Abstract
Background
Neurological deficits of alcohol use disorder (AUD) have been attributed to dysfunctions of specific brain structures. Studies of alcoholic patients and chronic alcohol exposure animal models consistently identify reduced hippocampal mass and cogntive dysfunctions as a key alcohol-induced brain adaptation. However, the precise substrate of chronic alcohol exposure that leads to structural and functional impairments of the hippocampus is largely unknown.
Methods
Using a calorie-matched alcohol feeding method, we tested whether chronic alcohol exposure targets neural stem cells and neurogenesis in the adult hippocampus. The effect of alcohol on proliferation of neural stem cells as well as cell fate determination and survival of newborn cells was evaluated via BrdU pulse and chase methods. A retrovirus-mediated single-cell labeling method was used to determine the effect of alcohol on the morphological development and circuitry incorporation of individual hippocampal newborn neurons. Finally, Novel Object Recognition and Y-maze tests were performed to examine whether disrupted neurogenesis is associated with hippocampus-dependent functional deficits in alcohol-fed mice.
Results
Chronic alcohol exposure reduced proliferation of neural stem cells and survival rate of newborn neurons; however, the fate determination of newborn cells remained unaltered. Moreover, the dendritic spine density of newborn neurons significantly decreased in alcohol-fed mice. Impaired spine formation indicates that alcohol interfered the synaptic connectivity of newborn neurons with excitatory neurons originating from a various areas of the brain. In the Novel Object Recognition test, alcohol-fed mice displayed deficits in the ability to discriminate the novel object.
Conclusions
Our study revealed that chronic alcohol exposure disrupted multiple steps of neurogenesis, including the production and development of newborn neurons. In addition, chronic alcohol exposure altered connectivity of newborn neurons with other input neurons. Decreased neurogenesis and aberrant integration of newborn neurons into hippocampal networks are closely associated with deficits in hippocampus-dependent cognitive functions of alcohol-fed mice.
Keywords: alcohol, neurogenesis, newborn neurons, dendritic spines, recognition memory
Introduction
Although the adult mammalian brains was once thought to be a fixed structure with no generation of new neurons, a recent discovery of adult hippocampal neurogenesis provided a new paradigm that the hippocampus is a plastic brain structure that dynamically develops during childhood and throughout adulthood (Gage, 2000, Suh et al., 2009). In particular, neural stem cells reside in the dentate gyrus of the hippocampus and continuously produce newborn neurons referred to as dentate granule cells (DGCs). Adult hippocampal neurogenesis is a multi-step process consisting of proliferation of neural stem cells, survival and differentiation of newborn cells, and functional integration of newborn neurons into hippocampal circuitry, and each step contributes to the structural and functional plasticity of the hippocampus (Eisch and Harburg, 2006). This hippocampal plasticity plays a key role in learning and memory, mood control, response to stress, social behavior, and addiction (Eisch and Harburg, 2006, Suh et al., 2009). Consequently, disregulated neurogenesis is commonly associated with cognitive, emotional, and addictive behavioral deficits found in many neurological and neuropsychiatric disorders, such as alcohol use disorders (AUD) (Nixon et al., 2010).
AUDs are characterized by uncontrollable consumption of excessive amount of alcohol (alcohol abuse) and continued addiction to alcohol physically and mentally (alcohol dependence). According to recent National Survey on Drug Use and Health (SAMHSA, 2013), approximately 17 million American adults meet the medical criteria for AUD. Unfortunately, about 700,000 adolescents aged 12 to 17 were diagnosed with AUD in 2013. The higher rate of AUD incidence among adolescents is a major public health issue because alcohol use during adolescence is a critical risk factor for AUD development at older ages, a binge drinking pattern is prevalent in adolescence, and amount of alcohol use during adolescence has been correlated with the severity of neuropsychological and neuropathological deficits found in adult stages (Clark et al., 2008, Grant et al., 2004).
Alcohol is a promiscuous drug that impairs various brain structures and functions (Geil et al., 2014). In particular, AUD is almost always associated with cognitive deficits in spatial learning and memory, declarative memory, and decision-making (Parsons, 1983, Sullivan and Pfefferbaum, 2005). This pattern strongly suggests that excessive alcohol consumption may negatively impact the structure and function of the hippocampus (Sullivan and Pfefferbaum, 2005, White and Swartzwelder, 2004). Indeed, studies of AUD patients as well as animal AUD models consistently identified the prefrontal cortex (PFC), entorhinal cortex, and hippocampus as the brain areas that are damaged by use of excessive alcohol (Crews et al., 2000, Obernier et al., 2002, Sullivan et al., 1995, Crews et al., 2005, De Bellis et al., 2000, Sullivan and Pfefferbaum, 2005). Structural impairments in the entorhinal cortex and hippocampus are very intriguing because these two structures are reciprocally connected (Amaral et al., 2007, Vivar et al., 2012).
Decreased neurogenesis and neuronal loss play a key role in alcohol-induced hippocampal adaptation (Morris et al., 2010, Broadwater et al., 2014, Taffe et al., 2010). In rat models of binge alcohol exposure, alcohol increased cell death and reduced hippocampal neurogenesis in the dentate gyrus of both adults and adolescents (Obernier et al., 2002, Nixon and Crews, 2002, Morris et al., 2010). However, the rate of decreased neurogenesis surpassed the rate of cell death, indicating that alcohol-induced neurogenesis may be the primary mechanism that causes the structural impairment of the dentate gyrus of the hippocampus (Morris et al., 2010, Nixon and McClain, 2010). Concomitantly with structural impairments, a variety of rodent AUD models showed that use of excessive alcohol affected the hippocampus-dependent functions such as spatial learning and memory, and contextual fear conditioning (Sircar et al., 2009, Schulteis et al., 2008).
Although the role of excessive alcohol exposure in hippocampal neurogenesis has been extensively studied in a rat binge model (Nixon and Crews, 2002, Morris et al., 2010), the effects of chronic alcohol exposure on neurogenesis have been studied in limited studies and conflicting results of chronic alcohol-induced neurogenesis have been reported (Pawlak et al., 2002, Rice et al., 2004, He et al., 2005). In this report, we investigated the effect of chronic alcohol exposure on structure and function of the hippocampus in order to make a direct association between alcohol-induced neurogenesis and cognition. In our chronic alcohol exposure model, mice were exposed to alcohol at adolescent age (postnatal day (PND) 42) and continued to consume alcohol during emerging adulthood (PND 70 to 77). Using a pair-fed mouse model in which both control and alcohol groups have an equivalent caloric intake over one month (He et al., 2005), we showed that chronic alcohol exposure decreased levels of hippocampal neurogenesis. Multiple steps of neurogenesis, including the production and survival of newborn cells, and the development and circuitry incorporation of newborn neurons, were impaired. In particular, the dendritic spine density of newborn DGCs was significantly reduced in alcohol-fed mice. In parallel to disregulated neurogenesis, chronic alcohol exposure also impaired recognition memory, a hippocampus-dependent function. Our results suggest that decreased neurogenesis and aberrant incorporation of newborn neurons results in long-lasting structural changes, which in turn leads to deficits in the hippocampus-dependent function in alcohol-fed mice. Thus, our study provides a critical insight into hippocampal neurogenesis as a critical neural substrate for chronic alcohol exposure, which may underlie the psychiatric features of alcohol use disorder.
