Abstract
Background
Decision making experts emphasize that understanding and using probabilistic information is important for making informed decisions about medical treatments involving complex risk-benefit tradeoffs. Yet empirical research demonstrates that individuals may not use probabilities when making decisions.
Objectives
To explore decision making and the use of probabilities for decision making from the perspective of women who were risk-eligible to enroll in the Study of Tamoxifen and Raloxifene (STAR).
Methods
We conducted narrative interviews with 20 women who agreed to participate in STAR and 20 women who declined. The project was based on a narrative approach. Analysis included the development of summaries of each narrative, and thematic analysis with developing a coding scheme inductively to code all transcripts to identify emerging themes.
Results
Interviewees explained and embedded their STAR decisions within experiences encountered throughout their lives. Such lived experiences included but were not limited to breast cancer family history, personal history of breast biopsies, and experiences or assumptions about taking tamoxifen or medicines more generally.
Conclusions
Women’s explanations of their decisions about participating in a breast cancer chemoprevention trial were more complex than decision strategies that rely solely on a quantitative risk-benefit analysis of probabilities derived from populations In addition to precise risk information, clinicians and risk communicators should recognize the importance and legitimacy of lived experience in individual decision making.
Introduction
Understanding and using probabilistic information is critical for making informed decisions about medical treatments that involve complex risk-benefit tradeoffs (1, 2). For some medical therapies, including the management of breast cancer risk, medical decision making involves using individualized risk estimates to evaluate such trade-offs. The United States (US) Food and Drug Administration (FDA) has approved the prescribing of tamoxifen and raloxifene for primary breast cancer risk reduction. However, both medications have potential side effects such as cataracts, hot flashes, and endometrial cancer. Concern about side effects can lead people to avoid preventive treatment entirely (3, 4), even when they understand the quantitative risks and benefits involved (1, 5). This may explain why the use of tamoxifen and raloxifene in the US is considerably lower than the number of women who would likely benefit from taking the drugs (6, 7). Such findings suggest that individuals may interpret probabilities differently from epidemiologically-based risk-benefit analyses. Indeed, some evidence suggests that individuals may not use probabilistic information when it is provided (8), or that they may use it in non-normative ways (9). This may be because understanding probabilities is difficult, both for the public and health care providers (10-12). Another possible explanation is that women at increased risk of developing breast cancer and health professionals have different understandings of risk, with women talking about ‘feeling at risk’ which they relate to bodily signs and symptoms rather than population-derived probabilities (13). Women’s perceptions of chemoprevention also differed from those of scientists and policy makers (14). However, past research did not focus on how women given the option of chemoprevention used individualized risk information in explaining their treatment decisions (3, 4, 13, 15). Addressing this question may inform the development of novel patient decision support tools.
The Study of Tamoxifen and Raloxifene (STAR)
FDA approval of raloxifene was based on the STAR clinical trial, which compared the effectiveness and side effect profiles of tamoxifen and raloxifene for reducing the risk of a primary invasive breast cancer in postmenopausal women aged 35 or older (16-18). STAR was conducted by the National Surgical Adjuvant Breast and Bowel Project NCI cooperative group. Risk eligibility was based on an adjusted Gail score (19) of 1.7 percent over five years. Participants were recruited from nearly 200 clinical centers throughout the US and Canada. From 1999-2005, 184,460 women were screened by completing a risk assessment The resulting two-page report (Figure 1-2) included the adjusted Gail score, the risks and benefits of STAR participation, and the risks and benefits of taking tamoxifen and raloxifene. Of those screened, 96,368 were risk-eligible and 19,747 (approximately 20%) were enrolled (17).
Figure 1.
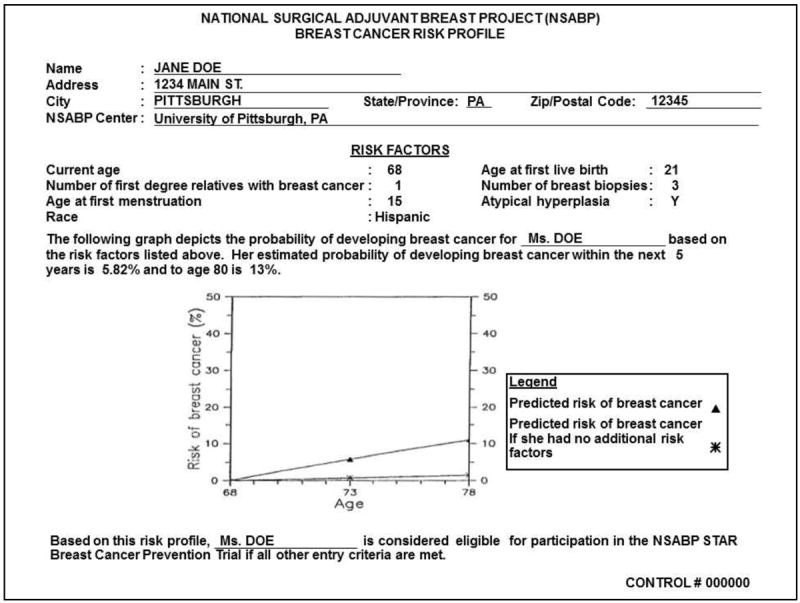
Example risk assessment result that potential STAR participants received
Figure 2.
