Abstract
Objective
To identify the issues needing to be resolved in order to design, implement and complete a definitive randomized controlled trial (RCT) of adjunctive corticosteroid use in children with septic shock.
Data Source
MEDLINE (1946- January 2015) and Embase (1947 to January 2015).
Study Selection and Synthesis
Pediatric studies which addressed adrenal function or steroid use in critically ill children with systemic inflammatory response syndrome (SIRS), sepsis or septic shock were reviewed and their relevant points discussed.
Results
There is considerable interest in the field of corticosteroids in pediatric septic shock which has not as yet translated into a much needed RCT. We found that the issues that need to be resolved include identification of the target population, achievement of individual and community equipoise, selection of a patient centered, clinically meaningful primary outcome measure and consideration of the adverse effects of corticosteroids.
Conclusions
We strongly believe that the time has come to conduct a trial on the use of corticosteroids in pediatric septic shock and that the question to be answered is: Will corticosteroids given to children with septic shock result in a benefit to some patients without resulting in harm to others? Answering this question will require a collaborative and committed effort on the parts of ethics boards, families, clinicians and researchers to actually make it happen once and for all and we propose an international planning meeting of interested parties to achieve agreement on these identified issues.
Keywords: cortisol, shock, septic shock, adrenal insufficiency, pediatric critical care, hydrocortisone
After more than 40 years of debating the role of corticosteroids in pediatric septic shock, we are still no closer to the answer. The question is, why? It does not appear to be due to a lack of interest in the topic as there have been more than 30 editorials, letters and reviews written on the subject just in the last two years. Instead, we believe that the major factors affecting the pediatric critical care community's inability to answer this question include difficulties in correctly identifying patients who may benefit from corticosteroid administration, an unfortunate lack of community and individual equipoise, agreement on a clinically meaningful outcome and the existence of significant barriers to conducting randomized controlled trials (RCTs) in pediatric critical care.
Who is the target population?
There are at least four distinct issues that need to be resolved in order to identify the appropriate target population for a trial of corticosteroids in pediatric septic shock. These include the definition of refractory shock, the early identification of patients with septic shock and perhaps most importantly, the identification of patients who are most likely to derive benefit from corticosteroid administration.
Refractory Shock
There are two separate issues in regard to using the presence of refractory shock as a criterion for inclusion of patients into a trial: 1) its definition and 2) whether this definition is the same as the threshold at which clinicians consider corticosteroid administration. The most recent Surviving Sepsis Guidelines use the term “catecholamine resistant shock” for patients who are still hypotensive following administration of 60 cc/kg of fluid, 10 micrograms/kg/min of dopamine and/or 0.05 to 0.3 micrograms/kg/min of epinephrine 1 and recommend consideration of corticosteroids at this point. Some researchers have, however, enrolled patients into corticosteroid and septic shock trials following 60 cc/kg of fluid alone 2 or following fluid plus 0.1 micrograms/kg/min of norepinephrine 3. A recent survey found that a slight majority of respondents (51.4%) stated that they would administer corticosteroids to patients in shock who were on one high dose vasoactive agent whereas almost all clinicians (91.4%) would administer corticosteroids for patients on two or more vasoactive agents.
The problem, however, with waiting for a patient to be on at least two vasoactive medications is that they may have already progressed to irreversible organ damage. This may explain why Annane and colleagues found a decrease in mortality with corticosteroid administration4 (enrolled patients within 8 hours) while the study from Sprung and colleagues did not find a difference in mortality rates but instead found an increase in adverse effects5 (enrolled patients up to 72 hours post shock). We recognize that the exact enrolment criteria will need to be agreed upon by a consensus of experts but would strongly advocate for a trial of targeted corticosteroid administration within 8 hours of initiation of a vasoactive agent and rapid weaning of the steroids as soon as the patient stabilizes and improves.
Identification of septic shock
Another practical problem is that there is no gold standard for the identification of septic shock in adults or children. Septic shock was conceptually defined for research purposes by experts at the International Pediatric Septic Consensus Conference as evidence of a systemic inflammatory response plus cardiovascular dysfunction in the context of a suspected or proven infection6. However, as elegantly demonstrated by Weiss et al in 20127, one third of patients diagnosed clinically with sepsis would not have be identified based on these consensus guidelines or International Classification of Diseases, Ninth Revision Codes. This raises a very important issue going forward as to whether it is better to answer the question in a restricted and narrowly defined population or to be more pragmatic and to include patients that clinicians suspect have septic shock and therefore try to answer the question such that the results are relevant to bedside clinicians. As such the specific inclusion and exclusion criteria for an RCT will need to be established by international consensus.
