Abstract
Background
Greater body mass index (BMI), a measure of overall adiposity, is associated with higher risk of postmenopausal breast cancer. The role of central adiposity, often measured by waist circumference, is less well understood especially among premenopausal women. We aimed to examine multiple measures of adiposity in relation to breast cancer in a prospective cohort study.
Methods
50,884 Sister Study cohort participants ages 35–74 were enrolled from 2003–2009. Inclusion criteria for the cohort included having a sister previously diagnosed with breast cancer. Trained study personnel measured height, weight, waist and hip circumference during a home visit and study participants completed a detailed questionnaire. Using Cox regression, we estimated multivariable hazard ratios (HR) and 95% confidence intervals (CIs) for breast cancer risk associated with adiposity measurements, considering tumor subtype and menopausal status.
Results
In total, 2,009 breast cancers were diagnosed during follow-up (mean=5.4 years). Weight, BMI, waist circumference and waist-hip-ratio were positively associated with overall breast cancer risk and HRs were greater among postmenopausal women, those with hormonally responsive tumors and non-current postmenopausal hormone users. In models that adjusted for BMI, waist circumference associations persisted among both postmenopausal women (81–88cm vs ≤80cm, HR=1.16, 95%CI 1.01, 1.35; >88cm vs ≤80cm, HR=1.30, 95% CI 1.10, 1.54) and premenopausal women (81–88cm vs ≤80cm, HR=1.56, 95%CI 1.19, 2.04; >88cm vs ≤80cm, HR=1.30, 95% CI 0.91, 1.87).
Conclusions
Findings from this large, prospective study with examiner-measured body size indicate that waist circumference is independently and positively associated with both premenopausal and postmenopausal breast cancer risk.
Keywords: Adiposity, breast neoplasms, waist circumference, body mass index
Overall adiposity, measured using body mass index (BMI, kg/m2), is an established risk factor for postmenopausal breast cancer1. This risk factor is particularly notable in the aging U.S. population where obesity rates among women over the age of 60 have increased from 31.5% to 38.1% in the last decade2. Additionally, the prevalence of abdominal obesity, measured by waist circumference, in the U.S. increased from 55.4% in 1999–2000 to 64.7% in 2011–2012 in women3. These trends underscore the increasing likelihood that body size, and in particular central adiposity, may be influential in breast cancer trends in coming years.
Previous studies have found the relationship between overall adiposity and breast cancer to vary by menopausal status, with a recent meta-analysis reporting a 15% higher risk of postmenopausal and a 7% non-significant lower risk of premenopausal breast cancer when comparing women with BMI ≥25 vs. <254. The potential inverse association of obesity among younger women is not well-understood, but may be the result of increased anovulatory cycles5 and lower levels of progesterone6. Greater postmenopausal adiposity is hypothesized to be associated with breast cancer risk via increased estrogen levels due to the peripheral conversion of androgens, which encourage cell growth and thus increase the likelihood of mutations or proliferation of initiated cells7.
Measures of central adiposity, such as waist circumference or waist-to-hip ratio (WHR), are associated with a host of hormonal and metabolic changes and may be a better predictor of breast cancer risk than overall adiposity8. Waist circumference has been found to be associated with higher levels of insulin-like growth factors or androgen levels in premenopausal women9, and thus central adiposity may be particularly relevant to premenopausal breast cancer risk10–12. Central adiposity has also been hypothesized to be a better measurement than BMI of metabolically active visceral fat among postmenopausal women5. Central and overall adiposity may be more closely related to specific tumor subtypes, such as estrogen receptor (ER)-negative or triple negative tumors10,13.
Less is known about the relationship between adiposity and premenopausal breast cancer and whether the associations between central adiposity measures and breast cancer vary by menopausal status at diagnosis and tumor hormone receptor status. In this study, we aimed to evaluate the relationship between prospectively collected adiposity measures and breast cancer risk by central (waist circumference and WHR) versus overall (BMI) adiposity, menopausal status at diagnosis and by tumor hormone receptor status in the prospective Sister Study cohort.
Methods
Study Design and Population
The National Institute of Environmental Health (NIEHS) Sister Study is a prospective observational cohort study designed to identify environmental risk factors for breast cancer. During 2003–2009, 50,884 female residents of the United States and Puerto Rico were recruited via the media, breast cancer professionals and advocates, the Internet, a network of recruitment volunteers, and a national advertising campaign conducted in both English and Spanish. Eligible participants were breast cancer-free at enrollment, ages 35–74, and had a sister who had been diagnosed with breast cancer. The study was approved by the Institutional Review Board of the National Institute of Environmental Health Sciences, NIH, and the Copernicus Group Institutional Review Board. Written informed consent was obtained from all participants. The data presented here was from Sister Study data release 3.1 (May 2014).
Study participants completed extensive phone and written questionnaires with detailed information on medical and family cancer history, as well as lifestyle factors and demographics, including postmenopausal hormone use. Premenopausal status was defined as reporting one or more menstrual cycles in the prior 12-month period. For this study, we excluded records from: (1) 128 women who were diagnosed with breast cancer prior to completion of study enrollment activities or had a missing date of diagnosis; (2) 205 women with incomplete information on adiposity measurements; (3) 3,385 women with unknown menopausal status (including 3,354 women who were less than 55 at interview and who reported that their menses stopped at the age of hysterectomy with ovarian conservation). Therefore, information from 47,166 women contributed to this analysis.