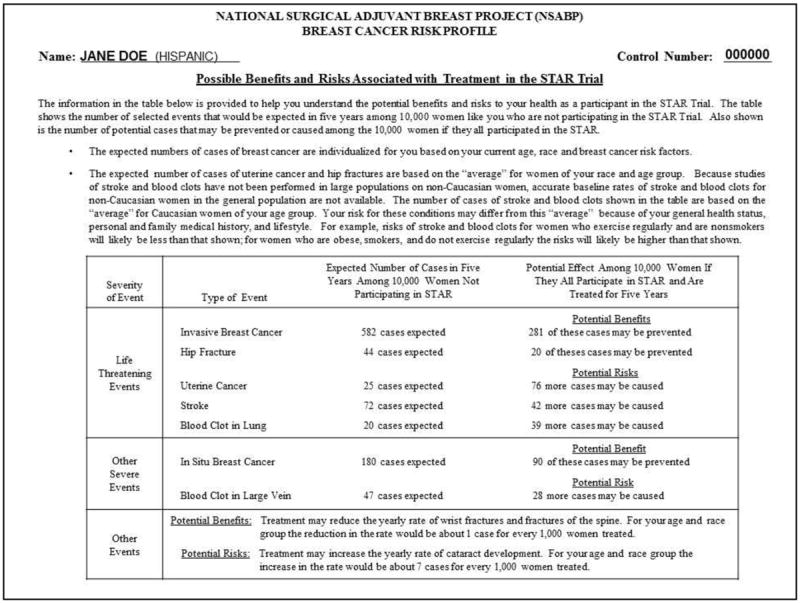
Risk/Benefit Information Sheet that potential STAR participants received
Theoretical Basis for the Research
Narrative theory asserts that people use narratives (stories) to make sense of events that occur (20). The way we tell a story provides insight into how we organize and create meaning in everyday life. Narratives reflect the meaning-making processes by reworking things that happen to us into tell-able stories (21). They describe past events, while making sense of them in the present. Through storytelling, narratives provide insight into how storytellers want others to view them. In this sense, narratives are always co-created by a particular situation, interviewer, and interviewee (22).
Study Purpose and Aim
The purpose of this study was to investigate the tension between the clinical importance of personalized risk information and patients’ use of that information when making medical decisions. The study aim was to examine how women considered at increased risk for breast cancer decided for or against participation in STAR, with a particular focus on the use or non-use of probabilistic information. Because both arms of the trial required taking a chemoprevention medication, a participant who decided to enroll in the trial also decided to accept chemopreventive therapy.
Methods
Study Design
We conducted a qualitative interview study using in-depth one-on-one narrative interviews (23, 24). Narrative interviewing is a type of qualitative interview intended to capture meaning-making about particular events from the interviewee’s point of view (22). Narrative interviewing is structured in such a way that the interviewer starts the conversation with a question related to the research aims that allows the interviewee to tell a story. This question is the same for every interview in order to ensure comparability across interviews (23). This question begins the conversation, but the flow of conversation is determined by the interviewee and the topics s/he introduces. As the narrative progresses, the interviewer responds to the interviewee’s statements in a way that is socially appropriate for conversation, but does not bias responses (e.g., “What happened next?”). After the narrative impulse is over and the interviewee has discussed all issues that come to mind, the interviewer inquires about topics that are relevant to the research question but were not raised by the interviewee.
Setting
We recruited participants from two STAR study sites. To reflect the diversity of potential STAR participants and of recruitment sites, we chose sites that had very different roles in the trial and different clientele. A Northeastern US site was chosen because it was involved in the overall organization of the trial and had a breast cancer risk assessment program. A Southern US site was chosen to increase sample diversity; it was part of efforts to improve minority participation in STAR and thus focused on recruiting African-American women (25). It did not have a high-risk program. Institutional review board approval was obtained from both clinics.
Sample
The inclusion criteria were being risk-eligible to participate in the STAR trial, speaking English, and receiving written risk information regarding STAR participation (Figures 1-2). Exclusion criteria included not being risk-eligible for STAR or not having received the risk information. Participation in the interviews was not contingent on agreement to participate in the clinical trial—both STAR participants and STAR decliners were included.
The written risk information consisted of two pages. The first page showed the Gail risk score (risk factor components, 5-year risk, lifetime risk) and was used to determine STAR risk eligibility. The risk information was communicated using absolute numeric estimates and a graph comparing the potential participant’s risk to the risk of the average woman. The second page used a table to summarize the possible benefits and risks associated with participating in the STAR trial, including the risks and benefits of taking tamoxifen and raloxifene (26).
Sampling approach
We invited risk-eligible women from the Southern and Northeastern clinics to be interviewed about their decision making regarding STAR participation. Recruitment into the interview study at the Southern site began in the winter of 2003-2004 and data collection was completed in the summer of 2004. Recruitment for the interview study at the Northeastern site began in spring 2004, and data collection was completed in May 2005. Due to the small number of STAR participants at the Southern site (n=5), narrative interviews were conducted with all of them. At the Northeastern site, the selection for interview partners was based on availability to schedule an interview. To interview STAR decliners, risk-eligible women who had not signed up for STAR were contacted either by the study coordinators at the sites or by CH, up to three times at different hours of the day. Among those who agreed to be interviewed, selection was then based on availability and scheduling of an interview (Table 1). Reasons given for STAR decliners to refuse an interview were time constraints, health issues, a general philosophy against participating in research, negative attitudes about chemoprevention, and concern about influence from the pharmaceutical industry.
Table 1.
