Abstract
Electronic cigarettes (ECIGs) have continued to gain popularity among the general public since their introduction in 2003. While all ECIGs work by heating a liquid solution into an aerosol that is then inhaled by the user, there are differences in engineering characteristics and appearance of the devices as well as how the liquid is stored and heated, its nicotine concentration, its ratio of propylene glycol and/or vegetable glycerin, and the flavorants added to the liquid. Some of the research areas previously examined with ECIGs include aerosol toxicant yield, user puffing behavior, physiological effects, subjective effects, abuse liability, and effects on smoking cessation. Much of this work used earlier device models that delivered very little nicotine to the user, and additional research needs to be conducted using consistent and reliable devices, assays, and methodologies in order to gain a clearer understanding of ECIGs and their implications for individual and public health. Furthermore, the effects that ECIGs have on smoking cessation and among vulnerable populations must be addressed empirically.
Keywords: electronic cigarettes, behavior, smoking cessation, device evolution
Introduction
Electronic cigarettes (ECIGs) heat a liquid solution and produce a smoke-free aerosol for user inhalation. The liquid solutions often contain nicotine (ready-to-use concentrations ≤ 48 mg/ml; higher concentrations available for dilution), propylene glycol and/or vegetable glycerin, flavorants, and other additives (Etter, 2012; Vaporzone Inc., 2014). Generally, ECIGs consist of a power source (e.g., battery) and a heating element (“atomizer”), and a reservoir for the liquid solution. All of these features have changed considerably over the past decade, and the product class is evolving constantly. This evolution is a challenge to understanding ECIG effects, and is driven by the growing popularity of ECIGs in a variety of populations.
In the U.S., ever use of ECIGS among adults increased from 3.3% in 2010 to 6.2% in 2011, including increases among current smokers from 6.8% to 9.8% within the same timeframe (King et al., 2013). Additionally, ever use among adult former smokers increased from 2.5% in 2010 to 9.6% in 2013 (King et al., 2013). In a 2012 survey, 7% of smokers aged 18–34 were also current ECIG users (Rath et al., 2012). Youth who never smoked conventional tobacco cigarettes but reported ECIG use increased more than three-fold from 79,000 in 2011 to 263,000 in 2013 (Bunnell et al., 2015). ECIG sales have continued to rise since their introduction to the U.S. market, with sales between 2012 to 2013 increasing from $273B to $636B (Giovenco et al., in press).
The potential effects of ECIGs are uncertain. That is, ECIGs may help smokers quit smoking conventional tobacco cigarettes, they may induce more people to use nicotine who might otherwise remain nicotine-naïve or among those who previously have quit smoking cigarettes entirely, and they may be associated with adverse health outcomes due to user inhalation of the liquid and aerosol constituents. The primary aim of this review is to examine what is known about ECIGs today and to highlight important issues about them that remain unresolved, particularly regarding the rapid evolution of this product and inconsistencies in the literature thus far. The review is not intended to be comprehensive; detailed syntheses can be found elsewhere (e.g., Breland et al., 2014; Grana, Benowitz, & Glantz, 2014; Pisinger & Døssing, 2014). Rather, the intent is to introduce a variety of relevant topics including the engineering of ECIGs and contents/effects of ECIG aerosol in preclinical assays, the nicotine delivery and effects of ECIGs in clinical laboratory studies, and the cessation outcomes associated with ECIGs in clinical trials. In so doing, important gaps in the literature emerge, including the need to take product evolution into consideration, uncertainty regarding long-term health outcomes, the influence of flavors on ECIG abuse liability, and the effect of ECIGs on vulnerable populations.
Current Knowledge of ECIGS
Engineering and contents/effects of ECIG aerosol in preclinical assays
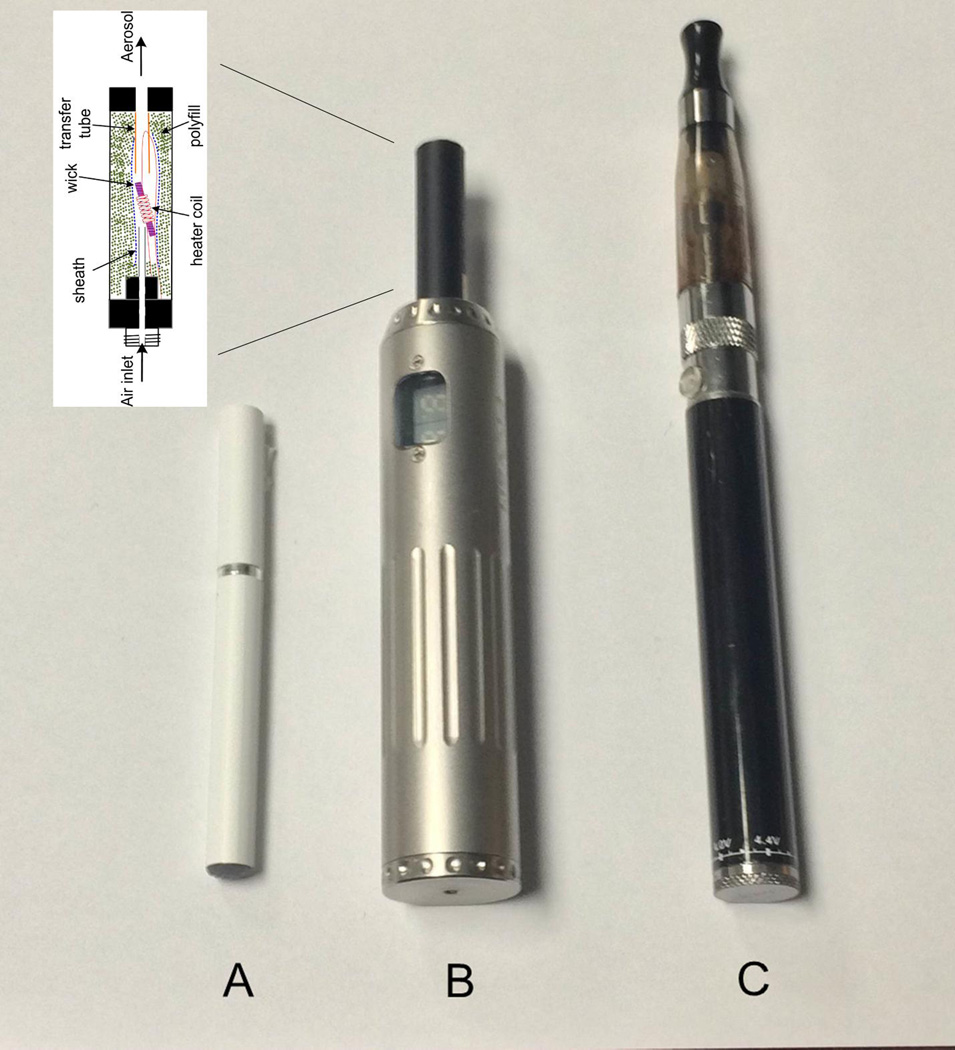