Incident breast cancer
Participants are asked to complete annual health updates and biennial surveys to update risk factor information. Women who reported an incident breast cancer diagnosis during the follow-up period were asked to allow their medical records to be released as well as to provide additional diagnostic and treatment details. Response rates have been greater than 94% over follow-up14. Medical records have been obtained for more than 80% of breast cancer diagnoses. Agreement between self-reported and medical record-abstracted data was high15; therefore, self-reported data was used when medical record data was missing. Tumor subtypes of interest were defined as ER+ and PR+ (ER+PR+) and ER− and PR− (ER−PR−).
Adiposity Measurements
Adiposity-related measures included waist circumference, hip circumference, WHR, and BMI. At time of enrollment, current height, weight, hip and waist circumferences were measured during home visits by trained study personnel for almost all participants. A small proportion of height measurements (0.2%) were not available and for these, self-reported measures were used. These measurements were used to derive current BMI and waist-to-hip ratio. BMI was evaluated as a continuous variable (1-unit increases) and also categorized by standard WHO definitions, underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), obese (30–34.9 kg/m2), severely obese (35–39.9 kg/m2) and morbidly obese (≥40 kg/m2)16. Classification of waist circumference was based on the criteria from the American Diabetes Association for abdominal obesity as normal (≤80cm), action level 1 (80.1–88cm), or action level 2 (>88cm)17. Height, weight and waist-to-hip ratio were classified in quartiles based on distribution in the study population.
Statistical analysis
Multivariable Cox proportional hazards models were used to estimate hazard ratios (HR) and 95% confidence intervals (CI) for the association between adiposity measures and breast cancer risk. Statistical models used age as the time scale and person-time was accrued from age at study enrollment. Follow-up extended until study participants had a breast cancer diagnosis or were censored at the date of last follow-up. In analyses of breast cancer subtypes defined by hormone receptor status, competing or undefined breast cancer subtypes were censored at the date of diagnosis. For instance, when the outcome of interest was ER+PR+ breast cancers, women who had ER− and/or PR− breast cancers were censored at their date of diagnosis. Similarly, for ER−PR− outcomes of interest, women with ER+ and/or PR+ tumors were censored at date of diagnosis. ER/PR status was less frequently reported in the medical records for in situ breast cancer, and mammographic detection of in situ disease may be influenced by adiposity. Therefore, analysis of subtypes was limited to invasive cancer and person-time was censored on the date of an in situ diagnosis. Data on in situ breast cancer according to the adiposity measures addressed here are provided in the supporting material (Supporting Table I).
In analyses investigating associations by menopausal status at the time of breast cancer diagnosis, women who became postmenopausal during the follow-up period were censored at month of menopause with respect to the outcome of premenopausal breast cancer. Consequently, the person-time that accumulated after menopause contributed to postmenopausal person-time at risk. The proportional hazard assumption was visually assessed using ln-ln survival plots as well as with the inclusion of an interaction term with survival time in the regression model, using an alpha of 0.05. There was no suggestion of time-variant associations.
Stratified models were used to assess postmenopausal hormone use (using three definitions: (1) ever, never; and (2) by type: estrogen, progesterone, or both and (3) current, not current), smoking history (ever smoker, never smoker) and race (white, black) as potential effect measure modifiers. Additionally, we evaluated a potential interaction between waist circumference (≤80cm, 81–88cm, >88cm) and BMI (<25 kg/m2, ≥25 kg/m2). Confounders were identified using the prior literature and a directed acyclic graph18. Multivariable-adjusted models included the following confounders: age, race (non-Hispanic white, black, Hispanic, other), education (less than high school, high school equivalent, some college, 4-year degree or higher), age at menarche, age at first birth (<21, 21–<25, 25–<29, 29–<32, ≥32), parity (nulliparous or 1, 2–3, 4+), breastfeeding history (total weeks), oral contraceptives (ever, never), postmenopausal hormone use (premenopausal, none, estrogen only, estrogen and progesterone, estrogen and estrogen and progesterone), age at menopause (premenopausal, <40, 40–50, 51–55, 55+), smoking history (total pack years), current alcohol consumption (never drinker, former drinker, current <1 drink/day, current 1 drink/day, current 1.1–1.9 drinks/day, current 2+ drinks day) and physical activity (current metabolic equivalent hours/week). Models estimating the independent association between waist circumference and WHR with breast cancer risk were further adjusted for BMI as a continuous variable. Missing data on adiposity measurements was less than 1%, thus study participants with missing data were excluded from the analysis. Two-sided tests were used with a p value of 0.05 to evaluate statistical significance. All analyses were performed using SAS version 9.3 software (SAS Institute, Inc., Cary, NC).
Results
Among 47,116 women, 2,009 breast cancer cases were diagnosed during a total of 252,117 person-years. Average follow-up time was approximately 5.4 years. Study participant characteristics are stratified by BMI categories at baseline (Table I). Women were most likely to fall into the BMI ranges of 18.5–24.9 (37.7%) and 25.0–29.9 (31.8%), respectively, rather than being classified as having a BMI<18.5 or BMI ≥30.
Table 1.
