Abstract
Many epithelial cancers are associated with chronic inflammation. However, the features of inflammation that are pro-carcinogenic are not fully understood. Tregs typically restrain overt inflammatory responses and maintain intestinal immune homeostasis. Their immune suppressive activity can inhibit inflammation-associated cancers. Paradoxically, we show that colonic Tregs initiate IL-17-mediated carcinogenesis in multiple intestinal neoplasia mice colonized with the human symbiote ETBF. Depletion of Tregs in ETBF-colonized C57BL/6 Foxp3DTR mice enhanced colitis but diminished tumorigenesis associated with shifting of mucosal cytokine profile from IL-17 to IFN-γ; inhibition of ETBF-induced colon tumorigenesis was dependent on reduced IL-17 inflammation and IFN-γ-independent. Treg enhancement of IL-17 production is cell-extrinsic. IL-2 blockade restored Th17 responses and tumor formation in Treg-depleted animals. Our findings demonstrate that Tregs limit the availability of IL-2 in the local microenvironment, allowing Th17 development necessary to promote ETBF-triggered neoplasia and thus unveil a new mechanism whereby Treg responses to intestinal bacterial infection can promote tumorigenesis.
Keywords: IBD, ETBF, CRC, Tregs, IL-17
INTRODUCTION
Colorectal cancer (CRC) remains the third most common cancer and third leading cause of cancer-related deaths in the United States(1). Chronic intestinal inflammation represents one of the main environmental risk factors associated with development of CRC, a feature that is strongly supported by the much higher incidence of CRC in patients suffering from inflammatory bowel disease (IBD) including both Crohn’s and ulcerative colitis (2-4). A role for the IL-23/Th17 inflammatory response in IBD patients is supported by GWAS studies demonstrating an extremely high association of Crohn’s disease with a specific polymorphism of the IL-23 receptor (IL23R) (5). Multiple murine models of immune-mediated carcinogenesis establish the IL-17 response as critical, particularly in the intestine (6-9). Even in CRC that is not overtly colitis-associated, inflammation represents a critical component of the tumor microenvironment (TME) and has a critical impact on the clinical outcome (10). Identification of a Th17 immune signature, defined by the increased expression of genes Il17a, Rorc and Il23r, has been linked with a poor prognosis in CRC patients (11). Because of these emerging insights into specific immune pathways that can promote both early as well as later stages of cancer progression, particularly in the gut, inflammatory triggers and molecular mechanisms initiating pathogenic Th17 immune responses are sought as therapeutic targets for intestinal inflammatory disorders and colon tumorigenesis.
Microbial symbionts are key determinants of intestinal inflammation. In mice, development of Th17 cells in intestinal lamina propria is dictated by the microbiota, and, in one model, colonization of germ free mice by segmented filamentous bacteria (SFB) is required for mucosal Th17 differentiation (12). It was further shown that the balance between intestinal Th1 and Th17 responses could be determined by the nature of the bacterium recognized (e.g. Listeria monocytogenes versus SFB, respectively) (13). Although SFB has been identified as a trigger for mucosal Th17 differentiation and is sufficient to promote autoimmunity in murine models (14), no microbial promoter of IL-17 has yet been formally associated with IBD or CRC in humans. However, there is now emerging data linking enterotoxigenic Bacteroides fragilis (ETBF), a human colonic bacterium associated worldwide with inflammatory diarrheal diseases (15, 16), to IBD(17, 18) and CRC in humans (19, 20). Furthermore, we recently showed that ETBF triggers chronic Stat3/IL-17-driven colitis in C57BL/6 mice and promotes rapid colon tumorigenesis in multiple intestinal neoplasia (Min) mice (heterozygous for the adenomatous polyposis coli (Apc) gene), which is dependent upon the IL-17 response based on abrogation of tumorigenesis by in vivo anti-IL-17 treatment but not anti-IFNγ treatment (6, 21, 22). Additionally, ETBF-induced Th17 colitis and colon tumorigenesis absolutely requires production of the metalloproteinase toxin, B. fragilis toxin (BFT) (21, 23).
In an attempt to further characterize the regulation of pro-carcinogenic IL-17 responses induced by ETBF colonization, we studied the role of Foxp3+ regulatory T cells (Tregs), expecting that they would diminish the magnitude of these responses and thus mitigate tumor formation. This is because depletion of Tregs ultimately leads to the development of colitis in mice not challenged with any colitogenic microbes (24-26). Furthermore, Treg specific deletion of Stat3 results in the ultimate development of spontaneous Th17 colitis (25). We found that ETBF colonization was characterized by the accumulation of Treg cells, as well as IL-17+ T cells, at the dominant site of ETBF tumorigenesis, the distal colon. Surprisingly, we observed that depletion of Tregs in ETBF-colonized Min mice led to the abrogation of tumorigenesis at the earliest stages. Colons of Treg-depleted ETBF-colonized animals were highly inflamed, demonstrating that colon Treg suppressive capacity remains intact. Notably, the inflammation in Treg-depleted ETBF-colonized animals that exhibited increased colitis but decreased tumorigenesis was characterized by elevated IFN-γ and profoundly decreased IL-17A in the lamina propria, resulting in loss of the characteristic Th17 colitis associated with ETBF colonization. We show that Tregs promote acute IL-17-driven colitis via local consumption of IL-2, which inhibits Th17 polarization while enhancing expansion of Th1 cells. While mucosal Tregs are initially required to promote Th17 polarization, they do not participate in the stabilization of the IL-17 response at later stages of ETBF colitis. Thus, we identify an unexpected role for Tregs in promoting the early stages of colon carcinogenesis.
RESULTS
Simultaneous expansion of mucosal Tregs and IL-17-producing cells precedes colon tumorigenesis in ETBF-colonized Min mice
ETBF colonization of four to five week old C57Bl/6 mice (Apc+/+) elicits acute, self-limited (~3-4 days) inflammatory diarrhea, associated with a robust mucosal IL-17 response. ETBF colonization then persists, leading to a chronic (≥1 year), asymptomatic Th17-mediated colitis (21, 22) that promotes distal colon tumorigenesis in Min mice (6) (Figure 1A). Since IL-17A is required for ETBF-triggered tumorigenesis, we sought to understand the regulation of inflammation and colon tumorigenesis in the context of ETBF colonization. Because mucosal Tregs are instrumental in tuning the intestinal Th17 response to counter microbial aggression (27) or, conversely, to limit excessive responses to microbial components(24, 28), we looked for a possible Treg response to ETBF. We found that early after ETBF colonization (day 7) IL-17+ and Foxp3+ T cell density (cells per gram of tissue) and percent of lymphocytes concurrently increased in the tumor-prone distal colon compared to uninfected mice (Figure 1B and 1C). The synchronized expansion of IL-17-producing and Treg cells following ETBF colonization suggests that Treg responses may play a role in modulating IL-17-driven immune procarcinogenesis in the colon.
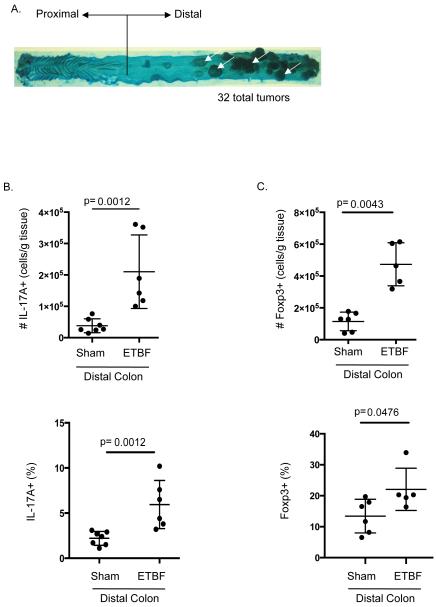
Figure 1. ETBF induces distal colon tumorigenesis with parallel increases in IL-17A+ and Foxp3+ cells.
