Abstract
Innate and adaptive immune cells within the microenvironment identify and eliminate cells displaying signs of malignant potential. Immunosurveillance effector Natural Killer (NK) cells and Cytotoxic T Lymphocyte (CTLs) identify malignant cells through germline receptors such as NKG2D and in the case of CTLs, presentation of antigen through the T cell receptor. Manipulation of immunosurveillance through altered tumor-identifying ligand expression or secretion, resistance to cytotoxicity, or compromised cytotoxic cell activity through immune tolerance mechanisms all contribute to failure of these systems to prevent cancer development. This review examines the diverse mechanisms by which alterations in the immune microenvironment can promote lymphomagenesis.
Keywords: Lymphomagenesis, tumorigenesis, microenvironment, NKG2D, Natural killer, immunosurveillance, cytotoxic, CD8, regulatory, PD-1, TGFβ, IL-10, anergy
Introduction
Through intrinsic cell mechanisms and the innate and adaptive immune system, the body has evolved a diverse array of defences protecting against errors within our own cells that may lead to oncogenic transformation. The immune system provides constant surveillance and editing out of cells it recognises as potentially malignant. Many interdependent layers and systems are involved in this process with the innate and adaptive immune systems working in concert. The most important effector cells in this process are innate Natural Killer (NK) cells relying on germ line receptors to recognise malignant cells and adaptive Cytotoxic T Lymphocytes (CTL) which use a combination of germline receptors and the V(D)J rearranged T cell receptor (TcR). However, the effectiveness of these cells’ antitumor effect is mediated by their ability to recognise tumor and the action of other immune cells within the microenvironment; amplifying or suppressing the immune response and in some cases active contributing to lymphomagenesis.
Cytotoxic effector cells play a central role in tumor immunosurveillance
Intrinsic mechanisms highlight potentially malignant cells to the immune system
Intrinsic safety mechanisms identify cells displaying malignant characteristics to the immune system. These mechanisms lead to tumor-associated antigen displayed upon cell major histocompatibility complexes (MHC) class I molecules and expression of MHC-related ligands allowing recognition by immune cells. These MHC-related ligands are poorly expressed on healthy cells but are frequently upregulated on tumor cells and play an important role in the immune recognition of malignancy.[1] NKG2D is a germline-encoded receptor which is stimulated by the upregulated MHC-related ligands and plays an important role, most significantly in NK-mediated immunosurveillance. It is expressed on NK cells, some activated CD8+ T cells, γδ T cells, NKT cells and certain CD4+ T cells. Excessive proliferation, mediated by control mechanisms protecting cells where proliferation is desirable, leads to upregulation of NKG2D ligands (NKG2D-L) and acquired sensitivity to NKG2D-dependent NK-mediated killing.[1, 2] Other triggers for NGK2D-L upregulation include oncogene-induced senescence and DNA damage repair mechanisms (reviewed [1]).
DNA damage is an important trigger for the upregulation of NKG2D ligands. When DNA damage occurs, protective mechanisms within the cell arrest the cell cycle and attempt to repair it. If the damage is irreparable the cell initiates p53-family mediated apoptotic cell death. [3] Cells undergoing DNA repair also generate signals highlighting that the cell has sustained damage; expression of ligands activating NKG2D and other receptors including DNAM-1, release of chemokines and release of damage-associated molecular pattern molecules (DAMPs).[4] (Figure 1) In Eμ-myc mouse models, inhibition of Ataxia Telangiectasia mutated protein kinase (ATM)-mediated DNA Damage Repair mechanisms has been shown to impair tumor immune control in a process that is dependent upon the presence of NK cells, CD4+ T lymphocytes and CD8+ CTLs.[3] DNAM-1 ligand (CD155) is upregulated on malignant cells in an ATM-dependent manner and inhibition of DNAM-1 impaired tumor control. Additionally, after initial tumor regression, malignant cells showed increased expression of NKG2D ligands.[3] A second study examining the Eμ-myc mouse model found early NKG2D-L upregulation upon lymphomagenesis. A third noted that early tumor NKG2D-L expression in association with active NK phenotypes correlated with subsequent downregulation of NKG2D-L by tumor cells, implying tumor cell adaptation in response to NK-mediated antitumor activity. Furthermore evidence of NK exhaustion with loss of effector function in later disease correlated with tumor cells re-expressing NKG2D ligands. [5, 6]
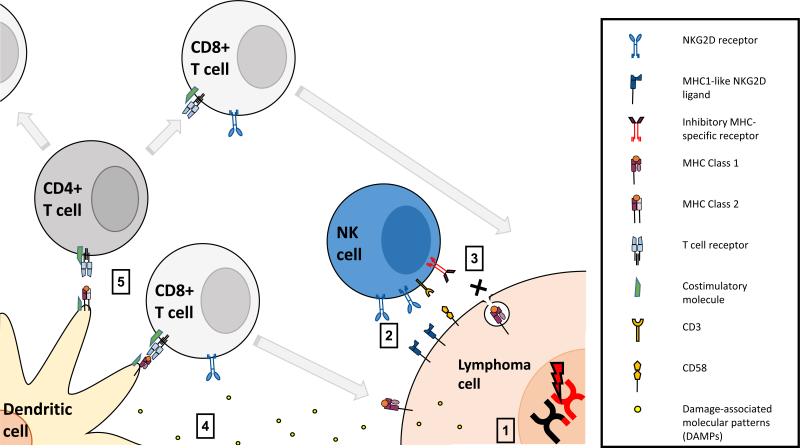
Figure 1. Activation of immunosurveillance in response to DNA damage repair mechanisms.
1. DNA damage triggers upregulation of MHC-like NKG2D ligands and release of damage-associated molecular patterns (DAMPs). 2. NKG2D and other receptors on Natural Killer cells and Cytotoxic T Lymphocytes assisted by adhesion interactions such as CD2-CD58 lead to NK-mediated killing of the tumor cell. 3. Tumor cell downregulation of MHC class 1 molecules to minimise immunogenicity is identified by inhibitory MHC-specific receptors allowing identification of “missing self” and giving NK cells the ability to identify and kill cells not expressing MHC class 1. 4. DAMPs activate dendritic cells stimulating them to present antigen and costimulatory molecules. 5. Dendritic cells activate CD8+ and CD4+ T cells initiating an orchestrated adaptive immune response.
Germline receptors assist recognition of malignant cells and play an important role in both innate and adaptive immunosurveillance
NKG2D and DNAM-1 are two of a number of receptors which play important roles in NK-mediated tumor immunosurveillance; others include natural cytotoxicity receptors which play a similar role to NKG2D, the SLAM family of receptors, adhesion molecules enabling immunological synapse formation (DNAM-1 is one of these) and inhibitory MHC-specific receptors. Inhibitory MHC-specific receptors are of great importance as they give NK cells the unique ability to identify and kill cells which have down-regulated MHC class I molecules – a process known as “missing self-recognition”.[1] Of note, the NKG2D receptor also plays an important role in CTLs where it functions as a costimulatory molecule facilitating tumor cell recognition. This has been demonstrated in H2-K(b)-MICA transgenic mice which over express NKG2D ligands leading to severe NKG2D dysfunction. Introduction of reactivated memory CD8 T cells expressing functional NKG2D in this model was associated with a substantially enhanced cytolytic antitumor response.[7]
The impact that defective NK and CTL function can have on protection against lymphomagenesis is seen in a number of primary immunodeficiencies, where there is an increased incidence of lymphomas, highlighting the important role these cells play in lymphoma prevention. WAS protein is involved in the signalling that controls actin cytoskeleton reorganisation and the establishment of immunological synapses. WAS deficiency leads to impaired synapse formation between CTL or NK cells and their target and also between T lymphocytes and antigen presenting cells. Additionally, WAS deficiency impairs NK cytotoxicity, chemotaxis and chemokinesis and more than 10% these patients develop malignancy, the predominant type being diffuse large B cell lymphoma (DLBCL).[8] Similarly, X-linked lymphoproliferative disease 1 is a rare congenital immunodeficiency caused by SH2D1A loss of function mutations leading to a deficiency of SLAM-associated protein (SAP). In NK cells SLAM-related receptors play an important role in the killing of haematopoetic target cells and signal via SAP family adaptor proteins.[1, 9, 10] In the absence of SAP inhibitory signals impaired NK and T cell cytolytic activity leads to an inability to control EBV infections and the development of B cell lymphomas.[11]
Adaptations to minimise exposure to germline immunosurveillance mechanisms are seen in established lymphomas and infections associated with lymphomagenesis
Manipulation of NKG2D immunosurveillance systems by infective elements in the microenvironment may contribute to lymphoma risk. NK cells play an important role in the elimination of viral infections. HIV, a virus strongly implicated in lymphomagenesis, promotes dysregulation of NK cell activity. Secretion of HIV-1 viral protein R upregulates NKG2D ligands through a DNA damage response on both infected and bystander cells leading to increased indiscriminate NK cell activity and accelerated depletion of CD4+ cells.[12] HIV nef meanwhile downmodulates NKG2D-L expression on infected cells and promotes other mechanisms of NK evasion including MHC class I downregulation.[13] HIV also promotes shedding of soluble NKG2D-Ls from infected cells leading to impaired NK activity and downregulation of NKG2D on NK cells.[14, 15] The net effect of these and other mechanisms of HIV-1 manipulation of NK activity is significant NK dysfunction (reviewed [16]). The impact that this has upon HIV lymphomagenesis is not yet fully evaluated.