Study participant descriptive characteristics by categories of body mass index, The Sister Study.
| Characteristic | Underweight BMI <18.5 |
Normal BMI 18.5–24.9 |
Overweight BMI 25.0–29.9 |
Obese BMI 30.0–34.9 |
Severely Obese BMI 35.0–39.9 |
Morbidly Obese BMI ≥40 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
N participants (% total participants) |
539 | 1.1 | 17,769 | 37.7 | 14,984 | 31.8 | 8,043 | 17.1 | 3,579 | 7.6 | 2,252 | 4.8 |
|
Age at enrollment, mean y (SD) |
54.4 | (9.8) | 54.6 | (9.3) | 56.3 | (9.0) | 56.2 | (8.8) | 55.5 | 8.6 | 54.5 | (8.4) |
|
N breast cancer cases (% total cases) |
19 | 1.0 | 720 | 35.8 | 646 | 32.2 | 382 | 19.0 | 145 | 7.2 | 97 | 4.8 |
|
Total person-years (% total PY) |
2,981 | 1.2 | 97,714 | 38.8 | 79,670 | 31.6 | 42,009 | 16.7 | 18,434 | 7.3 | 11,309 | 4.5 |
|
Age at menarche, mean y (SD) |
13.1 | (1.6) | 12.9 | (1.5) | 12.6 | (1.5) | 12.4 | (1.5) | 12.3 | (1.6) | 12.1 | (1.6) |
|
N parous at enrollment (% col) |
401 | 74.4 | 14,250 | 80.2 | 12,460 | 83.2 | 6,708 | 83.4 | 2,911 | 81.3 | 1,744 | 77.4 |
|
N premenopausal at enrollment (% col) |
199 | 36.9 | 6,758 | 38.0 | 4,437 | 29.6 | 2,313 | 28.8 | 1,069 | 29.9 | 752 | 33.4 |
|
Age at first life birth, mean y (SD) |
26.1 | (5.4) | 25.8 | (5.3) | 24.5 | (5.2) | 23.9 | (5.0) | 23.8 | (5.2) | 23.8 | (5.3) |
|
Total breastfeeding, mean weeks (SD) |
76.6 | (78.3) | 70.4 | (74.0) | 64.9 | (72.4) | 61.9 | (74.2) | 60.0 | (69.5) | 55.5 | (64.2) |
| Pack-years, mean (SD) | 15.5 | (16.6) | 12.4 | (13.9) | 15.3 | (15.5) | 16.6 | (16.7) | 16.7 | (16.5) | 17.0 | (16.8) |
|
Total MET hours physical activity per week, mean (SD) |
55.9 | (35.6) | 56.5 | (33.2) | 50.6 | (30.3) | 45.5 | (28.6) | 41.3 | (26.7) | 37.2 | (26.7) |
| N ever BC pill (% col) | 419 | 77.7 | 15,081 | 84.9 | 12,415 | 82.9 | 6,737 | 83.8 | 2,953 | 82.5 | 1,835 | 81.5 |
| N white (% col) | 491 | 91.1 | 15,989 | 90.0 | 12,540 | 83.7 | 6,404 | 79.6 | 2,742 | 76.6 | 1,650 | 73.3 |
| N ever alcohol (% col) | 511 | 94.8 | 17,217 | 96.9 | 14,384 | 96.0 | 7,707 | 95.8 | 3,410 | 95.3 | 2,146 | 95.3 |
The association between adiposity measurements and overall breast cancer risk and by select subgroups is displayed in Table II. Increasing weight category was associated with an higher overall breast cancer risk (>184 lbs. vs. <136 lbs., HR=1.32, 95% CI 1.15, 1.51). Estimates were greater among women with ER+PR+ tumors; women >157 lbs. had an over 60% increase in risk for ER+PR+ breast cancer compared to women <136 lbs. However, given the small numbers of hormone-receptor negative tumors in our study, HRs were more imprecise for women with ER−PR− tumors. Consistent trends were not observed for height.
Table 2.
Hazard Ratios (HR) and 95% Confidence Intervals (CIs) for the association between adiposity measurements and overall breast cancer risk and by select subgroups, The Sister Study.
| Characteristic | Person-years (N=252,117) |
Total breast cancer (N=2,009) |
HR (95% CI)a | HR(95%CI)b | ER+/PR+ (N=893) |
HR (95% CI)b | ER−/PR− (N=192) |
HR (95% CI)b |
|---|---|---|---|---|---|---|---|---|
| Weight (lbs) | ||||||||
| <136 | 63,022 | 422 | 1 | 1 | 168 | 1 | 37 | 1 |
| 137–156 | 64,169 | 487 | 1.13 (0.99,1.29) | 1.15 (1.00,1.31) | 216 | 1.29 (1.04,1.59) | 55 | 1.63 (1.06,2.52) |
| 157–183 | 62,885 | 569 | 1.38 (1.21,1.56) | 1.42 (1.25,1.62) | 258 | 1.69 (1.38,2.07) | 52 | 1.49 (0.96,2.33) |
| >184 | 62,042 | 531 | 1.30 (1.14,1.48) | 1.32 (1.15,1.51) | 251 | 1.62 (1.35,2.05) | 48 | 1.34 (0.84,2.14) |