Targeted corticosteroid administration
The theoretical paradigm behind the use of corticosteroids in patients with septic shock is the belief that some critically ill patients have an inadequate amount of cortisol at the tissue level to meet their physiological needs8. This condition has been variably termed relative adrenal insufficiency or critical illness related corticosteroid insufficiency 9 and may result from dysfunction of the hypothalamic-pituitary-adrenal axis at any of multiple levels from the hypothalamus to the cytoplasmic glucocorticoid receptor10. Multiple studies have attempted to define a patient's potential response to corticosteroid administration using cortisol levels (free and total) and adrenocorticotropic hormone stimulation tests, but these studies have provided inconsistent and inconclusive results11–13. This is likely due to the varied causes for this clinical condition as well as the inability to measure tissue cortisol levels and means that determination of inclusion criteria for a proposed RCT will have to be established by clinical consensus rather than biochemical criteria.
Should there be equipoise on the use of corticosteroids in pediatric septic shock?
In an attempt to answer to this question, we searched MEDLINE (1946- January 2015) and Embase (1947 to January 2015) for all pediatric studies using the keywords: adrenal function, corticosteroids, hydrocortisone, critical illness, critical care, systemic inflammatory response (SIRS), sepsis and septic shock. We excluded reviews, case series and case reports as well as studies that exclusively enrolled neonates or focused on patients with primary cardiogenic shock.
Benefit
Two early RCTs, involving a total of only 120 patients 14, 15, (see Table 1) suggested a mortality benefit to corticosteroid supplementation in patients with dengue fever while a third open label study suggested a decrease in time to shock reversal in septic patients in the developing world2. However, as pointed out in a recent systematic reviews on this topic16, these RCTs were limited by weak methodology, restricted patient populations and small sample sizes. Furthermore, the overall meta-analysis in the systematic review showed no difference in mortality rates between those who did and did not receive corticosteroids (RR 0.744 95% CI 0.475–1.165, P =0.197)16. Finally, a retrospective analysis of the largest pediatric sepsis trial to date (the RESOLVE trial)17 found that no definitive improvement in outcomes could be attributed to adjunctive corticosteroid therapy18.
Table 1.
Pediatric studies on corticosteroid therapy in pediatric shock.
| Suggested benefit | Did not endorse benefit or harm | Suggested harm | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Year | No. of patients | Study design | Author | Year | No. of patients | Study design | Author | Year | No. of patients | Study design |
| Min14 | 1975 | 98 | RCT* | Pongpanich19 | 1973 | 71 | RCT | Markovitz21 | 2005 | 6693 | Retrospective cohort |
| Futrakul15 | 1981 | 22 | RCT | Sumarmo34 | 1982 | 97 | RCT | Atkinson23 | 2014 | 496 | Retrospective cohort |
| Valoor2 | 2009 | 38 | RCT | Tassniyom35 | 1993 | 63 | RCT | Wong20 | 2014 | 180 | Retrospective cohort |
| Hebbar3 | 2011 | 97 | Retrospective cohort | Slusher36 | 1996 | 72 | RCT | Wong23 | 2015 | 132 | Prospective cohort |
| Madhi37 | 2014 | 410 | Retrospective cohort | Panpanich38 | 2006 | 284 | Systematic review | ||||
| Zimmerman18 | 2010 | 477 | Retrospective cohort | ||||||||
| Menon16 | 2013 | 447 | Systematic review | ||||||||
RCT = randomized controlled trial
Harm
None of the existing RCTs were powered to detect a difference in the incidence of adverse events but nevertheless one of them demonstrated a statistically significant increase in bleeding with corticosteroids19. More recently, there have been several considerably larger, albeit mostly retrospective studies suggesting an increased risk of mortality, secondary infections and suppression of adaptive immunity from corticosteroid administration especially in certain subgroups20–23. Given the lack of clear evidence for benefit and the increasing suggestions of harm with corticosteroid administration, there is no question that equipoise on this subject should indeed exist. In order to mitigate the risk of harm, however, there are two potential strategies which may be employed for a future RCT. The first is to exclude patients with septic shock who may be at higher risk of harm from corticosteroid administration by using a gene expression-based classification method to identify them20. An additional approach would be to administer corticosteroids early and wean them as soon as the patient was hemodynamically stable (this was not done in the two largest adult trials4, 5) so as to limit corticosteroid exposure to the minimum amount needed.
