Abstract
Background
Histologic subtypes of classical Hodgkin lymphoma (cHL) (e.g., nodular sclerosis (NS), mixed cellularity (MC), not otherwise specified (NOS)) are epidemiologically and prognostically distinctive. Therefore, unexplained, ongoing incidence rate declines for MC and increases for NOS require examination.
Methods
We analyzed detailed histology-specific HL incidence rates in 1992–2011 U.S. SEER data (n=21,372) and reviewed a regional subset of 2007–11 NOS pathology reports for insight into diagnostic practices.
Results
cHL rates were stable until 2007, then decreased for whites (annual percent change (APC) and 95% confidence interval, −3.6% (−5.6%, −1.5%)). NS rates declined after 2007 by 5.9% annually, with variation by gender, age, and race/ethnicity. In 1992–2011, MC rates declined (APC −4.0% (−4.7%, −3.3%)) whereas NOS rates rose (5.3% (4.5%, 6.2%)) overall and in most patient groups. 2007–11 NOS age-specific rates were more similar to MC rates for 1992–96 than 2007–11. Trends in combined rates were minimal, supporting increasing misclassification of MC, LD, and specific NS subtypes as NOS. Eighty-eight of 165 reviewed NOS pathology reports addressed classification choice. Twenty (12.1%) justified the classification, 21 (12.7%) described insufficient biopsy material, and coders missed specific subtype information for 27 (16.4%).
Conclusion
Recent NS rate declines largely represent true incidence changes. Long-term rate decreases for MC and other less-common subtypes, and increases for NOS (comprising ~30% of cHL cases in 2011), likely reflect changes in diagnostic and/or classification practice.
Impact
Diminishing histologic subtyping undermines future surveillance and epidemiologic study of HL. Guideline-based use of excisional biopsies and more coding quality control are warranted.
Keywords: Hodgkin lymphoma, histologic subtype, incidence, SEER, pathology
INTRODUCTION
Hodgkin lymphoma (HL) is a B-cell malignancy with complex, variable pathology (1) that has been classified by several histology schemes over time (2, 3). The 1966 “Rye” modification of the Lukes-Butler classification, which described reproducible, clinically correlated subtypes (nodular sclerosis (NS), mixed cellularity (MC), lymphocyte depletion (LD), lymphocyte predominance (LP)), was used for nearly 30 years (1, 2). In 1994, the Revised European-American Lymphoma (REAL) classification differentiated the etiologically distinct nodular LP (nLP) from classical HL (cHL) (4), which comprised NS, MC, LD and the new LP category lymphocyte-rich (LR). In 2001, this schema was accepted in the World Health Organization (WHO) classification of hematopoietic and lymphoid tumors (3). Its categories are captured by International Classification of Diseases for Oncology (ICD-O) codes, with which tumor registrars code HL histologic subtypes based on pathology reports (5).
In addition to having biological and prognostic differences (2, 3, 6–10), HL histologic subtypes show considerable epidemiologic variation (1, 11–18). For NS, the most common cHL subtype, incidence rates have been relatively stable over time (12, 19, 20), whereas studies have documented persistently declining rates for MC (12, 20, 21) and increasing rates of cHL not otherwise specified (12, 20), an ICD-O-3 category designating cHL without further histologic subtyping (hereafter called NOS). For MC, the second most common cHL subtype in the western world, decreasing rates could signal changes in the prevalence of risk factors (12, 21, 22), including lower socioeconomic status (23, 24), HIV infection (25), other immunosuppression (26, 27), and smoking (28); and in factors leading to the presence in some tumors of Epstein-Barr virus (EBV) (29, 30). For NOS, rate increases may reflect changes in diagnostic practice (e.g., use of smaller biopsies rendering precise diagnoses difficult) and/or in histologic classification (e.g., increasing reliance on cHL as the terminal category), leading to incorporation of cases that previously would have been coded as more specific histologic subtypes.
As histologic heterogeneity is central to HL etiology and the accurate monitoring of its occurrence, understanding the persistent yet unexplained incidence trends for MC and NOS is important. Therefore, we evaluated detailed HL incidence rates by histologic subtype over the past 20 years, using population-based U.S. National Cancer Institute (NCI) Surveillance, Epidemiology and End Results (SEER) cancer registry data to provide the large case series needed for informative study of this uncommon disease. Further, for insight into diagnostic and classification issues pertinent to NOS rate increases, we reviewed diagnostic pathology reports for a regional subset of NOS cases.
MATERIALS AND METHODS
We identified all new cases of primary cHL (ICD-O, 3rd Edition, morphology codes 9663–9667 (NS); 9652 (MC); 9651 (LR); 9653 (LD); 9650 (NOS)) and nLP (code 9659) diagnosed in the years 1992 through 2011 and included in the SEER 13 database (31). This database provides broad geographic coverage across 13 U.S. states and metropolitan areas, and data by detailed racial/ethnic groups (i.e., non-Hispanic white, Hispanic, non-Hispanic black, non-Hispanic Asian/Pacific Islander, and American Indian/Alaska Native, hereafter called white, Hispanic, black, API and AI/AN), which have differing HL epidemiologic profiles (11, 23, 24). For all 21,372 HL cases, we obtained registry data routinely abstracted or derived from the medical record on patient age, gender, race/ethnicity, tumor histology coded to ICD-O-3, tumor site, disease stage, and reporting SEER registry from the time of diagnosis.
To obtain information about recent diagnostic and classification practices for NOS, we used data from a quality-control review at the Greater Bay Area Cancer Registry (a participant in the SEER program) of all 286 incident HL cases reported to SEER as NOS for the diagnosis years 2007–11 (a period chosen because of the availability of pathology reports submitted to the registry electronically). Among these cases, the 165 (57.7%) with electronic pathology reports and reviewed by a registry quality-control specialist did not differ significantly from the 121 not reviewed, according to chi-square tests, by age (four groups, p=0.11), gender (p=0.21), race/ethnicity (p=0.22), tumor site (p=0.55), or disease stage (p=0.37), but did differ by year of diagnosis (p<0.001), due to increasing electronic reporting over time. From the pathology report text, the reviewer classified each NOS diagnosis by factors related to diagnostic practice. These included justification of the NOS classification, indication of specimen inadequacy for subtyping, subtype specification with insufficient definitiveness for coding per SEER requirements (32), subtype specification missed by coding registrars, biopsy type (excisional, core/fine needle aspiration (FNA)), biopsy site (lymph node, bone marrow), diagnosis facility (NCI-designated cancer center, integrated health system, other), location of final diagnosis (original diagnosing hospital, outside consultation), and existence of additional diagnostic studies.
Statistical Analysis
We computed average annual age-adjusted (to the 2000 US standard million population) HL incidence rates per 100,000 population and 95% confidence intervals (CI) for cHL overall, nLP, and cHL subtypes (as defined above), as well as for NS subtypes (the predominant category, NS NOS (code 9663), and the remaining categories NS cellular phase, grade 1, and grade 2 (codes 9664–9667). We calculated rates by 10-year age group to capture HL age heterogeneity, and for the age groups 0–14, 15–39, 40–54 and 55 years and above (called “children”, “adolescents/young adults (AYAs)”, “middle-aged adults” and “older adults”) to accommodate etiologic differences (33). We also calculated rates by gender, race/ethnicity (because of small numbers, we do not present AI/AN rates), stage (localized, regional, distant, unknown), tumor site (nodal, extranodal), year of diagnosis, and reporting SEER registry.
To evaluate time trends, we calculated annual incidence rates and conducted Joinpoint (segmented linear) regression analysis, which identifies specific significant changes in annual rates over time and calculates the annual percent change (APC) and associated 95% CI in rates within each trend segment (34). We also calculated incidence rates for four five-year time periods (1992–96, 1997–2001, 2002–06, 2007–11) for cHL, cHL subtypes and nLP (Supplemental Table 1), and for NS subtype groups (Supplemental Table 2). We compared five-year rates in order to a) summarize the overall magnitude of a change, b) quantify change when sample sizes prevented calculation of an APC, or c) compare rates for two specific five-year periods. For these purposes, we calculated incidence rate ratios (IRRs), considering as significant any differences for which the IRR 95% CI did not include 1; all IRRs presented reflect comparisons to the 1992–96 rate unless otherwise indicated.
To examine the possibility that the previously described opposing incidence trends for MC and NOS rates (12, 20) reflected increasing incorporation of true MC diagnoses into NOS over time, we 1) compared age-specific rates of MC and NOS in the earliest and latest five-year time periods (1992–96, 2007–11), using IRRs to evaluate whether differences in subtype rate patterns diminished, consistent with a case transfer; and 2) calculated rates, and used Joinpoint to examine trends, for MC and NOS combined, postulating that a lack of significant trends for this grouped category would support a case transfer. We repeated both analyses by also combining LD cases, since they may be considered grades of one subtype (2), and the NS subtype group 9664–9667, as pathology practices around their use have changed over time (35, 36).
We characterized NOS diagnostic practice using chi-square or Fisher’s exact tests to examine cases by registry patient and tumor characteristics and quality-control study factors, focusing on biopsy type because core/FNA biopsies have been associated with difficulties in diagnosing HL (37)).
We used SEER*Stat software (38) to calculate rates, Joinpoint Regression Program version 4.0.4 for Joinpoint analyses, and SAS version 9.3 for descriptive statistics. All analyses had the oversight of the institutional review board at the Cancer Prevention Institute of California.
RESULTS
Incidence trends for cHL and nLP
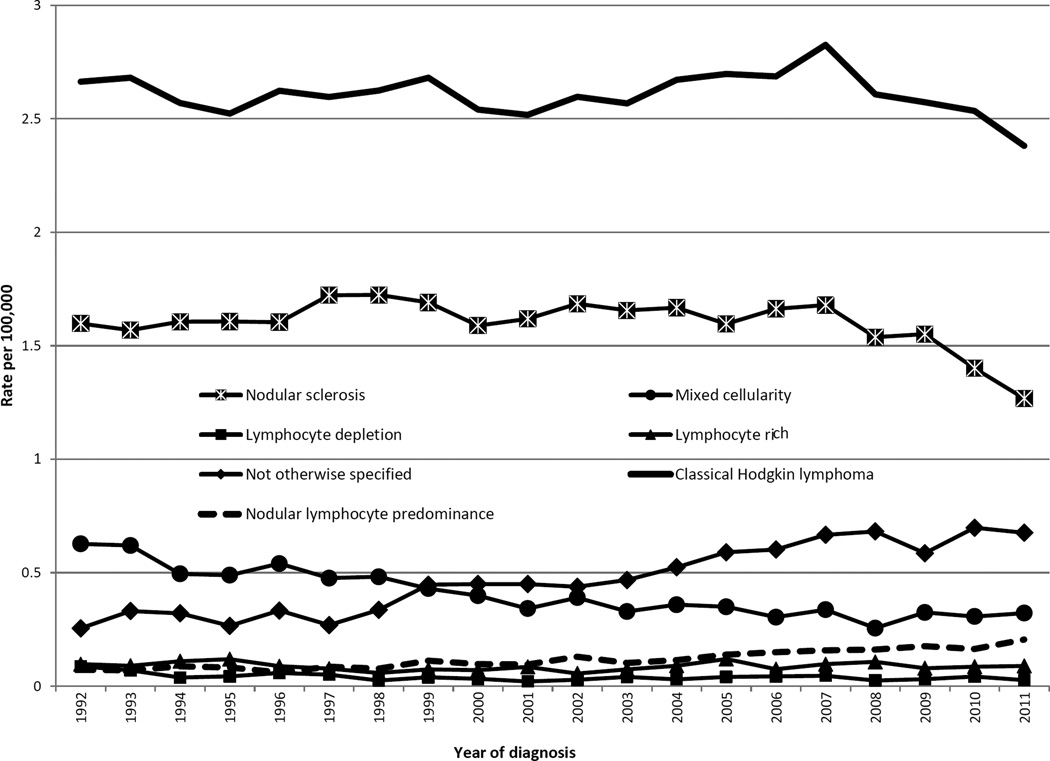
Overall age-adjusted incidence rates of cHL were stable until 2007, when they began to decline (Table 1, Figure 1). For whites, they decreased from 2007 at nearly 5% per year, whereas for APIs they rose throughout the study period, although with a plateau suggested in the 2000s (IRR, 2002–06: 1.39 (1.13, 1.70); 2007–2011: 1.36 (1.11, 1.66)). cHL rates decreased for ages 30–39 from 2008 at greater than 8% annually, but rose modestly for ages 80 and older and, starting in 2001, for ages 10–19. Rates decreased for localized disease, but increased modestly for regional and distant disease. For nodal disease, rates declined at 3.7% annually after 2007, whereas for extranodal disease, they rose throughout the study period.
