Abstract
Background
Colorectal cancer (CRC) screening is effective but underused. Guidelines for which tests are recommended and at what intervals depend on specific risks. We developed a tablet-based Cancer Risk Intake System (CRIS) that asks questions about risk prior to appointments and generates tailored printouts for patients and physicians summarizing and matching risk factors with guideline-based recommendations.
Methods
Randomized controlled trial among patients who: (1) used CRIS and they and their physicians received tailored printouts; (2) used CRIS to answer questions but received standard information about cancer screening while their physicians received a standard electronic chart prompt indicating they were age-eligible but not currently adherent for CRC screening; or (3) comprised a no-contact group that neither used CRIS nor received any information while their physicians received the standard prompt. Participation in testing was assessed via electronic medical record at 12 months.
Results
Participation in any CRC testing was three times higher for those who used the CRIS and received any printed materials, compared to no-contact controls (47% v. 16%; p < 0.0001). Among CRIS users ages 50 and older, participation in any testing was higher in the tailored group (53% v. 44%, p=0.023).
Conclusion
Use of CRIS and receipt of any information facilitated participation in testing. There was more testing participation in the CRIS-tailored than nontailored group.
Impact
Asking patients questions about their specific risk factors and giving them and their providers’ information just prior to an appointment may increase participation in CRC testing. Tailoring the information has some added benefit.
Keywords: Colorectal cancer, Cancer screening, Tailored intervention, Randomized controlled trial
INTRODUCTION
Colorectal cancer (CRC) screening has been found to reduce incidence, morbidity, and mortality(1;2), but is still underused(3–5). Several testing modalities are available; which ones are recommended and at what intervals depend on a person’s specific risks. For example, guideline-based recommendations for average-risk individuals include options of stool-based testing repeated yearly or colonoscopy every 10 years (if results are normal). In contrast, some individuals with elevated risk should only choose colonoscopy and may need to undergo the procedure at an earlier age or more often than their average-risk counterparts(6;7). However, delivery of risk-tailored recommendations is not easy. Identifying risk level and therefore appropriate test and schedule requires knowledge of multiple personal and familial factors (e.g., prior polyps or cancer, age at finding or diagnosis, and number and closeness of affected relatives(8) who are not routinely assessed and summarized in patients’ medical records(9).
To facilitate risk-tailored testing in the context of primary care, we developed a tablet-based Cancer Risk Intake System (CRIS) through which patients answer detailed questions about their CRC risk prior to an appointment. Tailoring algorithms in CRIS generate printed information for patients and their physicians summarizing their risk factors and matching them with guideline-based recommendations. CRIS also assesses patients’ concerns about CRC testing so that they can be summarized in the physician’s version of the tailored printout and briefly addressed in the patient’s version.
CRIS was based on the Information-Motivation-Behavioral Skills model, which posits three fundamental determinants of risk avoidance behavior: (1) risk reduction information, (2) motivation, and (3) behavioral skills.(10) To deliver risk and risk reduction information and enhance personal motivation, the printout was tailored to address personalized risks and perceived barriers to engaging in the recommended testing behavior. To increase social motivation, the intervention was designed to facilitate and supplement recommendation from the participant’s physician in the context of the office visit. CRIS was also designed to be used and the information delivered just prior to an office visit to facilitate behavioral skills for engaging in testing (i.e., receipt of a stool test kit or referral for colonoscopy).
We conducted a three-group randomized controlled trial among patients in a university-based primary care practice to test hypotheses regarding the two major aspects of CRIS — its provision of risk-tailored testing information to patients and their physicians and its extensive assessment of risk factors.
MATERIALS AND METHODS
Setting
Enrollment occurred in one Family Medicine and two General Internal Medicine practices affiliated with the University of Texas Southwestern (UTSW) Medical Center in Dallas. Nearly all patients had Medicare or commercial insurance that covered all or most of the costs of CRC testing. The UTSW Health System has a major colonoscopy program with timely access to this as a screening choice in addition to stool-based testing. The study was approved by the UTSW Institutional Review Board and registered on clinicaltrials.gov.