Bypassing NK-mediated immunosurveillance appears to be an important step in tumorigenesis that is achieved by varying means. In an early-arising prostate cancer mouse model downregulation of NKG2D-L was seen, permitting immune escape. In contrast, in Eu-myc mouse models aggressive NKG2D-sensitive tumors arose in NKG2D deficient mice but also in NKG2D WT mice where there was evidence of active NKG2D-mediated immunosurveillance. This suggests that a fraction of lymphoma cells escaped by employing an alternative mechanism of NKG2D-mediated immunosurveillance evasion or simply a rapid growth rate.[17] Possible mechanisms for evasion include shedding of NKG2D ligands or inactivation of effector cells.[18, 19] Adaptations such as the shedding of NKG2D ligand reduces the immunogenicity of the tumor cell and may also have distant effects potentially downregulating NKG2D on effector cells further abrogating the NK/CTL antitumor response, although the significance of this mechanism is not fully determined.[1] Alternatively, aggressive tumors may overwhelm NKG2D-mediated killing whilst remain sensitive. Further to the Eu-mycexample above, in mouse models of EBV LMP1+ lymphomas LMP1+ B cells were efficiently eliminated by T cells but rapid fatal lymphoproliferation resulted when immune surveillance was broken despite continuing to express NKG2D-L and remaining susceptible to its effects.[20] In these examples of overwhelming rapid proliferation sustained exposure of ligand to NKG2D+ cells can paradoxically alter NKG2D signalling induce a hyporesponsive state in immune effector cells hence suppressing the anti-tumor response, further tipping the balance in favour of lymphoma escape.[21, 22]
Many lymphomas show evidence of adaptations to minimise their vulnerability to NK and CTL immunosurveillance. (Figure 2) In DLBCL over 60% of cases show evidence of convergent mechanisms minimising NK and CTL immunogenicity. These include frequent co-selection of aberrant CD58 expression (a ligand for CD2 which is required for T and NK cell adhesion and activation) and β-2 microglobulin gene mutations leading to loss of expression of the HLA class 1 complex.[23] Classical Hodgkin Lymphoma provides an example of both NKG2D-L shedding and suppression of the effector cell. Enzymes able to shed NKG2D-L from cell membrane are expressed on Reed-Sternberg (RS) cells and mesenchymal stromal cells (MSC) and shed ligand has been detected in supernatant from both cell types. RS cells lacking NKG2D-L are resistant to CTL killing and sensitivity is partially restored with upregulation of NKG2D-L expression.[18] Additionally, investigators noted that after co-culture with MSCs, cytolytic activity against NKG2D-L+ cells was reduced apparently due to local TGF- β production leading to NKG2D downregulation upon T lymphocytes.[18, 24] Other examples include Adult T-cell Leukaemia/Lymphoma (ATLL) where interactions with epithelial cells lead to downregulation of NKG2D-L and evidence of downregulation on multiple T and B cell lymphoma lines. [25, 26]
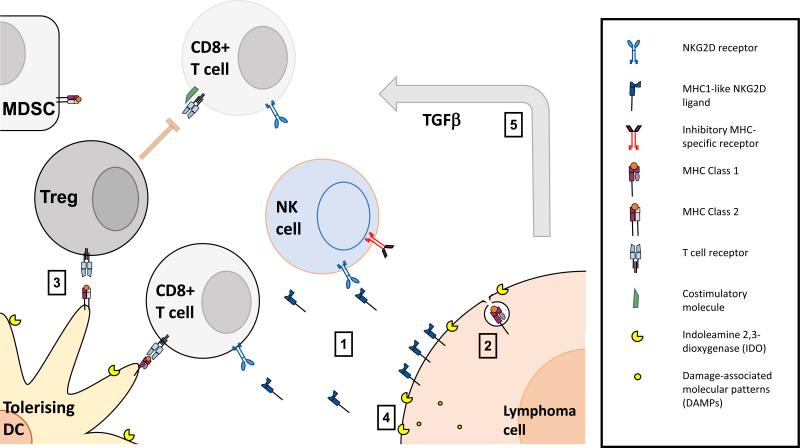
Figure 2. Lymphoma evasion mechanisms undermine the immunosurveillance response.
1. NKG2D ligand shedding overwhelms NK cells leading to downregulated NKG2D and a hypofunctional phenotype and reduced CD58 expression may impair ability for missing self recognition. 2. Downregulation of MHC Class 1 molecules reduce Cytotoxic T Lymphocyte ability to identify tumor cells. 3. If DAMPs are not present, dendritic cells present antigen without costimulatory molecules promoting immune tolerance and regulatory T phenotypes. 4. Indoleamine 2,3-dioxygenase (IDO) expression on tumor and tolerogenic dendritic cells impairs CTL activity 5. TGFβ secretion and myeloid-derived suppressor cell (MDSC) activity further skews towards tolerogenic phenotypes.
Natural Killer cells play an indispensable role in antitumor immunity through NGK2D-mediated activity and also their ability to recognise and kill cells which are missing self-antigen. However, early tumor development in RAG-deficient mice with no functional B, T or NKT cells and observations that lymphomagenesis risk in primary immunodeficiency is more closely related to T-cell number and dysfunction as opposed to immunodeficiency type demonstrates that innate antitumor activity alone is not sufficient for effective immunosurveillance.[8, 27, 28]
Cytotoxic T Lymphocytes: the principal effector cell in anti-tumor immunosurveillance
Cytotoxic T Lymphocytes play a central role as effector cells in tumor immunosurveillance (reviewed [29]). CTLs primarily identify cells with malignant potential through recognition through the T cell receptor of antigen presented through HLA class 1 complexes and target cells via 2 mechanisms, TNF receptor superfamily members 6 and 10 (TRAIL and Fas/CD95) or the perforin and granzyme pathway.[29]
Effective perforin-mediated cytotoxicity is important to CTL function. Perforin deficiency in mice leads to impaired control of transplanted lymphoma lines and increased rates and early tumorigenesis.[30] Perforin deficient mice have been noted to have a 1000-fold increased risk of lymphoid malignancy.[31] Severe perforin gene defects usually present early with aggressive haemophagocytic lymphohistiocytosis but patients with a less severe phenotype present later or have susceptibility to lymphoma.[32] EBV-positive Hodgkin lymphoma has been reported in an individual with biallelic STXBP2 mutations, a gene required for perforin-containing lytic granule exocytosis and in a separate study 8 of 29 patients diagnosed with lymphomas with features of HLH harboured mono- or biallelic mutations of the perforin gene.[33, 34] However, resistance to perforin-mediated cytotoxicity is not usually an important mechanism in lymphomagenesis and most lymphomas remain sensitive to its effects.[35] Adaptations to suppress CTL function play a more important role in immune evasion.
In contrast, evasion of CD95-mediated apoptosis frequently plays a role in lymphomagenesis and is achieved by a number of mechanisms. These include downregulation of surface receptors expression, secretion of soluble forms of CD95 and DcR3 (a soluble decoy receptor which binds CD95-L inhibiting CD95/CD95-L ligation) and cellular mechanisms. Soluble CD95 levels correlates with response to chemotherapy in DLBCL and DcR3 secretion is seen in DLBCL, EBV associated lymphomas and HTLV1 associated ATLL.[36-38] Acquiring resistance to CD95-mediated apoptosis is also an important step towards lymphoma escape in the development of mucosa-associated lymphoid tissue (MALT) lymphomas and gastric DLBCL.[39]
Mechanisms of cellular resistance to CD95 ligation are also important. On CD95 ligation a Fas death-induced signalling complex forms triggering apoptosis. [40] Maintenance of cellular Fas-associated death domain-like IL-1-converting enzyme-inhibitory protein (c-FLIP) levels inhibits this pathway and DLBCL and Hodgkin Lymphomas acquire resistance to CD95 ligation through elevated levels of c-FLIP.[41] Viral proteins also inhibit CD95-mediated apoptosis by this mechanism; HTLV-1 Tax elevates c-FLIP levels, HHV-8 (Kaposi-sarcoma associated herpesvirus) produces a viral FLICE-inhibitory protein (v-FLIP) and EBV also manipulates c-FLIP providing mechanisms by which these viruses contribute to lymphomagenesis.[42-45] Other important mechanisms of CD95-mediated apoptosis suppression relate to B-cell receptor and CD40 signalling reflecting the normal survival signals received by B cells on antigen encounter with T cell support in the germinal centre (discussed further below).[46]
Lymphomas adopt multiple strategies to suppress cytotoxic T lymphocyte activity
Resistance to mechanisms of CTL cytotoxic killing as described above provides some protection from immunosurveillance, however more frequent adaptations implicated in lymphomagenesis are directed towards suppression of cytotoxic cell activity through induction of anergy and exhausted cell phenotypes, secretion of immunosuppressive cytokines and manipulation of regulatory and immune tolerance mechanisms. (Figure 3)
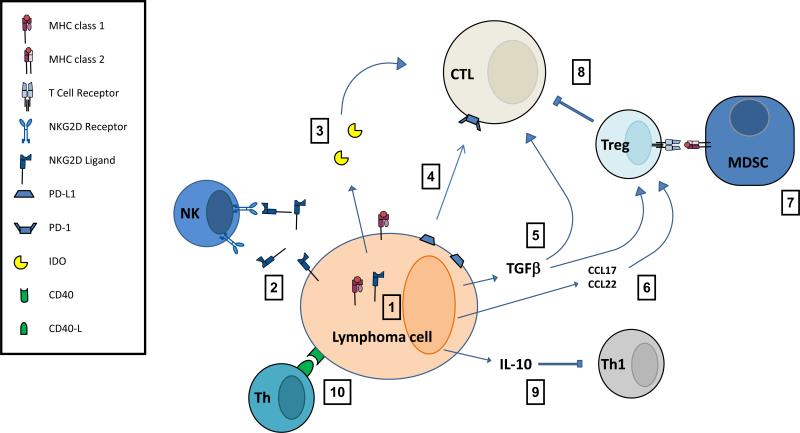
Figure 3. Overview of mechanisms by which lymphoma cells promote exhausted effector cell phenotypes and evade the anti-tumor response.
