Abstract
Background
Northeastern states of the US show more progress in reducing colorectal cancer (CRC) incidence and mortality rates than Southern states, resulting in considerable disparities. We quantified how the disparities in CRC rates between Louisiana (Southern state) and New Jersey (Northeastern state) would be affected if differences in risk factors, screening and stage-specific CRC relative survival between states were eliminated.
Methods
We used the MISCAN-Colon microsimulation model to estimate age-adjusted CRC incidence and mortality rates in Louisiana from 1995-2009 assuming Louisiana had the same 1) smoking and obesity prevalence; 2) CRC screening uptake; 3) stage-specific CRC relative survival; and 4) a combination of all three, as observed in New Jersey.
Results
In 2009 the observed CRC incidence and mortality rates in Louisiana were 141.4 cases and 61.9 deaths per 100,000 individuals, respectively. With the same risk factors and screening as New Jersey, the CRC incidence rate in Louisiana was reduced by 3.5% and 15.2%. New Jersey's risk factors, screening and survival reduced the CRC mortality rate in Louisiana by 3.0%, 10.8%, and 17.4%, respectively. With all trends combined, the modeled rates per 100,000 individuals in Louisiana became lower than the observed rates in New Jersey for both incidence (116.4 versus 130.0) and mortality (44.7 versus 55.8).
Conclusions
The disparities in CRC incidence and mortality rates between Louisiana and New Jersey could be eliminated if Louisiana could attain New Jersey levels of risk factors, screening and survival. Priority should be given to enabling Southern states to improve screening and survival rates.
Keywords: Colorectal cancer, screening, computer simulation, prevention and control
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer death in the United States (US). An estimated 132,700 CRC cases will be newly diagnosed and 49,700 persons will die of the disease in 2015.[1] While age-standardized CRC incidence and mortality rates have been decreasing in the Northeastern states of the US since the late 1970s/early 1980s, the decreases began later and were slower in the Southern states.[2] As a result, CRC incidence and mortality rates are now higher in Southern states than in Northeastern states, opposite to the patterns observed prior to 1980.[2]
Most cancer control plans and policies that affect cancer prevention and access to screening in the US are designed and implemented at the state level. The observed variation in CRC incidence and mortality trends between states provides important information for policy makers on the success of the implemented interventions and provides evidence that interventions in some states can be improved. Differences in risk factors, screening and treatment are the most likely candidates to explain the observed disparity in CRC incidence and mortality trends.[3] Screening has been hypothesized to be the most important driver.[2] However, the individual contributions of these factors to disparities have never been evaluated, and doing so could inform the design of future cancer control policies and interventions.
In this analysis, we determined to what extent improving risk factor prevalence, screening uptake and CRC relative survival could reduce observed disparities in CRC incidence and mortality rates between states. We chose Louisiana as an exemplary Southern state with unfavorable trends in CRC incidence and mortality, and New Jersey as an exemplary Northeastern state with more favorable trends, because for both states long term, high-quality cancer registry data are available through the Surveillance Epidemiology and End Results (SEER) Program and the National Program of Cancer Registries (NPCR).
Methods
We used the MISCAN-Colon microsimulation model[4] of the Cancer Intervention and Surveillance Modeling Network (CISNET) to quantify how the disparity in observed CRC rates between Louisiana and New Jersey would be affected if Louisiana were to attain risk factor prevalence (i.e. smoking and obesity), screening uptake and stage-specific relative survival for CRC as observed in New Jersey. Stage-specific survival was used as a proxy for differences in treatment between states.
MISCAN-Colon Model
Supporting material 1 describes the MISCAN model. Briefly, the model simulates the life histories of a large population of individuals from birth to death and has a natural history component that tracks the progression of underlying colorectal disease in the absence of screening. As each simulated individual ages, there is a chance that one or more adenomas may develop depending on age, sex, race and individual risk. Adenomas can progress in size from small (≤5 mm) to medium (6-9 mm) to large (≥10 mm), and some may eventually become malignant. A preclinical (i.e., not yet detected) cancer has a chance of progressing through stages I-IV and may be detected because of symptoms at any stage. With screening, adenomas and preclinical cancers may be detected depending on the sensitivity of the test and, for endoscopic tests, whether the lesion is within reach of the endoscope.