Interview screening process: Total numbers by study site
| Northeastern clinic | Southern clinic | Sites combined | |
|---|---|---|---|
| STAR participants | 39 | 5 | 44 |
| STAR participants invited to participate in interview | 39 | 5 | 44 |
| STAR participants who agreed to interview | 39 | 5 | 44 |
| STAR participants interviewed | 15 | 5 | 20 |
| STAR decliners | 156 | 29 | 185 |
| STAR decliners invited to participate in interview | 90 | 23 | 113 |
| STAR decliners who agreed to interview | 58 | 12 | 70 |
| STAR decliners interviewed | 15 | 5 | 20 |
Sample size
The sample size (n=40) was based on other qualitative studies (15, 27), the study aim, and feasibility. To detect possible differences between STAR participants and decliners in how they invested meaning in STAR participation/declining and how they discussed the risk-benefit information, we included equal numbers from each group.
Data collection
All interviews were conducted at the respective clinical centers by CH. The interviews were conducted in person, were audio-recorded, lasted between 30 minutes and two hours, and were conducted in accordance with established narrative interviewing guidelines (23). Participants were asked, “You have done a risk assessment for your statistical risk of developing breast cancer. Would you please tell me about it?” An interview guide listed several topics that were of particular interest to the investigators (Table 2). If participants did not address these topics during the initial narrative, the interviewer probed for them at the end of the interview. Narrative interviews can vary considerably in length because the interviews are dependent on the story telling of the individual. Similar events can take on very different meanings for an individual’s life, which may influence the length of an interview. If the interviewer attempts to standardize the interviews by encouraging participants to make short stories longer, or longer stories shorter, it risks biasing the results (28). Descriptive data were reported by the interviewees.
Table 2.
Topics of interest and example probes
Introductory question (asked of all participants):
|
Key topics and example probes (asked only if the participant does not address the key topic first):
|
Data Analysis
A professional transcriber transcribed the audio recordings. CH verified accuracy by cross-checking the transcripts. In general, narratives can be analyzed from a linguistic perspective, structurally and/or thematically, person-by-person, and/or across narratives (22). The choice of appropriate analysis tools depends on the research question. To examine the way in which interviewees decided for or against participation in STAR, with a particular focus on the use or non-use of probabilistic information, the analysis included a person-by-person analysis conducted by CH and EW including a summary of decision making approaches for each interview (29) and a thematic analysis across interviews and of emerging themes from the first interview question. Transcripts were coded thematically by KW and CH (22). Codes were developed inductively and iteratively. KW and CH read the transcripts and identified all segments in which the women spoke about deciding about STAR. Then themes were identified in the segments and a preliminary codebook was developed. To identify whether the codebook captured all the themes discussed, this codebook was applied to the next five interviews. Next, all transcripts were coded using the finalized codebook. Coders conferred regularly to discuss discrepancies.
Coding of themes related to decision making were compared and categorized. This led to the identification of categories of decision making, which were then connected to anthropological and psychological theories and let to the development of four major themes. Data, categories, codes, and analysis memos were managed using MAXQDA software. To reflect the study design, analysis was conducted separately for STAR participants and decliners.
To ensure credibility of results, materials and results were regularly presented and discussed in research team meetings, in a qualitative research group at the Charité - Universitätsmedizin Berlin, and at the respective clinical centers. The data were also searched for contradictory cases. Confirmability was established by including investigators from multiple disciplines.
Results
Interviewees were between 44 and 74 years of age and all had at least a high school diploma. The majority of women (83%) were white (Table 3). All of the interviewees, except two decliners from the Southern site, either had a family history of breast cancer or had experienced one or multiple breast biopsies. Of the 40 interviews, eight were conducted within a year of completing the risk assessment, fourteen up to three years after, and 18 within three to five years after completion of the risk assessment. Two of the major themes that illustrated the way participants made their decision about STAR participation emerged from the narratives (Table 4). One related to their personal or family experiences with breast cancer, breast biopsies, and medication use. The second involved weighing the possible health effects that could result from taking a chemopreventive agent or medications in general. In women’s narratives, the probabilistic risk information did not prove to be of importance for decision making. Two additional major themes were developed as a result of the probe into the risk assessment information: “gist” representations of risk (30) and relevance of risk information for individual decision making. For an overview of categories that gave rise to the four major themes presented see Table 5.
Table 3.
Sociodemographic Characteristics and 5-Year Gail Score of Study Sample (N=40)
| STAR Participants n (%) | STAR Decliners n (%) | Total n (%) | |
|---|---|---|---|
| Race | |||
| African-American | 5 (25.0) | 2 (10.0) | 7 (17.5) |
| Hispanic | 0 (0.0) | 1 (5.0) | 1 (2.5) |
| White | 15 (75.0) | 17 (85.0) | 32 (80.0) |
| Education | |||
| High school diploma/GED | 4 (20.0) | 2 (10.0) | 6 (15.0) |
| Some college | 9 (45.0) | 5 (25.0) | 14 (35.0) |
| College graduate | 3 (15.0) | 4 (20.0) | 7 (17.5) |
| Some graduate school or more | 4 (20.0) | 8 (40.0) | 12 (30.0) |
| Missing data | 0 (0.0) | 1 (5.0) | 1 (2.5) |
|
| |||
|
Mean (Range)
|
|||
| Age | 54.6 (44-64) | 57.2 (48-74) | 56 (44-74) |
| GAIL Scorea | 3.12 (1.7-6.3) | 3.94 (1.7-13.7) | 3.53 (1.7-13.7) |
>= 1.67 was the cut-point for risk-eligibility for STAR. Mean Gail scores of STAR participants and decliners were not statistically significantly different, t(38) = 1.249, p=0.219.
Table 4.