The wide variety of ECIG models differ by how the solution is stored, method of heater activation, electric power, and other device features. ECIGs that are sized and shaped like cigarettes are often referred to as “cig-alikes” (Figure 1A; Cassidy, 2011) and are often constructed as a single unit that is disposable. Other ECIGs do not resemble cigarettes in size or shape and use either a cartridge (“cartomizer”) or reservoir (“tank”) that screws onto a separate and rechargeable battery that may vary by voltage (Figure1B and 1C). The cartomizer or tank simultaneously stores the liquid and brings it into contact with the heating element; both can be refilled with solution and used often (Etter, 2012).
Figure 1.
Various ECIG models; A) pneumatically-operated cig-alike, B) cartomizer with variable-voltage battery, C) tank atomizer with manually-operated 3.3 volt battery. Schematic of inside of cartomizer provided by Dr. Alan Shihadeh. Detailed descriptions of other ECIG products can be found elsewhere (e.g., Farsalinos et al., 2014b; Polosa et al., 2014).
ECIG design and user behavior can each influence the amount of nicotine emitted per unit time from the mouth-end of an ECIG (i.e., the “nicotine flux”, Shihadeh & Eissenberg, 2015). With regard to design, increasing the power source voltage from 3.3 to 5.2 volts led to a 4–5 fold increase in nicotine flux (Talih et al., 2015). Device power includes heater resistance as well as power source voltage, and lowering the resistance also likely increases nicotine flux. Puff velocity does not influence nicotine flux, while doubling the duration of a puff produces a modest flux increase (e.g., at 3.3 volts, 4.9 µg/s for a 4-sec puff and 6.0 µg/s for an 8 sec puff; Talih et al., 2015). The role played by these and other variables, including liquid nicotine concentration, in influencing nicotine flux can be predicted using a mathematical model (Talih et al., 2015). However, nicotine is only one ECIG aerosol constituent, and the extent to which these and other device and user characteristics may influence ECIG toxicant yield await systematic investigation. To our knowledge, these are the only systematic studies that have examined how device variables and user behavior influence nicotine yield. As devices evolve, relevant variables could be entered the mathematic model to estimate nicotine flux.
Because ECIGs do not combust during normal operation like conventional tobacco cigarettes, the aerosol that they produce may contain fewer/lower levels of toxicants. Toxicants such as formaldehyde, acetaldehyde, acrolein, toluene, N’-nitrosonornicotine (NNN), and 4-methylnitrosoamino-1–3-pyridyl-1-butanone (NNK), as well as added flavorings are important to examine in ECIG aerosol because they may be contained in the liquid prior to heating or formed during heating (Farsalinos et al., 2014a; Goniewicz et al., 2014). Formaldehyde, acetaldehyde, and acrolein are carbonyl compounds that are cytotoxic, carcinogenic, and can cause pulmonary emphysema and dermatitis, while NNN and NNK are carcinogenic tobacco-specific nitrosamines (Smith, Livingston, & Doolittle, 1997). While trace amounts of these toxicants were found in the aerosol produced by ECIGs purchased in Poland and the U.K. that varied by brand and model as well as flavor and nicotine content, they were at levels 9- to 450-times lower compared to cigarettes (Goniewicz et al., 2014). Others have examined the toxicant levels in aerosols of sweet-flavored e-liquids, as these liquids are thought to be more attractive to youth and non-smoking adults (Farsalinos et al., 2014a). While the content of these flavorings (e.g., diacetyl and acetyl propionyl) are generally recognized as safe food additives for oral consumption, they have not been tested adequately for safety via inhalation. Compared to cigarette smoke, levels of diacetyl and acetyl propionyl were found to be 10- to 100- times lower in the e-liquid aerosol (Farsalinos et al., 2014a). As this product class continues to evolve, there is a growing need to develop methods that allow for accurate prediction and rapid testing of ECIG nicotine and other toxicant output, including added flavorants and preservatives that may be in the liquid, as well as how they relate to or produce toxicants.
