Abstract
Background
The P2X4 receptor is thought to be involved in regulating alcohol-consuming behaviors and ethanol (EtOH) has been reported to inhibit P2X4 receptors. Ivermectin is an anti-parasitic agent that acts as a positive allosteric modulator of the P2X4 receptor. The current study examined the effects of systemically- and centrally-administered ivermectin on alcohol drinking of replicate lines of high-alcohol-drinking (HAD-1/HAD-2) rats, and the effects of lentiviral-delivered short-hairpin RNAs (shRNAs) targeting P2rx4 on EtOH intake of female HAD2 rats.
Method
For the 1st experiment, adult male HAD-1 & HAD-2 rats were given 24-hr free-choice access to 15% EtOH vs. water. Dose-response effects of ivermectin (1.5 to 7.5 mg/kg i.p.) on EtOH intake were determined; the effects of ivermectin were then examined for 2% w/v sucrose intake over 5 consecutive days. In the 2nd experiment, female HAD-2 rats were trained to consume 15% EtOH under 2-hr limited access conditions, and dose-response effects of intracerebroventricular (ICV) administration of ivermectin (0.5 to 2.0 μg) were determined over 5 consecutive days. The 3rd experiment determined the effects of microinfusion of a lentivirus expressing P2rx4 shRNAs into the posterior ventral tegmental area (VTA) on 24-hr EtOH free-choice drinking of female HAD-2 rats.
Results
The highest i.p. dose of ivermectin reduced alcohol drinking (30-45%) in both rat lines, but did not alter sucrose intake. HAD-2 rats appeared to be more sensitive than HAD1 rats to the effects of ivermectin. ICV administration of ivermectin reduced 2-hr limited access intake (∼35%) of female HAD-2 rats; knockdown of P2rx4 expression in the posterior VTA reduced 24-hr free choice EtOH intake (∼20%).
Conclusion
Overall, the results of the current study support a role for P2X4 receptors within the mesolimbic system in mediating alcohol drinking behavior.
Keywords: alcohol drinking, high-alcohol-drinking rats, ivermectin, P2rx4, P2X4 receptor
Introduction
P2X4 receptors (P2X4Rs) are the most widely expressed functional ATP-gated purinergic receptor in the CNS (e.g. Coddou et al., 2011). These ionotropic receptors modulate signaling of several neurotransmitter systems, including GABA, glycine, glutamate, and nicotinic acetylcholine (Adelsberger et al., 2000; Krause et al., 1998; Tabakoff et al., 2009; Sattelle et al., 2009). Furthermore, these receptors are localized in key brain regions associated with alcohol consumption and reinforcement, such as the ventral tegmental area (VTA) and nucleus accumbens (NAc); i.e., the mesolimbic reward pathway (Pierce et al., 2006; Xiao et al., 2008). Findings indicate that intoxicating and anesthetic ethanol (EtOH) doses desensitize the response of P2X4R-expressing cell lines to ATP exposure (Li et al., 1993; Xiong et al., 2000; Davies et al., 2005; Ostrovskaya et al., 2011), suggesting EtOH alters the effects of these receptors on modulating neurotransmission. This effect likely occurs in part at P2X4R ectodomain-transmembrane segment interfaces (Lalo et al. 2007; Asatryan et al., 2008), as the inhibitory effect of EtOH on P2X4Rs may be reversed by point mutations in these areas (Popova et al., 2010).
Expression of P2rx4, the gene that codes for P2X4Rs, has been reported to correlate negatively with alcohol intake of recombinant inbred rat strains, which have low to moderate alcohol intakes (Tabakoff et al., 2009). In contrast, P2rx4 expression is significantly higher in the VTA of high-alcohol drinking replicate (HAD-2) rats compared to their low-alcohol-drinking (LAD-2) counterparts (McBride et al., 2012). However, opposite findings were observed in the VTA of HAD1 vs. LAD1 and alcohol-preferring (P) vs. non-preferring (NP) rats (McBride et al., 2012). In addition, other differences in innate expression of many other genes also exist in the VTA between the HAD2-LAD2 line-pair vs. the HAD1-LAD1 line-pair (McBride et al., 2012). Other differences between these line-pairs have been reviewed (Murphy et al., 2002).
Similar to EtOH, ivermectin (22,23-dihydroavermectin B1a + B1b) modulates neurotransmission, in part, through its actions at P2X4R ectodomain-transmembrane segment interfaces (Popova et al., 2010; Lalo et al. 2007). Ivermectin is an anti-parasitic agent used in veterinary and clinical medicine (e.g. Gonzalez et al., 2012). In addition to its anti-helminthic properties, ivermectin is a selective, positive allosteric modulator of ATP-evoked currents at P2X4Rs (Lalo et al. 2007; Priel and Silberberg, 2004). It typically enhances inhibitory neurotransmission and decreases excitatory transmission (Shan et al., 2001), although evidence suggests that ivermectin blocks some of the behavioral effects of GABA agonists as well (Davies et al., 2013). Similarly, molecular modeling suggests that ivermectin blocks some of the inhibitory effects of EtOH at P2X4Rs, possibly through interfering with EtOH at an overlapping site of action (Asatryan et al., 2010, 2014).
To date, few pre-clinical studies have investigated the effects of direct manipulation of P2X4Rs (including with ivermectin) on alcohol self-administration in rodents. These reports all found that a single systemic ivermectin exposure reduces alcohol self-administration (Kosten 2011; Yardley et al. 2012; Asatryan et al., 2014). Ivermectin was reported to interfere with EtOH-induced reinforcement and conditioned approach behaviors in Sprague-Dawley rats (Kosten, 2011), as well as two-bottle choice EtOH self-administration and preference under intermittent, 4-hr limited access and continuous access conditions in C57BL/6 mice (Yardley et al., 2012; Asatryan et al., 2014).
However, the effects of ivermectin have not been tested in rats genetically selected for high alcohol intake. These animals will consume sufficient alcohol to obtain pharmacologically relevant blood alcohol concentrations under limited access, continuous access and relapse-like conditions (Bell et al. 2006, 2011, 2012, 2014; Dhaher et al. 2012; Murphy et al. 1986, 2002). The present series of experiments were designed with the objective of testing the effects of ivermectin using repeated administration in suitable animal models of alcoholism (Bell et al., 2012; McBride et al., 2014). Given the growing literature indicating P2X4Rs are involved in alcohol drinking (Franklin et al., 2014), the current study hypothesized that systemic, intracerebroventricular, and CNS site-specific modulation of P2X4R activity would alter alcohol drinking by replicate rat lines selectively bred for high alcohol intakes (i.e., HAD-1 and HAD-2 rats).