The natural history part of the model was calibrated to pre-screening data from autopsy studies and 1995 age-specific CRC incidence from the Louisiana Tumor Registry (main assumptions presented in Table 1).[5] We included only first primary cases. Autopsy only and death certificate only cases, as well as tumors of the appendix were excluded. The model uses state-specific all-cause mortality life tables from the Cancer Survival in Five Continents study (CONCORD).[10] Stage-specific relative survival following CRC diagnosis from 1995 to 2009 for Louisiana and New Jersey were obtained from SEER data (Supporting material 2).[6] The prevalence of smoking and obesity over time, by state and by age was obtained from the Behavioral Risk Factor Surveillance System (BRFSS).[11] Smoking prevalence data were available from 1955 onwards, and obesity prevalence data were available from 1970 onwards (Supporting material 3). We assumed smoking and obesity prevalence before these years to be equal to the 1955 and 1970 levels respectively. The relative risk of smokers versus non-smokers was estimated to be 1.6, and the relative risk for obese (body mass index ≥30) versus non-obese individuals was estimated to be 1.4.[12-13] The prevalence of risk factors affected the risk for developing adenomas, subsequently an increase in risk factor prevalence would affect CRC incidence after an average lag time of approximately 20 years.[14]
Table 1.
Main natural history assumptions in the MISCAN-Colon model.
| Model parameter | Value | Source |
|---|---|---|
| Distribution of risk for adenomas over the general population | Gamma distributed, mean 1, variance 1.98 | Fit to multiplicity distribution of adenomas in autopsy studies:[7] Age 60 ≥1: 20% ≥2: 6% ≥3: 2% Age 90: ≥1: 37% ≥2: 17% ≥3: 9% |
| Adenoma incidence per year | Age and race dependent varying from 0-10% per year | Fit to adenoma prevalence in autopsy studies*, and cancer incidence in SEER registry in 1995.[6] |
| Probability that a new adenoma is progressive | Dependent on age at onset, varying from 0-89% | Fit to adenoma prevalence in autopsy studies*, and cancer incidence in SEER registry in 1975-1979 (pre-screening era).[6] |
| Regression of adenomas | No significant regression of adenomas | Expert opinion |
| Mean duration of preclinical cancer | 6.7 years | Estimated from large randomized controlled FOBT trials.[8] |
| Percent of non-progressive adenomas that stay 6-9mm, or become ≥10mm | 25% and 75% respectively | Fit to size distribution of adenomas in colonoscopy trial (percentages corrected for colonoscopy sensitivity): [9] ≤5mm: 73% 6-9mm: 15% ≥10mm: 12% |
| Percent of cancers that develops from 6-9mm adenoma and from 10≥mm adenoma | 30% of CRCs develop from 6-9 mm, 70% from 10≥mm | Expert opinion |
| Localization distribution of adenomas and cancer | Dependent on state and race: | Directly estimated from SEER in 1995.[6] |
| 10-year relative survival after clinical diagnosis of CRC | Dependent on period, stage, state and race (Supporting material 2) | Directly estimated from SEER in 1995-2008.[6] |
SEER: Surveillance, Epidemiology, and End Results; CRC: Colorectal cancer
References to autopsy studies provided in Supporting material 1.
The estimates for screening uptake over time were also obtained from BRFSS data (Supporting material 4).[11] We assumed no screening prior to 1978. For years in which no data were available, rates were extrapolated linearly. An overview of the test characteristics of screening tests used is provided in Supporting material 1.
The validity of the model has been tested previously using data from several large randomized screening and surveillance studies, such as the three large randomized controlled trials for fecal occult blood testing[8], the CoCap sigmoidoscopy study[15], and the National Polyp Study.[16] Additionally, the model was able to reproduce the observed CRC incidence and mortality trends in the US while accounting for secular trends in risk factor prevalence, screening practice, and chemotherapy treatment.[17]
Study Population
We used the model to simulate the Louisiana population from 1995 to 2009 (corrected for the impact of Hurricane Katrina) for both genders and all races combined. In a secondary analysis, we also simulated the black and white Louisiana populations separately. We did not analyze other racial groups or Hispanics separately due to small numbers in Louisiana. We restricted our analysis to the population aged 50 years and older, because this is the group for whom screening is recommended.[18-19]
Base case analysis
We simulated the Louisiana population with CRC risk factor prevalence, CRC screening uptake and stage-specific CRC relative survival as observed in Louisiana (Run 1). Alternatively, we modeled the Louisiana population assuming they had the same risk factors (Run 2), screening (Run 3), CRC survival (Run 4), and a combination of all three (Run 5) as observed in New Jersey.
