Summary
The MYC oncogene encodes a transcription factor, MYC, whose broad effects make its precise oncogenic role enigmatically elusive. The evidence to date suggests that MYC triggers selective gene expression amplification to promote cell growth and proliferation. Through its targets, MYC coordinates nutrient acquisition to produce ATP and key cellular building blocks that increase cell mass and trigger DNA replication and cell division. In cancer, genetic and epigenetic derangements silence checkpoints and unleash MYC’s cell growth- and proliferation-promoting metabolic activities. Unbridled growth in response to deregulated MYC expression creates dependence on MYC-driven metabolic pathways, such that reliance on specific metabolic enzymes provides novel targets for cancer therapy.
MYC function
c-MYC (termed MYC henceforth), like its family members N-MYC and L-MYC, is a transcription factor that dimerizes with MAX to bind DNA and regulate gene expression (1). A nuclear localization sequence, DNA binding domain, helix-loop-helix (HLH) dimerization domain, and transcriptional regulatory domain underlie this functional ability. MYC was first discovered as the cellular homolog of the retroviral v-myc oncogene identified from studies of oncogenic retroviruses (2–4). Soon after its discovery, chromosomal translocations that juxtapose MYC to immunoglobulin enhancers were documented in B cell Burkitt lymphomas (5). Classical in vitro assays using normal primary rat embryo fibroblasts then documented MYC’s transforming activity in cooperation with activated RAS and the sufficiency of these two oncogenes to transform normal cells (6). Tightly regulated in non-cancerous cells (Figure 1A), MYC is now known to be one of the most frequently deregulated oncogenes. It is frequently translocated in hematopoietic cancers and was found in a pan-cancer copy number analysis to be the third most amplified gene in human cancers (Figure 1B) (7, 8). Deregulated expression of MYC in transgenic murine tissues of many varieties can trigger tumorigenesis in those tissues, illustrating its transforming activity in vivo and supporting the notion that it is a human oncogene (9).
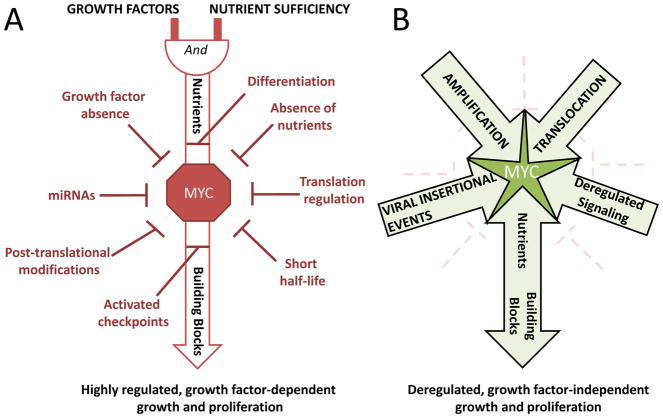
Figure 1. Schematic illustration of growth factor-dependent and growth factor-independent MYC activity.
A) In non-cancerous cells, growth signals and adequate nutrients are required for MYC activity. Multiple levels of feedback loops and checkpoints further control MYC activity. B) In cancerous cells, in contrast, checkpoint loss, gene amplification, chromosomal translocation, abnormal enhancer activation, or one or more other deregulated signaling events lead to growth factor-independent MYC metabolic activities and subsequent unconstrained cellular growth and proliferation.
Because of its oncogenic potential, the MYC proto-oncogene is tightly regulated in normal cells at the transcriptional and post-transcriptional levels (Figure 1A) (10). Post-transcriptionally, it is regulated by microRNAs and by translation of its mRNA (11–13). Post-translationally, Myc protein half-life and transcriptional activity are controlled by kinases, ubiquitin ligases, acetyl transferases and other interacting proteins (11–16), and indeed, oncogenic KRAS and ERK can upregulate Myc in part through enhanced protein stability (17–20). Recent studies show that long non-coding RNAs control MYC activity and protein stability altering post-translational modification (15, 21). Exquisite regulatory restraint is also achieved through the governance of MYC proto-oncogene enhancers that appear cell-type specific (22–25) (Figure 2A). Many growth-promoting signal transduction pathways downstream of ligand-membrane receptor engagement, such as Notch and EGFR converge on MYC, underscoring the centrality of MYC to cell growth regulation (26–32) (Figure 2A). Activation of the proto-oncogene is invariably dependent upon such stimulation by growth factors. In contrast, in cancer, MYC amplifications that increase MYC copy number, translocations that pair MYC with highly active enhancers, or viral insertional events in the MYC locus sever MYC from its dependence on growth factor signaling (1) (Figure 1B and 2B). Changes in the activity of MYC’s enhancers can likewise uncouple MYC expression from its normally required stimuli, affecting cancer risk and progression. For example, Notch-dependent enhancers of MYC appear to be intimately involved in activation of MYC in human T-cell lymphoma (22, 23). Other enhancers exhibit single nucleotide polymorphisms that affect transcriptional activator TCF-7 binding and predispose to prostate and colon cancer (33–36).
Figure 2.
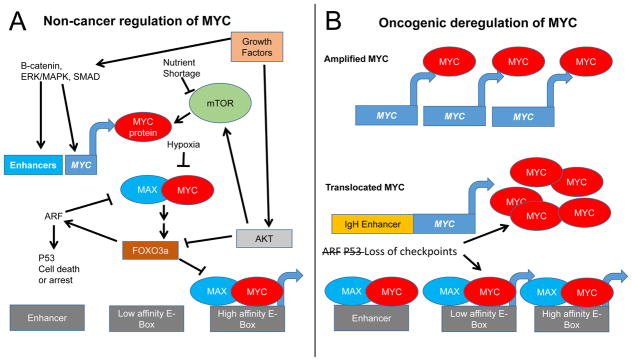
MYC regulation in non-cancerous and cancerous cells. A) In non-cancerous cells MYC expression is activated by growth factors through activation of enhancers. MYC protein, whose translation is enhanced by activated mTOR, dimerizes with MAX to form a heterodimer that activates transcription of genes containing high affinity E-boxes. Upon nutrient shortage or hypoxia, MYC translation, protein stability and MYC/MAX dimerization inhibited. Over-activation of MYC activates the ARF and p53 checkpoints resulting in cell death or arrest, while ARF can inhibit MYC function. Downstream of AKT, FOXO3a proteins counteract MYC activation. B) In cancer cells, constitutive activation of growth factor and mTOR signaling, loss of checkpoints, engagement of atypical enhancers, or amplification or translocation of MYC can increase levels of MYC to supraphysiologic levels independently of growth factors, causing MYC/MAX binding to lower affinity binding sites and enhancers in addition to high affinity sites. Loss of ARF or p53 checkpoints allows uncontrolled cell growth.
In non-cancerous cells, check-points further protect against deregulated MYC expression. As such, in experimental transgenic models, acute deregulated MYC expression does not induce cell proliferation; rather, it results in the activation of checkpoints including those through p53, ARF, BIM, and PTEN that can cause cell growth arrest or death (Figure 2A) (37–40). For example, in a MYC driven lymphoma model increased nuclear localization of the transcription factor FOXO3a can activate ARF to suppress growth (41). Further, ARF can bind MYC and directly inhibit its transcriptional activity (42). Loss of these checkpoints synergizes with MYC to promote transformation. Not surprisingly then, AKT, which can phosphorylate and inhibit FOXO3a, cooperates with MYC in neoplastic transformation (43). Genetic inactivation of FOXO3a can substitute for AKT activation and was documented to be sufficient to transform primary murine embryonic fibroblasts in cooperation with MYC (43) (Figure 2A). These observations are consistent with the finding that MYC-driven murine lymphomas are all virtually devoid of p53 or ARF, indicating that elimination of checkpoints are essential for MYC-mediated tumorigenesis (38). Indeed, human Burkitt’s lymphoma loses TP53 in up to 40% of cases (44, 45).
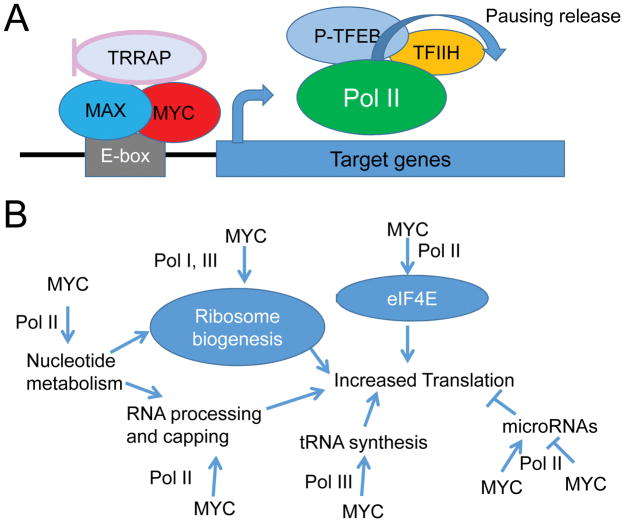
The MYC-MAX heterodimeric transcription factor has been documented to bind consensus DNA sites, termed E-boxes (5′-CACGTG-3′), with high affinity and non-consensus sites with lower affinities (Figure 2) (46). MYC binding to proximal gene promoter sequences relieves transcriptionally paused RNA polymerases and catalyzes transcriptional elongation (Figure 3A) (47). In this regard, it has been proposed that MYC is a general transcription factor which amplifies the expression of genes that are already expressed at basal level, seemingly without any specificity (“general amplification” model) (48–50). The general amplification model, however, does not account for the ability of MYC to repress genes, such as those activated by the transcription factor MIZ-1 (46). Thus, counter to this general amplifier viewpoint is the hypothesis that MYC targets are largely dictated by chromatin accessibility (51), which permits MYC to bind target genes and cooperate with other transcription factors to activate or repress gene expression selectively (“selective amplification”) (52, 53). That is, the degree by which MYC stimulates expression of a gene is dependent on other transcription factors bound to the gene and or to nearby enhancers.
Figure 3.
MYC enhances transcription and translation. A) The MYC/MAX dimer binds to E-boxes or lower affinity degenerate sequences to recruit histone acetylases or promote polymerase phosphorylation, thus release polymerase from pausing to amplify transcription. B) Acting on Pol I, Pol II and Pol III, MYC controls translation through upregulation of transcription of ribosomal subunits, tRNA, and nucleotide synthesis genes. MYC also stimulates translation by upregulating eukaryotic translation initiation factor 4E (eIF4E) and stimulating enzymes that control RNA processing and capping. MYC upregulation and downregulation of microRNAs also regulates the translation of microRNA targets.
Two recent papers provide evidence of both selective gene expression amplification that promotes cell growth and direct gene repression by MYC (53, 54). As non-dividing cells tightly control their expression of metabolic enzymes to tailor metabolism for homeostasis, it stands to reason that MYC activation would selectively amplify many metabolic genes required for the building blocks required for growth. Genes involved in non-proliferative cellular functions and cell cycle inhibition driven by MIZ-1 would, on the other hand, be repressed by MYC (54). Collectively, the studies suggest that MYC binds DNA to promote gene expression by relieving paused RNA polymerases. However, the means by which MYC binds DNA and activates or represses gene expression is influenced by chromatin accessibility that is marked by factors such as WDR5, which was recently shown to be required for MYC to bind its targets (55). Indeed, recent work suggests a centrally degenerate E-box motif in closed chromatin may be accessible to MYC employing a partially unfolded DNA-binding domain when assisted by other factors, adding a structural rationale to the idea that MYC’s ability to impact gene expression is a confluence of DNA consensus sequence affinity, chromatin accessibility, and interaction with other proteins (56). Overexpression of MYC can in turn upregulate chromatin modifiers to further alter chromatin accessibility, as suggested by the MYC mediated induction of the Polycomb complex member EZH2 (40, 57, 58). In addition, high levels of deregulated oncogenic MYC further perturb transcription by invading enhancer sequences, causing non-linear amplification of target gene expression and supporting constitutive biomass accumulation in cancer cells, which are driven by MYC (48, 53, 54). However, it is notable that high levels of MYC did not further increase biomass in U2OS cells, but instead induced genes involved in processes such as angiogenesis, metastasis and cell migration (54). U2OS cells are distinct, however, because ectopic MYC expression is detrimental, resulting in cell death rather than promoting cell growth and proliferation (48, 53, 54) (Figure 2B). Regardless of the exact function of MYC in regulating gene expression, studies to date support the notion that activation of MYC results in a genomic program that promotes ribosome biogenesis, cell growth and subsequently cell proliferation (8, 59).
In addition to its transcriptional role, MYC has roles both indirect and direct (i.e. independent of transcription) in regulating protein translation (Figure 3B). As will be discussed further in later sections, MYC transactivates genes encoding the RNA and protein components of ribosomes as well as the nutrient importers and nucleotide synthesis enzymes needed to support this ribosomal assembly. Additionally, MYC promotes cap-dependent translation through the direct stimulation of cap methylation and the transcriptional activation of translation initiation factors and genes involved in mRNA capping (60–66). The production of tRNAs is also stimulated through MYC’s effects on Pol III transcription (67).
Building a cell
T cell lymphocytes serve as a useful model for understanding the role of the transcriptional program driven by growth factor-regulated (non-cancerous) MYC in normal cells (68). Resting, non-proliferating cells, such as dormant stem cells or memory T cells, need nutrients for homeostasis. Maintenance of cell membrane potentials and protein synthesis are two major energy demanding cellular processes that must be sustained for survival (69). Energy and nutrients are also required during homeostasis for redox control and replacement of damaged macromolecules and organelles (70, 71). Intriguingly, in the case of resting memory T cells, a major source of energy for homeostasis is a futile cycle of oxidation of de novo synthesized lipids (72). Glucose and glutamine carbons are imported into cells and then converted to citrate for lipogenesis. In contrast to MYC-mediated proliferating cells, which use de novo lipogenesis to produce membranes for cell growth, resting T cells oxidize the de novo synthesized fatty acids for ATP production. Additional studies to determine whether dormant cancer cells use this previously unsuspected memory T cell futile cycle may reveal additional metabolic rewiring pathways used by cancer cells (73, 74).