A) Methylene blue-stained colon of 2 month ETBF-colonized Min mouse. The distal colon predominance of ETBF tumorigenesis is shown (white arrows).
B) Lamina propria lymphocytes (LPLs) were isolated from distal colons of C57Bl/6 mice 7 days after sham (PBS) or ETBF inoculation and 4 hr culture with Cell Stimulation Cocktail preceded intracellular staining (ICS) for IL-17A. Live CD3+ IL-17+ cell numbers were normalized by the mass of colon tissue after cleaning and before LPL isolation (top). Percent IL-17+ of live CD3+ lymphocytes (bottom). Each symbol represents one sample, and error bars represent one standard deviation from the mean in each direction. Shown are data from two separate experiments.
C) ICS for Foxp3 on distal colon LPLs from C57Bl/6 mice was performed fresh ex vivo without stimulation. Live CD3+ CD4+ Foxp3+ cell numbers normalized by mass of colon tissue as in B (top). Percent Foxp3+ of live CD3+ CD4+ lymphocytes (bottom). Each symbol represents one sample, and error bars represent one standard deviation from the mean in each direction. Shown are data from two separate experiments.
Tregs promote colonic neoplasia in ETBF-colonized Min mice
We previously showed that ETBF-induced colon tumorigenesis is IL-17-dependent since injection of an anti-IL17 blocking antibody inhibited tumor formation (6). Foxp3+ Treg function in intestinal mucosa is typically thought to suppress excessive effector immune responses to microbiota and protect the integrity of the intestinal barrier (29-31). Thus, we initially hypothesized that Tregs are necessary to limit ETBF-induced IL-17-mediated tumorigenesis. To assess the impact of Tregs on ETBF tumorigenesis, we crossed Min mice to Foxp3DTR-GFP mice that express the diphtheria toxin receptor (DTR) on Foxp3+ cells (32). Intraperitoneal administration of DT, therefore, selectively depletes Foxp3+ cells. Because autoimmunity develops when Tregs are systemically depleted for more than two weeks (32), we tested the impact of Treg depletion on early microadenoma induction by ETBF in Min mice, which develop microadenomas in the distal colon beginning one week after ETBF colonization. This early time window (day 13) allowed us to assess the effect of Foxp3+ cells on early ETBF tumorigenesis prior to any systemic effects of Treg depletion. DT or PBS was administered to Min × Foxp3DTR mice starting one day prior to ETBF inoculation and every other day thereafter until harvest at day 13. Not surprisingly, Treg-depletion significantly increased colonic inflammation (Figure 2A and 2B), emphasizing that the immunosuppressive function of colonic Tregs remains intact. Surprisingly, however, microadenoma formation was significantly reduced in Treg-depleted mice compared to Treg sufficient controls (Figure 2A and 2C).
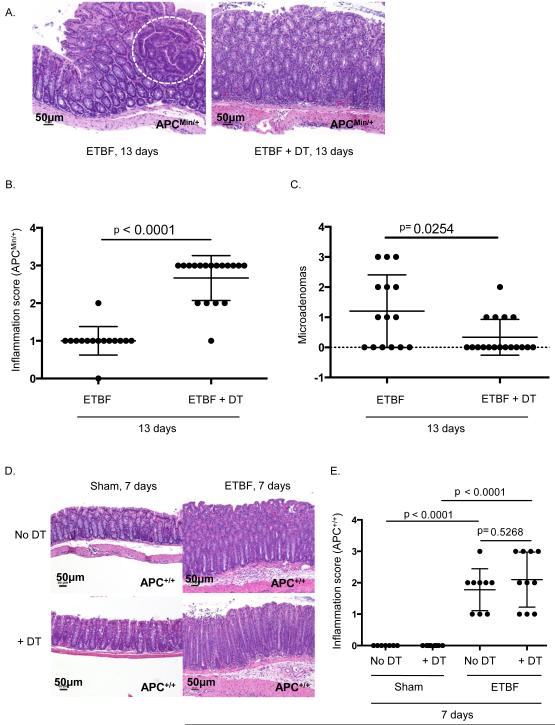
Figure 2. Treg depletion reduces microadenoma formation in Min mice.
A) B6.Foxp3DTRxMin mice were inoculated with ETBF on day 0. Either 150 μl purified sterile H2O or 50 ng/g diphtheria toxin (DT) was administered intraperitoneal (ip) on days −1, 0, 1, 3, and every other day until sacrifice and harvest. Colons were harvested on day 13, cleaned, rolled, and fixed in 10% formalin for histology & scoring. Images are of distal colon. Microadenoma is encircled. Scale bars are 50 μm.
B) Each symbol represents total inflammation score per Min mouse from Figure 2A. Data shown include 2 separate experiments with 5-12 mice per group per experiment. Bars indicate mean +/− SD.
C) Microadenomas counted per colon in (B).
D) B6.Foxp3DTR mice were inoculated with sham or ETBF on day 0. Either 150 μl purified sterile H2O or 50 ng/g diphtheria toxin (DT) was administered intraperitoneal (ip) on days −2, −1, 0, 1, 3, and 5 until sacrifice and harvest. Colons were harvested on day 7, cleaned, rolled, fixed in 10% formalin, paraffin embedded, and H&E stained for histology & scoring. Images are of distal colon. Scale bars are 50 μm.
E) Each symbol represents total inflammation score per mouse from Figure 2D. Data shown include 2 separate experiments with 2-6 mice per group per experiment. Bars indicate mean +/− SD.
During the acute colitis stage at one week, inflammation upon Treg depletion was similar to that of Treg-competent mice in sham (0.0 +/− 0.0, mean +/− SD in sham and sham + DT mice, p>0.999, N=7-8 mice per group) or ETBF-colonized mice (1.78 +/− 0.67 vs 2.1 +/− 0.88, ETBF vs ETBF + DT mice, p=0.5, N=9-10 mice per group)(Figure 2D and 2E). Of note, one week ETBF-colonized mice with or without Treg depletion displayed marked colitis compared to sham mice (p<0.0001 for both comparisons, Figure 2E).
Treg provide help for Th17 responses to ETBF colonization via a cell-extrinsic mechanism
Since IL-17 induction is an absolute requirement for ETBF-induced colon tumorigenesis (6) and microadenoma formation was significantly reduced in ETBF-colonized Treg-depleted mice, we asked whether Tregs in ETBF-colonized mice modified the mucosal Th1/Th17 balance in favor of protumoral Th17 effectors, potentially contributing to the development of distal colon tumorigenesis. To determine the impact of Tregs on the Th1/Th17 balance in ETBF-colonized mice, lamina propria lymphocytes (LPLs) were isolated after one week from the colons of sham or ETBF-colonized Foxp3DTR-GFP mice treated or not with DT and analyzed for cytokine production by intracellular staining (ICS). Indeed, in accordance with the unexpected finding that Treg depletion diminished early in situ tumor formation, depletion of Tregs in ETBF-induced colitis dramatically reduced the proportion of Th17 cells (5 +/− 2%, N=5) in the colonic lamina propria compared to ETBF-colonized Treg-sufficient mice (27 +/− 8%, N=5, P < 0.0001 Figure 3A and 3B). Notably, the effect of Treg depletion was not limited to Th17 cells as IL-17 production by other cell types (CD4−) was also mitigated (Supplemental Figure 1). Conversely, Foxp3+ Treg depletion in ETBF-colonized mice strongly enhanced the Th1 response with a marked increase in the proportion of IFNγ+ CD4+ T cells. Since IFNγ+ CD4+ T cells also increased in sham mice after Treg depletion, our data further support the concept that mucosal regulation of Th1 by Tregs is key to intestinal immune homeostasis (Figure 3A).