Materials and Methods
Subjects
All animal procedures were approved by the Cleveland Clinic Institutional Animal Care and Use Committee. Five-week-old C57BL/6 female mice were purchased from The Jackson Laboratory (Bar Harbor, Maine) and housed as pairs in a temperature- and humidity-controlled environment with an alternating 12 hour light and 12 hour dark cycle.
Blood alcohol
Blood alcohol contents (BACs) were measured 1, 3 or 5 weeks after onset of alcohol feeding. Three hours after the onset of the dark cycle, tail blood samples were collected in heparinized capillary tubes (Fisher, Waltham, MA), and centrifuged to separate plasma. BACs were measured following instructions of Ethanol L3K kit (Equal Diagnostics, Lexington, MA).
Animal Behavior tests
All animal behavior tests were performed in the Rodent Behavioral Core of The Cleveland Clinic. Eleven 5-week-old female mice (C57BL/6) were used per control and alcohol groups for behavioral analyses. Alcohol and pair-fed control mice consumed alcohol- and isocaloric-diet over 4 weeks. Then, normal solid laboratory chow was given to both control and alcohol-fed mice. Three days later, mice were subject to behavioral test in the order of: 1-day rotarod test, 3-day novel object recognition (NOR) test, and 1-day Y-maze test.
Rotarod
Sensorimotor functioning was tested using an accelerating rotarod (Rotamex-V, Columbus Instruments, Columbus, OH). Rotating rods (3 cm in diameter) were placed 46cm above a bedded platform. During the acclimation phase, the rotarod ran for 30 seconds at 4 rounds per minute (RPM). During 5 minutes of the trial phase, the rotarod accelerate from 4 to 40 RPM. Latency to fall was recorded by the photobeam sensors (0.1 second resolution). Mice completed three trials with 5 minutes inter-trial intervals.
Novel object recognition
A three-day protocol consisting of an open-arena habituation phase, training phase, and test phase was used. During the habituation phase (Day 1), the mouse was placed in an open arena (60cm × 50cm × 40cm) without any objects and allowed to explore for 10 minutes. During the training phase (Day 2), the mouse was exposed to two identical objects that were placed in the center of the container for 10 minutes. During the test phase (Day 3), a novel object replaced one of the familiar objects from the previous day, and the mouse was allowed to explore for 10 minutes. In each trial, both the arena and objects were thoroughly cleaned using 70% ethanol. Speed, distance, and time spent with each object were recorded using a video-assisted tracking system (Ethovision XT; Noldus Information Technology, Netherland).
Y-maze
The mouse was placed in the center of an opaque apparatus with three equal length arms (21 cm length × 8.5 cm width × 40 cm height) positioned at 120° from each other the apparatus, and was allowed to freely explore the three arms for 5 minutes. Speed, distance, and time spent in each arm were recorded using a video-assisted tracking system (Ethovision XT; Noldus Information Technology, Netherland). Spontaneous alteration was scored when the mouse visited all three arms without going into the same arm twice in a row. The apparatus was thoroughly cleaned using 70% ethanol between trials.
Statistical analysis
In all cases, analyses were conducted in Prism (GraphPad Prism, San Diego, CA), are reported as mean ± standard error of the mean (SEM) with differences considered significant at P < 0.05. In all data presentation, symbols were used to represent different confidence levels. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001.
Supporting Information
Bromodeoxyuridine (BrdU) labeling, tissue preparation, immunohistochemistry, quantification, retrovirus production and injection and dendritic analysis have previously reported (Suh et al., 2007, Zhao et al., 2006). Details regarding these methods as well as alcohol feeding paradigm are described in the supporting information.
Results
A pair-fed chronic alcohol exposure mouse model
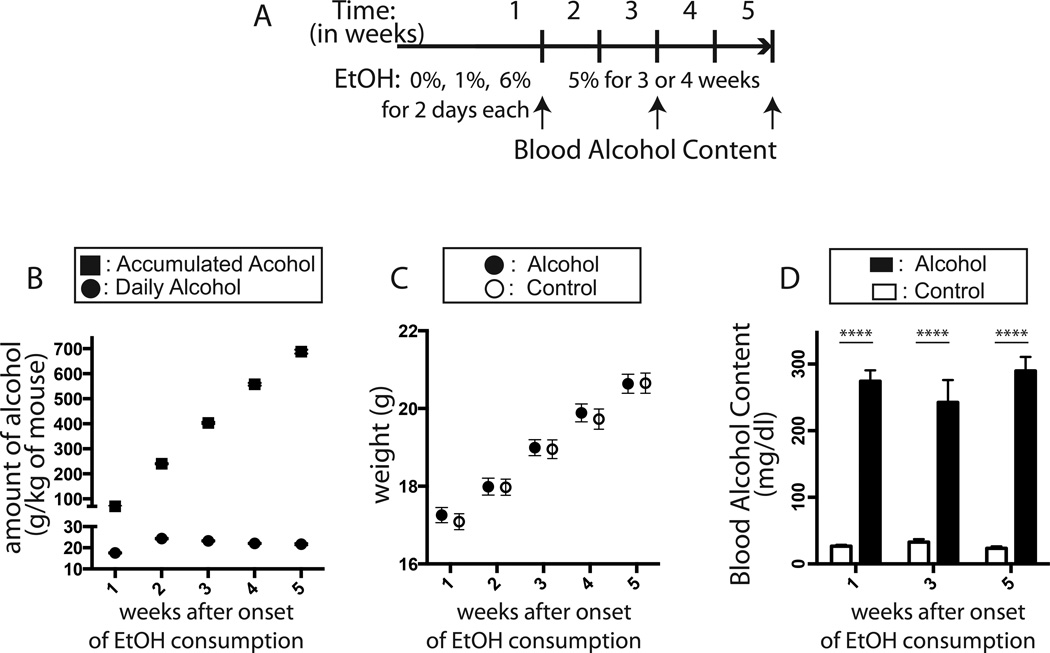
Mice had voluntary access to a nutritionally adequate liquid diet containing either ethanol or dextrose (Figure 1A). In one serving of the Lieber-DeCarli control diet (Lieber and DeCarli, 1982) 18% of energy is derived from protein, 35% from fat, and 47% from carbohydrate. In the alcohol diet (5% ethanol, V/V), ethanol provided 27% of dietary energy. Dextrin was used to isocalorically match the calories from ethanol in the control diet. During the 5-week alcohol-feeding period, mice consumed a significant amount of alcohol daily (n=26, 21.78 ± 0.25 g/kg of mouse/day) and the total amount of alcohol consumed reached 688 ± 7.8 g/kg of mouse (n=26, Figure 1B, Table 1). Alcohol-fed mice reached high peak BACs (250 mg/dl or higher) when measured 3 hours after the dark cycle at 1, 3 and 5 weeks after onset of alcohol feeding [two-way ANOVA, main effects of alcohol consumption, F(1,74) = 107.7, P < 0.0001; main effects of time, F(2,74) = 0.2110, p = 0.81] (Figure 1D, Table 1). This peak BAC value of 250 mg/dl corresponds to 0.25% of BAC, and is about 3 times higher than legal alcohol limit for driving (0.08%). Importantly, both alcohol and control mice gained comparable amount weight over the experimental period. A repeated measures ANOVA revealed significant main effects of time [F(4,292) = 501.2, p < 0.0001]; however, neither statistical differences between control and alcohol-fed mice [F(1,73) = 0.06, p = 0.81] nor significant interactions between time and alcohol consumption with regard to weight gain was found [F(4,292) = 0.46, p = 0.77] (Figure 1C, Table 1).