Table 1.
Joinpoint average percent change (APC)* and 95% confidence intervals (CI) for Hodgkin lymphoma incidence rates, by histologic subtype and patient and tumor characteristics, 1992–2011, SEER (13 registries)
| Classical Hodgkin lymphoma | Nodular lymphocyte predominance | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Nodular sclerosis | Mixed cellularity | Lymphocyte depletion | Lymphocyte rich | Not otherwise specified | |||||||||
| N=20,437 | N=12,722 | N=3,069 | N=303 | N=673 | N=3670 | N=935 | ||||||||
| Interval (yrs) | APC (95% CI) | Interval (yrs) | APC (95% CI) | Interval (yrs) | APC (95% CI) | Interval (yrs) | APC (95% CI) | Interval (yrs) | APC (95% CI) | Interval (yrs) | APC (95% CI) | Interval (yrs) | APC (95% CI) | |
| TOTAL | ||||||||||||||
| 1992 – 2002 | −0.3 (−0.8, 0.3) | 1992 – 2007 | 0.2 (−0.2, 0.6) | 1992 – 2011 | −4 (−4.7, −3.3) | 1992 – 2011 | −3.6 (−5.6, −1.5) | 1992 – 2011 | 0.1 (−1.5, 1.8) | 1992 – 2011 | 5.3 (4.5, 6.2) | 1992 – 2011 | 5.9 (5.1, 6.8) | |
| 2002 – 2007 | 1.5 (−0.5, 3.6) | 2007 – 2011 | −5.9 (−8.8, −2.9) | |||||||||||
| 2007 – 2011 | −3.6 (−5.6, −1.5) | |||||||||||||
| Sex | ||||||||||||||
| Male | 1992 – 2011 | −0.1 (−0.5, 0.3) | 1992 – 2007 | 0.6 (0, 1.3) | 1992 – 2011 | −4.1 (−5, −3.2) | 1992 – 2011 | −5.7 (−8.3, −3) | 1992 – 2011 | −1.7 (−3.5, 0.2) | 1992 – 2011 | 5.2 (3.9, 6.5) | 1992 – 2011 | 6.2 (5.2, 7.2) |
| 2007 – 2011 | −6.4 (−11, −1.5) | |||||||||||||
| Female | 1992 – 2011 | −0.1 (−0.6, 0.4) | 1992 – 2011 | −0.8 (−1.4, −0.2) | 1992 – 2011 | −3.6 (−4.9, −2.3) | 1992 – 2011 | 2.4 (−0.2, 5.2) | 1992 – 2011 | 5.8 (4.9, 6.7) | 1992 – 2011 | 5.0 (2.9, 7.2) | ||
| Race/Ethnicity | ||||||||||||||
| White | 1992 – 2004 | 0.1 (−0.3, 0.5) | 1992 – 2007 | 0.6 (0.1, 1) | 1992 – 2011 | −4.1 (−5, −3.2) | 1992 – 2011 | −3.5 (−6, −0.9) | 1992 – 1999 | −6.5 (−13.5, 1.1) | 1992 – 2011 | 5.3 (4.2, 6.4) | 1992 – 2011 | 6.5 (5.3, 7.8) |
| 2004 – 2007 | 2.7 (−4.0, 9.9) | 2007 – 2011 | −6.7 (−10.2, −3.2) | 1999 – 2011 | 2.7 (−0.8, 6.4) | |||||||||
| 2007 – 2011 | −4.7 (−6.8, −2.5) | |||||||||||||
| Black | 1992 – 2011 | 0.6 (−0.2, 1.5) | 1992 – 2008 | 1.5 (0.5, 2.6) | 1992 – 2011 | −4.1 (−5.9, −2.4) | 1992 – 2011 | 6.2 (4.2, 8.3) | 1992 – 2011 | 5.8 (3.7, 7.8) | ||||
| 2008 – 2011 | −15.6 (−27.2, −2.2) | |||||||||||||
| Hispanic | 1992 – 2011 | 0.1 (−0.7, 0.9) | 1992 – 2011 | 0 (−1, 1) | 1992 – 2011 | −3.4 (−4.8, −2.1) | 1992 – 2011 | 1.8 (−2.2, 6) | 1992 – 2011 | 4.4 (2.4, 6.4) | ||||
| API | 1992 – 2011 | 1.8 (0.5, 3.1) | 1992 – 2011 | 1.7 (−0.1, 3.6) | 1992 – 2011 | −3.1 (−6.1, −0.1) | ||||||||
| AI/AN | ||||||||||||||
| Unknown | ||||||||||||||
| Age group (10 years) | ||||||||||||||
| 00–09 years | 1992 – 2011 | 0.6 (−0.9, 2.0) | 1992 – 2011 | −0.7 (−4.0,2.8) | 1992 – 2011 | 0.5 (−2.1, 3.1) | ||||||||
| 10–19 years | 1992 – 1995 | −8.2 (−18.1, 2.9) | 1992 – 2011 | −0.8 (−1.8, 0.3) | 1992 – 2011 | −4.7 (−7, −2.4) | 1992 – 2011 | 6.7 (4.6, 8.9) | 1992 – 2011 | 6.3 (2.7, 10.1) | ||||
| 1995 – 1998 | 9.7 (−11.6, 36.1) | |||||||||||||
| 1998 – 2001 | −9.8 (−27.7, 12.6) | |||||||||||||
| 2001 – 2011 | 1.9 (0.1, 3.6) | |||||||||||||
| 20–29 years | 1992 – 1998 | −3 (−5.9, 0) | 1992 – 2011 | −0.7 (−1.3, −0.1) | 1992 – 1999 | −11 (−17.7, −3.7) | 1992 – 2011 | 5.6 (3.9, 7.4) | 1992 – 2011 | 4.4 (1.1, 7.8) | ||||
| 1998 – 2011 | 0.8 (−0.2, 1.8) | 1999 – 2011 | 2.2 (−2, 6.6) | |||||||||||
| 30–39 years | 1992 – 2008 | 0.5 (−0.1, 1) | 1992 – 2003 | 2 (0.6, 3.4) | 1992 – 2011 | −3.3 (−4.9, −1.8) | 1992 – 2011 | 0.7 (−2.4, 3.9) | 1992 – 2011 | 4.3 (2.7, 5.9) | 1992 – 2011 | 2.8 (0.5, 5.2) | ||
| 2008 – 2011 | −8.4 (−14.9, −1.4) | 2003 – 2011 | −4.6 (−7, −2.2) | |||||||||||
| 40–49 years | 1992 – 2011 | 0.2 (−0.5, 0.9) | 1992 – 2006 | 1.9 (0.6, 3.3) | 1992 – 2011 | −4 (−5.7, −2.2) | 1992 – 2011 | −3 (−6.8, 0.9) | 1992 – 2011 | 5.0 (3.0, 7.0) | 1992 – 2011 | 6.5 (3.5, 9.5) | ||
| 2006 – 2011 | −8.7 (−14.3, −2.7) | |||||||||||||
| 50–59 years | 1992 – 2011 | −0.7 (−1.6, 0.3) | 1992 – 2011 | −1.6 (−3, −0.3) | 1992 – 2011 | −5.5 (−7.3, −3.7) | 1992 – 2011 | 2 (−1.3, 5.4) | 1992 – 2011 | 6.2 (4.2, 8.2) | ||||
| 60−69 years | 1992 – 2011 | −0.7 (−1.6, 0.3) | 1992 – 1996 | 13.5 (−4.0, 34.1) | 1992 – 2011 | −4.3 (−6.2, −2.3) | 1992 – 2011 | 1.3 (−3.1, 5.8) | 1992 – 2011 | 3.8 (1.5, 6.0) | 1992 – 2011 | 7.6 (3.6, 11.7) | ||
| 1996 – 2011 | −3 (−4.8, −1) | |||||||||||||
| 70–79 years | 1992 – 2011 | 0.2 (−0.7, 1.1) | 1992 – 2011 | 0.8 (−1, 2.6) | 1992 – 2011 | −5 (−6.7, −3.3) | 1992 – 2011 | 4.1 (0.7, 7.7) | 1992 – 2011 | 5.2 (3.0, 7.5) | ||||
| 80+ years | 1992 – 2011 | 1.9 (0.7, 3.1) | 1992 – 2011 | 0.6 (−1.1, 2.3) | 1992 – 2011 | −1.8 (−3.7, 0) | 1992 – 2011 | 5.7 (3.6, 7.9) | ||||||
| Age groups | ||||||||||||||
| 00–14 years | 1992 – 2011 | 0 (−1.1, 1.1) | 1992 – 2011 | −1.1 (−2.8, 0.7) | 1992 – 2011 | −0.7 (−3, 1.7) | 1992 – 2011 | 8.5 (3.8, 13.3) | ||||||
| 15–39 years | 1992 – 2011 | −0.3 (−0.6, 0.1) | 1992 – 2008 | −0.1 (−0.6, 0.3) | 1992 – 2002 | −7.4 (−10.2, −4.5) | 1992 – 2011 | −0.7 (−3, 1.7) | 1992 – 2011 | −2.1 (−4.5, 0.3) | 1992 – 2011 | 5.2 (4.1, 6.3) | 1992 – 2011 | 3.7 (2.2, 5.3) |
| 2008 – 2011 | −6.7 (−11.9, −1.2) | 2002 – 2011 | 2.4 (−2.2, 7.1) | |||||||||||
| 40–54 years | 1992 – 2011 | −0.1 (−0.7, 0.6) | 1992 – 2004 | 1.9 (0.6, 3.3) | 1992 – 2011 | −4.7 (−6.1, −3.3) | 1992 – 2011 | −0.6 (−3.6, 2.5) | 1992 – 2011 | 5.7 (3.9, 7.6) | 1992 – 2011 | 6.5 (4.4, 8.6) | ||
| 2004 – 2011 | −5.4 (−8.3, −2.6) | |||||||||||||