We did not incorporate all known risk factors for CRC into the model, because data were not available. Therefore the simulated CRC incidence (mortality) rates do not fully correspond with the observed rates in Louisiana in 2009. Instead, we assumed that the simulated relative benefit of New Jersey risk factors, screening and CRC survival over Louisiana would be applicable to the observed CRC incidence and mortality. This assumption seems reasonable; three randomized controlled trials on biennial guaiac FOBT screening found similar percent mortality reductions ranging from 15% to 21% despite being carried out in populations with a different background CRC incidence level.[20]
We calculated the expected CRC incidence (mortality) rates in Louisiana for the scenarios by applying the percent difference in age-standardized incidence (mortality) rates between Run 1 and Run 2, 3, 4, or 5, respectively, to the observed CRC incidence and mortality rates for Louisiana in 2009.
The observed excess CRC risk was calculated as the absolute difference in observed CRC incidence (mortality) rates between Louisiana and New Jersey in 2009 (Formula 1, Supporting material 5).[21] Subsequently, the expected excess risk from each of the modeled scenarios was calculated as the absolute difference between the expected CRC incidence (mortality) rate from each scenario and the observed incidence (mortality) in New Jersey (Formula 2-5, Supporting material 5).
Sensitivity analyses
We performed four sensitivity analyses. First we performed an analysis in which Louisiana residents not only received less screening but also lower quality screening, assuming 25% lower adenoma detection rates with endoscopy. We then re-estimated the reduction in excess CRC risk due to differences in screening assuming New Jersey screening adherence. Second, we explored the robustness of our results to the assumption that equal access to care resulted in the same stage-specific relative CRC survival for Louisiana and New Jersey by assuming that 25% of the difference in relative survival between states could not be taken away with equal access to care. Third, we evaluated the impact on mortality disparity if equal access to care not only resulted in the same stage-specific relative CRC survival for Louisiana as for New Jersey, but also in the same stage distribution. Finally, in the base case CRC relative survival estimates by state were estimated using SEER*Stat.[6] SEER*Stat uses US life tables to estimate expected mortality in the absence of cancer. Louisiana death rates are higher than overall US death rates, while New Jersey rates are lower. Therefore we performed a sensitivity analysis in which we corrected the CRC relative survival for the differential background mortality in each state.
Results
In 1995, the observed Louisiana CRC incidence rate (167 cases per 100,000 persons aged 50 years and older) was approximately 19% lower than the New Jersey CRC incidence (205 cases per 100,000) (Figure 1). By 2009 the ordering had reversed, with CRC incidence in Louisiana being almost 10% higher than in New Jersey. For CRC mortality a similar pattern was observed (Figure 2). The observed excess in age-standardized CRC incidence and mortality rates in 2009 in Louisiana compared to New Jersey were 11.5 cases and 6.1 deaths per 100,000, respectively (Table 2 and 3).
Figure 1.
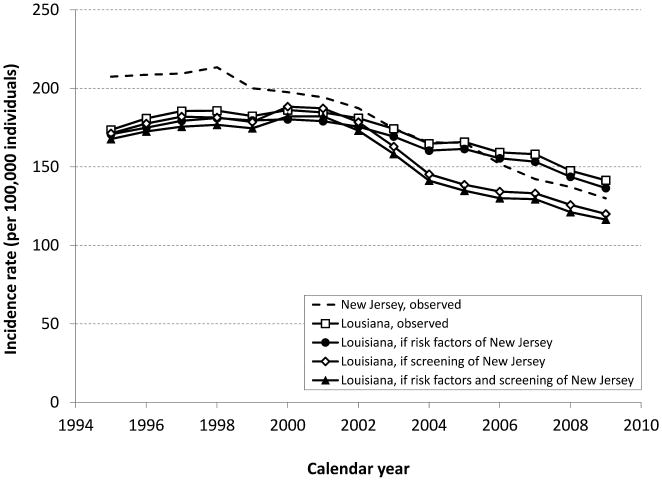
Age-standardized CRC incidence rates (per 100,000 individuals) in the 50+ year-old population from 1995-2009, as observed in Louisiana and New Jersey, and as expected in Louisiana if they would have had the same risk factors, and/or screening pattern as New Jersey.