Upon experimental growth stimulation with anti-CD3 and anti-CD8 antibodies, normal T cells are activated through the T-cell receptor, which transmits growth signals that induce metabolic changes required for proliferation. T cells from mice with floxed alleles of Myc have revealed that the transcriptionally driven metabolic reprogramming necessary for T cell proliferation requires Myc (68). Elimination of floxed Myc by Cre recombinase resulted in T cells that were unable to mount a growth response. This study corroborates previous studies documenting the role of MYC in driving a transcriptional program that promotes cell metabolism, growth, and proliferation, as will be discussed in detail in subsequent sections (68). Once stimulated, T cells begin to acquire nutrients, particularly glucose and glutamine, and convert them to the necessary components for making new DNA and RNA, new enzymes, new cytoskeleton, new membranes, new organelles, and new copies of genetic material (Figure 4). Ribosomes, which mediate translation of existing mRNAs in the resting cell for homeostasis, become especially critical during cell growth. Once a requisite cell size is reached and adequate nucleotide pools are achieved, the cell undergoes DNA replication while constantly monitoring the replicated DNA for errors and correcting them. After DNA is replicated, the cell then undergoes division using components, such as the cytoskeleton and membrane components, built from the raw nutrients that were required to overcome the checkpoints blocking cell cycle progression.
Figure 4.
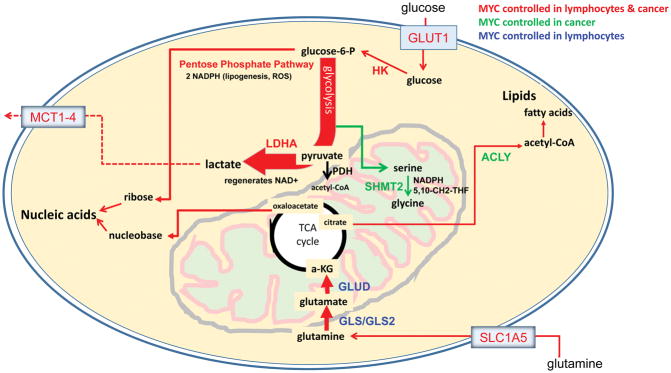
Myc-regulated metabolic pathways in cancer. Glucose is taken up by glucose transporters (GLUT) and phosphorylated by hexokinase (HK) to form glucose-6-phosphate (glucose-6-P). Glucose-6-P can then either enter glycolysis or the pentose phosphate pathway, which supports nucleotide synthesis by yielding two NADPH reducing equivalents and one ribose per molecule of glucose. The serine biosynthesis pathway branches off glycolysis, producing serine and glycine that likewise support nucleotide synthesis. Serine hydroxymethyltransferase 2 (SHMT2) converts serine to glycine which, in a series of coupled reactions, can be used to create nucleotide and epigenetic methyl donor 5,10-CH2-tetrahydrofolate and mitochondrial NADPH for redox control. Lactate dehydrogenase (LDHA) can regenerate NAD+ by converting glycolysis-derived pyruvate to lactate, which is then exported out of the cell by monocarboxylate transporters (MCT1-4). Alternatively, pyruvate can enter the TCA cycle in a pyruvate dehydrogenase (PDH)-dependent conversion to acetyl-CoA. TCA cycle citrate can be exported to the cytoplasm where it is converted to acetyl-CoA by ATP citrate lyase (ACLY). Cytoplasmic acetyl-CoA can then be channeled into lipogenesis. In addition to glucose, glutamine is an important fuel source in cancers. Glutamine is transported across the membrane by the glutamine transporter (SLC1A5/ASCT2) and converted to glutamate by glutaminase (GLS or GLS2). Glutamate can then be converted to the TCA cycle intermediate α-ketoglutarate (αKG) by glutamate dehydrogenase (GLUD) or aminotransferases.
Nutrient sensing, FOXO, HIF, and MYC
The availability of nutrients is essential for cell growth and proliferation. In fact, lower organisms have developed nutrient sensing mechanisms that are coordinated with growth. The yeast Saccharomyces cerevisiae has a remarkable glucose and glutamine sensing mechanism that involves signaling through Target of Rapamycin Complex (TORC) to inhibit two key transcriptional repressors of ribosome biogenesis, Dot6 and Tod6 (75). Nutrient insufficiency causes decreased TORC signaling, resulting in the inhibition of the production of ribosomes and cell growth through the activation of Dot6 and Tod6 (75). Yeast mutants lacking these functional repressors are rendered constitutive for cell growth, causing them to be ‘addicted’ to nutrients, such that withdrawal of glucose and glutamine culminates in non-viability. With nitrogen starvation in the presence of limited carbon sources, normal yeast cells undergo meiosis and sporulation that suspends yeast cells in a quiescent metabolic state (76). The slime mold Dictyostelium discoideum are amoeba-like unicellular organisms that have a life cycle of active feeding on bacteria. When nutrients become scarce, Dictyostelium aggregate to form a slug and then a fruiting body for sporulation in a TOR dependent manner (77, 78). The released spores can be re-animated into amoebae when nutrients become available again. These TOR regulated adaptive mechanisms are recapitulated in the higher organism Caenorhabditis elegans which can go into the dormant ‘dauer’ state with severe nutrient deprivation (79, 80). Hence, throughout evolution, organisms incorporate adaptive mechanisms in response to periods of feast and long periods of famine (81).
However, mammalian cells, owing to perfusion by the circulatory system, are constantly bathed in nutrients, particularly when food and oxygen are available. Under starvation a number of adaptive mechanisms have evolved that are cell intrinsic as well as non-cell autonomous (82). At the organismal level, fat depots and the liver are two major energy storage sources. White fat can be mobilized by lipolysis in response to starvation, while liver glycogen, synthesized from excess glucose, can be mobilized through glycogenolysis. While poorly vascularized tumors or acutely ischemic tissues may have disruption of nutrient availability, these organismal level processes and others normally maintain sufficient circulating levels of key nutrient sources, including a tightly regulated glucose level and more dynamic levels of circulating lipoprotein particles and glutamine, the most abundant plasma amino acid (83). With severe starvation or pathologic processes that disrupt tissue perfusion, cell autonomous nutrient-sensing mechanisms protect cells through pathways aimed at preserving adequate ATP pools. The mammalian Target of Rapamycin (mTOR), AMP kinase (AMPK), and GCN2 pathways are key to survival of cells under nutrient deprivation. Lack of amino acids attenuates mTOR activity through sensing by the Rag proteins on the lysosomal membrane (84), thereby both diminishes protein synthesis and cellular processes that would consume nutrients and ATP and relieves the inhibitory block on autophagy (84). Deprivation of amino acids also results in non-aminoacylated tRNAs that bind to and activate GCN2 and the stress response transcription factor ATF4, which mediates the unfolded protein response (85). When ATP is consumed and AMP is produced, AMPK is activated and its phosphorylation of key substrates(86), such as acetyl-CoA carboxylase (ACACA), diminishes fatty acid synthesis and other high energy consuming pathways. AMPK also increases glycolysis and activates autophagy to produce ATP while simultaneously inhibiting mTOR to slow energetically costly macromolecule synthesis (86).
In addition to its response to growth factor stimulation, MYC also appears to be involved in nutrient sensing downstream of different signaling pathways. As discussed, the yeast Saccharomyces cerevisiae senses glucose and glutamine to regulate ribosome biogenesis and cell growth (75). While yeasts do not have a MYC homolog, Drosophila dMyc is functionally equivalent to mammalian MYC. Intriguingly, with nutrient starvation, diminished TOR activity attenuates cell growth through diminished expression of dMyc. This pathway appears to involve TOR-dependent AKT phosphorylation and inactivation of FOXO transcription factors, which bind to and negatively regulate dMyc expression through a FOXO-responsive cis-element that senses nutrients through TOR and FOXO (87). Mutation of the dMyc FOXO-responsive cis-elements renders the mutant flies nonviable with nutrient depletion. Mammalian MYC activity also depends on nutrient status sensed through mTOR via mTOR’s regulation of MYC translation (Figure 2A) (14, 88). Further, PI3K/AKT inhibits mammalian FOXO, which when active antagonizes MYC through several mechanisms (89). FOXO3a can transactivate the MYC antagonist and transcriptional repressor, MXI-1 (also called MXD2), which dimerizes with MAX to bind and inhibit MYC target genes (90). Additionally, FOXO3a was documented to inhibit mitochondrial biogenesis by antagonizing MYC’s ability to activate genes involved in mitochondrial function (91, 92). Downstream of the mTOR complex 2 or MK5/PRAK, FOXO3a activity can also control MYC levels through induction of miR-34b/c (93, 94). Additionally, mTOR-dependent nutrient sensing controls MYC stability through the autophagy scaffolding protein AMBRA1 (95). When mTOR is inhibited by branched amino acid starvation, AMBRA1 promotes dephosphorylation of serine 62 of MYC by protein phosphatase 2 (PP2A). This dephosphorylation, which destabilizes MYC, is rescued by AMBRA1 downregulation (95). These studies collectively indicate that nutrient sufficiency and growth factor signaling are required for MYC to carry out its transcriptional program—a transcriptional program that serves to propagate the translational growth program triggered by mTOR, a key activator of cap-dependent translation (96).
In addition to nutrients, oxygen is also required for various metabolic enzymatic activities and for proper mitochondrial function. As such, limitation of oxygen, termed hypoxia, also regulates MYC function. In non-transformed cells, endogenous MYC function can be attenuated by hypoxia at several levels including protein stability and protein function. Under hypoxia, MYC protein levels are diminished by proteolytic degradation that can be accentuated by concurrent glucose deprivation (97, 98). Through substrate (O2) limitation, hypoxia diminishes hydroxylation of HIF-α subunits by prolyl hydroxylases (PHDs), which would otherwise lead to targeting of HIF-α subunits for rapid proteasomal degradation (99–101). Additionally, hypoxia-induced reactive oxygen species can contribute to the stabilization of HIF-1α (102). HIF-1α is pivotal for hypoxic survival through its transcriptional activation of target genes involved in glycolysis and attenuation of mitochondrial function. HIF-1α activates the expression of MXI-1, a MYC antagonist that attenuates MYC induced mitochondrial biogenesis (98, 103). Another study suggests that HIF-1α can compete directly with MYC by binding MAX (104). The complexity of the crosstalk between HIF and MYC is further heightened by the role of the HIF-1α target FOXO3a, which antagonizes MYC in a multitude of ways as discussed above (91, 92). As an attenuator of ROS (91), hypoxia-induced FOXO3a could reduce HIF-α stabilization, reminiscent of the negative feedback loop in which HIF-mediated activation of prolyl hydroxylases in turn decreases HIF-1α protein levels (105). Interestingly, however, overexpressed MYC can both overwhelm ROS-attenuating mechanisms induced by FOXO and bypass the repressive activity of HIF on MYC. HIF-1α and MYC, hence, can cooperate when MYC is overexpressed (106). By contrast, HIF-2α has been reported to promote MYC-MAX activity and therefore cooperates presumably with both endogenous and ectopic MYC (104). Nutrient and hypoxia sensing in non-transformed cells, therefore, are not equivalent to the rewiring of nutrient sensing and metabolism in MYC-transformed cells, which have lost many of the feedback regulatory loops that restrain cell growth under nutrient or oxygen deprivation.
MYC, intermediary metabolism, and macromolecular synthesis
Despite different levels, endogenous and oncogenic MYC appear to share target genes involved in several facets of intermediary metabolism from glycolysis and glutaminolysis to nucleotide and lipid synthesis (46). In this regard, we surmise that the posited gene expression amplifier function of MYC is compatible with MYC’s ability to alter metabolism across many cell types, particularly since nearly all cells basally express metabolic genes, such as those encoding enzymes required for glycolysis, mitochondrial function, and oxidative phosphorylation. When MYC is induced, metabolic genes that are already expressed would be further amplified to support the bioenergetic needs of the growing cell (46). In fact, many canonical MYC target genes involved in metabolism have conserved high affinity MYC consensus E-box binding sites in their proximal promoters (53, 107). Non-consensus sites to which MYC binds with lower affinities, particularly when MYC levels are high and deregulated, have also been identified (Figure 2B)(48). In addition to the presence of other transcription factors, the binding of MYC to any gene locus is dictated by open chromatin structure as well as the binding site affinity (53). Hence, it is hypothesized that MYC would occupy the highest affinity binding sites at lower levels of MYC (46). In retrospect, it does not seem surprising now that some of the earliest MYC-responsive genes identified are involved in metabolism, such as lactate dehydrogenase A (LDHA) and ornithine decarboxylase (ODC), enzymes involved in glycolysis and polyamine synthesis (108, 109). Both of these genes have canonical MYC E-boxes in their proximal promoter regions. As the low throughput candidate gene or subtraction cloning approaches are replaced by unbiased genome-wide gene expression and chromatin-immunoprecipitation (ChIP) analyses using next-generation sequencing, we now witness the entire spectrum of MYC’s transcriptional perturbation across the genome and its encoded metabolic pathways (48, 50, 53).