Does equipoise on the use of corticosteroids in shock actually exist?
We strongly believe that given the limited evidence for benefit for corticosteroid administration in septic shock and the suggestion of potential harms, equipoise should definitely exist on this issue. However, the evidence from both reported and observed clinical practices suggests that it does not.
Individual equipoise
In a recent survey, 76% of pediatric critical care physicians stated that they would be willing to randomize patients who were on two or more high dose vasoactive medications into a trial of corticosteroid efficacy24. However, 61% of the same physicians stated that they would start open label steroids in such patients if they were deteriorating. This dichotomy suggests that although most of us are in support of a trial of corticosteroids in pediatric septic shock, many of us may not actually be willing to randomize our most critically ill patients.
Community equipoise
This observation is further supported by a recent retrospective study which showed that pediatric critical care physicians often used corticosteroids in patients with fluid and catecholamine dependent shock25 and almost always used corticosteroids in patients with refractory shock24, 26 thus providing evidence of lack of equipoise. Interestingly, a recent survey reported that the possibility of adverse effects does not appear to influence clinicians' decision making when administering corticosteroids to children with septic shock26. Perhaps the final nail in the coffin for the existence of equipoise is the statement from the most recent Surviving Sepsis Guidelines which recommended “timely hydrocortisone therapy in children with fluid-refractory, catecholamine-resistant shock and suspected or proven absolute adrenal insufficiency” 1(Grade 1A). However, there are two significant issues with these recommendations. The first is that real-time adrenal axis is not available in many centres and even when it is there is no consensus on the diagnosis of suspected or proven absolute adrenal insufficiency in the critical care setting11. The second issue is that there has never been a large, adequately powered randomized controlled trial of corticosteroids in pediatric septic shock16 making one question the justification for the Grade 1A recommendation. We would strongly advocate for a well-designed, adequately powered RCT upon which to base future recommendations.
Clinically meaningful outcomes
The high mortality rates in adult septic shock patients (32.8% to 38.8%)4, 5 have made mortality a commonly used outcome measure in adult septic shock trials4, 5, 27 However, mortality rates for pediatric septic shock in the developed world range from 2% to 12% 25, 28 which makes mortality infeasible as a primary outcome measure. A recent retrospective study with an observed mortality rate of 11.7% in patients with septic shock showed that one would require 7682 patients to demonstrate a 2% absolute mortality reduction25. Given that the largest pediatric sepsis trial to date (RESOLVE)17 enrolled 477 patients, a sample size greater than 500 patients would be unrealistic. When Canadian pediatric intensive physicians were asked what they thought would be the single most relevant and feasible outcome measure for a trial of corticosteroids in pediatric septic shock, 65.7% selected a hemodynamic outcome (39.3%, time to discontinuation of all vasoactive drugs, and 23% time to hemodynamic stability). The same retrospective review suggested that a primary outcome measure of time on vasopressors would only require 106 to 420 patients depending on the clinically important difference chosen (24 hours or 12 hours respectively). Although organ dysfunction scores such as the PELOD-2 are scientifically valid and have merit29, they were considerably less popular (11.4%) leading to questions about their relevance to bedside clinicians. A decision regarding the most appropriate primary outcome measure will need to be made by consensus amongst experts, research networks and bedside clinicians.
Barriers, perceived or real, to clinical trials in pediatric critical care
There has been a significant increase in the number of publications on adrenal function and corticosteroid use in pediatric septic shock over the past 10 years (see Figure 1). However, as shown in Figure 1, there has not been a corresponding increase in the number of related pediatric RCTs. In the last 10 years only one published pediatric RCT involving 38 patients in the developing world2 compared to 16 adult trials involving several thousand patients. There are currently three pediatric trials listed under clinicaltrials.gov, two of which have not been updated since 2010 (NCT01047670, NCT00732277) and one pilot study which is currently recruiting (NCT02044159). There are numerous potential barriers to conducting pediatric critical care trials including obtaining consent from legal guardians and treating physicians30, limited patient numbers26, and the lack of equipoise but it is clear that with collaboration and commitment these barriers can be overcome and large pediatric critical care trials completed31.