Figure 2.
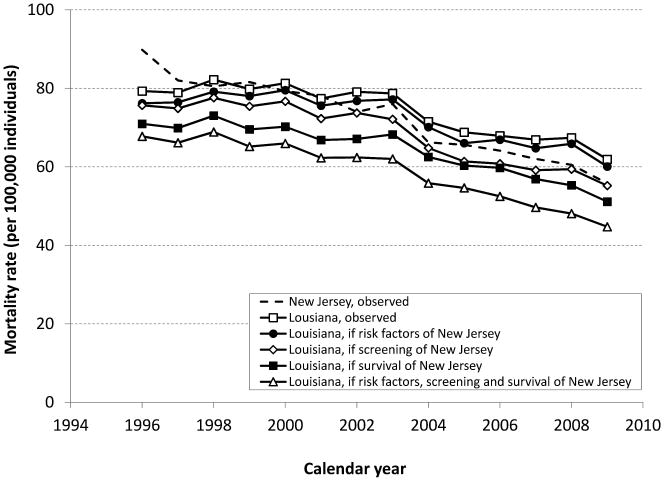
Age-standardized CRC mortality rates (per 100,000 individuals) in the 50+year old population from 1995-2009, as observed in Louisiana and New Jersey, and as expected in Louisiana if they would have had the same risk factors, screening pattern, and/or survival pattern as New Jersey.
Table 2.
Base case analysis: Disparities in CRC incidence between individuals aged 50 years and older in Louisiana and New Jersey, as observed in 2009.
| All races | Blacks | Whites | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Scenario | ASR | % reduction* | Excess rate† | ASR | % reduction* | Excess rate† | ASR | % reduction* | Excess rate† |
| New Jersey, observed | 130.0 | 132.0 | 131.2 | ||||||
| Louisiana, observed | 141.4 | 11.5 | 174.2 | 42.2 | 131.7 | 0.5 | |||
| -if risk factors of New Jersey | 136.5 | 3.5% | 6.5 | 171.3 | 1.6% | 39.3 | 128.4 | 2.5% | -2.8 |
| -if screening of New Jersey | 120.0 | 15.2% | -10.0 | 153.8 | 11.7% | 21.8 | 112.3 | 14.7% | -18.9 |
| -if risk factors, screening and survival of New Jersey | 116.4 | 17.7% | -13.6 | 151.1 | 13.2% | 19.1 | 110.3 | 16.2% | -20.8 |
ASR, Age-standardized rate per 100,000 individuals (2000 US standard population).
Percent reduction, compared to the incidence rate observed in Louisiana.
Excess CRC incidence rate in Louisiana compared to New Jersey
Table 3.
Base case analysis: Disparities in CRC mortality between individuals aged 50 years and older in Louisiana and New Jersey in 2009.
| All races | Blacks | Whites | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Scenario | ASR | % reduction* | Excess rate† | ASR | % reduction* | Excess rate† | ASR | % reduction* | Excess rate† |
| New Jersey, observed | 55.8 | 71.5 | 55.3 | ||||||
| Louisiana, observed | 61.9 | 6.1 | 79.9 | 8.4 | 56.5 | 1.2 | |||
| -if risk factors of New Jersey | 60.1 | 3.0% | 4.3 | 77.8 | 2.6% | 6.3 | 54.8 | 3.0% | -0.5 |
| -if screening of New Jersey | 55.2 | 10.8% | -0.6 | 73.1 | 8.5% | 1.6 | 49.3 | 12.7% | -6.0 |
| -if survival of New Jersey | 51.1 | 17.4% | -4.7 | 71.3 | 10.8% | -0.2 | 49.5 | 12.4% | -5.8 |
| -if risk factors, screening and survival of New Jersey | 44.7 | 27.8% | -11.1 | 65.3 | 18.3% | -6.2 | 43.4 | 23.2% | -11.9 |
ASR, Age-standardized rate per 100,000 individuals (2000 US standard population).