| Continuous (per 5 lbs) | 1.01 (1.00,1.02) | 1.01 (1.00,1.02) | 1.02 (1.01,1.03) | 1.01 (0.99,1.03) | ||||
| Height (in) | ||||||||
| <62 | 56,647 | 429 | 1.00 (0.88,1.13) | 1.00 (0.88,1.14) | 192 | 1.14 (0.94,1.39) | 40 | 1.06 (0.69,1.62) |
| 62–64 | 74,416 | 570 | 1 | 1 | 234 | 1 | 54 | 1 |
| 65–66 | 53,445 | 475 | 1.14 (1.01,1.29) | 1.13 (0.99,1.28) | 220 | 1.25 (1.03,1.51) | 45 | 1.33 (0.89,2.00) |
| >66 | 67,609 | 535 | 1.05 (0.94,1.19) | 1.05 (0.93,1.19) | 247 | 1.14 (0.95,1.38) | 53 | 1.24 (0.83,1.84) |
| Continuous | 1.01 (0.99,1.03) | 1.01 (0.99,1.03) | 1.01 (0.98,1.04) | 1.04 (0.98,1.10) | ||||
| Body mass index (kg/m2) | ||||||||
| <18.5 | 2,981 | 19 | 0.84 (0.53, 1.34) | 0.90 (0.56,1.44) | 5 | 0.61 (0.25,1.48) | 5 | 2.65 (1.06,6.58) |
| 18.5–24.9 | 97,714 | 720 | 1 | 1 | 296 | 1 | 71 | 1 |
| 25–29 | 79,670 | 646 | 1.12 (1.00, 1.24) | 1.14 (1.02,1.27) | 315 | 1.45 (1.23,1.71) | 58 | 0.98 (0.68,1.41) |
| 30–34.9 | 42,009 | 382 | 1.26 (1.11, 1.43) | 1.29 (1.13,1.47) | 162 | 1.42 (1.16,1.75) | 35 | 1.15 (0.75,1.76) |
| ≥35 | 29,743 | 242 | 1.12 (0.97, 1.30) | 1.13 (0.97,1.33) | 115 | 1.49 (1.18,1.88) | 23 | 0.97 (0.58,1.62) |
| Continuous (≥18.5) | 1.01 (1.00, 1.02) | 1.01 (1.00,1.02) | 1.02 (1.01,1.04) | 1.01(0.98,1.04) | ||||
| Waist circumference (cm) | ||||||||
| ≤80 | 99,948 | 705 | 1 | 1 | 285 | 1 | 68 | 1 |
| 81–88 | 53,235 | 443 | 1.20 (1.06, 1.35) | 1.24 (1.10,1.40) | 225 | 1.63 (1.36,1.96) | 44 | 1.28 (0.87,1.89) |
| >88 | 98,934 | 861 | 1.27 (1.15, 1.40) | 1.30 (1.17,1.45) | 383 | 1.53 (1.29,1.81) | 80 | 1.18 (0.83,1.67) |
| Continuous (per in) | 1.02 (1.01, 1.02) | 1.02 (1.01,1.03) | 1.03 (1.02,1.04) | 1.02 (0.99,1.04) | ||||
| Waist:Hip ratio | ||||||||
| <0.75 | 71,702 | 529 | 1 | 1 | 232 | 1 | 48 | 1 |
| 0.75–0.79 | 53,237 | 388 | 1.00 (0.88,1.15) | 1.01 (0.89,1.16) | 179 | 1.08 (0.88,1.32) | 38 | 1.07 (0.69,1.66) |
| 0.80–0.85 | 71,214 | 564 | 1.09 (0.97,1.23) | 1.10 (0.97,1.25) | 250 | 1.12 (0.93,1.35) | 53 | 1.16 (0.77,1.73) |
| ≥0.86 | 55,964 | 528 | 1.33 (1.18,1.50) | 1.35 (1.19,1.54) | 232 | 1.41 (1.16,1.71) | 53 | 1.46 (0.97,2.21) |
Adjusted for age
Adjusted for age, race, education, age at menarche, breastfeeding history, age at first birth, parity, postmenopausal hormone use, age at menopause, smoking history, alcohol consumption, and physical activity.
A non-linear increase in overall breast cancer risk was observed for increased categories of BMI. Estimates were stronger and monotonic for women with ER+PR+ invasive tumors (25–29 kg/m2, HR=1.45, 95% CI 1.23, 1.71; 30–34.9 kg/m2, HR=1.42, 95% CI 1.16, 1.75; ≥35 kg/m2, HR=1.49, 95% CI 1.18, 1.88, vs. 18.5–24.9 kg/m2). In contrast, this association was not apparent among women with invasive ER−PR− tumors. For women with ER−PR− tumors, the highest risk was observed for underweight women (HR=2.65, 95% CI 1.06, 6.58) compared to the normal weight category.
Higher overall breast cancer risk was observed with greater waist circumference and were more pronounced for ER+PR+ invasive tumors (81–88 cm vs. ≤80 cm, HR=1.63, 95% CI 1.36, 1.96; >88 cm vs. ≤80 cm 1.53, 95% CI 1.29, 1.81). More modest estimates, although with wide confidence intervals, were also observed for ER−PR− invasive tumors (81–88 cm vs. ≤80 cm, HR=1.28, 95% CI 0.87, 1.89; >88 cm vs. ≤80 cm, HR=1.18, 95% CI 0.83, 1.67). Women in the highest category of WHR, when compared to women in the lowest category, had a higher risk for overall breast cancer (HR=1.35, 95%CI 1.19, 1.54), and for ER+PR+ and ER−PR− tumors.
