Abstract
Background
There is a lack of information about the correlates of completing all three cancer screening tests among women living in Appalachia.
Methods
Cross-sectional telephone interviews were conducted (April-September 2013) among women (n=637) ages 51-75 from 12 Appalachia Ohio counties. Outcomes of within screening guidelines were verified by medical record. Multivariable logistic regression models identified correlates of being within guidelines for all three cancer screening tests.
Results
Screening rates were: mammography (32.1%), Pap test (36.1%), and a colorectal cancer test (30.1%). Only 8.6% of women were within guidelines for all tests. Having had a check-up in the past two years and having received a screening recommendation were significantly related to being within guidelines for all three tests (p<0.01). Participants with higher annual household incomes ($60,000+; OR=3.53, 95% CI: 1.49, 8.33) and conditions requiring regular medical visits (OR=3.16, 95% CI: 1.29, 7.74) were more likely to be within guidelines for all three screening tests.
Conclusion
Less than 10% of women had completed screening within guidelines for all three screening tests. Regular contact with the healthcare system and higher incomes were significant predictors of being within guidelines.
Impact
Within guidelines rates for the three recommended cancer screening tests is low among women in Appalachia Ohio. This finding illustrates the need for innovative interventions to improve rates of multiple cancer screening tests.
Keywords: Cancer, Cancer Screening, Early Detection of Cancer, Appalachian Region, Health Disparities
Introduction
Compared to cancer rates among women living in the United States(U.S.), women living in the Appalachian region have increased cancer incidence and mortality rates, as well as lower cancer screening rates (1-10). Reasons for cancer disparities among this underserved population are likely due to many social determinants of health included in the multilevel social determinants of health model (11). This model includes proximal, intermediate, and distal factors that may affect and individual's health. Examples of factors that may be especially relevant to the Appalachian population include lower socioeconomic status (SES), lower educational levels, smoking, limited sources for healthcare, lack of health insurance, culture, communication issues, genetics, or a combination of these factors (7, 12, 13).
Many of these factors individually or collectively play a role in determining health behaviors. Previous studies have suggested that some health behaviors are interrelated and tend to cluster. For example, there is evidence that individuals who complete a specific cancer screening test are more likely to complete other cancer screening tests (e.g. breast and cervical cancer screening) (14-18) or practice other preventive health behaviors (e.g. colorectal cancer screening and regular seat belt use) (19, 20). In addition, studies have documented that individuals who are changing one health behavior may be trying to change multiple behaviors simultaneously (e.g., diet and physical activity, smoking cessation and physical activity) (21-23). Several common health behavior theories (e.g. Health Belief Model (24), the Transtheoretical Model (25), Social Cognitive Theory (26)) have been used to explain the correlates of completing a singular cancer screening behavior (27-32), and multiple behavior change interventions (simultaneously or sequentially within a limited time frame) may potentially be important public health strategies (33).
Currently, we have limited information about the frequency and correlates of being within recommended guidelines for multiple cancer screening tests among women and there is insufficient evidence about potential mechanisms for achieving this multiple behavior change (34). The few studies that have reported on multiple cancer screening behaviors have suggested that common factors (e.g. education level) and theoretical constructs (e.g. intention) play an important role in changing multiple behaviors (14-18). More recently, a conceptual model for breast, cervical, and CRC screening has been proposed and focuses on multiple levels (policy, system, facility, provider, and individual) and individual-level steps including risk assessment, detection (routine screening and follow-up testing), diagnosis, and treatment (35).
The focus of this study is to describe multiple cancer screening behaviors (mammography for breast cancer, Pap test for cervical cancer, and fecal occult blood tests (FOBT) and colonoscopy for colorectal cancer (CRC)) among women living in Appalachian Ohio. This information may provide researchers valuable insight to plan future interventions to improve screening rates across multiple organ sites.
Materials and Methods
A telephone survey was conducted (April-September 2013) at the completion of a group randomized trial (GRT) designed to test a county-level intervention to improve CRC screening in 12 counties. The design of the intervention and the GRT has been previously described (27, 36). At the completion of the study, there were no significant differences between the two arms of the study in CRC screening rates (37). Therefore, the current report describes the cancer screening behaviors for breast, cervical and CRC among female participants living in all 12 Appalachia counties captured at the end of the study. The study was approved by The Ohio State University Institutional Review Board and written informed consent was obtained from all participants.