The effects of ECIG aerosols have been examined using a limited set of preclinical assays (Cervellati et al., 2014; Marini et al., 2014). For example, human skin and lung cells have been exposed to ECIG aerosol to examine possible cytotoxic effects and cytokine release (Cervellati et al., 2014). While the cytotoxicity of the ECIG aerosol was lower than the cytotoxic effects of cigarette smoke for both cell types, the aerosol’s cytotoxicity was directly related to the flavor additive used (i.e., balsamic flavor; Cervellati et al., 2014). Others have examined the particle number concentrations of ECIG aerosol and effects on exhaled nitric oxide among regular cigarette smokers (Marini et al., 2014). Total particle number concentrations were similar for nicotine-free ECIGs and conventional cigarettes (3.5 ± 0.4 × 109 and 3.1 ± 0.6 × 109, respectively) and higher for nicotine-containing ECIGs (5.1 ± 0.1 × 109; Marini et al., 2014). Acute respiratory effects showed immediate reductions in exhaled nitric oxide following ECIG use with and without nicotine, consistent with results from conventional cigarettes (Malinovschi et al., 2006; Min & Min, 2014; Marini et al., 2014).
ECIGs with and without nicotine and with and without flavors (i.e., menthol and tobacco flavors) did not induce any cytotoxic, genotoxic, or inflammatory effects (Misra et al., 2014). One explanation for the apparent discrepancy between these results and those reported elsewhere (i.e., Marini et al., 2014) is a difference is device type. Marini et al (2014) used a tank system while Misra et al (2014) used a “cig-alike”; the nicotine concentration of the liquids also differed across studies. These methodological differences make cross-study comparisons challenging and, more generally, the diversity of devices and assays used to evaluate ECIG effects preclinically highlights the need for standardization of methods. Consistency across studies would help in understanding how changes in products influence health risks, and involving commonly used devices and testing the effects of the aerosols they generate would increase result verisimilitude. Aerosols should be produced in a manner that mimics the way actual ECIG users generate aerosols under natural environment conditions. In particular, puff parameters used to simulate the behavior of combustible tobacco cigarette smokers likely are not representative of experienced ECIG users (see Behar et al., 2015; Hua et al 2013; Spindle et al., 2015). Given that puff parameters are one of several factors that can influence aerosol toxicant content (Talih et al., 2015), testing the toxicant content of aerosols that are generated using parameters that do not represent the actual behavior of ECIG users likely does not reveal the toxicants to which these users are exposed. In a related point, the rapid evolution and diversity of this product class also must be taken into consideration when evaluating preclinical effects; results from previous studies using early-generation, low power devices may not be representative of the effects of later-generation, more powerful devices; again, the power of the device influences toxicant yield (Talih et al., 2015). There is also a need for animal models to study ECIG effects in vivo.
Clinical laboratory studies
There is a growing literature examining the effects of ECIG use in the clinical laboratory, wherein either ECIG-naïve or ECIG-experienced participants use an ECIG under controlled conditions. Common outcome measures include nicotine delivery and other physiological effects, subjective response, and/or behavioral tasks.
Nicotine delivery
Initial studies of the nicotine delivery profile of various models of ECIGS showed that tobacco cigarette smokers naïve to ECIG use received little to no nicotine (Bullen et al., 2010; Vansickel et al., 2010). More recent studies with different ECIG designs and experienced ECIG users have reported nicotine delivery of approximately 2.1–8.3 ng/ml (Dawkins & Corcoran, 2014; Farsalinos et al., 2014b; Vansickel & Eissenberg, 2013), less than that delivered by a tobacco cigarette under similar conditions (e.g., 15–20 ng/ml; Vansickel et al., 2010). Comparisons between a cig-alike and a tank ECIG shows that plasma nicotine concentrations were higher by 35–72% in the tank model (Farsalinos et al., 2014b). However, some ECIGs are capable of delivering cigarette-like nicotine doses (Spindle et al., 2015). In this study, 13 ECIG-experienced overnight-abstinent participants used a dual heating-coil cartomizer with 1.5 Ohm resistance with their preferred battery and liquid. Mean plasma nicotine concentration increased following a 10-puff bout (mean = 19.2 ng/ml, SEM = 2.3) relative to baseline (mean = 2.4 ng/ml, SEM = 0.2; Spindle et al., 2015). In summary, while earlier ECIG models tested in 2010 failed to delivered nicotine, models tested in 2012–13 had evolved enough such that some nicotine was being delivered to experienced users and, currently, at least some ECIGs on the market are capable of delivering the nicotine delivered by a single cigarette (e.g., Spindle et al., 2015). The time course of this combustible cigarette-like nicotine delivery (5 minutes or less) is consistent with pulmonary rather than buccal drug absorption. This rapid evolution, from no nicotine delivery to cigarette-like doses in the 5–10 years since ECIGs entered the market, suggests that subsequent product development may lead to even greater nicotine delivery.
Physiological effects
ECIG effects on heart rate and pulmonary function have been examined in several studies. Cig-alikes that did not delivery nicotine also did not increase heart rate (Vansickel et al., 2010). However, “cartomizer” ECIGs that deliver nicotine also increase heart rate: after a 10-puff bout, heart rate significantly increased to a mean = 74.2 beats/min (SEM = 1.6) relative to a mean = 65.7 beats/min (SEM = 1.5) at baseline among experienced ECIG users (Spindle et al., 2015). While ECIGs can increase heart rate, they may do so to a lesser degree as compared to combustible tobacco, especially when using devices that inefficiently deliver nicotine to the user (Yan & D’Ruiz, 2015). Reports of studies examining the effects of ECIGs on pulmonary function are less conclusive. While some researchers have reported acute effects concordant with conventional cigarette use such as increases in total respiratory resistance (Vardavas et al., 2012), others have reported no lung function impairments (Chorti et al., 2012; Flouris et al., 2013). These inconsistent findings may be the result of variations in ECIG model including using devices with uncertain nicotine delivery profile as well as other sources of cross-study variability, such as user puffing behavior. Certainly, more evidence is needed to make definitive conclusions on the physiological effects of ECIGs as the devices continue to evolve.