Figure 1. Establishment of chronic alcohol exposure mouse model.
In the pair-fed alcohol model, mice consumed a high dose of alcohol daily during the experimental period. Both control and alcohol mice received the same amount of calories to minimize the possibility that different energy consumption affected adult hippocampal neurogenesis. Alcohol feeding schedule is displayed (A). Mice consumed a significant amount of alcohol daily (circle) during the feeding period (rectangle, accumulated) (B). Both control and alcohol-fed mice gained equivalent level of body weight (C). Throughout the period of chronic alcohol exposure, mice reached and maintained high level of peak BAC (D). All results are displayed in the form of mean ± SEM.
Table 1.
Summary of chronic alcohol exposure in a pair-feeding mouse model. All data are shown in the format of mean ± SEM.
| Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | ||
|---|---|---|---|---|---|---|
| Weight (g) |
Control Group (n=36) | 17.09 ± 0.21 | 17.95 ± 0.21 | 18.95 ± 0.24 | 19.73 ± 0.26 | 20.65 ± 0.26 |
| Alcohol Group (n=39) | 17.26 ± 0.19 | 17.99 ± 0.22 | 18.99 ± 0.21 | 19.89 ± 0.23 | 20.64 ± 0.25 | |
| Daily Alcohol Consumption (g/kg, n=26) |
17.56 ± 0.24 | 24.32 ± 0.3 | 23.28 ± 0.27 | 22.06 ± 0.31 | 21.70 ± 0.44 | |
| Accumulated Alcohol Consumption (g/kg, n=26) |
70.2 ± 0.1 | 240.5 ± 2.4 | 403.4 ± 3.8 | 557.8 ± 5.5 | 688 ± 7.8 | |
| Peak Blood Alcohol Contents (mg/dl) |
274.2 ± 16.3 (n=11) |
242.5 ± 33.5 (n=11) |
289.8 ± 20.9 (n=9) |
|||
Chronic alcohol exposure reduces production and survival of newborn neurons
To investigate the effects of short-term and long-term alcohol exposure on multiple steps of neurogenesis, three independent BrdU pulse and chase experimental paradigms were used (Figure 2A and G). Each experiment was performed with 4 control and 6 alcohol-fed mice and some experiments were repeated (Figure 2B, C, D and H). First, mice received BrdU 4 days (1% for 2 days, 6% for 2 days) after alcohol feeding (Day 6), and the brains were analyzed 24 hours (Day 7) after final BrdU administration. To investigate the effect of short-term alcohol exposure on proliferation of neural stem/progenitors, the expression of Ki67 and pH3 (phosphohistone H3), and BrdU incorporation were examined. Ki67 is a physiological marker for proliferating cells, pH3 is specifically expressed during M-phase, and BrdU incorporates into replicating chromosome during S-phase of cell cycle (McClain et al., 2011a). Short-term alcohol consumption decreased the number of BrdU+ and pH3+ cells [t-test, BrdU, p = 0.002, control = 3323 ± 76.03, alcohol = 2211 ± 190.8; pH3, p = 0.006, control = 102.0 ± 7.35, alcohol = 44 ± 11.56] (Figure 2C and D). Interestingly, however, the number of Ki67+ cells did not change [t-test, p = 0.3101, control = 1636 ± 130.6, alcohol = 1444 ± 101.4] (Figure 2B). These results suggested that short-term alcohol exposure interfered with proliferation by affecting cell cycle progression rather reducing the number of cycling cells (McClain et al., 2011a). Proliferation index was further investigated during abstinence following the short-term alcohol exposure. After the 4-day alcohol consumption (Day 6), BrdU were injected twice in 2-hour interval at 2, 4 and 7 days later (Day 8, 10, and 13, for each time point, control n =3, alcohol n = 3) and the brains were analyzed 2 hours after the last BrdU administration. The number of BrdU+ cells was examined in the dentate gyrus as well as the molecular layer and hilus of the hippocampus (Figure 2 E and F). Although there were no statistically significant effects of alcohol consumption [two-way ANOVA, F(1,12) = 1.341, p = 0.2693] and time [two-way ANOVA, F(2,12) = 0.2583, p = 0.7765], there was a significant interaction between these two variables [F(2,12) = 4.456, p = 0.0357] with regard to the number of BrdU+ cells in the dentate gyrus (Figure E). Post-hoc analysis showed that the effect of short-term alcohol use on proliferation lasted up to 2 days of abstinence, but proliferation index returned to the level of control mice by 4 days of abstinence (Figure E). Two-way ANOVA also revealed no main effects of time [F(2,12) = 2.055, p = 0.1708] and alcohol consumption [F(1,12) = 0.7671, p = 0.3938] on the number of BrdU+ cells in the molecular layer and hilus (Figure 2F).
Figure 2. Chronic alcohol exposure disrupted multiple steps of hippocampal neurogenesis.
Diagram displays the schedule of BrdU injection and analysis with respect to alcohol feeding paradigm (A and G). Arrows and arrowheads indicate the injection of BrdU and analysis, respectively. After short-term alcohol exposure (1% for 2 days and 6% for 2 days), quantitative analysis of Ki67+ (B), BrdU+ (C), and pH3+ (D) cells show the number of proliferating cells, cells that had gone through S-phase in 24 hours, and cells in G2/M phase, respectively. Following short-term alcohol exposure, the number of BrdU+ cells in the dentate gyrus (DG) (E), and molecular layer and hilus (F) of the hippocampus are analyzed to examine proliferation of cells during 7-day abstinence. BrdU injection to label and trace cells born before and after the onset of alcohol exposure shows the chronic alcohol exposure reduced production and decreased survival rate of newborn cells (J and H). However, chronic alcohol exposure does not alter fate determination of cells born before (K) and after (I) alcohol treatment. Quantitative analysis of Ki67+ cells shows that chronic alcohol exposure decreased the number of proliferating cells (L). Liquid food and sold food fed control mice show the equivalent number of Ki67+ cells (hatched box, L). Representative images show BrdU+ and Ki67+ cells that were used for counting (M). BrdU+ and Ki67+ cells (arrow) with a higher magnification were also displayed in insets (M). Z-stack confocal images show differentiation of newborn cells into neurons (BrdU/NeuN) or astrocytes (BrdU/S100β) (N). All results are displayed in the form of mean ± SEM.
