Abstract
Objective
Although there is increasing reliance on patient reported outcomes (PROs) for disease management, there is little known about the differences in PROs across juvenile idiopathic arthritis (JIA) categories. The purpose of the study is to assess PROs across JIA categories, including pain, quality of life, and physical function and to determine clinical factors associated with differences in these measures across categories.
Methods
This was a longitudinal cohort study of JIA patients at a tertiary care pediatric rheumatology clinic. Subjects, PROs, and clinical variables were identified by querying the electronic medical record. Mixed effects regression assessed pain, quality of life, and function.
Results
Subjects with enthesitis-related arthritis (ERA) and undifferentiated JIA had significantly more pain, poorer quality of life, and poorer physical function. The ERA and undifferentiated JIA categories, physician global disease activity assessment, female sex, and non-steroidal anti-inflammatory (NSAID) use were significantly associated with more pain, poorer quality of life, and poorer function. In models limited to ERA, female sex and tender enthesis count were significant predictors of decreased function.
Conclusion
ERA and undifferentiated JIA categories had poorer PROs than other JIA categories. Future studies should evaluate the impact of changes in PROs for different therapeutic algorithms.
Key Indexing Terms: juvenile arthritis, patient outcome assessment, pediatric, rheumatology, quality of life, pain
Introduction
Patient reported outcomes (PROs) provide insight to knowledge only known to the patient. The ability to open jars and tie shoelaces, the patient’s perception of limitations from disease, and the overall disease burden are some of the information that can be collected using PROs. Surveying the PROs of pain, quality of life, and physical function are being increasingly used in research and have rising importance in the clinical management of pediatric rheumatologic conditions (1). This is emphasized by the Food and Drug Administration to use PROs for medical product labeling, by the United States Affordable Care Act to create the Patient Centered Outcomes Research Institute (PCORI), and by the National Institute of Health development of the Patient Reported Outcomes Measurement Information System (PROMIS). It is not uncommon for discordance between patient- and physician- reported outcomes (2–5). This is not surprising as physicians and patients and their families conceptualize disease differently and value somewhat different outcomes. Thus, full evaluation of disease activity and treatment goals should include both physician assessment and parent or child reports. Despite this mounting importance, there is minimal literature on PROs such as pain, quality of life, and physical function in across categories of juvenile idiopathic arthritis.
Juvenile idiopathic arthritis (JIA) is a group of related chronic inflammatory arthritic conditions diagnosed in children less than 16 years of age. There are seven categories of JIA defined by the International League of Associations for Rheumatology (ILAR): oligoarticular, systemic, polyarticular rheumatoid factor (RF) positive, polyarticular RF negative, psoriatic, enthesitis related, and undifferentiated arthritis (6). Although JIA is a group of heterogeneous conditions, many of the categories are combined for both clinical treatment guidelines (7) as well as clinical trials (8, 9). In particular, those patients with polyarticular disease are often considered as a group regardless of distinguishing features such as the presence of rheumatoid factor, psoriasis, enthesitis, or sacroiliitis. Therefore, amongst these JIA categories differential PRO response patterns have not been systematically evaluated.
Investigation of associated clinical characteristics with PROs are not well described across JIA categories. Prior work leveraging national cross-sectional registry data from The Childhood Arthritis and Rheumatology Research Alliance (CARRA) as well as several small single-center study demonstrated that children with enthesitis-related arthritis have more pain and lower physical function than other JIA subtypes (10–12). However, all of these studies included limited patient-level factors.
This study utilizes a longitudinal cohort design to evaluate PROs. To our knowledge, this is one of the largest studies evaluating PROs and associated patient-level clinical factors (10, 13). Further understanding of PROs by leveraging information only known to the patient and the variables that influence these outcomes can allow for better understanding of JIA and allow improved disease management. The purpose of the study is 1) to characterize PROs, including pain, quality of life, and physical function in a sample of children with JIA; and 2) to determine clinical factors associated with increased pain, decreased quality of life, and decreased physical function.