Methods
Subjects
Adult, male HAD-1 and HAD-2 rats (n = 8/rat line/dose) were used for the intraperitoneal (i.p.) ivermectin experiments, and adult female HAD-2 (n = 6-7/group) rats were used for intracerebroventricular (ICV) and lentiviral experiments. Subjects were 90-120 days old at the start of the experiments. All rats received free access to standard laboratory chow and water throughout all of the experiments. The subjects were housed individually in hanging stainless steel wire-mesh cages (with Plexiglas platform) and maintained on a 12/12 hr light cycle, with lights off at 10:30 hrs for all but the viral vector experiment (19:00 hr when lights went out) in a temperature- (21°C) and humidity- (50%) controlled vivarium. Animals used in these procedures were maintained in facilities fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). All experimental procedures were approved by the Institutional Animal Care and Use Committee of the Indiana University Schools of Dentistry and Medicine (Indianapolis, IN) and are in accordance with the guidelines of the Institutional Animal Care and Use Committee of the National Institute on Drug Abuse, National Institutes of Health, and the Guide for the Care and Use of Laboratory Animals (Research Institute for Laboratory Animal Research, 2011).
Test Compounds
The EtOH solution was prepared as 15% v/v in tap water from 190-proof EtOH. The sucrose solution was prepared at a 2% w/v concentration in tap water. For peripheral experiments, ivermectin solution (10 mg/ml in 60% propylene glycol) (Norbrook Inc., Lenexa, KS) was diluted in 0.9% NaCl in water (sterile saline) to a concentration that would allow for an i.p. injection volume of 1 ml/kg of body weight, in doses ranging from 1.5-7.5 mg/kg (c.f. Trailovic and Nedeljkovic, 2011). The vehicle consisted of propylene glycol (Sigma-Aldrich Co. LLC, St. Louis, MO, USA) in sterile saline, at a concentration equivalent to that present in the highest ivermectin dose used for each experiment. For ICV experiments, the ivermectin solution was diluted in artificial cerebrospinal fluid (aCSF: 120 mM NaCl, 4.75mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM NaHCO3, 2.5 mM CaCl2, 10 mM D-glucose, pH 7.2-7.4) in doses ranging from 0.5 to 2.0 μg. Vehicle was composed of propylene glycol in aCSF in a concentration equivalent to the highest dose.
For lentiviral vector experiments, 19 nucleotide targeting siRNAs were designed using the Dharmacon siDesign Center (GE Healthcare, Pittsburgh, PA). Oligonucleotides encoding shRNAs were cloned into the lentiviral vector pLL3.7 as described previously (Lasek et al., 2007). Lentiviral plasmids expressing shRNAs were screened for the ability to knockdown expression of P2rx4 in cell culture. The most effective targeting sequence was chosen for microinfusion studies into the rat VTA and exhibited approximately 50% knockdown of P2rx4 expression. The 19 nucleotide targeting sequence was: 5′-GCACACTCACCAAGGCGTA-3′. High-titer lentivirus was produced in 293FT cells as described previously (Lasek et al., 2007).
Effects of i.p. Ivermectin on EtOH and sucrose intakes
Rats were given 15% EtOH for 6 weeks to stabilize EtOH intakes. The animals were provided free-choice access to EtOH 24 hr/day (including weekends), except during periods of husbandry and when weights were being assessed. Water and food were always available. Following stabilization of EtOH drinking, each line of rats was balanced across doses for equivalent EtOH intakes, according to average daily EtOH intake obtained from the last five days of the acquisition phase (n = 8/line/dose). Subjects then received random, double-blind assignment to one of 5 groups: 1.5, 3.0, 5.0 or 7.5 mg/kg ivermectin, or vehicle. All of the current doses are below the 10 mg/kg dose level that has been reported to induce CNS toxicity (Lerchner et al., 2007). Each dose was tested in a separate group of rats. Due to the slow clearance of ivermectin and its slow passage across the blood-brain barrier (Merck et al., 1988; Yardley et al., 2012), 24-hr EtOH drinking was assessed at 4 and 24 hr following ivermectin administration. Rats were injected (i.p.) for 5 consecutive days.
A separate group of EtOH-naïve male HAD-1 rats was used to assess the effects of ivermectin on 24-hr sucrose intake. These animals were provided two weeks of 24-hr access to 2% w/v sucrose and water. Rats then were administered (i.p.) 3.0, 5.0 or 7.5 mg/kg ivermectin, or vehicle (n = 7/group) for 5 consecutive days. Injections occurred 30 min prior to onset of the dark cycle; subjects were assessed for sucrose and water intake at 24 hr following ivermectin injection. For all subjects, body weights were recorded daily (Mon.-Fri.).
Effects of ICV Ivermectin on EtOH intake
A separate group of female HAD-2 rats were used for the ICV experiment. These rats were given a standardized alcohol drinking acquisition phase, during which EtOH access was limited as follows: 14 days with 24-hr free-choice access; followed by 1 week (5 days) of 8-hr access; 1 week (5 days) of 4-hr access; and 2 weeks (5-days/week) with 2-hr access. All alcohol drinking sessions began at the start of the dark phase. Subjects did not have EtOH access on weekends, with the exception of the initial 14-day, 24-hr free-choice access period. Water and food were always available.
Stereotaxic Surgery
While under isoflurane anesthesia, female HAD-2 rats were implanted unilaterally with 22-gauge guide cannulae (Plastics One, Roanoke, VA, USA) aimed 3 mm above the lateral ventricle (AP -1.0, ML +1.5, DV -4.0, 0.0° offset from vertical; Paxinos and Watson 1998). Cannulae were secured to the skull with cranioplastic cement. Subjects were allowed to recover from surgery for 5 days. Next, rats underwent 5 days of 2-hr access to EtOH to establish postsurgical baseline EtOH drinking levels.
Microinjection Procedure
Hamilton syringes (Hamilton Company, Reno, NV, USA) were placed on a microinfusion pump (Harvard Apparatus, Hilliston, MA, USA), and connected by polyethylene (PE) tubing (Becton Dickins and Company, Sparks, MD, USA) to a 26-gauge internal cannula (Plastics One, Roanoke, VA, USA). The infusates were loaded into the PE tubing, and primed for delivery through the internal cannulae. The internal cannula extended 3 mm beyond the guide cannula into the lateral ventricle. The microinfusion pump was programmed to deliver 1 μl infusate, at a 0.2 μl/min flow rate, over a period of 5 minutes.
On the final day of the 5-day postsurgical baseline EtOH drinking assessment period, rats were “mock” infused with aCSF 30 minutes prior to EtOH access to allow habituation to the process and sensation of infusion. Next, subjects were assigned at random to receive ivermectin at doses of 0.5, 1.0, 1.5 or 2.0 μg, or aCSF, by ICV infusion, at 0.2 μg/min flow rate for 5 minutes, daily across the 5-day test period. Following the infusions, the internal cannulae remained inserted for an additional 2.5 minutes to allow dispersal of the infusate. Microinjections occurred 30 min prior to EtOH access, which coincided with the beginning of the dark cycle. Each dose was tested in a separate group of rats. For all subjects, body weights and 24-hr water consumption were recorded daily (Mon.-Fri.) prior to the start of testing.
Histological Placements
Following testing, methylene blue dye (Sigma-Aldrich Co. LLC, St. Louis, MO, USA) was infused through the internal cannula, and animals were euthanized with CO2 inhalation and decapitated. Brains were immediately removed and frozen at −80°C. Brains were sliced in 40-μm sections on a cryostat vibratome (Leica Microsystems Inc., Bannockburn, IL), and infusion locations were verified within the lateral ventricle, according to the Paxinos and Watson (1998) rat brain atlas. Data from animals exhibiting surgical placements in areas outside of the lateral ventricle were excluded.