1. Downregulation of NKG2D ligands and MHC class 1 molecules reduces of lymphoma cells by NK cells and Cytotoxic T lymphocytes. 2. Secretion of NKG2D ligand reduces cell expression and promotes anergy in NK cells through hyperstimulation. 3. Secreted Indoleamine 2,3-dioxygenase (IDO) degrades tryptophan limiting CTL cytotoxic activity. 4. PD-L1 expression promotes exhausion in CTLs. 5. TGFβ promotes exhausted CTL phenotype and CD4+ T cell differentiation to T Regulatory cells. 6. CCL17 and CCL22 secretion attracts more T regulatory cells promoting a tolorogenic microenvironment. 7. Myeloid-derived suppressor cells increase Treg activity by presenting tumor associated antigen in a tolorogenic manner. 8. Treg anti-inflammatory activity further suppresses CTL function. 9. IL-10 secretion suppresses Th1 responses which would promote the anti-tumor response. 10. CD4+ T helper cells are co-opted into providing support to the lymphoma cell increasing its resistance to immune surveillance.
Suppression of CTL activity: Induction of anergy
Induction of CD8+ T cell anergy leading to compromised antitumor activity plays an important role in established lymphomas, particularly in Hodgkin Lymphoma and Primary Mediastinal B cell Lymphoma (PMBCL). [47] It has also been implicated in lymphomagenesis and is seen in a number of microenvironments associated with increased rates of lymphomagenesis.
The programmed cell death 1 (PD-1 or CD279) receptor is involved in the suppression of immune responses through the induction of programmed cell death in antigen specific T cells and promotion of a regulatory T cell phenotype.[48] There is significant evidence pointing to the importance of this mechanism in established lymphoma; PDL1 expression on lymphoma cells induces T cell defects in chronic lymphocytic leukaemia, follicular lymphoma and DLBCLs.[49] In B cell non-Hodgkin lymphomas (NHL) increased PD-1 expression and induced exhaustion in CD8+ T Lymphocytes is seen in TGF-β rich microenvironments.[50] Additionally, Hodgkin Reed-Sternberg cells and malignant cells in PMBCL modulate of the immune microenvironment through expression of PD-1 ligands.[51, 52] There is also exciting evidence of the impact that manipulation of this pathway may have in the treatment of lymphoma with promising results in early trials in relapsed Hodgkin Lymphoma.[53] Furthermore there is early direct evidence that the PD-L1 pathway is importan1 at the point of lymphomagenesis.[54]
Elevated PD-1 expression is also seen in microenvironments associated with lymphomagenesis. Chronic viral infections such as HTLV-1 increase PD-1 expression and CD8+ T cell exhaustion, and PD-1 induced T cell exhaustion remains important in patients with ATLL suggesting that this mechanism might influence lymphomagenesis.[55] Similarly, in HIV infection multiple lines of evidence demonstrate upregulation of inhibitory molecules including PD-1 and cytotoxic T cell exhaustion.[56] EBV infection may also induce PD-L1 expression.[52] T cell dysfunction and compromised immunosurveillance may also contribute to lymphomagenesis in the elderly; studies of PD-L1/PD-1 mediated CD8 T cell dysfunction in CLL mouse models note aging as a confounding factor with distinct but significant increases in PD1+T cells and T cell dysfunction in both CLL and aging control mice.[57] Consistent with this elevated PD-1 expression is noted in aging and T cell dysfunction is identified as a factor contributing to increased rates of lymphomagenesis in the elderly.[58, 59]
Other important markers of an exhausted phenotype include LAG-3 and TIM-3. CD4+ T cells expressing LAG-3 in EBV-positive Hodgkin Lymphoma lead to selective loss of IFN-γ in LMP1 specific CD8+ T cell subsets, suggesting a mechanism by which EBV may contribute to lymphoma survival.[60] TIM-3 is associated with CD8+ T cell dysfunction in advancing HIV and disease progression and similarly observed in HCV-related CD8+ T cell dysfunction .[61] Viral suppression of CTL function in this manner may contribute to an environment conducive for lymphomagenesis. TIM-3 expression is also contributes to non-Hodgkin lymphoma microenvironments; TIM-3 expressed on lymphoma-derived epithelial cells in B cell lymphoma mouse models also inhibited Th1 polarisation and CD4+ Th cell activation promoting lymphoma progression.[62] Furthermore, TIM-3 expression was increased in both CD4+ and CD8+ T cells in newly diagnosed DLBCL and further increased with advanced disease, suggesting a relationship with the development of DLBCL.[63] Certain polymorphisms in the TIM-3 gene additionally appear to confer an increased risk of NHL, which is further amplified in HIV positive patients.[64] However, evidence detailing the relationship between TIM-3 and its function in T cell exhaustion is not as clear-cut as in PD-1 (reviewed [61]).
Suppression of CTL activity: Cytokines
TGF-β
Cytokine secretion modulates cytotoxic immunosurveillance and immunosuppressive cytokines such as TGF-β play an important role in mediating the CTL response in several lymphomas. TGF-β affects T cell suppression in established B-cell Non-Hodgkin Lymphoma; TGF-β stimulation upregulates CD70 on effector memory T cells leading to an exhausted phenotype with higher PD-1 expression and preferentially induces FoxP3 expression in naïve T cells.[50] TGF-β also induces repression of genes encoding cytotoxic mediators such as perforin, granzyme B, CD95 and INF-γ.[29] There is also in vivo evidence that environmental T cells in Hodgkin Lymphoma are under the inhibitory influence of TGF-β.[65]
The suppressive environment promoted by TGF-β in lymphoma appears to be advantageous to the tumor cell, dampening the cytotoxic antitumor response; however, TGF-β usually functions as a negative regulator of lymphocytes inhibiting proliferation and initiating apoptotic programs.[66]In an Eμ-myc mouse model apoptotic lymphomatous cells stimulated macrophages to produce TGF-β which decelerated lymphoma progression; TGF-β production appears therefore to initially suppress lymphomagenesis.[67] However, acquiring tumor cell resistance to its effects is frequently an important step towards escape. In the germinal centre autocrine TGF-β production controls B cell proliferation whereas in Burkitt's cell lines it functions as an autocrine growth factor.[45, 68] In EBV positive Hodgkin lymphoma downregulation of TGF-β target genes contributes to resistance to apoptosis.[69]MYC-transformation also confers an aberrant response to TGF-β proapoptotic signals.[70] Furthermore there is evidence that both EBNA1 and EBNA2 in latent of EBV infection confer resistance to TGF-β mediated apoptosis.[45, 71, 72] A TGF-β-rich microenvironment therefore may suppress lymphomagenesis until the point that a mechanism of resistance to apoptotic signals is acquired whereupon it becomes advantageous.
IL-10
IL-10 similarly has a complex relationship with lymphomagenesis. Variations in IL-10 and TNF loci have been associated with an increased risk of B cell lymphoma.[73] Additionally, patients with IL-10 or IL-10 receptor (IL-10R) deficiency developed B-cell non-Hodgkin lymphoma at an early age. IL-10 or IL-10R deficiency leads to severe early-onset inflammatory bowel disease however the lymphomas, observed in 5 cases, were similar in nature and occurred at sites distant to intestinal inflammation. It was noted that they were DLBCL-like of germinal centre origin and associated with low intratumoral T-cell infiltration and T cells were deficient in the cytotoxic effector Granzyme B implying that impaired immunosurveillance was a likely factor in the development of these lymphomas.[74]
IL-10 plays multiple roles within the immune system. Within the lymphoid compartment IL-10 enhances B cell proliferation, survival, differentiation and MHC-II expression and isotype switching. It induces proliferation and cytotoxicity in thymocytes and CD8+ T cells and induces cytotoxic effector molecules and IFN-γ in CD8+ T cells and CD8-derived IFN-γ upregulates tumor MHC-II expression. These T-cell effects lead to improved tumor immunosurveillance. Other functions of IL-10 are anti-inflammatory, suppressing IL-12 and IL-23 production and by this mechanism reducing Th1 CD4+ lymphocyte production of IFN-γ and TNFα and Th17 CD4+ lymphocyte production of IL-17; effects that might impair the antitumor response .[75]
High levels of IL-10 production are seen in HIV-associated Burkitt's Lymphoma and IL-10 antisense oligonucleotides inhibit tumor growth.[68] IL-10 is secreted in mantle cell lymphoma microenvironments, by Reed-Sternberg cells in Hodgkin Lymphoma and by both tumor cells and associated macrophages in Burkitt's lymphoma.[76-78] This may be advantageous as it promotes local immunosuppression by modulation of T cell response and macrophage engulfment of apoptotic tumor cells in a non-immunogenic manner.[78] This is not the case for all lymphomas however and lower concentrations of IL-10 are seen in the follicular lymphoma microenvironment where other means of local immunosuppression are more prominent.[79, 80] Evidence of IL-10 effects in early lymphomagenesis is less comprehensive. However, elevated IL-10 levels are seen in mouse models of hepatitis C virus-related lymphomagenesis and increased responsiveness to IL-10 and upregulation of IL-10R in tumor cells is seen in EBV lymphomagenesis and murine models of Myc-induced lymphomagenesis respectively.[81-83] Additionally polymorphisms leading to lower IL-10 levels in HIV patients are associated with a lower risk of non-Hodgkin lymphoma.[84]
In light of the above despite the lymphomagenesis risk with a complete deficiency of IL-10, the immunosuppressive effects of locally elevated IL-10 levels appear generally conducive to lymphomagenesis.