| 55+ years | 1992 – 2011 | 0.1 (−0.4, 0.6) | 1992 – 2011 | −0.3 (−1.2, 0.7) | 1992 – 2011 | −4.2 (−5.1, −3.3) | 1992 – 2011 | −5.8 (−8.6, −2.8) | 1992 – 2001 | −5 (−9.9, 0.2) | 1992 – 2011 | 5.0 (3.5, 6.4) | 1992 – 2011 | 7.2 (4.7, 9.8) |
| 2001 – 2004 | 31 (−26.9, 134.5) | |||||||||||||
| 2004 – 2011 | −5.8 (−11, −0.3) | |||||||||||||
| Stage | ||||||||||||||
| Localized | 1992 – 2011 | −3.2 (−3.9, −2.5) | 1992 – 1998 | 1.2 (−3.9, 6.6) | 1992 – 2011 | −6.5 (−7.7, −5.4) | 1992 – 2011 | −3.4 (−5, −1.8) | 1992 – 2011 | 2.3 (0.9, 3.8) | 1992 – 2011 | 2.8 (1.8, 3.9) | ||
| 1998 – 2011 | −5.6 (−7.4, −3.8) | |||||||||||||
| Regional | 1992 – 2011 | 0.7 (0.2, 1.1) | 1992 – 2007 | 0.7 (0.1, 1.3) | 1992 – 2011 | −3.1 (−4.1, −2.1) | 1992 – 2011 | 0.9 (−1, 2.8) | 1992 – 2011 | 9.2 (7.7, 10.7) | 1992 – 2011 | 8.4 (6.9, 9.8) | ||
| 2007 – 2011 | −4.4 (−8.7, 0.1) | |||||||||||||
| Distant | 1992 – 2009 | 1.7 (0.9, 2.5) | 1992 – 2009 | 1.9 (1.1, 2.7) | 1992 – 2002 | −6.9 (−9.9, −3.9) | 1992 – 2011 | 5.0 (3.3, 6.8) | ||||||
| 2009 – 2011 | −10 (−27.3, 11.5) | 2009 – 2011 | −20.4 (−38.2, 2.7) | 2002 – 2011 | 0.7 (−2.9, 4.4) | - | ||||||||
| NA and unstaged | 1992 – 2011 | −0.6 (−1.9, 0.8) | 1992 – 2011 | 0.2 (−1.9, 2.3) | 1992 – 2011 | −0.5 (−2.4, 1.4) | ||||||||
| Tumor site | - | |||||||||||||
| Nodal | 1992 – 2003 | −0.3 (−0.7, 0.1) | 1992 – 2007 | 0.2 (−0.2, 0.6) | 1992 – 2011 | −4.1 (−4.9, −3.4) | 1992 – 2011 | −4.3 (−6.3, −2.1) | 1992 – 2011 | −0.2 (−1.9, 1.5) | 1992 – 2011 | 5.2 (4.2, 6.2) | 1992 – 2011 | 6.0 (5.0, 7.0) |
| 2003 – 2007 | 1.8 (−1.2, 5) | 2007 – 2011 | −6.2 (−9.1, −3.1) | |||||||||||
| 2007 – 2011 | −3.7 (−5.6, −1.8) | |||||||||||||
| Extranodal | 1992 – 2011 | 3.6 (2.3, 5) | 1992 – 2011 | 2.3 (0.1, 4.5) | ||||||||||
| Registry | ||||||||||||||
| Connecticut | 1992 – 2011 | −0.6 (−1.2, 0) | 1992 – 2003 | 2.2 (0.5, 3.9) | 1992 – 2001 | −12.8 (−18.3, −6.9) | 1992 – 2011 | −1.1 (−5.2, 3.2) | 1992 – 2011 | 5.0 (2.8, 7.2) | ||||
| 2003 – 2011 | −7.1 (−9.8, −4.3) | 2001 – 2011 | 3.0 (−3.9, 10.5) | |||||||||||
| Detroit | 1992 – 2011 | −0.3 (−1.2, 0.6) | 1992 – 2008 | 0.7 (−0.5, 2) | 1992 – 2011 | −2.7 (−4.5, −0.9) | 1992 – 2011 | 0 (−3.2, 3.4) | 1992 – 2011 | 2.6 (0.7, 4.7) | 1992 – 2011 | 6.4 (3.0, 10.0) | ||
| 2008 – 2011 | −17.0 (−30.8, −0.4) | |||||||||||||
| Hawaii | 1992 – 2011 | 1.3 (−0.5, 3.3) | 1992 – 2011 | 2.0 (−0.4, 4.4) | 1992 – 2011 | 5.5 (1.7, 9.4) | ||||||||
| Iowa | 1992 – 2011 | 0.1 (−0.7, 1) | 1992 – 2011 | −0.4 (−1.4, 0.5) | 1992 – 2011 | −2.1 (−4.4, 0.3) | 1992 – 2011 | 6.9 (3.8, 10.2) | 1992 – 2011 | 4.3 (0.3, 8.5) | ||||
| New Mexico | 1992 – 2011 | −0.6 (−1.8, 0.5) | 1992 – 2011 | −3.9 (−5.7, −2.0) | 1992 – 2011 | 1.3 (−3.5, 6.2) | 1992 – 2011 | 4.5 (1.0, 8.1) | ||||||
| Seattle | 1992 – 2011 | −0.1 (−0.7, 0.5) | 1992 – 2009 | 1.0 (−0.1, 2.1) | 1992 – 2011 | −4.3 (−6.2, −2.3) | 1992 – 2011 | −1.4 (−4.6, 2.0) | 1992 – 2011 | 5.3 (3.7, 6.9) | 1992 – 2011 | 4.9 (2.6, 7.2) | ||
| 2009 – 2011 | −20.9 (−43.9, 11.6) | |||||||||||||
| Utah | 1992 – 2011 | 0.7 (−0.3, 1.6) | 1992 – 2011 | 0.9 (−0.4, 2.2) | 1992 – 2011 | −2.1 (−6.2, 2.3) | 1992 – 2011 | 3.3 (−0.4, 7.2) | ||||||
| Atlanta | 1992 – 2011 | −0.2 (−1.4, 0.9) | 1992 – 2011 | −0.7 (−2.1, 0.7) | 1992 – 2011 | −4.6 (−7, −2.2) | 1992 – 2011 | 4.4 (1.2, 7.7) | ||||||
| San Francisco- Oakland | 1992 – 2011 | 0.3 (−0.6, 1.1) | 1992 – 2011 | −1.3 (−2.5, −0.1) | 1992 – 1999 | 4.1 (−3.1, 11.8) | 1992 – 2011 | 7.7 (5.1, 10.3) | ||||||
| 1999 – 2002 | −40.5 (−71.9, 25.9) | |||||||||||||
| 2002 – 2007 | 27.4 (1.1, 60.5) | |||||||||||||
| 2007 – 2011 | −21.7 (−38.4, −0.5) | |||||||||||||
| San Jose-Monterey | 1992 – 2011 | 0.1 (−0.9, 1.1) | 1992 – 2011 | −0.5 (−2.1, 1.2) | 1992 – 2011 | −5.8 (−8.6, −2.9) | 1992 – 2011 | 4.6 (2.5, 6.8) | ||||||
| Los Angeles | 1992 – 2011 | −0.1 (−0.7, 0.5) | 1992 – 2011 | −0.1 (−0.7, 0.6) | 1992 – 2011 | −3.5 (−4.7, −2.2) | 1992 – 2011 | −6.6 (−9.6, −3.5) | 1992 – 2011 | 1.0 (−1.7, 3.8) | 1992 – 2011 | 5.2 (3.5, 6.9) | 1992 – 2011 | 3.4 (1.0, 5.8) |
| Alaska Natives | ||||||||||||||
| Rural Georgia | ||||||||||||||
Joinpoint cannot process records when a dependent variablehas a value of 0.
Figure 1.
Annual age-adjusted incidence rates of Hodgkin lymphoma by histologic subtype and year of diagnosis, 1992–2011, SEER (13 registries)
For nLP, rates increased nearly 6% per year (Table 1), more than doubling between 1992–96 and 2007–11 (IRR: 2.32 (1.89, 2.85)). Increases occurred for both genders, all races (significantly for whites and blacks), all age groups, and all disease stages.
Incidence trends for cHL subtypes
NS
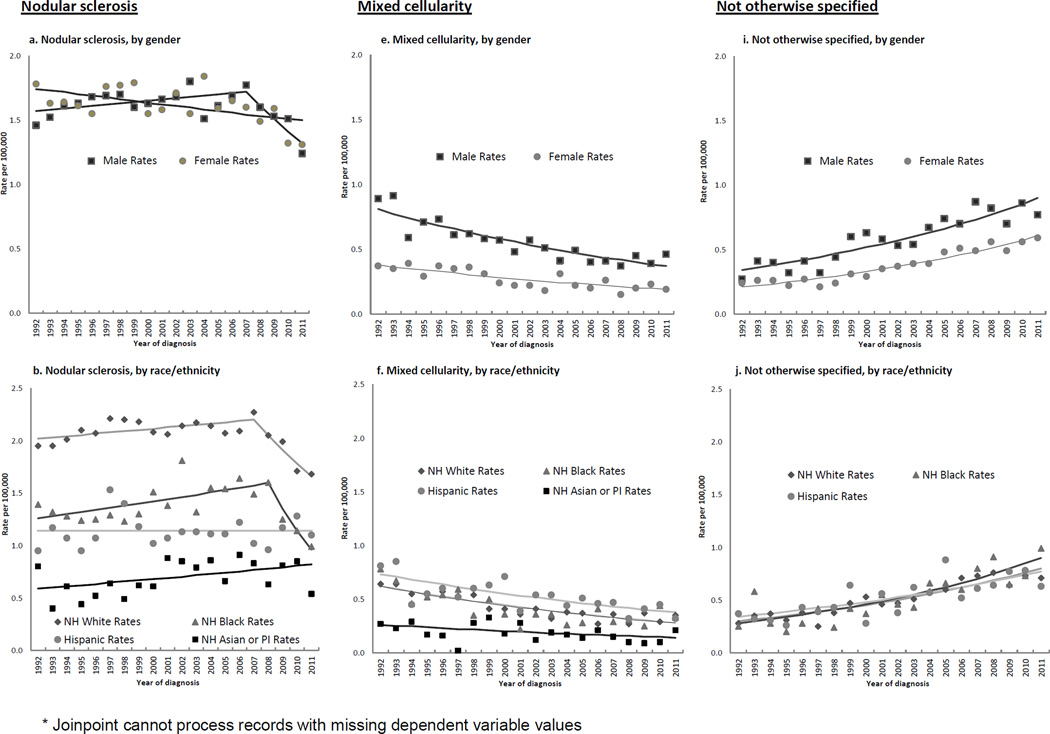
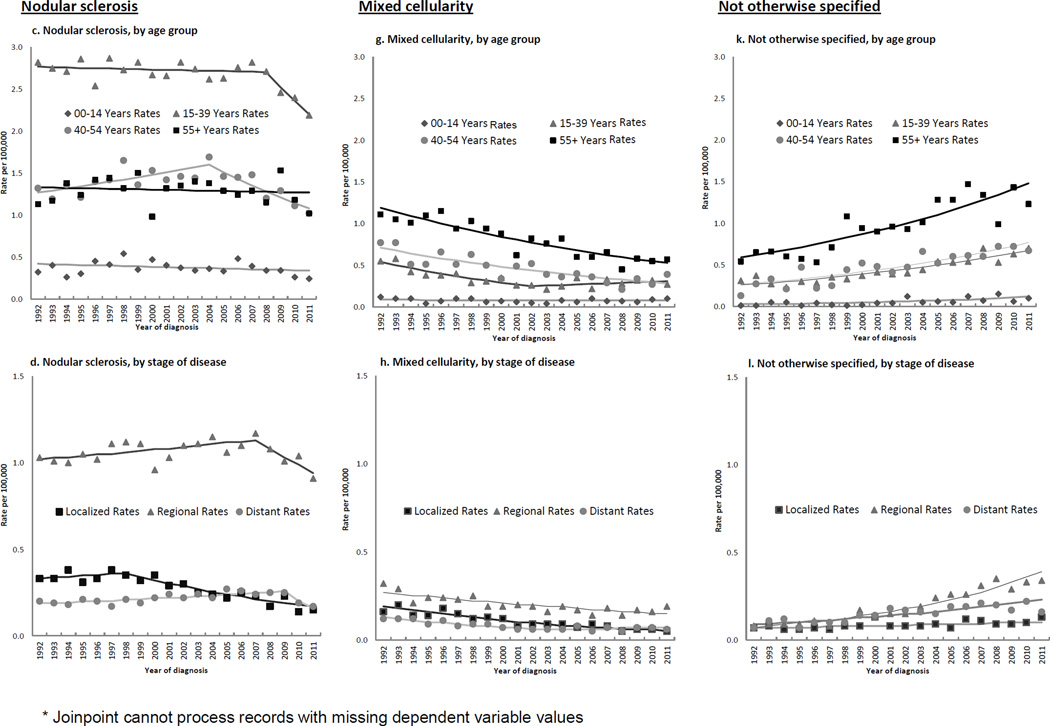
NS rates overall were stable until 2007, then declined 6% on average per year. Table 1 and Figures 2a–d show that rates decreased for females throughout the study period but for males only after 2007 (2a); rates decreased for whites and blacks after 2007–08, did not change for Hispanics, and increased for APIs over the study years (Figure 2b). Rates decreased across most age groups (Figure 2c), at greater than 8% annually under age 10 (from 2001) and at ages 40–49 (from 2006) (Table 1). For AYAs, females experienced a significant, although slight, rate decline throughout (APC for 1992–2011, −0.9 (−1.5, −0.4)), whereas rates for males suggested a recent drop (APC for 1992–2008, 0.4 (−0.4, 1.2); 2008–2011, −8.3 (−17.9, 2.3)). For middle-aged adults, rates declined in 2004 following a modest increase. Rates decreased for localized disease as of 1998. They decreased for nodal disease from 2007, but rose steadily for extranodal disease since 1992.