Subjective effects
Suppression of tobacco/nicotine abstinence symptoms, direct positive effects, and adverse events of ECIGs has also been examined. While ECIGs have been shown to reduce tobacco/nicotine abstinence symptoms reported by combustible tobacco smokers (Bullen et al., 2010, Dawkins et al., 2013a, 2013b; Vansickel et al., 2010), whether nicotine delivery or behavioral conditioning stimuli may be causing these reductions separately or additively remains unclear. ECIGs can decrease tobacco/nicotine abstinence symptom severity without actually delivering nicotine to the user (Vansickel et al., 2010), and there are interactions between gender and nicotine-containing ECIGs where women showed reduced desire to smoke regardless of the nicotine content, but nicotine-containing ECIGs were more effective in men (Dawkins et al., 2012). Furthermore, experienced ECIG users show significantly decreased symptom severity after using their preferred liquid in controlled 10-puff bouts (Spindle et al., 2015) and after 10 directed puffs plus ad libitum use for 60 minutes (Dawkins & Corcoran, 2014) following 12 hours of tobacco/nicotine abstinence. Others have found no effects of ECIG use on reducing cigarette craving among regular smokers (Norton et al., 2014). ECIGs that mimic the nicotine delivery of a conventional combustible cigarette would perhaps have the greatest effect on reducing abstinence symptoms in cigarette smokers, though this speculation awaits systematic investigation.
Topography
Puff topography is the quantitative measurement of puff behavior including puff number, duration, volume, flow rate, and interpuff interval. The puff topography of experienced ECIG users recently has been measured objectively (Spindle et al., 2015). Relative to tobacco cigarette smokers from a previous study under similar conditions (Kleykamp et al., 2008), experienced ECIG users inhaled twice the volume per puff, had a three times longer puff duration, and a lower flow rate. These results are consistent with earlier exploratory studies suggesting that the topography of an experienced ECIG user is markedly different than that of a conventional cigarette user, with ECIG users taking puffs twice as long as cigarette smokers (Farsalinos et al., 2013; Hua, Yip, & Talbot, 2013). While more work is needed, these initial three studies of ECIG user topography provide a first approximation of the type of puffing behavior that should be used to generate ECIG aerosol for subsequent toxicity testing.
Cognition and abuse liability
Cognitive effects of ECIGs have also been examined. First-generation ECIG models with 18 mg/ml nicotine concentration have been reported to improve working memory performance relative to placebo cig-alikes in nicotine-dependent smokers following ad libitum ECIG use (Dawkins et al., 2012). In another study, abstaining conventional smokers using an 18 mg/ml nicotine concentration ECIG ad libitum (later-generation tank model) significantly improved in their prospective time-based, but not event-based, memory compared to those using a placebo ECIG (Dawkins et al., 2013a, 2013b). However, this work that has been conducted with a limited set of devices likely is not conclusive. Similarly, only one study has examined the abuse liability of a single ECIG (Vansickel et al., 2012). A more systematic investigation that takes into account the nicotine delivery profile and other characteristics of a range of devices would likely be more informative and help to elucidate the possible cognitive effects and the abuse liability of ECIGs.
Clinical trials & smoking cessation
Uncontrolled designs have hinted at ECIG effects and smoking cessation (e.g., Caponnetto et al., 2011; Polosa et al., 2011) but two randomized control trials have examined this issue rigorously (Bullen et al., 2013; Caponnetto et al., 2013). In one, tobacco cigarette smokers with no intention to quit were randomized to one of three double-blind “cig-alike: ECIG conditions for 12 weeks: 7.2 mg/ml nicotine concentration for all 12 weeks, 7.2 mg/ml nicotine concentration for 6 weeks followed by 5.4 mg/ml nicotine concentration for weeks 7 through 12, or placebo ECIG for all 12 weeks (Caponnetto et al., 2013). While there were significant decreases in cigarettes smoked per day and expired breath CO at study visits compared to baseline, there were no consistent differences across ECIG conditions. Overall, over 20% of the sample reduced their smoking by week 12, with 10% reporting reduction at a 52 week follow-up. Approximately 10% of the sample ceased all combustible tobacco use in the study.
Another trial examined the extent to which cig-alike ECIGs were more effective than nicotine patches at maintaining tobacco abstinence in tobacco cigarette smokers who were motivated to quit (Bullen et al., 2013). Smokers were randomized to a nicotine patch, an active ECIG (16 mg/ml), or a placebo ECIG. Approximately 7% of those randomized to the active ECIG condition were verified as abstinent from smoking at 6 months, compared to 6% in the nicotine patch condition and 4% in the placebo ECIG condition. There were no significant differences in abstinence rates between the active ECIG and nicotine patch conditions.
While these studies did not report differences among ECIG conditions (Caponnetto et al., 2013) or increased abstinence greater than that obtained with nicotine patch (Bullen et al., 2013), this lack of an effect may be a reflection of uncertain nicotine delivery with the ECIG devices/liquid nicotine used. Indeed, in one of the two trials (Bullen et al., 2013), the ECIGs delivered about 2 ng/ml nicotine, far less than a tobacco cigarette. Thus, the ability of ECIGs that approximate the nicotine delivery of a combustible cigarette to maintain tobacco cigarette abstinence remains uncertain, although a recent Cochrane review found that use of active (nicotine-containing) ECIGs in these two RCTs led to increased long-term cessation and a reduction in the number of combustible cigarettes smoked as compared to placebo ECIGs (McRobbie et al., 2014). However, the review also noted that the small sample size suggested caution when interpreting the reliability and generalizability of these findings.