The effects of chronic alcohol exposure on survival and fate determination of newborn cells were examined. Using two different BrdU pulse and chase methods, we traced the survival and fate of cells that were born one week before (Day -6, Figure 2J - L) and after (Day 6, Figure 2H and I) onset of alcohol treatment (Figure 2G). Four-week chronic alcohol consumption reduced survival of cells that were born before as well as after the onset of alcohol consumption, suggesting that both reduced proliferation and survival rate accounted for the reduction in newborn cells [Figure 2J, t-test, cells born before alcohol onset, p = 0.021, control = 604.5 ± 36.77, alcohol = 503.0 ± 15.91; Figure 2H, t-test, cells born after alcohol onset, p = 0.0003, control = 625.5 ± 39.37, alcohol = 288.0 ± 31.46] (Figure 2M). The reduction of Ki-67+ cells, a marker for cycling cells, indicates that chronic alcohol reduction also reduced the number of proliferating cells [one-way ANOVA, F(2,12) = 8.828, p = 0.0044; post-hoc analysis between control and alcohol-fed mice, control = 1460 ± 98.54, alcohol = 958.3 ± 84.11, p < 0.05] (Figure 2M and L). In our experimental paradigm, solid chow-fed and liquid diet-fed mice showed the equivalent number of Ki67+ cells, suggesting that long-term consumption of liquid food did not affect the number of proliferating cells [solid chow = 1577 ± 154.4, control = 1460 ± 98.54]. Multipotent neural stem cells differentiate into either neurons or astrocytes (Suh et al., 2007). The effect of alcohol on fate determination was assessed via co-labeling of BrdU with a cell type-specific marker (either neuron-specific, NeuN, or astrocyte-specific, S100β) (Figure 2N). The ratio of neuronal and astrocyte differentiation of newborn cells did not differ, suggesting that chronic alcohol exposure did not affect cell fate determination of cells that are born immediately before and after the onset of alcohol consumption (Figure 2I and K).
Chronic alcohol disrupts synaptic connectivity of newborn neurons
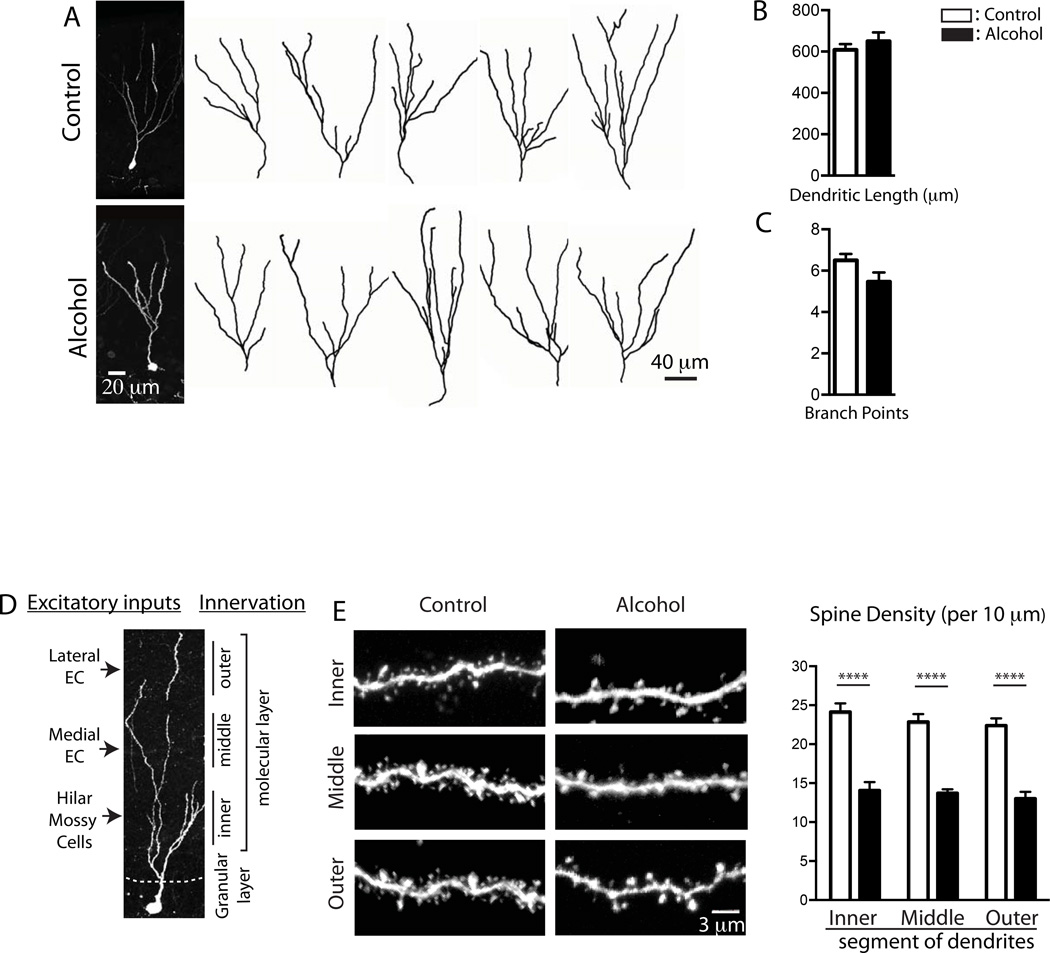
To examine the alcohol effect on the structural development of newborn neurons, GFP-expressing retrovirus was injected to label newborn neurons. Following one week of recovery, mice were fed with alcohol for 4 weeks (control n = 4, alcohol n = 4). The dendritic arborization of individual GFP+ neurons was reconstructed with confocal microscopy (Figure 3A). These GFP+ cells represent age-synchronized 5 weeks old newborn neurons that underwent maturation under the presence of alcohol. Alcohol-fed mice showed no difference in the total length of dendrites [t-test, p = 0.4293, control = 609.0 ± 27.34 µm, n = 15; alcohol = 650 ± 43.22 µm, n = 15) and branching points (t-test, p = 0.071, control = 6.5 ± 0.31, n = 14; alcohol = 5.467 ± 0.45, n = 15) compared to control mice, suggesting that a chronic alcohol consumption did not interfere with dendritic arborization (Figure 3B and C). Dendritic spines are an anatomical structure where glutamatergic excitatory synapses are formed, and have been implicated in synaptic function, plasticity and connectivity in excitatory neuronal circuits. Since excitatory neurons originating from the lateral, medial entorhinal cortex and hilus project their axons in a topological manner to outer-, middle- and inner-third segments of dendrites (Figure 3D)(Amaral et al., 2007), the spine density along the different location of dendrites was examined to estimate excitatory inputs from the different brain areas. Two-way ANOVA revealed the significant main effect of alcohol consumption on the density of dendritic spines [F(1, 94) = 159.3, p < 0.0001]; however, it did not yield a significant main effect of the position of dendrites [F(2, 94) = 1.118, p = 0.3312] and alcohol consumption × position of dendrites interactions [F(2, 94) = 0.1308, p = 0.8776] (Figure 3E). Post-hoc analysis (Sidak’s multiple comparisons test) showed that chronic alcohol exposure reduced spine density in the outer-, middle- and inner-third segments of dendrites of newborn neurons, indicating that alcohol interfered with the synaptic connectivity of newborn DGCs with excitatory neurons originated from the entorhinal cortex and hilus (Figure 3E).