Codebook for inductive thematic analysis by level of analysis
| Level of analysis | Categories | Subcategories | Codes | Subcodes |
|---|---|---|---|---|
| Answer to the opening question of the interview | ||||
| Not applicable (N/A) | N/A | Personal disease stories | N/A | |
| N/A | N/A | Family history of breast cancer | N/A | |
| N/A | N/A | Reflections on risk (reliability and nature of statistical information) | N/A | |
|
| ||||
| Across cases analysis | ||||
| Illness experience | N/A | Family history of breast cancer | Mother | |
| N/A | Sister | |||
| N/A | Other female relative | |||
| N/A | Friend | |||
| N/A | Mother death | |||
| N/A | Family history of other diseases | N/A | ||
|
| ||||
| Risk | N/A | Remembered about risk score | Number | |
| N/A | Gist (average, above average, below average) | |||
| N/A | Perception of breast cancer risk | High | ||
| N/A | Low | |||
| N/A | Reflection on risk (reliability and nature of statistical information) | Will get disease | ||
|
| ||||
| Medicines | N/A | Medication intake | Side effects | |
| N/A | Effects of medicine intake | Bodily effects | ||
| N/A | Psychological effects | |||
| N/A | Being pro-active | N/A | ||
|
| ||||
| HCP/Clinic – woman relationship | N/A | Counselling | Discussion of side effects | |
|
| ||||
| Person-by-person analysis | ||||
| Decision making | Deliberate decision | To have a choice | ||
| Weighing risks | Tamoxifen | |||
| Experiences with drugs in general | ||||
| Assumptions/Experiences with other implicated diseases | ||||
| Intuitive decision | Clear-cut case | N/A | ||
| Something decides for you | ||||
| Best option | ||||
| I have to | ||||
Table 5.
Categories in relationship to major themes
| Major themes | Categories |
|---|---|
| Personal experience | Illness experience |
| Medicines | |
| General situation | |
| Answer to first question | |
| HCP/Clinic-woman relationship | |
|
| |
| Weighing risks and benefits | Decision making |
| Risk | |
|
| |
| Evaluating the “gist” | Risk |
|
| |
| Relevance of risk information for personal decision making | Answer to the first question |
| Risk | |
| General situation | |
| Decision making | |
In general, STAR participants and decliners discussed the same themes and responded similarly to the probes about the probabilistic information. They were also similar in terms of the time lag between completion of the risk assessment and the interview. However, their narratives differed on the experiential level, e.g. how a family member experienced tamoxifen intake. In sum, providing information about the risks and benefits of STAR participation and breast cancer chemoprevention initiated a decision making process for or against STAR participation that was guided by personal experiences, attitudes, and beliefs. To reflect our sampling strategy and illustrate our findings, we present exemplars for each theme below.
Personal Experiences
The most prominent experiences related to a family history of breast cancer or medication intake, or a personal experience of breast biopsies. How they made sense of such events appeared to influence their thinking about STAR participation. For example, a STAR decliner had a sister who developed cognitive problems during tamoxifen treatment following a breast cancer diagnosis. This experience influenced her decision making profoundly.
“…then we got into the debate about tamoxifen. Well, first of all, (…) I was very frightened of because of the side effects and what a highly…I mean, it’s not a baby aspirin; it’s a complex drug. Did I want to pour more stuff in to cut risk, you know. It was like slicing away at risk and they said, “Oh, but this is like a 50 percent reduction.” Well, all I know is my sister said what bad side effects she had and she attributed it a lot to tamoxifen. It was enough to scare me to try and be very, very cautious. (…)
(P5, STAR decliner, 52 years old, Gail score 3.94)
A STAR participant who had a long family history of deaths from breast cancer situated her decision making within these experiences. This experience was the first thing she mentioned.
“What happened is I come from an extraordinarily long line of breast cancer victims. I say “victims” because they haven’t all been survivors, unfortunately. My maternal great-grandmother, my maternal grandmother, my mother and all three of her sisters died of breast cancer. Of the four girls—my mother and her three sisters—they produced four more girls; two of them have had breast cancer, including my sister. One had opted for a prophylactic double mastectomy and that leaves me.”
(P 27, STAR participant, 48 years old, Gail score 4.17)
Weighing the Risks and Benefits
Lived experiences in domains other than family history were implicated in the women’s decision making processes (Table 4). When weighing the risks and benefits of participation, women took the information that was given to them (Figures 1-2) and then situated it within their experiences, including assumptions and prior knowledge they had about these events. Women did not discuss “risk” as a numerical estimate of the likelihood of an event or as a weighing of probabilities. Risk was used in terms of “something that could happen.” These different possible events were not considered as standing on equal terms. Rather, they were evaluated according to assumptions about the event’s severity and which event would be experientially worse. Thus, the weighing of risks meant thinking about different possibilities and using feelings, sentiments, and lived experiences.
“And there were risks associated with the drugs. One of the risks was that you could get cataracts or you could get uterine cancer, and so my mind is saying to me, “Well, I might not get breast cancer but I might get uterine cancer. What good is that?” And actually breast cancer I think is a lot easier to detect a lot of times, especially when you’re getting mammogram on a regular basis, as I am. And then the other one was cataracts and I really didn’t want cataracts either.”
(STAR decliner, 58 years old, Gail score 5.34)
“I looked at what was told was the risks of taking tamoxifen, I looked at what my own known personal risk of developing the disease is, and I also looked at what the consequences of not doing anything would be, and the benefits—possible benefits—of participation, for me, so far outweighed any detrimental possible effects of possibly taking tamoxifen (…). (…) But you do not go into this stupid (…). They don’t sugar-coat it, so I knew and I was going to take whatever risks participation might present because in my mind they were less risky than just letting what I assumed to be the natural progression things go forward.”