Figure 1.
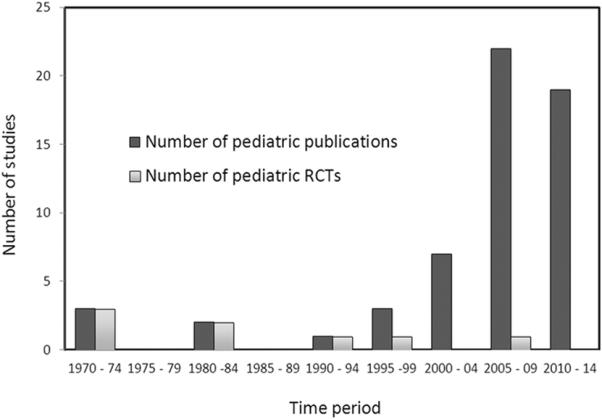
Studies on adrenal function and corticosteroid use in pediatric critical illness over time. RCT = randomized controlled trial.
Approach to a future pediatric RCT
It is clear that conducting this trial will require an international coordinated effort between multiple research networks. The specific issues that will need to be agreed upon include the target population, specific inclusion and exclusion criteria, adverse events reporting plan and stopping rules and the primary outcome measure. Practical issues such as multi-lateral funding, use of deferred consent models and development of strategies to ensure individual and community equipoise will also need to be addressed. We propose an international planning meeting of interested parties to accomplish these goals.
Conclusion
We, along with others32, 33, strongly believe that the time has come to determine if corticosteroids given to children with clinically suspected septic shock result in benefit to some patients without resulting in harm to the others? Given the potential barriers to conducting RCTs in pediatric critical care, however, answering this question will require a collaborative and committed effort on the parts of ethics boards, families, clinicians and researchers to actually make it happen once and for all.
Reference List
- 1.Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013 Feb;39(2):165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valoor HT, Singhi S, Jayashree M. Low-dose hydrocortisone in pediatric septic shock: an exploratory study in a third world setting. Pediatr Crit Care Med. 2009 Jan;10(1):121–5. doi: 10.1097/PCC.0b013e3181936ab3. [DOI] [PubMed] [Google Scholar]
- 3.Hebbar KB, Stockwell JA, Fortenberry JD. Clinical effects of adding fludrocortisone to a hydrocortisone-based shock protocol in hypotensive critically ill children. Intensive Care Med. 2011 Mar;37(3):518–24. doi: 10.1007/s00134-010-2090-3. [DOI] [PubMed] [Google Scholar]
- 4.Annane D, Sebille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002 Aug 21;288(7):862–71. doi: 10.1001/jama.288.7.862. [DOI] [PubMed] [Google Scholar]
- 5.Sprung CL, Annane D, Keh D, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008 Jan 10;358(2):111–24. doi: 10.1056/NEJMoa071366. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005 Jan;6(1):2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 7.Weiss SL, Parker B, Bullock ME, et al. Defining pediatric sepsis by different criteria: discrepancies in populations and implications for clinical practice. Pediatr Crit Care Med. 2012 Jul;13(4):e219–e226. doi: 10.1097/PCC.0b013e31823c98da. [DOI] [PubMed] [Google Scholar]
- 8.Marik PE, Zaloga GP. Adrenal insufficiency during septic shock. Crit Care Med. 2003 Jan;31(1):141–5. doi: 10.1097/00003246-200301000-00022. [DOI] [PubMed] [Google Scholar]
- 9.Annane D. Defining critical illness-related corticosteroid insufficiency: one step forward! Crit Care Med. 2010 Feb;38(2):721–2. doi: 10.1097/CCM.0b013e3181c54620. [DOI] [PubMed] [Google Scholar]
- 10.Peeters RP, Hagendorf A, Vanhorebeek I, et al. Tissue mRNA expression of the glucocorticoid receptor and its splice variants in fatal critical illness. Clin Endocrinol (Oxf) 2009 Jul;71(1):145–53. doi: 10.1111/j.1365-2265.2008.03443.x. [DOI] [PubMed] [Google Scholar]