Participants
Participants were recruited from randomly selected households using commercially available lists of residents aged 51-80 living in one of 12 Appalachian Ohio counties included in the study. We used a proportional sampling scheme that reflected the county gender proportions from the 2000 Census because more males than females were represented on the lists (38). Of the 2,467 (38.7%) potentially eligible and contacted households, 1,376 (55.8%) refused participation in the study. Among the 1,091 (44.2%) who completed the survey, 641 (58.8%) of the participants were females.
Selected residents were mailed a packet containing a recruitment letter, consent form, and answer responses to be used during the telephone interviews. Trained interviewers called the potential participants and assessed study eligibility. Eligibility criteria included: being 51-75 years old; able to read and speak English; being a resident of one of the 12 counties; having a working telephone number; being average-risk for CRC (having no prior history of CRC, familial/hereditary cancer syndrome, polyps, or inflammatory bowel disease); no contraindication to CRC screening; not pregnant; and being able to provide consent.
If eligible, the telephone interview was completed in approximately 10-15 minutes. After the interview, participants were sent a $10 gift card in appreciation of their time. A participant was sent a medical record review(MRR) form if they reported at least one cancer screening test completed within recommended guidelines (39). The signed medical release forms were sent to the participants' healthcare providers to confirm self-reported dates of completed cancer screening tests.
Measures
Outcomes
After a brief description of each cancer screening test (Pap test, mammography, and CRC screening test: FOBT, flexible sigmoidoscopy, or colonoscopy), participants were asked if they completed each test. If a participant reported completing a screening test, they were asked the date of their last screening test. If participants were unable to remember the test date, they were asked to approximate the date with a categorical response (e.g., less than 10 years ago, more than 10 years ago for colonoscopy).
Participants were classified as being within recommended American Cancer Society screening guidelines (40) for: 1) Breast: mammography was completed in the past year,2) Cervical: a Pap test was completed within the past three years, and 3) CRC: FOBT in the last year; flexible sigmoidoscopy in the last 5 years; or colonoscopy in the last 10 years. Participants had to be within recommended guidelines for mammography, Pap test, and a CRC screening test for them to be classified as “yes” for our primary outcome of being within guidelines for all three tests.
Demographic Characteristics
The information collected included:age, race [White, Black or African American, Asian, Native Hawaiian or Pacific Islander, Native American or Alaska Native, more than one race], marital status [single/never married, married or living as married, divorced, separated, widowed], education [8th grade or less, some high school, high school graduate, some college, associate degree, college graduate, graduate or professional degree], annual household income [<$10,000, $10,000–$19,999, $20,000–$29,999, $30,000–$39,999, $40,000–$49,999, $50,000–$59,999, $60,000–$69,999, >$70,000], employment status [full or part-time, unemployed or disabled, retired or volunteer], and health insurance [Medicaid, Medicare, private].
General Health and Healthcare Utilization
Self-rated health status was measured by a single item on a Likert scale [poor; fair; good; very good; excellent] (41). Having medical conditions requiring the participant to have regular medical visits was documented [yes/no], and if the participant responded “yes,” the medical condition was documented.
Participants were asked if they had a regular health care provider [yes/no], and if the response was “yes,” the name and location of the health care provider were documented. Participants were also asked when they underwent a regular check-up [within the last year; between 1 and 2 years ago; more than 2 years ago; did not remember].
Smoking Behavior
Participants' smoking status was determined using two items (42). Each participant was asked: ‘Have you smoked at least 100 cigarettes in your entire life? [yes/no]. If a participant responded “yes,” they were asked: Do you now smoke cigarettes every day, some days, or not at all? Participants were categorized as never smokers (never smoked at least 100 cigarettes), former smokers (smoked at least 100 cigarettes, but not currently smoking), and current smokers (smoked at least 100 cigarettes and smokes on some or every day).
Patient-Provider Cancer Screening Communication
An item was also included to assess patient-provider communication about all three cancer screening tests. For each test (mammography, Pap test, and CRC tests: FOBT, flexible sigmoidoscopy, and colonoscopy) the participant was asked if a doctor ever asked them to complete the test [yes/no].
Data Analysis
As previously mentioned, a review of the medical record was only conducted if a participant reported being within guidelines for one of the three cancer screening tests. This decision was based on strong evidence that participants who reported not having a CRC screening test had no documentation of the test based on MRR (39).