Summary
In summary, ECIGs have evolved rapidly from early devices that resembled cigarettes (cig-alikes) into devices that use separate tanks or cartomizers and connect to batteries of varying voltage. A mathematical model exits that can predict ECIG aerosol nicotine yield (Talih et al., 2015). Preclinical studies have examined in vitro toxicant exposure from both the liquid that goes into the ECIG as well as the aerosol produced (e.g., Behar et al., 2014; Cervellati et al., 2014). Clinical laboratory studies have also examined acute effects such as nicotine delivery (e.g., Spindle et al., 2015; Yan & D’Ruiz, 2015; Dawkins and Corcoran, 2014; Bullen et al., 2010), physiological effects (e.g. Yan & D’Ruiz, 2015), subjective effects (e.g., Bullen et al., 2010; Norton et al., 2014), topography (e.g., Spindle et al., 2015), cognition (e.g., Dawkins et al., 2013a, 2013b), and abuse liability (e.g., Vansickel et al., 2012). RCTs have also been conducted to examine smoking cessation with ECIGs (McRobbie et al., 2014). The majority of this research has been conducted with a limited number of ECIG types and a large representation of early-generation cig-alike models that likely or demonstrably under delivered or inconsistently delivered nicotine to the user. While variability within the ECIG product class increases regularly, the scientific literature thus far has lagged behind. If every device available on the marked must be tested separately, then this lag is likely to continue. Instead, the purpose of this non-comprehensive review is to make the case that another approach is needed, wherein scientific work regarding ECIGs is driven less by specific devices and more by questions that can be answered in a generalizable manner. For example, in considering ECIG nicotine delivery, questions such as “How does liquid nicotine concentration interact with device power to influence plasma nicotine concentration?” might extend knowledge more than questions such as “What is the nicotine delivery profile of this particular device with this particular liquid in it?”. The field is ready for a systematic determination of the factors that influence ECIG effects.
Future Directions
Based on the existing literature and the rapid evolution of ECIG devices, more research is clearly warranted. In addition to the overwhelming need to understand ECIG health effects, ECIGs need to be examined for their ability to help with cessation efforts among existing smokers and their potential effects with vulnerable populations.
Cessation
ECIGs theoretically could be useful as smoking cessation tools, as they mimic nicotine and non-nicotine stimuli associated with smoking. While numerous surveys suggest that ECIGs have potential for helping smokers quit tobacco (see Breland et al., 2014), the slower delivery and lower dose of nicotine available from some ECIGs, relative to tobacco cigarettes, may have influenced the results obtained in the few randomized control trials examining cessation outcomes (Bullen et al., 2013; Caponnetto et al., 2013). Generally, randomized control trials examining the effects of ECIGs on smoking cessation should not begin until the devices and e-liquids/aerosols have been tested so that, at the least, the nicotine delivery profile of the device/liquid combination to be used in the trial is well-known. All other things being equal, the greatest cessation effect is likely to be observed when a device/liquid combination mimics the nicotine delivery of a tobacco cigarette. Also, trial design needs to take into account the extent to which ECIGs are likely to be used for nicotine cessation generally, or tobacco cigarette cessation specifically. Trials that are designed to examine effects on nicotine cessation generally will require a follow-up period where participants are expected to be free of tobacco cigarettes, ECIGs, and all other nicotine products. Trials that are designed to examine effects on tobacco cigarette cessation specifically may have a follow-up period where participants continue to use ECIGs. This latter trial design allows the study of ECIGs as a harm reduction product, a controversial approach that, if adopted, should include a wide array of health-related measures during the follow-up period.
Vulnerable populations
While ECIGs primarily have been studied with otherwise healthy populations, effects on vulnerable populations such as children and adolescents, pregnant and recently postpartum women, and individuals with other substance use or severe mental health disorders also should be examined. While over 200,000 youth who were never smokers reported ECIG use in 2013 (Bunnell et al., 2015), how many of these youth will go on to be regular ECIG users, become regular tobacco smokers, or be dual users is still unclear, especially as ECIG models continue to evolve as their popularity increases. Cross-sectional studies with middle- and high-school students have shown that sweet flavored liquids are most preferred among current ECIG users, current cigarette smokers are more likely to use nicotine-containing liquids compared to ever or never smokers, and current smokers preferred ECIGs to conventional cigarettes (Krishnan-Sarin et al., 2015). Additionally, high school students were more likely to experiment with ECIGs because of the availability of appealing flavors and less likely to use ECIGs to aid with smoking cessation compared to college-aged students (Kong et al., 2015). While the majority of research with youth has been in the form of focus groups and cross-sectional surveys, longitudinal monitoring is warranted to examine long-term outcomes.
Women of childbearing age, specifically pregnant and recently postpartum women, are also a vulnerable population that has been understudied regarding ECIG use and effects. 40% of obstetricians report asking their patients about non-combustible tobacco use during prenatal care, 29% stated that they believed ECIGs to be safer than cigarettes, and over 13% reported that ECIGs had no health effects (England et al., 2014). Because there are currently no data on the effects of ECIG use during pregnancy, whether or not ECIGs are safer or more harmful than conventional tobacco use on maternal and infant health remains unknown. Certainly, while nicotine readily crosses the placenta and there are numerous effects on the developing fetus from cigarette smoking (Cnattingius, 2004; Suter et al., 2015), the effects that inhaled propylene glycol and vegetable glycerin may have on fetal development remain to be seen.