Figure 2.
Annual age-adjusted incidence rates*, and Joinpoint trend lines, of selected Hodgkin lymphoma histologic subtypes by patient and tumor characteristics, 1992–2011, SEER (13 registries)
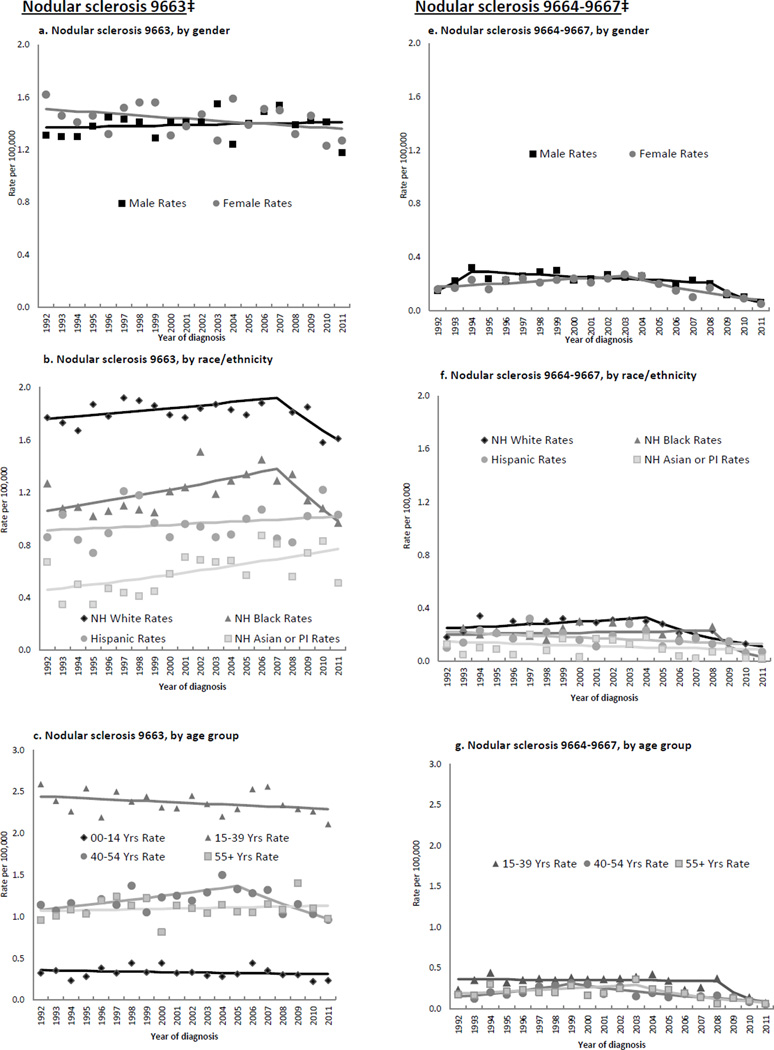
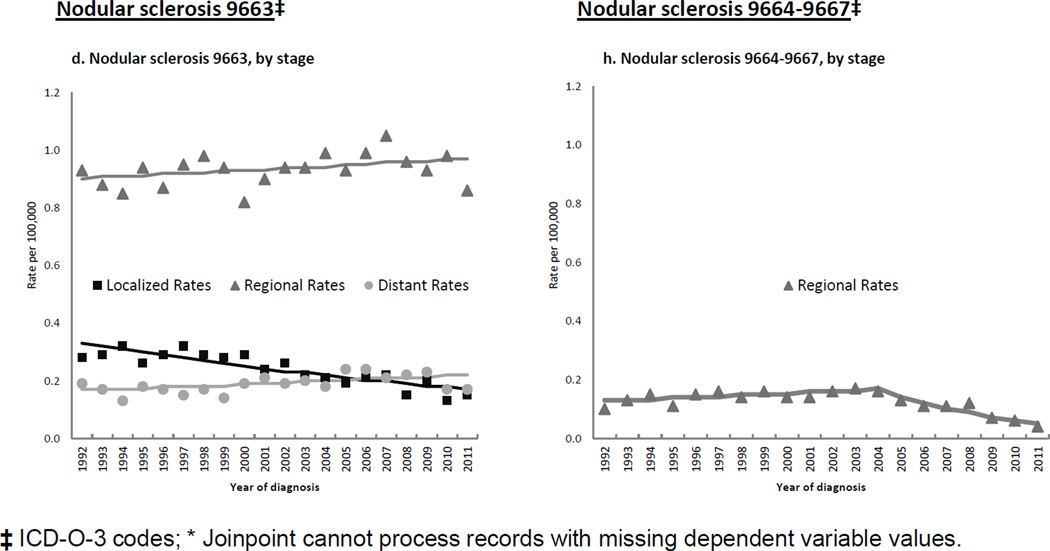
Table 2 and Figures 3a–d show that for the NS 9663 subtype (comprising 87.5% of all NS cases), rate patterns were quite similar to those seen for NS overall. For the remaining NS subtypes group, significant, large rate declines occurred for males and females (Figure 3e); whites (Figure 3f); AYAs, middle-aged and older adults (Figure 3g); localized and regional stages (Figure 3h); and nodal disease. Many of these declines commenced in the mid 2000s.
Table 2.
Joinpoint average percent change (APC)* and 95% confidence intervals (CI) for nodular sclerosis Hodgkin lymphoma incidence rates, by subtype and patient and tumor characteristics, 1992–2011, SEER (13 registries)
| Nodular sclerosis | ||||
|---|---|---|---|---|
| Not otherwise specified (9663‡) | Cellular phase, Grades 1 and
2 (9664–9667‡) |
|||
| N= 11,135 | N= 1,587 | |||
| Interval (yrs) | APC (95% CI) | Interval (yrs) | APC (95% CI) | |
| TOTAL | ||||
| 1992–2009 | 0.2 (−0.2, 0.6) | 1992–2004 | 2.1 (−0.8, 5) | |
| 2009–2011 | −8 (−18.1, 3.3) | 2004–2011 | −15 (−22.1, −7.2) | |
| Sex | ||||
| Male | 1992–2011 | 0.2 (−0.4, 0.7) | 1992–1994 | 38 (−6.6, 103.9) |
| 1994–2008 | −2.4 (−4.2, −0.7) | |||
| 2008–2011 | −34.1 (−49.9, −13.3) | |||
| Female | 1992–2011 | −0.6 (−1.2, 0.1) | 1992–2003 | 3.6 (0.4, 6.9) |
| 2003–2011 | −13.6 (−19.4, −7.4) | |||
| Race/ Ethnicity | ||||
| White | 1992–2007 | 0.6 (0.1, 1.1) | 1992–2004 | 2.4 (−0.6, 5.4) |
| 2007–2011 | −4.5 (−8.2, −0.6) | 2004–2011 | −14.6 (−22.2, −6.1) | |
| Black | 1992–2007 | 1.8 (0.6, 3) | 1992–2008 | 0.9 (−2.2, 4) |
| 2007–2011 | −8.1 (−15.7, 0) | 2008–2011 | −50.8 (−77.8, 9) | |
| Hispanic | 1992–2011 | 0.6 (−0.5, 1.7) | 1992–2011 | −2.9 (−6.2, 0.5) |
| API | 1992–2011 | 2.8 (0.8, 4.7) | 1992–2011 | −2.9 (−8.2, 2.8) |
| Age group (10 years) | ||||
| 00–09 years | 1992–2011 | 0.5 (−2.4, 3.5) | ||
| 10–19 years | 1992–2011 | −0.6 (−1.7, 0.5) | 1992–2011 | −2.5 (−5.2, 0.3) |
| 20–29 years | 1992–2011 | −0.4 (−1, 0.3) | 1992–2008 | −0.7 (−2.5, 1.2) |
| 2008–2011 | −40 (−60.7, −8.5) | |||
| 30–39 years | 1992–2007 | 0.7 (−0.3, 1.6) | 1992–2003 | 6.7 (2.2, 11.4) |
| 2007–2011 | −7.1 (−13.7, 0) | 2003–2011 | −17.7 (−25.3, −9.4) | |
| 40–49 years | 1992–2006 | 2.4 (1, 3.7) | 1992–2000 | 8.9 (−1.6, 20.5) |
| 2006–2011 | −7 (−12.7, −0.8) | 2000–2011 | −11.5 (−18.6, −3.7) | |
| 50–59 years | 1992–2011 | −1.7 (−3, −0.3) | 1992–2011 | −1.6 (−5.1, 1.9) |
| 60–69 years | 1992–2011 | −0.9 (−2.5, 0.7) | ||
| 70–79 years | 1992–2011 | 2 (0.2, 3.8) | ||
| 80+ years | 1992–2011 | 1.3 (−0.6, 3.2) | ||
| Age groups | ||||
| 00–14 years | 1992–2011 | −0.8 (−2.5, 1) | ||
| 15–39 years | 1992–2011 | −0.3 (−0.8, 0.1) | 1992–2008 | −0.3 (−2.4, 1.9) |
| 1992–2005 | 1.8 (0.5, 3.2) | 2008–2011 | −40.9 (−61.9, −8.2) | |
| 40–54 years | 2005–2011 | −5.5 (−9.4, −1.4) | 1992–1999 | 11.1 (1.6, 21.4) |
| 1999–2011 | −9.4 (−13.2, −5.4) | |||
| 55+ years | 1992–2011 | 0.3 (−0.6, 1.2) | 1992–2003 | 4 (−0.5, 8.7) |
| 2003–2011 | −15.9 (−21.8, −9.5) | |||
| Stage | ||||
| Localized | 1992–2011 | −3.5 (−4.5, −2.5) | ||
| Regional | 1992–2011 | 0.4 (−0.1, 0.8) | 1992–2004 | 2.3 (−0.1, 4.7) |
| 2004–2011 | −15.3 (−20.6, −9.5) | |||
| Distant | 1992–2011 | 1.5 (0.3, 2.7) | ||
| NA and unstaged | 1992–2011 | −0.5 (−2.9, 1.9) | ||
| Tumor site | ||||
| Nodal | 1992–2009 | 0.1 (−0.3, 0.5) | 1992–2004 | 2 (−1, 5.1) |
| 2009–2011 | −8.4 (−18.3, 2.7) | 2004–2011 | −15.1 (−21.5, −8.3) | |
| Extranodal | 1992–2011 | 2.3 (0.4, 4.2) | ||
| Registry | ||||
| Connecticut | 1992–2003 | 2.5 (0.7, 4.3) | 1992–2011 | −4.5 (−8.3, −0.5) |
| 2003–2011 | −6.3 (−9.2, −3.3) | |||
| Detroit | 1992–2011 | −0.4 (−1.8, 1.1) | 1992–2011 | −0.8 (−4.7, 3.2) |
| Hawaii | 1992–2011 | 1.8 (−0.7, 4.4) | ||
| Iowa | 1992–2011 | −0.1 (−1.1, 0.8) | 1992–2011 | −2.9 (−6.2, 0.6) |
| New Mexico | 1992–2011 | −3.1 (−5.1, −1) | ||
| Seattle | 1992–2011 | 1 (−0.3, 2.3) | −5.9 (−10.8, −0.7) | |
| Utah | 1992–2011 | 1.4 (0.1, 2.8) | ||
| Atlanta | 1992–2011 | −0.7 (−1.9, 0.6) | ||
| San Francisco- Oakland | 1992–2011 | −0.9 (−2.3, 0.5) | ||
| San Jose-Monterey | 1992–2011 | −0.4 (−1.9, 1.2) | ||
| Los Angeles | 1992–2011 | 0.2 (−0.7, 1.1) | 1992–2004 | 5.6 (1.2, 10.2) |
| 2004–2011 | −16 (−26.6, −3.9) | |||
| Alaska Natives | ||||
| Rural Georgia | ||||
ICD-O-3 codes;
Joinpoint cannot process records when a dependent variablehas a value of 0.