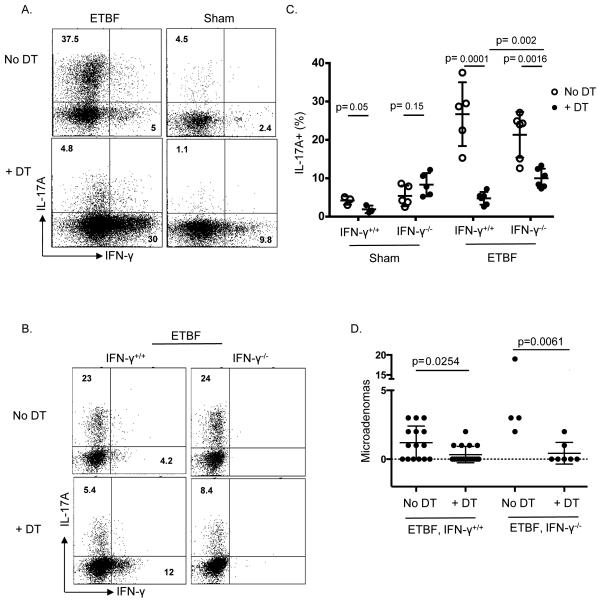
Figure 3. Treg depletion mitigates the Th17 response to ETBF in favor of a Th1 response.
A) Colon LPLs from 1-2 B6.Foxp3DTR mice per group were harvested on day 7 following inoculation with ETBF or Sham on day 0. Either 150 μl purified sterile H2O or 50ng/g DT was administered ip on days −2, −1, 1, 3, & 5. Cells were stimulated ex vivo, followed by ICS. Plots show viable CD3+ CD4+ Foxp3- LPLs and are a representation of 4-5 separate experiments.
B) Aggregate data from combined experiments showing percentage of viable CD3+ CD4+ Foxp3- LPLs that are IL-17A+. Each symbol represents 1-2 Treg-depleted B6.Foxp3DTR mice (****, +DT) or Treg-sufficient mice (○, No DT), and error bars represent 1 standard deviation from the mean in each direction. Holm-Sidak method for multiple comparisons was used to compare No DT vs + DT groups.
C) B6.Foxp3DTR and B6.Foxp3DTRxIFNγ−/− mice were inoculated with ETBF on day 0. Either 150ul purified sterile H2O or 50 ng/g DT was administered ip on days −2, −1, 1, 3, & 5. Colonic LPLs from 1-2 mice per group were harvested on day 7. Cells were stimulated ex vivo, followed by ICS. Dot plots show viable CD3+ CD4+ Foxp3- LPLs and are representative of 4-5 experiments.
D) B6.Foxp3DTR × IFNγ−/− × Min mice were inoculated with ETBF on day 0. Either 150 μl purified sterile H2O or 50 ng/g DT was administered ip on days −1, 0, 1, 3, and every other day until sacrifice and harvest. Colons were harvested on day 13, cleaned, rolled, and fixed in 10% formalin, paraffin embedded and H&E stained for microadenoma counting per colon. Each symbol represents 1 colon, and error bars represent 1 standard deviation from the mean in each direction. 2-5 mice per group per experiment. Combined data from 2 experiments. IFNγ+/+ × Min microadenoma counts from Figure 2B are also shown for easier side-by-side comparison.
Since STAT1/IFN-γ signaling is well established as an inhibitor of the STAT3/Th17 pathway (33), we explored the possibility that loss of IL-17 production upon Treg depletion was indirectly due to inhibition by the increased Th1 response. We crossed Foxp3DTR mice to IFN-γ−/− mice and demonstrated that, even though T cell-generated IL-17 did trend slightly higher in IFN-γ−/− mice compared to IFN-γ+/+ mice after Treg depletion, in the absence of IFN-γ, Treg depletion still mitigated the proportion of Th17 cells in LPLs (Figure 3B and 3C). This result demonstrated that decreased IL-17 production in Treg-depleted mice is mostly not due to increases in IFN-γ. Because Treg cells are viewed as components of the TME that suppress anti-tumor immunity and promote tumor growth (34), it is possible that Treg depletion impaired ETBF tumorigenesis by unleashing a robust anti-tumoral IFN-γ response. Thus, we asked whether increased IFN-γ driven inflammation in the absence of Tregs might promote potent anti-tumor immunity. Depletion of Tregs in Min × Foxp3DTR × IFN-γ−/− mice reduced microadenoma numbers similar to those observed in Treg-depleted Min × Foxp3DTR mice, establishing that decreased Treg-mediated IL-17 production, and not increased IFN-γ, is most likely responsible for reduced neoplasia (Figure 3D).
While these results suggest that Tregs are providing cell-extrinsic “help” for the differentiation of naïve CD4+ LPL to Th17, it is possible that the effects of Foxp3+ cell depletion could be cell-intrinsic, i.e. via depletion of Foxp3+ precursors to the colonic Th17 cells. Indeed, there is evidence that Th17 cells and peripherally induced Tregs may differentiate from a common Foxp3+RORγt+ precursor, or Th17 cells may result from “trans-differentiation” of Tregs (35, 36). Consistent with a potential intrinsic mechanism of Foxp3 in cells differentiating into Th17 cells, we indeed observed the presence of IL-17+ Foxp3+ CD4+ T cells in colon tumors (Supplemental Figure 2).
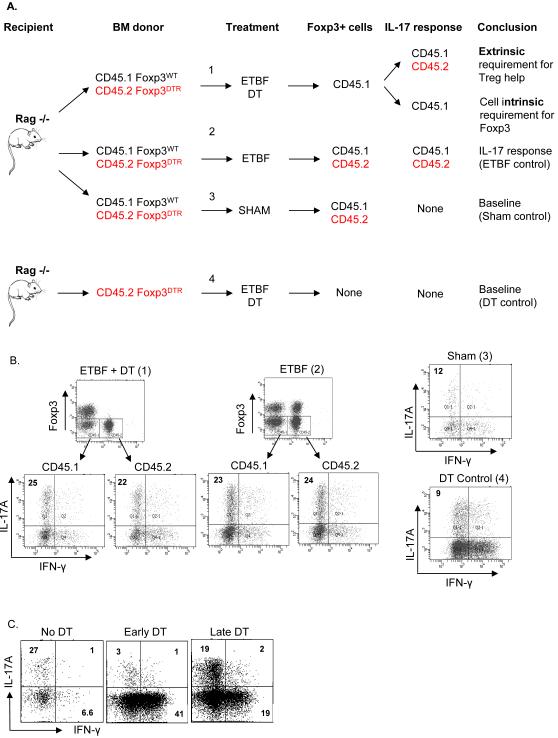
To distinguish whether Foxp3 marked precursors to Th17 cells (intrinsic mechanism) or if Foxp3+ Treg help was necessary for Th17 differentiation (extrinsic mechanism) in ETBF colitis, we transferred both CD45.2+ Foxp3DTR and CD45.1+ Foxp3WT bone marrow (BM) at a 1:1 ratio into sub-lethally irradiated RAG1−/− recipients, establishing mixed BM chimera mice. This approach allowed us to selectively deplete CD45.2+ Foxp3DTR Tregs following DT injection, and it enabled us to monitor the origin (CD45.1+ or CD45.2+ BM) of the Th17 cells during ETBF colitis (Figure 4A). Upon CD45.2+ Foxp3+ depletion, a cell intrinsic mechanism would only diminish Th17 responses among CD45.2+ T cells while a cell extrinsic mechanism would not diminish Th17 responses among either CD45.1+ or CD45.2+ T cells. When CD45.2+ Foxp3DTR cells were depleted in the mixed BM chimeras, we found that neither CD45.1+ (i.e. CD45.2−) nor CD45.2+ Th17 cells were decreased compared to Treg sufficient mixed BM chimeras (Figure 4B). In these experiments, CD45.2+ Tregs were fully depleted, but CD45.1+ Tregs were unaffected (Figure 4B). As a positive control for the action of DT, we depleted total Tregs from ETBF-colonized CD45.2+ Foxp3DTR BM chimeras. As expected, this resulted in a Th17 proportion in LPLs similar to the sham mixed BM chimera mice (Figure 4B). In order to determine at which point Tregs enhanced ETBF-induced Th17 responses, DT was administered one day post ETBF inoculation (late depletion), in contrast to the experiments described above in which DT was given two days prior to ETBF inoculation. In striking contrast to pre-inoculation Treg depletion, late depletion of Tregs resulted in a Th17 response that was comparable to that of Treg sufficient ETBF-colonized mice (Figure 4C). Taken together, these results suggest that Foxp3+ Tregs provided extrinsic support for initial Th17 differentiation, but not maintenance, in response to ETBF. Regardless of when Treg depletion was initiated (early versus late), the absence of Tregs resulted in an increased proportion of Th1 cells (Figure 4C).