Materials and Methods
Human subjects protections
The protocol for the conduct of this study was approved by the Committee for the Protection of Human Subjects at the Children’s Hospital of Philadelphia.
Study design and subjects
This is a retrospective longitudinal cohort study. Subjects were included if they were evaluated at our single tertiary pediatric rheumatology clinic between June 2010 and December 2012, had a diagnosis of JIA according to ILAR criteria (6), and had less than three years disease duration. All available visits for subjects were included in the analysis. Potential subjects were identified by querying the electronic medical record for patients with a diagnosis of JIA as indicated by the ICD-9-CM codes 696.x, 714.xx, and 720.x. All JIA diagnoses and category assignment were confirmed using the JIA calculator software package (14).
PROs
Pain, quality of life, and physical function were assessed at each clinic visit as part of routine clinical care. All patients were provided with paper questionnaires to complete upon entering clinic. These paper questionnaires are reviewed by the clinician, scored, and entered into our electronic medical record. Pain over the past week was assessed with the question, “How do you rate your/your child’s pain due to his/her illness in the past week?” with an ordinal numeric rating scale. This 11-point pain scale ranges from 0 to 10 with “no pain” listed at 0 and “very severe pain” listed at 10 (15–17). Studies have shown the ordinal scale is comparable to the 10-cm linear scale (15). Quality of life was evaluated using the Pediatric Rheumatology Quality of Life scale (PRQL) (18). This 10 question tool assesses quality of life over the past 4 weeks and includes domains of physical health and psychological health and has been validated for use in children with JIA in the United States (19). Scores range from 0 to 30 with a higher scores indicating worse quality of life. Physical function was estimated with the Childhood Health Assessment Questionnaire (CHAQ); this two-page, 30 question survey assesses the 8 domains of dressing and grooming, arising, eating, walking, hygiene, reach, grip, and activities (20). CHAQ scores range from 0 to 3 with a higher score indicating worse function. Parents rated health status by answering the question, “Considering all the ways that your rheumatic condition affects you/your child, how do you rate how he/she is doing in the past week?” using an 11-point ordinal numeric rating scale.
Physician reported global disease activity assessment
Physician global disease activity assessment was reported by the physician at the end of each clinic visit as part of routine clinical care using an 11-point ordinal numeric rating scale ranging from 0 to 10 with 0 corresponding to “very well” and 10 corresponding to “very poor”.
Clinical disease data collection
Demographics (sex, age at diagnosis, ANA, RF, HLA B27 status) were abstracted from the electronic medical record. Clinical disease elements (active joint count, tender enthesis count) and current medication use at each visit were also abstracted from the electronic medical record. Intraarticular glucocorticoid exposure was carried forward by one visit to account for exposure at the prior visit.
Statistical analysis
Differences in baseline demographics and clinical characteristics were evaluated using the Kruskal-Wallis equality of populations rank test or chi-square test as appropriate. Median and interquartile range (IQR) or mean and 95% confidence interval (CI) were used as appropriate. Less than 25% of PROs and less than 15% of physician reported outcomes were missing in our data set. All clinic variables, including age, disease duration, medication exposure, and physical examination findings were complete.
Model development
We tested the association of clinical attributes with pain over time using mixed-effects ordinal logistic regression (proportional odds) with random intercepts. Quality of life (PRQL) and physical function (CHAQ) were evaluated over time using mixed-effects linear regression with random intercepts. Variables with p<0.20 in univariate analysis were included in multivariate analysis. Variables in multivariate models were considered statistically significant if p<0.05. Subject was a random effect in all models. Fixed effects examined for all PRO outcomes in univariate analysis included: JIA category, physician global disease activity assessment, disease duration (in years), subject age, female sex, active joint count, non-steroidal anti-inflammatory (NSAID), systemic glucocorticoid, disease-modifying anti-rheumatic drug (DMARD), biologic, and intraarticular glucocorticoid use. For examination of significant features associated with pain, quality of life, and function within the ERA and undifferentiated JIA categories, fixed effects tested in univariate analysis included: physician global disease activity assessment, disease duration (in years), subject age, female sex, active joint count, tender enthesis count, sacroiliac tenderness, HLA B27 positivity (ERA only), NSAID, systemic glucocorticoid, DMARD, biologic, and intraarticular glucocorticoid use.