Figure 3.
Annual age-adjusted incidence rates*, and Joinpoint trend lines, of nodular sclerosis Hodgkin lymphoma subtypes by patient and tumor characteristics, 1992–2011, SEER (13 registries)
MC
MC rates decreased over the study period, approximately halving by 2007–11 (IRR: 0.56 (0.5, 0.62)). Figures 2e–h show 3%–6% annual declines for both genders (Figure 2e), all racial/ethnic groups (Figure 2f), all age groups except 0–9 and 20–29 (Figure 2g), and all stages (Figure 2h). APCs showed that rates also declined in almost all regional registries (Table 1).
LD
Decreases occurred in rates overall, and for males, whites, older adults, localized and distant stages (IRR, 2007–11: 0.59 (0.36, 0.95)) and nodal disease.
LR
LR incidence declined significantly among older adults (Table 1), children (IRR, 2002–06: 0.35 (0.11, 0.93)), and persons ages 20–29 (IRR, 2002–06: 0.53 (0.28, 0.98), IRR, 2007–11: 0.51 (0.27, 0.95)). Rates declined for localized disease throughout and rose for distant-stage disease (IRR, 2007–11: 2.84 (1.22, 7.40)).
NOS
Across the study period, NOS rates doubled overall (IRR, 2007–11: 2.19 (1.98, 2.42)) and increased more than six-fold for APIs (IRR, 2007–11: 6.53 (3.09, 16.21)). Increases occurred in almost every patient subgroup (Figures 2i–l) at 4%–6% per year, with a three-fold higher rate by 2007–2011 for children (IRR: 3.59 (1.82, 7.76) (2j)), and significant rises in almost all SEER regions (data not shown). Figure 1 illustrates a crossover of MC and NOS rates occurring around 1999.
Comparison of MC and NOS rates over time
For the period 1992–96, NOS rates were approximately half of MC rates overall (IRR, NOS vs. MC: 0.55 (0.49, 0.61)) and for most patient and tumor characteristics (Supplemental Table 1). For the period 2007–11, NOS rates were approximately double MC rates overall (IRR, NOS vs. MC: 2.14 (1.94, 2.35)) and across study characteristics. The later NOS rates were quite similar to the earlier MC rates overall (IRR, NOS 2007–11 vs. MC 1992–96: 1.19 (1.1, 1.3)), although they were higher for females (IRR: 1.53 (1.33, 1.76)), whites and blacks (IRR: 1.21 (1.09, 1.34), and 1.38 (1.07, 1.78), respectively), and extranodal disease (IRR 5.59 (2.97, 11.69)). The Supplemental Figure illustrates similarities in 2007–11 NOS and 1992–96 MC age-specific rate curves except for AYAs (IRR, NOS 2007–11 vs. MC 1996–96: 1.33 (1.16, 1.54) and over age 80 (IRR: 1.76 (1.26, 2.48)). For combined MC/NOS rates, Table 3 (first column) shows minimal (<1%) annual change overall) but significant increases for females and blacks after 2000, for whites and APIs over the study period, and for AYAs starting in 1998 after a prior decline. Very similar patterns occurred in combined rates also including LD (Table 3, second column). With the addition of NS 9664–9667 (Table 3, third column), trends in combined rates were seen only for persons over age 80, and for localized and regional disease.
Table 3.
Joinpoint average percent change (APC)* and 95% confidence intervals (CI) for Hodgkin lymphoma incidence rates, by combined histologic subtypes and patient and tumor characteristics, 1992–2011, SEER (13 registries)
| Combined Rates | ||||||
|---|---|---|---|---|---|---|
| Mixed cellularity
+ Not otherwise specified |
Mixed cellularity
+ Lymphocyte depletion + Not otherwise specified |
Mixed cellularity
+ Lymphocyte depletion + Not otherwise specified + Nodular sclerosis 9664–9667‡ |
||||
| N=6,739 | N=7,042 | N=8,629 | ||||
| Interval (yrs) | APC (95% CI) | Interval (yrs) | APC (95% CI) | Interval (yrs) | APC (95% CI) | |
| TOTAL | ||||||
| 1992 – 2011 | 0.9 (0.3, 1.5) | 1992 – 1995 | −6.7 (−15.4, 2.8) | 1992–2011 | 0.1 (−0.4, 0.5) | |
| 1995 – 2011 | 1.4 (0.6, 2.1) | |||||
| Sex | ||||||
| Male | 1992 – 2011 | 0.5 (−0.1, 1.2) | 1992 – 2011 | 0.3 (−0.4, 1) | 1992–2011 | −0.3 (−0.8, 0.3) |
| Female | 1992 – 2000 | −1 (−3.5, 1.6) | 1992 – 2000 | −1.5 (−4.1, 1.1) | 1992–2011 | 0.5 (−0.1, 1.1) |
| 2000 – 2011 | 3.2 (1.8, 4.6) | 2000 – 2011 | 3.3 (1.8, 4.8) | |||
| Race/Ethnicity | ||||||
| White | 1992 – 2011 | 0.8 (0.2, 1.4) | 1992 – 2011 | 0.7 (0, 1.3) | 1992–2011 | 0 (−0.5, 0.4) |
| Black | 1992 – 2000 | −4.2 (−9, 0.9) | 1992 – 2011 | 1.5 (−0.1, 3.0) | 1992–2011 | 0.9 (−0.5, 2.2) |
| 2000 – 2011 | 5.0 (2.0, 8.1) | |||||
| Hispanic | 1992 – 2011 | 0.5 (−0.7, 1.7) | 1992 – 2011 | 0.1 (−1.1, 1.4) | 1992–2011 | −0.3 (−1.3, 0.8) |
| API | 1992 – 2011 | 3.0 (1.1, 4.9) | 1992 – 2011 | 2.1 (0.3, 4) | 1992–2011 | 1.0 (−0.7, 2.7) |
| AI/AN | ||||||
| Unknown | ||||||
| Age group (10 years) | ||||||
| 00–09 years | 1992 – 2011 | 2.3 (−0.8, 5.6) | 1992 – 2011 | 2.3 (−0.8, 5.6) | 1992–2011 | 1.2 (−1.7, 4.1) |
| 10–19 years | 1992 – 2004 | −2.3 (−4.7, 0.2) | 1992 – 2003 | −3.1 (−5.5, −0.6) | 1992–2011 | 0.3 (−0.9, 1.5) |
| 2004 – 2011 | 9.2 (3.8, 14.9) | 2003 – 2011 | 8.4 (4.5, 12.4) | |||
| 20–29 years | 1992 – 1996 | −14.2 (−24.9, −2) | 1992 – 1996 | −13.8 (−24.5, −1.7) | 1992–2011 | 0.4 (−0.8, 1.8) |
| 1996 – 2011 | 4.6 (2.8, 6.4) | 1996 – 2011 | 4.6 (2.9, 6.4) | |||
| 30–39 years | 1992 – 2011 | 0.9 (−0.4, 2.1) | 1992 – 2011 | 0.6 (−0.6, 1.8) | 1992–2011 | −0.1 (−1.0, 0.9) |
| 40–49 years | 1992 – 2011 | 0.8 (−0.3, 1.9) | 1992 – 2011 | 0.8 (−0.3, 1.9) | 1992–2011 | −0.1 (−1.1, 0.8) |
| 50–59 years | 1992 – 2011 | 0.1 (−0.9, 1.2) | 1992 – 2011 | 0 (−1.1, 1.1) | 1992–2011 | −0.1 (−1.1, 0.9) |
| 60–69 years | 1992 – 2011 | 0 (−1.4, 1.4) | 1992 – 2011 | −0.4 (−1.7, 1.0) | 1992–2011 | −0.7 (−2.1, 0.7) |
| 70–79 years | 1992 – 2011 | 0.3 (−1.0, 1.6) | 1992 – 2011 | −0.4 (−1.6, 0.9) | 1992–2011 | −0.8 (−1.8, 0.2) |
| 80+ years | 1992 – 2011 | 2.5 (1.0, 4.0) | 1992 – 2011 | 2.5 (0.9, 4.2) | 1992–2011 | 2.0 (0.4, 3.7) |
| Age groups | ||||||
| 00–14 years | 1992 – 2011 | 2.9 (0.9, 4.9) | 1992 – 2011 | 2.9 (0.9, 4.9) | 1992–2011 | 1.8 (−0.1, 3.6) |
| 15–39 years | 1992 – 1998 | −6.1 (−11, −0.9) | 1992 – 1998 | −6.0 (−10.4, −1.4) | 1992–2011 | 0.1 (−0.7, 0.8) |
| 1998 – 2011 | 3.8 (2.1, 5.5) | 1998 – 2011 | 3.7 (2.2, 5.2) | |||
| 40–54 years | 1992 – 2011 | 0.5 (−0.4, 1.5) | 1992 – 2011 | 0.5 (−0.4, 1.5) | 1992–2011 | −0.1 (−0.9, 0.7) |
| 55+ years | 1992 – 2011 | 0.6 (−0.1, 1.3) | 1992 – 2011 | 0.3 (−0.5, 1.0) | 1992–2011 | −0.1 (−0.8, 0.6) |
| Stage | ||||||
| Localized | 1992 – 2011 | −2.0 (−3.2, −0.9) | 1992 – 2011 | −2.2 (−3.3, −1.1) | 1992–2011 | −2.9 (−4, −1.9) |
| Regional | 1992 – 2000 | −1.2 (−3.5, 1.2) | 1992 – 1994 | −14.5 (−33.1, 9.2) | 1992–2011 | 1.1 (0.6, 1.7) |
| 2000 – 2011 | 4.4 (2.9, 5.8) | 1994 – 2011 | 3.0 (2.2, 3.7) | |||
| Distant | 1992 – 2011 | 1.4 (0.6, 2.3) | 1992 – 2011 | 1.0 (0.1, 2) | 1992–2011 | 0.7 (−0.2, 1.6) |
| NA and unstaged | 1992 – 2011 | −0.6 (−2.0, 0.9) | 1992 – 2011 | −0.5 (−1.7, 0.7) | 1992–2011 | −0.7 (−2.0, 0.6) |
| Tumor site | ||||||
| Nodal | 1992 – 1997 | −3.8 (−7.9, 0.6) | 1992 – 1997 | −4.1 (−8.1, 0.1) | 1992–2011 | 0 (−0.5, 0.4) |
| 1997 – 2011 | 1.8 (0.9, 2.7) | 1997 – 2011 | 1.6 (0.7, 2.6) | |||
| Extranodal | 1992–2011 | 1.9 (0, 3.9) | ||||
| Registry | ||||||
| Connecticut | 1992 – 1995 | −20.5 (−35.8, −1.6) | 1992 – 1995 | −21.6 (−36.8, −2.6) | 1992–1995 | −16.1 (−29.1, −0.8) |
| 1995 – 2011 | 2.8 (0.9, 4.7) | 1995 – 2011 | 3.1 (1.3, 5.1) | 1995–2011 | 1.5 (0, 3) | |
| Detroit | 1992 – 2011 | 0.2 (−1.2, 1.5) | 1992 – 2011 | 0.1 (−1.3, 1.6) | 1992–2011 | −0.2 (−1.4, 1) |
| Hawaii | 1992 – 2011 | 0.6 (−1.7, 2.9) | 1992 – 2011 | 0.2 (−2.1, 2.6) | 1992–2011 | 0.3 (−1.9, 2.7) |
| Iowa | 1992 – 2011 | 1.8 (0, 3.6) | 1992 – 2011 | 1.4 (−0.2, 3.1) | 1992–2011 | 0.5 (−1.1, 2.1) |
| New Mexico | 1992 – 1998 | −10.2 (−22.3, 3.9) | 1992 – 1999 | −10.1 (−21.3, 2.7) | 1992–1998 | −10 (−18.9, −0.1) |
| 1998 – 2005 | 17.1 (3, 33.1) | 1999 – 2003 | 32.3 (−11, 96.6) | 1998–2005 | 12.7 (2.5, 24) | |
| 2005 – 2011 | −9.0 (−18.8, 2) | 2003 – 2011 | −5.5 (−12.1, 1.6) | 2005–2011 | −8.8 (−16.8, 0.1) | |
| Seattle | 1992 – 2011 | 0.2 (−1.2, 1.5) | 1992 – 2011 | −0.1 (−1.3, 1.2) | 1992–2011 | −1.3 (−2.4, −0.1) |
| Utah | 1992 – 2011 | 1.0 (−1.8, 3.9) | 1992 – 2011 | 0.9 (−1.8, 3.7) | 1992–2011 | 0.3 (−1.9, 2.5) |
| Atlanta | 1992 – 2011 | 1.1 (−0.9, 3.3) | 1992 – 2011 | 0.7 (−1.3, 2.7) | 1992–2011 | 0.3 (−1.5, 2.1) |
| San Francisco- Oakland | 1992 – 2011 | 3.0 (1.2, 4.8) | 1992 – 2011 | 2.9 (1.1, 4.7) | 1992–2011 | 1.6 (−0.1, 3.2) |
| San Jose-Monterey | 1992 – 2011 | 0.9 (−0.7, 2.6) | 1992 – 2011 | 0.9 (−0.8, 2.5) | 1992–2011 | 0.4 (−1, 1.9) |
| Los Angeles | 1992 – 2011 | 0.1 (−1.0, 1.3) | 1992 – 2011 | −0.3 (−1.4, 0.8) | 1992–2011 | −0.5 (−1.5, 0.6) |
| Alaska Natives | ||||||
| Rural Georgia | ||||||
ICD-O-3 codes;
Joinpoint cannot process records when a dependent variable has a value of 0.