Suppression of CTL activity: Indoleamine 2,3-dioxygenase (IDO)
A further, increasingly recognised mechanism of Cytotoxic T cell and Natural Killer activity suppression is via expression of Indoleamine 2,3-dioxygenase (IDO), an enzyme which through degradation of tryptophan limits inflammatory responses and reduces effector T cell proliferation and NKG2D expression.[85, 86] IDO is an important immunosuppressive microenvironmental factor in a number of lymphomas including DLBCL, Hodgkin Lymphoma and ATLL.[87-89] In EBV-associated Hodgkin lymphoma IDO is expressed by Reed Sternberg cells and microenvironmental dendritic cells.[89, 90] Increased IDO expression is also seen in lymphomagenic microenvironments; Helicobacter pylori infection increases IDO expression and numbers of CD4+CD25+ T regulatory cells and EBV induces IDO expression on microenvironmental macrophages.[91, 92]
CD4+ T helper cells augment immunosurveillance but can be manipulated by lymphoma cells into supporting and protecting the tumor
Microenvironmental CD4+ T helper cells play multiple roles in lymphomagenesis, both contributing to immunosurveillance and detracting from it by providing support and survival signals to malignant cells and contributing to immune tolerance as T regulatory cells.
CD4+ T helper cells provide essential survival signals to lymphoma cells
CD4+ T cells can actively support lymphomagenesis by providing survival signals to malignant cells. Extrinsic support by CD4+ T cells of antigen-stimulated germinal centre B cells amplifies the immune response stimulating proliferation, immunoglobulin switching, antibody production and rescue from apoptosis. In addition to stimulation and amplification, this interaction provides a safety mechanism ensuring autoreactive or cells carrying mutations with malignant potential do not survive. However, there are frequent examples of T cells being co-opted into providing anti-apoptotic signals; assisting with lymphomagenesis and subsequent proliferation.
Germinal centre (GC) B cells are dependent upon survival signals from their microenvironment. Unusually for B cells they have a pre-formed Fas death-induced signalling complex requiring constant inhibition by cFLIP to escape apoptosis. cFLIP is rapidly degraded so constant production triggered by external support provides a mechanism which ensures that a cell will die if external help is not provided.[40] This is of particular importance within the germinal centre as B cells undergo somatic hypermutation of immunoglobulin loci and class switch recombination to generate high-affinity antibody and hence are at particularly high risk of acquiring a lymphomagenic mutation. This process is mediated by the enzyme activation-induced deaminase (AID).[93] AID targets ssDNA at sites where transcription is stalled and can damage the genome outside the immunoglobulin loci resulting in mutations that may predispose to lymphoma.[94-96]
Survival signals are received through B-cell receptor (BcR) stimulation by foreign or autoantigen but these alone are insufficient to escape apoptosis, in fact activation of the BcR in the absence of T-cell help in GC B cells has a pro-apoptotic effect.[45] CD40 ligation by CD40L is one of the principle mechanisms by which T-cells promote B cell survival, and this mechanism also suppresses CD95-mediated apoptotic pathways by maintenance of cFLIP.[40, 45]
Many GC origin lymphomas retain the need for ongoing survival signals from the microenvironment. In Follicular Lymphoma anti-apoptotic molecules such as Bcl-2 are upregulated and in EBV-associated lymphomas (such as in EBV-associated cHL) LMP1 and LMP2 mimic CD40 and BcR signalling and suppress TGF-β and CD95 signalling pathways.[45, 97] These mechanisms reduce the dependence of the cell on extrinsic support increasing the probability of survival. However, in both FL and EBV-associated cHL these mechanisms and others are complemented by extrinsic support from environmental T cells via CD40/CD40L ligation, an interaction that both FL and cHL Reed-Sternberg cells remain dependent upon.[98, 99] Within the normal germinal centre T-cell support is obtained through the presentation of antigen by the B cell on MHC class II molecules stimulating the activated CD4+ T cell's TcR with costimulation via CD40/CD40L and other costimulatory molecules. Both Follicular Lymphoma and Hodgkin Lymphoma cells show evidence of downregulation of MHC class II molecules; despite this both malignancies are able to elicit T cell support.[51, 100] Of note, gene expression profiles in Follicular lymphoma-associated T cells, which contain significant populations of T follicular helper (TFH) and T follicular regulatory cells, demonstrated that FL-associated TFH cells overexpressed CD40L and IL-4 when compared to healthy lymph node TFH cells.[101]
The majority of lymphomas originate from follicular B cells which generate high-affinity antibody by a T-cell dependent mechanism within the germinal centre. However, not all B cells depend upon this interaction; marginal zone B cells are among a population of B cells capable of generating an antibody response in the absence of T cell help and marginal zone-like cells have been shown to undergo T cell independent somatic hypermutation.[102, 103] However, examinations of ectopic lymphoid structure in salivary glands of patients with Sjögrens Syndrome who are at risk of MALT-type lymphoma revealed germinal centre-like structures supporting the possibility of T-cell dependent reactions.[104] Gastric MALT lymphoma cells express somatically mutated immunoglobulin. [105] Data suggests that MALT lymphoma proliferation is still highly dependent upon the presence of T cells. Interestingly this dependence appears to be independent of CD40-CD40L interactions.[106] Gastric MALT lymphomas occur in an altered microenvironment created by the buffering of stomach acid by Helicobacter pylori and the infiltration of lymphocytes into an environment where they could not usually survive. [107] In a process driven by molecular mimicry on the part of H.pylori polyreactive neoplastic B-cells are dependent upon autoantigenic stimulation from the gastric mucosa, a process fuelled by APRIL (a proliferation induced ligand) production by microenvironmental macrophages.[108-110] B-cells are thought to receive cognate help from cross-reactive Hp-specific T-cells. Furthermore, Hp-specific T helper clones derived from MALT lymphomas have been shown to increase their B-cell help in a dose-dependent manner in response to H. pylori exposure whereas cytotoxic T-cell control of proliferating B-cells is impaired. This contrasts to chronic gastritis where T helper assistance is attenuated at higher doses and B cell proliferation is controlled by cytotoxic T-cells.[111]
CD4+ T helper cells can promote the anti-tumor response
The above paragraphs highlight examples of CD4+ T cells providing survival signals and supporting lymphomagenesis, however, activated CD4+ T cells also play an important role in the antitumor response, augmenting immunosurveillance. After recognition of tumor antigen presented on MHC class 2 molecules CD4+ T cells improve Dendritic Cell (DC) capacity to induce a cytotoxic T lymphocyte (CTL) response, stimulate clonal expansion of activated CTLs through IL-2 secretion and enhance macrophage and Natural Killer immunosurveillance through production of IFN-γ. [29] Evidence of the selective pressure this exerts on tumor cells is seen in convergent evolution of different lymphomas. MHC class II molecule expression is downregulated across different lymphomas by differing mechanisms. In classical Hodgkin Lymphoma (cHL) and Primary Mediastinal B Cell Lymphoma (PMBCL) this is achieved via disruption of the MHC II transactivator (CIITA), in immune privileged sites such as the testes or central nervous system (CNS) it is caused by protein level homozygous deletion and in Activated B-Cell Type DLBCL (ABC-DLBCL) it is achieved through partial differentiation to the plasma cell stage.[112-114]
Suppression of CTL activity: Regulatory T cells and immune tolerance mechanisms are manipulated to allow tumor growth
CD4+ T regulatory cells are an important subset of primarily CD4+ T helper cells which play a vital role in suppressing autoreactive cells and maintain peripheral immune tolerance. However, their importance in cancer medicine is increasingly recognised and adaptations to manipulate immune tolerance mechanisms are prominent features of lymphomas, frequently developing in the early stages of lymphomagenesis.[115]
Tolerance mechanisms have evolved to control self-reactive T-cells generated during the normal immune response. Naive T-cells, confined to haematopoetic and lymphoid tissue are activated by antigen presented by professional antigen presenting dendritic cells. High-affinity autoreactive T-cells are eliminated in the thymus, however, this process is incomplete and some functional low-affinity autoreactive T-cells and high-affinity cells reactive to rare antigen not represented in the thymus escape. (reviewed [116]). Peripheral inactivation is achieved by tolerizing DCs or by the action of regulatory T cells. DCs that encounter antigen associated with pathogen-associated molecular patterns (PAMPs) upregulate costimulatory molecules enabling them to fully activate CTLs in concert with activated CD4+ T cells. Self-reactive CD4+ and CTLs that escape central tolerance exist in the periphery and under certain conditions can be activated by DCs. Immunogenic tumor cell death, releasing factors such as HMGB1 and calreticulin (damage-associated molecular patterns or DAMPs) especially in association with necrotic tissue can also prime dendritic cells. DCs not primed with PAMPs or DAMPs and in the presence of immunosuppressive mediators such as TGF-β can display self-antigen but due to lack of expression of costimulatory molecules such as B7 family receptors and CD40 this interaction promotes T cell anergy and apoptosis.[29] A second mechanism of peripheral tolerance is mediated by T regulatory (TReg) cells which prevent auto-reactive T cell activation, expansion and function through multiple mechanisms depriving responder T cells of activating signals and through inactivation of responder APCs and T cells (reviewed [117]). Although TReg cells are activated by specific antigen via the TcR their action leads to local immunosuppression independent of effector cell antigen specificity.