Eligibility criteria
A UTSW electronic medical records (EMR) system administrator developed a query to identify potentially eligible patients with upcoming appointments. We identified patients who, according to their records, were eligible for but not currently adherent to routine screening guidelines (i.e., no record of a stool test within the previous year, sigmoidoscopy within 5 years, or colonoscopy within the previous 10 years). We selected 75 years as the upper age limit, as testing is considered reasonable for people whose age and comorbid conditions do not limit life expectancy.(11) For the lower age of patients using CRIS, we selected 25 — the age by which the National Comprehensive Cancer Network then recommended beginning colonoscopy among carriers of mutations for the most common hereditary cancer (Lynch) syndrome.(12) (Newer NCCN guidelines now extend that age down to 20 years.(13)) For the tailored and nontailored CRIS groups, we invited patients ages 25–49 who were potentially eligible for colon cancer testing due to family history of CRC or a personal history of inflammatory bowel disease or adenomatous polyp based on patient self-report; whether they were actually in need of testing was determined through CRIS’ more extensive risk questions. If patients these ages used CRIS but no testing was recommended, they were thanked and received a gift card but were not included in the final sample for analyses. We excluded those previously diagnosed with colorectal cancer and, due to the nature of data collection and intervention, those who had no telephone access, were unable to speak or read English, or had severely impaired hearing or speech. For the no-contact control group, we did not select patients younger than 50 because there was no opportunity to ask questions to determine whether their risks warranted earlier-age CRC testing.
Study design: The study included three groups
Patients in group 1 used the CRIS and they and their physicians received tailored printouts summarizing their risk factors and recommendations based on these factors. Those in group 2 used CRIS to answer questions but received standard information about multiple types of cancer screening (e.g., content from an American Cancer Society cancer screening brochure), while their physicians received standard electronic chart prompts for those 50 and over indicating they were age-eligible but not currently adherent. Group 3 was a true no-contact group that neither used CRIS nor received any information and whose physicians received the standard electronic prompts during their visits. Because we did not collect risk data from the no-contact controls, we were not able to determine whether the tests they received were guideline-concurrent for their personal risk levels.
Analyses reported here were guided by our hypotheses that, within 12 months of the baseline visit:
The CRIS tailored recipients would have higher rates of risk-appropriate and any type of testing, compared to non-tailored recipients (group 1 v. 2), and
Those who used the CRIS and received any type of information (groups 1 & 2) would have higher participation in any type of testing, compared to controls (group 3).
CRIS’ assessment of risk factors identified a small number of people in need of testing younger than the age of 50. Because it is possible that these subgroups — those with risks warranting testing at an earlier age as opposed to those in the normal screening age group — might react differently to tailored information, we assessed participation in testing separately for participants aged 50 and older and those younger than 50.
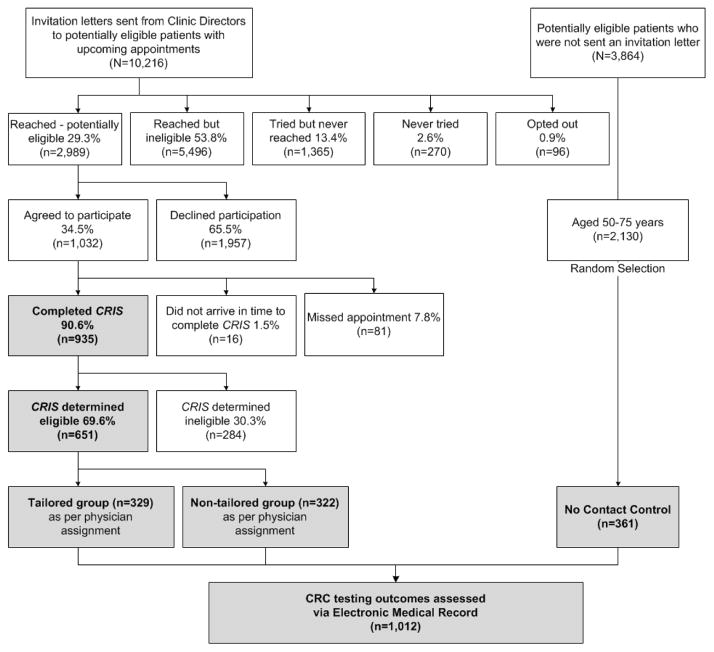
Figure 1 shows details regarding patient identification, recruitment, and participation rates. Patients in the CRIS tailored and nontailored groups were contacted and consented to be in the study. They accepted invitations to arrive 30 minutes prior to their appointments to use CRIS to enter their risk factor data and receive information — either tailored or nontailored, depending on group assignment — about CRC testing. Patients in the no-contact control group had no interaction with the study team and received only usual care when they presented for appointments. They did not use CRIS to input data about their personal risk factors nor did they receive any printed information. Consistent with usual care at UTSW, Epic records for those 50 and older displayed physician prompts indicating they were age-eligible but nonadherent for CRC screening. Therefore, their physicians received the same routine prompts as those in the nontailored CRIS group.
Figure 1.