Review of medical records was missing on 31.1% for Pap tests, 15.9% for mammograms, and 15.1% for CRC screening tests. In addition, there was missing information on 9.4% for income. Fully conditional multiple imputation was used to impute screening test outcomes and income (43). Multiple imputation provides unbiased estimates of covariate effects in regression models when the reason for missingness is related to the observed data, whereas an analysis of just the complete cases may result in substantial bias (44). Among participants who reported a screening test, individuals who had medical record data on all three tests compared to those with missing medical record data were more likely to have private health insurance (p<0.05). Four participants missing age or insurance status (needed for the imputation model) were omitted from analyses; leaving 637 participants. A total of 30 imputed datasets were created.
A backward selection methodology was used to identify correlates of being within guidelines for all three tests. First, a full model was fit for each of the 30 imputations and the results combined using SAS PROC MIANALYZE. Next, the least significant variable was eliminated, and the process was repeated until only variables significant at the 0.1 level remained. Multiple degree of freedom tests were used to determine whether to retain the multilevel categorical variables of income and employment status. All analyses were conducted using SAS v9.3 (SAS Institute, Cary, NC).
Results
Study participants
Demographic characteristics of the 637 female participants are listed in Table 1.Participants had a mean age of 62.8 years, were predominantly non-Hispanic white (95.0%), were married or living as married (75.8%), had some college education (56.2%), had an annual household income less than $60,000 (75.3%), and were mostly retired/volunteers, unemployed, or disabled (61.5%). Almost half (46.0%) of the women reported having private health insurance and the majority self-rated their health as at least good (83.7%). The majority of women reported never being a smoker (57.8%), had a medical condition requiring regular medical visits (71.0%), had a check-up in the past two years (88.9%), and had received a doctor recommendation for all three cancer screening tests (70.6%).
Table 1. Characteristics of female participants (n=637).
| Characteristic | Level | Frequency (%) |
|---|---|---|
| Age (years) | Mean (SD) | 62.8 (6.5) |
| Race/Ethnicity: non-Hispanic White | Yes | 605 (95.0%) |
| No | 32 (5.0%) | |
| Marital status: Married/Living as married | Yes | 483 (75.8%) |
| No | 154 (24.2%) | |
| Education: At least some college | Yes | 358 (56.2%) |
| No | 279 (43.8%) | |
| Annual household income (dollars) | <30,000 | 227 (39.3%) |
| 30,000-60,000 | 208 (36.0%) | |
| >60,000 | 142 (24.6%) | |
| Employment status | Full/Part time | 245 (38.5%) |
| Unemployed/Disabled | 118 (18.5%) | |
| Retired/Volunteer | 274 (43.0%) | |
| Insurance: Private | Yes | 293 (46.0%) |
| No | 344 (54.0%) | |
| Self-rate health: Excellent/very good/good | Yes | 533 (83.7%) |
| No | 104 (16.3%) | |
| Smoking status: Never smoker | Yes | 368 (57.8%) |
| No | 269 (42.2%) | |
| Medical condition requiring regular doctor visits | Yes | 452 (71.0%) |
| No | 185 (29.0%) | |
| Checkup in last two years | Yes | 566 (88.9%) |
| No | 71 (11.1%) | |
| Doctor recommendation for all three cancer screening tests | Yes | 450 (70.6%) |
| No | 187 (29.4%) |
Cancer screening
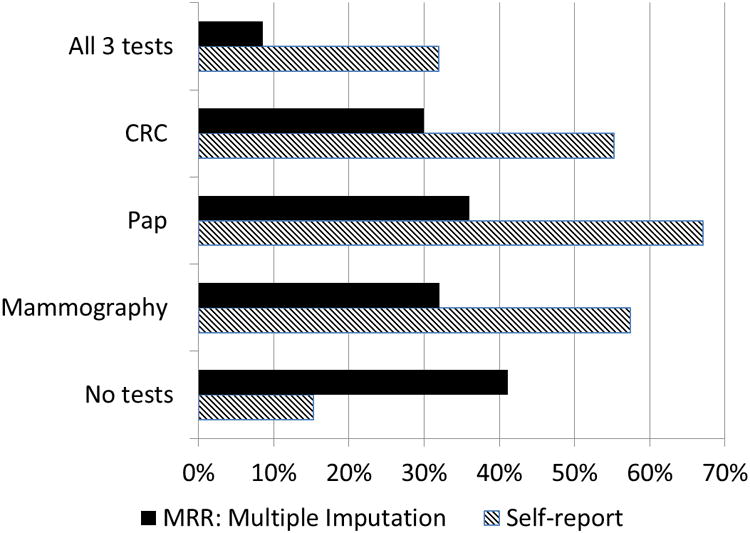
Percentage of women within cancer screening guidelines for all three cancer screening tests, for each test, and for no tests are shown in Figure 1. The average percentage of women within guidelines across 30 imputed data sets was: 36.1% for Pap test, 32.1% for mammography, and 30.1% for a CRC test. Importantly, although almost a third of the women (31.9%) self-reported being within recommended screening guidelines for all three tests, based on MRR with imputation, only 8.6% had completed all three tests within guidelines.