Severity of pain was classified into mild (>0 to <3.5), moderate (>3.5 to <6.0), and severe (>6.0) (21). Severity of decreased function was classified into mild (>0 to <0.13), moderate (>0.13 to <1.75), and severe (>1.75) (22).
Correlation of physician rating with PROs
Correlation between physician global disease activity assessment with patient/parent rating of pain, quality of life, physician function, and global activity assessment were performed using Spearman rank correlation coefficient. Spearman rho coefficient of <0.4, >0.4 to <0.7, and >0.7 indicated poor, moderate, and high correlation (23). All analyses were performed using Stata 13.1 (StataCorp, College Station, Texas).
Results
Patients
During the study period there were 398 patients evaluated at 1577 visits. 152 (38%) of patients had newly-diagnosed JIA. Age and sex distribution within JIA categories were in accordance with previously published JIA cohorts (Table 1). As expected, oligoarticular JIA was the most prevalent JIA category, followed by ERA and polyarticular RF-negative. There were significant differences in pain, quality of life, and function across JIA categories (all p<0.01). Physician assessment of disease trended towards significance (p=0.06) and patient assessment of disease activity was significantly different across categories (p<0.01).
Table 1.
Baseline patient characteristics across JIA categories in 398 JIA subjects.
Baseline characteristics of children with new and prevalent JIA diagnoses who are included in the study. Median (IQR) are reported unless otherwise stated.
| Oligo | Systemic | Poly RF+ | Poly RF− | Psoriatic | ERA | Undiff | p-value | |
|---|---|---|---|---|---|---|---|---|
| N (%) | 147 (37) | 30 (8) | 12 (3) | 59 (14) | 27 (7) | 92 (23) | 31 (8) | -- |
| Age (years) | 5.9 (2.7, 11.2) | 6.9 (3.1, 13.1) | 13.3 (10.1, 15.8) | 7.1 (3.0, 10.7) | 11.5 (6.3, 14.0) | 13.2 (10.7, 15.4) | 10.8 (7.9, 13.3) | <0.01 |
| Female# | 109 (75) | 15 (50) | 12 (100) | 48 (81) | 20 (75) | 36 (39) | 21 (68) | <0.01 |
| AJC | 0 (0, 2) | 0 (0, 1) | 4 (0, 8) | 0 (0, 8) | 0 (0, 3) | 0 (0, 1) | 0 (0, 1) | <0.01 |
| Duration (years) | 0.2 (0, 1.1) | 0.4 (0, 1.9) | 0.4 (0, 2.2) | 0.3 (0, 1.2) | 0.1 (0, 2.1) | 0.1 (0, 0.9) | 0 (0, 1.7) | 0.46 |
| Physician disease activity | 0 (0, 2) | 0 (0, 2) | 0 (0, 2) | 1 (0, 2) | 1 (0, 2) | 1 (0, 2) | 0 (0, 2) | <0.01 |
| Patient/parent disease activity | 0 (0, 2) | 0 (0, 2) | 0 (0, 2) | 1 (0, 2) | 1 (0, 3) | 1 (0, 4) | 1 (0, 5) | <0.01 |
N (%) are reported.
Oligo = oligoarticular. Poly RF+ = polyarticular rheumatoid factor positive. Poly RF− = polyarticular rheumatoid factor negative. ERA = enthesitis-related arthritis. Undiff = undifferentiated. AJC = active joint count.