(STAR participant, 48 years old, Gail score 4.7)
The above quotes illustrate the point that neither of the women evaluated the risks and benefits of treatment using a formal examination of probabilistic information. Rather, they discussed events that could happen and placed them in the context of their lived experiences. Despite similar approaches to making this decision, they arrived at very different conclusions about whether or not to undergo treatment.
Evaluating the “Gist”
In general, most women did not mention the risk estimates that they were provided with until the interviewer probed specifically for this information. In response, some of the participants distilled the complex probabilistic information into its bottom-line qualitative meaning, or “gist” (30), as illustrated in the examples below. Women then evaluated these gist representations by placing them in the context of what they knew from their personal experiences. Finally, they drew an overarching conclusion about the risk numbers that also took the form of gist representations. Ultimately, the women indicated that their decisions were based less on the risk information provided than on what they already knew about themselves, their experiences, and the world as they perceived it.
The following participant seemed to partially remember the probability information. Nevertheless, she uses her family’s experiences with cancer to draw a conclusion about what to do.
“Like, two to three percent above and then by age (…) no, no, it was like two to three percent was the risk of getting breast cancer and then it was a ten percent risk by age 80; I can’t remember if that was cumulative or how they phrased it. (…) the numbers were definitelynot high enough to make me think that I would gain anything from participating in STAR. (…) So (…) and on my father’s side of the family, even though he had colon cancer, which was not what he died of, and his sister didn’t have breast cancer, my cousins have not had breast cancer. So, you know, I’d like to think that maybe I’m not really as much at risk as the paperwork said I was.”
(P17, STAR decliner, 56 years old, Gail score 2.54)
“No I don’t [remember the actual number], but I do remember (…) it was just slightly above. Because (…) my sister hasn’t had breast cancer but I have cousins that have had breast cancer. I had one cousin that died of breast cancer and I just don’t want to (…). To me, I felt like, you know, that it’s in my family so I did feel, you know, more at risk personally. I don’t care what the number said, to be honest, you know. [laughs] (…) Actually, statistically I wasn’t all that high.”
(P26, STAR participant, 48 years old, Gail score 1.74)
These are exemplars of the typical way the interviewees spoke about the risk information, which suggests that that they did not use it for personal decision making.
Relevance of Risk Information for Individual Decision Making
One way for the women to talk about the risk results was to situate them within their own experiences. Another was to talk about the gist information in relation to the nature of probabilistic information and statistics, and the extent to which population-based risk modeling is a legitimate way to provide information to individuals. The women’s comments reflect the idea that, on the individual level, probabilistic information is binary: you either do or do not develop cancer. For this reason, the probabilistic information was not considered an important ground to base a decision on, especially if it did not overlap with their own assumptions regarding their risk.
“I believe it was average and then I don’t know how they came up with it being slightly higher. Was I surprised? (…) I was surprised that at first (…) yeah, I was surprised that I was at average risk; I thought I’d be higher. In fact, I still thought I’d be higher, yet because she’s [her mother] postmenopausal they explained that the risk wouldn’t be that high (…). But these are just statistics and I can’t really go by statistics, you know? All because it says I may not be, you know, at average risk or you’re a little higher than average risk; that doesn’t mean anything. You know, your body has its own mind what’s going to happen to it, so I can’t really say I really trust the number that they gave me, you know?”
(P19, STAR decliner, 48 years old, Gail score 1.79)
“Well, I had already suspected (…) I mean, it wasn’t a surprise because I knew from my family history already that I was at a high risk. (…) So it just validated what I thought. (…) It [the risk assessment] didn’t seem to mean much to me because it was hard to translate it into what actually (…) I guess it really didn’t (…) you know, you just know you’re at a higher risk, you don’t really know. It can’t tell you if you’re going to get it, cancer or not, so (…).”
(P29, STAR participant, 52 years old, Gail score 4.01)
Both women expressed difficulty in translating the probabilistic information into a useable form, in large part due to the inherent uncertainty about whether they, personally, would develop breast cancer. However, as demonstrated above, women came to very different conclusions about whether or not to participate in STAR, despite common stories about how they arrived at their decisions.
Discussion
Oftentimes, the goal of numerical risk communication in medical settings is to guide individuals’ risk perceptions to reflect the numerical risk calculations and thus make appropriate health decisions when there are complex risk tradeoffs (31). However, the way in which the interviewees discussed their STAR participation decisions were far richer and more complex than a straightforward evaluation of probabilistic information. The decision process was triggered by an information session that included clinical and probabilistic information, but the actual decision was embedded in their life histories and lived experiences, which were used to guide, explain, and make sense of their decisions.
The narratives indicate that probabilistic information was generally not used to rationalize decision making. Risk information, either as gist representations or as probabilities, were not used in telling the story of their STAR participation/declining. However, none of the women were unaware of their high-risk status. Rather, they were aware of the possibility of developing breast cancer due in large part to a long history of lived experiences.
This is consistent with what the affect heuristic would predict (32). Each personal or family experience with breast cancer and/or chemopreventive medication likely generated a subjective “affective tag.” The more experiences the women lived through, and the more affectively-laden the experiences were, the more powerful the affective tags became (33). Thus, the differences between STAR participants and decliners lay not in the different events that they experienced but in how they experienced them, and in their beliefs, feelings, and assumptions about breast cancer, risk, and medication intake.
The interviews also reflect the tendency for decision makers to reduce complex information to basic gist representations (30). Gist representations facilitate the translation of probability estimates into personally relevant information, which can be used when making decisions (1). However, the narratives indicate that although women formulated gist representations of the probability information, they evaluated their overall personal risk using a more comprehensive approach. It was not the gist representations of the probabilistic information that the women based their decisions on, but rather their lived experiences of breast cancer and medication intake. Indeed, the gist representations and probabilistic information were devalued and dismissed in individual decision making if they did not conform with interviewees’ experiences and assumptions.