Figure 3. Chronic alcohol exposure disrupted development of dendritic spines of newborn neurons.
A. Z-stack confocal images of 5-week-old, GFP expressing newborn DGCs are acquired, projected into 2-dimension and represented. Using IMARIS software, the total dendritic length (B) and the number of branching points (C) are measured. A cartoon explains the topological innervation of excitatory neurons originating from the lateral, medial entorhinal cortex and hilus into the outer-, middle- and inner-third of dendrites of newborn DGCs (D). Spine density was examined in the inner, middle and outer third of dendrites. Chronic alcohol exposure reduces the density of dendritic spines of newborn DGCs (E). All results are displayed in the form of mean ± SEM.
Chronic alcohol impairs hippocampus-dependent cognitive function
Eleven 5-week-old mice female mice were used per control and alcohol groups for behavioral analyses. Alcohol and control mice consumed alcohol- and isocaloric-diet over 4 weeks. Then, normal solid laboratory chow was given to both control and alcohol-fed mice. Three days later, mice were subject to behavioral tests.
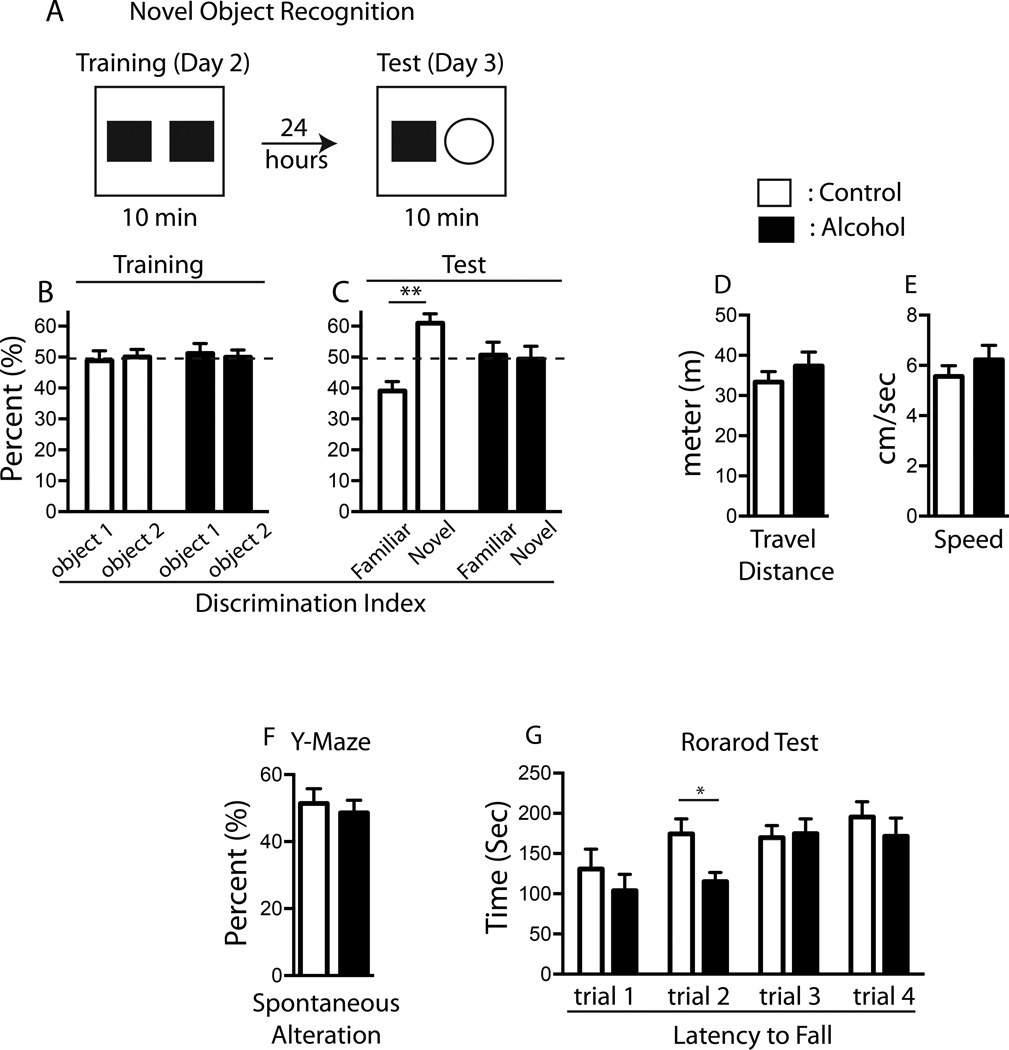
Two behavior tests, NOR and Y-maze, were used to examine whether hippocampus-dependent cognitive function is sensitive to chronic alcohol exposure. In the NOR test that measures animals’ recognition memory, mice in both control and alcohol groups spent an equivalent amount time with two identical objects (familiar object) during the training phase [two-way ANOVA: treatment × object F(1,40) = 0.1962, p = 0.6602; object F(1,40) = 0.1517, p = 0.6990; treatment F(1,40) = 0.0, p > 0.9999] (Figure 4A and B). When one familiar object was replaced with a novel object during the test phase, statistical analysis revealed significant interactions of alcohol consumption × object with regard to recognition ability of mice, but no main effects of these two variables [two-way ANOVA: alcohol consumption × object F(1,40) = 4.213, p = 0.0467; object F(1,40) = 0.2.983, p = 0.0918; treatment F(1,40) = 9.591e-015, p > 0.9999]. Multiple comparisons test revealed that the control mice exhibited a strong preference for the novel object [novel object, 57.847 ± 4.146 %; familial object, 42.153 ± 4.146 %, p = 0.0144]; however, mice in the alcohol group failed to discriminate the novel object and instead spent comparable time with novel and familiar objects [novel object, 49.325 ± 4.159 %; familial object, 50.675 ± 4.159 %, p = 0.8206], suggesting that alcohol impaired the hippocampus-dependent recognition memory (Figure 4C). There was no difference in locomotor activity and exploratory behavior between control and alcohol-fed mice as assessed by travel distance [t-test, p = 0.2101, control = 31.38 ± 3.06 m; alcohol = 37.36 ± 3.45 m] (Figure 4D) and speed (t-test, p = 0.3723, control = 5.557 ± 0.4338 cm/sec; alcohol = 6.227 ± 0.5756 cm/sec)(Figure 4E) during testing phase.
Figure 4. Chronic alcohol exposure impairs the hippocampus-dependent cognitive function.