Pain
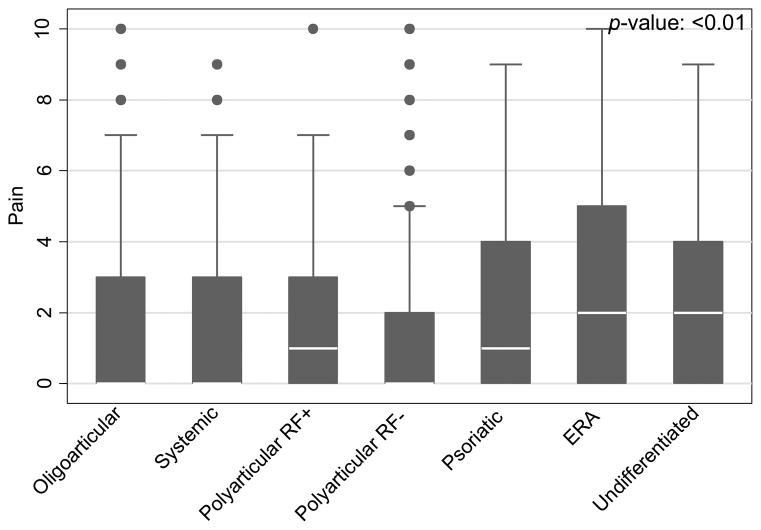
Pain prevalence and pain intensity significantly varied across JIA categories (p<0.01). Patients with ERA and undifferentiated JIA reported a significantly higher prevalence of pain, and patients with ERA, psoriatic, and undifferentiated arthritis report moderate and severe pain more frequently than other subtypes (p<0.01). Median pain intensity over the past week was highest in ERA (2; IQR 0, 5) and undifferentiated (2; IQR 0, 4) and was lowest in oligoarticular and systemic (both 0; IQR 0, 3) and polyarticular RF-negative (0; IQR 0, 2) (Figure 1). Statistically significant predictors of pain in a multivariate model included ERA (β: 1.30; 95% CI: 0.72, 1.89) and undifferentiated JIA (β: 1.06; 95% CI: 0.32, 1.80), physician global disease activity assessment (β: 0.64; 95% CI: 0.54, 0.74), female sex (β: 0.85; 95% CI: 0.41, 1.28), NSAID (β: 0.58; 95% CI: 0.28, 0.88), and intraarticular glucocorticoid use (β: 0.58; 95% CI: 0.10, 1.07) (Table 2). There was no significant interaction between biologic and DMARD exposure (p=0.13) and no significant association of biologic or DMARD exposure and pain.
Figure 1.
Box plot of pain scores across JIA categories. RF = rheumatoid factor. ERA = enthesitis related arthritis. Number of visits for oligoarticular: 451; systemic: 91; polyarticular rheumatoid factor positive (RF+): 42; polyarticular rheumatoid factor negative (RF−): 213; psoriatic: 80; enthesitis-related arthritis (ERA): 257; undifferentiated 92.
Table 2.
Factors associated with pain, quality of life, and physical function across JIA categories.
Multivariate analysis of clinical variables associated with patient reported outcomes across JIA categories.