CRIS Study CONSORT diagram
Because the decision whether to order CRC testing and which test to select is ultimately controlled by the primary care physician, to decrease the risks of contamination, we made physician the unit of randomization. This meant that patients were pre-identified as potential CRIS-tailored intervention or nontailored group participants before they were invited, depending on the group to which their physician was assigned. There were a total of 14,080 patients with upcoming appointments with no evidence of screening adherence in their medical records. Of these, 3,864 were reserved for drawing the no-contact control sample and 10,216 received letters from the clinics signed by the medical director saying they might be invited for participation in a “study of beliefs and practices about cancer prevention and early detection” (but not specifically about CRC). Letters provided a toll-free number to opt out of contact (n = 96).(14) One week after the mailing, research assistants began calling patients who received letters to explain the study, verify eligibility and, if the patient agreed, arrange a meeting at the clinic 30 minutes prior to the appointment.
As shown in Figure 1, we reached and invited 2,989 patients who were potentially eligible. Of these, 1,032 agreed to participate and 935 arrived in time to use the CRIS program. Research assistants met patients, obtained consent, and facilitated use of the touch-screen tablet through which CRIS collected risk data and either informed they were not in need of screening (n = 284) or generated printouts recommending risk-based screening (n = 651). The RA handed participants tailored (n=329) or nontailored (n = 322) printouts. Twenty-one patients had complicated medical histories for which programming in the CRIS algorithms could not recommend a specific testing regimen; therefore, the final analytical sample for risk-appropriate screening was n = 630.
Of the 2,130 patients in the no-contact group who were ages 50–75, we randomly selected 363. We defined baseline visit for the no-contact control group as the first appointment date when they became eligible for study participation. Two were excluded (one did not have an electronic medical record and one patient was a member of the study team). Final analytical sample for the no-contact group was 361 patients.
Interventions and measures
The CRIS touch-screen program collected: demographics, personal medical history including previous testing, detailed family history, and concerns about testing. Average time to complete CRIS was ≤ 10 minutes. CRIS then used these data to determine risk-appropriate testing option(s) and generate patient and physician printouts tailored to this information (or a nontailored printout for the comparison group and standard electronic chart prompts for their physicians). Algorithms linking responses to risk questions with guideline-based recommendations were developed in a structured process by an expert panel including representatives from primary care, gastroenterology, genetic counseling, and health communications and included extensive pre-testing. We created iterative questions measuring risk factors such as family history of CRC and inflammatory bowel disease. For example, if the patient had inflammatory bowel disease, follow-up questions assessed disease duration (because guidelines recommend special testing for an 8- to 10-year history) and, if she had family history, there were questions about degree of relation and age at diagnosis. For each risk category, we created recommendation messages based on national guidelines.
The one-page CRIS-tailored printouts included a summary of patients’ personal risks, appropriate test(s) based on risk factors, and concerns about testing. The message for those at average risk was “you may choose to have a stool blood test, a colonoscopy, or another option your doctor recommends,” whereas those whose risks warranted colonoscopy did not include the stool test option. The physician also received a version of the CRIS printout that summarized the patient’s individual risk factors, the guideline-recommended testing option(s), and any concerns or barriers to screening that were reported. Patients in the CRIS nontailored group received nontailored content from an American Cancer Society brochure about cancer screening in general. Physicians of patients in the nontailored and no-contact control group received our system’s routine electronic chart prompts indicating patient age of 50 and over and no recent CRC testing documented in the record.
Outcome assessment
All patients from the three groups (n = 1,012) had their EMRs audited 12 months postbaseline to determine whether they had received risk-appropriate CRC testing and any type of CRC testing (Hypothesis 1). We first performed analyses including all subjects in the tailored and nontailored groups, including those who were under the age of 50. Then, we ran a second set of analyses excluding the 57 patients under 50 in the tailored and nontailored groups, such that the age ranges in the three groups would be comparable. For these analyses, we created two yes/no variables. For risk-appropriate testing, the binary outcome values were: (1) participation in any risk-appropriate CRIS-recommended test, or (2) either no testing or participation in a nonrecommended test (e.g., stool-based test for an individual whose risk profile indicated need for colonoscopy). For “any testing,” values for the binary outcome were: (1) participation in any testing, or (2) no participation in testing within 12 months.
Statistical analysis
For Hypothesis 1 (participation between patients in the tailored v. nontailored groups who used the CRIS), mixed-effects logistic regression models were fitted with group assignment as the main independent variable. Clinic physician was included as a random cluster effect. Multivariate analyses were conducted to identify significant factors associated with risk-appropriate screening and any screening using backward selection mixed-effect logistic regression. All the demographic variables and group assignment were included as covariates in multivariate analyses using physician as the random cluster effect.