Effects of P2rx4 shRNA on EtOH intake of HAD-2 rats
Female HAD-2 rats had 10 weeks of 24 hr free-choice access to 15% EtOH prior to surgery. EtOH (g/kg) and water (ml/kg) intakes were assessed 5 days/wk (Mon-Fri). Body weights were measured 3 days/wk. Food and water were freely available. Following 10 weeks of 24-hr EtOH access, EtOH drinking levels were assessed and animals received pseudo-random assignment to one of three treatment groups: active P2rx4 shRNA, a control shRNA group as described previously (Lasek et al., 2007), or a third group that did not undergo the surgical procedure (n = 7/group). Groups were balanced for equal EtOH intakes (mean ± S.E.M.: 7.2 ± 0.6 g/kg/24 hrs). HAD-2 rats were used for this experiment because our laboratory previously reported that P2rx4 expression is innately higher in the VTA of HAD-2 relative to LAD-2 rats (McBride et al., 2012).
During stereotaxic surgeries each animal received bilateral infusions aimed at the posterior VTA (AP -5.6, ML +2.1, DV -7.5, 10.0° angle; Paxinos and Watson, 1998). Animals were infused with either 3 μl of lentivirus expressing P2rx4 shRNA (1.5 μl/side) or a lentivirus expressing a control shRNA at a flow rate of 0.2 μl/minute. The infusate was allowed 10 minutes to diffuse into the extracellular space. Animals were quarantined for 72 hr where they had unrestricted access to water, food and EtOH before being returned to their home cages for an additional 6 weeks.
Verification of the effects of shRNA on P2X4Rs in the VTA
Brains were sectioned in the coronal plane on a cryostat; 20 μm sections containing the VTA (Bregma -5.3 through Bregma -6.3), were mounted on slides and then fixed with 4% paraformaldehyde. Twelve to 16 sections from each animal were processed immunohistochemically using antigen retrieval and double fluorescence labeling for GFP (mouse anti-green fluorescent protein monoclonal antibody, 1:1000, Millipore Corp, Billerica, MA, USA), which is expressed from the lentiviral vectors, and for P2X4R (rabbit polyclonal antibody, 2 μg/ml, Abcam, Cambridge, MA, USA). Analysis of the stained sections was then done on an Olympus AX70 microscope (Olympus America, Center Valley, PA, USA) equipped with filter cubes for fluorescent visualization of Dylight 594 (excitation of 591 nm and emission of 616 nm) and Alexa Fluor 488 (excitation of 495 nm and emission of 519 nm), with an Olympus CC-12 camera digital imaging system and CellSense software (Olympus America, Center Valley, PA, USA). Visualization of P2X4R was performed using Dylight 594-conjugated AffiniPure Donkey anti-rabbit IgG (1:500, Jackson ImmunoResearch Laboratories (West Grove, PA, USA)) and for GFP with Alexa Fluor 488-conjugated AffiniPure Donkey anti-mouse IgG (1:200, Jackson ImmunoResearch Laboratories). The images from sections within the VTA between Bregma -5.3 through Bregma -5.8 were imported into Adobe Photoshop, the VTA on each section outlined, and all P2X4R-positive cells within this outline were counted. The cell counts for each animal reflect the average number of P24XR-positive cells counted/section.
Data analyses and statistics
Water, sucrose, and EtOH intakes were determined from changes in the weight of glass drinking bottles. EtOH intakes were then converted to g of absolute (i.e., corrected for specific gravity) EtOH consumed/kg of body weight/unit of time. Water and sucrose were analyzed as ml fluid consumed/kg of body weight/unit of time.
EtOH, sucrose and water intakes were analyzed as cumulative intake at each time point. A 4-way (line × dose × day × time) ANOVA with repeated measures for day and time was conducted for EtOH intakes. If a significant line effect was detected (which was the case), the data for each rat line were analyzed separately. A 3-way (dose × day × time) ANOVA (with repeated measures for day and time) was conducted for (a) EtOH intakes at 4 and 24 hr post-ivermectin treatment; and (b) 4 and 24-hr water intakes. Two-way (dose × day) ANOVAs (with repeated measures for day) were performed for body weights, for each rat line.
For sucrose intakes, 3-way (dose × day × time) ANOVAs (with repeated measures for day and time), were conducted for (a) sucrose intakes at 24 hours post-ivermectin treatment (cumulative); and (b) water intakes at 24 hours post-ivermectin treatment, and 2-way ANOVAs (dose × day) were conducted for body weights.
For all i.p. ivermectin-exposure experiments, if a significant interaction term or overall dose effect was obtained, then individual one-way ANOVAs were conducted on the first test day and for the average intake across the last 4 days of the test-phase for (a) EtOH at 4 and 24 hours or sucrose intakes at 24 hours following ivermectin treatment; (b) water intakes at 4 and 24 hours following ivermectin treatment and (c) body weights. Dunnett's multiple comparison t-tests were used to determine individual significant dose effects, relative to vehicle control-values.
For the ICV experiment, 2-way (dose × day) ANOVAs (with repeated measures for day) were conducted for (a) 2-hr EtOH intakes; (b) 2- and 24-hr water intakes; and (c) body weight. If a significant interaction term or overall dose effect was obtained, then individual one-way ANOVAs were conducted on the first test day and the average intake across the last 4 days of test-phase. Dunnett's multiple comparison t-tests were used to determine individual significant dose effects, relative to control-values.
Two-way (group × week) ANOVAs were utilized to evaluate the effects of P2rx4 shRNA expression on EtOH and water intakes, as well as body weights, relative to the control vector or non-injected controls during the final 4 weeks of the alcohol drinking. Significant overall effects were analyzed further with one-way ANOVAs, followed by Dunnett's multiple comparisons testing. A mixed linear regression model was used to compare cell counts between animals that received the active P2rx4 shRNA and the control shRNA.
Results
Effects of i.p. Ivermectin Administration
Cumulative EtOH intakes were recorded at 4 and 24 hr following ivermectin injections. Approximately 35% of the total daily EtOH intake was consumed within the first 4 hr. For HAD-1 rats, the 4-way ANOVA revealed a significant 4-way interaction [F(16,140) = 2.530, p < 0.01], as well as significant main effects for dose [F(4,35) = 5.491, p < 0.01], day [F(4,140) = 3.290, p < 0.05], and time [F(1,35) = 799.056, p < 0.001].
For HAD-1 rats (Figs. 1a & 1b), the highest (7.5 mg/kg) ivermectin dose reduced EtOH drinking on the 1st day at 4 (∼55%) and 24 (∼40%) hr relative to vehicle-values (p < 0.05). Across the last 4-day test, the highest (7.5 mg/kg) ivermectin dose reduced (∼35%) alcohol drinking at 4 and 24 hr following treatment, relative to vehicle-values (p-values < 0.05).
Fig 1.