Percent reduction, compared to the mortality rate observed in Louisiana.
Excess CRC mortality rate in Louisiana compared to New Jersey
If Louisiana had the same smoking and obesity prevalence as observed in New Jersey, the expected CRC incidence rate would have been 136.5 per 100,000 in 2009, 3.5% lower than the observed rate for Louisiana (Figure 1 and Table 2). The expected CRC mortality rate in 2009 would have been 60.1 per 100,000 (3.0% lower than observed, Figure 2 and Table 3). In this scenario Louisiana would still have an excess of 6.5 cases and 4.3 deaths per 100,000 compared to New Jersey.
If Louisiana would have had the same screening uptake or stage-specific relative CRC survival as New Jersey, CRC mortality would drop to 55.2 and 51.1 per 100,000 respectively in 2009, 10.8% and 17.4% lower than the observed rate in Louisiana. With the same trends in smoking and obesity, screening, and stage-specific relative CRC survival as New Jersey combined, CRC mortality in Louisiana would have been 27.8% lower than the observed rate of 61.9 per 100,000 in Louisiana. In addition, this reversed the disparity between the states; Louisiana would have 13.6 cases and 11.1 deaths per 100,000 less as currently observed in New Jersey.
The observed disparity in CRC incidence and mortality between Louisiana and New Jersey was considerably higher for blacks (42.2 excess cases and 8.4 excess deaths per 100,000 persons) compared to whites (0.5 excess cases and 1.2 excess deaths) (Tables 2 and 3). Interestingly, the potential reduction in CRC mortality if Louisiana had similar risk factor, screening and survival as New Jersey was lower for blacks than for whites; 18.3% and 23.2%, respectively.
Sensitivity analyses
Our findings were robust for assumptions concerning quality of endoscopy, residual survival differences and stage distribution (Table 4). Lower-quality endoscopy slightly attenuated the potential reduction in excess mortality from 27.8% (base case) to 26.8%. Residual difference in stage-specific relative CRC survival and correcting for differential background mortality between Louisiana and New Jersey decreased the potential reduction in CRC mortality to 24.0% and 25.0%, respectively. Stage distribution had virtually no effect.
Table 4.
Sensitivity analyses: Disparities in CRC mortality between individuals aged 50 years and older in Louisiana and New Jersey in 2009, under alternative model assumptions.
| Lower quality endoscopies | Residual survival difference | Same survival and stage distribution | Survival corrected for background mortality | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scenario | ASR | % reduction* | Excess rate† | ASR | % reduction† | Excess rate* | ASR | % reduction* | Excess rate† | ASR | % reduction* | Excess rate† |
| New Jersey, observed | 55.8 | 55.8 | 55.8 | 55.8 | ||||||||
| Louisiana, observed | 61.9 | 6.1 | 61.9 | 6.1 | 61.9 | 6.1 | 61.9 | |||||
| -if risk factors of New Jersey | 60.2 | 2.7% | 4.4 | 60.1 | 3.0% | 4.3 | 60.1 | 3.0% | 4.3 | 60.0 | 3.0% | 4.2 |
| -if screening of New Jersey | 56.1 | 9.3% | 0.3 | 55.2 | 10.8% | -0.6 | 55.2 | 10.8% | -0.6 | 54.6 | 11.7% | -1.2 |
| -if survival of New Jersey | 50.7 | 18.1% | -5.1 | 53.6 | 13.3% | -2.2 | 50.8 | 17.9% | -5.0 | 53.4 | 13.7% | -2.4 |
| -if risk factors, screening and survival of New Jersey | 45.3 | 26.8% | -10.5 | 47.0 | 24.0% | -8.8 | 44.6 | 27.9% | -11.2 | 46.4 | 25.0% | -9.4 |
ASR, Age-standardized rate per 100,000 individuals (2000 US standard population).
Percent reduction, compared to the mortality rate observed in Louisiana.