Regulatory T cells play a significant role in early lymphoma immune escape. TReg depletion in early tumor progression in a B cell lymphoma mouse model led to 70% disease free survival whereas no effect was seen with depletion in mice with established disease.[118] Additionally CTLA-4 gene polymorphisms affect patient susceptibility to developing gastric MALT lymphoma suggesting a link between TReg function and lymphomagenesis risk. [119] Of note CTLA-4 is also found on CD8+ CTLs and variations in levels affect both Treg function and CTL immunosurveillance providing another possible explanation for this finding.[120] As expected, increased numbers of intratumoral TReg cells in B cell non-Hodgkin lymphoma are associated with decreased proliferation, cytotoxic granule production and cytotoxicity of CD8+ T cells demonstrating the impact this cell's presence has on immunosurveillance.[121] Among a series of mechanisms, malignant B cells in NHL promote T cell differentiation towards a TReg phenotype and suppress effector TH cells through TGF-β secretion. Additionally, they promote a TReg rich microenvironment through the secretion Treg attracting cytokines including CCL17 and CCL22.[50, 106] NHL tumor IDO expression, which attenuates local CTL effector function also correlates with TReg numbers and increases TReg infiltration and differentiation.[122] Increased infiltration of TReg is seen in multiple lymphomas including gastric MALT lymphoma and Follicular lymphoma.[106, 123] In gastric MALT lymphoma the presence of intratumoral TReg cells creates a highly suppressive microenvironment and depletion of these cells blocks tumor growth as efficiently as depletion of all CD4+ T cells.[106]
Antigen Presenting Cells play an increasingly recognised role in promoting a tumorigenic environment
A further category of cells that may play an important role in promoting an immunosuppressive microenvironment are myeloid-derived suppressor cells (MDSCs) (reviewed [124]). Data exploring the extent of their role in lymphomagenesis is limited but mouse lymphoma MDSCs secrete cytokines and chemokines promoting TReg recruitment and an immunosuppressive environment.[125] MDSCs may play an important role in the induction of tumor tolerance functioning as tolerogenic antigen presenting cells presenting to tumor-specific TRes cells.[126] Populations of this cell subset are inversely proportional to NK cell numbers in NHL and increase with NK cell depletion suggesting that they may be regulated by NK cells, a hypothesis supported by the observation that MDSCs express NKG2D ligands and activate NK cells to produce high amounts of IFN-γ.[127, 128]
Other antigen presenting cells such as dendritic cells play an important but less well defined role in lymphomagenesis. Dendritic cells (DCs) appear to foster local T-cell tolerance and the formation of an inflammatory milieu. Deletion of DCs delays progression of lymphoma in an Eμ-Myc model.[129] This mechanism is dependent upon DC expression of the C/EBPβ transcription factor which controlled lymphoma-associated cytokine expression and led to lymphoma-educated DCs suppressing T cell responses.[129] Further evidence of a potential role for DC-lymphocyte interactions is the strong predisposition to germinal-centre B type lymphomagenesis in mice deficient in CD137, a costimulatory receptor involved in DC and germinal centre B cell crosstalk.[130] Furthermore, in established T-cell lymphoproliferations monocyte-derived cells were actively recruited to the tumor microenvironment where they were unable to mature into DCs due to tumor IL-10 secretion and protected tumor cells from serum starvation or doxorubicin induced cell death.[131]
Conclusions
This review has examined some of the diverse mechanisms by which immune cells within the microenvironment identify or fail to identify and destroy potentially lymphomagenic cells and how malignant cells can recruit assistance from non-malignant immune cells allowing them to subvert this process. Furthermore it touches upon some of the mechanisms by which pathogens and other processes can alter interactions in the immune microenvironment and in doing so inhibit immune control and promote lymphomagenesis. These mechanisms are compounded by the further inhibitory effects of established lymphoma cells on the tumor microenvironment, transforming the affected lymph node from an area promoting lymphoma cell eradication to one conducive to lymphoma growth. The impact of the tumor microenvironment upon lymphoma growth is highlighted by the clinical success of novel agents such as anti-PD1 or anti-PDL1 antibodies, which by targeting both tumor and surrounding immune cells and interrupting the tolerogenic mechanisms hijacked by the lymphoma can help bring the malignancy back under immune control.
Acknowledgments
Supported by grants from the National Cancer Institute (P01 CA95426; JGG) and from the Baker Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
JGG declares the following conflicts of interest
Honoraria from Roche/Genentech, Celgene, Pharmacyclics, Janssen, Gilead, JGT has no COI to declare
References
- 1.Marcus A, et al. Recognition of tumors by the innate immune system and natural killer cells. Adv Immunol. 2014;122:91–128. doi: 10.1016/B978-0-12-800267-4.00003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu XV, et al. Ras activation induces expression of Raet1 family NK receptor ligands. J Immunol. 2012;189(4):1826–34. doi: 10.4049/jimmunol.1200965. [DOI] [PubMed] [Google Scholar]
- 3.Tang ML, Gasser S. ATM activation mediates anticancer immunosurveillance by natural killer and T cells. Oncoimmunology. 2013;2(6):e24438. doi: 10.4161/onci.24438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gasser S, Raulet D. The DNA damage response, immunity and cancer. Semin Cancer Biol. 2006;16(5):344–7. doi: 10.1016/j.semcancer.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Unni AM, Bondar T, Medzhitov R. Intrinsic sensor of oncogenic transformation induces a signal for innate immunosurveillance. Proc Natl Acad Sci U S A. 2008;105(5):1686–91. doi: 10.1073/pnas.0701675105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenner CD, et al. Requirements for control of B-cell lymphoma by NK cells. Eur J Immunol. 2010;40(2):494–504. doi: 10.1002/eji.200939937. [DOI] [PubMed] [Google Scholar]
- 7.Andre MC, et al. Impaired tumor rejection by memory CD8 T cells in mice with NKG2D dysfunction. Int J Cancer. 2012;131(7):1601–10. doi: 10.1002/ijc.26191. [DOI] [PubMed] [Google Scholar]
- 8.Salavoura K, et al. Development of cancer in patients with primary immunodeficiencies. Anticancer Res. 2008;28(2B):1263–9. [PubMed] [Google Scholar]
- 9.Benoit L, et al. Defective NK cell activation in X-linked lymphoproliferative disease. J Immunol. 2000;165(7):3549–53. doi: 10.4049/jimmunol.165.7.3549. [DOI] [PubMed] [Google Scholar]
- 10.Dong Z, et al. Essential function for SAP family adaptors in the surveillance of hematopoietic cells by natural killer cells. Nat Immunol. 2009;10(9):973–80. doi: 10.1038/ni.1763. [DOI] [PubMed] [Google Scholar]
- 11.Meazza R, et al. XLP1 inhibitory effect by 2B4 does not affect DNAM-1 and NKG2D activating pathways in NK cells. Eur J Immunol. 2014;44(5):1526–34. doi: 10.1002/eji.201344312. [DOI] [PubMed] [Google Scholar]
- 12.Richard J, et al. Viral protein R upregulates expression of ULBP2 on uninfected bystander cells during HIV-1 infection of primary CD4+ T lymphocytes. Virology. 2013;443(2):248–56. doi: 10.1016/j.virol.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cerboni C, et al. Human immunodeficiency virus 1 Nef protein downmodulates the ligands of the activating receptor NKG2D and inhibits natural killer cell-mediated cytotoxicity. J Gen Virol. 2007;88(Pt 1):242–50. doi: 10.1099/vir.0.82125-0. [DOI] [PubMed] [Google Scholar]
- 14.Matusali G, et al. Soluble ligands for the NKG2D receptor are released during HIV-1 infection and impair NKG2D expression and cytotoxicity of NK cells. FASEB J. 2013;27(6):2440–50. doi: 10.1096/fj.12-223057. [DOI] [PubMed] [Google Scholar]
- 15.Nolting A, et al. MHC class I chain-related protein A shedding in chronic HIV-1 infection is associated with profound NK cell dysfunction. Virology. 2010;406(1):12–20. doi: 10.1016/j.virol.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sowrirajan B, Barker E. The natural killer cell cytotoxic function is modulated by HIV-1 accessory proteins. Viruses. 2011;3(7):1091–111. doi: 10.3390/v3071091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guerra N, et al. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28(4):571–80. doi: 10.1016/j.immuni.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zocchi MR, et al. High ERp5/ADAM10 expression in lymph node microenvironment and impaired NKG2D ligands recognition in Hodgkin lymphomas. Blood. 2012;119(6):1479–89. doi: 10.1182/blood-2011-07-370841. [DOI] [PubMed] [Google Scholar]