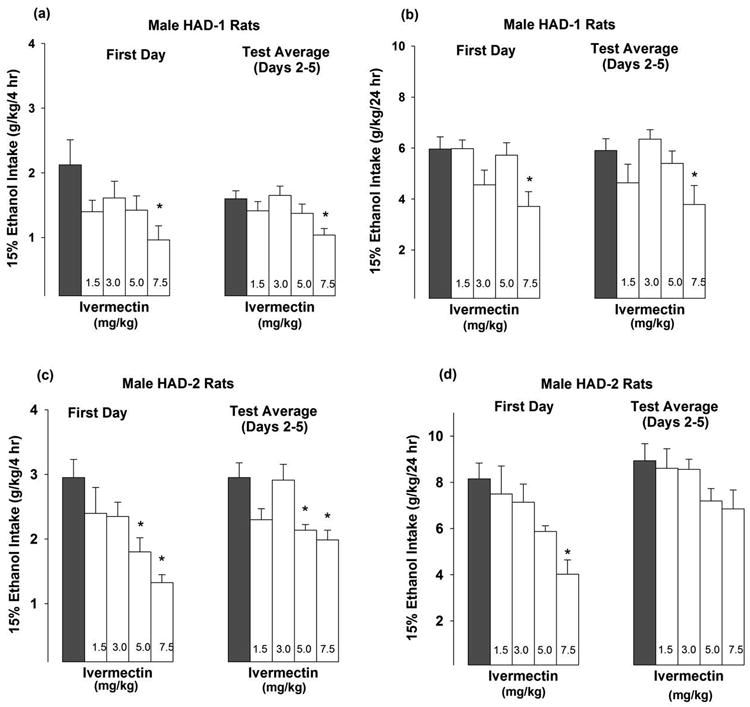
EtOH intake (g/kg) by male HAD-1 rats during (a) 4 hr or (b) 24 hr session, and by male HAD-2 rats during (c) 4 hr or (d) 24 hr sessions following ivermectin treatment. Rats (n = 8/dose) with 24-hr continuous access to 15% EtOH vs. water were administered 1.5, 3.0, 5.0 or 7.5 mg/kg ivermectin (i.p; open white bars), or a vehicle (i.p; solid black bars.) once daily for 5 consecutive days. EtOH intakes are presented on the 1st test day and the test average across days 2-5. Symbols indicate that the ivermectin group differed from the vehicle group (Dunnet's t-test; * p < 0.05).
For HAD-2 rats, the ANOVA did not reveal a 4-way interaction. However, there were significant main effects for dose [F(4,35) = 5.784, p < 0.001], day [F(4,140) = 7.420, p < 0.001], and time [F(1,35) = 665.101, p < 0.001]. For EtOH intake (Figs. 1c & 1d), the highest (7.5 mg/kg) ivermectin dose reduced EtOH drinking by 50-55% on the 1st day at 4 and 24 hr relative to vehicle-values (p < 0.01). In addition, on the 1st test day, the 5.0 mg/kg ivermectin dose also reduced (∼40%) EtOH drinking, at the 4-hr time point, relative to vehicle-values (p < 0.05; Fig. 1c). Across the last 4 days of alcohol drinking test, the 5.0 and 7.5 mg/kg ivermectin doses reduced (∼30%) EtOH drinking at the 4-hr time point (p < 0.05; Fig. 1c) but there was only ∼21 % reduction for 24-hr time point (p = 0.13) (Fig. 1d).
For 24-hr sucrose intakes by HAD-1 males, the omnibus 3-way ANOVA did not reveal dose × day × time or dose × day interactions, nor were there significant main effects for dose (Table 1).
Table 1. Sucrose intake (ml/kg) by male HAD-1 rats 24 hr following ivermectin treatment.
| Ivermectin | n | First Day | Test Average (Days 2-5) |
|---|---|---|---|
| Vehicle | 8 | 56 ± 2 | 61 ± 6 |
| 3.0 mg/kg | 8 | 53 ± 2 | 57 ± 5 |
| 5.0 mg/kg | 8 | 58 ± 2 | 56 ± 4 |
| 7.5 mg/kg | 8 | 55 ± 3 | 48 ± 5 |
Sucrose intake (ml/kg) by male HAD-1 rats 24 hr following ivermectin treatment. Rats (n = 7/dose) with 24-hr continuous access to 2% w/v sucrose were administered 1.5, 3.0, 5.0 or 7.5 mg/kg ivermectin, or a vehicle (i.p.) once daily for 5 consecutive days. Sucrose intakes are presented on the 1st test day and the test average across days 2-5. No differences were present between the groups.
ICV Ivermectin Administration
The aCSF infusion given on the day prior to testing for effects of ivermectin revealed that microinjection of the vehicle did not alter EtOH intake by HAD-2 females, relative to baseline EtOH drinking days. During testing, the 2-way ANOVA did not reveal a dose × day interaction; however, there was a significant main effect of dose [F(4,26) = 6.287, p < 0.001]. Simple effect analyses revealed that infusion with 1.5 or 2.0 μg doses of ivermectin reduced alcohol drinking by approximately 35% on the 1st test day (p-values < 0.05) and infusions with 0.5, 1.0, 1.5 or 2.0 μg doses of ivermectin reduced alcohol drinking by approximately 23-33% across the last 4 days of alcohol drinking test relative to vehicle-values (p-values < 0.05; Fig. 2). Two of the animals given the 1.5 μg dose of ivermectin were lost during the experiment, and their data were not included in the analyses. We did not observe any problems with the 2.0 μg dose or any of the lower doses of ivermectin.
Fig 2.
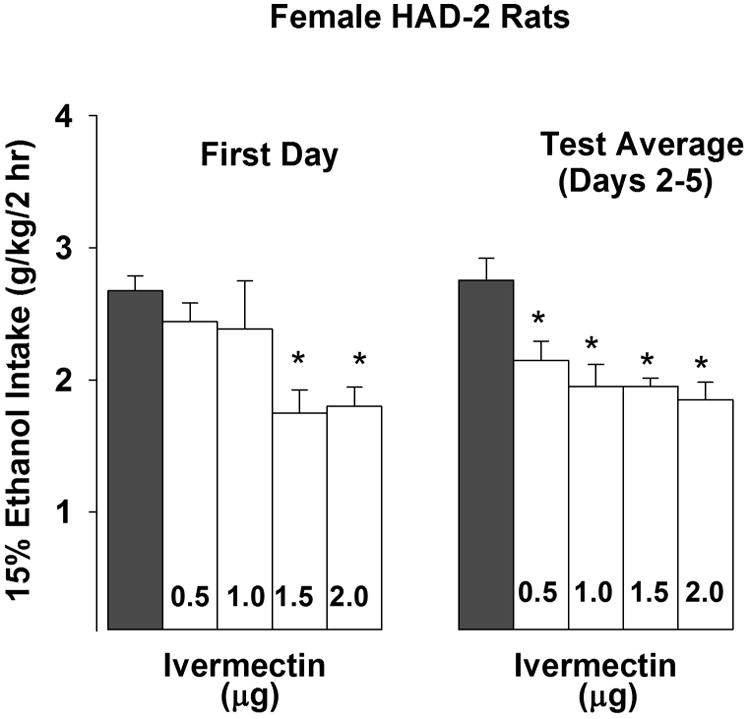
Female HAD-2 rats (n = 6-8/group) were trained to consume 15% EtOH under 2-hr limited access conditions. During the test week, rats were administered 0.5, 1.0, 1.5 or 2.0 μg ivermectin (ICV; open white bars), or vehicle (ICV; solid black bars) into the lateral ventricle once daily for 5 consecutive days. EtOH access was initiated 30 min following the infusions. EtOH intakes (g/kg) are presented on the 1st test day and the test average across days 2-5. Symbols above a bar indicate ivermectin-treated groups that differed from the vehicle group (Dunnett's t-test, * p < 0.05).