The women evaluated the risk information provided to them in light of what they knew about their situation. Sometimes, such a patient perspective is portrayed as in need of explanation or correction (34). Such a view neglects the fact that probabilistic risk information is itself a value-laden construct (35). For example, decisions about what information is included in epidemiological datasets are strongly influenced by cultural and social factors (36). Personalized health risk assessment tools may be especially problematic, as they are not an objective measure of an individual but of a population fraction (37). In this context, women’s skepticism of the probabilistic information could be considered reasonable. It has been argued that patients may lack an understanding of epidemiological risk knowledge, which hinders its use in decision making (38). Most of the women in our study had been in risk counseling for some time. Consequently, one may argue that they understood their risk. However, for their personal decision making, they favored their own knowledge and assumptions about breast cancer risk. Considering the real limitations of risk prediction algorithms (39, 40), this may be a reasonable distinction to make.
Nevertheless, the women did weigh the risks and benefits of trial participation. They simply adopted a more comprehensive and personally meaningful approach to defining risks and benefits than calculating probabilities. The importance of lived experiences for STAR decision making is consistent with other research that indicates how health behaviors and decision making are situated within complex social contexts. For example, “daily-lived experiences” were found to profoundly influence hypertension self-management (41). Using lived experiences to make clinical trial participation decisions meaningful similarly demonstrates that health decision making is highly contextualized and embedded within women’s overall experiences. These results strengthen the argument that it is important to ensure that preference-based decisions, such as the decision to undergo breast cancer chemoprevention, are consistent with patients’ preferences and values (42).
Limitation and Future Directions
Participants were recruited for STAR participation between 1999 and 2004; thus in some cases there was up to 5 years between completing the STAR risk assessment screening/recruitment and the time of interview. This might have introduced discrepancies between how they would have explained their decision at the time of STAR recruitment and at the time of interview. However, interviews conducted within a year of STAR recruitment did not show different patterns in the discussion of probabilities than interviews conducted later.
Because the purpose of analyzing the narratives was to learn how interviewees want us to understand their decision making (22), the particular circumstances of how a story is told years later shows what is important to the narrator. So indeed, this may be one reason why the interviewees did not focus on the risk probabilities in their narratives.
Another consideration is that the decision was undertaken in the context of a clinical trial. Women making chemoprevention decisions in a regular clinical setting might report different experiences. However, the eligibility criteria for trial entry and the criteria for chemoprevention in the clinical setting are similar, which suggests that women in the clinical setting may have similar lived experiences. Nevertheless, research should verify the extent to which women in modern clinical settings do or do not report making decisions in terms similar to those described here. The recent recommendation by the United States Preventive Services Task Force that clinicians engage in shared decision making with high-risk women about chemoprevention may alter the decision landscape (43). Furthermore, since more than 93% of STAR participants were white (17), additional research should investigate chemoprevention decision making among racial and ethnic minorities and women with limited formal education and/or income.
Conclusion
These women’s stories stand in the context of a tension between an objective probabilistic approach to chemoprevention decisions (44-48) and an approach that allows for lived experiences and intuitive information processing (1, 30, 49, 50). They demonstrate that it would be naïve to expect women to make decisions based solely on numerical risk-benefit tables, since probabilities depict only one aspect of a reality that might or might not occur. If clinicians and risk communicators are to facilitate informed decision making in the context of chemoprevention of primary breast tumors, they need to recognize the importance and legitimacy of lived experience in decision making, in addition to precise personalized risk information.
Acknowledgments
We want to particularly thank Joan James for her continued support for the study, her input, and her hospitality. Without her this study would not have been possible.
Funding: This research was supported by funding from the National Cancer Institute Cancer Prevention Fellowship Program (Holmberg, Waters) and by the Barnes-Jewish Hospital Foundation (Waters). The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report.