- 19.Groh V, et al. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419(6908):734–8. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 20.Zhang B, et al. Immune surveillance and therapy of lymphomas driven by Epstein-Barr virus protein LMP1 in a mouse model. Cell. 2012;148(4):739–51. doi: 10.1016/j.cell.2011.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coudert JD, et al. Altered NKG2D function in NK cells induced by chronic exposure to NKG2D ligand-expressing tumor cells. Blood. 2005;106(5):1711–7. doi: 10.1182/blood-2005-03-0918. [DOI] [PubMed] [Google Scholar]
- 22.Oppenheim DE, et al. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat Immunol. 2005;6(9):928–37. doi: 10.1038/ni1239. [DOI] [PubMed] [Google Scholar]
- 23.Challa-Malladi M, et al. Combined genetic inactivation of beta2-Microglobulin and CD58 reveals frequent escape from immune recognition in diffuse large B cell lymphoma. Cancer Cell. 2011;20(6):728–40. doi: 10.1016/j.ccr.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Espinoza JL, et al. Human microRNA-1245 down-regulates the NKG2D receptor in natural killer cells and impairs NKG2D-mediated functions. Haematologica. 2012;97(9):1295–303. doi: 10.3324/haematol.2011.058529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyatake Y, et al. Protective roles of epithelial cells in the survival of adult T-cell leukemia/lymphoma cells. Am J Pathol. 2013;182(5):1832–42. doi: 10.1016/j.ajpath.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 26.Pende D, et al. Major histocompatibility complex class I-related chain A and UL16-binding protein expression on tumor cell lines of different histotypes: analysis of tumor susceptibility to NKG2D-dependent natural killer cell cytotoxicity. Cancer Res. 2002;62(21):6178–86. [PubMed] [Google Scholar]
- 27.Shankaran V, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410(6832):1107–11. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 28.Canioni D, et al. Lymphoproliferative disorders in children with primary immunodeficiencies: immunological status may be more predictive of the outcome than other criteria. Histopathology. 2001;38(2):146–59. doi: 10.1046/j.1365-2559.2001.01039.x. [DOI] [PubMed] [Google Scholar]
- 29.Topfer K, et al. Tumor evasion from T cell surveillance. J Biomed Biotechnol. 2011;2011:918471. doi: 10.1155/2011/918471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van den Broek ME, et al. Decreased tumor surveillance in perforin-deficient mice. J Exp Med. 1996;184(5):1781–90. doi: 10.1084/jem.184.5.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smyth MJ, et al. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J Exp Med. 2000;192(5):755–60. doi: 10.1084/jem.192.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pachlopnik Schmid J, et al. Inherited defects in lymphocyte cytotoxic activity. Immunol Rev. 2010;235(1):10–23. doi: 10.1111/j.0105-2896.2010.00890.x. [DOI] [PubMed] [Google Scholar]
- 33.Machaczka M, et al. Development of classical Hodgkin's lymphoma in an adult with biallelic STXBP2 mutations. Haematologica. 2013;98(5):760–4. doi: 10.3324/haematol.2012.073098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clementi R, et al. A proportion of patients with lymphoma may harbor mutations of the perforin gene. Blood. 2005;105(11):4424–8. doi: 10.1182/blood-2004-04-1477. [DOI] [PubMed] [Google Scholar]
- 35.Godal R, et al. Lymphomas are sensitive to perforin-dependent cytotoxic pathways despite expression of PI-9 and overexpression of bcl-2. Blood. 2006;107(8):3205–11. doi: 10.1182/blood-2005-07-2880. [DOI] [PubMed] [Google Scholar]
- 36.Bedewy AM, et al. Assessing DcR3 expression in relation to survivin and other prognostic factors in B cell non-Hodgkin's lymphoma. Ann Hematol. 2013;92(10):1359–67. doi: 10.1007/s00277-013-1775-4. [DOI] [PubMed] [Google Scholar]
- 37.Ohshima K, et al. Amplification and expression of a decoy receptor for fas ligand (DcR3) in virus (EBV or HTLV-I) associated lymphomas. Cancer Lett. 2000;160(1):89–97. doi: 10.1016/s0304-3835(00)00567-x. [DOI] [PubMed] [Google Scholar]
- 38.Hara T, et al. Serum soluble Fas level determines clinical outcome of patients with diffuse large B-cell lymphoma treated with CHOP and R-CHOP. J Cancer Res Clin Oncol. 2009;135(10):1421–8. doi: 10.1007/s00432-009-0586-4. [DOI] [PubMed] [Google Scholar]
- 39.Seeberger H, et al. Loss of Fas (CD95/APO-1) regulatory function is an important step in early MALT-type lymphoma development. Lab Invest. 2001;81(7):977–86. doi: 10.1038/labinvest.3780310. [DOI] [PubMed] [Google Scholar]
- 40.van Eijk M, Medema JP, de Groot C. Cutting edge: cellular Fas-associated death domain-like IL-1-converting enzyme-inhibitory protein protects germinal center B cells from apoptosis during germinal center reactions. J Immunol. 2001;166(11):6473–6. doi: 10.4049/jimmunol.166.11.6473. [DOI] [PubMed] [Google Scholar]
- 41.van Houdt IS, et al. Expression of c-FLIP is primarily detected in diffuse large B-cell lymphoma and Hodgkin's lymphoma and correlates with lack of caspase 8 activation. Histopathology. 2007;51(6):778–84. doi: 10.1111/j.1365-2559.2007.02882.x. [DOI] [PubMed] [Google Scholar]
- 42.Krueger A, et al. HTLV-1 Tax protects against CD95-mediated apoptosis by induction of the cellular FLICE-inhibitory protein (c-FLIP). Blood. 2006;107(10):3933–9. doi: 10.1182/blood-2005-06-2567. [DOI] [PubMed] [Google Scholar]
- 43.Belanger C, et al. Human herpesvirus 8 viral FLICE-inhibitory protein inhibits Fas-mediated apoptosis through binding and prevention of procaspase-8 maturation. J Hum Virol. 2001;4(2):62–73. [PubMed] [Google Scholar]
- 44.Ballon G, et al. Kaposi sarcoma herpesvirus (KSHV) vFLIP oncoprotein induces B cell transdifferentiation and tumorigenesis in mice. J Clin Invest. 2011;121(3):1141–53. doi: 10.1172/JCI44417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spender LC, Inman GJ. Inhibition of germinal centre apoptotic programmes by epstein-barr virus. Adv Hematol. 2011;2011:829525. doi: 10.1155/2011/829525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schattner EJ, Friedman SM, Casali P. Inhibition of Fas-mediated apoptosis by antigen: implications for lymphomagenesis. Autoimmunity. 2002;35(4):283–9. doi: 10.1080/0891693021000002072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamamoto R, et al. PD-1-PD-1 ligand interaction contributes to immunosuppressive microenvironment of Hodgkin lymphoma. Blood. 2008;111(6):3220–4. doi: 10.1182/blood-2007-05-085159. [DOI] [PubMed] [Google Scholar]
- 48.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–42. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramsay AG, et al. Multiple inhibitory ligands induce impaired T-cell immunologic synapse function in chronic lymphocytic leukemia that can be blocked with lenalidomide: establishing a reversible immune evasion mechanism in human cancer. Blood. 2012;120(7):1412–21. doi: 10.1182/blood-2012-02-411678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang ZZ, et al. TGF-beta upregulates CD70 expression and induces exhaustion of effector memory T cells in B-cell non-Hodgkin's lymphoma. Leukemia. 2014;28(9):1872–84. doi: 10.1038/leu.2014.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steidl C, et al. MHC class II transactivator CIITA is a recurrent gene fusion partner in lymphoid cancers. Nature. 2011;471(7338):377–81. doi: 10.1038/nature09754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Green MR, et al. Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: implications for targeted therapy. Clin Cancer Res. 2012;18(6):1611–8. doi: 10.1158/1078-0432.CCR-11-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ansell SM, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med. 2015;372(4):311–9. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Y, et al. Contribution of PD-L1 to oncogenesis of lymphoma and its RNAi-based targeting therapy. Leuk Lymphoma. 2012;53(10):2015–23. doi: 10.3109/10428194.2012.673228. [DOI] [PubMed] [Google Scholar]
- 55.Kozako T, et al. PD-1/PD-L1 expression in human T-cell leukemia virus type 1 carriers and adult T-cell leukemia/lymphoma patients. Leukemia. 2009;23(2):375–82. doi: 10.1038/leu.2008.272. [DOI] [PubMed] [Google Scholar]