Figure 4. Mixed chimeras reveal a cell extrinsic requirement for Foxp3 in Th17 differentiation.
A) Schematic of the experiment designed to determine whether Foxp3 expression is a cell extrinsic or intrinsic requirement for Th17 differentiation.
B) 107 total bone marrow cells from B6.Foxp3DTR CD45.2 mice only (DT control), or mixed 1:1 with bone marrow cells from B6.Foxp3WT CD45.1 mice, were transferred retro-orbitally into Rag1−/− recipients that had received 300 rads irradiation 5-6 hours prior. Following 6 weeks for hematopoietic cell reconstitution, mice were inoculated with ETBF or sham on day 0, and either 150 μl purified sterile H2O or 50 ng/g DT was administered ip on days −2, −1, 1, 3, & 5. Colonic LPLs from 1-2 mice were harvested on day 7 following ETBF or Sham inoculation. Cells were stimulated ex vivo followed by ICS. Dot plots show viable CD3+ CD4+ LPLs and are representative of 2 experiments.
C) Colon LPLs from 1 B6.Foxp3DTR mouse per group were harvested on day 7 following inoculation with ETBF or sham on day 0. For no depletion and early depletion, purified sterile H2O or DT, respectively, was administered ip on days −2, −1, 1, 3, & 5. For late depletion, DT was administered ip on days 1, 3, & 5. Cells were stimulated ex vivo, followed by ICS. Plots show viable CD3+ CD4+ Foxp3- LPLs and are representative of 2 mice per group.
Tregs promote Th17 development in the colon via consumption of IL-2
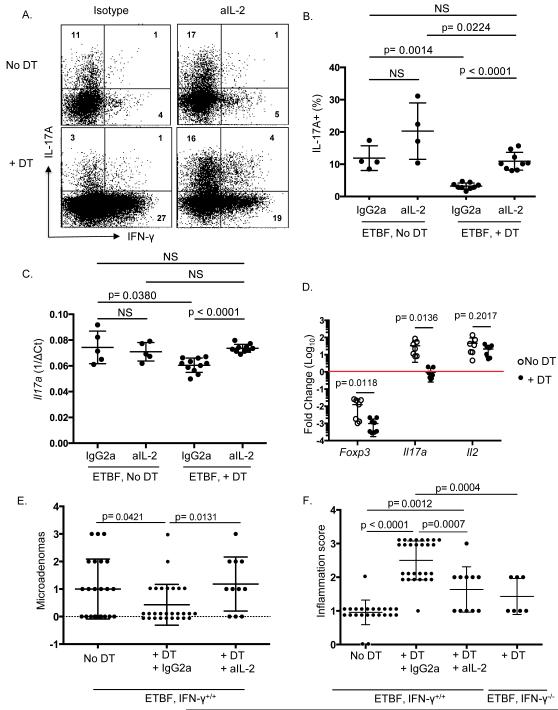
Tregs, which express high levels of IL-2 receptors, particularly when activated, do not produce endogenous IL-2. Thus, they are extremely dependent on exogenous sources of IL-2 for their survival. Treg proliferation and activation upon ETBF colonization may therefore deprive the local inflammatory environment of IL-2. Since IL-2 down-regulates IL-17 production via STAT5 signaling, Tregs may promote Th17 differentiation by limiting the amount of local IL-2 available to uncommitted T cells (37-39). In contrast, IL-2 may promote proliferation of Th1 cells (40). To determine whether Treg promotion of Th17 differentiation upon ETBF colonization occurs via limiting local IL-2, ETBF-colonized Treg-depleted Foxp3DTR mice were injected with a blocking antibody against IL-2 (S4B6-1) and LPLs were isolated after 7 days of ETBF colonization. Figure 5 shows that anti-IL-2 treatment indeed restored the Th17 response to ETBF in the absence of Treg cells. In contrast, when Treg cells were present, anti-IL-2 treatment minimally increased the proportion of Th17 cells (Figure 5A & B). In keeping with the notion that IL-2 expands Th1 cells, anti-IL-2 treatment partially mitigated the increase in IFNγ-producing cells upon Treg depletion (Figure 5A). When Tregs were depleted, the expression of total IL-17 mRNA was similarly restored when anti-IL-2 mAb was administered (Figure 5C). The decreased availability of IL-2 likely resulted from cytokine consumption by Tregs (“sink effect”) and not inhibition of IL-2 production by effector cells as IL-2 mRNA expression was indeed similar between effector T cells (CD11b- Foxp3- CD4+ or CD8+) sorted from LPLs of ETBF-colonized Treg-sufficient and Treg-deficient mice (Figure 5D). As expected, expression of Foxp3 was low in both groups of effector T cells, and IL-17 mRNA was decreased in effector T cells sorted from Treg-depleted mice compared to effector T cells from Treg-sufficient mice (Figure 5D).
Figure 5. Anti-IL-2 restores the Th17 response to ETBF in the absence of Foxp3+ Tregs.
A) 4-9 B6.Foxp3DTR mice per group were inoculated with ETBF. Either 150 μl purified sterile H2O or 50 ng/g DT was administered ip on days −2, −1, 1, 3, & 5, and either rat anti-mouse IL-2 (S4B6-1) or rat IgG2a isotype (JES3-19F) was delivered ip daily (day −2 through day 6). Colonic LPLs from each mouse were harvested on day 7. Cells were stimulated ex vivo, followed by ICS. Representative dot plots show viable CD3+ CD4+ Foxp3- LPLs from 1 mouse per group. aIL-2, anti-mouse IL-2.
B) Aggregate data from 2-3 mice per group showing percentage of viable CD3+ CD4+ Foxp3- LPLs that are IL-17A+. Each symbol represents one B6.Foxp3DTR mouse, and error bars represent 1 standard deviation from the mean in each direction. Isotype-treated animals were not different from untreated animals, so these two animal groups were combined.
C) Tissue for RNA isolation was taken from the middle colon from each mouse in Figure 5B and Taqman qRT-PCR was performed for IL-17A. ^Ct was calculated by subtracting Ct of Gapdh from Ct of IL-17A and averaging 2 technical replicates. Each symbol represents one mouse, and error bars represent 1 standard deviation from the mean in each direction.