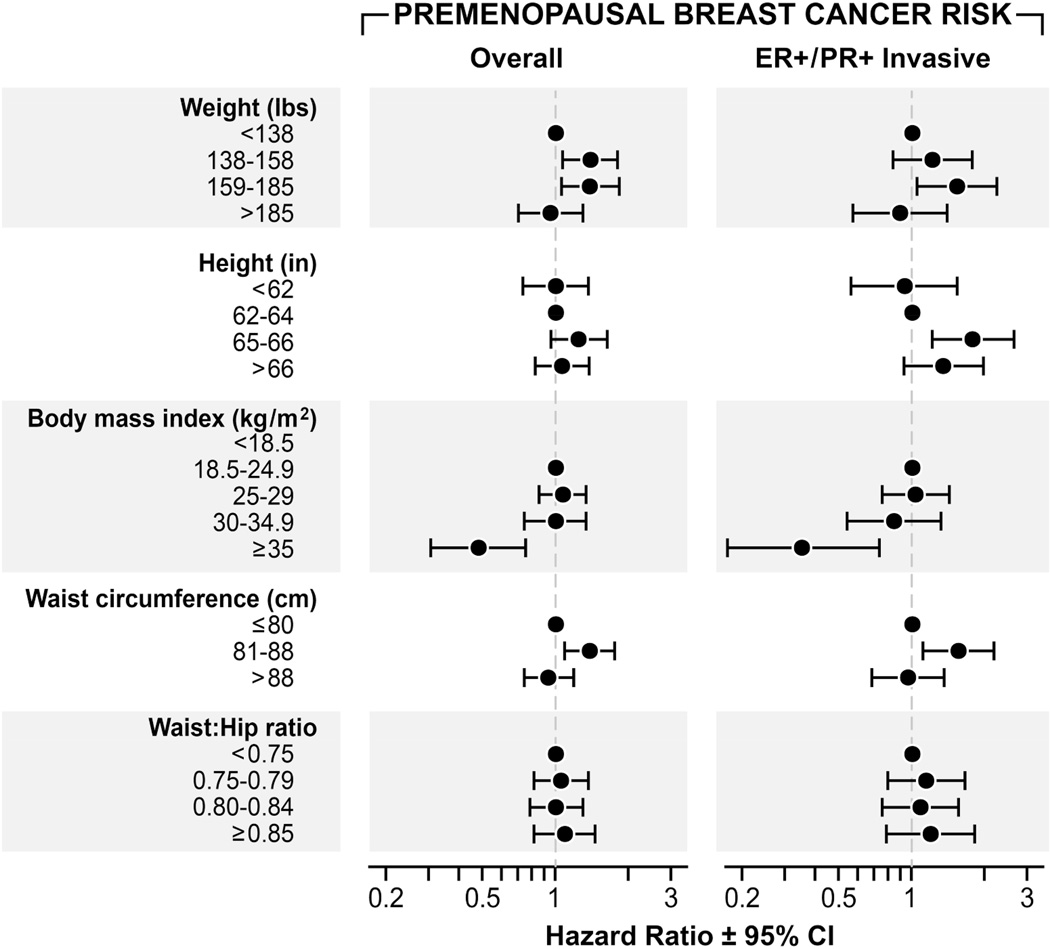
When analyses were limited to postmenopausal breast cancer (Figure I, Supporting Table II), the trends of adiposity measures with breast cancer risk were very similar to those observed in all women. Women in the highest category of weight did not have an elevated premenopausal breast cancer risk compared to those with weights <136 lbs. (HR=0.95, 95% CI 0.69, 1.31), although higher HRs were noted for women between 137–184 lbs. (Figure 2, Supporting Table III). A trend towards lower HRs was observed with increasing BMI for premenopausal breast cancer. For example, an inverse association was observed between BMI ≥35 kg/m2 and premenopausal ER+PR+ tumors (HR=0.35, 95% CI 0.17, 0.74) relative to those with a BMI 18.5–24.9 kg/m2. There was no clear association with waist circumference and premenopausal breast cancer. While an elevated HR was noted for premenopausal women with waist circumference between 81–88 cm, this trend did not continue for women with waist circumference >88 cm. Due to small sample sizes (n=33), we were unable to examine the adiposity measurements in association with premenopausal ER−PR− tumors.
Figure I.
Hazard Ratios (HR) and 95% Confidence Intervals (CIs) for the association between adiposity measurements and postmenopausal breast cancer risk, The Sister Study.
Figure II.
Hazard Ratios (HR) and 95% Confidence Intervals (CIs) for the association between adiposity measurements and premenopausal breast cancer risk, The Sister Study.
Estimates for central adiposity after adjustment for overall adiposity are displayed in Table III. Adjustment for BMI did not attenuate the positive association between waist circumference or WHR and overall breast cancer. Interestingly, in premenopausal women, positive associations between breast cancer risk and higher categories of waist circumference (81–88 cm vs. <80 cm, HR=1.56, 95%CI 1.19, 2.04; >88 cm vs. <80 cm 1.30, 95%CI 0.91, 1.87) and WHR (≥0.86 vs. <0.75, HR=1.26, 95% CI 0.91, 1.73) remained as strong or became even more pronounced after adjustment for BMI.
Table 3.
Hazard Ratios (HR) and 95% Confidence Intervals (CIs) for the association between breast cancer and central adiposity with adjustment for BMI, The Sister Study.
| Overall | Postmenopausal | Premenopausal | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Person- years |
Total breast cancer |
HR(95%CI)a | Person- years |
Breast cancer |
HR (95% CI)a | Person- years |
Breast cancer |
HR (95% CI)a |
| Waist circumference (cm) | |||||||||
| ≤80 | 99,948 | 705 | 1 | 72,084 | 505 | 1 | 25,660 | 193 | 1 |
| 81–88 | 53,235 | 443 | 1.25 (1.10,1.42) | 41,981 | 340 | 1.16 (1.01,1.35) | 10,244 | 99 | 1.56 (1.19,2.04) |
| >88 | 98,934 | 861 | 1.33 (1.14,1.54) | 80,266 | 737 | 1.30 (1.10,1.54) | 16,851 | 121 | 1.30 (0.91,1.87) |
| Continuous (per in) | 1.03 (1.01,1.05) | 1.03 (1.01,1.05) | 1.02 (0.98,1.06) | ||||||
| Waist:Hip ratio | |||||||||
| <0.75 | 71,702 | 529 | 1 | 51,656 | 384 | 1 | 18,426 | 140 | 1 |
| 0.75–0.79 | 53,237 | 388 | 1.00 (0.88,1.15) | 40,117 | 286 | 0.97 (0.82,1.13) | 11,907 | 98 | 1.11 (0.84,1.46) |
| 0.80–0.85 | 71,214 | 564 | 1.08 (0.95,1.22) | 56,157 | 457 | 1.06 (0.91,1.23) | 13,814 | 104 | 1.10 (0.84,1.45) |
| ≥0.86 | 55,964 | 528 | 1.31 (1.14,1.50) | 46,400 | 455 | 1.28 (1.10,1.49) | 8,607 | 71 | 1.26 (0.91,1.73) |
Adjusted for age, race, education, age at menarche, breastfeeding history, age at first birth, parity, postmenopausal hormone use, age at menopause, smoking history, alcohol consumption, physical activity and BMI.