High proportions of individuals with substance use disorders and mental illness are current smokers (71% and 35%, respectively; CDC, 2013; Richter et al., 2002). To our knowledge, no studies to date have examined short- and long-term effects of ECIG use with either population. Only one study to date examined ECIG use prevalence among opioid-maintained smokers, and reported that around 34% of individuals used ECIGs in the past 30 days (Stein et al., 2015). Curiosity and help with nicotine cessation were the most endorsed reasons for ECIG use (41% and 26%, respectively; Stein et al., 2015). Future studies should state clearly whether or not ECIG use prevalence mimics, is lower, or is higher than conventional cigarette use rates. Furthermore, what effects ECIG use has on illicit drug use, mental illness symptomatology, cigarette use, and cessation rates among these populations remains to be seen.
Concluding Remarks
While ECIGs have been increasing in popularity and device/liquids have been evolving rapidly, the development of standardized preclinical and clinical methods to examine their effects has been slower. Recent advances include the characterization of user puffing behavior that can be used to generate aerosol for toxicity testing and the development of a mathematical model that predicts the amount of nicotine that is emitted from the mouth-end of an ECIG. While the literature reviewed here was not exhaustive, and may reflect some selection bias, it nonetheless demonstrate the inconsistencies in the literature thus far, many of which are due to using devices that of uncertain nicotine delivery profiles. Priority areas for further work include identifying a set of pre-clinical assays that are relevant for testing the toxicity of ECIG aerosols, developing mathematical models for other toxicants in addition to nicotine, understanding the long-term health effects of daily inhalation of hundreds of propylene glycol/vegetable glycerin-laced puffs, investigating the effects of ECIGs that deliver nicotine as effectively as a tobacco cigarette using outcome measures related to health as well as cessation, and a better understanding of ECIG effects, including abuse liability, in a variety of vulnerable populations, including youth and individuals with substance use disorders. Much has been written about ECIGs as a technology that can improve the individual health of smokers and public health generally. While this technology evolves, so must the science that can be used to evaluate empirically the extent to which ECIGs realize the individual and public health promises made about them.
Highlights.
We discuss the current state of the evolution of ECIGs.
Device characteristics should be considered when evaluating the literature.
Standardized preclinical and clinical methods are warranted to examine effects.
Public health implications should take device variability into consideration.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Behar RZ, Hua M, Talbot P. Puffing topography and nicotine intake of electronic cigarette users. PLoS One. 2015;10(2):e0117222. doi: 10.1371/journal.pone.0117222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breland AB, Spindle T, Weaver M, Eissenberg T. Science and electronic cigarettes: current data, future needs. Journal of Addictive Medicine. 2014;8(4):223–233. doi: 10.1097/ADM.0000000000000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen C, Howe C, Laugesen M, McRobbie H, Parag V, Williman J, Walker N. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382:1629–1637. doi: 10.1016/S0140-6736(13)61842-5. [DOI] [PubMed] [Google Scholar]
- Bullen C, McRobbie H, Thornley S, Glover M, Lin R, Laugesen M. Effect of an electronic nicotine delivery device (e cigarette) on desire to smoke and withdrawal, user preferences and nicotine delivery: randomized cross-over trial. Lancet. 2010;382:1629–1637. doi: 10.1136/tc.2009.031567. [DOI] [PubMed] [Google Scholar]
- Bunnell RE, Agaku IE, Arrazola RA, Apelberg BJ, Caraballo RS, Corey CG, Coleman BN, Dube SR, King BA. Intentions to smoke cigarettes among never-smoking U.S. middle and high school electronic cigarette users, National Youth Tobacco Survey, 2011–2013. Nicotine and Tobacco Research. 2015;17(2):228–235. doi: 10.1093/ntr/ntu166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caponnetto P, Campagna D, Cibella F, Morjaria JB, Caruso M, Russo C, Polosa R. Efficiency and safety of an electronic cigarette (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLOS ONE. 2013;8(6):e66317. doi: 10.1371/journal.pone.0066317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caponnetto P, Polosa R, Russo C, Leotta C, Campagna D. Successful smoking cessation with electronic cigarettes in smokers with a documented history of recurring relapses: a case series. Journal of Medical Case Reports. 2011;5(1):585. doi: 10.1186/1752-1947-5-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy S. How electronic cigarettes work. 2011 Retrieved from http://science.howstuffworks.com. [Google Scholar]
- CDC. Vital Signs: Focusing on People with Mental Illness. 2013 Retrieved from: http://www.cdc.gov/vitalsigns/SmokingAndMentalIllness.