For Hypothesis 2, comparing receipt of any CRC test between all patients who used the CRIS and received either a tailored or nontailored printout (groups 1 and 2, n = 651) v. the no-contact group (n = 361), we fitted a mixed-effects logistic regression model, with group assignment as the main independent variable and physician as the random cluster effect. Age and gender were also included as covariates. P-values ≤ 0.05 were considered to be statistically significant. Analyses were performed using SAS software, Version 9.2 of the SAS System for Windows (SAS Institute Inc., Cary, NC, USA, 2008). PROC GLIMMIX in SAS was used to fit the mixed-effects logistic regression models.
RESULTS
Patient characteristics
Sample characteristics are shown in Table 1 (for all ages) and Table 2 (≥50). Overall, participants were 62.9% female, with no significant difference in gender among the three groups (p = 0.08). There was significant difference in mean age among the three groups (p = 0.0002) adjusting for multiple comparisons by Bonferroni correction (α=0.017=0.05/3), with the tailored group being slightly younger than patients in the no-contact control group (p < 0.0001, 57.5 v. 60.3, respectively). The proportions of patients under the age of 50 in the tailored and nontailored groups were small and did not differ significantly (10.6% and 6.8% respectively; p=0.086). Although race/ethnicity and education level were similar in the tailored and nontailored groups, with the majority of participants being Non-Hispanic White and having four or more years of college, employment was significantly higher among tailored than nontailored recipients (72.6% v. 59.3%; p=0.0003). (Because the control group provided no patient-reported data, we do not have information about their race/ethnicity, educational attainment, or employment.)
Table 1.
Patient Characteristics (all ages)
| Tailored N=329 n (%) |
Non-Tailored N=322 n (%) |
No Contact N=361 n (%) |
Overall N=1,012 n (%) |
p-valuea | |
|---|---|---|---|---|---|
| Age, years, Mean (STD)b | 57.5 (±8.6) | 59.1 (±7.9) | 60.3 (±6.5) | 59.0 (±7.7) | 0.04 |
| Age, under <50 | 35 (10.6) | 22 (6.8) | - | 57 (8.8) | 0.086 |
| Sex, Female | 205 (62.3) | 216 (67.1) | 216 (59.8) | 637 (62.9) | 0.08 |
| Race/Ethnicity | |||||
| NH White | 205 (62.3) | 218 (67.7) | -- | 423 (65.0) | 0.53 |
| NH Black | 76 (23.1) | 63 (19.6) | -- | 139 (21.4) | |
| Hispanic | 31 (9.4) | 26 (8.1) | -- | 57 (8.8) | |
| Asian/NA/Hawaiian/PI | 13 (4.0) | 10 (3.1) | -- | 23 (3.5) | |
| Other/Refused | 4 (1.2) | 5 (1.6) | -- | 9 (1.4) | |
| Education | |||||
| < 4 years of college | 163 (49.5) | 151 (46.9) | -- | 314 (48.2) | 0.45 |
| ≥ 4 years of college | 165 (50.2) | 171 (53.1) | -- | 336 (51.6) | |
| Refused | 1 (0.3) | 0 (0.0) | -- | 1 (0.2) | |
| Employment | |||||
| Full- or Part-time | 239 (72.6) | 191 (59.3) | -- | 430 (66.1) | 0.00 |
| Unemployed | 89 (27.1) | 131 (40.7) | -- | 220 (33.8) | |
| Refused | 1 (0.3) | 0 (0.0) | -- | 1 (0.2) | |
STD = Standard deviation; NH = Non-Hispanic; NA = Native American; PI = Pacific Islander.
p-value based on the comparison of Tailored and Non-Tailored groups with a generalized linear mixed effects model.
Measured at first eligible appointment date.
Table 2.
Patient Characteristics (age ≥50)
| Tailored N=294 n (%) |
Non-Tailored N=300 n (%) |
No Contact N=361 n (%) |
Overall N=955 n (%) |
p-valuea | |
|---|---|---|---|---|---|
| Age, years, Mean (STD)b | 59.2 (±7.2) | 60.2 (±6.9) | 60.3 (±6.5) | 59.9 (±6.9) | 0.102 |
| Sex, Female | 180 (61.2) | 197 (65.7) | 216 (59.8) | 593 (62.1) | 0.697 |
| Race/Ethnicity | |||||
| NH White | 181 (61.6) | 205 (68.3) | -- | 386 (65.0) | 0.319 |
| NH Black | 67 (22.8) | 56 (18.7) | -- | 123 (20.7) | |
| Hispanic | 30 (10.2) | 26 (8.7) | -- | 56 (9.4) | |
| Asian/NA/Hawaiian/PI | 12 (4.1) | 8 (2.7) | -- | 20 (3.4) | |
| Other/Refused | 4 (1.4) | 5 (1.7) | -- | 9 (1.5) | |
| Education | |||||
| < 4 years of college | 151 (51.4) | 142 (47.3) | -- | 293 (49.3) | 0.290 |
| ≥ 4 years of college | 142 (48.3) | 158 (52.7) | -- | 300 (50.5) | |
| Refused | 1 (0.3) | 0 (0.0) | -- | 1 (0.2) | |
| Employment | |||||
| Full- or Part-time | 220 (71.4) | 177 (59.0) | -- | 387 (65.2) | 0.001 |
| Unemployed | 83 (28.2) | 123 (41.0) | -- | 206 (34.7) | |
| Refused | 1 (0.3) | 0 (0.0) | -- | 1 (0.2) | |
STD = Standard deviation; NH = Non-Hispanic; NA = Native American; PI = Pacific Islander.