Associations were generally not evident among current postmenopausal hormone users, whereas results among non-current hormone users were similar to overall results (Supporting Table IV). We did not observe evidence of additional effect measure modification between adiposity measurements and breast cancer by other postmenopausal hormone use definitions, smoking, race or between BMI and waist circumference (data not shown).
Discussion
This study is notable because of the large, prospective study design and the use of examiner-collected adiposity measurements. We observed higher breast cancer risk in association with measurements of body size and adiposity. The magnitude of these associations were often strongest among women with ER+PR+ tumors, as seen in other studies13,19–23; and less consistent or not evident among women with ER−PR−. We report differential associations of overall adiposity by menopausal status, consistent with previous studies. In postmenopausal women, we found evidence of even more pronounced associations for measures of both overall and central adiposity. In premenopausal women, we observed a significant inverse association between the highest category of overall obesity and breast cancer.
Waist circumference was found to be an important predictor of breast cancer risk, independent of overall adiposity. In statistical models of waist circumference and breast cancer risk among postmenopausal women, additional adjustment for BMI (to determine if associations with central adiposity were independent of overall adiposity) did not result in an attenuation of observed associations. This suggests that waist circumference is an important, independent predictor of postmenopausal breast cancer risk. Few studies have considered whether central obesity is associated with postmenopausal breast cancer beyond its correlation with overall obesity. Those that have done so have been inconsistent, although most studies reported that waist circumference estimates were attenuated with consideration of BMI8,13,19,24–26. However, our finding that the association between waist circumference and premenopausal obesity is apparent only after adjusting for BMI is consistent with some studies8,25–27, but not all28, and merits replication.
Waist circumference is an accurate measure of central adiposity, particularly among older women5. A challenge in most studies of waist circumference is the use of self-reported waist circumference measures which may not be accurate. Biological mechanisms of central adiposity likely differ by menopausal status. Adjustment for overall adiposity did not attenuate waist circumference findings for postmenopausal women. In contrast, the observed point estimates remained similar or became more pronounced after BMI adjustment in premenopausal women. In postmenopausal women, central adiposity may be a better estimate of overall adiposity and a more accurate predictor of estrogen-producing visceral fat5. In contrast, centrally obese premenopausal women may have lower estradiol levels compared to women with lower waist circumference29. This suggests that the biologic mechanism for the higher premenopausal breast cancer risk in association with central adiposity measures may not solely be related to estrogen. This is consistent with estrogen responsive tumors being less common in premenopausal compared to postmenopausal women30. Rather, metabolic conditions may be mechanistically important. Central adiposity is an independent predictor of both hyperinsulinemia and levels of IGF-1, which have been previously found to be related to premenopausal breast cancer risk12,31.
Despite a current overall plateau in the previously increasing rates of BMI among most demographic groups, waist circumference is increasing in the U.S.3. The reason for this re-distribution of body weight is unknown, but is has been hypothesized that sleep deprivation, exposure to endocrine disrupting chemicals or medication use32 and even intake of diet soda33 may be driving this increase in central adiposity. Therefore, our finding that waist circumference was independently important for both pre- and postmenopausal women may be of great consequence from a public health standpoint.
We observed an interaction of current postmenopausal hormone use and the adiposity metrics with breast cancer risk. Findings were consistent with previous studies in which associations tend to be more pronounced among non-users of postmenopausal hormones34. However, no statistically significant interaction was observed based on type of hormone use or by smoking or race.
This study has many strengths; importantly, adiposity measurements were collected by trained study personnel. Additionally, these measurements were taken prior to diagnosis and therefore any fluctuations in body size in response to the onset of breast cancer would not have impacted these measurements. We were also able to consider a number of adiposity measurements, which may help to elucidate the biologic mechanism. The large sample size of the Sister Study permitted us to consider the breast cancer associations with anthropometric measures according to hormone receptor and menopausal status.
This study has some limitations. Despite the large sample size, confidence intervals for all ER−PR− tumors combined were wide—making it difficult to draw conclusions. Similarly, fewer women were diagnosed with premenopausal breast cancer which may have contributed to some instability in measures of association. Although we had examiner-provided measurements of adiposity that were collected using a standard protocol, there is still the possibility of non-differential misclassification across multiple examiners which may have resulted in a bias towards the null. The women in the Sister Study, by enrollment criteria, have a family history of breast cancer and therefore have a two-fold increased risk of breast cancer compared to women without a family history. Women in the Sister Study have a very similar distribution of breast cancer risk factors compared to women in the general population suggesting these results may still be generalizable to women without a family history35.
In conclusion, this study confirms previous reports that have found a positive association of postmenopausal breast cancer with overall adiposity as well as inverse associations between overall adiposity and premenopausal breast cancer. Adiposity associations were most apparent among women who were not currently using postmenopausal hormones. Central adiposity was an independent predictor of postmenopausal, and possibly premenopausal, breast cancer risk in this study population, and is an important risk factor to consider as waist circumferences continues to expand in the United States.