Review of original pathology reports for NOS-coded cases
Among the 165 reviewed pathology reports, 88 (53.3%) contained information providing insight into the choice of the NOS code; these cases were different (p≤0.05) than the 77 without such information on disease stage (17.4% vs. 6.4% early stage), biopsy type (39.1% vs. 12.8% excisional), biopsy site (92.0% vs. 80.8% lymph node), facility type (33.3% vs. 20.5% NCI-designated cancer center), and location of final diagnosis (34.5% vs. 11.5% outside consultation). Pathology report text directly justified the NOS classification for 20 cases (12.1%), described biopsy material as insufficient for further subtyping for 21 (12.7%) cases, and described a more specific subtype but without the definitive terminology required by SEER for coding for 14 (8.5%). For 27 cases (16.4%), coders had missed specific subtypes; for five cases (3.0%), they overlooked non-HL diagnoses (four non-Hodgkin lymphomas, one neuroendocrine tumor). Regarding biopsy type, core/FNA biopsies had been used in 121 cases (73.3%) overall and in 20 of the 21 (95.2%) cases described as having inadequate specimens; the only study factor significantly associated with biopsy type overall was stage, with core/FNA biopsies used in the diagnosis of 40.0%, 74.2%, and 85.1% cases with local, regional and distant stage disease, respectively.
DISCUSSION
In the most recent two decades of SEER data, cHL incidence rates overall were stable until 2007, then showed the first downturn in many years (12). Among cHL subtypes, NS had rates that were unchanged over the first 15 study years before declining, with variation in trends by gender and age; similar patterns and variation for the most common NS subtype (code 9663) are suggestive of a true incidence change. In contrast, rate decreases for MC and increases for NOS were seen largely irrespective of patient gender, age group, race/ethnicity, tumor stage and site, and SEER registry—a uniformity suggestive of artifactual changes. Growing classification of true MC as NOS over time was suggested by the opposing directions of the MC and NOS incidence trends, the similarity of age-specific incidence rates of NOS for 2007–11 and MC for 1992–96, and the minimal temporal increases in combined MC/NOS rates overall. Further, the uniform declines in the less common subtypes LD and NS codes 9664–9667, and the virtual absence of trends in rates also including these subtypes, support similar misclassification as NOS. Our review of NOS pathology reports provided evidence of likely contributing pathology practices, including prevalent use of non-excisional biopsies and stated insufficiency of biopsy specimens for histologic diagnosis, and of limitations in coding accuracy. For nLP, rate increases over time and across patient subgroups are consistent with the new designation of nLP as a separate disease entity in 1994 (3), although a true incidence rise cannot be ruled out. Together, these findings identify histology-specific HL incidence time trends with both etiologic significance (i.e., rate declines in the main NS category) and practical implications (i.e., misclassification of MC, LD, and NS 9664–9667 as NOS, and inadequate quality control of coding). The former change appears to have impacted overall cHL incidence and should be instructive to HL etiology. The latter changes reveal diminishing utility of the WHO classification system for HL, with nearly 30% of cHL diagnoses not subclassified by 2011.
Prior studies of HL incidence trends reported stable or slightly declining overall rates since the 1990s (12, 21, 39). This pattern, noted also in the first 15 years of our study period (40, 41), contrasts with larger HL rate decreases in earlier periods (42–48). As those latter trends were attributed to improving diagnostic differentiation of HL and non-Hodgkin lymphomas (44, 45), the cHL rate stability documented here may reflect the clearer differentiation among broad lymphoma types since implementation of the 1994 REAL and 2001 WHO classifications. For NS, previous studies reported no recent change in rates overall in the U.S. (12, 21, 39), whereas NS rates rose modestly in Australia through 2006 (20) and significantly in Japan through 2008 (21). For young-adult HL (which mostly comprises NS), several investigations found increasing incidence (48–51); in Australia, NS rates rose 5% annually for 15–24 year olds over the period 1997–2006 (20). However, in more recent SEER data (through 2010), rates for ages 20–44 declined since the mid-2000s, with some gender variation (52), as we also noted. For MC and NOS, our findings of decreasing and increasing rates, respectively, extend previous reports for the U.S. (12, 21) and Australia (20), although they differ from MC rate increases reported for Japan (21).
Trends in histology-specific HL rates could be a consequence of secular changes in risk factors. For the predominant NS category, recent declines could reflect changes in environments fostering early-life social isolation, which has been established to affect HL risk in young adults (53–59). Some of these factors (e.g., family size and birth order) have had decreasing importance to HL risk, likely because of changing prevalences resulting from demographic shifts (54, 60–64). However, preschool attendance has emerged as a protective factor (54). For the AYA birth cohorts in this study period, the rising percentages of children under age 5 in daycare or preschool (i.e., 8%, 15%, 20%, 30%, 31%, 28%, and 35% in 1965, 1977, 1982, 1984–85, 1988, 1991 and 1993, respectively (65)) are consistent with the observed declines in AYA NS rates. However, these attendance data do not speak to the gender differences in NS rate declines, which are consistent with well described but little understood gender differences in young-adult HL incidence, including cohort effects (52).
For MC, which is associated with lower socioeconomic status (23, 24), a general rise in the standard of living could have contributed to the rate decline, although SES changes in the U.S. over the study period have not been substantial. Changes in HIV infection prevalence also could have affected observed rates (25, 66). Since 1992, the incidence of new HIV infections in the U.S. increased and then stabilized (67); highly active anti-retroviral therapies introduced in the mid-1990s may have lessened HL risk, although their impact remains unclear (25). However, the occurrence of MC rate decreases across gender and age groups, and the low proportion of HIV-positive MC cases (10% of males (25)), suggest that the observed MC trends are not largely attributable to HIV infection. In SEER data restricted to California (38% of all study cases), overall MC rates (n=7,485) decreased whether the 515 cases with HIV/AIDS (68) were included (APC=−4.2 (−5.3, −3.1) for 1992–2011) or excluded (APC =−4.1 (−5.2, −3.1) for 1992–2011). Long-standing decreases in cigarette smoking prevalence (69) could have contributed to the observed rate decreases (28). MC rates also could be declining due to increasing westernization of immigrants, in whom MC risk is elevated (24, 70). However, we observed no differences in MC trends between whites and Hispanics or APIs, populations with large immigrant subgroups (70, 71). Further, in California data (70), MC rates declined between 1988–92 and 2000–04 similarly for whites, US-born Hispanics, and foreign-born Hispanics (data not shown).
Thus, artifact likely underlies the trends noted here, reflecting changes in diagnostic and/or classification practice. Changes in diagnostic practice have occurred with the advent of core needle biopsies and FNA in place of excisional biopsies (72). While less invasive, these new methods yield a smaller quantity and often lower quality of tumor tissue, specifically the tissue preserving tumor architecture; indeed, our review of NOS pathology reports found specimen inadequacy for subtyping mentioned in 12% of cases. This consequence of core/FNA biopsies could result in poorer diagnostic specificity for HL (73), given the difficulties of diagnosing HL with these methods (37). Diagnostic specificity due to lower quality specimens would be more likely to occur with MC than NS, since characteristic morphology of the latter often is retained in the needle biopsy. Gradual adoption of the new biopsy methods is consistent with our findings of similarities in later NOS to earlier MC rates, presumably as the decreasing ability to diagnose MC (and LD (74)) led to greater use of the NOS classification. A similar explanation may hold for cellular phase NS (code 9664, which also was excluded from the WHO classification in 2008), whereas the drop-off in the use of NS grade subtypes (codes 9665, 9667) likely reflects its lack of clinical relevance with modern chemotherapy (35, 36). Changes in classification practice also could have contributed to the trends observed here, if the WHO classification has been increasingly interpreted as requiring only distinction between cHL and nLP, given clinical requirements (72). Our finding that nearly half of the reviewed NOS diagnoses were recorded without further comment, while not directly addressing the pathologists’ rationale, is consistent with such a trend.
This study used a large database that permitted detailed evaluation of histology-specific rates, and whose high-quality population-based data with standardized coding yielded reliable findings generalizable to similar populations. Our pathology report review provided preliminary insights into diagnostic and classification practices for NOS. However, we were unable to evaluate the impact on histology rate trends of facility type, a factor previously associated with diagnostic accuracy of lymphomas (75), or of facility type and case volume, factors related to the specificity of histologic typing for other cancers (76). As our NOS quality-control review occurred in a single regional registry for a recent time period, it cannot speak to more widespread diagnostic practices or trends over time.
Diminishing specificity of histologic subtyping for cHL has important implications. For epidemiology, it renders histology-specific incidence rates difficult to interpret and confounds secular trends. Despite lacking a biological definition, NOS must be included as a subtype in HL research, as it is now the second most common cHL category. The potential to HL etiology of subtype differences in gene expression profiling (6) and transcriptional analyses of HL malignant cells (8) may be reduced. Finally, although subtypes are not included in clinical decision-making, their varying survival patterns suggest that more tailored therapies might be reconsidered (9, 10, 15, 77).
For all these purposes, accurate histologic subtyping is needed, which requires use of excisional biopsies as indicated and comprehensive central registry quality control. Less invasive biopsy techniques have patient benefits, and should be used for screening (i.e., to rule out malignancy), follow-up biopsies, deep lesions, or when an open biopsy is clinically contraindicated (e.g., by age, comorbidity, etc.). However, in other situations, excisional biopsies should be used as the first choice for the initial diagnosis, in accordance with National Comprehensive Cancer Network guidelines (78). Our finding of NOS coding in error in a California registry may reflect state budget-driven reductions in central registry quality control practices and indicates that enhanced quality control would be of benefit, at least for HL.