- Cervellati F, Muresan XM, Sticozzi C, Gambari R, Montagner G, Forman HJ, Torricelli C, Maioli E, Valacchi G. Comparative effects between electronic and cigarette smoke in human keratinocytes and epithelial lung cells. Toxicology in Vitro. 2014;28:999–1005. doi: 10.1016/j.tiv.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorti M, Poulianiti K, Jamurtas A, et al. Effects of active and passive electronic and tobacco cigarette smoking on lung function. Toxicology Letters. 2012;211(S64):64. [Google Scholar]
- Cnattingius S. The epidemiology of smoking during pregnancy: smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine and Tobacco Research. 2004;6(S2):S125–S140. doi: 10.1080/14622200410001669187. [DOI] [PubMed] [Google Scholar]
- Dawkins L, Corcoran O. Acute electronic cigarette use: nicotine delivery and subjective effects in regular users. Psychopharmacology. 2014;231:401–407. doi: 10.1007/s00213-013-3249-8. [DOI] [PubMed] [Google Scholar]
- Dawkins L, Turner J, Crowe E. Nicotine derived from the electronic cigarette improves time-based prospective memory in abstinent smokers. Psychopharmacology. 2013a;227:377–384. doi: 10.1007/s00213-013-2983-2. [DOI] [PubMed] [Google Scholar]
- Dawkins L, Turner J, Roberts A, et al. “Vaping” profiles and preferences: an online survey of electronic cigarette users. Addiction. 2013b;108:1115–1125. doi: 10.1111/add.12150. [DOI] [PubMed] [Google Scholar]
- Dawkins L, Turner J, Hasna S, Soar K. The electronic-cigarette: effects on desire to smoke, withdrawal symptoms, and cognition. Addictive Behaviors. 2012;37:970–973. doi: 10.1016/j.addbeh.2012.03.004. [DOI] [PubMed] [Google Scholar]
- England LJ, Anderson BL, Tong VT, Mahoney J, Coleman-Cowger VH, Melstrom P, Schulkin J. Screening practices and attitudes of obstetricians-gynecologists toward new and emerging tobacco products. American Journal of Obstetrics and Gynecology. 2014;211(6):695.e1–695.e7. doi: 10.1016/j.ajog.2014.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter JF. The electronic cigarette: An alternative to tobacco? Geneva, Switzerland: Jean-Francois Etter; 2012. [Google Scholar]
- Farsalinos KE, Kistler KA, Gillman G, Voudris V. Evaluation of electronic cigarette liquids and aerosol for the presence of selected inhalation toxins. Nicotine and Tobacco Research. 2014a doi: 10.1093/ntr/ntu176. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Voudris V. Evaluation of electronic cigarette use (vaping) topography and estimation of liquid consumption: implications for research protocol standards definition and for public health authorities’ regulation. International Journal of Environmental Research and Public Health. 2013;10:2500–2514. doi: 10.3390/ijerph10062500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos KE, Spyrou A, Tsimopoulou K, Stefapoulos C, Romagna G, Voudris V. Nicotine absorption from electronic cigarette use: comparison between first and new-generation devices. Scientific Reports. 2014b;4(4133):1–7. doi: 10.1038/srep04133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flouris AD, Chorti MA, Poulianiti KP, Jamurtas AZ, Kostikas K, Tzatzarakis MN, Wallace Hayes A, Tsatsakis AM, Koutedakis Y. Acute impact of active and passive electronic cigarette smoking on serum cotinine and lung function. Inhalation Toxicology. 2013;25:91–101. doi: 10.3109/08958378.2012.758197. [DOI] [PubMed] [Google Scholar]
- Giovenco DP, Hammond D, Corey CG, Ambrose BK, Delnevo CD. E-cigarette market trends in traditional U.S. retail channels, 2012–2013. Nicotine and Tobacco Research. doi: 10.1093/ntr/ntu282. (in press). epub ahead of publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, Prokopowicz A, Jablonska-Czapla M, Rosik-Dulewska C, Havel C, Jacob P, Benowitz N. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tobacco Control. 2014;23:133–139. doi: 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129(19):1972–1986. doi: 10.1161/CIRCULATIONAHA.114.007667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua M, Yip H, Talbot P. Mining data on used of electronic nicotine delivery systems (ENDS) from YouTube videos. Tobacco Control. 2013;22:723–728. doi: 10.1136/tobaccocontrol-2011-050226. [DOI] [PubMed] [Google Scholar]
- King BA, Alam S, Promoff G, Arrazola R, Dube SR. Awareness and ever-use of electronic cigarettes among U.S. adults, 2010–2011. Nicotine and Tobacco Research. 2013;15(9):1623–1627. doi: 10.1093/ntr/ntt013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleykamp BA, Jennings JM, Sams C, Weaver MF, Eissenberg T. The influence of transdermal nicotine on tobacco/nicotine abstinence and the effects of a concurrently administered cigarette in women and men. Experimental and Clinical Psychopharmacology. 2008;16(2):99–112. doi: 10.1037/1064-1297.16.2.99. [DOI] [PubMed] [Google Scholar]
- Kong G, Morean M, Cavallo D, Camenga D, Krishnan-Sarin S. Reasons for electronic cigarette experimentation and discontinuation among adolescents and young adults. Nicotine and Tobacco Research. 2015;17(7):847–854. doi: 10.1093/ntr/ntu257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Morean ME, Camenga DR, Cavallo DA, Kong G. E-cigarette use among high school and middle school adolescents in Connecticut. Nicotine and Tobacco Research. 2015;17(7):810–818. doi: 10.1093/ntr/ntu243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinovschi A, Janson C, Holmkvist T, Norback D, Merilainen P, Hogman M. Effect of smoking on exhaled nitric oxide and flow-independent nitric oxide exchange parameters. European Respiratory Journal. 2006;28:339–345. doi: 10.1183/09031936.06.00113705. [DOI] [PubMed] [Google Scholar]
- McRobbie H, Bullen C, Hartmann-Boyce J, Hajek P. Electronic cigarettes for smoking cessation and reduction. Cochrane Database of Systematic Reviews. 2014;12 doi: 10.1002/14651858.CD010216.pub2. [DOI] [PubMed] [Google Scholar]