Supplementary Material
This prospective study with examiner-measured body size found that waist circumference is independently and positively associated with both premenopausal and postmenopausal breast cancer risk after accounting for overall adiposity. This finding is potentially important for future breast cancer trends as average waist circumferences continue to increase in the United States.
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z01-ES044005), the Avon Foundation (02-2012-085), the National Center for Advancing Translational Sciences (KL2-TR001109) and by the UNC Lineberger Cancer Control Education Program (R25 CA57726);
Footnotes
There are no financial disclosures.
References
- 1.American Cancer Society. Atlanta, GA: American Cancer Society; 2014. Breast Cancer Facts and Figures 2014S. [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ford ES, Maynard LM, Li C. Trends in mean waist circumference and abdominal obesity among US adults, 1999–2012. JAMA. 2014;312(11):1151–1153. doi: 10.1001/jama.2014.8362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheraghi Z, Poorolajal J, Hashem T, Esmailnasab N, Doosti Irani A. Effect of body mass index on breast cancer during premenopausal and postmenopausal periods: a meta-analysis. PLoS One. 2012;7(12):e51446. doi: 10.1371/journal.pone.0051446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4(8):579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 6.Dowsett M, Folkerd E. Reduced progesterone levels explain the reduced risk of breast cancer in obese premenopausal women: a new hypothesis. Breast Cancer Res Treat. 2015;149(1):1–4. doi: 10.1007/s10549-014-3211-4. [DOI] [PubMed] [Google Scholar]
- 7.Cleary MP, Grossmann ME. Minireview: Obesity and breast cancer: the estrogen connection. Endocrinology. 2009;150(6):2537–2542. doi: 10.1210/en.2009-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang Z, Willett WC, Colditz GA, Hunter DJ, Manson JE, Rosner B, Speizer FE, Hankinson SE. Waist circumference, waist:hip ratio, and risk of breast cancer in the Nurses' Health Study. Am J Epidemiol. 1999;150(12):1316–1324. doi: 10.1093/oxfordjournals.aje.a009963. [DOI] [PubMed] [Google Scholar]
- 9.Bezemer ID, Rinaldi S, Dossus L, van Gils CH, Peeters PH, van Noord PA, Bueno-de-Mesquita HB, Johnsen SP, Overvad K, Olsen A. C-peptide, IGF-I, sex-steroid hormones and adiposity: a cross-sectional study in healthy women within the European Prospective Investigation into Cancer and Nutrition (EPIC) Cancer Causes & Control. 2005;16(5):561–572. doi: 10.1007/s10552-004-7472-9. [DOI] [PubMed] [Google Scholar]
- 10.Fagherazzi G, Chabbert-Buffet N, Fabre A, Guillas G, Boutron-Ruault MC, Mesrine S, Clavel-Chapelon F. Hip circumference is associated with the risk of premenopausal ER−/PR− breast cancer. Int J Obes (Lond) 2012;36(3):431–439. doi: 10.1038/ijo.2011.66. [DOI] [PubMed] [Google Scholar]
- 11.Hankinson SE, Eliassen AH. Circulating sex steroids and breast cancer risk in premenopausal women. Horm Cancer. 2010;1(1):2–10. doi: 10.1007/s12672-009-0003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Renehan AG, Harvie M, Howell A. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and breast cancer risk: eight years on. Endocr Relat Cancer. 2006;13(2):273–278. doi: 10.1677/erc.1.01219. [DOI] [PubMed] [Google Scholar]
- 13.Sellers TA, Davis J, Cerhan JR, Vierkant RA, Olson JE, Pankratz VS, Potter JD, Folsom AR. Interaction of waist/hip ratio and family history on the risk of hormone receptor-defined breast cancer in a prospective study of postmenopausal women. Am J Epidemiol. 2002;155(3):225–233. doi: 10.1093/aje/155.3.225. [DOI] [PubMed] [Google Scholar]
- 14.Nichols HB, Baird DD, DeRoo LA, Kissling GE, Sandler DP. Tubal ligation in relation to menopausal symptoms and breast cancer risk. Br J Cancer. 2013;109(5):1291–1295. doi: 10.1038/bjc.2013.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.NIEHS Sister Study. Sister Study Breast Cancer Concordance for Release 3.2. 2015 www.sistersutdy.niehs.nih.gov.