Glycolysis and Glutaminolysis
Cancer cells show profound metabolic changes that provide the energy and building blocks to sustain proliferation (Figure 4). The first noted change in cancer metabolism was the increased conversion of glucose to lactate discovered by Otto Warburg over 90 years ago (110, 111). Later studies indicated that glutamine, the most abundant circulating free amino acid in human plasma, can act as a source of carbon and nitrogen in cancer cells (112). In glutaminolysis, the enzyme glutaminase converts glutamine taken up by the cell to glutamate, which in turn, is converted by glutamate dehydrogenase or transaminases to α-ketoglutarate that is further catabolized in the TCA cycle (Figure 4). Similar to MYC dependent activated lymphocytes (68), MYC-transformed cells were found to have increased glucose and glutamine utilization and increased expression of key glycolytic and glutaminolytic enzymes (107, 108, 113–116). Previous studies documenting MYC’s regulation of glycolytic and glutaminolytic genes are now corroborated by genome-wide RNA-seq and ChIP-seq studies (48). MYC, in essence, regulates virtually all genes involved in glycolysis and many in glutaminolysis (113, 114, 117, 118). It also is particularly notable that MYC appears to not only drive expression of these genes but also favor specific mRNA splice variants, such as glycolysis-impacting PKM2 over PKM1 (119). Although resting cells also express many enzymes in these metabolic pathways, cells that are stimulated to grow must take up nutrients and catabolize these nutrients to make key building blocks. To achieve this end, MYC drives transcription to ultimately generate not only the enzymes that directly constitute these metabolic pathways but also the plasma membrane nutrient transporters needed to supply them. Key MYC targets include glucose membrane transporters such as GLUT1 (or SLC2A1) and glutamine transporter SLC1A5 (or ASCT2) (113, 114), loss of function of which diminishes cell proliferation and highlights their critical importance in cell growth (113, 115). Likewise, knock-down or inhibition of key enzymes in glycolysis or glutaminolysis diminishes cell growth.
MYC-driven buildup of glycolytic intermediates also fuels pathways that share intermediates with glycolysis. The pentose phosphate pathway (PPP), which uses glycolysis-derived glucose-6-phosphate as a starting substrate, produces the reducing equivalent NADPH and the nucleotide synthesis substrate ribose (Figure 4). Through upregulation of enzymes in the pathway, MYC has been shown to increase shunting of glucose to the PPP in both cancer and lymphocytes (68, 120). The glycolytic intermediate 3-phosphoglycerate can be shunted to synthesize serine (Figure 4), which in the mitochondria can be converted to glycine while producing 5,10-methylenetetrahydrofolate (5,10-CH2-THF) by the mitochondrial enzyme serine hydroxymethyltransferase 2 (SHMT2). 5,10-CH2-THF can then be converted to formate by methylenetetrahydrofolate dehydrogenase 2 (MTHFD2), which also generates the reducing equivalent NADPH. Folate plays a key role in nucleotide synthesis and the production of carbon donors that regulate epigenetic marks (121). While the role of MYC in regulating lymphocyte serine biosynthesis is poorly understood (68), serine biosynthesis increased in cancer by MYC through the elevation of the expression enzymes in the pathway (120, 122–125).
As both glucose and glutamine oxidation in the mitochondria generate reactive oxygen species, sufficient levels of the anti-oxidant tripeptide glutathione (L-glutamyl-L-cysteinyl-glycine) or peroxiredoxins (which are induced by MYC (126)), must be maintained to effectively titrate and attenuate these otherwise damaging byproducts. Glutamine-derived glutamate and glucose-derived glycine are themselves substrates for the synthesis of glutathione. Additionally, the NADPH derived from glucose through the PPP and serine metabolism, as well as glutamine-derived NADPH (via malic enzyme (127, 128)), is essential to the regeneration of glutathione, in addition to its role in reductive biosynthesis and redox homeostasis. Indeed, MYC-regulated SHMT2-dependent NADPH production was shown to be required for redox control and cell survival of MYC transformed cells in hypoxia (129). Intriguingly, SHMT2 was identified as the only gene that could partially rescue the slowed growth of Myc-null fibroblasts in an expression screen (130). Additionally, genetic reduction of MTHFD2 leads to oxidative stress, illustrating the importance of this pathway in a model cell culture system (131). Glucose and glutamine, hence, play a vital role in the production of ATP, reducing equivalents for biosynthesis, and building blocks for growing cells.
Metabolism of glucose and glutamine also produces toxic byproducts and acids that must be eliminated (Figure 4). Lactic acid produced from glucose is extruded by monocarboxylic acid transporters MCT1, a MYC target, and MCT4, a target of HIF-1α (132, 133). Inhibition of either of these transporters can markedly diminish cell growth or in vivo tumorigenesis (132, 133). Glutaminolysis involves the production of ammonia, which can be toxic. The mode by which cells eliminate ammonia metabolically is not well understood other than what we know about the urea cycle, which is found in specialized cells. Some cells express glutamine synthetase that can produce glutamine from glutamate and ammonia, while transaminases can deaminate glutamine and glutamate without producing ammonia (134, 135). Additional studies are necessary for fuller understanding of ammonia metabolism in cancer cells.
De novo nucleotide synthesis
The increased nucleotide synthesis required by cancers to maintain proliferation necessitates the coupling of nucleotide synthesis and the reprogramming of metabolism. MYC coordinately regulates nucleotide synthesis enzymes and other metabolic enzymes to achieve this increased nucleotide production (Figure 4). MYC binds to and regulates many genes involved in purine and pyrimidine synthesis (136, 137). Importantly, the generation of glycine from glucose and aspartate from glucose or glutamine are major contributors to the synthesis of purines and pyrimidines, respectively. In purine metabolism, MYC binds to the bi-directional promoter of phosphoribosyl pyrophosphate amidotransferase (PPAT) and phosphoribosylaminoimidazole carboxylase, phosphoriboxylaminoimidazole succinocarboxamide synthetase (PAICS), but regulates these genes differentially as they serve at different steps in purine synthesis. In pyrimidine synthesis, MYC is known to regulate carbamoyl-phosphate synthetase (CAD), one of the earliest identified MYC target genes (138). In addition, MYC directly regulates dihydroorate dehydrogenase (DHODH), an enzyme that couples with the mitochondrial electron transport chain to oxidize dihydroorotate to orotic acid, a pyrimidine precursor. In a time-series study of the human P493-6 lymphoma model cell line, these target genes had variable responses to MYC induction with phosphoribosylformylglycinamidine synthase (PFAS) in purine synthesis being most highly activated (136). Many of these genes were also induced by MYC in vivo using a transgenic model of inducible MYC in mouse liver (136). High-throughput genome-wide ChIP-seq experiments corroborated MYC binding to these genes as documented earlier by ChIP-PCR (48, 139, 140). Knockdown of MYC in various cell lines also resulted in diminished nucleotide gene expression providing the converse evidence for nucleotide metabolic genes as MYC targets. Further, ectopic expression of the MYC targets thymidylate synthetase (TS), inosine-5′-monophophate dehydrogenase (IMPDH2), and PRPS2 diminished the proliferative arrest caused by MYC knock-down, illustrating the functional role of nucleotide biosynthesis in MYC-induced cell growth (137).
In addition to glucose and glutamine, de novo synthesis of nucleotides also requires folate as co-factor for various enzymatic steps (121). De novo synthesis of purines occurs on the PPP-derived ribose-5-phosphate scaffold, which is derived from glucose. Biochemical activation of ribose to phosphoribose pyrophosphate (PRPP) by the enzyme phosphoribosyl pyrophosphate synthetase (PRPS2) and ATP prepares the scaffold for addition of a nitrogen from glutamine, which initiates the purine scaffold that is sequentially built up with glycine, additional glutamine nitrogens, and single carbons transferred from the folate carrier. MYC coordinates the increase in PPP activity, glycine and folate synthesis and glutamine uptake to fuel nucleotide production.
Nucleotide synthesis was recently linked to MYC-regulated protein synthesis for cell growth (Figure 3B). MYC has been shown to control protein synthesis through its involvement in ribosome biogenesis (detailed below), induction of eukaryotic translation initiation factors—including eukaryotic translation initiation factor eIF4E, an early documented MYC target gene (66, 141)—and its direct promotion of mRNA cap methylation(61). In essence, MYC regulates both ribosomal biosynthesis and components of the cap-dependent translation to control machinery to stimulate mRNA capping and protein synthesis (60, 61, 64–66, 141–145). Haploinsufficiency of the ribosomal protein-encoding Rpl24 gene was documented to diminish MYC-induced lymphomagenesis in Eμ-Myc transgenic mice, demonstrating the critical role of ribosome function and translation for MYC-induced tumorigenesis (146). A recent study found that Eμ-Myc mice with normalized translation rates dues to Rpl24 haploinsufficiency show reduced nucleotide pools in B cells expressing oncogenic MYC. Profiling of protein levels of nucleotide synthesis enzymes found that PRPS2 was unique in that its protein levels were markedly diminished in Rpl24 haploinsufficient MYC-driven cells. Although Prps2 is a direct transcriptional target of MYC (137), its translation is intriguingly regulated by a pyrimidine-rich translational element (PRTE) in the 5′ untranslated region of the Prps2 mRNA, which makes it proportionately sensitive to increased translation rates driven by MYC downstream of eIF4E activation(147). As mentioned, PRPS2 catalyzes a critical step in purine synthesis by converting ribose-5-phosphate to PRPP. Importantly, Prps2 knockdown is synthetically lethal in MYC-overexpressing cells, such that loss of PRPS2 prolonged the survival of transgenic mice with MYC-induced lymphoma. These studies illustrate that MYC-overexpressing cells are dependent on balanced translation and nucleotide synthesis, which are coupled through the regulation of PRPS2 translation.
Lipid synthesis
Membrane genesis is essential for a growing cell, and, correspondingly, MYC plays a key role in stimulating fatty acid and cholesterol synthesis. In addition to stimulating TCA cycle genes responsible for producing citrate, which is a critical precursor of fatty acids and cholesterol, MYC also activates the expression of the enzymes ATP citrate lyase (ACLY), acetyl-CoA carboxylase alpha (ACACA), fatty acid synthase (FASN), and stearoyl-CoA desaturase (SCD), which are all involved in fatty acid synthesis from citrate (148–150). ACLY converts citrate to acetyl-CoA in the cytosol and ACACA generates malonyl-CoA from acetyl-CoA. FASN catalyzes fatty acid chain elongation from the malonyl scaffold, while SCD monosaturates long chain fatty acids, for example converting palmitate to oleate. In addition, tracer studies document the role of MYC in driving labeled glucose carbons into fatty acids (149). Because glutamine can contribute to the TCA cycle, lipids also contain carbons derived from glutamine. As discussed above, the production of NADPH by the pentose phosphate, serine biosynthesis, and malic enzyme pathways is crucial to this reductive biosynthetic ability of MYC-driven cells.
Intriguingly, the ability of MYC to induce mitochondrial biogenesis (see below) and function appears to affect lipid metabolism indirectly. With loss of MYC function in knockout fibroblasts or in a neuroblastoma cell model, decreased mitochondrial production is associated with accumulation of lipid vacuoles, indicating that under unique circumstances MYC can stimulate fatty acid oxidation through increased mitochondrial biogenesis (150, 151), implying that MYC may play both anabolic and catabolic roles in lipid metabolism.
MYC, metabolism and organelle biogenesis
As a growth-promoting transcription factor, MYC stimulates metabolic pathways that support formation of new organelles, particularly ribosomes and mitochondria, which in turn is required for ATP generation and the production of many substrates for cell growth (152). The means by which a cell grows largely depends upon sufficient mitochondria, to produced building blocks and energy, and ribosomes to increase genomic output through translation.
Ribosomes
MYC is a unique transcription factor that stimulates transcription driven by all three RNA polymerases (I, II, and III) to produce components of the ribosome: rRNA, ribosomal proteins, and small 5S rRNAs (153, 154). MYC’s global ability to amplify gene expression allows MYC to relieve transcriptionally paused genes involved in ribosome biogenesis (142, 155). This role of MYC in the production of ribosomes is highlighted in Drosophila, as a hypomorphic dMyc allele causes the diminutive mutant fly phenotype (156–158). The small body and cell size associated with the diminutive fly phenocopies flies that belong to the large complementation group of small flies termed Minutes (159). Minutes are largely comprised of flies that have hypomorphic ribosomal protein genes. Thus, dMyc is linked to ribosome biogenesis through this group of Minute flies.
The links between MYC and ribosomes established in flies are further underscored by the enrichment of canonical MYC E-boxes in the promoters of ribosome biogenesis genes, conserved not only in flies but also down to the unicellular eukaryote, Nemostella (160). The connection between MYC and ribosome biogenesis is also recapitulated in mammals. Early studies of the P493-6 inducible-MYC human B lymphoma model cell line demonstrated that MYC could increase cell size independent of cell proliferation (161). MYC was shown by various studies of P493-6 cells to directly induce genes involved in ribosome biogenesis. In addition, acute adenoviral-mediated ectopic expression of MYC in mouse liver resulted in significant hepatocellular hypertrophy within a matter of days associated with highly elevated expression of ribosomal protein genes (162). MYC’s role in ribosome biogenesis is further illustrated by a cell-type-independent MYC target gene signature and the association of heightened ribosomal biogenesis in connection with MYC expression in human prostate cancer (163, 164). These studies collectively illustrate the ability of MYC to stimulate ribosome biogenesis, which is now corroborated by genome-wide studies of MYC target genes (48, 50, 53, 54). Intriguingly, haploinsufficiency of Myc prolongs mouse lifespan and is associated with a decrease in ribosome biogenesis (165). As treatment of mice with rapamycin, which inhibits mTORC1, or metformin, which inhibits mitochondrial Complex I activity can also increase lifespan, this study links lowered metabolic demands with longevity and further highlights the role of ribosomes in mediating phenotypic effects of MYC (166–168).