Excess CRC mortality rate in Louisiana compared to New Jersey
Discussion
This study shows that removing differences in smoking and obesity prevalence, screening uptake, and stage-specific relative CRC survival would eliminate observed disparities in CRC incidence and mortality rates between Louisiana and New Jersey. Screening had the biggest impact on CRC incidence: the observed CRC incidence in Louisiana could be reduced by 15.2% by increasing CRC screening up to the level of New Jersey. Stage specific CRC relative survival had the largest impact on CRC mortality, the observed CRC mortality could be reduced by 17.4% by improving the survival to the level of New Jersey. Eliminating differences in the prevalence of smoking and obesity had a relatively modest impact on CRC incidence (3.5% reduction) and mortality (3.0% reduction).
The reason that the impact of smoking and obesity is modest results from the relatively small impact of the individual risk factors on CRC incidence and mortality (relative risk of 1.6 and 1.4 respectively) and the fact that the prevalence of these risk factors were similar between the two states (Supporting material 3).
Together, eliminating differences in risk factors, screening and survival not only completely eliminates the excess CRC incidence and mortality in Louisiana but reverses the pattern. This may sound surprising, but given that in the early 1990's New Jersey had higher incidence and mortality rates than Louisiana[2], it makes sense that the background CRC risk in Louisiana is actually lower than in New Jersey.
The disparity in CRC incidence and mortality rates between Louisiana and New Jersey mainly exists for blacks, and not for whites (Tables 2 and 3). When simply looking at the 2009 rates, one could argue that the disparity between the two states is therefore a result of a difference in population distribution by race. However, when looking at the patterns since 1995 it is clear that population distribution is not the explanation; for both races, the observed CRC incidence and mortality rates decreased less in Louisiana than in New Jersey. This finding is corroborated by our modeling, showing that CRC incidence and mortality rates in Louisiana could be reduced to a similar extent in blacks and whites if risk factors, screening and survival were the same as in New Jersey. Interestingly, the potential reduction was even somewhat higher in whites than in blacks. This finding is probably explained by the slight increase in CRC incidence and mortality in Louisiana blacks in the late 1990's, which cannot be explained by the factors investigated in this study. This means that other differences between Louisiana and New Jersey (e.g. other lifestyle factors such as red meat consumption or physical inactivity; gender differences or differences in proportion of Hispanic population) are contributing to the difference in CRC incidence and mortality between these two states. Consequently, some excess in CRC rates in blacks remained after removing differences in smoking, obesity, screening, and survival in Louisiana compared to New Jersey.
In our primary analysis, we considered screening uptake, assuming equal access to and quality of screening, between Louisiana and New Jersey. The lower population density and larger geographic area of Louisiana might make achieving equal access more difficult. In addition, quality of endoscopy has been shown to be dependent on the skill of the endoscopist performing the procedure, with colonoscopy being performed by gastroenterologists being more sensitive for cancer than colonoscopy by non-gastroenterologists.[22] The number of certified gastroenterologists differs widely between states in the US. In Louisiana there were only 3.9 gastroenterologists per 100,000 residents in 2013 compared to 6.7 in New Jersey.[23] This pattern is mirrored in the other Southern and Northeastern states.[24]
Two limitations are noteworthy. First, we assumed that smoking and obesity prevalence only affected the risk for CRC by increasing adenoma incidence. This assumption is supported by the similar relative risk of these risk factors for developing adenomas and CRC.[12-13] However, greater adenoma progression may also play a role in the increased risk. In that case, eliminating differences in risk factors will have a relatively larger impact on disparities in CRC rates, while eliminating differences in screening may have a smaller effect. Second, we have not explicitly considered state differences in treatment but used state differences in stage-specific relative CRC survival as a proxy. Data on use and quality of CRC treatment by state are sparse, especially for the population below 65 years old. One study suggested that Louisiana patients surgically treated for stage III colon cancer were significantly less likely to receive adjuvant chemotherapy than patients from other states.[25] However, if part of the state differences in survival cannot be explained by differences in (the quality of) treatment, for example because Louisiana residents could have more comorbidities and are therefore unable to receive guideline therapy, we have overestimated the potential for reducing disparities in CRC mortality. We explored the impact of our assumption in a sensitivity analysis and found that the effect was limited.