- 56.Larsson M, et al. Molecular signatures of T-cell inhibition in HIV-1 infection. Retrovirology. 2013;10:31. doi: 10.1186/1742-4690-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McClanahan F, et al. Mechanisms of PD-L1/PD-1 mediated CD8 T-cell dysfunction in the context of aging-related immune defects in the Eμ-TCL1 CLL mouse model. 2015 doi: 10.1182/blood-2015-02-626754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mannick JB, et al. mTOR inhibition improves immune function in the elderly. Sci Transl Med. 2014;6(268):268ra179. doi: 10.1126/scitranslmed.3009892. [DOI] [PubMed] [Google Scholar]
- 59.Sarkozy C, Salles G, Falandry C. The biology of aging and lymphoma: a complex interplay. Curr Oncol Rep. 2015;17(7):457. doi: 10.1007/s11912-015-0457-x. [DOI] [PubMed] [Google Scholar]
- 60.Gandhi MK, et al. Expression of LAG-3 by tumor-infiltrating lymphocytes is coincident with the suppression of latent membrane antigen-specific CD8+ T-cell function in Hodgkin lymphoma patients. Blood. 2006;108(7):2280–9. doi: 10.1182/blood-2006-04-015164. [DOI] [PubMed] [Google Scholar]
- 61.Ferris RL, Lu B, Kane LP. Too much of a good thing? Tim-3 and TCR signaling in T cell exhaustion. J Immunol. 2014;193(4):1525–30. doi: 10.4049/jimmunol.1400557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang X, et al. Lymphoma endothelium preferentially expresses Tim-3 and facilitates the progression of lymphoma by mediating immune evasion. J Exp Med. 2010;207(3):505–20. doi: 10.1084/jem.20090397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xiao T, et al. Tim-3 expression is increased on peripheral T cells from diffuse large B cell lymphoma. Tumour Biol. 2014;35(8):7951–6. doi: 10.1007/s13277-014-2080-0. [DOI] [PubMed] [Google Scholar]
- 64.Song H, et al. T-cell immunoglobulin- and mucin-domain-containing molecule 3 genetic variants and HIV+ non-Hodgkin lymphomas. Inflammation. 2013;36(4):793–9. doi: 10.1007/s10753-013-9605-3. [DOI] [PubMed] [Google Scholar]
- 65.Chemnitz JM, et al. RNA fingerprints provide direct evidence for the inhibitory role of TGFbeta and PD-1 on CD4+ T cells in Hodgkin lymphoma. Blood. 2007;110(9):3226–33. doi: 10.1182/blood-2006-12-064360. [DOI] [PubMed] [Google Scholar]
- 66.Sebestyen A, et al. Rapamycin can restore the negative regulatory function of transforming growth factor beta 1 in high grade lymphomas. Cytokine. 2015;73(2):219–224. doi: 10.1016/j.cyto.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 67.Reimann M, et al. Tumor stroma-derived TGF-beta limits myc-driven lymphomagenesis via Suv39h1-dependent senescence. Cancer Cell. 2010;17(3):262–72. doi: 10.1016/j.ccr.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 68.Fassone L, et al. The role of cytokines in the pathogenesis and management of AIDS-related lymphomas. Leuk Lymphoma. 2000;38(5-6):481–8. doi: 10.3109/10428190009059266. [DOI] [PubMed] [Google Scholar]
- 69.Flavell JR, et al. Down-regulation of the TGF-beta target gene, PTPRK, by the Epstein-Barr virus encoded EBNA1 contributes to the growth and survival of Hodgkin lymphoma cells. Blood. 2008;111(1):292–301. doi: 10.1182/blood-2006-11-059881. [DOI] [PubMed] [Google Scholar]
- 70.Wiese KE, et al. The role of MIZ-1 in MYC-dependent tumorigenesis. Cold Spring Harb Perspect Med. 2013;3(12):a014290. doi: 10.1101/cshperspect.a014290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Campion EM, et al. Repression of the proapoptotic cellular BIK/NBK gene by Epstein-Barr virus antagonizes transforming growth factor beta1-induced B-cell apoptosis. J Virol. 2014;88(9):5001–13. doi: 10.1128/JVI.03642-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wood VH, et al. Epstein-Barr virus-encoded EBNA1 regulates cellular gene transcription and modulates the STAT1 and TGFbeta signaling pathways. Oncogene. 2007;26(28):4135–47. doi: 10.1038/sj.onc.1210496. [DOI] [PubMed] [Google Scholar]
- 73.Hosgood HD, 3rd, et al. IL10 and TNF variants and risk of non-Hodgkin lymphoma among three Asian populations. Int J Hematol. 2013;97(6):793–9. doi: 10.1007/s12185-013-1345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Neven B, et al. A Mendelian predisposition to B-cell lymphoma caused by IL-10R deficiency. Blood. 2013;122(23):3713–22. doi: 10.1182/blood-2013-06-508267. [DOI] [PubMed] [Google Scholar]
- 75.Oft M. No immunosurveillance in human IL-10R deficiency. Blood. 2013;122(23):3702–3. doi: 10.1182/blood-2013-10-531657. [DOI] [PubMed] [Google Scholar]
- 76.Bernard S, et al. Inhibitors of BCR signalling interrupt the survival signal mediated by the micro-environment in mantle cell lymphoma. Int J Cancer. 2015;136(12):2761–74. doi: 10.1002/ijc.29326. [DOI] [PubMed] [Google Scholar]
- 77.Beck A, et al. Expression of cytokine and chemokine genes in Epstein-Barr virus-associated nasopharyngeal carcinoma: comparison with Hodgkin's disease. J Pathol. 2001;194(2):145–51. doi: 10.1002/path.867. [DOI] [PubMed] [Google Scholar]
- 78.Ogden CA, et al. Enhanced apoptotic cell clearance capacity and B cell survival factor production by IL-10-activated macrophages: implications for Burkitt's lymphoma. J Immunol. 2005;174(5):3015–23. doi: 10.4049/jimmunol.174.5.3015. [DOI] [PubMed] [Google Scholar]
- 79.Calvo KR, et al. IL-4 protein expression and basal activation of Erk in vivo in follicular lymphoma. Blood. 2008;112(9):3818–26. doi: 10.1182/blood-2008-02-138933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Myklebust JH, et al. High PD-1 expression and suppressed cytokine signaling distinguish T cells infiltrating follicular lymphoma tumors from peripheral T cells. Blood. 2013;121(8):1367–76. doi: 10.1182/blood-2012-04-421826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tsukiyama-Kohara K, et al. Hepatitis C virus-related lymphomagenesis in a mouse model. ISRN Hematol. 2011;2011:167501. doi: 10.5402/2011/167501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hatton O, et al. Syk activation of phosphatidylinositol 3-kinase/Akt prevents HtrA2-dependent loss of X-linked inhibitor of apoptosis protein (XIAP) to promote survival of Epstein-Barr virus+ (EBV+) B cell lymphomas. J Biol Chem. 2011;286(43):37368–78. doi: 10.1074/jbc.M111.255125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yu D, et al. Functional validation of genes implicated in lymphomagenesis: an in vivo selection assay using a Myc-induced B-cell tumor. Ann N Y Acad Sci. 2005;1059:145–59. doi: 10.1196/annals.1339.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wong HL, et al. Cytokine signaling pathway polymorphisms and AIDS-related non-Hodgkin lymphoma risk in the multicenter AIDS cohort study. AIDS. 2010;24(7):1025–33. doi: 10.1097/QAD.0b013e328332d5b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Song H, et al. IDO metabolite produced by EBV-transformed B cells inhibits surface expression of NKG2D in NK cells via the c-Jun N-terminal kinase (JNK) pathway. Immunol Lett. 2011;136(2):187–93. doi: 10.1016/j.imlet.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 86.Andersen MH. The targeting of immunosuppressive mechanisms in hematological malignancies. Leukemia. 2014;28(9):1784–92. doi: 10.1038/leu.2014.108. [DOI] [PubMed] [Google Scholar]
- 87.Masaki A, et al. Prognostic Significance of Tryptophan Catabolism in Adult T-cell Leukemia/Lymphoma. Clin Cancer Res. 2015 doi: 10.1158/1078-0432.CCR-14-2275. [DOI] [PubMed] [Google Scholar]
- 88.Hara T, Tsurumi H. [Indoleamine 2, 3-dioxygenasase (IDO) as a prognostic factor of hematological malignancies]. Rinsho Byori. 2011;59(12):1133–43. [PubMed] [Google Scholar]
- 89.Choe JY, et al. Indoleamine 2,3-dioxygenase (IDO) is frequently expressed in stromal cells of Hodgkin lymphoma and is associated with adverse clinical features: a retrospective cohort study. BMC Cancer. 2014;14:335. doi: 10.1186/1471-2407-14-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ninomiya S, et al. Indoleamine 2,3-dioxygenase in tumor tissue indicates prognosis in patients with diffuse large B-cell lymphoma treated with R-CHOP. Ann Hematol. 2011;90(4):409–16. doi: 10.1007/s00277-010-1093-z. [DOI] [PubMed] [Google Scholar]
- 91.Liu WL, et al. Epstein-Barr virus infection induces indoleamine 2,3-dioxygenase expression in human monocyte-derived macrophages through p38/mitogen-activated protein kinase and NF-kappaB pathways: impairment in T cell functions. J Virol. 2014;88(12):6660–71. doi: 10.1128/JVI.03678-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Raitala A, et al. Helicobacter pylori-induced indoleamine 2,3-dioxygenase activity in vivo is regulated by TGFB1 and CTLA4 polymorphisms. Mol Immunol. 2007;44(5):1011–4. doi: 10.1016/j.molimm.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 93.Frieder D, et al. Antibody diversification: mutational mechanisms and oncogenesis. Immunol Res. 2006;35(1-2):75–88. doi: 10.1385/IR:35:1:75. [DOI] [PubMed] [Google Scholar]