Mitochondria
Mitochondria are essential organelles not only because respiration and oxidative phosphorylation produce ATP, but also because they serve as vital hubs for a number of biosynthetic pathways including nucleotide, fatty acid, cholesterol, amino acid, and heme synthesis (169, 170). Iron is required for mitochondrial function and a key MYC responsive gene is the transferrin receptor, TFRC, which imports iron essential for cell growth (171). Further, mitochondria are important to support transcription, since mitochondrial mass directly influences an overall rate of transcription in a cell (126, 171–174). Thus, as a cell grows, the number of mitochondria must also increase. While factors that control mitochondrial biogenesis for homeostasis have been well-established, the means by which mitochondrial biogenesis occurs in response to cell growth has only more recently been proposed (169). Unlike resting cells, which maintain mitochondrial homeostasis through peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α) and peroxisome proliferator-activated receptors (PPARs), proliferating cells appear to be under the control of MYC, which coordinately activates the expression of PGC1β and the mitochondrial DNA polymerase gamma, as well as many components of the mitochondria, for mitochondrial growth or biogenesis (98, 113, 116, 126, 149, 173, 175, 176). Importantly, key components of the mitochondrial machinery that are induced by MYC seem to be vital because knocking down or inhibiting these elements is synthetically lethal in MYC-overexpressing cells (177, 178). In this regard, anti-retroviral agents for treatment of HIV, which can inhibit mitochondrial DNA polymerase gamma, may have activity against MYC-driven tumors (179).
MYC controls many components of intermediary metabolic pathways that are confined to the mitochondrion, thereby affecting mitochondrial function. Enzymes in these pathways include glutaminase (GLS), which converts glutamine to glutamate for use in the TCA cycle. IMPDH2 and DHODH are MYC targets that produce mitochondrial enzymes involved in purine and pyrimidine synthesis (see above). Components of the mitochondrial folate pathways, such as SHMT2 and MTHFD2, are MYC targets along with the vast majority of the TCA cycle enzymes that reside in the mitochondrion. In addition, MYC may also control mitochondrial dynamics, altering the rates of mitochondrial fusion and fission that also influence mitochondrial function (180). Correspondingly, loss of Myc function through Cre recombinase treatment of T cells with floxed allele of Myc or in knockout fibroblasts was associated with severely deficient mitochondrial mass and morphology (173). A key function of MYC, therefore, is the coordination of cell and mitochondrial growth, which is potentially exploitable for therapy.
Other organelles
Much less is understood about how MYC influences the generation or maintenance of other organelles, but it appears that MYC does not increase the genesis of all organelles. In particular, it appears that MYC suppresses lysosomal biogenesis and autophagy in several experimental systems. MYC can antagonize MIZ-1 functions, which include the maintenance of autophagic flux, a process requiring functional lysosomes (181). Further, genes that are involved in lysosome biogenesis, such as that encoding the transcription factor TFEB, which stimulates lysosome biogenesis, appear suppressed in the MYC-expressing P493-6 cell line (182). The curbing of the activities of organelles that are not critical for cell growth, such as lysosomes, underscores the coordinating activity of MYC in stimulating growth. The role of MYC in the Golgi apparatus or nuclear formation is not well understood.
MYC, metabolism, and cell cycle progression
As organelle biogenesis proceeds to support growth, nucleotide pools accumulate in preparation for entry into S phase and DNA synthesis. MYC, however, does not only indirectly regulate the cell cycle through metabolism, it also directly triggers cell cycle progression, particularly during the G1 restriction point, by activating genes such as cyclin D and cyclin-dependent kinase 4 (CDK4) (183–186). In addition, MYC activates the expression of E2F transcription factors, creating a feed-forward loop that promotes progression into S phase (187). MYC and E2F together activate key DNA replication genes, such as the family of minichromosome maintenance complex (MCM) genes, to initiate and sustain DNA replication (140, 188). The induction of the microRNA cluster, miR-17-92, by MYC attenuates E2F1 function as cells enter S phase, seemingly to adjust the rate of DNA replication. Elimination of the miR-17-92 loop results in DNA replication stress following serum-induced MYC expression and cell proliferation (189, 190). In addition, MYC may have roles in initiation of DNA replication independent of its transcriptional activities, as it has been noted to localize to early sites of DNA replication and bind numerous components of the pre-replicative complex (191). The coupling of G1 to S-phase entry involves glycolysis and glutaminolysis in synchronized HeLa cells, which are known to have high MYC expression (192–194). Progression in the G1 phase toward S phase requires glycolysis and the activity of 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3), which is inactivated by the anaphase-promoting complex/cyclosome-Cdh1 (APC/C-Cdh1). Beyond the restriction point, committed HeLa cells appear dependent on glutaminase (GLS), which was also shown to be inactivated by APC/C-Cdh1 as cells progress from S to G2/M phase (193). We surmise then that accumulation of nucleotides and entry into S phase are coupled to ensure that DNA replication occurs with the highest fidelity, since nucleotide pool imbalance can result in undesirable mutations (195).
Oncogenic MYC-mediated metabolic rewiring and cancer therapy
The very first notable success in targeting metabolism for cancer therapy involves the use of anti-folates by Sidney Farber to effectively treat childhood acute lymphocytic leukemia (ALL) with aminopterin in 1948 (196). Farber, knowing that folates stimulated bone marrow and leukemic cell growth, sought anti-folates and pioneered clinical studies that first failed with pteroylglutamic conjugates of folate—because these were in fact folate agonists rather than antagonists. The emergence of the antagonist, aminopterin, from the laboratory, however, transformed the treatment of childhood ALL with significant numbers of temporary clinical remissions in the initial 16 children who were treated. One of the children, Einar Gustafson who remained in remission and was the initial ‘Jimmy’ that inspired the extant fundraising Jimmy Fund, in fact, had the MYC-driven malignancy, Burkitt’s lymphoma (196). Aminopterin inhibits dihydrofolate reductase (DHFR), and it is now replaced by methotrexate and more potent derivatives that are still mainstays in the cancer therapeutic armamentarium. As discussed above, the production of single carbon folate compounds requires the production of serine from glucose through a series of enzymes whose genes are directly regulated by MYC (122–124). Moreover, the sensitivity of human cancer cell lines to methotrexate has been linked to the MYC target gene signature and specifically to genes that are involved in folate metabolism (123).
As previously discussed, endogenous and ectopic MYC share many common targets that are involved in metabolism. The key question, then, is whether oncogenic MYC rewires metabolism distinctly from endogenous MYC. As noted early, endogenous normal MYC expression is upregulated only when growth factor signaling is activated and nutrients are available. Either growth factor withdrawal or nutrient insufficiency can inhibit endogenous MYC expression. These feedback loops, however, are ineffective to control MYC expression when it is deregulated, for example, by chromosomal translocation or gene amplification. In instances where the feedback loops are broken, deregulated MYC enforces a constitutive cellular growth program independent of nutrient availability, particularly when accompanied by loss of checkpoints such as TP53. While MYC driven metabolism in normal cells can be turned off by lack of either growth factors or nutrients, cancer cells that are unable to turn off MYC, are addicted to nutrients such as glucose and glutamine (Figure 5A–D). While the metabolic programs are generally similar in cells expressing low levels of MYC and high levels of MYC (197)(Figure 5E), the addiction of constitutive MYC activated cancer cells to metabolism creates therapeutic vulnerabilities.
Figure 5.
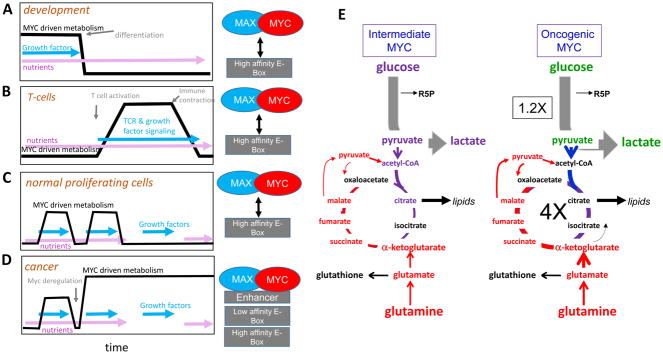
The effect of nutrient and growth factor availability on MYC driven metabolism. A) During cellular differentiation, loss of growth factor stimulation can turn off MYC- driven metabolism even in the presence of nutrients. B) In T cells, receptor stimulation and growth factors can drive MYC signaling, but withdraw of receptor stimulation and checkpoints turn off MYC-driven metabolism even in the presence of nutrients. C) In normal proliferating cells, MYC-driven metabolism is activated in the presence of both nutrients and growth factors, but individual exposure to nutrients or growth factors is not sufficient to activate MYC-driven metabolism. D) In cancer, MYC deregulation and loss of checkpoints leaves cells unable to turn off MYC-driven metabolism independent of growth factors and nutrient availability. The inability to turn off MYC-driven metabolism creates therapeutic vulnerabilities to metabolic inhibitors. E) Comparison of glucose and glutamine metabolism of cells expressing intermediate levels of MYC and oncogenic levels of MYC shows oncogenic levels of MYC cause small increase (1.2 fold) in lactate production and a large increase (4 fold) in TCA cycle flux (197).
With this framework in mind, it is surmised that loss of specific enzymatic activities would be synthetically lethal to MYC-overexpressing cells. Indeed, a screen using human fibroblasts overexpressing MYC revealed that loss of glucose metabolism genes (ALDOA and PDK1), nucleotide metabolism genes (CTPS), or transporters (SLC1A4 and SLC25A6) were synthetically lethal (198, 199). A recent limited screen for synthetic lethality aimed at the extended MYC transcription factor network revealed that MONDOA (178), a partner of MLX, is required for MYC-overexpressing cells. MLX binds the MXD proteins, which in turn interact with the MYC partner MAX, thus extending the MYC network (1). Because MONDOA had been linked to regulation of glucose metabolism, investigators performed a synthetic lethal screen directed at MONDOA and MYC target genes involved in metabolism (178). This revealed that individual losses of genes encoding glutamine/glutamate transporters (SLC1A5 and SLC3A2), purine metabolism enzymes (PFAS and CAD), cystathionine-beta-synthase (CBS), a mitochondrial transcription factor (TFAM), a glycolysis enzyme (ENO3), and lipogenesis enzymes (FASN and SCD) were synthetically lethal for MYC-overexpressing cells (178). These studies collectively indicate that MYC-overexpressing cells are metabolically addicted; hence, small molecule inhibitors of specific enzymes could be potentially applicable in cancer therapy.
Because MYC drives both glycolysis and glutaminolysis in vitro and in various in vivo models, targeting glycolytic and glutaminolytic enzymes has been of significant research interest. Knockout of hexokinase 2 (Hk2) in genetically engineered mice significantly blunts tumorigenesis in vivo, suggesting that HK2 is an attractive therapeutic target (200). Genetic inhibition of LDHA using siRNA, for example, diminishes tumor growth in several models of tumorigenesis including one driven by MYC (201–203). Genetic knockout of Ldha also diminishes tumorigenesis in transgenic models of cancers (204, 205). A tool compound that inhibits LDHA was documented to inhibit a MYC-driven lymphoma xenograft (206). Because other oncogenes could also cause glycolytic addiction, inhibition of LDH or LDHA could have a broad-based application across many cancers. To this end, there have been many attempts to generate new LDH inhibitors (207–211).
Pharmacological inhibition of glutaminase (GLS) has also been documented to curb tumor progression of an inducible MYC-driven human lymphoma xenograft model in a cell-autonomous fashion (212, 213). Further, loss of one copy of murine Gls decreases MYC-induced liver tumorigenesis, and a tool-compound inhibitor of GLS prolongs survival of these mice(214). These studies provided the foundation for the development of a drug-candidate glutaminase inhibitor that is now undergoing Phase I clinical studies in humans (215). Likewise, inhibition of the lactate exporter MCT1 can significantly inhibit MYC-mediated lymphomagenesis, and MCT1 inhibitors are likewise in clinical trials (132). NAMPT, an enzyme involved in NAD+ synthesis and a target of MYC, could also be another significant clinical target considering that the NAMPT inhibitor FK866 can profoundly inhibit MYC-induced lymphomagenesis and proliferation of lymphoma cell lines driven by other oncogenes (206). Additional inhibitors of NAMPT are being developed and some have already been studied clinically (216). Thus, it appears that MYC and potentially other oncogene-driven cancers are dependent on metabolic enzymes that could be explored and exploited for cancer therapy. In this regard, PIK3CA-, BRAF-, and RAS-driven cancers are dependent on MYC, such that resistance mechanisms to targeted inhibitors may result from MYC amplification or re-wiring of cellular metabolism (217–219). Given the extent to which cancer cells rewire metabolism, it stands to reason that combination therapy would be the most promising metabolic inhibition strategy in the clinic. For example, combination strategies, such as PI3K and MYC inhibition for breast cancer, BRAF and mitochondrial Complex I inhibition (phenformin) for melanoma, BRAF and pyruvate dehydrogenase inhibition for melanoma, mTOR and glutaminase inhibition for glioblastoma, and HSP90 and glutaminase inhibition for mTOR activated cancer, appear to have profound preclinical impact on tumorigenesis (220–223). This exciting area of cancer metabolism research has generated deeper and richer understanding of the relationships between oncogenic drivers and metabolism that will guide the field toward successful clinical applications of basic discoveries.