Figure 1. Cancer screening behaviors by self-report and medical record review (MRR; average across imputed datasets) among female participants.
Correlates of cancer screening
Using only the participants with completed MRR, significant predictors of being within guidelines for all three tests included receipt of a provider recommendation for each screening test and having a checkup within the last two years (both p-values <0.01). However, due to small cell counts, these predictors could not be included in the imputation model. After omitting these predictors from consideration (Table 2), participants who had higher annual household incomes ($60,000+ vs. <$30,000; OR=3.53, 95% CI: 1.49, 8.33), and those with a medical condition requiring regular doctor visits (OR=3.16, 95% CI: 1.29, 7.74) were more likely to be within guidelines for all three cancer screening tests. Further, individuals who were retired/volunteers were more likely than those who were unemployed/disabled to be within guidelines for all three cancer screening tests (OR=3.16, 95%CI:1.07,9.33).
Table 2. Final predictive model for being within guidelines for all three cancer screening tests from imputed models (n=637)a.
| Variable | Odds Ratio (95% CI) | |
|---|---|---|
| Annual Household Income (dollars) | <30,000 (referent) | 1.0 |
| 30,000-60,000 | 1.80 (0.78, 4.20) | |
| >60,000 | 3.53 (1.49, 8.33) | |
| Medical condition requiring regular doctor visits | No(referent) | 1.0 |
| Yes | 3.16 (1.29, 7.74) | |
| Employment status | Unemployed/Disabled (referent) | 1.0 |
| Full/Part Time | 1.78 (0.56, 5.65) | |
| Retired/Volunteer | 3.16 (1.07,9.33) | |
Note: Having had a checkup in the last two years and doctor recommendation for all three cancer screening tests were both significant univariately (Fisher's p=0.0088, p<0.0001). However, these variables could not be included in the imputation model due to small cell counts (multivariable model omits these predictors).
Discussion
Residents of Appalachian Ohio continue to have increased cancer disparities that may partially be explained by the lower cancer screening rates within recommended guidelines. This is one of the few studies to address multiple cancer screening tests among an underserved population (14-18), and the first to report findings from a random sample of rural women for each cancer screening test and all three cancer screening tests verified by MRR.
The predictors of being within recommended guidelines for all three cancer screening tests in this study are similar to many studies focused on correlates of individual cancer screening test completion (27-29, 45). Our findings suggest that women within recommended guidelines for all three cancer screening tests were more likely to have had a provider recommendation for each screening test, had a regular check up in the past two years, had a medical condition that required regular medical visits, and had higher annual household incomes. In addition, there was a trend for retired individuals/volunteers to be within guidelines for all three cancer screening tests.
Although the findings in this study are consistent with other studies that have found lower cancer screening rates among Appalachian residents (2, 16, 27, 29), we found that only 8.6% of the women were up to date with all three recommended cancer screening tests, even though 31.9% self-reported being within screening guidelines for all three tests. This finding highlights the importance of medical record verification of cancer screening behaviors reported by study participants and is consistent with previous studies (39, 46-50).
Our findings suggest that there is significant work that remains to improve cancer screening rates among this underserved population. Even though the concept of multiple health behavior change may have benefits (e.g. can address common concerns across cancer screening tests, allow for testing theories across behaviors, potential to reduce office visits, etc.), it also has theoretical, evaluative, and other challenges in research and in practice (34, 51, 52). A few examples of potential challenges to intervening on multiple cancer screening tests include: barriers vary depending on the specific test, the recommended time period for screening intervals vary depending on the test, an individual's cancer risk may vary across different organ sites, individuals may have limited access to resources, and some individuals may become overwhelmed by discussing multiple cancer screening tests with a healthcare provider at the same time. The existence of a one-stop cancer screening program is rare and numerous questions remain about its potential effectiveness, practicality, and cost. Co-variation is a phenomenon that fits with the one-stop shopping model (33). This has been defined as “taking effective action on one behavior increases the odds of taking effective action on a second behavior” (33). There are three forms of co-variation that can occur when an effective action is taken on one treated behavior: 1) increases the odds of effective action on a second treated behavior; 2) is associated with a change in an untreated behavior; or 3) accompanied by change in a second behavior that receives minimal treatment (33). For example, in a previous study evaluating a behavioral intervention study designed to increase mammography use, we found that women also increased Pap test completion (53). An example of co-variation focused on screening barriers is if a patient navigator was successful in assisting an individual with transportation to complete one cancer screening test and then that individual is able to use the same transportation solution to complete a second cancer screening test.