D) 6-12 B6.Foxp3DTR mice per group were inoculated with ETBF on day 0. Either 150 μl purified sterile H2O or 50 ng/g DT was administered ip on days −2, −1, 1, 3, & 5. Colonic LPLs from 1-3 mice per group were harvested on day 7 for fluorescence-associated cell sorting. Effector T cells (CD11b-, Foxp3-, CD4+ or CD8+) were sorted from Treg-depleted mice (****, +DT) and from Treg-sufficient mice ((, No DT). RNA was isolated from Trizol and Taqman qRT-PCR was performed for indicated genes. Gapdh was used as housekeeping control for total RNA quantity (^Ct), and average of 2 technical replicates were used for each sample. CD4+ Foxp3+ Tregs were sorted from Treg-sufficient mice as the reference sample for calculating ^^Ct. Fold change = 2^-(^^Ct). Each symbol represents one mouse (or pooled group), and error bars represent 1 standard deviation from the mean in each direction. Holm-Sidak method for multiple comparisons was used to compare No DT vs + DT groups.
E) B6.Foxp3DTRxMin mice were inoculated with ETBF on day 0. Either 150 μl purified sterile H2O or 50 ng/g DT was administered ip on days −1, 0, 1, 3, and every other day until sacrifice and harvest. Either rat anti-mouse IL-2 (S4B6-1) or rat IgG2a isotype (JES3-19F) was delivered ip daily (day −1 through day 13). Colons were harvested on day 13, cleaned, rolled, and fixed in 10% formalin for histology & scoring. Microadenomas were counted per colon and include 2 separate experiments with 3-12 mice per group per experiment. DT animals treated with isotype Ab (N=10) were not different from DT only-treated animals (N=18), so DT animals treated with or without isotype Ab were combined. Each symbol represents one mouse, and error bars represent 1 standard deviation from the mean in each direction. IFNγ−/− × Min + DT microadenoma counts from Figure 3D are also shown for easier side-by-side comparison.
F) Inflammation scores per colon in (E).
Importantly, when Treg-depleted Min × Foxp3DTR mice were treated with anti-mouse IL-2 mAb, microadenoma formation was restored (Figure 5E), demonstrating that the IL-17-dependent protumoral activity of mucosal Tregs in the ETBF-driven TME requires IL-2 deprivation. Despite the restoration of microadenomas upon anti-IL-2 treatment of Foxp3-depleted mice, colonic inflammation was reduced compared to Treg-depleted isotype-treated Min × Foxp3DTR mice (Figure 5F). The enhanced colitis of Treg depletion was also reduced in Treg-depleted IFNγ−/− mice (Figure 5F). These results dissociate colitis from tumorigenesis in that Th1-mediated colitis upon Treg depletion (Figure 3A) does not induce microadenomas as does Th17-mediated colitis (Figure 2C). Thus, we conclude that Treg cells facilitate Th17 differentiation in the inflammatory microenvironment of the ETBF-colonized colon by limiting excess IL-2 that can otherwise prevent the development of a pro-carcinogenic Th17-mediated immune response.
DISCUSSION
In an effort to further understand the mechanisms of ETBF-driven procarcinogenic colitis, we investigated the impact of mucosal Treg cells on the inflammatory response to ETBF murine colonization. These studies led to the surprising conclusion that Tregs are critical to initiate the Th17 colitis necessary for tumor induction. Through consumption of IL-2, Tregs inhibit the development of Th1 colitis, which is not procarcinogenic, and shift T cell differentiation to Th17 in the lamina propria of ETBF-colonized mice. This helper role of Tregs for Th17 development occurs only at the very initial stages of ETBF-mediated colitis.
Recent reports showed that mucosal Tregs, which commonly orchestrate mucosal immune homeostasis by restraining inflammatory responses to microbiota in the gut, can cooperate with immune effector cells to protect against and eradicate infections (27, 38). Th17 cells, by their production of IL-17, are predicted to be important to the mucosal defense against ETBF, resulting in the recruitment of bactericidal polymorphonuclear and phagocytic mononuclear cells. Consistent with this idea, IL-17−/− mice colonized with ETBF exhibit increased morbidity and mortality compared to wild-type mice (C Dejea, C Sears, unpublished). However, the mucosal Th17 defenses against ETBF are not antiseptic and thus do not prevent chronic colitis associated with the persistence of both ETBF and mucosal IL-17 production (22). In conjunction with a host genetic predisposition, such as the Apc mutation in Min mice, ETBF-induced mucosal IL-17 is instrumental in promoting marked distal colon tumorigenesis (6). Importantly, a predominant IL-17 response is associated with worse survival in human CRC (11). ETBF represents the first common human commensal identified as a potent trigger for murine colon tumorigenesis (6, 19). Surprisingly, ETBF triggers its carcinogenic IL-17 response, in part, through the accumulation of mucosal Tregs. This is in stark contrast to the mucosal immune homeostasis proposed as resulting from nontoxigenic B. fragilis-mediated Treg induction(41, 42). Our results show that ETBF-mediated colitis drives the accumulation of Foxp3+ Tregs that, despite their suppression of Th1 mucosal inflammation, consume IL-2 resulting in Th17 polarization that is critical to ETBF tumorigenesis. IL-2 is a potent inhibitor of IL-17 production via competition between STAT5 (IL-2 signaling) and STAT3 (IL-17 signaling) for binding to the Il17 promoter (43). Further, Tregs are incapable of producing IL-2 and are highly dependent on exogenous sources of IL-2, indispensable to their survival (44, 45). Thus, Tregs capture IL-2 in their environment via their elevated constitutive expression of the high affinity IL-2 receptor, CD25. By modulating levels of exogenous IL-2, Tregs release the STAT5 inhibitory effect on Th17 differentiation of uncommitted CD4+ T cells or suppress already-committed effector T cells by IL-2 deprivation (45, 46). Although Tregs have also been shown to inhibit IL-2 production by effector T cells (endogenous IL-2) (45, 47), we have shown herein that ETBF-induced Tregs do not alter IL-2 transcription by lamina propria effector T cells. Importantly, our results establish that Tregs strictly intervened at the initiation stage of Th17 differentiation, but they are not required for the stabilization of the colonic Th17 immune signature since late Treg depletion does not impair the Th17 response to ETBF infection (Figure 4C). Our findings described here may be related to those of Pandiyan et al. who showed in a murine model of oral Candida albicans that poorly suppressive Tregs were important to initiate the anti-fungal Th17 response, but they eventually resumed their suppressive function and inhibited pathogenic Th17 effectors (38).
Because Treg and Th17 cells demonstrate functional plasticity, we experimentally ruled out the possibility that ablation of Foxp3 limited Th17 cells generated via elimination of Foxp3+ progenitors. Tregs have been reported to convert into IL-17 or IFN-γ producing cells (48-50), and Th17 cells have been reported to up-regulate Tbet and produce IFN-γ(51, 52) or up-regulate Foxp3 and acquire suppressive function (36, 53). Xu et al demonstrated that, in the presence of IL-6, Foxp3+ Tregs can be induced to become Th17 cells(48). Moreover, IL-17+RORγt+ Tregs have been detected in mouse and human cancer and have been shown to be highly pathogenic(54). Even though a small proportion of IL-17+Foxp3+ T cells was detected in the colons of ETBF-colonized mice (Supplemental Figure 2), our use of mixed BM chimeras demonstrated that ablation of CD45.2+ BM derived Foxp3+ Tregs did not impair the generation of CD45.2+ Th17 cells when CD45.1+ Tregs were present. This indicated Tregs act to promote Th17 differentiation via an extrinsic mechanism in the ETBF murine model.