- 11.Menon K, Ward RE, Lawson ML, Gaboury I, Hutchison JS, Hebert PC. A prospective multicenter study of adrenal function in critically ill children. Am J Respir Crit Care Med. 2010 Jul 15;182(2):246–51. doi: 10.1164/rccm.200911-1738OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casartelli CH, Garcia PC, Branco RG, Piva JP, Einloft PR, Tasker RC. Adrenal response in children with septic shock. Intensive Care Med. 2007 Sep;33(9):1609–13. doi: 10.1007/s00134-007-0699-7. [DOI] [PubMed] [Google Scholar]
- 13.Zimmerman JJ, Donaldson A, Barker RM, et al. Real-time free cortisol quantification among critically ill children. Pediatr Crit Care Med. 2011 Sep;12(5):525–31. doi: 10.1097/PCC.0b013e3181fe4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Min M, U T, Aye M, Shwe TN, Swe T. Hydrocortisone in the management of dengue shock syndrome. Southeast Asian J Trop Med Public Health. 1975 Dec;6(4):573–9. [PubMed] [Google Scholar]
- 15.Futrakul P, Vasanauthana S, Poshyachinda M, Mitrakul C, Cherdboonchart V, Kanthirat V. Pulse therapy in severe form of dengue shock syndrome. J Med Assoc Thai. 1981 Oct;64(10):485–91. [PubMed] [Google Scholar]
- 16.Menon K, McNally D, Choong K, Sampson M. A systematic review and meta-analysis on the effect of steroids in pediatric shock. Pediatr Crit Care Med. 2013 Jun;14(5):474–80. doi: 10.1097/PCC.0b013e31828a8125. [DOI] [PubMed] [Google Scholar]
- 17.Nadel S, Goldstein B, Williams MD, et al. Drotrecogin alfa (activated) in children with severe sepsis: a multicentre phase III randomised controlled trial. Lancet. 2007 Mar 10;369(9564):836–43. doi: 10.1016/S0140-6736(07)60411-5. [DOI] [PubMed] [Google Scholar]
- 18.Zimmerman JJ, Williams MD. Adjunctive corticosteroid therapy in pediatric severe sepsis: observations from the RESOLVE study. Pediatr Crit Care Med. 2011 Jan;12(1):2–8. doi: 10.1097/PCC.0b013e3181d903f6. [DOI] [PubMed] [Google Scholar]
- 19.Pongpanich B, Bhanchet P, Phanichyakarn P, Valyasevi A. Studies on dengue hemorrhagic fever. Clinical study: an evaluation of steroids as a treatment. J Med Assoc Thai. 1973 Jan;56(1):6–14. [PubMed] [Google Scholar]
- 20.Wong HR, Cvijanovich NZ, Allen GL, et al. Corticosteroids are associated with repression of adaptive immunity gene programs in pediatric septic shock. Am J Respir Crit Care Med. 2014 Apr 15;189(8):940–6. doi: 10.1164/rccm.201401-0171OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Markovitz BP, Goodman DM, Watson RS, Bertoch D, Zimmerman J. A retrospective cohort study of prognostic factors associated with outcome in pediatric severe sepsis: what is the role of steroids? Pediatr Crit Care Med. 2005 May;6(3):270–4. doi: 10.1097/01.PCC.0000160596.31238.72. [DOI] [PubMed] [Google Scholar]
- 22.Wong HR, Cvijanovich NZ, Anas N, et al. Developing a clinically feasible personalized medicine approach to pediatric septic shock. Am J Respir Crit Care Med. 2015 Feb 1;191(3):309–15. doi: 10.1164/rccm.201410-1864OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atkinson SJ, Cvijanovich NZ, Thomas NJ, et al. Corticosteroids and pediatric septic shock outcomes: a risk stratified analysis. PLoS One. 2014;9(11):e112702. doi: 10.1371/journal.pone.0112702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menon K, McNally JD, Choong K, et al. A survey of stated physician practices and beliefs on the use of steroids in pediatric fluid and/or vasoactive infusion-dependent shock. Pediatr Crit Care Med. 2013 Jun;14(5):462–6. doi: 10.1097/PCC.0b013e31828a7287. [DOI] [PubMed] [Google Scholar]