Conclusion and future outlook
MYC has been an enigmatic oncogene in that it seems to affect all cellular processes, most prominently cell metabolism and ribosome biogenesis. At the molecular level, this enigma could be resolved by considering evidence that suggests MYC is a general modulator of gene expression with its targets dictated by binding-site sequence affinity and chromatin accessibility and its directionality and magnitude of impact determined by the transcriptional potential of gene loci that are co-regulated by other transcription factors. All cells, including stem cells, are metabolically active and basally express metabolic genes whose chromatin is open. Thus, quiescent stem cells in the proliferative compartments of tissues are poised to proliferate upon stimulation by growth factors that trigger MYC expression. Upon such MYC activation, metabolic genes, which are already expressed, are further amplified to support the bioenergetic needs of the growing cell in its exit from the stem cell pool and differentiation down a cell lineage. Normal MYC is restrained in this role not only by growth factor presence but also nutrient availability. Oncogenic MYC, on the other hand, not only drives metabolic genes already regulated by endogenous MYC, but—owing to its deregulated expression—appears to invade enhancer sequences that further amplify gene expression in a non-linear fashion. This skewed gene-expression amplification leads to non-stoichiometric expression of biochemical pathways, activation of the unfolded protein response (UPR) pathway, and dependence on MONDOA, which all render MYC-overexpressing cells susceptible to synthetic lethality when specific metabolic pathways are inhibited. Here, basic science research in cancer metabolism has led to a number of new insights and identification of therapeutic opportunities, which hopefully will prove to advance the treatment of cancer patients. The field now looks to a future of new drugs targeting metabolism, which in combination with other drugs and modalities hold the promise of being impactful in the clinic.
Statement of Significance.
MYC’s expression and activity are tightly regulated in normal cells by multiple mechanisms, including a dependence upon growth factor stimulation and replete nutrient status. In cancer, genetic deregulation of MYC expression and loss of checkpoint components, such as TP53, permit MYC to drive malignant transformation. However, because of the reliance of MYC-driven cancers on specific metabolic pathways, synthetic lethal interactions between MYC overexpression and specific enzyme inhibitors provide novel cancer therapeutic opportunities.
Acknowledgments
We would like to apologize to researchers whose work we could not cite due to space considerations.
Grant Support
This work was supported by the Abramson Family Cancer Research Institute, Leukemia & Lymphoma Society Grant 6106-14 (CVD), NCI grants R01CA057341 (CVD) R01CA051497 (CVD) and P30CA16620 (CVD). Z.E.W. was supported by NCI grant 5T32CA009140-40. Z.E.S. is supported by NCI grant 5F32CA174148. B.J.A. is supported by NCI grant 1F32CA180370.
Footnotes
The authors declare no conflict of interest.
Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Conacci-Sorrell M, McFerrin L, Eisenman RN. An overview of MYC and its interactome. Cold Spring Harbor perspectives in medicine. 2014;4:a014357. doi: 10.1101/cshperspect.a014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duesberg PH, Bister K, Vogt PK. The RNA of avian acute leukemia virus MC29. Proceedings of the National Academy of Sciences of the United States of America. 1977;74:4320–4. doi: 10.1073/pnas.74.10.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheiness D, Fanshier L, Bishop JM. Identification of nucleotide sequences which may encode the oncogenic capacity of avian retrovirus MC29. Journal of virology. 1978;28:600–10. doi: 10.1128/jvi.28.2.600-610.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bister K, Jansen HW. Oncogenes in retroviruses and cells: biochemistry and molecular genetics. Advances in cancer research. 1986;47:99–188. doi: 10.1016/s0065-230x(08)60199-2. [DOI] [PubMed] [Google Scholar]
- 5.Dalla-Favera R, Bregni M, Erikson J, Patterson D, Gallo RC, Croce CM. Human c-myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proceedings of the National Academy of Sciences of the United States of America. 1982;79:7824–7. doi: 10.1073/pnas.79.24.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Land H, Parada LF, Weinberg RA. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983;304:596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- 7.Zack TI, Schumacher SE, Carter SL, Cherniack AD, Saksena G, Tabak B, et al. Pan-cancer patterns of somatic copy number alteration. Nature genetics. 2013;45:1134–40. doi: 10.1038/ng.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dang CV. MYC on the path to cancer. Cell. 2012;149:22–35. doi: 10.1016/j.cell.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morton JP, Sansom OJ. MYC-y mice: from tumour initiation to therapeutic targeting of endogenous MYC. Molecular oncology. 2013;7:248–58. doi: 10.1016/j.molonc.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levens D. You Don’t Muck with MYC. Genes & cancer. 2010;1:547–54. doi: 10.1177/1947601910377492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lal A, Navarro F, Maher CA, Maliszewski LE, Yan N, O’Day E, et al. miR-24 Inhibits cell proliferation by targeting E2F2, MYC, and other cell-cycle genes via binding to “seedless” 3′UTR microRNA recognition elements. Molecular cell. 2009;35:610–25. doi: 10.1016/j.molcel.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim HH, Kuwano Y, Srikantan S, Lee EK, Martindale JL, Gorospe M. HuR recruits let-7/RISC to repress c-Myc expression. Genes & development. 2009;23:1743–8. doi: 10.1101/gad.1812509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sampson VB, Rong NH, Han J, Yang Q, Aris V, Soteropoulos P, et al. MicroRNA let-7a down-regulates MYC and reverts MYC-induced growth in Burkitt lymphoma cells. Cancer Res. 2007;67:9762–70. doi: 10.1158/0008-5472.CAN-07-2462. [DOI] [PubMed] [Google Scholar]
- 14.Wall M, Poortinga G, Hannan KM, Pearson RB, Hannan RD, McArthur GA. Translational control of c-MYC by rapamycin promotes terminal myeloid differentiation. Blood. 2008;112:2305–17. doi: 10.1182/blood-2007-09-111856. [DOI] [PubMed] [Google Scholar]
- 15.Tseng YY, Moriarity BS, Gong W, Akiyama R, Tiwari A, Kawakami H, et al. PVT1 dependence in cancer with MYC copy-number increase. Nature. 2014;512:82–6. doi: 10.1038/nature13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farrell AS, Sears RC. MYC degradation. Cold Spring Harbor perspectives in medicine. 2014:4. doi: 10.1101/cshperspect.a014365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sears R, Leone G, DeGregori J, Nevins JR. Ras enhances Myc protein stability. Molecular cell. 1999;3:169–79. doi: 10.1016/s1097-2765(00)80308-1. [DOI] [PubMed] [Google Scholar]
- 18.Sears R, Nuckolls F, Haura E, Taya Y, Tamai K, Nevins JR. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes & development. 2000;14:2501–14. doi: 10.1101/gad.836800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magudia K, Lahoz A, Hall A. K-Ras and B-Raf oncogenes inhibit colon epithelial polarity establishment through up-regulation of c-myc. The Journal of cell biology. 2012;198:185–94. doi: 10.1083/jcb.201202108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee SH, Hu LL, Gonzalez-Navajas J, Seo GS, Shen C, Brick J, et al. ERK activation drives intestinal tumorigenesis in Apc(min/+) mice. Nature medicine. 2010;16:665–70. doi: 10.1038/nm.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hung CL, Wang LY, Yu YL, Chen HW, Srivastava S, Petrovics G, et al. A long noncoding RNA connects c-Myc to tumor metabolism. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:18697–702. doi: 10.1073/pnas.1415669112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yashiro-Ohtani Y, Wang H, Zang C, Arnett KL, Bailis W, Ho Y, et al. Long-range enhancer activity determines Myc sensitivity to Notch inhibitors in T cell leukemia. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E4946–53. doi: 10.1073/pnas.1407079111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herranz D, Ambesi-Impiombato A, Palomero T, Schnell SA, Belver L, Wendorff AA, et al. A NOTCH1-driven MYC enhancer promotes T cell development, transformation and acute lymphoblastic leukemia. Nature medicine. 2014;20:1130–7. doi: 10.1038/nm.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi J, Whyte WA, Zepeda-Mendoza CJ, Milazzo JP, Shen C, Roe JS, et al. Role of SWI/SNF in acute leukemia maintenance and enhancer-mediated Myc regulation. Genes & development. 2013;27:2648–62. doi: 10.1101/gad.232710.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiang JF, Yin QF, Chen T, Zhang Y, Zhang XO, Wu Z, et al. Human colorectal cancer-specific CCAT1-L lncRNA regulates long-range chromatin interactions at the MYC locus. Cell research. 2014;24:513–31. doi: 10.1038/cr.2014.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roussel MF, Cleveland JL, Shurtleff SA, Sherr CJ. Myc rescue of a mutant CSF-1 receptor impaired in mitogenic signalling. Nature. 1991;353:361–3. doi: 10.1038/353361a0. [DOI] [PubMed] [Google Scholar]
- 27.Bowman T, Broome MA, Sinibaldi D, Wharton W, Pledger WJ, Sedivy JM, et al. Stat3-mediated Myc expression is required for Src transformation and PDGF-induced mitogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:7319–24. doi: 10.1073/pnas.131568898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barone MV, Courtneidge SA. Myc but not Fos rescue of PDGF signalling block caused by kinase-inactive Src. Nature. 1995;378:509–12. doi: 10.1038/378509a0. [DOI] [PubMed] [Google Scholar]
- 29.Lau LF, Nathans D. Expression of a set of growth-related immediate early genes in BALB/c 3T3 cells: coordinate regulation with c-fos or c-myc. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:1182–6. doi: 10.1073/pnas.84.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palomero T, Lim WK, Odom DT, Sulis ML, Real PJ, Margolin A, et al. NOTCH1 directly regulates c-MYC and activates a feed-forward-loop transcriptional network promoting leukemic cell growth. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:18261–6. doi: 10.1073/pnas.0606108103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weng AP, Millholland JM, Yashiro-Ohtani Y, Arcangeli ML, Lau A, Wai C, et al. c-Myc is an important direct target of Notch1 in T-cell acute lymphoblastic leukemia/lymphoma. Genes & development. 2006;20:2096–109. doi: 10.1101/gad.1450406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chou YT, Lin HH, Lien YC, Wang YH, Hong CF, Kao YR, et al. EGFR promotes lung tumorigenesis by activating miR-7 through a Ras/ERK/Myc pathway that targets the Ets2 transcriptional repressor ERF. Cancer research. 2010;70:8822–31. doi: 10.1158/0008-5472.CAN-10-0638. [DOI] [PubMed] [Google Scholar]
- 33.Grisanzio C, Freedman ML. Chromosome 8q24-Associated Cancers and MYC. Genes & cancer. 2010;1:555–9. doi: 10.1177/1947601910381380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmadiyeh N, Pomerantz MM, Grisanzio C, Herman P, Jia L, Almendro V, et al. 8q24 prostate, breast, and colon cancer risk loci show tissue-specific long-range interaction with MYC. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9742–6. doi: 10.1073/pnas.0910668107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tuupanen S, Turunen M, Lehtonen R, Hallikas O, Vanharanta S, Kivioja T, et al. The common colorectal cancer predisposition SNP rs6983267 at chromosome 8q24 confers potential to enhanced Wnt signaling. Nature genetics. 2009;41:885–90. doi: 10.1038/ng.406. [DOI] [PubMed] [Google Scholar]
- 36.Yochum GS, Sherrick CM, Macpartlin M, Goodman RH. A beta-catenin/TCF-coordinated chromatin loop at MYC integrates 5′ and 3′ Wnt responsive enhancers. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:145–50. doi: 10.1073/pnas.0912294107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eischen CM, Roussel MF, Korsmeyer SJ, Cleveland JL. Bax loss impairs Myc-induced apoptosis and circumvents the selection of p53 mutations during Myc-mediated lymphomagenesis. Molecular and cellular biology. 2001;21:7653–62. doi: 10.1128/MCB.21.22.7653-7662.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zindy F, Eischen CM, Randle DH, Kamijo T, Cleveland JL, Sherr CJ, et al. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes & development. 1998;12:2424–33. doi: 10.1101/gad.12.15.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muthalagu N, Junttila MR, Wiese KE, Wolf E, Morton J, Bauer B, et al. BIM is the primary mediator of MYC-induced apoptosis in multiple solid tissues. Cell reports. 2014;8:1347–53. doi: 10.1016/j.celrep.2014.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaur M, Cole MD. MYC acts via the PTEN tumor suppressor to elicit autoregulation and genome-wide gene repression by activation of the Ezh2 methyltransferase. Cancer research. 2013;73:695–705. doi: 10.1158/0008-5472.CAN-12-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bouchard C, Lee S, Paulus-Hock V, Loddenkemper C, Eilers M, Schmitt CA. FoxO transcription factors suppress Myc-driven lymphomagenesis via direct activation of Arf. Genes & development. 2007;21:2775–87. doi: 10.1101/gad.453107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qi Y, Gregory MA, Li Z, Brousal JP, West K, Hann SR. p19ARF directly and differentially controls the functions of c-Myc independently of p53. Nature. 2004;431:712–7. doi: 10.1038/nature02958. [DOI] [PubMed] [Google Scholar]