Effects of lentiviral-delivered P2rx4 shRNA in the posterior VTA on EtOH intake of HAD-2 rats
There was a significant effect for group [F(2,302) = 11.851, p < 0.001], but no group × week interaction or main effect for week. Simple effect analyses revealed a significant effect of group on EtOH drinking during the 2nd and 4th test weeks [F's >3.946, p's < 0.05], as well as a marginally significant effect during the 3rd test week. Over the final 4 weeks of the test period, the average EtOH intake in the active viral vector group was reduced approximately 30% relative to the scrambled control vector group (p < 0.001) and approximately 20% compared to the non-injected group (p < 0.01; Fig. 3a). No statistical differences were present between the scrambled vector and non-injected control groups (p = 0.211).
Fig 3.
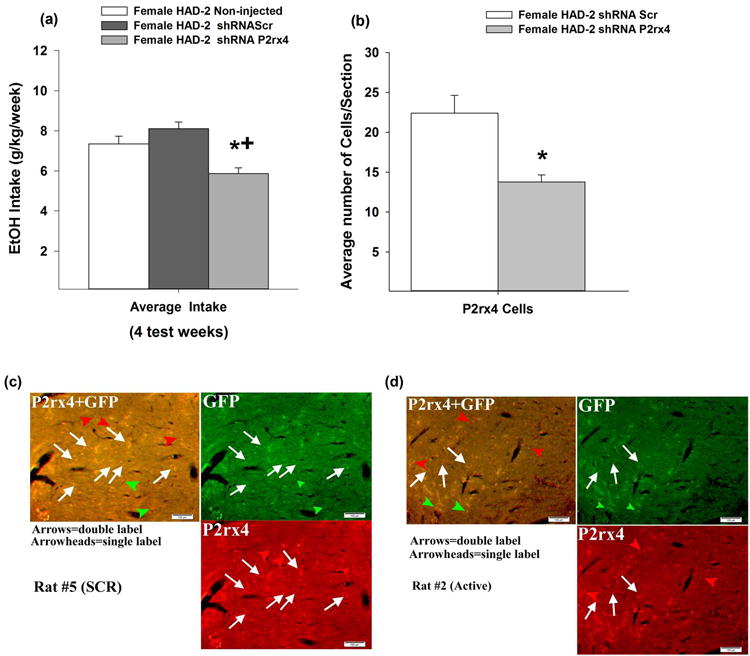
EtOH-experienced female HAD-2 rats were infused with an inactive or active shRNA targeting knockdown of P2rx4 gene expression in the posterior VTA. (a) EtOH (g/kg) during 24-hour continuous access averaged over the final 4 test weeks (n = 7/group). * indicates a significant difference from no injection group; + indicates a significant difference from scrambled vector group (Dunnett's t-test, p < 0.05) (b) * indicates a significant difference between the average number of P2X4R- positive cells counted/section within the posterior VTA in the P2rx4 shRNA group compared to control (Scr) shRNA group. (c) These images are general representations of the dual P2X4/GFP staining of the VTA for the inactive shRNA group, and (d) these images are general representations of the dual P2X4/GFP staining of the VTA for the active shRNA group.
For 24 hr water intake, there were significant effects for group [F(2,253) = 10.611, p < 0.001] and week [F(3,253) = 5.214, p < 0.01], but no group × week interaction. Over the final 4 weeks of the test period, water intake was reduced (∼20%) in the active (83.3 ml/kg ± 3.3) and scrambled (82.9 ml/kg ± 4.0) vector groups, relative to the non-injected group (101.2 ml/kg ± 3.9). Infusion with the active P2rx4 shRNA-expressing virus did not alter the weekly or average body weights over the period, relative to either of the control groups (data not shown).
Dual immunofluorescent staining of GFP and P2X4R in virus-infected VTA (Figs. 3c and 3d) demonstrated that the active P2rx4 shRNA virus caused a significant decrease (∼40%) in P2X4R-positive cells within the VTA compared to the control shRNA virus (p = 0.0006; Fig. 3b). There were no significant differences for GFP-positive cell counts between groups (p = 0.44). Random effects were included for each animal and a random effect of side (nested within animal). However, the sides examined did not have a significant effect (p = 0.99; for sections/animal, 9.1 vs. 7.6; for sections right side/animal, 4.7 vs. 3.7; and for sections left side/animal, 4.4 vs. 3.0 for shRNA P2rx4 vs. shRNA Scr). There was some viral infusion into the anterior VTA in 3 out of 7 scrambled rats and in 2 out of 7 of the rats given the active vector. Laser capture microdissection/quantitative-PCR analysis (LCM/qPCR) of GFP-positive cells also revealed a ∼36% decrease in P2rx4 gene expression with the active vector (6.35± 0.13 relative P2rx4/Gapdh) within the VTA compared to P2rx4 expression in virus-infected cells expressing control (9.95± 0.22 relative P2rx4/Gapdh) shRNA (data not shown).
Discussion
The major findings of this study are that (1) repeated i.p. administration of ivermectin, at the highest dose tested (7.5 mg/kg), significantly reduced EtOH intake but did not alter sucrose intake; (2) ivermectin was more effective in reducing EtOH intake by HAD-2 vs. HAD-1 rats during the 4 hour time point; (3) ICV administration of ivermectin also effectively reduced EtOH drinking; and (4) microinfusion of virus expressing shRNA targeting P2rx4 into the posterior VTA of HAD-2 rats reduced EtOH drinking as well. Notably, both male and female HAD rats were tested and these reductions in EtOH intake were consistent across the sexes, suggesting the effects observed are not sex-dependent. Overall, the data support a role for P2X4Rs in mediating alcohol drinking by HAD rats.
The highest (7.5 mg/kg) i.p. ivermectin dose was the most effective at reducing EtOH drinking. Across the two 5-day test-phases of EtOH drinking, this dose reduced 4- and 24-hr EtOH intake in HAD-1 and HAD-2 rats by approximately 30-40% (Figs. 1a-1c). Previous reports indicated that acute ivermectin doses below 2.5 mg/kg were ineffective in reducing EtOH intake of C57BL/6 mice (Yardley et al., 2012). In line with this, the current study also necessitated a higher dose (at least 5 mg/kg) before significant alterations in EtOH drinking became apparent.