In a large, population-based series of HL cases diagnosed over 20 recent years, we have found that histologic subtyping is diminishing. This pattern, which runs counter to the well-established heterogeneity of HL by histologic subtype (79), has an important impact on future HL research, particularly surveillance and epidemiologic studies, and investigations of treatments targeted to reduce survival disparities across subtypes. Adherence to current biopsy best practices for HL, together with improved quality control of lymphoma subtype coding, may help remediate this troubling trend.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Meg McKinley, Christina Schwarz, Rita Leung, Daphne Lichtensztajn, Shawky Matta, Kathleen Davidson-Allen, and David O. Nelson from the Cancer Prevention Institute of California; David Press, formerly from the Cancer Prevention Institute of California; and Richard Ambinder, from Johns Hopkins University, for their help with this study. The collection of cancer incidence data used in this study was supported by the California Department of Health Services as part of the statewide cancer-reporting program mandated by California Health and Safety Code, Section 103885; by the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program under contracts N01-PC-35136 awarded to the Cancer Prevention Institute of California, N02-PC-15105 awarded to the Public Health Institute, HHSN261201000140C awarded to the Cancer Prevention Institute of California, HHSN261201000035C awarded to the University of Southern California, and HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreements U55/CCR921930-02 awarded to the Public Health Institute and U58DP003862-01 awarded to the California Department of Public Health. The ideas and opinions expressed herein are those of the authors, and endorsement by the State of California Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended nor should be inferred.
Footnotes
Disclosure of conflicts of interest
None of the coauthors has actual, potential, or perceived conflicts of interest related to this paper
AUTHORSHIP CONTRIBUTIONS
Sally Glaser, Christina Clarke and Theresa Keegan designed the study. Sally Glaser, Christina Clarke, Theresa Keegan and Ellen Chang interpreted the results and contributed to manuscript writing and review. Dennis Weisenburger provided pathology expertise and contributed to manuscript writing and review.
REFERENCES
- 1.Caporaso NE, Goldin LR, Anderson WF, Landgren O. Current insight on trends, causes, and mechanisms of Hodgkin's lymphoma. Cancer Journal. 2009;15:117–123. doi: 10.1097/PPO.0b013e3181a39585. [DOI] [PubMed] [Google Scholar]
- 2.Eberle FC, Mani H, Jaffe ES. Histopathology of Hodgkin's lymphoma. Cancer Journal. 2009;15:129–137. doi: 10.1097/PPO.0b013e31819e31cf. [DOI] [PubMed] [Google Scholar]
- 3.Agostinelli C, Pileri S. Pathobiology of Hodgkin lymphoma. Mediterranean Journal of Hematology and Infectious Diseases. 2014;6:e2014040. doi: 10.4084/MJHID.2014.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuppers R. The biology of Hodgkin's lymphoma. Nat Rev Cancer. 2009;9:15–27. doi: 10.1038/nrc2542. [DOI] [PubMed] [Google Scholar]
- 5.Turner JJ, Morton LM, Linet MS, Clarke CA, Kadin ME, Vajdic CM, et al. InterLymph hierarchical classification of lymphoid neoplasms for epidemiologic research based on the WHO classification (2008): update and future directions. Blood. 2010;116:e90–e98. doi: 10.1182/blood-2010-06-289561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devilard E, Bertucci F, Trempat P, Bouabdallah R, Loriod B, Giaconia A, et al. Gene expression profiling defines molecular subtypes of classical Hodgkin's disease. Oncogene. 2002;21:3095–3102. doi: 10.1038/sj.onc.1205418. [DOI] [PubMed] [Google Scholar]
- 7.Mani H, Jaffe ES. Hodgkin lymphoma: An update on its biology with new insights into classification. Clin Lymph Res. 2009;9:206–216. doi: 10.3816/CLM.2009.n.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tiacci E, Döring C, Brune V, van Noesel CJM, Klapper W, Mechtersheimer G, et al. Analyzing primary Hodgkin and Reed-Sternberg cells to capture the molecular and cellular pathogenesis of classical Hodgkin lymphoma. Blood. 2012;120:4609–4620. doi: 10.1182/blood-2012-05-428896. [DOI] [PubMed] [Google Scholar]
- 9.Koshy M, Rich SE, Mahmood U, Kwok Y. Declining use of radiotherapy in stage I and II Hodgkin’s disease and its effect on survival and secondary malignancies. Int J Rad Oncol*Biol*Physics. 2012;82:619–625. doi: 10.1016/j.ijrobp.2010.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olszewski AJ, Shrestha R, Castillo JJ. Treatment selection and outcomes in early-stage classical Hodgkin lymphoma: Analysis of the National Cancer Data Base. J Clin Oncology. 2015;33:625–633. doi: 10.1200/JCO.2014.58.7543. [DOI] [PubMed] [Google Scholar]
- 11.Evens AM, Antillón M, Aschebrook-Kilfoy B, Chiu BC-H. Racial disparities in Hodgkin's lymphoma: a comprehensive population-based analysis. Annals of Oncology. 2012;23:2128–2137. doi: 10.1093/annonc/mdr578. [DOI] [PubMed] [Google Scholar]
- 12.Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood. 2006;107:265–276. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glaser SL. Recent incidence and secular trends in Hodgkin's disease and its histologic subtypes. J Chronic Dis. 1986;39:789–798. doi: 10.1016/0021-9681(86)90081-0. [DOI] [PubMed] [Google Scholar]
- 14.Glaser SL, Chang ET, Clarke CA, Keegan TH. Epidemiology. In: Engert A, Horning SJ, editors. Hematologic Malignancies: Hodgkin Lymphoma. Berlin: Springer-Verlag; 2011. [Google Scholar]
- 15.Ali S, Olszewski AJ. Disparate survival and risk of secondary non-Hodgkin lymphoma in histologic subtypes of Hodgkin lymphoma: a population-based study. Leukemia & Lymphoma. 2014;55:1570–1577. doi: 10.3109/10428194.2013.847938. [DOI] [PubMed] [Google Scholar]
- 16.Allemani C, Sant M, De Angelis R, Marcos-Gragera R, Coebergh JW the EWG. Hodgkin disease survival in Europe and the U.S. Cancer. 2006;107:352–360. doi: 10.1002/cncr.21995. [DOI] [PubMed] [Google Scholar]
- 17.Pulte D, Jansen L, Gondos A, Emrich K, Holleczek B, Katalinic A, et al. Improved population level survival in younger Hodgkin lymphoma patients in Germany in the early 21st century. British Journal of Haematology. 2014;164:851–857. doi: 10.1111/bjh.12722. [DOI] [PubMed] [Google Scholar]
- 18.Clarke CA, Glaser SL, Prehn AW. Age-specific survival after Hodgkin's disease in a population-based cohort (United States) Cancer Causes Control. 2001;12:803–812. doi: 10.1023/a:1012240222032. [DOI] [PubMed] [Google Scholar]
- 19.Shenoy P, Maggioncalda A, Malik N, Flowers CR. Incidence patterns and outcomes for Hodgkin lymphoma patients in the United States. Adv Hematol. 2011;2011:725219. doi: 10.1155/2011/725219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Leeuwen MT, Turner JJ, Joske DJ, Falster MO, Srasuebkul P, Meagher NS, et al. Lymphoid neoplasm incidence by WHO subtype in Australia 1982–2006. International Journal of Cancer. 2014;135:2146–2156. doi: 10.1002/ijc.28849. [DOI] [PubMed] [Google Scholar]
- 21.Chihara D, Ito H, Matsuda T, Shibata A, Katsumi A, Nakamura S, et al. Differences in incidence and trends of haematological malignancies in Japan and the United States. British Journal of Haematology. 2014;164:536–545. doi: 10.1111/bjh.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang K-C, Chen PC-H, Jones D, Su I-J. Changing patterns in the frequency of Hodgkin lymphoma subtypes and Epstein–Barr virus association in Taiwan. Cancer Science. 2008;99:345–349. doi: 10.1111/j.1349-7006.2007.00667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clarke CA, Glaser SL, Keegan THM, Stroup A. Neighborhood socioeconomic status and Hodgkin lymphoma incidence in California. Cancer Epidemiol Biomarkers Prev. 2005;14:1441–1447. doi: 10.1158/1055-9965.EPI-04-0567. [DOI] [PubMed] [Google Scholar]
- 24.Cozen W, Katz J, Mack TM. Risk patterns of Hodgkin's disease in Los Angeles vary by cell type. Cancer Epidemiol Biomarkers Prev. 1992;1:261–268. [PubMed] [Google Scholar]
- 25.Shiels MS, Koritzinsky EH, Clarke CA, Suneja G, Morton LM, Engels EA. Prevalence of HIV Infection among U.S. Hodgkin Lymphoma Cases. Cancer Epidemiology Biomarkers & Prevention. 2014;23:274–281. doi: 10.1158/1055-9965.EPI-13-0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frisch M, Biggar RJ, Engels EA, Goedert JJ. Association of cancer with AIDS-related immunosuppression in adults. JAMA. 2001;285:1736–1745. doi: 10.1001/jama.285.13.1736. [DOI] [PubMed] [Google Scholar]
- 27.Quinlan S, Landgren O, Morton L, Engels E. Hodgkin lymphoma among US solid organ transplant recipients. Transplantation. 2010;90:1011–1015. doi: 10.1097/TP.0b013e3181f5c3a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamper-Jorgensen M, Rostgaard K, Glaser SL, Zahm SH, Cozen W, Smedby KE, et al. Cigarette smoking and risk of Hodgkin lymphoma and its subtypes: a pooled analysis from the International Lymphoma Epidemiology Consortium (InterLymph) Ann Oncol. 2013;24:2245–2255. doi: 10.1093/annonc/mdt218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glaser SL, Lin RJ, Stewart SL, Ambinder RF, Jarrett RF, Brousset P, et al. Epstein-Barr virus-associated Hodgkin's disease: epidemiologic characteristics in international data. Int J Cancer. 1997;70:375–382. doi: 10.1002/(sici)1097-0215(19970207)70:4<375::aid-ijc1>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 30.Jarrett RF, Krajewski AS, Angus B, Freeland J, Taylor PR, Taylor GM, et al. The Scotland and Newcastle epidemiological study of Hodgkin's disease: impact of histopathological review and EBV status on incidence estimates. J Clin Pathol. 2003;56:811–816. doi: 10.1136/jcp.56.11.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 13 Regs Research Data, Nov 2013 Sub (1992–2011) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2012 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2014, based on the November 2013 submission [Google Scholar]
- 32.Ruhl J, Adamo M, Dickie L. Hematopoietic and Lymphoid Neoplasm Coding Manual. Bethesda, MD: National Cancer Institute; 2015. [Google Scholar]
- 33.Hjalgrim H, Engels EA. Infectious aetiology of Hodgkin and non-Hodgkin lymphomas: a review of the epidemiological evidence. J Int Medicine. 2008;264:537–548. doi: 10.1111/j.1365-2796.2008.02031.x. [DOI] [PubMed] [Google Scholar]
- 34.Kim H-J, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Statistics in Medicine. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 35.Masih AS, Weisenburger DD, Vose JM, Bast MA, Armitage JO. Histologic grade does not predict prognosis in optimally treated, advanced-stage nodular sclerosing Hodgkin's disease. Cancer. 1992;69:228–232. doi: 10.1002/1097-0142(19920101)69:1<228::aid-cncr2820690137>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 36.van Spronsen DJ, Vrints LW, Hofstra G, Crommelin MA, Coebergh JW, Breed WP. Disappearance of prognostic significance of histopathological grading of nodular sclerosing Hodgkin's disease for unselected patients, 1972–92. Br J Haematol. 1997;96:322–327. doi: 10.1046/j.1365-2141.1997.d01-2010.x. [DOI] [PubMed] [Google Scholar]