- 43.Bouchard C, Marquardt J, Bras A, Medema RH, Eilers M. Myc-induced proliferation and transformation require Akt-mediated phosphorylation of FoxO proteins. The EMBO journal. 2004;23:2830–40. doi: 10.1038/sj.emboj.7600279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmitz R, Young RM, Ceribelli M, Jhavar S, Xiao W, Zhang M, et al. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature. 2012;490:116–20. doi: 10.1038/nature11378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmitz R, Ceribelli M, Pittaluga S, Wright G, Staudt LM. Oncogenic mechanisms in Burkitt lymphoma. Cold Spring Harbor perspectives in medicine. 2014:4. doi: 10.1101/cshperspect.a014282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolf E, Lin CY, Eilers M, Levens DL. Taming of the beast: shaping Myc-dependent amplification. Trends in cell biology. 2015;25:241–8. doi: 10.1016/j.tcb.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rahl PB, Lin CY, Seila AC, Flynn RA, McCuine S, Burge CB, et al. c-Myc regulates transcriptional pause release. Cell. 2010;141:432–45. doi: 10.1016/j.cell.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin CY, Loven J, Rahl PB, Paranal RM, Burge CB, Bradner JE, et al. Transcriptional amplification in tumor cells with elevated c-Myc. Cell. 2012;151:56–67. doi: 10.1016/j.cell.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levens D. Cellular MYCro economics: Balancing MYC function with MYC expression. Cold Spring Harbor perspectives in medicine. 2013:3. doi: 10.1101/cshperspect.a014233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nie Z, Hu G, Wei G, Cui K, Yamane A, Resch W, et al. c-Myc is a universal amplifier of expressed genes in lymphocytes and embryonic stem cells. Cell. 2012;151:68–79. doi: 10.1016/j.cell.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guccione E, Martinato F, Finocchiaro G, Luzi L, Tizzoni L, Dall’Olio V, et al. Myc-binding-site recognition in the human genome is determined by chromatin context. Nature cell biology. 2006;8:764–70. doi: 10.1038/ncb1434. [DOI] [PubMed] [Google Scholar]
- 52.Dang CV. GENE REGULATION Fine-tuned amplification in cells. Nature. 2014;511:417–8. doi: 10.1038/nature13518. [DOI] [PubMed] [Google Scholar]
- 53.Sabo A, Kress TR, Pelizzola M, de Pretis S, Gorski MM, Tesi A, et al. Selective transcriptional regulation by Myc in cellular growth control and lymphomagenesis. Nature. 2014;511:488–92. doi: 10.1038/nature13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walz S, Lorenzin F, Morton J, Wiese KE, von Eyss B, Herold S, et al. Activation and repression by oncogenic MYC shape tumour-specific gene expression profiles. Nature. 2014;511:483–7. doi: 10.1038/nature13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomas LR, Wang Q, Grieb BC, Phan J, Foshage AM, Sun Q, et al. Interaction with WDR5 Promotes Target Gene Recognition and Tumorigenesis by MYC. Molecular cell. 2015 doi: 10.1016/j.molcel.2015.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soufi A, Garcia MF, Jaroszewicz A, Osman N, Pellegrini M, Zaret KS. Pioneer transcription factors target partial DNA motifs on nucleosomes to initiate reprogramming. Cell. 2015;161:555–68. doi: 10.1016/j.cell.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soufi A, Donahue G, Zaret KS. Facilitators and impediments of the pluripotency reprogramming factors’ initial engagement with the genome. Cell. 2012;151:994–1004. doi: 10.1016/j.cell.2012.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sander S, Bullinger L, Klapproth K, Fiedler K, Kestler HA, Barth TF, et al. MYC stimulates EZH2 expression by repression of its negative regulator miR-26a. Blood. 2008;112:4202–12. doi: 10.1182/blood-2008-03-147645. [DOI] [PubMed] [Google Scholar]
- 59.Dang CV, Eisenman RN. MYC and the Pathway to Cancer. 1. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2014. [Google Scholar]
- 60.van Riggelen J, Yetil A, Felsher DW. MYC as a regulator of ribosome biogenesis and protein synthesis. Nature reviews Cancer. 2010;10:301–9. doi: 10.1038/nrc2819. [DOI] [PubMed] [Google Scholar]
- 61.Cole MD, Cowling VH. Specific regulation of mRNA cap methylation by the c-Myc and E2F1 transcription factors. Oncogene. 2009;28:1169–75. doi: 10.1038/onc.2008.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cowling VH, Cole MD. The Myc transactivation domain promotes global phosphorylation of the RNA polymerase II carboxy-terminal domain independently of direct DNA binding. Molecular and cellular biology. 2007;27:2059–73. doi: 10.1128/MCB.01828-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fernandez-Sanchez ME, Gonatopoulos-Pournatzis T, Preston G, Lawlor MA, Cowling VH. S-adenosyl homocysteine hydrolase is required for Myc-induced mRNA cap methylation, protein synthesis, and cell proliferation. Molecular and cellular biology. 2009;29:6182–91. doi: 10.1128/MCB.00973-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin CJ, Cencic R, Mills JR, Robert F, Pelletier J. c-Myc and eIF4F are components of a feedforward loop that links transcription and translation. Cancer research. 2008;68:5326–34. doi: 10.1158/0008-5472.CAN-07-5876. [DOI] [PubMed] [Google Scholar]
- 65.Lin CJ, Nasr Z, Premsrirut PK, Porco JA, Jr, Hippo Y, Lowe SW, et al. Targeting synthetic lethal interactions between Myc and the eIF4F complex impedes tumorigenesis. Cell reports. 2012;1:325–33. doi: 10.1016/j.celrep.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rosenwald IB, Rhoads DB, Callanan LD, Isselbacher KJ, Schmidt EV. Increased expression of eukaryotic translation initiation factors eIF-4E and eIF-2 alpha in response to growth induction by c-myc. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:6175–8. doi: 10.1073/pnas.90.13.6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bywater MJ, Pearson RB, McArthur GA, Hannan RD. Dysregulation of the basal RNA polymerase transcription apparatus in cancer. Nature reviews Cancer. 2013;13:299–314. doi: 10.1038/nrc3496. [DOI] [PubMed] [Google Scholar]
- 68.Wang R, Dillon CP, Shi LZ, Milasta S, Carter R, Finkelstein D, et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35:871–82. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiological reviews. 1997;77:731–58. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- 70.Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nature reviews Drug discovery. 2013;12:931–47. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- 71.Cairns RA, Harris I, McCracken S, Mak TW. Cancer cell metabolism. Cold Spring Harbor symposia on quantitative biology. 2011;76:299–311. doi: 10.1101/sqb.2011.76.012856. [DOI] [PubMed] [Google Scholar]
- 72.O’Sullivan D, van der Windt GJ, Huang SC, Curtis JD, Chang CH, Buck MD, et al. Memory CD8(+) T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity. 2014;41:75–88. doi: 10.1016/j.immuni.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lagadinou ED, Sach A, Callahan K, Rossi RM, Neering SJ, Minhajuddin M, et al. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell stem cell. 2013;12:329–41. doi: 10.1016/j.stem.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Samudio I, Harmancey R, Fiegl M, Kantarjian H, Konopleva M, Korchin B, et al. Pharmacologic inhibition of fatty acid oxidation sensitizes human leukemia cells to apoptosis induction. The Journal of clinical investigation. 2010;120:142–56. doi: 10.1172/JCI38942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lippman SI, Broach JR. Protein kinase A and TORC1 activate genes for ribosomal biogenesis by inactivating repressors encoded by Dot6 and its homolog Tod6. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:19928–33. doi: 10.1073/pnas.0907027106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Neiman AM. Sporulation in the budding yeast Saccharomyces cerevisiae. Genetics. 2011;189:737–65. doi: 10.1534/genetics.111.127126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Loomis WF. Cell signaling during development of Dictyostelium. Developmental biology. 2014;391:1–16. doi: 10.1016/j.ydbio.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee S, Comer FI, Sasaki A, McLeod IX, Duong Y, Okumura K, et al. TOR complex 2 integrates cell movement during chemotaxis and signal relay in Dictyostelium. Molecular biology of the cell. 2005;16:4572–83. doi: 10.1091/mbc.E05-04-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang Y, Ezemaduka AN, Tang Y, Chang Z. Understanding the mechanism of the dormant dauer formation of C. elegans: from genetics to biochemistry. IUBMB life. 2009;61:607–12. doi: 10.1002/iub.211. [DOI] [PubMed] [Google Scholar]
- 80.Jia K, Chen D, Riddle DL. The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development. 2004;131:3897–906. doi: 10.1242/dev.01255. [DOI] [PubMed] [Google Scholar]
- 81.Brandhorst S, Choi IY, Wei M, Cheng CW, Sedrakyan S, Navarrete G, et al. A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell metabolism. 2015 doi: 10.1016/j.cmet.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Berg JM, Tymoczko JL, Stryer L. Biochemistry. 5. New York: W. H. Freeman and Company; 2002. [Google Scholar]
- 83.Hensley CT, Wasti AT, DeBerardinis RJ. Glutamine and cancer: cell biology, physiology, and clinical opportunities. The Journal of clinical investigation. 2013;123:3678–84. doi: 10.1172/JCI69600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–93. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Donnelly N, Gorman AM, Gupta S, Samali A. The eIF2alpha kinases: their structures and functions. Cellular and molecular life sciences: CMLS. 2013;70:3493–511. doi: 10.1007/s00018-012-1252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes & development. 2011;25:1895–908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Teleman AA, Hietakangas V, Sayadian AC, Cohen SM. Nutritional control of protein biosynthetic capacity by insulin via Myc in Drosophila. Cell metabolism. 2008;7:21–32. doi: 10.1016/j.cmet.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 88.Csibi A, Lee G, Yoon SO, Tong H, Ilter D, Elia I, et al. The mTORC1/S6K1 pathway regulates glutamine metabolism through the eIF4B-dependent control of c-Myc translation. Current biology: CB. 2014;24:2274–80. doi: 10.1016/j.cub.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 89.Peck B, Ferber EC, Schulze A. Antagonism between FOXO and MYC Regulates Cellular Powerhouse. Frontiers in oncology. 2013;3:96. doi: 10.3389/fonc.2013.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Delpuech O, Griffiths B, East P, Essafi A, Lam EW, Burgering B, et al. Induction of Mxi1-SR alpha by FOXO3a contributes to repression of Myc-dependent gene expression. Molecular and cellular biology. 2007;27:4917–30. doi: 10.1128/MCB.01789-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ferber EC, Peck B, Delpuech O, Bell GP, East P, Schulze A. FOXO3a regulates reactive oxygen metabolism by inhibiting mitochondrial gene expression. Cell death and differentiation. 2012;19:968–79. doi: 10.1038/cdd.2011.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jensen KS, Binderup T, Jensen KT, Therkelsen I, Borup R, Nilsson E, et al. FoxO3A promotes metabolic adaptation to hypoxia by antagonizing Myc function. The EMBO journal. 2011;30:4554–70. doi: 10.1038/emboj.2011.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Masui K, Tanaka K, Akhavan D, Babic I, Gini B, Matsutani T, et al. mTOR complex 2 controls glycolytic metabolism in glioblastoma through FoxO acetylation and upregulation of c-Myc. Cell metabolism. 2013;18:726–39. doi: 10.1016/j.cmet.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kress TR, Cannell IG, Brenkman AB, Samans B, Gaestel M, Roepman P, et al. The MK5/PRAK kinase and Myc form a negative feedback loop that is disrupted during colorectal tumorigenesis. Molecular cell. 2011;41:445–57. doi: 10.1016/j.molcel.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 95.Cianfanelli V, Fuoco C, Lorente M, Salazar M, Quondamatteo F, Gherardini PF, et al. AMBRA1 links autophagy to cell proliferation and tumorigenesis by promoting c-Myc dephosphorylation and degradation. Nature cell biology. 2015;17:20–30. doi: 10.1038/ncb3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Laplante M, Sabatini DM. Regulation of mTORC1 and its impact on gene expression at a glance. Journal of cell science. 2013;126:1713–9. doi: 10.1242/jcs.125773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Okuyama H, Endo H, Akashika T, Kato K, Inoue M. Downregulation of c-MYC protein levels contributes to cancer cell survival under dual deficiency of oxygen and glucose. Cancer research. 2010;70:10213–23. doi: 10.1158/0008-5472.CAN-10-2720. [DOI] [PubMed] [Google Scholar]
- 98.Zhang H, Gao P, Fukuda R, Kumar G, Krishnamachary B, Zeller KI, et al. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer cell. 2007;11:407–20. doi: 10.1016/j.ccr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 99.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ackerman D, Simon MC. Hypoxia, lipids, and cancer: surviving the harsh tumor microenvironment. Trends in cell biology. 2014;24:472–8. doi: 10.1016/j.tcb.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat Rev Cancer. 2008;8:967–75. doi: 10.1038/nrc2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, et al. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. The Journal of biological chemistry. 2000;275:25130–8. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 103.Corn PG, Ricci MS, Scata KA, Arsham AM, Simon MC, Dicker DT, et al. Mxi1 is induced by hypoxia in a HIF-1-dependent manner and protects cells from c-Myc-induced apoptosis. Cancer biology & therapy. 2005;4:1285–94. doi: 10.4161/cbt.4.11.2299. [DOI] [PubMed] [Google Scholar]
- 104.Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC. HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer cell. 2007;11:335–47. doi: 10.1016/j.ccr.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.D’Angelo G, Duplan E, Boyer N, Vigne P, Frelin C. Hypoxia up-regulates prolyl hydroxylase activity: a feedback mechanism that limits HIF-1 responses during reoxygenation. The Journal of biological chemistry. 2003;278:38183–7. doi: 10.1074/jbc.M302244200. [DOI] [PubMed] [Google Scholar]
- 106.Dang CV, Kim JW, Gao P, Yustein J. The interplay between MYC and HIF in cancer. Nat Rev Cancer. 2008;8:51–6. doi: 10.1038/nrc2274. [DOI] [PubMed] [Google Scholar]