Mice treated with alcohol for 4 weeks exhibit disrupted recognition memory in Novel Object Recognition test (A). Both control and alcohol fed groups do not show any preference when two identical objects (object 1 and object 2) are presented (B). However, while control mice show a significant preference for a novel object, mice in alcohol group failed to discriminate a novel object (C). Control and alcohol mice show no difference in travel distance (D) and speed (E) when assessed during the test phase. No difference in Y-maze spontaneous alteration is found between control and alcohol mice (F). Rotarod test shows that chronic alcohol exposure does not interfere with animals’ sensorimotor function (G). All results are displayed in the form of mean ± SEM.
In Y-maze test, spontaneous alteration (occurs when a mouse enters all three arms consecutively) was measured to determine hippocampus-dependent spatial working memory. No difference in the frequency of spontaneous alterations was found between control and alcohol groups [t-test, p = 0.3134, control = 51.43 ± 4.347; alcohol = 48.57 ± 3.810](Figure 4F). Statistically similar Rotarod performance among both alcohol and control groups eliminated the possibility that alcohol may affect sensorimotor coordination and motor learning ability of mice [A repeated measures ANOVA: interaction of alcohol consumption × trial F(3,60) = 1.513, p = 0.2205; time F(3,60) = 7.7459, p < 0.0001; alcohol consumption F(1, 20) = 1.737, p = 0.2025] (Figure 4G).
Discussion
Adolescence represents a psychologically and developmentally unique stage. In response to alcohol exposure, adolescents adapt behaviors differently from adults (White and Swartzwelder, 2004, Nixon and McClain, 2010, Spear, 2014). Compared to adults, adolescents have decreased response to negative alcohol effects that may limit excessive alcohol intake such as social inhibition, sedation, motor impairments, and hangover, concomitantly with increased sensitivity to rewarding/hedonic effects of alcohol. The adolescent brain also uniquely responds to alcohol intoxication, displaying elevated vulnerability to alcohol-induced structural impairments and neurodegeneration (Spear, 2014). These unique behavioral responses may promote excessive intakes of alcohol, which results in more severe brain structural and functional impairments during adolescence. Anatomical and functional deficits in the brain, in turn, reinforce uncontrollable intake of excessive amount of alcohol (Crews et al., 2005). While the role of adolescent binge alcohol exposure in alcohol-induced brain adaptation has been well studied, the effects of adolescence onset, chronic alcohol exposure on brain plasticity have not been clearly understood. In this study, we investigated whether plasticity of adolescent brains is vulnerable to chronic alcohol exposure, which may lead to long-lasting structural, functional and behavioral alterations.
To understand the effect of chronic alcohol consumption on hippocampal neurogenesis and cognition, the ad libitum alcohol feeding model of Lieber-DeCarli liquid diet with calorie-matched controls was used (Lieber and DeCarli, 1982). In this alcohol regimen, mice consumed a significant amount of alcohol daily over four weeks and peak BAC reached a high level (≥ 250 mg/kg). Subsequent study demonstrated that this dose of alcohol exposure and BAC level achieved in our experiment was sufficient to induce alcohol-dependent neurogenesis (Nixon, 2006). Our pair-feeding method has advantages in controlling of additional factors that can influence neurogenesis such as procedural stress, and reduced caloric intake and weight loss associated with chronic alcohol exposure (Gil-Mohapel et al., 2010). Stress and energy intake are known factors that can affect hippocampal neurogenesis, which makes it difficult to distinguish the direct effect of alcohol from the secondary effects associated with procedures (Maruszak et al., 2014, Gould and Tanapat, 1999). In our study, both control and fair-fed mice gained the equivalent amount of calories and the comparable level of weight. Moreover, a previous report showed that long-term consumption of liquid-diet did not induce corticosterone expression, a surrogate marker for stress, further suggesting that procedural stress is expected to be minimal to none in our liquid-diet feeding method (Patten et al., 2013). It is worthy to note that solid food deprivation associated with liquid-diet feeding may influence neurogenesis (Patten et al., 2013). However, the number of proliferating cells did not alter between fair-fed (liquid food) and chow-fed (solid food) mice in our study. Thus, by precisely controlling energy intake between control and alcohol groups, our model provides physiologically and nutritionally adequate experimental conditions that allow us to directly examine the effects of chronic alcohol exposure on hippocampal neurogenesis.
Using this alcohol regime, we demonstrated that altered neurogenesis contributed to anatomical as well as functional impairments in the hippocampus of alcohol-fed mice. Short-term alcohol feeding (1% for 2 days and 6% for 2 days) reduced the number of BrdU+ and pH3+ cells while the number of Ki67+ cells remained unaltered. This indicates that short-term alcohol exposure reduced the proportion of proliferating cells in S (BrdU+) and G2/M (pH3+) phases, but did not affect the number of cycling cells (Ki67+). This result is reminiscent of previous results showing binge alcohol exposure in adolescent rats reduced BrdU+ cells with unchanged number of Ki67+ and pH3+ cells (McClain et al., 2011a). Subsequent studies revealed that binge alcohol exposure accelerated cell cycle progression by shortening S-phase instead of affecting the number of proliferating cells (McClain et al., 2011a). Additional studies are needed to determine whether our short-term alcohol exposure also shortened cell cycle or arrested cell cycle progression in G1 and/or G2 phases. We further examined whether increased cell proliferation is observed in abstinence following short-term alcohol exposure (Nixon and Crews, 2004). The sequential burst of reactive microglia, activated astrocytes and neurogenesis has been observed at 2, 4, and 7 days of abstinence in binge alcohol exposure model (McClain et al., 2011b, Kelso et al., 2011, Nixon and Crews, 2004). This cellular response during abstinence has been hypothesized to play a critical role in structural and functional recovery. However, our short-term alcohol feeding in adolescent mice did not recapitulate the burst of proliferation during abstinence, suggesting that difference in influencing factors such as dose of alcohol exposure, BAC level, nutrition level, and/or species of laboratory animals may account for the different results (Nixon, 2006).
In addition to the reduced production of newborn neurons, our chronic alcohol exposure disrupted the development and synaptic connectivity of newborn DGCs. Spines are a structure where excitatory synapses are formed. Therefore, the number and morphology of spines are excellent indicators of synaptic connectivity and neural circuit formation of newborn neurons (Zhao et al., 2006). The entorhinal cortex is the major source for the cortical information necessary for the hippocampus to carry out its function. Anatomically, neurons originating from the entorhinal cortex send their axons to the dentate gyrus of the hippocampus, making direct synaptic connections with DGCs (Amaral et al., 2007). Excitatory neurons originating from the different parts of the entorhinal cortex innervate into the dendritic DGCs in a topographic manner: excitatory neurons from lateral and medial entorhinal cortex (LEC and MEC) make syanpses onto spines at the superficial- and medial-third of the dendrites of newborn neurons, respectively (Witter, 2007). Mossy cells located in the hilus make additional excitatory synapses at the inner-third of dendrites. Parrellel to topographic innervation, regional specific neural circuits convey functionally distinct information to the hippocampus (Witter, 2007). LEC provides information to encode the temporal order of contextual memory while MEC relays information for spatial memory (Moser et al., 2008, McNaughton et al., 2006). Thus, our observation that the reduced spine formation throughout all dendritic spine segments of newborn neurons indicates that alcohol disrupts synaptic connectivity of newborn DGCs with excitatory neurons, thereby impaires spatial and contextual memory function.