Given that most women (88.9%) in this study reported having a check-up in the last two years and that having a medical condition requiring regular doctor visits was predictive of being within guidelines for all three cancer screening tests, a comprehensive cancer screening intervention based in a clinical setting and tailored for an individual woman's screening barriers may be the logical next step to be tested. Such an integrative and innovative approach to cancer screening may be efficient and successful if the developed intervention focused on specific constructs within established behavioral theories (34). For example, it is well recognized that self-efficacy is a critical construct in several health behavior theories and has been found to be crucial to changing several behaviors (34).
This study has limitations. First, all participants had to have a working telephone to participate in the study. The response rate was 44%. Although a higher rate was attempted by calling individuals multiple times, participation rates for national telephone surveys have also documented a downward trend in individuals' willingness to respond to survey research (54). The participants also had to be age 51-75 and average-risk for CRC to be enrolled in the intervention study. Although this could not be avoided, we do not believe that it takes away from the findings of the current report. We were not able to determine if some women were not within screening guidelines for cervical cancer screening because of having had a hysterectomy, as this information was not collected. We were not able to determine common barriers for completing tests across organ sites since the main study was focused on CRC screening, and we did not collect information on barriers for mammography and Pap test completion. We did not have permission to review medical records from all participants or data was missing from the medical records, thus we imputed missing data. Finally, the participants in this report resided in only 12 counties in Ohio Appalachia. Although these findings do not represent women living in all Appalachian counties, many demographic characteristics of the participants reflect the demographics of the entire Appalachian population. In spite of limitations, this study included a population-based sample from an underserved population with documented cancer disparities. In addition, we verified reported cancer screening behaviors by MRR.
In addition to verifying all screening tests by MRR, future studies evaluating the uptake of cancer screening across multiple organ sites should include an objective risk assessment for each cancer, screening history for each test, motivation to complete each screening test, and a detailed measurement of barriers at different levels (individual, provider, system) for each screening test. Developing interventions for multiple cancer screening tests raises several practice-based issues, such as if all three cancer tests should be addressed simultaneously or sequentially in an intervention, and if screening tests are addressed sequentially, in what order should the tests be addressed so that women would be within recommended screening guidelines for all cancer screening tests.
In conclusion, among women aged 51-75 years living in 12 Appalachia Ohio counties, we found that only 8.6% of the respondents were screened within recommended guidelines for breast, cervical and CRC. This finding helps to explain the higher cancer incidence and mortality rates in this geographic region of the United States. Moreover, the results stress the need for interventions to improve the uptake of multiple cancer screening tests among individuals living in Appalachia. Since women with physician recommendation, higher incomes, or who had a medical condition that required regular medical visits were more likely to be within recommended guidelines for all three cancer screening tests, improving access and utilization of healthcare, and improving physician-patient communication about cancer screening are important strategies to focus on to increase cancer screening rates and reduce cancer disparities among this population.
Acknowledgments
Grant Support: The authors would like to acknowledge support from the following grants: National Institutes of Health: R24MD002785 (to E.D. Paskett), P30 CA016058 (Behavioral Measurement Shared Resource at The Ohio State University Comprehensive Cancer Center), and The Ohio State Clinical and Translational Science Award (NIH/NCRR UL1RR025755).
Footnotes
Disclosure of Potential Conflicts of Interest: There are no conflicts of interest.
