6 (2.8, 15.6) |
| No erlotinib in last regimen (14) | 1 (7%) | 5 (36%) | 3.0 (2.9, NA) |
Figure 1.
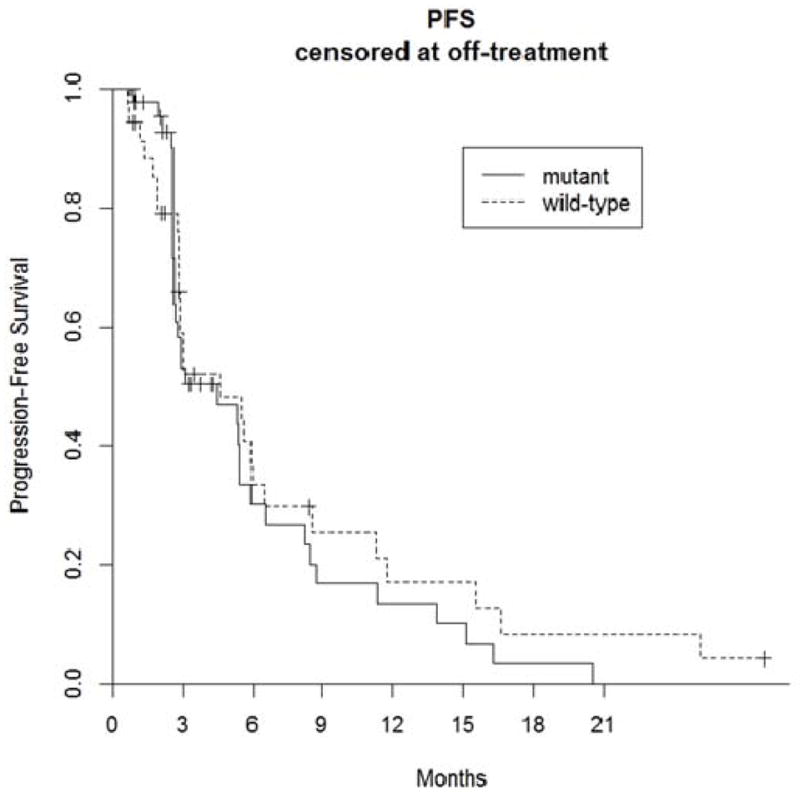
Progression-Free Survival Kaplan-Meier Curves (by EGFR mutational status);
Figure 2.
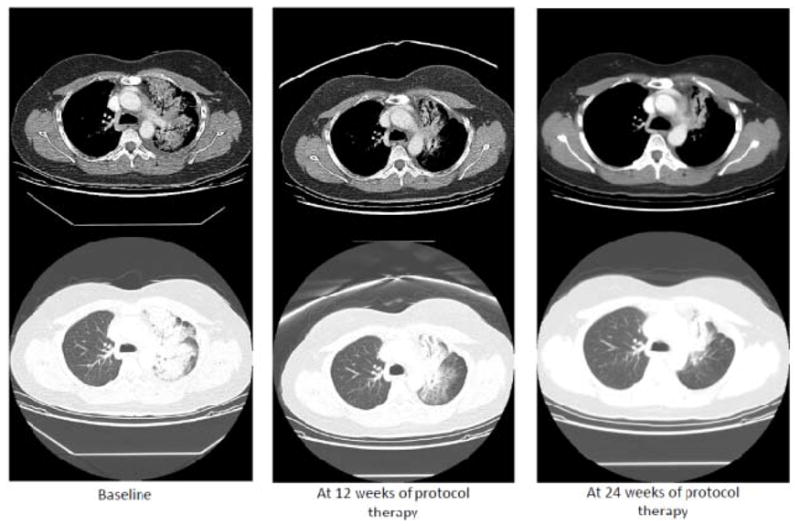
Example of a patient who responded to protocol therapy. This was 50 year old Asian-American female with lung adenocarcinoma that harbored an EGFR Exon 19 deletion. Prior therapies included: 1) carboplatin/paclitaxel/bevacizumab modified to carboplatin/docetaxel/bevacizumab and carboplatin/gemcitabine/bevacizumab due to allergic reactions; 2) single agent pemetrexed; 3) erlotinib + pemetrexed; and 4) single agent erlotinib. She experienced a partial response to treatment with MK-2206 and erlotinib, as shown in the computed tomography images.