Different aspects of morphological deficits of DGCs have been reported in various chronic alcohol exposure models. In contrast to the normal dendritic arborization of newborn neurons found in our study, chronic alcohol exposure in rats reduced dendritic growth of immature neurons. This difference may be due to the methodology used in two studies (He et al., 2005). We used a retrovirus to label neurons in an age-synchronized manner. Since retrovirus is transiently available in the system and transduces GFP exclusively in the proliferating cells upon injection, this method ensures that GFP+ cells represent an age-synchronized, homogeneous population of newborn neurons (5-week old) (Zhao et al., 2006). He et al. used DCX as a marker to investigate dendritic development. DCX is expressed in broad developmental stages of newborn neurons, ranging from 1- to 3-week old newborn DGCs (Brown et al., 2003). Different ages of newborn neurons investigated and the heterogeneity of DCX+ cell population may account for different results between two studies. Nevertheless, both studies predict that chronic alcohol exposure longer periods will impair the structure and function of the hippocampus more severely. In several reports, however, exposure to alcohol longer than six months in rats did not show any deficits in spatial learning and memory (Lukoyanov et al., 2000). Enhanced dendritic arborization and spine density may compensate the loss of DGCs in this model (Lukoyanov et al., 2000, Cadete-Leite et al., 1988). Investigation of such compensatory mechanism will be needed to understand how brain adapts in response to chronic alcohol exposure.
To understand the effect of chronic alcohol exposure on the hippocampus-dependent function, recognition and spatial working memory were examined (Saxe et al., 2006, Jessberger et al., 2009). While both NOR and Y-maze tests rely upon the animal’s innate exploratory preference for novel objects or environments, each test requires different memory retention periods; NOR is dependent upon long-term recognition memory whereas Y-maze tests short-term working memory. In our experiments, chronic alcohol exposure impaired recognition memory (in NOR) but not working memory (in Y-maze). Interestingly, the pattern of cognitive deficits observed in our study coincides with behavioral phenotypes caused by specific ablation of hippocampal neurogenesis; blockage of hippocampal neurogenesis impaired the performance in NOR, but not in Y-maze (Saxe et al., 2006, Jessberger et al., 2009). These results support the idea that disrupted hippocampal neurogenesis is involved in cognitive impairments associated with chronic alcohol exposure.
The present study demonstrates that chronic alcohol exposure targets multiple steps of hippocampal neurogenesis, not only reducing the production of newborn neurons but also inducing abnormal structural development and synapse formation of newborn DGCs. As structural reorganization in the specific brain areas has been hypothesized to cause long-lasting cognitive and behavioral changes associated with drug addiction (Russo et al., 2010), our study supports the emerging view that altered plasticity of adolescent hippocampus contributes to long-lasting structural and functional changes, which are associated with alcohol-induced cognitive behaviors. We will further seek to understand mechanisms by which alcohol regulates neurogenesis and cognitive function, which is essential to understand and prevent AUD.
Supplementary Material
Acknowledgement
The Authors thank NIAAA funded P20 Cleveland Clinic Alcohol Center for providing resources and intellectual discussion (AA017837, L. N.). This study was supported by the National Institute of Alcohol Abuse and Alcoholism (R01AA022377, H.S.), Whitehall Foundation (H. S.), and American Federation for Aging Research (H. S.). We also thank Dr. Bruce Lamb and the Rodent Behavior Core for data analysis and behavioral tests. Authors thank Sharvari Dharmaiah for technical assistance.
References
- Amaral DG, Scharfman HE, Lavenex P. The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies) Prog Brain Res. 2007;163:3–22. doi: 10.1016/S0079-6123(07)63001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwater MA, Liu W, Crews FT, Spear LP. Persistent loss of hippocampal neurogenesis and increased cell death following adolescent, but not adult, chronic ethanol exposure. Dev Neurosci. 2014;36:297–305. doi: 10.1159/000362874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Cadete-Leite A, Tavares MA, Uylings HB, Paula-Barbosa M. Granule cell loss and dendritic regrowth in the hippocampal dentate gyrus of the rat after chronic alcohol consumption. Brain Res. 1988;473:1–14. doi: 10.1016/0006-8993(88)90309-5. [DOI] [PubMed] [Google Scholar]
- Clark DB, Thatcher DL, Tapert SF. Alcohol, psychological dysregulation, and adolescent brain development. Alcohol Clin Exp Res. 2008;32:375–385. doi: 10.1111/j.1530-0277.2007.00601.x. [DOI] [PubMed] [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC, 3rd, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000;24:1712–1723. [PubMed] [Google Scholar]
- Crews FT, Buckley T, Dodd PR, Ende G, Foley N, Harper C, He J, Innes D, Loh El W, Pfefferbaum A, Zou J, Sullivan EV. Alcoholic neurobiology: changes in dependence and recovery. Alcoholism, clinical and experimental research. 2005;29:1504–1513. doi: 10.1097/01.alc.0000175013.50644.61. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, Kersh A, Keshavan MS. Hippocampal volume in adolescent-onset alcohol use disorders. The American journal of psychiatry. 2000;157:737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Harburg GC. Opiates, psychostimulants, and adult hippocampal neurogenesis: Insights for addiction and stem cell biology. Hippocampus. 2006;16:271–286. doi: 10.1002/hipo.20161. [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Geil CR, Hayes DM, Mcclain JA, Liput DJ, Marshall SA, Chen KY, Nixon K. Alcohol and adult hippocampal neurogenesis: Promiscuous drug, wanton effects. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54C:103–113. doi: 10.1016/j.pnpbp.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Mohapel J, Boehme F, Kainer L, Christie BR. Hippocampal cell loss and neurogenesis after fetal alcohol exposure: insights from different rodent models. Brain research reviews. 2010;64:283–303. doi: 10.1016/j.brainresrev.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P. Stress and hippocampal neurogenesis. Biol Psychiatry. 1999;46:1472–1479. doi: 10.1016/s0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug and alcohol dependence. 2004;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- He J, Nixon K, Shetty AK, Crews FT. Chronic alcohol exposure reduces hippocampal neurogenesis and dendritic growth of newborn neurons. The European journal of neuroscience. 2005;21:2711–2720. doi: 10.1111/j.1460-9568.2005.04120.x. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Jr, Consiglio A, Lie DC, Squire LR, Gage FH. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16:147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso ML, Liput DJ, Eaves DW, Nixon K. Upregulated vimentin suggests new areas of neurodegeneration in a model of an alcohol use disorder. Neuroscience. 2011;197:381–393. doi: 10.1016/j.neuroscience.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber CS, Decarli LM. The feeding of alcohol in liquid diets: two decades of applications and 1982 update. Alcoholism, clinical and experimental research. 1982;6:523–531. doi: 10.1111/j.1530-0277.1982.tb05017.x. [DOI] [PubMed] [Google Scholar]