- 107.Kim JW, Zeller KI, Wang Y, Jegga AG, Aronow BJ, O’Donnell KA, et al. Evaluation of myc E-box phylogenetic footprints in glycolytic genes by chromatin immunoprecipitation assays. Molecular and cellular biology. 2004;24:5923–36. doi: 10.1128/MCB.24.13.5923-5936.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shim H, Dolde C, Lewis BC, Wu CS, Dang G, Jungmann RA, et al. c-Myc transactivation of LDH-A: implications for tumor metabolism and growth. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:6658–63. doi: 10.1073/pnas.94.13.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bello-Fernandez C, Packham G, Cleveland JL. The ornithine decarboxylase gene is a transcriptional target of c-Myc. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:7804–8. doi: 10.1073/pnas.90.16.7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–70. [PubMed] [Google Scholar]
- 111.Warburg O, Posener K, Negelein E. Ueber den Stoffwechsel der Tumoren. Biochemische Zeitschrift. 1924;152:319–44. [Google Scholar]
- 112.DeBerardinis RJ, Cheng T. Q’s next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene. 2010;29:313–24. doi: 10.1038/onc.2009.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458:762–5. doi: 10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:18782–7. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Osthus RC, Shim H, Kim S, Li Q, Reddy R, Mukherjee M, et al. Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. The Journal of biological chemistry. 2000;275:21797–800. doi: 10.1074/jbc.C000023200. [DOI] [PubMed] [Google Scholar]
- 116.Fan Y, Dickman KG, Zong WX. Akt and c-Myc differentially activate cellular metabolic programs and prime cells to bioenergetic inhibition. The Journal of biological chemistry. 2010;285:7324–33. doi: 10.1074/jbc.M109.035584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yuneva MO, Fan TW, Allen TD, Higashi RM, Ferraris DV, Tsukamoto T, et al. The metabolic profile of tumors depends on both the responsible genetic lesion and tissue type. Cell metabolism. 2012;15:157–70. doi: 10.1016/j.cmet.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hu S, Balakrishnan A, Bok RA, Anderton B, Larson PE, Nelson SJ, et al. 13C-pyruvate imaging reveals alterations in glycolysis that precede c-Myc-induced tumor formation and regression. Cell metabolism. 2011;14:131–42. doi: 10.1016/j.cmet.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.David CJ, Chen M, Assanah M, Canoll P, Manley JL. HnRNP proteins controlled by c-Myc deregulate pyruvate kinase mRNA splicing in cancer. Nature. 2010;463:364–8. doi: 10.1038/nature08697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Morrish F, Isern N, Sadilek M, Jeffrey M, Hockenbery DM. c-Myc activates multiple metabolic networks to generate substrates for cell-cycle entry. Oncogene. 2009;28:2485–91. doi: 10.1038/onc.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Locasale JW. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat Rev Cancer. 2013;13:572–83. doi: 10.1038/nrc3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tedeschi PM, Markert EK, Gounder M, Lin H, Dvorzhinski D, Dolfi SC, et al. Contribution of serine, folate and glycine metabolism to the ATP, NADPH and purine requirements of cancer cells. Cell death & disease. 2013;4:e877. doi: 10.1038/cddis.2013.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vazquez A, Tedeschi PM, Bertino JR. Overexpression of the mitochondrial folate and glycine-serine pathway: a new determinant of methotrexate selectivity in tumors. Cancer research. 2013;73:478–82. doi: 10.1158/0008-5472.CAN-12-3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vazquez A, Markert EK, Oltvai ZN. Serine biosynthesis with one carbon catabolism and the glycine cleavage system represents a novel pathway for ATP generation. PloS one. 2011;6:e25881. doi: 10.1371/journal.pone.0025881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sun L, Song L, Wan Q, Wu G, Li X, Wang Y, et al. cMyc-mediated activation of serine biosynthesis pathway is critical for cancer progression under nutrient deprivation conditions. Cell research. 2015 doi: 10.1038/cr.2015.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wonsey DR, Zeller KI, Dang CV. The c-Myc target gene PRDX3 is required for mitochondrial homeostasis and neoplastic transformation. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:6649–54. doi: 10.1073/pnas.102523299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19345–50. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M, et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496:101–5. doi: 10.1038/nature12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ye J, Fan J, Venneti S, Wan YW, Pawel BR, Zhang J, et al. Serine catabolism regulates mitochondrial redox control during hypoxia. Cancer discovery. 2014;4:1406–17. doi: 10.1158/2159-8290.CD-14-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nikiforov MA, Chandriani S, O’Connell B, Petrenko O, Kotenko I, Beavis A, et al. A functional screen for Myc-responsive genes reveals serine hydroxymethyltransferase, a major source of the one-carbon unit for cell metabolism. Molecular and cellular biology. 2002;22:5793–800. doi: 10.1128/MCB.22.16.5793-5800.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fan J, Ye J, Kamphorst JJ, Shlomi T, Thompson CB, Rabinowitz JD. Quantitative flux analysis reveals folate-dependent NADPH production. Nature. 2014;510:298–302. doi: 10.1038/nature13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Doherty JR, Yang C, Scott KE, Cameron MD, Fallahi M, Li W, et al. Blocking lactate export by inhibiting the Myc target MCT1 Disables glycolysis and glutathione synthesis. Cancer research. 2014;74:908–20. doi: 10.1158/0008-5472.CAN-13-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Brahimi-Horn MC, Bellot G, Pouyssegur J. Hypoxia and energetic tumour metabolism. Current opinion in genetics & development. 2011;21:67–72. doi: 10.1016/j.gde.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 134.Kung HN, Marks JR, Chi JT. Glutamine synthetase is a genetic determinant of cell type-specific glutamine independence in breast epithelia. PLoS genetics. 2011;7:e1002229. doi: 10.1371/journal.pgen.1002229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Polletta L, Vernucci E, Carnevale I, Arcangeli T, Rotili D, Palmerio S, et al. SIRT5 regulation of ammonia-induced autophagy and mitophagy. Autophagy. 2015 doi: 10.1080/15548627.2015.1009778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liu YC, Li F, Handler J, Huang CR, Xiang Y, Neretti N, et al. Global regulation of nucleotide biosynthetic genes by c-Myc. PloS one. 2008;3:e2722. doi: 10.1371/journal.pone.0002722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mannava S, Grachtchouk V, Wheeler LJ, Im M, Zhuang D, Slavina EG, et al. Direct role of nucleotide metabolism in C-MYC-dependent proliferation of melanoma cells. Cell Cycle. 2008;7:2392–400. doi: 10.4161/cc.6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Miltenberger RJ, Sukow KA, Farnham PJ. An E-box-mediated increase in cad transcription at the G1/S-phase boundary is suppressed by inhibitory c-Myc mutants. Molecular and cellular biology. 1995;15:2527–35. doi: 10.1128/mcb.15.5.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Consortium EP. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zeller KI, Zhao X, Lee CW, Chiu KP, Yao F, Yustein JT, et al. Global mapping of c-Myc binding sites and target gene networks in human B cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17834–9. doi: 10.1073/pnas.0604129103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jones RM, Branda J, Johnston KA, Polymenis M, Gadd M, Rustgi A, et al. An essential E box in the promoter of the gene encoding the mRNA cap-binding protein (eukaryotic initiation factor 4E) is a target for activation by c-myc. Molecular and cellular biology. 1996;16:4754–64. doi: 10.1128/mcb.16.9.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kim S, Li Q, Dang CV, Lee LA. Induction of ribosomal genes and hepatocyte hypertrophy by adenovirus-mediated expression of c-Myc in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11198–202. doi: 10.1073/pnas.200372597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pourdehnad M, Truitt ML, Siddiqi IN, Ducker GS, Shokat KM, Ruggero D. Myc and mTOR converge on a common node in protein synthesis control that confers synthetic lethality in Myc-driven cancers. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:11988–93. doi: 10.1073/pnas.1310230110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ruggero D. The role of Myc-induced protein synthesis in cancer. Cancer research. 2009;69:8839–43. doi: 10.1158/0008-5472.CAN-09-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Stumpf CR, Ruggero D. The cancerous translation apparatus. Current opinion in genetics & development. 2011;21:474–83. doi: 10.1016/j.gde.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Barna M, Pusic A, Zollo O, Costa M, Kondrashov N, Rego E, et al. Suppression of Myc oncogenic activity by ribosomal protein haploinsufficiency. Nature. 2008;456:971–5. doi: 10.1038/nature07449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cunningham JT, Moreno MV, Lodi A, Ronen SM, Ruggero D. Protein and nucleotide biosynthesis are coupled by a single rate-limiting enzyme, PRPS2, to drive cancer. Cell. 2014;157:1088–103. doi: 10.1016/j.cell.2014.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Priolo C, Pyne S, Rose J, Regan ER, Zadra G, Photopoulos C, et al. AKT1 and MYC induce distinctive metabolic fingerprints in human prostate cancer. Cancer research. 2014;74:7198–204. doi: 10.1158/0008-5472.CAN-14-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Morrish F, Noonan J, Perez-Olsen C, Gafken PR, Fitzgibbon M, Kelleher J, et al. Myc-dependent mitochondrial generation of acetyl-CoA contributes to fatty acid biosynthesis and histone acetylation during cell cycle entry. The Journal of biological chemistry. 2010;285:36267–74. doi: 10.1074/jbc.M110.141606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Edmunds LR, Sharma L, Kang A, Lu J, Vockley J, Basu S, et al. c-Myc programs fatty acid metabolism and dictates acetyl-CoA abundance and fate. The Journal of biological chemistry. 2014;289:25382–92. doi: 10.1074/jbc.M114.580662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zirath H, Frenzel A, Oliynyk G, Segerstrom L, Westermark UK, Larsson K, et al. MYC inhibition induces metabolic changes leading to accumulation of lipid droplets in tumor cells. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:10258–63. doi: 10.1073/pnas.1222404110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Iritani BM, Delrow J, Grandori C, Gomez I, Klacking M, Carlos LS, et al. Modulation of T-lymphocyte development, growth and cell size by the Myc antagonist and transcriptional repressor Mad1. The EMBO journal. 2002;21:4820–30. doi: 10.1093/emboj/cdf492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gomez-Roman N, Grandori C, Eisenman RN, White RJ. Direct activation of RNA polymerase III transcription by c-Myc. Nature. 2003;421:290–4. doi: 10.1038/nature01327. [DOI] [PubMed] [Google Scholar]
- 154.Grandori C, Gomez-Roman N, Felton-Edkins ZA, Ngouenet C, Galloway DA, Eisenman RN, et al. c-Myc binds to human ribosomal DNA and stimulates transcription of rRNA genes by RNA polymerase I. Nature cell biology. 2005;7:311–8. doi: 10.1038/ncb1224. [DOI] [PubMed] [Google Scholar]
- 155.Guo QM, Malek RL, Kim S, Chiao C, He M, Ruffy M, et al. Identification of c-myc responsive genes using rat cDNA microarray. Cancer research. 2000;60:5922–8. [PubMed] [Google Scholar]
- 156.Johnston LA. Socializing with MYC: cell competition in development and as a model for premalignant cancer. Cold Spring Harbor perspectives in medicine. 2014;4:a014274. doi: 10.1101/cshperspect.a014274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.de la Cova C, Johnston LA. Myc in model organisms: a view from the flyroom. Seminars in cancer biology. 2006;16:303–12. doi: 10.1016/j.semcancer.2006.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Johnston LA, Prober DA, Edgar BA, Eisenman RN, Gallant P. Drosophila myc regulates cellular growth during development. Cell. 1999;98:779–90. doi: 10.1016/s0092-8674(00)81512-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lambertsson A. The minute genes in Drosophila and their molecular functions. Advances in genetics. 1998;38:69–134. doi: 10.1016/s0065-2660(08)60142-x. [DOI] [PubMed] [Google Scholar]
- 160.Brown SJ, Cole MD, Erives AJ. Evolution of the holozoan ribosome biogenesis regulon. BMC genomics. 2008;9:442. doi: 10.1186/1471-2164-9-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Schuhmacher M, Staege MS, Pajic A, Polack A, Weidle UH, Bornkamm GW, et al. Control of cell growth by c-Myc in the absence of cell division. Current biology: CB. 1999;9:1255–8. doi: 10.1016/s0960-9822(99)80507-7. [DOI] [PubMed] [Google Scholar]
- 162.Schlosser I, Holzel M, Murnseer M, Burtscher H, Weidle UH, Eick D. A role for c-Myc in the regulation of ribosomal RNA processing. Nucleic acids research. 2003;31:6148–56. doi: 10.1093/nar/gkg794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ji H, Wu G, Zhan X, Nolan A, Koh C, De Marzo A, et al. Cell-type independent MYC target genes reveal a primordial signature involved in biomass accumulation. PloS one. 2011;6:e26057. doi: 10.1371/journal.pone.0026057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Koh CM, Gurel B, Sutcliffe S, Aryee MJ, Schultz D, Iwata T, et al. Alterations in nucleolar structure and gene expression programs in prostatic neoplasia are driven by the MYC oncogene. The American journal of pathology. 2011;178:1824–34. doi: 10.1016/j.ajpath.2010.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hofmann JW, Zhao X, De Cecco M, Peterson AL, Pagliaroli L, Manivannan J, et al. Reduced expression of MYC increases longevity and enhances healthspan. Cell. 2015;160:477–88. doi: 10.1016/j.cell.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Neff F, Flores-Dominguez D, Ryan DP, Horsch M, Schroder S, Adler T, et al. Rapamycin extends murine lifespan but has limited effects on aging. The Journal of clinical investigation. 2013;123:3272–91. doi: 10.1172/JCI67674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–5. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, et al. Metformin improves healthspan and lifespan in mice. Nature communications. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Scarpulla RC. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiological reviews. 2008;88:611–38. doi: 10.1152/physrev.00025.2007. [DOI] [PubMed] [Google Scholar]