References
- 1.Hall HI, Rogers JD, Weir HK, Miller DS, Uhler RJ. Breast and cervical carcinoma mortality among women in the Appalachian region of the U.S., 1976-1996. Cancer. 2000;89:1593–602. doi: 10.1002/1097-0142(20001001)89:7<1593::aid-cncr25>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 2.Hall HI, Uhler RJ, Coughlin SS, Miller DS. Breast and cervical cancer screening among Appalachian women. Cancer Epidemiol Biomarkers Prev. 2002;11:137–42. [PubMed] [Google Scholar]
- 3.Haynes M, Smedley B. The unequal burden of cancer: An assessment of NIH research and programs for ethnic minorities and the medically underserved [Internet] Washington: The National Academies Press; 1999. Available from http://www.nap.edu/catalog/6377/the-unequal-burden-of-cancer-an-assessment-of-nih-research. [PubMed] [Google Scholar]
- 4.Hopenhayn C, King JB, Christian A, Huang B, Christian WJ. Variability of cervical cancer rates across 5 Appalachian states, 1998-2003. Cancer. 2008;113:2974–80. doi: 10.1002/cncr.23749. [DOI] [PubMed] [Google Scholar]
- 5.Horner MJ, Altekruse SF, Zou Z, Wideroff L, Katki HA, Stinchcomb DG. U.S. geographic distribution of prevaccine era cervical cancer screening, incidence, stage, and mortality. Cancer Epidemiol Biomarkers Prev. 2011;20:591–9. doi: 10.1158/1055-9965.EPI-10-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lengerich EJ, Tucker TC, Powell RK, Colsher P, Lehman E, Ward AJ, et al. Cancer incidence in Kentucky, Pennsylvania, and West Virginia: Disparities in Appalachia. J Rural Health. 2005;21:39–47. doi: 10.1111/j.1748-0361.2005.tb00060.x. [DOI] [PubMed] [Google Scholar]
- 7.Paskett ED, Fisher JL, Lengerich EJ, Schoenberg NE, Kennedy SK, Conn ME, et al. Disparities in underserved white populations: The case of cancer-related disparities in Appalachia. Oncologist. 2011;16:1072–81. doi: 10.1634/theoncologist.2011-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wingo PA, Tucker TC, Jamison PM, Martin H, McLaughlin C, Bayakly R, et al. Cancer in Appalachia, 2001-2003. Cancer. 2008;112:181–92. doi: 10.1002/cncr.23132. [DOI] [PubMed] [Google Scholar]
- 9.Yabroff KR, Lawrence WF, King JC, Mangan P, Washington KS, Yi B, et al. Geographic disparities in cervical cancer mortality: What are the roles of risk factor prevalence, screening, and use of recommended treatment? J Rural Health. 2005;21:149–57. doi: 10.1111/j.1748-0361.2005.tb00075.x. [DOI] [PubMed] [Google Scholar]
- 10.Yao N, Lengerich EJ, Hillemeier MM. Breast cancer mortality in Appalachia: Reversing patterns of disparity over time. J Health Care Poor Underserved. 2012;23:715–25. doi: 10.1353/hpu.2012.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warnecke RB, Oh A, Breen N, Gehlert S, Paskett E, Tucker KL, et al. Approaching health disparities from a population perspective: the National Institutes of Health Centers for Population Health and Health Disparities. Am J Public Health. 2008;98:1608–15. doi: 10.2105/AJPH.2006.102525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behringer B, Friedell GH. Appalachia: Where place matters in health. Prev Chronic Dis. 2006 Oct;3(4):A113. Epub 2006 Sep 15. [PMC free article] [PubMed] [Google Scholar]
- 13.Ludke RL, Obermiller PJ, editors. Appalachian health and well-being. Lexington (KY): University Press of Kentucky; 2012. [Google Scholar]
- 14.Guerrero-Preston R, Chan C, Vlahov D, Mitchell MK, Johnson SB, Freeman H. Previous cancer screening behavior as predictor of endoscopic colon cancer screening among women aged 50 and over, in NYC 2002. J Community Health. 2008;33:10–21. doi: 10.1007/s10900-007-9067-3. [DOI] [PubMed] [Google Scholar]
- 15.Reiter PL, Linnan LA. Cancer screening behaviors of African American women enrolled in a community-based cancer prevention trial. J Womens Health. 2011;20:429–38. doi: 10.1089/jwh.2010.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoenberg NE, Studts CR, Hatcher-Keller J, Buelt E, Adams E. Patterns and determinants of breast and cervical cancer non-screening among Appalachian women. Women Health. 2013;53:552–71. doi: 10.1080/03630242.2013.809400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wirth MD, Brandt HM, Dolinger H, Hardin JW, Sharpe PA, Eberth JM. Examining connections between screening for breast, cervical and prostate cancer and colorectal cancer screening. Colorectal Cancer. 2014;3:253–63. doi: 10.2217/crc.14.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlos RC, Fendrick AM, Patterson SK, Bernstein SJ. Associations in breast and colon cancer screening behavior in women. Acad Radiol. 2005;12:451–8. doi: 10.1016/j.acra.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 19.Mayer-Oakes SA, Atchison KA, Matthias RE, De Jong FJ, Lubben J, Schweitzer SO. Mammography use in older women with regular physicians: What are the predictors? Am J Prev Med. 1996;12:44–50. [PubMed] [Google Scholar]