Acute (1st day) i.p. ivermectin exposure reduced 24-hr free-choice EtOH drinking of HAD-1 and HAD-2 rats. A single administration of 2.5-10 mg/kg ivermectin was reported to reduce two-bottle choice intake and preference for 10% EtOH by C57BL/6 mice, using 24-hr continuous access conditions (Yardley et al., 2012; Asatryan et al. 2014). Kosten (2011) similarly reported that a single 10 mg/kg ivermectin injection reduced the amount of work that Sprague-Dawley rats would expend to obtain access to EtOH during a 3-hr operant test of alcohol self-administration. Taken together, it is likely that, despite the slow CNS penetration of ivermectin (Yardley et al., 2012; Asatryan et al., 2014), it is effective at reducing EtOH intake on the 1st day of treatment. Moreover, in the current study, the EtOH-reducing effects of the high ivermectin dose were present across the 5-day test-phase, suggesting tolerance did not develop to ivermectin administration. Somewhat in agreement with this idea, Yardley et al. (2012) reported that 7-day repeated systemic administration of a sub-threshold (1.25 mg/kg) dose of ivermectin reduced 24-hr EtOH intake by C57BL/6 mice.
Consistent with reports that systemic administration of 2.5 or 10.0 mg/kg ivermectin doses results in significant brain ivermectin levels that correlate with alcohol drinking reductions (Yardley et al., 2012), we found that ivermectin produced similar reductions in EtOH drinking when administered centrally or peripherally. Thus, it is likely that the effects of ivermectin in reducing alcohol drinking may be, at least in part, central nervous system driven. EtOH intakes across the last 4 days of ICV test period were reduced by 33% by infusion with 2 μg ivermectin, relative to vehicle-values (Fig. 2). Ivermectin crosses the blood brain barrier at a very slow rate; a dose of 5 mg/kg or 10 mg/kg (i.p.) reaches 1.81 ng×h/mg to 2.2 ng×h/mg, respectively, in brain tissue (Asatryan et al., 2014;Yardley et al., 2012). Since we did not examine the concentration of ivermectin in brain tissue, we are not certain if the brain tissue levels of ivermectin following ICV administration are similar to levels obtained with i.p. injections. However, considering the route of administration, it is possible that brain tissue levels of ivermectin following ICV administration may be higher than brain tissue levels following i.p. administration.
The results of the current study indicate that ivermectin does not significantly alter sucrose intake (Table 1), water intake or body weight (data not shown). Similarly, Yardley et al. (2012) reported that ivermectin did not alter operant sucrose responding in male mice. Previous reports indicated that ivermectin does not significantly alter water intake (Asatryan et al. 2014) or that it increases water intake (Yardley et al. 2012).
Ivermectin has been shown to induce motor impairments, such as somnolence and ataxia, at doses above 10 mg/kg (i.v.), whereas lower doses (up to 7.5 mg/kg, i.v.), did not induce signs of motor or CNS depression (Trailovic and Nedeljovic, 2011). The current study did not exceed 7.5 mg/kg dosing. In addition, ivermectin-induced motor impairments decrease with the passage of time (Trailovic et al., 2011). Further, ivermectin did not alter consumption of another rewarding stimulus, sucrose. Therefore, it is unlikely that locomotor disruptions were responsible for ivermectin-induced reductions in EtOH drinking at 24 hours following the first treatment. However, taken together with the decreased alcohol drinking present 4 hours post-injection on the first day of ivermectin administration (i.e. prior to the reported latency for ivermectin to enter the brain [Merck et al., 1988; Yardley et al., 2012]), it is possible that peripheral effects such as sedation contributed, in part, to the early drinking decreases seen in the present study.
Ivermectin appeared to be more effective in reducing EtOH intake by HAD-2 compared to HAD-1 rats at the 4hr time point, suggesting possible biological differences in the response to ivermectin between the replicate lines. P2rx4 expression is higher in the VTA of HAD-2 rats compared to LAD-2 rats. This difference in P2rx4 expression was not observed between the HAD-1 vs. LAD-1 lines (McBride et al., 2012). This may indicate that P2X4Rs play a more important role in regulating EtOH intake by HAD-2 rats vs. HAD-1 rats. Overall, these findings highlight the significance of genetic heterogeneity in assessing pharmacodynamic responses to medications for the treatment of alcohol use disorders, as previously emphasized (Bell et al., 2012; Heilig et al., 2011).
Since P2X4Rs are considered to be a strong candidate for the central mechanism of ivermectin's actions, the current study examined the potential involvement of P2rx4 expression within the posterior VTA of HAD-2 rats in mediating EtOH drinking behavior. Our findings indicated that micro-infusing an shRNA that decreases P2rx4 gene expression resulted in a significant (∼40%) reduction in the number of P2X4R-positive cells within the VTA (Fig. 3b) and a significant reduction in EtOH intake by HAD-2 rats over a 4 week period (Fig. 3a). Interestingly, water consumption was reduced in animals infused with the active or inactive shRNA vector, compared to those not receiving an injection. This may indicate that the surgical process itself induced adipsic processes. In contrast, EtOH drinking decreases were specific to animals given the active shRNA, suggesting that one possible central mechanism underlying ivermectin's ability to reduce EtOH intake involves P2X4Rs within the posterior VTA. Others have reported that P2X4Rs can play an important role in the reward circuitry by regulating the release of dopamine (Krugel et al., 2003; Kittner et al., 2004) or glutamate (Krugel et al., 2004; Khakh, 2009) within the VTA and nucleus accumbens. It is not known if knockdown of P2rx4 expression in the HAD1 line will reduce EtOH intake, or if there is a sex difference in the effects of the shRNA in the HAD2 line to reduce EtOH intake. Future studies will need to examine the effects of knocking down P2rx4 expression in male HAD2 rats, since female rats were used in the present study.
Ivermectin has been reported to block EtOH-induced inhibition of P2X4 receptors (Asatryan et al., 2010). One reason why ivermectin and P2rx4 shRNA both reduced EtOH intake of HAD-2 rats may be related to the ability of ivermectin to reduce the effects of EtOH at P2X4Rs. Similarly, the shRNA reduced the number of P2X4Rs in the pVTA at which EtOH could act. With both treatments, the actions of EtOH would be reduced, thereby reducing its rewarding effects and reducing EtOH intake. The shRNA results indicate that the VTA is one site of action in which P2X4 receptors act to modulate ethanol consumption. It is possible, however, that the VTA is not the only site where P2X4 receptors act to control alcohol consumption. Systemic and ICV administration of ivermectin also reduced alcohol consumption and, as a result, ivermectin may be acting in multiple brain regions to affect drinking. Ivermectin has direct actions at P2X4Rs, and as a result likely alters several neuronal signaling systems that are modulated by P2X4Rs that have been reported to be involved in alcohol drinking, such as GABA, glutamate, and dopamine (Tabakoff et al., 2009; Xiao et al., 2008; Vavra et al., 2011).
Furthermore, ivermectin also independently alters several other neural systems associated with alcohol drinking. For example, ivermectin potentiates activation of glycine receptors (Wang and Lynch, 2012), which have been identified as one of a few known primary targets for alcohol (Vengeliene et al., 2008). In addition, ivermectin enhances α7 nicotinic acetylcholine receptor functioning (Daly, 2005). Due to the inhibiting actions of alcohol on these receptor subtypes (Davis and de Fiebre, 2006), pharmacological enhancement of α7 cholinergic receptor signaling has been a recent focus for the treatment of alcohol use disorders (Rahman and Prendergast, 2012). As the current study did not employ site-specific administration of the ivermectin, it is not possible to identify CNS sites underlying ivermectin's actions. However, based upon the viral vector results, the posterior VTA qualifies as one potential site.