- 170.Wallace DC. Mitochondria and cancer. Nat Rev Cancer. 2012;12:685–98. doi: 10.1038/nrc3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.O’Donnell KA, Yu D, Zeller KI, Kim JW, Racke F, Thomas-Tikhonenko A, et al. Activation of transferrin receptor 1 by c-Myc enhances cellular proliferation and tumorigenesis. Molecular and cellular biology. 2006;26:2373–86. doi: 10.1128/MCB.26.6.2373-2386.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rouault TA. Mammalian iron-sulphur proteins: novel insights into biogenesis and function. Nature reviews Molecular cell biology. 2015;16:45–55. doi: 10.1038/nrm3909. [DOI] [PubMed] [Google Scholar]
- 173.Li F, Wang Y, Zeller KI, Potter JJ, Wonsey DR, O’Donnell KA, et al. Myc stimulates nuclearly encoded mitochondrial genes and mitochondrial biogenesis. Molecular and cellular biology. 2005;25:6225–34. doi: 10.1128/MCB.25.14.6225-6234.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Morrish F, Hockenbery D. MYC and mitochondrial biogenesis. Cold Spring Harbor perspectives in medicine. 2014:4. doi: 10.1101/cshperspect.a014225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kim J, Lee JH, Iyer VR. Global identification of Myc target genes reveals its direct role in mitochondrial biogenesis and its E-box usage in vivo. PloS one. 2008;3:e1798. doi: 10.1371/journal.pone.0001798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ahuja P, Zhao P, Angelis E, Ruan H, Korge P, Olson A, et al. Myc controls transcriptional regulation of cardiac metabolism and mitochondrial biogenesis in response to pathological stress in mice. The Journal of clinical investigation. 2010;120:1494–505. doi: 10.1172/JCI38331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sheth A, Escobar-Alvarez S, Gardner J, Ran L, Heaney ML, Scheinberg DA. Inhibition of human mitochondrial peptide deformylase causes apoptosis in c-myc-overexpressing hematopoietic cancers. Cell death & disease. 2014:5. doi: 10.1038/cddis.2014.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Carroll PA, Diolaiti D, McFerrin L, Gu HW, Djukovic D, Du JH, et al. Deregulated Myc Requires MondoA/Mlx for Metabolic Reprogramming and Tumorigenesis. Cancer cell. 2015;27:271–85. doi: 10.1016/j.ccell.2014.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Weinberg SE, Chandel NS. Targeting mitochondria metabolism for cancer therapy. Nat Chem Biol. 2015;11:9–15. doi: 10.1038/nchembio.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Graves JA, Wang Y, Sims-Lucas S, Cherok E, Rothermund K, Branca MF, et al. Mitochondrial structure, function and dynamics are temporally controlled by c-Myc. PloS one. 2012;7:e37699. doi: 10.1371/journal.pone.0037699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wolf E, Gebhardt A, Kawauchi D, Walz S, von Eyss B, Wagner N, et al. Miz1 is required to maintain autophagic flux. Nature communications. 2013;4:2535. doi: 10.1038/ncomms3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Schuhmacher M, Eick D. Dose-dependent regulation of target gene expression and cell proliferation by c-Myc levels. Transcription. 2013;4:192–7. doi: 10.4161/trns.25907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bretones G, Delgado MD, Leon J. Myc and cell cycle control. Biochimica et biophysica acta. 2014 doi: 10.1016/j.bbagrm.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 184.Hermeking H, Rago C, Schuhmacher M, Li Q, Barrett JF, Obaya AJ, et al. Identification of CDK4 as a target of c-MYC. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:2229–34. doi: 10.1073/pnas.050586197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bouchard C, Dittrich O, Kiermaier A, Dohmann K, Menkel A, Eilers M, et al. Regulation of cyclin D2 gene expression by the Myc/Max/Mad network: Myc-dependent TRRAP recruitment and histone acetylation at the cyclin D2 promoter. Genes & development. 2001;15:2042–7. doi: 10.1101/gad.907901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Beier R, Burgin A, Kiermaier A, Fero M, Karsunky H, Saffrich R, et al. Induction of cyclin E-cdk2 kinase activity, E2F-dependent transcription and cell growth by Myc are genetically separable events. The EMBO journal. 2000;19:5813–23. doi: 10.1093/emboj/19.21.5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dong P, Maddali MV, Srimani JK, Thelot F, Nevins JR, Mathey-Prevot B, et al. Division of labour between Myc and G1 cyclins in cell cycle commitment and pace control. Nature communications. 2014:5. doi: 10.1038/ncomms5750. [DOI] [PMC free article] [PubMed]
- 188.Zeller KI, Jegga AG, Aronow BJ, O’Donnell KA, Dang CV. An integrated database of genes responsive to the Myc oncogenic transcription factor: identification of direct genomic targets. Genome biology. 2003;4:R69. doi: 10.1186/gb-2003-4-10-r69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Aguda BD, Kim Y, Piper-Hunter MG, Friedman A, Marsh CB. MicroRNA regulation of a cancer network: consequences of the feedback loops involving miR-17-92, E2F, and Myc. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:19678–83. doi: 10.1073/pnas.0811166106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Pickering MT, Stadler BM, Kowalik TF. miR-17 and miR-20a temper an E2F1-induced G1 checkpoint to regulate cell cycle progression. Oncogene. 2009;28:140–5. doi: 10.1038/onc.2008.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Dominguez-Sola D, Ying CY, Grandori C, Ruggiero L, Chen B, Li M, et al. Non-transcriptional control of DNA replication by c-Myc. Nature. 2007;448:445–51. doi: 10.1038/nature05953. [DOI] [PubMed] [Google Scholar]
- 192.Moncada S, Higgs EA, Colombo SL. Fulfilling the metabolic requirements for cell proliferation. The Biochemical journal. 2012;446:1–7. doi: 10.1042/BJ20120427. [DOI] [PubMed] [Google Scholar]
- 193.Colombo SL, Palacios-Callender M, Frakich N, Carcamo S, Kovacs I, Tudzarova S, et al. Molecular basis for the differential use of glucose and glutamine in cell proliferation as revealed by synchronized HeLa cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:21069–74. doi: 10.1073/pnas.1117500108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Colombo SL, Palacios-Callender M, Frakich N, De Leon J, Schmitt CA, Boorn L, et al. Anaphase-promoting complex/cyclosome-Cdh1 coordinates glycolysis and glutaminolysis with transition to S phase in human T lymphocytes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:18868–73. doi: 10.1073/pnas.1012362107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mathews CK. DNA precursor metabolism and genomic stability. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2006;20:1300–14. doi: 10.1096/fj.06-5730rev. [DOI] [PubMed] [Google Scholar]
- 196.Miller DR. A tribute to Sidney Farber-- the father of modern chemotherapy. British journal of haematology. 2006;134:20–6. doi: 10.1111/j.1365-2141.2006.06119.x. [DOI] [PubMed] [Google Scholar]
- 197.Murphy TA, Dang CV, Young JD. Isotopically nonstationary 13C flux analysis of Myc-induced metabolic reprogramming in B-cells. Metabolic engineering. 2013;15:206–17. doi: 10.1016/j.ymben.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cermelli S, Jang IS, Bernard B, Grandori C. Synthetic lethal screens as a means to understand and treat MYC-driven cancers. Cold Spring Harbor perspectives in medicine. 2014:4. doi: 10.1101/cshperspect.a014209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Toyoshima M, Howie HL, Imakura M, Walsh RM, Annis JE, Chang AN, et al. Functional genomics identifies therapeutic targets for MYC-driven cancer. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:9545–50. doi: 10.1073/pnas.1121119109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Patra KC, Wang Q, Bhaskar PT, Miller L, Wang ZB, Wheaton W, et al. Hexokinase 2 Is Required for Tumor Initiation and Maintenance and Its Systemic Deletion Is Therapeutic in Mouse Models of Cancer (vol 24, pg 213, 2013) Cancer cell. 2013;24:399. doi: 10.1016/j.ccr.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer cell. 2006;9:425–34. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 202.Seth P, Grant A, Tang J, Vinogradov E, Wang X, Lenkinski R, et al. On-target inhibition of tumor fermentative glycolysis as visualized by hyperpolarized pyruvate. Neoplasia. 2011;13:60–71. doi: 10.1593/neo.101020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wang ZY, Loo TY, Shen JG, Wang N, Wang DM, Yang DP, et al. LDH-A silencing suppresses breast cancer tumorigenicity through induction of oxidative stress mediated mitochondrial pathway apoptosis. Breast cancer research and treatment. 2012;131:791–800. doi: 10.1007/s10549-011-1466-6. [DOI] [PubMed] [Google Scholar]
- 204.Xie H, Hanai J, Ren JG, Kats L, Burgess K, Bhargava P, et al. Targeting lactate dehydrogenase--a inhibits tumorigenesis and tumor progression in mouse models of lung cancer and impacts tumor-initiating cells. Cell metabolism. 2014;19:795–809. doi: 10.1016/j.cmet.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wang YH, Israelsen WJ, Lee D, Yu VW, Jeanson NT, Clish CB, et al. Cell-state-specific metabolic dependency in hematopoiesis and leukemogenesis. Cell. 2014;158:1309–23. doi: 10.1016/j.cell.2014.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, et al. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:2037–42. doi: 10.1073/pnas.0914433107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kolappan S, Shen DL, Mosi R, Sun J, McEachern EJ, Vocadlo DJ, et al. Structures of lactate dehydrogenase A (LDHA) in apo, ternary and inhibitor-bound forms. Acta crystallographica Section D, Biological crystallography. 2015;71:185–95. doi: 10.1107/S1399004714024791. [DOI] [PubMed] [Google Scholar]
- 208.Deiab S, Mazzio E, Messeha S, Mack N, Soliman KF. High-Throughput Screening to Identify Plant Derived Human LDH-A Inhibitors. European journal of medicinal plants. 2013;3:603–15. doi: 10.9734/ejmp/2013/5995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Billiard J, Dennison JB, Briand J, Annan RS, Chai D, Colon M, et al. Quinoline 3-sulfonamides inhibit lactate dehydrogenase A and reverse aerobic glycolysis in cancer cells. Cancer & metabolism. 2013;1:19. doi: 10.1186/2049-3002-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kohlmann A, Zech SG, Li F, Zhou T, Squillace RM, Commodore L, et al. Fragment growing and linking lead to novel nanomolar lactate dehydrogenase inhibitors. Journal of medicinal chemistry. 2013;56:1023–40. doi: 10.1021/jm3014844. [DOI] [PubMed] [Google Scholar]
- 211.Granchi C, Roy S, Giacomelli C, Macchia M, Tuccinardi T, Martinelli A, et al. Discovery of N-hydroxyindole-based inhibitors of human lactate dehydrogenase isoform A (LDH-A) as starvation agents against cancer cells. Journal of medicinal chemistry. 2011;54:1599–612. doi: 10.1021/jm101007q. [DOI] [PubMed] [Google Scholar]
- 212.Le A, Lane AN, Hamaker M, Bose S, Gouw A, Barbi J, et al. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell metabolism. 2012;15:110–21. doi: 10.1016/j.cmet.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wang JB, Erickson JW, Fuji R, Ramachandran S, Gao P, Dinavahi R, et al. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer cell. 2010;18:207–19. doi: 10.1016/j.ccr.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Xiang Y, Stine ZE, Xia J, Lu Y, O’Connor RS, Altman BJ, et al. Targeted inhibition of tumor-specific glutaminase diminishes cell-autonomous tumorigenesis. The Journal of clinical investigation. 2015;125:2293–306. doi: 10.1172/JCI75836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Gross MI, Demo SD, Dennison JB, Chen L, Chernov-Rogan T, Goyal B, et al. Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Molecular cancer therapeutics. 2014;13:890–901. doi: 10.1158/1535-7163.MCT-13-0870. [DOI] [PubMed] [Google Scholar]
- 216.Tan B, Young DA, Lu ZH, Wang T, Meier TI, Shepard RL, et al. Pharmacological inhibition of nicotinamide phosphoribosyltransferase (NAMPT), an enzyme essential for NAD+ biosynthesis, in human cancer cells: metabolic basis and potential clinical implications. The Journal of biological chemistry. 2013;288:3500–11. doi: 10.1074/jbc.M112.394510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zhuang D, Mannava S, Grachtchouk V, Tang WH, Patil S, Wawrzyniak JA, et al. C-MYC overexpression is required for continuous suppression of oncogene-induced senescence in melanoma cells. Oncogene. 2008;27:6623–34. doi: 10.1038/onc.2008.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Soucek L, Whitfield JR, Sodir NM, Masso-Valles D, Serrano E, Karnezis AN, et al. Inhibition of Myc family proteins eradicates KRas-driven lung cancer in mice. Genes & development. 2013;27:504–13. doi: 10.1101/gad.205542.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ilic N, Utermark T, Widlund HR, Roberts TM. PI3K-targeted therapy can be evaded by gene amplification along the MYC-eukaryotic translation initiation factor 4E (eIF4E) axis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:E699–708. doi: 10.1073/pnas.1108237108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Yuan P, Ito K, Perez-Lorenzo R, Del Guzzo C, Lee JH, Shen CH, et al. Phenformin enhances the therapeutic benefit of BRAF(V600E) inhibition in melanoma. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:18226–31. doi: 10.1073/pnas.1317577110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Parmenter TJ, Kleinschmidt M, Kinross KM, Bond ST, Li J, Kaadige MR, et al. Response of BRAF-mutant melanoma to BRAF inhibition is mediated by a network of transcriptional regulators of glycolysis. Cancer discovery. 2014;4:423–33. doi: 10.1158/2159-8290.CD-13-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Tanaka K, Sasayama T, Irino Y, Takata K, Nagashima H, Satoh N, et al. Compensatory glutamine metabolism promotes glioblastoma resistance to mTOR inhibitor treatment. The Journal of clinical investigation. 2015 doi: 10.1172/JCI78239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Li J, Csibi A, Yang S, Hoffman GR, Li C, Zhang E, et al. Synthetic lethality of combined glutaminase and Hsp90 inhibition in mTORC1-driven tumor cells. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:E21–9. doi: 10.1073/pnas.1417015112. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]