References
- 1.Fagerlin A, Zikmund-Fisher BJ, Nair V, Derry HA, McClure JB, Greene S, et al. Women’s decisions regarding tamoxifen for breast cancer prevention: responses to a tailored decision aid. Breast Cancer Res Treat. 2010 Feb;119(3):613–20. doi: 10.1007/s10549-009-0618-4. Epub 2009/11/13. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zikmund-Fisher BJ. The right tool is what they need, not what we have: a taxonomy of appropriate levels of precision in patient risk communication. Medical care research and review : MCRR. 2013 Feb;70(1 Suppl):37S–49S. doi: 10.1177/1077558712458541. [DOI] [PubMed] [Google Scholar]
- 3.Melnikow J, Paterniti D, Azari R, Kuenneth C, Birch S, Kuppermann M, et al. Preferences of Women Evaluating Risks of Tamoxifen (POWER) study of preferences for tamoxifen for breast cancer risk reduction. Cancer. 2005;103(10):1996–2005. doi: 10.1002/cncr.20981. [DOI] [PubMed] [Google Scholar]
- 4.Heisey R, Pimlott N, Clemons M, Cummings S, Drummond N. Women’s views on chemoprevention of breast cancer: qualitative study. Can Fam Physician. 2006 May;52:624–5. [PMC free article] [PubMed] [Google Scholar]
- 5.Waters EA, Weinstein ND, Colditz GA, Emmons K. Aversion to side effects in preventive medical treatment decisions. British Journal of Health Psychology. 2007;12:383–401. doi: 10.1348/135910706X115209. [DOI] [PubMed] [Google Scholar]
- 6.Waters EA, Cronin KA, Graubard BI, Han PK, Freedman AN. Prevalence of tamoxifen use for breast cancer chemoprevention among U.S. women. Cancer Epidemiology, Biomarkers, & Prevention. 2010;19(2):443–6. doi: 10.1158/1055-9965.EPI-09-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waters EA, McNeel TS, Stevens WM, Freedman AN. Use of tamoxifen and raloxifene for breast cancer chemoprevention in 2010. Breast Cancer Res Treat. 2012 May 24; doi: 10.1007/s10549-012-2089-2. Epub 2012/05/25. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hogarth R, Kunreuther H. Decision making under ignorance: Arguing with yourself. Journal of Risk and Uncertainty. 1995;10(1):15–36. [Google Scholar]
- 9.Pachur T, Galesic M. Strategy Selection in Risky Choice: The Impact of Numeracy, Affect, and Cross-Cultural Differences. Journal of Behavioral Decision Making. 2013;26(3):260–71. [Google Scholar]
- 10.Gigerenzer G. HIV screening: helping clinicians make sense of test results to patients. BMJ. 2013;347 doi: 10.1136/bmj.f5151. [DOI] [PubMed] [Google Scholar]
- 11.Wegwarth O, Schwartz LM, Woloshin S, Gaissmaier W, Gigerenzer G. Do physicians understand cancer screening statistics? A national survey of primary care physicians in the United States. Annals of internal medicine. 2012;156(5):340–9. doi: 10.7326/0003-4819-156-5-201203060-00005. [DOI] [PubMed] [Google Scholar]
- 12.Galesic M, Garcia-Retamero R. Statistical numeracy for health: a cross-cultural comparison with probabilistic national samples. Arch Intern Med. 2010 Mar 8;170(5):462–8. doi: 10.1001/archinternmed.2009.481. Epub 2010/03/10. eng. [DOI] [PubMed] [Google Scholar]
- 13.Salant T, Ganschow PS, Olopade OI, Lauderdale DS. “Why take it if you don’t have anything?” breast cancer risk perceptions and prevention choices at a public hospital. Journal of general internal medicine. 2006;21(7):779–85. doi: 10.1111/j.1525-1497.2006.00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paterniti DA, Melnikow J, Nuovo J, Henderson S, DeGregorio M, Kuppermann M, et al. “I’m going to die of something anyway”: women’s perceptions of tamoxifen for breast cancer risk reduction. Ethn Dis. 2005 Summer;15(3):365–72. [PubMed] [Google Scholar]
- 15.Altschuler A, Somkin CP. Women’s decision making about whether or not to use breast cancer chemoprevention. Women & health. 2005;41(2):81–95. doi: 10.1300/J013v41n02_06. [DOI] [PubMed] [Google Scholar]
- 16.Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Wolmark N. The study of tamoxifen and raloxifene: preliminary enrollment data from a randomized breast cancer risk reduction trial. Clinical Breast Cancer. 2002 Jun;3(2):153–9. doi: 10.3816/CBC.2002.n.020. [DOI] [PubMed] [Google Scholar]
- 17.Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. Jama. 2006;295(23):2727–41. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 18.Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: Preventing Breast Cancer. Cancer Prevention Research. 2010 Jun 1;3(6):696–706. doi: 10.1158/1940-6207.CAPR-10-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gail MH, Costantino JP, Pee D, Bondy M, Newman L, Selvan M, et al. Projecting individualized absolute invasive breast cancer risk in African American women. Journal of the National Cancer Institute. 2007 Dec 5;99(23):1782–92. doi: 10.1093/jnci/djm223. [DOI] [PubMed] [Google Scholar]
- 20.Ricoeur P. Narrative Time. In: Mitchel WJT, editor. On narrative. Chicago: 1981. pp. 165–186. [Google Scholar]
- 21.Good B. Medicine, rationality and experience: an anthropological perspective. Cambridge University Press; 1994. [Google Scholar]
- 22.Riessman CK. Narrative methods for the human sciences. Los Angeles: Sage; 2008. [Google Scholar]
- 23.Honer A. Lebensweltliche Ethnographie: ein explorativ-interpretativer Forschungsansatz am Beispiel von Heimwerker-Wissen. Wiesbaden: Deutscher Universitäts-Verlag; 1993. [Google Scholar]
- 24.Schütze F. Biographieforschung und narratives Interview. Neue Praxis. 1983;13(3):183–293. [Google Scholar]
- 25.McCaskill-Stevens W, Wilson JW, Cook ED, Edwards CL, Gibson RV, McElwain DL, et al. National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene trial: advancing the science of recruitment and breast cancer risk assessment in minority communities. Clinical trials (London, England) 2013 Apr;10(2):280–91. doi: 10.1177/1740774512470315. Epub 2013/01/22. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz LM, Woloshin S. The Drug Facts Box: Improving the communication of prescription drug information. Proceedings of the National Academy of Sciences of the United States of America. 2013 Aug 20;110(Suppl 3):14069–74. doi: 10.1073/pnas.1214646110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ziebland S, Herxheimer A. How patients’ experiences contribute to decision making: illustrations from DIPEx (personal experiences of health and illness) Journal of nursing management. 2008 May;16(4):433–9. doi: 10.1111/j.1365-2834.2008.00863.x. Epub 2008/04/15. eng. [DOI] [PubMed] [Google Scholar]