p-value based on the comparison of Tailored and Non-Tailored groups adjusting for cluster effect (clinic provider) with a generalized linear mixed effects model.
Measured at first eligible appointment date.
Hypotheses 1, that there would be significantly higher participation in risk-appropriate or any type of CRC testing at 12 months post appointment in the CRIS-tailored v. the nontailored group, was partially upheld among participants ages 50 and older
As shown in Table 3, the full CRIS tailored and nontailored groups had similar participation in risk-appropriate testing (47.9% and 41.6%, respectively; p = 0.11) and in any type of testing (50% and 44%, respectively; p = 0.3). In the tailored and nontailored groups combined, only 3.3% of those screened had a test that differed from what the CRIS intervention recommended (e.g., they had a stool-based test when, according to guidelines, they should have had a colonoscopy). Among those at average risk for CRC who could have chosen either test option, 77.1% had a colonoscopy.
Table 3.
Mixed-effects logistic regression model analysis of CRIS group effect on colorectal cancer (CRC) testing within 12 months of baseline visit (all ages)
| 12-month CRC testing outcome | Tailored N=329 n (%) |
Non-Tailored N=322 n (%) |
p-valuea |
|---|---|---|---|
| 0.11 | |||
| Risk-appropriate testing | 150 (48) | 132 (42) | |
| No Risk-appropriate testing | 163 (52) | 185 (58) | |
| 0.13 | |||
| Any testing | 164 (50) | 141 (44) | |
| No testing | 165 (50) | 181 (56) |
p-value tested the group effect on the CRC testing while adjusting for cluster effect (clinic provider).
Twenty-one patients had complicated medical histories for which programming in the CRIS algorithms could not recommend a specific testing regimen; therefore the final analytical sample for risk-appropriate testing was n = 630.
However, as shown in Table 4, when analyses included only those ages 50 and older in the two CRIS groups, participation in any type of testing was significantly higher among the tailored than the nontailored group (53% v. 44%; p=.023). Although participation in risk-appropriate testing approached significance in univariate analysis (51% v. 43%; p=0.068), the p value was 0.11 when age entered the step-wise regression.
Table 4.
Mixed-effects logistic regression model analysis of CRIS group effect on colorectal cancer (CRC) testing within 12 months of baseline visit (age ≥50)
| 12-month CRC testing outcome | Tailored N=294 n (%) |
Non-Tailored N=300 n (%) |
p-valuea |
|---|---|---|---|
| 0.11 | |||
| Risk-appropriate testing ≥ 50 | 144 (51) | 128 (43) | |
| No Risk-appropriate testing ≥ 50 | 141 (49) | 170 (57) | |
| 0.023 | |||
| Any testing ≥ 50 | 156 (53) | 131 (44) | |
| No testing ≥ 50 | 138 (47) | 169 (56) |
p-value tested the group effect on the CRC testing while adjusting for cluster effect (clinic provider).
Eleven patients had complicated medical histories for which programming in the CRIS algorithms could not recommend a specific testing regimen; therefore the final analytical sample for risk-appropriate testing was n = 630.
Table 5 shows results for the very small group (n=57) of tailored and nontailored group members younger than 50, between which no differences in testing were found.
Table 5.
Mixed-effects logistic regression model analysis of CRIS group effect on colorectal cancer (CRC) testing within 12 months of baseline visit (age <50)
| 12-month CRC screening outcome | Tailored N=35 n (%) |
Non-Tailored N=22 n (%) |
p-value* |
|---|---|---|---|
| 0.852 | |||
| Risk-appropriate Screeningb<50 | 6 (21) | 4 (21) | |
| No Risk-appropriate Screening<50 | 22 (79) | 15 (79) | |
| 0.146 | |||
| Any Screening<50 | 8 (23) | 10 (45) | |
| No Screening<50 | 27 (77) | 12 (55) |
p-value tested the group effect on the CRC testing while adjusting for cluster effect (clinic provider).