| Variable | Pain β (95% CI) |
Quality of Life β (95% CI) |
Physical Function β (95% CI) |
|---|---|---|---|
| JIA category^ | |||
| Systemic | 0.56 (−0.22, 1.34) | 1.12 (−0.13, 2.37) | 0.14 (−0.01, 0.27) |
| Polyarticular RF+ | 0.51 (−0.57, 1.60) | −0.22 (−2.03, 1.59) | 0.10 (−0.10, 0.30) |
| Polyarticular RF− | −0.11 (−0.73, 0.50) | 0.16 (−0.79, 1.11) | 0.08 (−0.02, 0.19) |
| Psoriatic | 0.49 (−0.30, 1.27) | 0.79 (−0.47, 2.05) | 0.02 (−0.12, 0.16) |
| ERA | 1.30 (0.72, 1.89)* | 1.81 (0.89, 2.74)* | 0.15 (0.06, 0.24)* |
| Undifferentiated | 1.06 (0.32, 1.80)* | 2.61 (1.43, 3.79)* | 0.15 (0.02, 0.28)* |
| Physician disease activity | 0.64 (0.54, 0.74)* | 0.60 (0.46, 0.73)* | 0.06 (0.04, 0.07)* |
| Disease duration, years | −0.11 (−0.29, 0.07) | −0.16 (−0.41, 0.10) | −0.01 (−0.03, 0.02) |
| Older age, each year | 0.02 (−0.02, 0.06) | 0.01 (−0.06, 0.07) | -- |
| Female | 0.85 (0.41, 1.28)* | 1.33 (0.66, 2.01)* | 0.10 (0.03, 0.18)* |
| Active joint count | 0.03 (−0.04, 0.10) | 0.14 (0.05, 0.22)* | 0.01 (0.01, 0.02)* |
| NSAIDs | 0.58 (0.28, 0.88)* | 0.72 (0.31, 1.13)* | 0.07 (0.03, 0.11)* |
| Systemic glucocorticoids | -- | 0.04 (−0.69, 0.77) | −0.01 (−0.09, 0.07) |
| DMARDs | −0.22 (−0.55, 0.10) | 0.01 (−0.45, 0.48) | −0.01 (−0.06, 0.04) |
| Biologics | 0.08 (−0.27, 0.42) | −0.32 (−0.81, 0.16) | −0.02 (−0.07, 0.03) |
| Intraarticular glucocorticoids | 0.58 (0.10, 1.07)* | −0.27 (−0.95, 0.41) | 0.07 (−0.01, 0.14) |
For JIA categories, oligoarticular JIA is the reference standard.
p<0.05.
Since ERA and undifferentiated JIA categories had significantly greater prevalence and intensity of pain, we investigated additional clinical factors associated with pain within these categories. Clinical attributes significantly associated with increased pain in children with ERA in a multivariate model included physician global disease activity assessment (β: 0.68; 95% CI: 0.46, 0.89), female sex (β: 1.42; 95% CI: 0.69, 2.15), and tender enthesis count (β: 0.16; 95% CI: 0.05, 0.28). Longer disease duration (β: −0.53; 95% CI: −0.91, −0.15) and HLA B27 positivity (β: −0.98; 95% CI: −1.67, −0.30) were inversely associated with pain (Table 3). Physician global disease activity assessment was the only variable significantly associated with pain in undifferentiated JIA (β: 0.86; 95% CI: 0.43, 1.28) (Table 4).
Table 3.
Factors associated with pain, quality of life, and physical function in children with enthesitis-related arthritis.
Multivariate analysis of factors associated with patient reported outcomes in children with enthesitis-related arthritis.
| Variable | Pain β (95% CI) |
Quality of Life β (95% CI) |
Physical Function β (95% CI) |
|---|---|---|---|
| Physician disease activity | 0.68 (0.46, 0.89)* | 0.44 (0.15, 0.74)* | 0.01 (−0.02, 0.03) |
| Disease duration, years | −0.53 (−0.91, −0.15)* | −0.70 (−1.34, −0.07)* | −0.02 (−0.08, 0.04) |
| Female | 1.42 (0.69, 2.15)* | 1.96 (0.47, 3.44)* | 0.28 (0.10, 0.45)* |
| Active joint count | −0.03 (−0.15, 0.10) | -- | 0.01 (−0.01, 0.02) |
| Tender enthesis count | 0.16 (0.05, 0.28)* | 0.28 (0.08, 0.48)* | 0.06 (0.04, 0.08)* |
| Sacroiliac tenderness | 0.18 (−0.55, 0.90) | 0.84 (−0.65, 2.33) | 0.04 (−0.14, 0.21) |
| HLA B27+ | −0.98 (−1.67, −0.30)* | -- | −0.06 (−0.23, 0.11) |
| NSAIDs | −0.13 (−0.70, 0.43) | -- | -- |
| Systemic glucocorticoids | 0.02 (−1.03, 1.06) | -- | 0.16 (0.01, 0.31)* |
| Biologics | 0.17 (−0.49, 0.82) | -- | −0.01 (−0.11, 0.10) |
p<0.05.
Table 4.
Factors associated with pain, quality of life, and physical function in children with undifferentiated arthritis.