- Lukoyanov NV, Brandao F, Cadete-Leite A, Madeira MD, Paula-Barbosa MM. Synaptic reorganization in the hippocampal formation of alcohol-fed rats may compensate for functional deficits related to neuronal loss. Alcohol. 2000;20:139–148. doi: 10.1016/s0741-8329(99)00069-5. [DOI] [PubMed] [Google Scholar]
- Maruszak A, Pilarski A, Murphy T, Branch N, Thuret S. Hippocampal neurogenesis in Alzheimer’s disease: is there a role for dietary modulation? J Alzheimers Dis. 2014;38:11–38. doi: 10.3233/JAD-131004. [DOI] [PubMed] [Google Scholar]
- Mcclain JA, Hayes DM, Morris SA, Nixon K. Adolescent binge alcohol exposure alters hippocampal progenitor cell proliferation in rats: effects on cell cycle kinetics. The Journal of comparative neurology. 2011a;519:2697–2710. doi: 10.1002/cne.22647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcclain JA, Morris SA, Deeny MA, Marshall SA, Hayes DM, Kiser ZM, Nixon K. Adolescent binge alcohol exposure induces long-lasting partial activation of microglia. Brain Behav Immun. 2011b;25(Suppl 1):S120–S128. doi: 10.1016/j.bbi.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcnaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB. Path integration and the neural basis of the ‘cognitive map’Nature reviews . Neuroscience. 2006;7:663–678. doi: 10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- Morris SA, Eaves DW, Smith AR, Nixon K. Alcohol inhibition of neurogenesis: a mechanism of hippocampal neurodegeneration in an adolescent alcohol abuse model. Hippocampus. 2010;20:596–607. doi: 10.1002/hipo.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser EI, Kropff E, Moser MB. Place cells, grid cells, and the brain’s spatial representation system. Annual review of neuroscience. 2008;31:69–89. doi: 10.1146/annurev.neuro.31.061307.090723. [DOI] [PubMed] [Google Scholar]
- Nixon K. Alcohol and adult neurogenesis: roles in neurodegeneration and recovery in chronic alcoholism. Hippocampus. 2006;16:287–295. doi: 10.1002/hipo.20162. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. Journal of neurochemistry. 2002;83:1087–1093. doi: 10.1046/j.1471-4159.2002.01214.x. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Temporally specific burst in cell proliferation increases hippocampal neurogenesis in protracted abstinence from alcohol. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:9714–9722. doi: 10.1523/JNEUROSCI.3063-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K, Mcclain JA. Adolescence as a critical window for developing an alcohol use disorder: current findings in neuroscience. Curr Opin Psychiatry. 2010;23:227–232. doi: 10.1097/YCO.0b013e32833864fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K, Morris SA, Liput DJ, Kelso ML. Roles of neural stem cells and adult neurogenesis in adolescent alcohol use disorders. Alcohol. 2010;44:39–56. doi: 10.1016/j.alcohol.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obernier JA, Bouldin TW, Crews FT. Binge ethanol exposure in adult rats causes necrotic cell death. Alcohol Clin Exp Res. 2002;26:547–557. [PubMed] [Google Scholar]
- Parsons OA. Cognitive dysfunction and recovery in alcoholics. Substance and alcohol actions/misuse. 1983;4:175–190. [PubMed] [Google Scholar]
- Patten AR, Moller DJ, Graham J, Gil-Mohapel J, Christie BR. Liquid diets reduce cell proliferation but not neurogenesis in the adult rat hippocampus. Neuroscience. 2013;254:173–184. doi: 10.1016/j.neuroscience.2013.09.024. [DOI] [PubMed] [Google Scholar]
- Pawlak R, Skrzypiec A, Sulkowski S, Buczko W. Ethanol-induced neurotoxicity is counterbalanced by increased cell proliferation in mouse dentate gyrus. Neurosci Lett. 2002;327:83–86. doi: 10.1016/s0304-3940(02)00369-5. [DOI] [PubMed] [Google Scholar]
- Rice AC, Bullock MR, Shelton KL. Chronic ethanol consumption transiently reduces adult neural progenitor cell proliferation. Brain Res. 2004;1011:94–98. doi: 10.1016/j.brainres.2004.01.091. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends in neurosciences. 2010;33:267–276. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samhsa. National Survey on Drug Use and Health. 2013 [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulteis G, Archer C, Tapert SF, Frank LR. Intermittent binge alcohol exposure during the periadolescent period induces spatial working memory deficits in young adult rats. Alcohol. 2008;42:459–467. doi: 10.1016/j.alcohol.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sircar R, Basak AK, Sircar D. Repeated ethanol exposure affects the acquisition of spatial memory in adolescent female rats. Behavioural brain research. 2009;202:225–231. doi: 10.1016/j.bbr.2009.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Adolescents and alcohol: acute sensitivities, enhanced intake, and later consequences. Neurotoxicol Teratol. 2014;41:51–59. doi: 10.1016/j.ntt.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh H, Consiglio A, Ray J, Sawai T, D’Amour KA, Gage FH. In Vivo Fate Analysis Reveals the Multipotent and Self-Renewal Capacities of Sox2(+) Neural Stem Cells in the Adult Hippocampus. Cell Stem Cell. 2007;1:515–528. doi: 10.1016/j.stem.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh H, Deng W, Gage FH. Signaling in adult neurogenesis. Annu Rev Cell Dev Biol. 2009;25:253–275. doi: 10.1146/annurev.cellbio.042308.113256. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Anterior hippocampal volume deficits in nonamnesic, aging chronic alcoholics. Alcoholism, clinical and experimental research. 1995;19:110–122. doi: 10.1111/j.1530-0277.1995.tb01478.x. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology. 2005;180:583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Taffe MA, Kotzebue RW, Crean RD, Crawford EF, Edwards S, Mandyam CD. Long-lasting reduction in hippocampal neurogenesis by alcohol consumption in adolescent nonhuman primates. Proc Natl Acad Sci U S A. 2010;107:11104–11109. doi: 10.1073/pnas.0912810107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivar C, Potter MC, Choi J, Lee J, Stringer TP, Callaway EM, Gage FH, Suh H, Van Praag H. Monosynaptic inputs to new neurons in the dentate gyrus. Nature Communications. 2012:1–11. doi: 10.1038/ncomms2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM, Swartzwelder HS. Hippocampal function during adolescence: a unique target of ethanol effects. Ann N Y Acad Sci. 2004;1021:206–220. doi: 10.1196/annals.1308.026. [DOI] [PubMed] [Google Scholar]
- Witter MP. The perforant path: projections from the entorhinal cortex to the dentate gyrus. Progress in brain research. 2007;163:43–61. doi: 10.1016/S0079-6123(07)63003-9. [DOI] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.