Ten patients had complicated medical histories for which programming in the CRIS algorithms could not recommend a specific testing regimen; therefore the final analytical sample for risk-appropriate screening was n = 47.
Hypotheses 2, that there would be significantly higher participation in any testing among patients who used CRIS and received any printout (e.g., tailored and nontailored groups) v. those in the no-contact control group, was confirmed
Participation in any type of testing by 12 months post visit was only 16% in the no-contact control group. There were significant differences in the proportion of having any type of testing between CRIS users and no-contact controls in all patients (p<0.0001) and patients with ages 50–75 (p<0.0001).
DISCUSSION
CRIS is not the first tailored intervention to promote colon cancer testing within the context of primary care(15–22). Most of these previous CRC screening-promotion interventions have assessed multiple behavioral determinants and delivered tailored messages developed to enhance perceived benefits and reduce barriers to undergoing any of the US Preventive Task Force-recommended screening options. None of which we are aware have assessed risks thoroughly enough to determine whether resulting test participation is risk-appropriate. The unique focus of CRIS is its extensive assessment of risk factors and complex algorithms tailoring risk information to guidelines for specific test modality and schedule rather than a “one size fits all” approach that recommends the same test for everyone regardless of risk level. The strategy is to encourage either stool-based or endoscopic screening for individuals at average risk, but only colonoscopy for those with elevated risks — perhaps starting earlier or repeating more often, depending on specific risk factors.
Our unique study design allowed us to compare three groups of patients: those who: (1) used the CRIS to answer questions and they and their physicians received tailored information before their visit, (2) used CRIS and received nontailored information while their physicians received standard electronic prompts, and (3) did not use CRIS or receive any information and whose physicians received the standard prompts. We were therefore able to compare the effect of tailored v. nontailored information as well as using the CRIS and receiving any information v. no contact or information for patients.
Findings showed a benefit in using CRIS and receiving any information. Patients in the tailored and nontailored groups were roughly three times more likely to undergo any type of testing than were members of the control group. There may be several reasons why there was such a large difference between those who actively participated in the study and used CRIS v. those who were not contacted. Researchers have previously suggested that recruitment into screening studies and data collection may interact with an intervention, such that the intervention effect, assessed at follow-up, may be influenced by or even depend upon the presence of a pretest.(23)
Participating in the study may have produced a Hawthorne effect(24) in which patients who received information about screening and gave permission for their records to be evaluated by study staff behaved differently because they knew they would be observed. Another potential explanation for differences between the CRIS groups and the no-contact group is that answering multiple questions about risk factors and previous testing history may have acted as an intervention or had a priming effect prior to the visit. Indeed, previous studies have shown that mere measurement may promote health behaviors(25), and a number of randomized intervention trials have found changes in cancer screening outcomes among individuals in comparison groups who completed data collection but did not receive tailored interventions.(16;26;27)
Patients’ use of the CRIS right before the office visit and getting any printed information about screening may have prompted them to think more about screening before their visits and this, rather than whether their information was tailored, may explain why active study participants who used the CRIS were so much more likely to receive testing than were the no-contact controls. CRIS users may have also been more likely to talk with their providers about screening or be more open to physician recommendations. In our evaluation of an earlier version of CRIS that addressed multiple risks, screening, and prevention behaviors, CRIS users reported more screening-related discussions with their providers(28), compared to a usual-care group. Whatever the explanation, these findings highlight the importance of including a true control group in experimental designs.
The difference we found between participation in testing among those 50 and older in the tailored v. nontailored groups was real but much smaller. These findings suggest that CRIS’ extensive assessment of risk factors was more important than its provision of risk-tailored information to patients and physicians.
Strengths and limitations
Findings of the study highlight the importance of including a true control group, which minimizes threats to validity from pretest sensitization and Hawthorne effects(29). Whereas the expense of data collection often precludes use of such a design, our use of electronic medical records for drawing the sample and assessing the outcomes enabled us to employ it. However, although the no-contact control group is an important and strong feature of the research design, it was not perfect. For the no-contact group, we could not select and include patients younger than 50 because there was no opportunity to ask questions to determine whether their risks warranted earlier-age CRC testing. Therefore, findings from this group can only be generalized to those ages 50 and over.
Limitations of the study also include the clinical setting in which it was conducted. All patients were insured, with ready access to colonoscopy and physicians inclined to recommend the procedure. Because the vast majority of those screened had colonoscopies, which are guideline-concordant with any risk level, there was little opportunity to find differences in receipt of nonrecommended tests. From these findings, we cannot determine whether, in lower-resource settings or those with a “FIT-first” strategy, receipt of tailored recommendations would have resulted in different rates of any screening and guideline-concordant testings, driven by stool testing.