- 25.Menon K, Dayre MJ, Choong K, Lawson ML, Ramsay T, Wong HR. A Cohort Study of Pediatric Shock: Frequency of Corticosteriod Use and Association with Clinical Outcomes. Shock. 2015 Feb 13; doi: 10.1097/SHK.0000000000000355. [DOI] [PubMed] [Google Scholar]
- 26.Duffett M, Choong K, Vanniyasingam T, Thabane L, Cook DJ. Making decisions about medications in critically ill children: a survey of canadian pediatric critical care clinicians. Pediatr Crit Care Med. 2015 Jan;16(1):21–8. doi: 10.1097/PCC.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 27.Briegel J, Forst H, Haller M, et al. Stress doses of hydrocortisone reverse hyperdynamic septic shock: a prospective, randomized, double-blind, single-center study. Crit Care Med. 1999 Apr;27(4):723–32. doi: 10.1097/00003246-199904000-00025. [DOI] [PubMed] [Google Scholar]
- 28.Kissoon N, Carcillo JA, Espinosa V, et al. World Federation of Pediatric Intensive Care and Critical Care Societies: Global Sepsis Initiative. Pediatr Crit Care Med. 2011 Sep;12(5):494–503. doi: 10.1097/PCC.0b013e318207096c. [DOI] [PubMed] [Google Scholar]
- 29.Leteurtre S, Duhamel A, Salleron J, Grandbastien B, Lacroix J, Leclerc F. PELOD-2: an update of the PEdiatric logistic organ dysfunction score. Crit Care Med. 2013 Jul;41(7):1761–73. doi: 10.1097/CCM.0b013e31828a2bbd. [DOI] [PubMed] [Google Scholar]
- 30.Menon K, Ward RE, Gaboury I, et al. Factors affecting consent in pediatric critical care research. Intensive Care Med. 2012 Jan;38(1):153–9. doi: 10.1007/s00134-011-2412-0. [DOI] [PubMed] [Google Scholar]
- 31.Lacroix J, Hebert PC, Hutchison JS, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007 Apr 19;356(16):1609–19. doi: 10.1056/NEJMoa066240. [DOI] [PubMed] [Google Scholar]
- 32.Leclerc F, Botte A, Chene G, Leteurtre S. Adjunctive corticosteroid therapy in pediatric severe sepsis: many unsolved questions. Pediatr Crit Care Med. 2011 Jan;12(1):101–2. doi: 10.1097/PCC.0b013e3181d9c72b. [DOI] [PubMed] [Google Scholar]
- 33.Zimmerman JJ. Expanding the conversation regarding adjunctive corticosteroid therapy for pediatric septic shock*. Pediatr Crit Care Med. 2013 Jun;14(5):541–3. doi: 10.1097/PCC.0b013e31828a8165. [DOI] [PubMed] [Google Scholar]
- 34.Sumarmo, Talogo W, Asrin A, Isnuhandojo B, Sahudi A. Failure of hydrocortisone to affect outcome in dengue shock syndrome. Pediatrics. 1982 Jan;69(1):45–9. [PubMed] [Google Scholar]
- 35.Tassniyom S, Vasanawathana S, Chirawatkul A, Rojanasuphot S. Failure of high-dose methylprednisolone in established dengue shock syndrome: a placebo-controlled, double-blind study. Pediatrics. 1993 Jul;92(1):111–5. [PubMed] [Google Scholar]
- 36.Slusher T, Gbadero D, Howard C, et al. Randomized, placebo-controlled, double blinded trial of dexamethasone in African children with sepsis. Pediatr Infect Dis J. 1996 Jul;15(7):579–83. doi: 10.1097/00006454-199607000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Madhi F, Levy C, Deghmane AE, Bechet S, Cohen R, Taha MK. [Impact of corticosteroids in the immediate management of invasive meningococcal disease associated with hyperinvasive strains of the ST-11 clonal complex in children] Arch Pediatr. 2014 Mar;21(3):258–64. doi: 10.1016/j.arcped.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 38.Panpanich R, Sornchai P, Kanjanaratanakorn K. Corticosteroids for treating dengue shock syndrome. Cochrane Database Syst Rev. 2006;3:CD003488. doi: 10.1002/14651858.CD003488.pub2. [DOI] [PubMed] [Google Scholar]