- Marini S, Buonanno G, Stabile L, Ficco G. Short-term effects of electronic and tobacco cigarettes on exhaled nitric oxide. Toxicology and Applied Pharmacology. 2014;278(1):9–15. doi: 10.1016/j.taap.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Min J, Min K. Cadmium, smoking, and reduced levels of exhaled nitric oxide among US adults. International Journal of Hygiene and Environmental Health. 2014;217:323–327. doi: 10.1016/j.ijheh.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Misra M, Leverette RD, Cooper BT, Bennett MB, Brown SE. Comparative in vitro toxicity profile of electronic and tobacco cigarettes, smokeless tobacco and nicotine replacement therapy products: e-liquids, extracts and collected aerosols. International Journal of Environmental Research and Public Health. 2014;11:11325–11347. doi: 10.3390/ijerph111111325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton KJ, June KM, O’Connor RJ. Initial puffing behaviors and subjective responses differ between an electronic nicotine delivery system and traditional cigarettes. Tobacco Induced Diseases. 2014;12(17):1–8. doi: 10.1186/1617-9625-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisinger C, Døssing M. A systematic review of health effects of electronic cigarettes. Preventive Medicine. 2014;69:248–260. doi: 10.1016/j.ypmed.2014.10.009. [DOI] [PubMed] [Google Scholar]
- Polosa R, Caponnetto P, Morjaria JB, Papale G, Campagna D, Russo C. Effect of an electronic nicotine delivery device (e-Cigarette) on smoking reduction and cessation: a prospective 6-month pilot study. BMC Public Health. 2011;11:786. doi: 10.1186/1471-2458-11-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polosa R, Caponnetto P, Maglia M, Morjaria JB, Russo C. Success rates with nicotine personal vaporizers: a prospective 6-month pilot study of smokers not intending to quit. BMC Public Health. 2014;14:1159. doi: 10.1186/1471-2458-14-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath JM, Villanti AC, Abrams DB, Vallone DM. Patterns of tobacco use and dual use in US young adults: the missing link between youth prevention and adult cessation. Journal of Environmental Public Health. 2012;2012:679134. doi: 10.1155/2012/679134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter KP, Ahluwalia HK, Mosier MC, Nazir N, Ahluwalia JS. A population-based study of cigarette smoking among illicit drug users in the United States. Addiction. 2002;97(7):861–869. doi: 10.1046/j.1360-0443.2002.00162.x. [DOI] [PubMed] [Google Scholar]
- Shihadeh A, Eissenberg T. Electronic cigarette effectiveness and abuse liability: predicting and regulating “nicotine flux”. Nicotine and Tobacco Research. 2015;17(2):158–162. doi: 10.1093/ntr/ntu175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CJ, Livingston SD, Doolittle DJ. An international literature survey of IARC group I carcinogens reported in mainstream cigarette smoke. Food and Chemical Toxicology. 1997;35:1107–1130. doi: 10.1016/s0278-6915(97)00063-x. [DOI] [PubMed] [Google Scholar]
- Spindle TR, Breland AB, Karaoghlanian NV, Shihadeh AL, Eissenberg T. Preliminary results of an examination of electronic cigarette user puff topography: the effect of a mouthpiece-based topography measurement device on plasma nicotine and subjective effects. Nicotine and Tobacco Research. 2015;17(2):142–149. doi: 10.1093/ntr/ntu186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MD, Caviness CM, Grimone K, Audet D, Borges A, Anderson BJ. E-cigarette knowledge, attitudes, and use in opioid dependent smokers. Journal of Substance Abuse Treatment. 2015;52:73–77. doi: 10.1016/j.jsat.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter MA, Mastrobattista J, Sachs M, Aagaard K. Is there evidence for potential harm of electronic cigarette use in pregnancy? Birth Defects Research Part A: Clinical and Molecular Teratology. 2015;103(3):186–195. doi: 10.1002/bdra.23333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talih S, Balhas Z, Eissenberg T, Salman R, Karaoghlanian N, El Hellani A, Baalbaki R, Saliba N, Shihadeh A. Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette yield: measurements and model predictions. Nicotine and Tobacco Research. 2015;17(2):150–157. doi: 10.1093/ntr/ntu174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vansickel AR, Cobb CO, Weaver MF, Eissenberg T. A clinical laboratory model for evaluating the acute effects of electronic cigarettes: nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiology, Biomarkers & Prevention. 2010;19:1945–1953. doi: 10.1158/1055-9965.EPI-10-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vansickel AR, Eissenberg T. Electronic cigarettes: effective nicotine delivery after acute administration. Nicotine and Tobacco Research. 2013;15(1):267–270. doi: 10.1093/ntr/ntr316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vansickel AR, Weaver MF, Eissenberg T. Clinical laboratory assessment of the abuse liability of an electronic cigarette. Addiction. 2012;107:1493–1500. doi: 10.1111/j.1360-0443.2012.03791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaporzone, Inc. Vaperzone’s website for e-cigarettes and e-liquids. [Accessed November 13, 2014]; Available at: http://www.vaporzone.com. [Google Scholar]
- Vardavas CI, Anagnostopoulos N, Kougias M, Evangelopoulou V, Connolly GN, Behrakis PK. Short-term pulmonary effects of using an electronic cigarette: impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest. 2012;141:1400–1406. doi: 10.1378/chest.11-2443. [DOI] [PubMed] [Google Scholar]
- Yan XS, D’Ruiz C. Effects of using electronic cigarettes on nicotine delivery and cardiovascular function in comparison with regular cigarettes. Regulatory Toxicology and Pharmacology. 2015;71:24–34. doi: 10.1016/j.yrtph.2014.11.004. [DOI] [PubMed] [Google Scholar]