- 16.World Health Organization. Obesity: preventing and managing the global epidemic. World Health Organization; 2000. [PubMed] [Google Scholar]
- 17.Ardern CI, Janssen I, Ross R, Katzmarzyk PT. Development of health-related waist circumference thresholds within BMI categories. Obes Res. 2004;12(7):1094–1103. doi: 10.1038/oby.2004.137. [DOI] [PubMed] [Google Scholar]
- 18.Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC medical research methodology. 2008;8:70. doi: 10.1186/1471-2288-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaudet MM, Carter BD, Patel AV, Teras LR, Jacobs EJ, Gapstur SM. Waist circumference, body mass index, and postmenopausal breast cancer incidence in the Cancer Prevention Study-II Nutrition Cohort. Cancer Causes Control. 2014;25(6):737–745. doi: 10.1007/s10552-014-0376-4. [DOI] [PubMed] [Google Scholar]
- 20.Macinnis RJ, English DR, Gertig DM, Hopper JL, Giles GG. Body size and composition and risk of postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev. 2004;13(12):2117–2125. [PubMed] [Google Scholar]
- 21.Canchola AJ, Anton-Culver H, Bernstein L, Clarke CA, Henderson K, Ma H, Ursin G, Horn-Ross PL. Body size and the risk of postmenopausal breast cancer subtypes in the California Teachers Study cohort. Cancer causes & control : CCC. 2012 doi: 10.1007/s10552-012-9897-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki R, Orsini N, Saji S, Key TJ, Wolk A. Body weight and incidence of breast cancer defined by estrogen and progesterone receptor status--a meta-analysis. Int J Cancer. 2009;124(3):698–712. doi: 10.1002/ijc.23943. [DOI] [PubMed] [Google Scholar]
- 23.Ritte R, Lukanova A, Berrino F, Dossus L, Tjonneland A, Olsen A, Overvad TF, Overvad K, Clavel-Chapelon F, Fournier A, Fagherazzi G, Rohrmann S, Teucher B, Boeing H, Aleksandrova K, Trichopoulou A, Lagiou P, Trichopoulos D, Palli D, Sieri S, Panico S, Tumino R, Vineis P, Quiros JR, Buckland G, Sanchez MJ, Amiano P, Chirlaque MD, Ardanaz E, Sund M, Lenner P, Bueno-de-Mesquita B, van Gils CH, Peeters PH, Krum-Hansen S, Gram IT, Lund E, Khaw KT, Wareham N, Allen NE, Key TJ, Romieu I, Rinaldi S, Siddiq A, Cox D, Riboli E, Kaaks R. Adiposity, hormone replacement therapy use and breast cancer risk by age and hormone receptor status: a large prospective cohort study. Breast Cancer Res. 2012;14(3):R76. doi: 10.1186/bcr3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morimoto LM, White E, Chen Z, Chlebowski RT, Hays J, Kuller L, Lopez AM, Manson J, Margolis KL, Muti PC, Stefanick ML, McTiernan A. Obesity, body size, and risk of postmenopausal breast cancer: the Women's Health Initiative (United States) Cancer Causes Control. 2002;13(8):741–751. doi: 10.1023/a:1020239211145. [DOI] [PubMed] [Google Scholar]
- 25.Lahmann PH, Hoffmann K, Allen N, van Gils CH, Khaw KT, Tehard B, Berrino F, Tjonneland A, Bigaard J, Olsen A, Overvad K, Clavel-Chapelon F, Nagel G, Boeing H, Trichopoulos D, Economou G, Bellos G, Palli D, Tumino R, Panico S, Sacerdote C, Krogh V, Peeters PH, Bueno-de-Mesquita HB, Lund E, Ardanaz E, Amiano P, Pera G, Quiros JR, Martinez C, Tormo MJ, Wirfalt E, Berglund G, Hallmans G, Key TJ, Reeves G, Bingham S, Norat T, Biessy C, Kaaks R, Riboli E. Body size and breast cancer risk: findings from the European Prospective Investigation into Cancer And Nutrition (EPIC) Int J Cancer. 2004;111(5):762–771. doi: 10.1002/ijc.20315. [DOI] [PubMed] [Google Scholar]
- 26.Sonnenschein E, Toniolo P, Terry MB, Bruning PF, Kato I, Koenig KL, Shore RE. Body fat distribution and obesity in pre- and postmenopausal breast cancer. Int J Epidemiol. 1999;28(6):1026–1031. doi: 10.1093/ije/28.6.1026. [DOI] [PubMed] [Google Scholar]
- 27.Harvie M, Hooper L, Howell AH. Central obesity and breast cancer risk: a systematic review. Obes Rev. 2003;4(3):157–173. doi: 10.1046/j.1467-789x.2003.00108.x. [DOI] [PubMed] [Google Scholar]
- 28.Robinson WR, Tse CK, Olshan AF, Troester MA. Body size across the life course and risk of premenopausal and postmenopausal breast cancer in Black women, the Carolina Breast Cancer Study, 1993–2001. Cancer Causes & Control. 2014;25(9):1101–1117. doi: 10.1007/s10552-014-0411-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freeman EW, Sammel MD, Lin H, Gracia CR. Obesity and reproductive hormone levels in the transition to menopause. Menopause. 2010;17(4):718–726. doi: 10.1097/gme.0b013e3181cec85d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clarke CA, Keegan TH, Yang J, Press DJ, Kurian AW, Patel AH, Lacey JV., Jr Age-specific incidence of breast cancer subtypes: understanding the black-white crossover. J Natl Cancer Inst. 2012;104(14):1094–1101. doi: 10.1093/jnci/djs264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amadou A, Hainaut P, Romieu I. Role of obesity in the risk of breast cancer: lessons from anthropometry. J Oncol. 2013;2013:906495. doi: 10.1155/2013/906495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elobeid MA, Desmond RA, Thomas O, Keith SW, Allison DB. Waist circumference values are increasing beyond those expected from BMI increases. Obesity (Silver Spring) 2007;15(10):2380–2383. doi: 10.1038/oby.2007.282. [DOI] [PubMed] [Google Scholar]
- 33.Nettleton JA, Lutsey PL, Wang Y, Lima JA, Michos ED, Jacobs DR. Diet soda intake and risk of incident metabolic syndrome and type 2 diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes care. 2009;32(4):688–694. doi: 10.2337/dc08-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munsell MF, Sprague BL, Berry DA, Chisholm G, Trentham-Dietz A. Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol Rev. 2014;36(1):114–136. doi: 10.1093/epirev/mxt010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weinberg CR, Shore DL, Umbach DM, Sandler DP. Using risk-based sampling to enrich cohorts for endpoints, genes, and exposures. Am J Epidemiol. 2007;166(4):447–455. doi: 10.1093/aje/kwm097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.