Conclusions
The results of this study taken in conjunction with previous studies suggest that CNS P2X4Rs are involved in regulating alcohol drinking, and may present a potential molecular target for the treatment of alcohol use disorders.
Acknowledgments
Drs. Franklin and Hauser share first authorship on this manuscript. The authors would like to thank Thomas H. Ewing, Yun Zhang and Donghong He for their technical support. The authors would also like to thank Dr. Paula Hoffman for her critical reading of the manuscript and for her help in getting the immunohistochemical analysis completed. None of the authors has a conflict of interest associated with this research. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of NIAAA or NIH.
Sources of Support: (NIAAA) grant AA007611, INIA grants AA013522 and AA016654, and NIAAA contract HHSN267200700037C
References
- Adelsberger H, Lepier A, Dudel J. Activation of rat recombinant alpha(1)beta(2)gamma(2S) GABA(A) receptor by the insecticide ivermectin. Eur J Pharmacol. 2000;394:163–170. doi: 10.1016/s0014-2999(00)00164-3. [DOI] [PubMed] [Google Scholar]
- Asatryan L, Yardley MM, Khoja S, Trudell JR, Hyunh N, Louie SG, Petasis NA, Alkana RL, Davies DL. Avermectins differentially affect ethanol intake and receptor function: implications for developing new therapeutics for alcohol use disorders. Int J Neuropsychopharmacology. 2014;17:907–916. doi: 10.1017/S1461145713001703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asatryan L, Popova M, Perkins D, Trudell JR, Alkana RL, Davies DL. Ivermectin antagonizes ethanol inhibition in purinergic P2X4 receptors. J Pharmacol Exp Ther. 2010;334:720–728. doi: 10.1124/jpet.110.167908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asatryan L, Popova M, Woodward JJ, King BF, Alkana RL, Davies DL. Roles of ectodomain and transmembrane regions in ethanol and agonist action in purinergic P2X2 and P2X3 receptors. Neuropharmacology. 2008;55:835–843. doi: 10.1016/j.neuropharm.2008.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Engleman EA, Toalston JE, McBride WJ. Scheduled access alcohol drinking by alcohol-preferring (P) and high-alcohol-drinking (HAD) rats: modeling adolescent and adult binge-like drinking. Alcohol. 2014;48:225–234. doi: 10.1016/j.alcohol.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Sable HJ, Colombo G, Hyytia P, Rodd ZA, Lumeng L. Animal models for medications development targeting alcohol abuse using selectively bred rat lines: neurobiological and pharmacological validity. Pharmacol Biochem Behav. 2012;103:119–155. doi: 10.1016/j.pbb.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Smith RJ, Toalston JE, Franklin KM, McBride WJ. Modeling binge-like ethanol drinking by peri-adolescent and adult P rats. Pharmacol Biochem Behav. 2011;100:90–97. doi: 10.1016/j.pbb.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Sable HJ, Schultz JA, Hsu CC, Lumeng L, Murphy JM, McBride WJ. Daily patterns of ethanol drinking in peri-adolescent and adult alcohol-preferring (P) rats. Pharmacol Biochem Behav. 2006;83:35–46. doi: 10.1016/j.pbb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Coddou C, Yan Z, Obsil T, Huidobro-Toro JP, Stojilkovic SS. Activation and regulation of purinergic P2X receptor channels. Pharmacol Rev. 2011;63:641–683. doi: 10.1124/pr.110.003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly JW. Nicotinic agonists, antagonists, and modulators from natural sources. Cell Mol Neurobiol. 2005;25:513–552. doi: 10.1007/s10571-005-3968-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaher R, McConnell KK, Rodd ZA, McBride WJ, Bell R. Daily patterns of ethanol drinking in adolescent and adult, male and female, high alcohol drinking (HAD) replicate lines of rats. Pharmacol Biochem Behav. 2012;102:540–548. doi: 10.1016/j.pbb.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DL, Bortolato M, Finn DA, Ramaker MJ, Barak S, Ron D, Liang J, Olsen RW. Recent advances in the discovery and preclinical testing of novel compounds for the prevention and/or treatment of alcohol use disorders. Alcohol Clin Exp Res. 2013;37:8–15. doi: 10.1111/j.1530-0277.2012.01846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DL, Kochegarov AA, Kuo ST, Kulkarni AA, Woodward JJ, King BF, Alkana RL. Ethanol differentially affects ATP-gated P2X(3) and P2X(4) receptor subtypes expressed in Xenopus oocytes. Neuropharmacology. 2005;49:243–253. doi: 10.1016/j.neuropharm.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Davis TJ, de Fiebre CM. Alcohol's actions on neuronal nicotinic acetylcholine receptors. Alcohol Res Health. 2006;29:179–185. [PMC free article] [PubMed] [Google Scholar]
- Franklin KM, Asatryan L, Jakowec MW, Trudell JR, Bell RL, Davies DL. P2X4 receptors (P2X4Rs) represent a novel target for the development of drugs to prevent and/or treat alcohol use disorders. Front Neurosci. 2014;8:176. doi: 10.3389/fnins.2014.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez P, Gonzalez FA, Ueno K. Ivermectin in human medicine, an overview of the current status of its clinical applications. Curr Pharm Biotechnol. 2012;13:1103–1109. doi: 10.2174/138920112800399248. [DOI] [PubMed] [Google Scholar]
- Heilig M, Goldman D, Berrettini W, O'Brien CP. Pharmacogenetic approaches to the treatment of alcohol addiction. Nat Rev Neurosci. 2011;12:670–684. doi: 10.1038/nrn3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS. ATP-gated P2X receptors on excitatory nerve terminals onto interneurons initiate a form of asynchronous glutamate release. Neuropharmacology. 2009;56:216–222. doi: 10.1016/j.neuropharm.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Kittner H, Hoffmann E, Krugel U, Illes P. P2 receptor-mediated effects on the open field behaviour of rats in comparison with behavioural responses induced by the stimulation of dopamine D2-like and by the blockade of ionotrophic glutamate receptors. Behav Brain Res. 2004;149:197–208. doi: 10.1016/s0166-4328(03)00227-4. [DOI] [PubMed] [Google Scholar]
- Kosten TA. Pharmacologically targeting the P2rx4 gene on maintenance and reinstatement of alcohol self-administration in rats. Pharmacol Biochem Behav. 2011;98:533–538. doi: 10.1016/j.pbb.2011.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause RM, Buisson B, Bertrand S, Corringer PJ, Galzi JL, Changeux JP, Bertrand D. Ivermectin: a positive allosteric effector of the alpha7 neuronal nicotinic acetylcholine receptor. Mol Pharmacol. 1998;53:283–294. doi: 10.1124/mol.53.2.283. [DOI] [PubMed] [Google Scholar]