DISCUSSION
Although EGFR TKIs are approved and clinically useful in both EGFR-mutated and EGFR-wild type NSCLC, acquired resistance is a universal phenomenon and is a focus of active clinical investigation. In lung cancer patients whose tumors harbor activating EGFR mutations – often in-frame deletions in exon 19 (del19) or a point mutation in exon 21 (L858R) – the median PFS with EGFR inhibitors is typically less than 12 months.(9) In patients with EGFR wild type lung cancer, median PFS is even more modest, typically around 2–3 months. (10) Furthermore, in EGFR wild type tumors, clinical benefit is primarily “cytostatic” with prolonged disease control, in contrast to “cytotoxic” effects in EGFR mutants that result in a dramatic response. (10, 11) Mechanisms of acquired resistance in the EGFR mutant population include the development of a secondary EGFR mutation in exon 20 (T790M); this mutation accounts for approximately half of all EGFR-resistant cases. Other less well-characterized non-T790M mutations have been reported such as D761Y and L747S, but their frequency is often below 5%.(9) In a substantial proportion of EGFR mutated NSCLC (~20–30%), an alternative mechanism of EGFR TKI resistance revolves around aberrant bypass signaling through the HGF-MET pathway, which subsequently signals through AKT to mediate cell proliferation and survival Our current clinical trial aimed to exploit a potential AKT-mediated resistance mechanism in both EGFR mutant and wild type lung cancers.
In this stratified clinical trial, we found that in patients with EGFR wild type NSCLC cancers, the combination of MK2206 and erlotinib met pre-determined criteria for clinical activity to warrant further clinical investigation. This patient stratum met its primary endpoint target of DCR at 12 weeks > 20%; specifically, DCR was found to be 43%, much higher than originally anticipated. In contrast, in EGFR mutated NSCLC, the primary endpoint of RECIST response rate > 20% was not met; the observed response rate was only 9%. However, it must be pointed out that the 12-week DCR of 40% can still be considered a sign of clinical benefit and suggests activity for this doublet in a yet unidentified molecular subset. The results of this trial appear somewhat comparable to the results of the phase III Lux Lung 1 trial which randomized patients who have had prior chemotherapy and at least 12 weeks of EGFR TKI therapy to either afatinib, an irreversible EGFR kinase inhibitor, or placebo.(12) In that trial (which also did not pre-screen patients for EGFR mutation status prior to study entry), PFS was 3.3 months in the afatinib arm versus 1.1 months in the placebo arm. Disease control rates at 8 weeks in the afatinib and placebo arms were respectively 58% and 19%. In the phase III LUX-Lung 8 trial, PFS and DCR were significantly better in patients with relapsed/refractory squamous cell carcinoma of the lung – essentially tumors that are EGFR wild type – who were treated with afatinib than in those treated with erlotinib. (13)
Our clinical trial attempted to use AKT inhibition as a means to restore erlotinib sensitivity in lung cancer patients who had previously shown clinical benefit from erlotinib. The relatively modest efficacy results seen here for the combination of MK-2206 and erlotinib call attention to the incomplete inhibition of the complex bypass and redundant signaling mechanisms that underlie non-T790M-mediated EGFR TKI resistance. It is apparent that dual AKT and EGFR inhibition was insufficient to induce substantial cytotoxic responses; rather cytostatic responses were observed in both patient strata, mirroring subsequent preclinical observations. (14) Notably, in a recently reported “basket trial” of various targeted therapies directed against specific molecular phenotypes, a low frequency of genetic alterations in the PIK3CA/AKT/PTEN pathway was observed; of seven patients whose thoracic cancer had an alteration in this pathway, single agent MK2206 was not found to yield tumor response. (15) It is conceivable that there exists a critical number of signaling pathways that when inhibited simultaneously or sequentially with targeted agents will lead to clinically relevant tumor cell death or apoptosis, as long as toxicity is not excessive. For instance, a biomarker driven trial of MK-2206 and selumetinib in colorectal cancer reported the infeasibility of combining these agents due to overlapping toxicities that prevented dose escalation of each agent to achieve exposures presumably required for clinical activity.(16)
The limited resources available to this publically-funded trial precluded comprehensive molecular profiling of tumor tissue collected immediately prior to study entry. At the time this study was initiated, serial biopsy of tumor tissue for molecular phenotyping in refractory lung cancer was not yet considered standard-of-care and therefore not typically reimbursed by third party payers. Access to such tissue would have provided important information regarding which molecular subsets were most associated with clinical benefit from MK-2206/erlotinib therapy. For example, it is not clear whether the subset of patients enjoying disease control at 12 weeks represent those patients whose tumors are employing bypass molecular pathways that signal through AKT. For the few remarkable responders on this trial (see Figure 2), such molecular information would have been valuable.