- 94.Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2012;30:429–57. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 95.Pham P, et al. Processive AID-catalysed cytosine deamination on single-stranded DNA simulates somatic hypermutation. Nature. 2003;424(6944):103–7. doi: 10.1038/nature01760. [DOI] [PubMed] [Google Scholar]
- 96.Di Noia JM, Neuberger MS. Molecular mechanisms of antibody somatic hypermutation. Annu Rev Biochem. 2007;76:1–22. doi: 10.1146/annurev.biochem.76.061705.090740. [DOI] [PubMed] [Google Scholar]
- 97.Karube K, et al. Monoclonal B cell lymphocytosis and “in situ” lymphoma. Semin Cancer Biol. 2014;24:3–14. doi: 10.1016/j.semcancer.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 98.Aldinucci D, et al. The role of CD40/CD40L and interferon regulatory factor 4 in Hodgkin lymphoma microenvironment. Leuk Lymphoma. 2012;53(2):195–201. doi: 10.3109/10428194.2011.605190. [DOI] [PubMed] [Google Scholar]
- 99.Rawal S, et al. Cross talk between follicular Th cells and tumor cells in human follicular lymphoma promotes immune evasion in the tumor microenvironment. J Immunol. 2013;190(12):6681–93. doi: 10.4049/jimmunol.1201363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Green MR, et al. Mutations in early follicular lymphoma progenitors are associated with suppressed antigen presentation. Proc Natl Acad Sci U S A. 2015;112(10):E1116–25. doi: 10.1073/pnas.1501199112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ame-Thomas P, et al. Characterization of intratumoral follicular helper T cells in follicular lymphoma: role in the survival of malignant B cells. Leukemia. 2012;26(5):1053–63. doi: 10.1038/leu.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Defrance T, Taillardet M, Genestier L. T cell-independent B cell memory. Curr Opin Immunol. 2011;23(3):330–6. doi: 10.1016/j.coi.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 103.Scheeren FA, et al. T cell-independent development and induction of somatic hypermutation in human IgM+ IgD+ CD27+ B cells. J Exp Med. 2008;205(9):2033–42. doi: 10.1084/jem.20070447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bombardieri M, et al. Activation-induced cytidine deaminase expression in follicular dendritic cell networks and interfollicular large B cells supports functionality of ectopic lymphoid neogenesis in autoimmune sialoadenitis and MALT lymphoma in Sjogren's syndrome. J Immunol. 2007;179(7):4929–38. doi: 10.4049/jimmunol.179.7.4929. [DOI] [PubMed] [Google Scholar]
- 105.Craig VJ, et al. Gastric MALT lymphoma B cells express polyreactive, somatically mutated immunoglobulins. Blood. 2010;115(3):581–91. doi: 10.1182/blood-2009-06-228015. [DOI] [PubMed] [Google Scholar]
- 106.Craig VJ, et al. B-cell receptor signaling and CD40 ligand-independent T cell help cooperate in Helicobacter-induced MALT lymphomagenesis. Leukemia. 2010;24(6):1186–96. doi: 10.1038/leu.2010.76. [DOI] [PubMed] [Google Scholar]
- 107.Ferreri AJ, Govi S, Ponzoni M. Marginal zone lymphomas and infectious agents. Semin Cancer Biol. 2013;23(6):431–40. doi: 10.1016/j.semcancer.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 108.Amedei A, et al. Molecular mimicry between Helicobacter pylori antigens and H+, K+ -- adenosine triphosphatase in human gastric autoimmunity. J Exp Med. 2003;198(8):1147–56. doi: 10.1084/jem.20030530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Suarez F, et al. Infection-associated lymphomas derived from marginal zone B cells: a model of antigen-driven lymphoproliferation. Blood. 2006;107(8):3034–44. doi: 10.1182/blood-2005-09-3679. [DOI] [PubMed] [Google Scholar]
- 110.Munari F, et al. Tumor-associated macrophages as major source of APRIL in gastric MALT lymphoma. Blood. 2011;117(24):6612–6. doi: 10.1182/blood-2010-06-293266. [DOI] [PubMed] [Google Scholar]
- 111.D'Elios MM, et al. Impaired T-cell regulation of B-cell growth in Helicobacter pylori--related gastric low-grade MALT lymphoma. Gastroenterology. 1999;117(5):1105–12. doi: 10.1016/s0016-5085(99)70395-1. [DOI] [PubMed] [Google Scholar]
- 112.Scott DW, Gascoyne RD. The tumour microenvironment in B cell lymphomas. Nat Rev Cancer. 2014;14(8):517–34. doi: 10.1038/nrc3774. [DOI] [PubMed] [Google Scholar]
- 113.Rimsza LM, et al. Loss of major histocompatibility class II expression in non-immune-privileged site diffuse large B-cell lymphoma is highly coordinated and not due to chromosomal deletions. Blood. 2006;107(3):1101–7. doi: 10.1182/blood-2005-04-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Roberts RA, et al. Loss of major histocompatibility class II gene and protein expression in primary mediastinal large B-cell lymphoma is highly coordinated and related to poor patient survival. Blood. 2006;108(1):311–8. doi: 10.1182/blood-2005-11-4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vesely MD, et al. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–71. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 116.Gilboa E. The risk of autoimmunity associated with tumor immunotherapy. Nat Immunol. 2001;2(9):789–92. doi: 10.1038/ni0901-789. [DOI] [PubMed] [Google Scholar]
- 117.Yamaguchi T, Wing JB, Sakaguchi S. Two modes of immune suppression by Foxp3(+) regulatory T cells under inflammatory or non-inflammatory conditions. Semin Immunol. 2011;23(6):424–30. doi: 10.1016/j.smim.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 118.Elpek KG, et al. CD4+CD25+ T regulatory cells dominate multiple immune evasion mechanisms in early but not late phases of tumor development in a B cell lymphoma model. J Immunol. 2007;178(11):6840–8. doi: 10.4049/jimmunol.178.11.6840. [DOI] [PubMed] [Google Scholar]
- 119.Cheng TY, et al. Association of T-cell regulatory gene polymorphisms with susceptibility to gastric mucosa-associated lymphoid tissue lymphoma. J Clin Oncol. 2006;24(21):3483–9. doi: 10.1200/JCO.2005.05.5434. [DOI] [PubMed] [Google Scholar]
- 120.Miska J, et al. Autoimmunity-mediated antitumor immunity: tumor as an immunoprivileged self. Eur J Immunol. 2012;42(10):2584–96. doi: 10.1002/eji.201242590. [DOI] [PubMed] [Google Scholar]
- 121.Yang ZZ, et al. Attenuation of CD8(+) T-cell function by CD4(+)CD25(+) regulatory T cells in B-cell non-Hodgkin's lymphoma. Cancer Res. 2006;66(20):10145–52. doi: 10.1158/0008-5472.CAN-06-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu XQ, et al. Up-regulated expression of indoleamine 2,3-dioxygenase 1 in non-Hodgkin lymphoma correlates with increased regulatory T-cell infiltration. Leuk Lymphoma. 2014;55(2):405–14. doi: 10.3109/10428194.2013.804917. [DOI] [PubMed] [Google Scholar]
- 123.Ame-Thomas P, Tarte K. The yin and the yang of follicular lymphoma cell niches: role of microenvironment heterogeneity and plasticity. Semin Cancer Biol. 2014;24:23–32. doi: 10.1016/j.semcancer.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 124.De Veirman K, et al. Myeloid-derived suppressor cells as therapeutic target in hematological malignancies. Front Oncol. 2014;4:349. doi: 10.3389/fonc.2014.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schlecker E, et al. Tumor-infiltrating monocytic myeloid-derived suppressor cells mediate CCR5-dependent recruitment of regulatory T cells favoring tumor growth. J Immunol. 2012;189(12):5602–11. doi: 10.4049/jimmunol.1201018. [DOI] [PubMed] [Google Scholar]
- 126.Serafini P, et al. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008;68(13):5439–49. doi: 10.1158/0008-5472.CAN-07-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sato Y, et al. Characterization of the myeloid-derived suppressor cell subset regulated by NK cells in malignant lymphoma. Oncoimmunology. 2015;4(3):e995541. doi: 10.1080/2162402X.2014.995541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nausch N, et al. Mononuclear myeloid-derived “suppressor” cells express RAE-1 and activate natural killer cells. Blood. 2008;112(10):4080–9. doi: 10.1182/blood-2008-03-143776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rehm A, et al. Dendritic cell-mediated survival signals in Emu-Myc B-cell lymphoma depend on the transcription factor C/EBPbeta. Nat Commun. 2014;5:5057. doi: 10.1038/ncomms6057. [DOI] [PubMed] [Google Scholar]
- 130.Middendorp S, et al. Mice deficient for CD137 ligand are predisposed to develop germinal center-derived B-cell lymphoma. Blood. 2009;114(11):2280–9. doi: 10.1182/blood-2009-03-208215. [DOI] [PubMed] [Google Scholar]
- 131.Wilcox RA, et al. Monocytes promote tumor cell survival in T-cell lymphoproliferative disorders and are impaired in their ability to differentiate into mature dendritic cells. Blood. 2009;114(14):2936–44. doi: 10.1182/blood-2009-05-220111. [DOI] [PMC free article] [PubMed] [Google Scholar]