Our study showed that, in the absence of Tregs, ETBF colonization induced a strong inflammatory response associated with increased IFN-γ production. Although commonly associated with anti-tumor host defenses, we demonstrated that the IFN-γ/Th1-type immune response was not responsible for reduced colon neoplasia in Treg depleted-ETBF-colonized Min mice. This result highlights the impact of IL-17 in tumor initiation. The increased IFN-γ response to Treg depletion is not surprising, especially in the gut where there is constant exposure to inflammatory stimuli such as pathogen-associated molecular patterns on commensal bacteria. In fact, Treg cell depletion has recently been shown to increase the inflammatory cytokines IFN-γ and IL-17 in the intestines (24), and adoptive transfer of Treg cells can cure experimental models of inflammatory bowel disease(26, 55). These data emphasize that Tregs exercise strong immunosuppressive function on effector T cells in other settings. However, in ETBF colonization, mucosal Tregs polarize a robust IL-17 mucosal immunity while suppressing Th1 immunity. We noted that the inflammation score one week following ETBF colonization, in contrast to inflammation following 13 days ETBF, remained similar with or without Tregs; however, the character of this inflammation differed, with Tregs promoting IL-17 while repressing IFN-γ.
Our findings allow a better understanding of the Th17 polarization induced by ETBF colonization and its contribution to colon tumorigenesis. However, the cellular and molecular mechanisms by which ETBF selectively increases Tregs in the distal murine colon remain unknown. As BFT, which profoundly alters the biology of colonic epithelial cells (23), is required for ETBF-driven oncogenic IL-17 production, we postulate that epithelial-derived signals, following the rapid cleavage of E-cadherin induced by BFT (56), contribute to the recruitment of effector immune cells, including Tregs. It has recently been shown that diminished epithelial barrier function induced the production of alarmins that directly recruit and activate Tregs(57). For example, IL-33 stimulated Tregs and opposed the restraining effect of proinflammatory IL-23 on Tregs in microbiota-induced chronic colitis. Additional inflammatory signals, including those potentially provided by BFT-altered epithelial cells, may play a critical role in the Treg response to ETBF and promotion of protumoral IL-17 production. Furthermore, while the precise mechanism by which IL-17 contributes to ETBF-mediated tumorigenesis is still unclear, IL-17 has been shown to directly influence colon epithelial cell signaling, survival, and proliferation. (58) The elucidation of IL-17-induced epithelial-derived signaling pathways will contribute to a better understanding of the regional distribution of ETBF-induced colon tumors and may eventually provide therapeutic targets to control deleterious mucosal IL-17 and Treg responses.
METHODS
Mice and reagents
C57BL/6 Foxp3DTR-GFP mice were provided by A. Rudensky (Memorial Sloan-Kettering Cancer Center, New York, New York). C57BL/6 Rag1−/−, CD45.1, and IFN-γ−/− mice were purchased from Jackson Laboratories (Bar Harbor, ME). ApcΔ716 (Min) mice were obtained from Drs. David Huso and Bert Vogelstein. IFN-γ−/− × Foxp3DTR-GFP mice were obtained in our facility by crossing C57BL/6 Foxp3DTR-GFP mice with IFN-γ−/− mice. Min × Foxp3DTR-GFP mice were obtained in our facility by crossing C57BL/6 Foxp3DTR-GFP mice with Min mice. In some experiments, bone marrow (BM) chimera mice were established by retro-orbital injection of 107 BM cells from donor mice into sub-lethally irradiated (300 rad) recipient C57BL/6 Rag1−/− mice. Irradiated mice were rested 6 hours before BM cell injection. Reconstituted mice were maintained on prophylactic antibiotic treatment (Sulfatrim) prior to ETBF inoculation (see below). In accordance with the Association for the Assessment and Accreditation of Laboratory Animal Care International, mice were maintained under specific pathogen-free conditions and studied according to protocols approved by the Johns Hopkins University Animal Care and Use Committee.
Aqua cell viability dye was purchased from Life Technologies (Grand Island, NY). Flow cytometry antibodies against CD3, CD4, CD45.2, Foxp3, IL-17A, and IFN-γ were purchased from Affymetrix/eBioscience (San Diego, CA). Intracellular staining (ICS) was performed with eBioscience Foxp3 staining kit according to the manufacturer’s instructions. Anti-IL-2 mAb (clone S4B6) and isotype control (rat IgG2a) were purified from hybridomas purchased at American Type Culture Collection (Manassas, VA).
ETBF mouse models
ETBF strain 86-5443-2-2 was used in this study (6, 21). B. fragilis strains were grown anaerobically on BHI medium plates containing 37 g of brain heart infusion base (Difco Laboratories, Detroit, MI) per liter along with 5 g of yeast extract (Difco) per liter, 0.1 mg of vitamin K per liter, 0.5 mg of hemin per liter, 50 mg of L-cysteine, and 6 μg of clindamycin per liter (all from Sigma, St. Louis, MO). A single colony was inoculated into BHI broth and grown anaerobically overnight at 37°C. Pelleted, washed bacteria were resuspended in 0.1 N sodium bicarbonate buffer and adjusted to an optical density corresponding to ~109 colony forming units (CFU)/ml for mouse inoculations. Three to 4 week old mice were treated with clindamycin and streptomycin (0.1 g/L and 5 g/L, respectively, in water bottles) 5 days prior to ETBF inoculation by gavage (~108 bacteria in PBS). BM chimera mice were inoculated with ETBF 6 weeks after sub-lethal irradiation. All strains are resistant to these antibiotic treatments. Mice were sacrificed 7 days after colonization, unless otherwise noted.
Treg depletion and IL-2 neutralization
Mice received 50 ng intraperitoneal (ip) injections of diphtheria toxin (DT; Sigma-Aldrich, MO) per g body weight on days one and two prior to ETBF inoculation (D-2, D-1) and on days 1, 3 and 5 after ETBF inoculation (D+1, D+3, D+5). Colons were harvested from mice 7 days after ETBF inoculation. In some experiments, IL-2 was neutralized by ip injection of 0.5 mg/mouse of anti-IL-2 mAb (clone S4B6) or isotype control (rat IgG2a) 2 days prior and every day after ETBF inoculation until mouse sacrifice. For tumor experiments, mice received 50 ng DT per g body weight on D-1, D0, D+1, D+3, and every other day until sacrifice and harvest on D+13.
Lamina propria lymphocytes (LPL) isolation
Dissected colons were flushed with 20 ml Ca2+, Mg2+ free PBS 1X and cut longitudinally. Tissues were cut in <0.5 mm pieces and washed 3 times for 20 min in 37°C 2mM EDTA, 10% FCS, 25mM Hepes, HBSS buffer. Tissue pieces were subsequently digested 30 min in 5% FCS RPMI in presence of 400 Unit/ml Liberase (Roche Diagnostic, Indianapolis, IN) and 0.2 mg/ml DNAse 1 (Roche Diagnostic). Mononuclear cells were isolated by 20/40/80 Percoll gradient separation (GE Healthcare Life Science, Pittsburgh, PA).
Flow cytometry and fluorescence-associated cell sorting (FACS)
Colons from 1-2 mice per group were processed to obtain lamina propria lymphocytes (LPLs) as previously described (59). Mononuclear cells collected by Percoll gradient separation were cultured 4hr in Iscove’s Modified Dulbecco’s Medium (IMDM) with 5% FCS and in the presence of Cell Stimulation Cocktail (plus protein transport inhibitors) (eBioscience). Cells were then washed and stained for cell surface markers followed by fixation and permeabilization (Foxp3 Fixation buffer, eBioscience). ICS was performed for IFN-γ, IL-17A and Foxp3. Flow cytometry acquisition was performed on LSRII cytometer (BD Bioscience) and data was analyzed using FACSDiva 6.1.3 software. In some experiments, FACS was performed on FACSAria II (BD Bioscience).