In conclusion, this NCI-sponsored trial strongly suggests that AKT pathway inhibition merits further clinical evaluation in erlotinib-refractory EGFR-wild type NSCLC. Further investigations as to the optimal therapeutic strategies in erlotinib-refractory EGFR mutant NSCLC are critical. Most recently, early phase trials of third generation EGFR TKIs (CO1896, AZD9291) that inhibit the T790M resistance mutation have shown remarkable responses in this patient subset.(17–18) However, the optimal approach to tumors that mediate erlotinib resistance through non-T790M mechanisms remains inadequately addressed. As of this writing, there is still no agent or combination of agents that has been FDA-approved for the treatment of acquired resistance to erlotinib. Thus we believe that the work described here has continued clinical relevance and helps set the stage for future work that combines various signal transduction inhibitors in EGFR TKI-refractory NSCLC patients. Furthermore, this experience provides proof-of-concept and feasibility for the incorporation of AKT pathway inhibition as a viable therapeutic strategy, especially in light of continued clinical development of other AKT inhibitors such as AZD5363 and GSK2141795.
STATEMENT OF TRANSLATIONAL RELEVANCE.
MK-2206 is a potent allosteric inhibitor of AKT with anti-proliferative activity alone and in combination with other agents such as the EGFR inhibitor erlotinib in preclinical models. In vitro investigations in lung cancer cell lines showed that in selected erlotinib-sensitive cell lines (whether EGFR mutated or not), stimulation with HGF, the ligand for MET, reverses the cytotoxic and cytostatic effects of erlotinib. MK-2206 was able to overcome HGF-mediated resistance to erlotinib, partially restoring erlotinib activity. Here we report the results of a phase II trial of MK-2206 plus erlotinib in previously erlotinib-treated NSCLC patients, stratified by EGFR mutational status. Combination therapy was found to be tolerable. Importantly, clinically relevant activity was observed: the combination met the protocol-defined primary endpoint in erlotinib-pretreated patients with EGFR wild type NSCLC. The results of this trial provide support for further clinical evaluation of AKT pathway inhibition in EGFR wild type NSCLC.
Acknowledgments
Grant/Contract Support: This investigation was supported by NCI-CTEP N01 CM-00038 awarded to the University of California Davis (Phase II headquarters of the California Cancer Consortium; Principal Investigator: P Lara).
We acknowledge the contributions of Will Holland to the preclinical modeling studies and to Stella Khoo for her administrative support.
Footnotes
Clinical Trials.gov registration # NCT01294306
Presented in part at the 45rd Annual Meeting of the American Society of Clinical Oncology, June 1–5, 2014, Chicago, IL.
Conflict of interest: None.