Another feature of the clinical setting is that the prevalence of screening was already relatively high and growing (from 62% in 2012 to 81% in 2014 in the General Internal Medicine practice). Patients eligible for the trial due to nonadherence might have over-represented the most recalcitrant who have previously refused or failed to complete CRC testing. From this study, we cannot determine whether rates would have been triple among those who used the CRIS or the tailored/nontailored difference in testing would have been similarly in a more “screening-naive” setting.
Another limitation is that risk factor assessment and generation of tailored screening recommendations occurred in “real time” just prior to a primary care visit. Had the CRIS assessment been completed days or weeks prior to the appointment, the difference between groups who did v. did not use CRIS might not have been as strong, or the difference between those receiving tailored v. nontailored information may have been stronger.
Among participants ages 50 to 75, participation in any testing was significantly higher among the tailored group and difference in risk-appropriate testing approached significance. We looked separately at this age group for two reasons. First, they were mostly different in size. Also, because one’s risk level had to be higher-than-average to qualify for receipt of a testing recommendation, we thought the under-50 individuals might differ meaningfully from their counterparts in the routine-screening age group. Our study was limited by the fact that we had enough power to identify significant differences in the over-50, but not the younger-than-50 sample.
Conclusions
In our study setting, asking patients specifics about their family and personal risk factors and giving them and their providers any information about testing just prior to an appointment increased their likelihood of participation in CRC testing after the visit. Among those 50 and over, risk tailored CRC testing information given to patients and their physicians seems to have an additional benefit.
Acknowledgments
Financial Support: All authors were supported by the National Institutes of Health R01 CA122330.
Footnotes
Trial registration ID: NCT02368236
Dr. Gupta has received consulting fees from Exact Sciences. No conflicts of interest have been declared by any other authors.
References
- 1.Levin B, Lieberman DA, McFarland B, Andrews KS, Brooks D, Bond J, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–95. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 2.U S. Preventive Services Task Force. Screening for Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2008;149:627–38. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 3.Smith RA, Manassaram-Baptiste D, Brooks D, Cokkinides V, Doroshenk M, Saslow D, et al. Cancer screening in the United States, 2014: a review of current American Cancer Society guidelines and current issues in cancer screening. CA: A Cancer Journal for Clinicians. 2014;64:30–51. doi: 10.3322/caac.21212. [DOI] [PubMed] [Google Scholar]
- 4.Holden DJ, Jonas DE, Porterfield DS, Reuland D, Harris R. Systematic review: enhancing the use and quality of colorectal cancer screening. Ann Intern Med. 2010;152:668–76. doi: 10.7326/0003-4819-152-10-201005180-00239. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Use of colorectal cancer tests - United States, 2002, 2004, and 2006. MMWR Morb Mortal Wkly Rep. 2008;57:253–8. [PubMed] [Google Scholar]
- 6.Dove-Edwin I, Sasieni P, Adams J, Thomas HJ. Prevention of colorectal cancer by colonoscopic surveillance in individuals with a family history of colorectal cancer: 16 year, prospective, follow-up study. BMJ. 2005;331:1047. doi: 10.1136/bmj.38606.794560.EB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Acheson LS. Recording, interpreting, and updating the family history of cancer: implications for cancer prevention. JAMA. 2011;306:208–10. doi: 10.1001/jama.2011.980. [DOI] [PubMed] [Google Scholar]
- 8.Winawer S, Fletcher R, Rex D, Bond J, Burt R, Ferrucci J, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale-Update based on new evidence. Gastroenterology. 2003;124:544–60. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- 9.Welch BM, Dere W, Schiffman JD. Family health history: The case for better tools. JAMA. 2015;313:1711–2. doi: 10.1001/jama.2015.2417. [DOI] [PubMed] [Google Scholar]
- 10.Fisher WA, Fisher JD, Harman J. The information-motivation-behavioral skills model: A general social psychological approach to understanding and promoting health behavior. In: Suls J, Wallston K, editors. Social Psychological Foundations of Health and Illness. Massuchessetts: Blackwell; 2003. pp. 82–106. [Google Scholar]