- Krugel U, Kittner H, Franke H, Illes P. Purinergic modulation of neuronal activity in the mesolimbic dopaminergic system in vivo. Synapse. 2003;47:134–142. doi: 10.1002/syn.10162. [DOI] [PubMed] [Google Scholar]
- Krugel U, Spies O, Regenthal R, Illes P, Kittner H. P2 receptors are involved in the mediation of motivation-related behavior. Purinergic Signal. 2004;1:21–29. doi: 10.1007/s11302-004-4745-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalo U, Verkhratsky A, Pankratov Y. Ivermectin potentiates ATP-induced ion currents in cortical neurones: evidence for functional expression of P2X4 receptors? Neurosci Lett. 2007;421:158–162. doi: 10.1016/j.neulet.2007.03.078. [DOI] [PubMed] [Google Scholar]
- Lasek LW, Janak PH, He L, Whistler JL, Heberlein U. Downregulation of mu opioid receptor by RNA interference in the ventral tegmental area reduces ethanol consumption in mice. Genes Brain Behav. 2007;6:728–735. doi: 10.1111/j.1601-183X.2007.00303.x. [DOI] [PubMed] [Google Scholar]
- Lerchner W, Xiao C, Nashmi R, Slimko EM, van Trigt L, Lester HA, Anderson DJ. Reversible silencing of neuronal excitability in behaving mice by a genetically targeted, ivermectin-gated Cl- channel. Neuron. 2007;54:35–49. doi: 10.1016/j.neuron.2007.02.030. [DOI] [PubMed] [Google Scholar]
- Li C, Aguayo L, Peoples RW, Weight FF. Ethanol inhibits a neuronal ATP-gated ion channel. Mol Pharmacol. 1993;44:871–875. [PubMed] [Google Scholar]
- McBride WJ, Kimpel MW, McClintick JN, Ding ZM, Hyytia P, Colombo G, Edenberg HJ, Lumeng L, Bell RL. Gene expression in the ventral tegmental area of 5 pairs of rat lines selectively bred for high or low ethanol consumption. Pharmacol Biochem Behav. 2012;102:275–285. doi: 10.1016/j.pbb.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Rodd ZA, Bell RL, Lumeng L, Li TK. The alcohol-preferring (P) and high-alcohol-drinking (HAD) rats-Animal models of alcoholism. Alcohol. 2014;48:209–215. doi: 10.1016/j.alcohol.2013.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merck, Sharp, Dohme . Ivermectin. Div of Merck & Co Ltd; W. P. Pennsylvania: 1988. Poison Control Monograph. [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, Lumeng L, Li TK. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet. 2002;32:363–388. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Gatto GJ, Waller MB, McBride WJ, Lumeng L, Li TK. Effects of scheduled access on ethanol intake by the alcohol-preferring (P) line of rats. Alcohol. 1986;3:331–336. doi: 10.1016/0741-8329(86)90010-8. [DOI] [PubMed] [Google Scholar]
- Ostrovskaya O, Asatryan L, Wyatt L, Popova M, Li K, Peoples RW, Alkana RL, Davies DL. Ethanol is a fast channel inhibitor of P2X4 receptors. J Pharmacol Exp Ther. 2011;337:171–179. doi: 10.1124/jpet.110.176990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Popova M, Asatryan L, Ostrovskaya O, Wyatt LR, Li K, Alkana RL, Davies DL. A point mutation in the ectodomain-transmembrane 2 interface eliminates the inhibitory effects of ethanol in P2X4 receptors. J Neurochem. 2010;112:307–317. doi: 10.1111/j.1471-4159.2009.06460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priel A, Silberberg SD. Mechanism of ivermectin facilitation of human P2X4 receptor channels. J Gen Physiol. 2004;123:281–293. doi: 10.1085/jgp.200308986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S, Prendergast MA. Cholinergic receptor system as a target for treating alcohol abuse and dependence. Recent Pat CNS Drug Discov. 2012;7:145–150. doi: 10.2174/157488912800673173. [DOI] [PubMed] [Google Scholar]
- Research Institute for Laboratory Animal Research. Guide for the Care and Use of Laboratory Animals. 8th. The National Academies Press; Washington, D.C: 2011. [Google Scholar]
- Sattelle DB, Buckingham SD, Akamatsu M, Matsuda K, Pienaar IS, Jones AK, Sattelle BM, Almond A, Blundell CD. Comparative pharmacology and computational modelling yield insights into allosteric modulation of human alpha7 nicotinic acetylcholine receptors. Biochem Pharmacol. 2009;78:836–843. doi: 10.1016/j.bcp.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Shan Q, Haddrill JL, Lynch JW. Ivermectin, an unconventional agonist of the glycine receptor chloride channel. J Biol Chem. 2001;276:12556–12564. doi: 10.1074/jbc.M011264200. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Saba L, Printz M, Flodman P, Hodgkinson C, Goldman D, Koob G, Richardson HN, Kechris K, Bell RL, Hubner N, Heinig M, Pravenec M, Mangion J, Legault L, Dongier M, Conigrave KM, Whitfield JB, Saunders J, Grant B, Hoffman PL WHO/ISBRA Study on State and Trait Markers of Alcoholism. Genetical genomic determinants of alcohol consumption in rats and humans. BMC Biol. 2009;7:70. doi: 10.1186/1741-7007-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trailovic SM, Ivanovic SR, Varagic VM. Ivermectin effects on motor coordination and contractions of isolated rat diaphragm. Res Vet Sci. 2011;91:426–433. doi: 10.1016/j.rvsc.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Trailovic SM, Nedeljkovic JT. Central and peripheral neurotoxic effects of ivermectin in rats. J Vet Med Sci. 2011;73:591–599. doi: 10.1292/jvms.10-0424. [DOI] [PubMed] [Google Scholar]
- Vavra V, Bhattacharya A, Zemkova H. Facilitation of glutamate and GABA release by P2X receptor activation in supraoptic neurons from freshly isolated rat brain slices. Neuroscience. 2011;188:1–12. doi: 10.1016/j.neuroscience.2011.04.067. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. Br J Pharmacol. 2008;154:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Lynch JW. A comparison of glycine- and ivermectin-mediated conformational changes in the glycine receptor ligand-binding domain. Int J Biochem Cell Biol. 2012;44:335–340. doi: 10.1016/j.biocel.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Xiao C, Zhou C, Li K, Davies DL, Ye JH. Purinergic type 2 receptors at GABAergic synapses on ventral tegmental area dopamine neurons are targets for ethanol action. J Pharmacol Exp Ther. 2008;327:196–205. doi: 10.1124/jpet.108.139766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong K, Li C, Weight FF. Inhibition by ethanol of rat P2X(4) receptors expressed in Xenopus oocytes. Br J Pharmacol. 2000;130:1394–1398. doi: 10.1038/sj.bjp.0703439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardley MM, Wyatt L, Khoja S, Asatryan L, Ramaker MJ, Finn DA, Alkana RL, Huynh N, Louie SG, Petasis NA, Bortolato M, Davies DL. Ivermectin reduces alcohol intake and preference in mice. Neuropharmacology. 2012;63:190–201. doi: 10.1016/j.neuropharm.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]