Quantitative RT-PCR
Total RNA was isolated from sorted cells or whole tissue using TRIzol® reagent from Life Technologies (Grand Island, NY) according to manufacturer’s instructions. 1μg of RNA was reverse transcribed using High-Capacity RNA-to-cDNA™ Kit (Applied Biosystems®). 40 cycles of TaqMan® Gene Expression qRT-PCR was performed on 1 ng RNA per sample for indicated genes. ^Ct was calculated by subtracting Ct of Gapdh from Ct of target gene and averaging 2 technical replicates.
Histology and microadenoma counts
Colons were dissected and preserved in 10% buffered formalin. Histologic examination was performed after hematoxylin and eosin (H&E) staining of 5 μm sections. To facilitate longitudinal examination of the full-length colon, colons were ‘Swiss-rolled’ prior to embedding and sectioning. Total colon inflammation was scored as previously described(21). Microadenoma counts on formalin-fixed paraffin-embedded H&E colon tissue sections from 2-week ETBF-colonized Min mice, and all histopathology scoring, were performed by a pathologist (DH).
Statistical analysis
Comparison of means was done by unpaired, two-tailed Mann-Whitney U testing, unless otherwise indicated. A p value of ≤ 0.05 was considered to designate a significant difference.
Supplementary Material
SIGNIFICANCE.
Tregs promote an oncogenic immune response to a common human symbiote associated with inflammatory bowel disease and colorectal cancer. Our data defines mechanisms by which mucosal Tregs, despite suppressing excessive inflammation, promote the earliest stages of immune pro-carcinogenesis via enhancement of IL-17 production at the expense of IFN-γ production.
ACKNOWLEDGEMENTS
We greatly appreciate the support of the SKCCC Flow Core, particularly Ada Tam and Richard L. Blosser.
Funding for this work was provided by the National Institutes of Health (RO1CA151325 CLS, DMP; R01DK080817 CLS; P30DK0889502 GI Core; P30CA006973 SKCCC core; T32AI007247 ALG).
Abbreviations
- IBD
inflammatory bowel disease
- CRC
colorectal cancer
- ETBF
enterotoxigenic Bacteroides fragilis
- BFT
B. fragilis toxin
- Min
multiple intestinal neoplasia
- Treg
regulatory T cell
- Th17
T helper 17 cell
- SFB
segmented filamentous bacteria
- LPL
lamina propria lymphocyte
- ICS
intracellular staining
Footnotes
The authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
A.L. Geis contributed to study concept and design; performed data acquisition, analysis, and interpretation; and wrote the manuscript. H. Fan, X. Wu and S. Wu assisted with the animal experiments. J.L. Wolfe contributed to study concept. D. Huso did the pathology examinations. C.L. Sears, F. Housseau, and D.M. Pardoll provided study mentorship and supervision, including study concept/design, data interpretation and writing of the manuscript. All authors approved the manuscript.
References
- 1.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–17. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 2.Herrinton LJ, Liu L, Levin TR, Allison JE, Lewis JD, Velayos F. Incidence and mortality of colorectal adenocarcinoma in persons with inflammatory bowel disease from 1998 to 2010. Gastroenterology. 2012;143:382–9. doi: 10.1053/j.gastro.2012.04.054. [DOI] [PubMed] [Google Scholar]
- 3.Bansal P, Sonnenberg A. Risk factors of colorectal cancer in inflammatory bowel disease. Am J Gastroenterol. 1996;91:44–8. [PubMed] [Google Scholar]
- 4.Gillen CD, Walmsley RS, Prior P, Andrews HA, Allan RN. Ulcerative colitis and crohn's disease: A comparison of the colorectal cancer risk in extensive colitis. Gut. 1994;35:1590–2. doi: 10.1136/gut.35.11.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Meglio P, Di Cesare A, Laggner U, Chu CC, Napolitano L, Villanova F, et al. The IL23R R381Q gene variant protects against immune-mediated diseases by impairing IL-23-induced Th17 effector response in humans. PLoS One. 2011;6:e17160. doi: 10.1371/journal.pone.0017160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–22. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McAllister F, Bailey JM, Alsina J, Nirschl CJ, Sharma R, Fan H, et al. Oncogenic kras activates a hematopoietic-to-epithelial IL-17 signaling axis in preinvasive pancreatic neoplasia. Cancer Cell. 2014;25:621–37. doi: 10.1016/j.ccr.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizzo A, De Mare V, Rocchi C, Stolfi C, Colantoni A, Neurath MF, et al. Smad7 induces plasticity in tumor-infiltrating Th17 cells and enables TNF-alpha-mediated killing of colorectal cancer cells. Carcinogenesis. 2014;35:1536–46. doi: 10.1093/carcin/bgu027. [DOI] [PubMed] [Google Scholar]
- 9.Kirchberger S, Royston DJ, Boulard O, Thornton E, Franchini F, Szabady RL, et al. Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model. J Exp Med. 2013;210:917–31. doi: 10.1084/jem.20122308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 11.Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71:1263–71. doi: 10.1158/0008-5472.CAN-10-2907. [DOI] [PubMed] [Google Scholar]
- 12.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–98. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Y, Torchinsky MB, Gobert M, Xiong H, Xu M, Linehan JL, et al. Focused specificity of intestinal TH17 cells towards commensal bacterial antigens. Nature. 2014;510:152–6. doi: 10.1038/nature13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–27. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sack RB, Myers LL, Almeido-Hill J, Shoop DS, Bradbury WC, Reid R, et al. Enterotoxigenic bacteroides fragilis: Epidemiologic studies of its role as a human diarrhoeal pathogen. J Diarrhoeal Dis Res. 1992;10:4–9. [PubMed] [Google Scholar]
- 16.Sears CL, Islam S, Saha A, Arjumand M, Alam NH, Faruque AS, et al. Association of enterotoxigenic bacteroides fragilis infection with inflammatory diarrhea. Clin Infect Dis. 2008;47:797–803. doi: 10.1086/591130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prindiville TP, Sheikh RA, Cohen SH, Tang YJ, Cantrell MC, Silva J., Jr Bacteroides fragilis enterotoxin gene sequences in patients with inflammatory bowel disease. Emerg Infect Dis. 2000;6:171–4. doi: 10.3201/eid0602.000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Basset C, Holton J, Bazeos A, Vaira D, Bloom S. Are helicobacter species and enterotoxigenic bacteroides fragilis involved in inflammatory bowel disease? Dig Dis Sci. 2004;49:1425–32. doi: 10.1023/b:ddas.0000042241.13489.88. [DOI] [PubMed] [Google Scholar]
- 19.Boleij A, Hechenbleikner EM, Goodwin AC, Badani R, Stein EM, Lazarev MG, et al. The bacteroides fragilis toxin gene is prevalent in the colon mucosa of colorectal cancer patients. Clin Infect Dis. 2015;60:208–15. doi: 10.1093/cid/ciu787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toprak NU, Yagci A, Gulluoglu BM, Akin ML, Demirkalem P, Celenk T, et al. A possible role of bacteroides fragilis enterotoxin in the aetiology of colorectal cancer. Clin Microbiol Infect. 2006;12:782–6. doi: 10.1111/j.1469-0691.2006.01494.x. [DOI] [PubMed] [Google Scholar]