- 20.Shapiro JA, Seeff LC, Nadel MR. Colorectal cancer-screening tests and associated health behaviors. Am J Prev Med. 2001;21:132–7. doi: 10.1016/s0749-3797(01)00329-4. [DOI] [PubMed] [Google Scholar]
- 21.Gillman MW, Pinto BM, Tennstedt S, Glanz K, Marcus B, Friedman RH. Relationships of physical activity with dietary behaviors among adults. Prev Med. 2001;32:295–301. doi: 10.1006/pmed.2000.0812. [DOI] [PubMed] [Google Scholar]
- 22.Ussher MH, Taylor A, Faulkner G. Exercise interventions for smoking cessation. Cochrane Database Syst Rev. 2012 Jan 18;1:CD002295. doi: 10.1002/14651858.CD002295.pub4. [DOI] [PubMed] [Google Scholar]
- 23.Emmons KM, McBride CM, Puleo E, Pollak KI, Clipp E, Kuntz K, et al. Project PREVENT: A randomized trial to reduce multiple behavioral risk factors for colon cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1453–9. doi: 10.1158/1055-9965.EPI-04-0620. [DOI] [PubMed] [Google Scholar]
- 24.Champion VL, Skinner CS. The Health Belief Model. In: Glanz K, Rimer BK, Viswanath K, editors. Health Behavior and Health Education: Theory, Research, and Practice. 4th. San Francisco: Jossey-Bass; 2008. pp. 45–65. [Google Scholar]
- 25.Prochaska JO, Redding CA, Evers KE. The Transtheoretical Model and Stages of Change. In: Glanz K, Rimer BK, Viswanath K, editors. Health Behavior and Health Education: Theory, Research, and Practice. 4th. San Francisco: Jossey-Bass; 2008. pp. 97–121. [Google Scholar]
- 26.McAlister AL, Perry CL, Parcel GS. How Individuals, Environments, and Health Behaviors Interact: Social Cognitive Theory. In: Glanz K, Rimer BK, Viswanath K, editors. Health Behavior and Health Education: Theory, Research, and Practice. 4th. San Francisco: Jossey-Bass; 2008. pp. 169–188. [Google Scholar]
- 27.Paskett ED, Llanos AA, Young GS, Pennell ML, Lee CJ, Katz ML. Correlates of colorectal cancer screening among residents of Ohio Appalachia. J Community Health. 2013;38:609–18. doi: 10.1007/s10900-013-9683-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Studts CR, Tarasenko YN, Schoenberg NE. Barriers to cervical cancer screening among middle-aged and older rural Appalachian women. J Community Health. 2013;38:500–12. doi: 10.1007/s10900-012-9639-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amonkar MM, Madhavan S. Compliance rates and predictors of cancer screening recommendations among Appalachian women. J Health Care Poor Underserved. 2002;13:443–60. doi: 10.1353/hpu.2010.0582. [DOI] [PubMed] [Google Scholar]
- 30.Eheman CR, Leadbetter S, Benard VB, Blythe Ryerson A, Royalty JE, Blackman D, et al. National breast and cervical cancer early detection program data validation project. Cancer. 2014 Aug 15;120(Suppl 16):2597–603. doi: 10.1002/cncr.28825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown ML, Klabunde CN, Cronin KA, White MC, Richardson LC, McNeel TS. Challenges in meeting healthy people 2020 objectives for cancer-related preventive services, national health interview survey, 2008 and 2010. Prev Chronic Dis. 2014 Feb 27;11:E29. doi: 10.5888/pcd11.130174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edwards JB, Tudiver F. Women's preventive screening in rural health clinics. Womens Health Issues. 2008;18:155–66. doi: 10.1016/j.whi.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Prochaska JO. Multiple health behavior research represents the future of preventive medicine. Prev Med. 2008;46:281–5. doi: 10.1016/j.ypmed.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 34.Noar SM, Chabot M, Zimmerman RS. Applying health behavior theory to multiple behavior change: Considerations and approaches. Prev Med. 2008;46:275–80. doi: 10.1016/j.ypmed.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Beaber EF, Kim JJ, Schapira MM, Tosteson AN, Zauber AG, Geiger AM, et al. Unifying screening processes within the PROSPR consortium: a conceptual model for breast, cervical, and colorectal cancer screening. J Natl Cancer Inst. 2015 May 7;107(6):djv120. doi: 10.1093/jnci/djv120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katz ML, Keller B, Tatum CM, Fickle DK, Midkiff C, Carver S, et al. Progress in Community Health Partnerships: Research, Education, and Action Forthcoming. 2015. Community members' input into cancer prevention campaign development and experience being featured in the campaign. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krok-Schoen J, Katz ML, Oliveri J, Young G, Pennell M, Reiter P, et al. A media and clinic intervention to increase colorectal cancer screening in Ohio Appalachia. 2015 doi: 10.1155/2015/943152. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.United States Census Bureau [Internet] Census 2000 Gateway. Available from: http://www.census.gov/main/www/cen2000.html.