References
- 1.Lwin Z, Riess JW, Gandara D. The continuing role of chemotherapy for advanced non-small cell lung cancer in the targeted therapy era. Journal of thoracic disease. 2013;5:S556–64. doi: 10.3978/j.issn.2072-1439.2013.08.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gandara DR, Li T, Lara PN, et al. Acquired resistance to targeted therapies against oncogene-driven non-small-cell lung cancer: approach to subtyping progressive disease and clinical implications. Clinical lung cancer. 2014;15:1–6. doi: 10.1016/j.cllc.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirsch FR, Janne PA, Eberhardt WE, et al. Epidermal growth factor receptor inhibition in lung cancer: status 2012. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2013;8:373–84. doi: 10.1097/JTO.0b013e31827ed0ff. [DOI] [PubMed] [Google Scholar]
- 4.Iida M, Brand TM, Campbell DA, et al. Targeting AKT with the allosteric AKT inhibitor MK-2206 in non-small cell lung cancer cells with acquired resistance to cetuximab. Cancer Biol Ther. 2013;14:481–91. doi: 10.4161/cbt.24342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirai H, Sootome H, Nakatsuru Y, et al. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Molecular cancer therapeutics. 2010;9:1956–67. doi: 10.1158/1535-7163.MCT-09-1012. [DOI] [PubMed] [Google Scholar]
- 6.Tolcher AW, Yap TA, Fearen I, Taylor A, Carpenter C, Brunetto AT, et al. A phase I study of MK-2206, an oral potent allosteric Akt inhibitor (Akti), in patients (pts) with advanced solid tumor (ST) Journal of Clinical Oncology. 2009;27:3503. [Google Scholar]
- 7.Mack PC, Farneth N, Mahaffey C, Lara PN, Gandara DR. Impact of AKT inhibitor MK-2206 on erlotinib resistance in non-small cell lung cancer (NSCLC) Journal of Clinical Oncology. 2011;29:7573. [Google Scholar]
- 8.Molife LR, Yan L, Vitfell-Rasmussen J, Zernhelt AM, Sullivan DM, Cassier PA, et al. Phase 1 trial of the oral AKT inhibitor MK-2206 plus carboplatin/paclitaxel, docetaxel, or erlotinib in patients with advanced solid tumors. Journal of hematology & oncology. 2014;7:1. doi: 10.1186/1756-8722-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen KS, Kobayashi S, Costa DB. Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancers dependent on the epidermal growth factor receptor pathway. Clinical lung cancer. 2009;10:281–9. doi: 10.3816/CLC.2009.n.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. The New England journal of medicine. 2005;353:123–32. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 11.Cappuzzo F, Ciuleanu T, Stelmakh L, Cicenas S, Szczésna A, Juhász E, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11:521–29. doi: 10.1016/S1470-2045(10)70112-1. [DOI] [PubMed] [Google Scholar]
- 12.Miller VA, Hirsh V, Cadranel J, Chen YM, Park K, Kim S-W, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncology. 2012;13:528– 38. doi: 10.1016/S1470-2045(12)70087-6. [DOI] [PubMed] [Google Scholar]
- 13.Goss GD, Felip E, Cobo M, Lu S, Syrigos K, Lee K, et al. A randomized, open-label, phase III trial of afatinib (A) vs erlotinib (E) as second-line treatment of patients (pts) with advanced squamous cell carcinoma (SCC) of the lung following first-line platinum-based chemotherapy: LUX-Lung 8 (LL8). Proceedings of the European Society for Medical Oncology (ESMO); 2014; p. Abstract 1222O. [Google Scholar]
- 14.Holland WS, Chinn DS, Lara PN, Gandara DR, Mack PC. Effects of AKT inhibition on HGF mediated erlotinib resistance in non-small cell lung cancer cell lines. J Cancer Res Clin Oncol. 2015;41:615–26. doi: 10.1007/s00432-014-1855-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez-Chavez A, Thomas A, Rajan A, Raffeld M, Morrow B, Kelly R, et al. Molecular profiling and targeted therapy for advanced thoracic malignancies: a biomarker-derived, multiarm, multihistology phase II basket trial. J Clin Oncol. 2015;33:1000–7. doi: 10.1200/JCO.2014.58.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Do K1, Speranza G, Bishop R, Khin S, Rubinstein L, Kinders RJ, et al. Biomarker-driven phase 2 study of MK-2206 and selumetinib (AZD6244, ARRY-142886) in patients with colorectal cancer. Invest New Drugs. 2015 doi: 10.1007/s10637-015-0212-z. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sequist LV, Soria JC, Goldman JW, Wakelee HA, Gadgeel SM, Varga A, et al. Rociletinib in EGFR-mutated non-small-cell lung cancer. N Engl J Med. 2015;372:1700–9. doi: 10.1056/NEJMoa1413654. [DOI] [PubMed] [Google Scholar]
- 18.Jänne PA, Yang JC, Kim DW, Planchard D, Ohe Y, Ramalingam SS, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med. 2015;372:1689–99. doi: 10.1056/NEJMoa1411817. [DOI] [PubMed] [Google Scholar]