- 21.Rhee KJ, Wu S, Wu X, Huso DL, Karim B, Franco AA, et al. Induction of persistent colitis by a human commensal, enterotoxigenic bacteroides fragilis, in wild-type C57BL/6 mice. Infect Immun. 2009;77:1708–18. doi: 10.1128/IAI.00814-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wick EC, Rabizadeh S, Albesiano E, Wu X, Wu S, Chan J, et al. Stat3 activation in murine colitis induced by enterotoxigenic bacteroides fragilis. Inflamm Bowel Dis. 2014;20:821–34. doi: 10.1097/MIB.0000000000000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sears CL, Geis AL, Housseau F. Bacteroides fragilis subverts mucosal biology: From symbiont to colon carcinogenesis. J Clin Invest. 2014;124:4166–72. doi: 10.1172/JCI72334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boehm F, Martin M, Kesselring R, Schiechl G, Geissler EK, Schlitt HJ, et al. Deletion of Foxp3+ regulatory T cells in genetically targeted mice supports development of intestinal inflammation. BMC Gastroenterol. 2012;12:97. doi: 10.1186/1471-230X-12-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, et al. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–91. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mottet C, Uhlig HH, Powrie F. Cutting edge: Cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939–43. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, Friedrich C, Hagemann SC, Korte WH, Goharani N, Cording S, et al. Regulatory T cells promote a protective Th17-associated immune response to intestinal bacterial infection with C. rodentium. Mucosal Immunol. 2014;7:1290–301. doi: 10.1038/mi.2014.17. [DOI] [PubMed] [Google Scholar]
- 28.Geuking MB, Cahenzli J, Lawson MA, Ng DC, Slack E, Hapfelmeier S, et al. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity. 2011;34:794–806. doi: 10.1016/j.immuni.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 29.Huter EN, Stummvoll GH, DiPaolo RJ, Glass DD, Shevach EM. Cutting edge: Antigen-specific TGF beta-induced regulatory T cells suppress Th17-mediated autoimmune disease. J Immunol. 2008;181:8209–13. doi: 10.4049/jimmunol.181.12.8209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 31.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 32.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–7. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka K, Ichiyama K, Hashimoto M, Yoshida H, Takimoto T, Takaesu G, et al. Loss of suppressor of cytokine signaling 1 in helper T cells leads to defective Th17 differentiation by enhancing antagonistic effects of IFN-gamma on STAT3 and smads. J Immunol. 2008;180:3746–56. doi: 10.4049/jimmunol.180.6.3746. [DOI] [PubMed] [Google Scholar]
- 34.Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127:759–67. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- 35.Tartar DM, VanMorlan AM, Wan X, Guloglu FB, Jain R, Haymaker CL, et al. FoxP3+RORgammat+ T helper intermediates display suppressive function against autoimmune diabetes. J Immunol. 2010;184:3377–85. doi: 10.4049/jimmunol.0903324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valmori D, Raffin C, Raimbaud I, Ayyoub M. Human RORgammat+ TH17 cells preferentially differentiate from naive FOXP3+Treg in the presence of lineage-specific polarizing factors. Proc Natl Acad Sci U S A. 2010;107:19402–7. doi: 10.1073/pnas.1008247107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–81. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 38.Pandiyan P, Conti HR, Zheng L, Peterson AC, Mathern DR, Hernandez-Santos N, et al. CD4(+)CD25(+)Foxp3(+) regulatory T cells promote Th17 cells in vitro and enhance host resistance in mouse candida albicans Th17 cell infection model. Immunity. 2011;34:422–34. doi: 10.1016/j.immuni.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y, Haines CJ, Gutcher I, Hochweller K, Blumenschein WM, McClanahan T, et al. Foxp3(+) regulatory T cells promote T helper 17 cell development in vivo through regulation of interleukin-2. Immunity. 2011;34:409–21. doi: 10.1016/j.immuni.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 40.Fujimura K, Oyamada A, Iwamoto Y, Yoshikai Y, Yamada H. CD4 T cell-intrinsic IL-2 signaling differentially affects Th1 and Th17 development. J Leukoc Biol. 2013;94:271–9. doi: 10.1189/jlb.1112581. [DOI] [PubMed] [Google Scholar]
- 41.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–5. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 42.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A. 2010;107:12204–9. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang XP, Ghoreschi K, Steward-Tharp SM, Rodriguez-Canales J, Zhu J, Grainger JR, et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011;12:247–54. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D, et al. Stability of the regulatory T cell lineage in vivo. Science. 2010;329:1667–71. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–96. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8:1353–62. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 47.Jenabian MA, Seddiki N, Yatim A, Carriere M, Hulin A, Younas M, et al. Regulatory T cells negatively affect IL-2 production of effector T cells through CD39/adenosine pathway in HIV infection. PLoS Pathog. 2013;9:e1003319. doi: 10.1371/journal.ppat.1003319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: Regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178:6725–9. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 49.Kitani A, Xu L. Regulatory T cells and the induction of IL-17. Mucosal Immunol. 2008;1(Suppl 1):S43–6. doi: 10.1038/mi.2008.51. [DOI] [PubMed] [Google Scholar]
- 50.Feng T, Cao AT, Weaver CT, Elson CO, Cong Y. Interleukin-12 converts Foxp3+ regulatory T cells to interferon-gamma-producing Foxp3+ T cells that inhibit colitis. Gastroenterology. 2011;140:2031–43. doi: 10.1053/j.gastro.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sujino T, Kanai T, Ono Y, Mikami Y, Hayashi A, Doi T, et al. Regulatory T cells suppress development of colitis, blocking differentiation of T-helper 17 into alternative T-helper 1 cells. Gastroenterology. 2011;141:1014–23. doi: 10.1053/j.gastro.2011.05.052. [DOI] [PubMed] [Google Scholar]
- 52.Martin-Orozco N, Chung Y, Chang SH, Wang YH, Dong C. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur J Immunol. 2009;39:216–24. doi: 10.1002/eji.200838475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ye J, Su X, Hsueh EC, Zhang Y, Koenig JM, Hoft DF, et al. Human tumor-infiltrating Th17 cells have the capacity to differentiate into IFN-gamma+ and FOXP3+ T cells with potent suppressive function. Eur J Immunol. 2011;41:936–51. doi: 10.1002/eji.201040682. [DOI] [PubMed] [Google Scholar]
- 54.Blatner NR, Mulcahy MF, Dennis KL, Scholtens D, Bentrem DJ, Phillips JD, et al. Expression of RORgammat marks a pathogenic regulatory T cell subset in human colon cancer. Sci Transl Med. 2012;4:164ra159. doi: 10.1126/scitranslmed.3004566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu H, Hu B, Xu D, Liew FY. CD4+CD25+ regulatory T cells cure murine colitis: The role of IL-10, TGF-beta, and CTLA4. J Immunol. 2003;171:5012–7. doi: 10.4049/jimmunol.171.10.5012. [DOI] [PubMed] [Google Scholar]
- 56.Wu S, Lim KC, Huang J, Saidi RF, Sears CL. Bacteroides fragilis enterotoxin cleaves the zonula adherens protein, E-cadherin. Proc Natl Acad Sci U S A. 1998;95:14979–84. doi: 10.1073/pnas.95.25.14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schiering C, Krausgruber T, Chomka A, Frohlich A, Adelmann K, Wohlfert EA, et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature. 2014;513:564–8. doi: 10.1038/nature13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang K, Kim MK, Di Caro G, Wong J, Shalapour S, Wan J, et al. Interleukin-17 receptor a signaling in transformed enterocytes promotes early colorectal tumorigenesis. Immunity. 2014;41:1052–63. doi: 10.1016/j.immuni.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harris TJ, Grosso JF, Yen HR, Xin H, Kortylewski M, Albesiano E, et al. Cutting edge: An in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoimmunity. J Immunol. 2007;179:4313–7. doi: 10.4049/jimmunol.179.7.4313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.