The Patient Protection and Affordable Care Act (ACA, Pub.L. 111-148, 2010) may be an important step towards the reduction of health disparities between states although Louisiana has yet to expand the state Medicaid program. The ACA aims to improve access to quality health care for all Americans. Furthermore, all new health plans must cover certain preventive services including CRC screening without charging a deductible, co-pay or coinsurance. Several studies have shown that in situations with equal access to care, such as military medical centers, Department of Defense facilities or clinical trials, no differences in screening uptake or CRC treatments exist.[26-28] A notable example is universal CRC screening coverage in Delaware that eliminated the black-white disparities in CRC mortality rates.[29]
In conclusion, this study shows that the disparities in CRC incidence and mortality rates between Louisiana and New Jersey could be eliminated if Louisiana could attain New Jersey levels of risk factors, screening and CRC relative survival. Priority should be given to enabling Southern states to improve screening and survival rates.
Supplementary Material
Acknowledgments
Funding: This research was financially supported by the National Cancer Institute at the National Institutes of Health (U01-CA-152959) and by the intramural Research Program of the American Cancer Society.
The New Jersey State Cancer Registry and Louisiana Tumor Registry are supported by the National Program of Cancer Registries of the Centers for Disease Control and Prevention (5U58DP003931-02 and 1U58DP005390) and the Surveillance, Epidemiology, and End Results program of the National Cancer Institute (N01-PC-2013-00021, HHSN261201300016I and HHSN26100003).
The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, and approval of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of any of the funding sources.
Footnotes
Conflicts of interest. None
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Naishadham D, Lansdorp-Vogelaar I, Siegel R, Cokkinides V, Jemal A. State disparities in colorectal cancer mortality patterns in the United States. Cancer Epidemiol Biomarkers Prev. 2011;20(7):1296–302. doi: 10.1158/1055-9965.EPI-11-0250. [DOI] [PubMed] [Google Scholar]
- 3.DeLancey JO, Thun MJ, Jemal A, Ward EM. Recent trends in Black-White disparities in cancer mortality. Cancer Epidemiol Biomarkers Prev. 2008;17(11):2908–12. doi: 10.1158/1055-9965.EPI-08-0131. [DOI] [PubMed] [Google Scholar]
- 4.Loeve F, Boer R, van Oortmarssen GJ, van Ballegooijen M, Habbema JD. The MISCAN-COLON simulation model for the evaluation of colorectal cancer screening. Comput Biomed Res. 1999;32(1):13–33. doi: 10.1006/cbmr.1998.1498. [DOI] [PubMed] [Google Scholar]
- 5.Louisiana State University School of Public Health. [Accessed September 25, 2014];Louisiana Tumor Registry. Available at: http://sph.lsuhsc.edu/louisiana-tumor-registry.
- 6.National Cancer Institute. [Accessed September 25, 2014];SEER*Stat Software, version 5.3.1. Surveillance Research Program. Available at: http://www.seer.cancer.gov.
- 7.Koretz RL. Malignant polyps: are they sheep in wolves' clothing? Ann Intern Med. 1993;118(1):63–8. doi: 10.7326/0003-4819-118-1-199301010-00011. [DOI] [PubMed] [Google Scholar]
- 8.Lansdorp-Vogelaar I, van Ballegooijen M, Boer R, Zauber A, Habbema JD. A novel hypothesis on the sensitivity of the fecal occult blood test: Results of a joint analysis of 3 randomized controlled trials. Cancer. 2009;115(11):2410–9. doi: 10.1002/cncr.24256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stoop EM, de Haan MC, de Wijkerslooth TR, Bossuyt PM, van Ballegooijen M, Nio CY, et al. Participation and yield of colonoscopy versus non-cathartic CT colonography in population-based screening for colorectal cancer: a randomised controlled trial. Lancet Oncol. 2012;13(1):55–64. doi: 10.1016/S1470-2045(11)70283-2. [DOI] [PubMed] [Google Scholar]
- 10.Baili P, Micheli A, De Angelis R, Weir HK, Francisci S, Santaquilani M, et al. Life tables for world-wide comparison of relative survival for cancer (CONCORD study) Tumori. 2008;94(5):658–68. doi: 10.1177/030089160809400503. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. [Accessed September 25, 2014];Behavioral Risk Factor Surveillance System. Available at: http://www.cdc.gov/brfss/index.htm.