- 28.Riessman CK. Divorce Talk: Women and Men Make Sense of Personal Relationships. Rutgers University Press; 1990. [Google Scholar]
- 29.Miles MB, Huberman AM. Qualitative data analysis : an expanded sourcebook. 2. XIV. Thousand Oaks, Calif. [u.a.]: Sage; 1994. p. 338 S. Nachdr. [Google Scholar]
- 30.Reyna VF. A theory of medical decision making and health: fuzzy trace theory. Med Decis Making. 2008 Nov-Dec;28(6):850–65. doi: 10.1177/0272989X08327066. Epub 2008/11/19. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edwards AG, Naik G, Ahmed H, Elwyn GJ, Pickles T, Hood K, et al. Personalised risk communication for informed decision making about taking screening tests. The Cochrane database of systematic reviews. 2013;2 doi: 10.1002/14651858.CD001865.pub3. CD001865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finucane ML, Alhakami A, Slovic P, Johnson SM. The affect heuristic in judgments of risks and benefits. Journal of Behavioral Decision Making. 2000;13(1):1–17. [Google Scholar]
- 33.Damásio AR. Descartes’ error: emotion, reason, and the human brain: Quill. 1994 [Google Scholar]
- 34.Linnenbringer E, Roberts JS, Hiraki S, Cupples LA, Green RC. “I know what you told me, but this is what I think:” perceived risk of Alzheimer disease among individuals who accurately recall their genetics-based risk estimate. Genetics in medicine : official journal of the American College of Medical Genetics. 2010 Apr;12(4):219–27. doi: 10.1097/GIM.0b013e3181cef9e1. Epub 2010/02/09. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holmberg C, Bischof C, Bauer S. Making Predictions: Computing Populations. Science, Technology & Human Values. 2013 May 1;38(3):398–420. [Google Scholar]
- 36.Aronowitz R. Framing disease: an underappreciated mechanism for the social patterning of health. Soc Sci Med. 2008;67(1):1–9. doi: 10.1016/j.socscimed.2008.02.017. Epub 2008 Apr 1. [DOI] [PubMed] [Google Scholar]
- 37.Holmberg C, Parascandola M. Individualised risk estimation and the nature of prevention. Health, Risk & Society. 2010 Oct 01;12(5):441–52. [Google Scholar]
- 38.Han PK, H N, Neilson M, Roy B, Kungel T, Gutheil C, Diefenbach M, Hansen M. The value of personalised risk information: a qualitative study of the perceptions of patients with prostate cancer. BMJ Open. 2013;3(9) doi: 10.1136/bmjopen-2013-003226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rockhill B. Theorizing about causes at the individual level while estimating effects at the population level: implications for prevention. Epidemiology. 2005 Jan;16(1):124–9. doi: 10.1097/01.ede.0000147111.46244.41. [DOI] [PubMed] [Google Scholar]
- 40.Rockhill B. The privatization of risk. American journal of public health. 2001 Mar;91(3):365–8. doi: 10.2105/ajph.91.3.365. Epub 2001/03/10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bokhour BG, Cohn ES, Cortes DE, Solomon JL, Fix GM, Elwy AR, et al. The role of patients’ explanatory models and daily-lived experience in hypertension self-management. J Gen Intern Med. 2012 Dec;27(12):1626–34. doi: 10.1007/s11606-012-2141-2. Epub 2012/07/24. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elwyn G, Edwards A, Kinnersley P. Shared decision-making in primary care: the neglected second half of the consultation. The British journal of general practice : the journal of the Royal College of General Practitioners. 1999 Jun;49(443):477–82. Epub 1999/11/24. eng. [PMC free article] [PubMed] [Google Scholar]
- 43.Force USPST. [2014 Mar 22 2014];Medications for Risk Reduction of Primary Breast Cancer in Women. Topic Page Available from: http://www.uspreventiveservicestaskforce.org/uspstf/uspsbrpv.htm.
- 44.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. Journal of the National Cancer Institute. 1998;90(18):1371–88. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 45.Freedman AN, Graubard BI, Rao SR, McCaskill-Stevens W, Ballard-Barbash R, Gail MH. Estimates of the number of US women who could benefit from tamoxifen for breast cancer chemoprevention. Journal of the National Cancer Institute. 2003;95(7):526–32. doi: 10.1093/jnci/95.7.526. [DOI] [PubMed] [Google Scholar]
- 46.Freedman AN, Graubard BI, Rao SR, McCaskill-Stevens W, Ballard-Barbash R, Gail MH. How many US women are eligible to use tamoxifen for breast cancer chemoprevention? How many women would benefit. The American Journal of Oncology Review. 2004;3:47. [Google Scholar]
- 47.Freedman AN, Yu B, Gail MH, Costantino JP, Graubard BI, Vogel VG, et al. Benefit/Risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older. J Clin Oncol. 2011 Jun 10;29(17):2327–33. doi: 10.1200/JCO.2010.33.0258. Epub 2011/05/04. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Port ER, Montgomery LL, Heerdt AS, Borgen PI. Patient reluctance toward tamoxifen use for breast cancer primary prevention. Annals of Surgical Oncology. 2001;8(7):580–5. doi: 10.1007/s10434-001-0580-9. [DOI] [PubMed] [Google Scholar]
- 49.Fagerlin A, Zikmund-Fisher BJ, Ubel PA. “If I’m better than average, then I’m ok?”: Comparative information influences beliefs about risk and benefits. Patient education and counseling. 2007 Dec;69(1-3):140–4. doi: 10.1016/j.pec.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ropka ME, Keim J, Philbrick JT. Patient decisions about breast cancer chemoprevention: a systematic review and meta-analysis. J Clin Oncol. 2010 Jun 20;28(18):3090–5. doi: 10.1200/JCO.2009.27.8077. Epub 2010/05/12. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]