- 37.Das DK, Francis IM, Sharma PN, Sathar SA, John B, George SS, et al. Hodgkin's lymphoma: Diagnostic difficulties in fine-needle aspiration cytology. Diagnostic Cytopathology. 2009;37:564–573. doi: 10.1002/dc.21064. [DOI] [PubMed] [Google Scholar]
- 38.Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov < http://www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2012 Sub (2000–2010) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2011 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2013, based on the November 2012 submission [Google Scholar]
- 39.Adamson P, Bray F, Costantini AS, Tao M-H, Weiderpass E, Roman E. Time trends in the registration of Hodgkin and non-Hodgkin lymphomas in Europe. European Journal of Cancer. 2007;43:391–401. doi: 10.1016/j.ejca.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 40.Proctor IE, McNamara C, Rodriguez-Justo M, Isaacson PG, Ramsay A. Importance of expert central review in the diagnosis of lymphoid malignancies in a regional cancer network. J Clin Oncol. 2011;29:1431–1435. doi: 10.1200/JCO.2010.31.2223. [DOI] [PubMed] [Google Scholar]
- 41.Strobbe L, van der Schans SAM, Heijker S, Meijer JWR, Mattijssen EJMV, Mandigers CMPW, et al. Evaluation of a panel of expert pathologists: review of the diagnosis and histological classification of Hodgkin and non-Hodgkin lymphomas in a population-based cancer registry. Leukemia & Lymphoma. 2014;55:1018–1022. doi: 10.3109/10428194.2013.827787. [DOI] [PubMed] [Google Scholar]
- 42.Polednak AP. Trends in cancer incidence in Connecticut, 1935–1991. Cancer. 1994;74:2863–2872. doi: 10.1002/1097-0142(19941115)74:10<2863::aid-cncr2820741020>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 43.Hartge P, Devesa SS, Fraumeni JFJ. Hodgkin's and non-Hodgkin's lymphomas. Cancer Surv. 1994;19–20:423–453. [PubMed] [Google Scholar]
- 44.Glaser SL, Swartz WG. Time trends in Hodgkin's disease incidence. The role of diagnostic accuracy. Cancer. 1990;66:2196–2204. doi: 10.1002/1097-0142(19901115)66:10<2196::aid-cncr2820661026>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 45.Hjalgrim H, Askling J, Pukkala E, Hansen S, Munksgaard L, Frisch M. Incidence of Hodgkin's disease in Nordic countries. The Lancet. 2001;358:297–298. doi: 10.1016/S0140-6736(01)05498-8. [DOI] [PubMed] [Google Scholar]
- 46.Foss Abrahamsen A, Egeland T, Hansen S, Langholm R, Holte H, Kvaløy S. Hodgkin’s disease in a national and hospital population: trends over 20 years. European Journal of Cancer. 1997;33:2380–2383. doi: 10.1016/s0959-8049(97)00342-0. [DOI] [PubMed] [Google Scholar]
- 47.Liu S, Semenciw R, Waters C, Shi Wu W, Mao Y. Time trends and sex patterns in Hodgkin's disease incidence in Canada, 1970–1995. Canadian Journal of Public Health. 2000;91:188–192. doi: 10.1007/BF03404269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sjöberg J, Halthur C, Kristinsson SY, Landgren O, Axdorph Nygell U, Dickman PW, et al. Progress in Hodgkin lymphoma: a population-based study on patients diagnosed in Sweden from 1973-20092012. doi: 10.1182/blood-2010-08-302604. [DOI] [PubMed] [Google Scholar]
- 49.Alston RD, Geraci M, Eden TOB, Moran A, Rowan S, Birch JM. Changes in cancer incidence in teenagers and young adults (ages 13 to 24 years) in England 1979‒2003. Cancer. 2008;113:2807–2815. doi: 10.1002/cncr.23901. [DOI] [PubMed] [Google Scholar]
- 50.Ariad S, Lipshitz I, Benharroch D, Gopas J, Barchana M. A sharp rise in the incidence of Hodgkin's lymphoma in young adults in Israel. Isr Med Assoc J. 2009;11:453–455. [PubMed] [Google Scholar]
- 51.Hjalgrim H, Seow A, Rostgaard K, Friborg J. Changing patterns of Hodgkin lymphoma incidence in Singapore. Int J Cancer. 2008;123:716–719. doi: 10.1002/ijc.23504. [DOI] [PubMed] [Google Scholar]
- 52.Zhu C, Bassig B, Shi K, Boyle P, Guo H, Zheng T. Different time trends by gender for the incidence of Hodgkin’s lymphoma among young adults in the USA: a birth cohort phenomenon. Cancer Causes & Control. 2014;25:923–931. doi: 10.1007/s10552-014-0391-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gutensohn (Mueller) N, Cole P. Childhood social environment and Hodgkin's disease. New Engl J Med. 1981;304:135–140. doi: 10.1056/NEJM198101153040302. [DOI] [PubMed] [Google Scholar]
- 54.Chang ET, Zheng T, Weir EG, Borowitz M, Mann RB, Spiegelman D, et al. Childhood social environment and Hodgkin's lymphoma: new findings from a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2004;13:1361–1370. [PubMed] [Google Scholar]
- 55.Alexander FE, Ricketts TJ, McKinney PA, Cartwright RA. Community lifestyle characteristics and incidence of Hodgkin's disease in young people. Int J Cancer. 1991;48:10–14. doi: 10.1002/ijc.2910480103. [DOI] [PubMed] [Google Scholar]
- 56.Bernard SM, Cartwright RA, Darwin CM, Richards ID, Roberts B, O'Brien C, et al. Hodgkin's disease: case control epidemiological study in Yorkshire. Br J Cancer. 1987;55:85–90. doi: 10.1038/bjc.1987.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bonelli L, Vitale V, Bistolfi F, Landucci M, Bruzzi P. Hodgkin's disease in adults: association with social factors and age at tonsillectomy. A case-control study. Int J Cancer. 1990;45:423–427. doi: 10.1002/ijc.2910450307. [DOI] [PubMed] [Google Scholar]
- 58.Westergaard T, Melbye M, Pedersen JB, Frisch M, Olsen JH, Andersen PK. Birth order, sibship size and risk of Hodgkin's disease in children and young adults: a population-based study of 31 million person-years. Int J Cancer. 1997;72:977–981. doi: 10.1002/(sici)1097-0215(19970917)72:6<977::aid-ijc10>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 59.Chang ET, Montgomery SM, Richiardi L, Ehlin A, Ekbom A, Lambe M. Number of siblings and risk of Hodgkin's lymphoma. Cancer Epidemiol Biomarkers Prev. 2004;13:1236–1243. [PubMed] [Google Scholar]
- 60.Glaser SL, Clarke CA, Stearns CB, Dorfman RF. Age variation in Hodgkin's disease risk factors in older women: evidence from a population-based case-control study. Leuk Lymphoma. 2001;42:997–1004. doi: 10.3109/10428190109097719. [DOI] [PubMed] [Google Scholar]
- 61.Glaser SL, Clarke CA, Nugent RA, Stearns CB, Dorfman RF. Social class and risk of Hodgkin's disease in young-adult women in 1988–94. Int J Cancer. 2002;98:110–117. doi: 10.1002/ijc.10164. [DOI] [PubMed] [Google Scholar]
- 62.Hjalgrim H, Smedby KE, Rostgaard K, Molin D, Hamilton-Dutoit S, Chang ET, et al. Infectious mononucleosis, childhood social environment, and risk of Hodgkin lymphoma. Cancer Res. 2007;67:2382–2388. doi: 10.1158/0008-5472.CAN-06-3566. [DOI] [PubMed] [Google Scholar]
- 63.Roswall N, Olsen A, Christensen J, Rugbjerg K, Mellemkjær L. Social inequality and incidence of and survival from Hodgkin lymphoma, non-Hodgkin lymphoma and leukaemia in a population-based study in Denmark, 1994–2003. European Journal of Cancer. 2008;44:2058–2073. doi: 10.1016/j.ejca.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 64.Crump C, Sundquist K, Sieh W, Winkleby MA, Sundquist J. Perinatal and family risk factors for Hodgkin lymphoma in childhood through young adulthood. Am J Epidemiol. 2012;176:1147–1158. doi: 10.1093/aje/kws212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Child Trends Inc. Trends in the well-being of America's children and youth: 1996. U.S. Department of Health and Human Services; 1996. [Google Scholar]
- 66.Glaser SL, Clarke CA, Gulley ML, Craig FD, DiGiuseppe JA, Dorfman RF, et al. Population-based patterns of human immunodeficiency virus-related Hodgkin lymphoma in the Greater San Francisco Bay Area, 1988–1998. Cancer. 2003;98:300–309. doi: 10.1002/cncr.11459. [DOI] [PubMed] [Google Scholar]
- 67.Hall HI, Song R, Rhodes P, Prejean J, An Q, Lee LM, et al. Estimation of HIV incidence in the United States. JAMA : the journal of the American Medical Association. 2008;300:520–529. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Clarke C, Glaser SL. Population-based surveillance of HIV-associated cancers: utility of cancer registry data. J Acquir Immune Defic Syndr. 2004;36:1083–1091. doi: 10.1097/00126334-200408150-00012. [DOI] [PubMed] [Google Scholar]
- 69.CDC. [cited 2015];2015 http://www.cdc.gov/tobacco/data_statistics/tables/trends/cig_smoking/index.htm.
- 70.Glaser SL, Clarke CA, Chang ET, Yang J, Gomez SL, Keegan TH. Hodgkin lymphoma incidence in California Hispanics: Influence of nativity and tumor Epstein–Barr virus. Cancer Causes & Control. 2014;25:709–725. doi: 10.1007/s10552-014-0374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Surveillance, Epidemiology, and End Results Website. [cited 2009 March 31, 2014];SEER*Stat Software Version 8.1.5 - March 31, 2014. 2014 Available from: http://seer.cancer.gov/seerstat/
- 72.Zhang J-R, Raza AS, Greaves TS, Cobb CJ. Fine-needle aspiration diagnosis of Hodgkin lymphoma using current WHO classification—Re-evaluation of cases from 1999–2004 with new proposals. Diagnostic Cytopathology. 2006;34:397–402. doi: 10.1002/dc.20439. [DOI] [PubMed] [Google Scholar]
- 73.Wilkins BS. Pitfalls in lymphoma pathology: avoiding errors in diagnosis of lymphoid tissues. Journal of Clinical Pathology. 2011;64:466–476. doi: 10.1136/jcp.2010.080846. [DOI] [PubMed] [Google Scholar]
- 74.Grosso LE, Collins BT, Dunphy CH, Ramos RR. Lymphocyte-depleted Hodgkin's disease: Diagnostic challenges by fine-needle aspiration. Diagnostic Cytopathology. 1998;19:66–69. doi: 10.1002/(sici)1097-0339(199807)19:1<66::aid-dc14>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 75.Bowen JM, Perry AM, Laurini JA, Smith LM, Klinetobe K, Bast M, et al. Lymphoma diagnosis at an academic centre: rate of revision and impact on patient care. British Journal of Haematology. 2014;166:202–208. doi: 10.1111/bjh.12880. [DOI] [PubMed] [Google Scholar]
- 76.Gansler T, Fedewa SA, Flanders D, Virgo KS, Ward EM. "Lumping" vs "splitting" in oncologic pathology: association with cancer center type and case volume. J Registry Manag. 2012;39:43–52. [PubMed] [Google Scholar]
- 77.Andersen MD, Kamper P, Nielsen PS, Bendix K, Riber-Hansen R, Steiniche T, et al. Tumour-associated mast cells in classical Hodgkin lymphoma: Correlation with histological subtype, other tumour-infiltrating inflammatory cell subsets, and outcome. European J Haematol. 2015 doi: 10.1111/ejh.12583. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 78.National Comprehensive Cancer Network N. 2014 Available from: http://www.nccn.org/professionals/physician_gls/pdf/hodgkins.pdf. [Google Scholar]
- 79.Levy A, Armon Y, Gopas J, Ariad S, Prinsloo I, Shpilberg O, et al. Is classical Hodgkin's disease Indeed a single entity? Leukemia & Lymphoma. 2002;43:1813–1818. doi: 10.1080/1042819021000006286. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.