- 12.Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Kearney J, et al. A prospective study of cigarette smoking and risk of colorectal adenoma and colorectal cancer in U.S. men. J Natl Cancer Inst. 1994;86(3):183–91. doi: 10.1093/jnci/86.3.183. [DOI] [PubMed] [Google Scholar]
- 13.Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med. 1995;122(5):327–34. doi: 10.7326/0003-4819-122-5-199503010-00002. [DOI] [PubMed] [Google Scholar]
- 14.Atkin WS, Edwards R, Kralj-Hans I, Wooldrage K, Hart AR, Northover JM, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375(9726):1624–33. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- 15.Loeve F, Boer R, van Ballegooijen M, van Oortmarssen GJ, Habbema JDF. Final report MISCAN-COLON microsimulation model for colorectal cancer: report to the National Cancer Institute Project No NO1-CN55186. Rotterdam: Department of Public Health, Erasmus University; 1998. [Google Scholar]
- 16.Loeve F, Boer R, Zauber AG, Van Ballegooijen M, Van Oortmarssen GJ, Winawer SJ, et al. National Polyp Study data: evidence for regression of adenomas. Int J Cancer. 2004;111(4):633–9. doi: 10.1002/ijc.20277. [DOI] [PubMed] [Google Scholar]
- 17.Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544–73. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(9):627–37. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 19.Levin B, Lieberman DA, McFarland B, Smith RA, Brooks D, Andrews KS, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58(3):130–60. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 20.Hewitson P, Glasziou P, Watson E, Towler B, Irwig L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol. 2008;103(6):1541–9. doi: 10.1111/j.1572-0241.2008.01875.x. [DOI] [PubMed] [Google Scholar]
- 21.Harper S, Lynch J. NCI Cancer Surveillance Monograph Series, Number 6. Bethesda, MD: National Cancer Institute; 2005. Methods for Measuring Cancer Disparities: Using Data Relevant to Healthy People 2010 Cancer-Related Objectives. NIH Publication No. 05-5777. [Google Scholar]
- 22.Rex DK, Rahmani EY, Haseman JH, Lemmel GT, Kaster S, Buckley JS. Relative sensitivity of colonoscopy and barium enema for detection of colorectal cancer in clinical practice. Gastroenterology. 1997;112(1):17–23. doi: 10.1016/s0016-5085(97)70213-0. [DOI] [PubMed] [Google Scholar]
- 23.American Board of Internal Medicine. [Accessed September 25, 2014];Candidates Certified by US State/Territory. Available at: https://www.abim.org/pdf/data-candidates-certified/State-Number-of-Certificates-Issued.pdf.
- 24.Moayyedi P, Tepper J, Hilsden R, Rabeneck L. International comparisons of manpower in gastroenterology. Am J Gastroenterol. 2007;102(3):478–81. doi: 10.1111/j.1572-0241.2006.00973.x. [DOI] [PubMed] [Google Scholar]
- 25.Cress RD, Sabatino SA, Wu XC, Schymura MJ, Rycroft R, Stuckart E, et al. Adjuvant Chemotherapy for Patients with Stage III Colon Cancer: Results from a CDC-NPCR Patterns of Care Study. Clin Med Oncol. 2009;3:107–19. doi: 10.4137/cmo.s2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hassan MO, Arthurs Z, Sohn VY, Steele SR. Race does not impact colorectal cancer treatment or outcomes with equal access. Am J Surg. 2009;197(4):485–90. doi: 10.1016/j.amjsurg.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 27.Hofmann LJ, Lee S, Waddell B, Davis KG. Effect of race on colon cancer treatment and outcomes in the Department of Defense healthcare system. Dis Colon Rectum. 2010;53(1):9–15. doi: 10.1007/DCR.0b013e3181bdcdb2. [DOI] [PubMed] [Google Scholar]
- 28.Sanoff HK, Sargent DJ, Green EM, McLeod HL, Goldberg RM. Racial differences in advanced colorectal cancer outcomes and pharmacogenetics: a subgroup analysis of a large randomized clinical trial. J Clin Oncol. 2009;27(25):4109–15. doi: 10.1200/JCO.2009.21.9527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grubbs SS, Polite BN, Carney J, Jr, Bowser W, Rogers J, Katurakes N, et al. Eliminating racial disparities in colorectal cancer in the real world: it took a village. J Clin Oncol. 2013;31(16):1928–30. doi: 10.1200/JCO.2012.47.8412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