- 11.U S. Preventive Services Task Force. Screening for colorectal cancer: recommendation and rationale. Ann Intern Med. 2002;137:129–31. doi: 10.7326/0003-4819-137-2-200207160-00014. [DOI] [PubMed] [Google Scholar]
- 12.Lynch HT, Shaw TG, Lynch JF. Inherited predisposition to cancer: a historical overview. Am J Med Genet C Semin Med Genet. 2004;129C:5–22. doi: 10.1002/ajmg.c.30026. [DOI] [PubMed] [Google Scholar]
- 13.National Comprehensive Cancer Network. NCCN Guidelines Version 2.2012 Lynch Syndrome. 2012 [Google Scholar]
- 14.Junghans C, Feder G, Hemingway H, Timmis A, Jones M. Recruiting patients to medical research: double blind randomised trial of “opt-in” versus “opt-out” strategies. BMJ. 2005;331:940. doi: 10.1136/bmj.38583.625613.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rawl SM, Skinner CS, Perkins SM, Springston J, Wang HL, Russell KM, et al. Computer-delivered tailored intervention improves colon cancer screening knowledge and health beliefs of African-Americans. Health Educ Res. 2012;27:868–85. doi: 10.1093/her/cys094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myers RE, Sifri R, Hyslop T, Rosenthal M, Vernon SW, Cocroft J, et al. A randomized controlled trial of the impact of targeted and tailored interventions on colorectal cancer screening. Cancer. 2007;110:2083–91. doi: 10.1002/cncr.23022. [DOI] [PubMed] [Google Scholar]
- 17.Costanza ME, Luckmann R, Stoddard AM, White MJ, Stark JR, Avrunin JS, et al. Using tailored telephone counseling to accelerate the adoption of colorectal cancer screening. Cancer Detect Prev. 2007;31:191–8. doi: 10.1016/j.cdp.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Menon U, Belue R, Wahab S, Rugen K, Kinney AY, Maramaldi P, et al. A randomized trial comparing the effect of two phone-based interventions on colorectal cancer screening adherence. Ann Behav Med. 2011;42:294–303. doi: 10.1007/s12160-011-9291-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christy SM, Perkins SM, Tong Y, Krier C, Champion VL, Skinner CS, et al. Promoting colorectal cancer screening discussion: a randomized controlled trial. Am J Prev Med. 2013;44:325–9. doi: 10.1016/j.amepre.2012.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myers RE, Sifri R, Daskalakis C, DiCarlo M, Geethakumari PR, Cocroft J, et al. Increasing colon cancer screening in primary care among african americans. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greiner KA, Daley CM, Epp A, James A, Yeh HW, Geana M, et al. Implementation intentions and colorectal screening: a randomized trial in safety-net clinics. Am J Prev Med. 2014;47:703–14. doi: 10.1016/j.amepre.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lairson DR, DiCarlo M, Deshmuk AA, Fagan HB, Sifri R, Katurakes N, et al. Cost-effectiveness of a standard intervention versus a navigated intervention on colorectal cancer screening use in primary care. Cancer. 2014;120:1042–9. doi: 10.1002/cncr.28535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glenn BA, Bastani R, Maxwell AE. The perils of ignoring design effects in experimental studies: lessons from a mammography screening trial. Psychol Health. 2013;28:593–602. doi: 10.1080/08870446.2012.756880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.James L, Vo H. Hawthorne Effect. In: Salkind NJ, editor. Encyclopedia of Research Design. Thousand Oaks, CA: SAGE Publications, Inc; 2010. pp. 562–4. [Google Scholar]
- 25.Godin G, Sheeran P, Conner M, Germain M. Asking questions changes behavior: mere measurement effects on frequency of blood donation. Health Psychol. 2008;27:179–84. doi: 10.1037/0278-6133.27.2.179. [DOI] [PubMed] [Google Scholar]
- 26.Champion VL, Rawl SM, Bourff SA, Champion KM, Smith LG, Buchanan AH, et al. Randomized trial of DVD, telephone, and usual care for increasing mammography adherence. J Health Psychol. 2014 Jul 28; doi: 10.1177/1359105314542817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vernon SW, Bartholomew LK, McQueen A, Bettencourt JL, Greisinger A, Coan SP, et al. A randomized controlled trial of a tailored interactive computer-delivered intervention to promote colorectal cancer screening: Sometimes more is just the same. Ann Behav Med. 2011;41:284–99. doi: 10.1007/s12160-010-9258-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skinner CS, Rawl SM, Moser BK, Buchanan AH, Scott LL, Champion VL, et al. Impact of the Cancer Risk Intake System on patient-clinician discussions of tamoxifen, genetic counseling, and colonoscopy. J Gen Intern Med. 2005;20:360–5. doi: 10.1111/j.1525-1497.2005.40115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cunningham CJL, Weathington BL, Pittenger DJ. Correlated-Groups Designs. In: Cunningham CJL, Weathington BL, Pittenger DJ, editors. Understanding and Conducting Research in the Health Sciences. 1. Hoboken, NJ: John Wiley & Sons, Inc; 2013. pp. 367–89. [Google Scholar]