- 39.Reiter PL, Katz ML, Oliveri JM, Young GS, Llanos AA, Paskett ED. Validation of self-reported colorectal cancer screening behaviors among Appalachian residents. Public Health Nurs. 2013;30:312–22. doi: 10.1111/phn.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith RA, Manassaram-Baptiste D, Brooks D, Cokkinides V, Doroshenk M, Saslow D, et al. Cancer screening in the United States, 2014: A review of current American cancer society guidelines and current issues in cancer screening. CA Cancer J Clin. 2014;64:30–51. doi: 10.3322/caac.21212. [DOI] [PubMed] [Google Scholar]
- 41.Mossey JM, Shapiro E. Self-rated health: A predictor of mortality among the elderly. Am J Public Health. 1982;72:800–8. doi: 10.2105/ajph.72.8.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Centers for Disease Control and Prevention [Internet] National health and nutrition examination survey. Sample personal questionnaire: Smoking and tobacco use; 2011-2012. Available from: http://www.cdc.gov/nchs/nhanes.htm.
- 43.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16:219–42. doi: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 44.Ibrahim JG, Chu H, Chen MH. Missing data in clinical studies: Issues and methods. J Clin Oncol. 2012;30:3297–303. doi: 10.1200/JCO.2011.38.7589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schoenberg NE, Kruger TM, Bardach S, Howell BM. Appalachian women's perspectives on breast and cervical cancer screening. Rural Remote Health. 2013;13:2452. [PMC free article] [PubMed] [Google Scholar]
- 46.Madlensky L, McLaughlin J, Goel V. A comparison of self-reported colorectal cancer screening with medical records. Cancer Epidemiol Biomarkers Prev. 2003;12:656–9. [PubMed] [Google Scholar]
- 47.Shokar NK, Vernon SW, Carlson CA. Validity of self-reported colorectal cancer test use in different racial/ethnic groups. Fam Pract. 2011;28:683–8. doi: 10.1093/fampra/cmr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Allgood KL, Rauscher GH, Whitman S, Vasquez-Jones G, Shah AM. Validating self-reported mammography use in vulnerable communities: Findings and recommendations. Cancer Epidemiol Biomarkers Prev. 2014;23:1649–58. doi: 10.1158/1055-9965.EPI-13-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cronin KA, Miglioretti DL, Krapcho M, Yu B, Geller BM, Carney PA, et al. Bias associated with self-report of prior screening mammography. Cancer Epidemiol Biomarkers Prev. 2009;18:1699–705. doi: 10.1158/1055-9965.EPI-09-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Howard M, Agarwal G, Lytwyn A. Accuracy of self-reports of pap and mammography screening compared to medical record: A meta-analysis. Cancer Causes Control. 2009;20:1–13. doi: 10.1007/s10552-008-9228-4. [DOI] [PubMed] [Google Scholar]
- 51.Prochaska JJ, Nigg CR, Spring B, Velicer WF, Prochaska JO. The benefits and challenges of multiple health behavior change in research and in practice. Prev Med. 2010;50:26–9. doi: 10.1016/j.ypmed.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prochaska JJ, Velicer WF, Nigg CR, Prochaska JO. Methods of quantifying change in multiple risk factor interventions. Prev Med. 2008;46:260–5. doi: 10.1016/j.ypmed.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Katz ML, Tatum CM, Degraffinreid CR, Dickinson S, Paskett ED. Do cervical cancer screening rates increase in association with an intervention designed to increase mammography usage? J Womens Health. 2007;16:24–35. doi: 10.1089/jwh.2006.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Galea S, Tracy M. Participation rates in epidemiologic studies. Ann Epidemiol. 2007;17:643–53. doi: 10.1016/j.annepidem.2007.03.013. [DOI] [PubMed] [Google Scholar]