Multivariate analysis of factors associated with patient reported outcomes in children with undifferentiated JIA.
| Variable | Pain β (95% CI) |
Quality of Life β (95% CI) |
Physical Function β (95% CI) |
|---|---|---|---|
| Physician disease activity | 0.86 (0.43, 1.28)* | 1.07 (0.36, 1.78)* | 0.05 (0.01, 0.10)* |
| Female | 1.74 (−0.06, 3.54) | 4.56 (1.96, 7.17)* | 0.31 (0.05, 0.57)* |
| Active joint count | 0.17 (−0.33, 0.66) | 0.44 (−0.46, 1.35) | 0.09 (0.03, 0.14)* |
| NSAIDs | 1.13 (−0.19, 2.44) | 3.27 (1.23, 5.31)* | 0.22 (0.07, 0.37)* |
| Systemic glucocorticoids | -- | 0.24 (−3.41, 3.89) | 0.01 (−0.23, 0.24) |
| DMARDs | -- | 0.97 (−1.09, 3.04) | -- |
| Biologics | -- | -- | −0.10 (−0.28, 0.07) |
p<0.05.
Quality of life
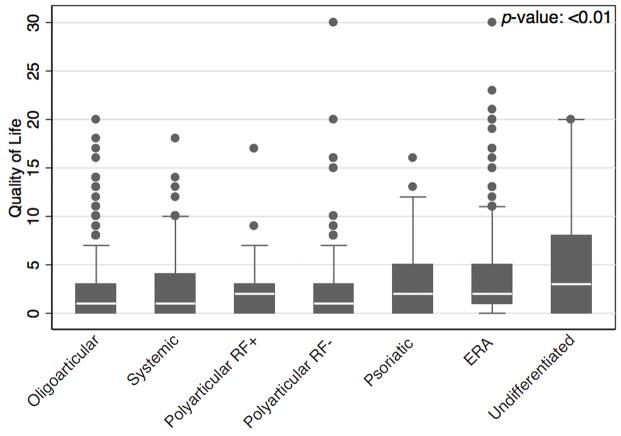
Children with ERA and psoriatic arthritis had a higher prevalence of decreased quality of life within the past 4 weeks (p<0.01) and undifferentiated JIA reports the highest median value of decreased quality of life (Figure 2). Variables associated with decreased quality of life in multivariate analysis included ERA (β: 1.81; 95% CI: 0.89, 2.74), undifferentiated JIA (β: 2.61; 95% CI: 1.43, 3.79), physician global disease activity assessment (β: 0.60; 95% CI: 0.46, 0.73), female sex (β: 1.33; 95% CI: 0.66, 2.01), active joint count (β: 0.14; 95% CI: 0.05, 0.22) and NSAID use (β: 0.72; 95% CI: 0.31, 1.13) (Table 2). There was no significant interaction between biologic and DMARD exposure (p=0.64) and no significant association of biologic exposure and quality of life.
Figure 2.
Box plot of quality of life values across JIA categories. Number of visits for oligoarticular: 449; systemic: 89; polyarticular rheumatoid factor positive (RF+): 43; polyarticular rheumatoid factor negative (RF−): 220; psoriatic: 81; enthesitis-related arthritis (ERA): 250; undifferentiated 94.
ERA and undifferentiated categories were associated with higher prevalence and more impaired quality of life than other JIA categories. In multivariate analysis of children with ERA, significant predictors of decreased quality of life included physician global disease activity assessment (β: 0.44; 95% CI: 0.15, 0.74), disease duration (β: −0.70; 95% CI: −1.34, −0.07), female sex (β: 1.96; 95% CI: 0.47, 3.44), and tender enthesis count (β: 0.28; 95% CI: 0.08, 0.48) (Table 3). For children with undifferentiated JIA, significant predictors of decrease quality of life were physician global disease activity assessment (β: 1.07; 95% CI: 0.36, 1.78), female sex (β: 4.56; 95% CI: 1.96, 7.17), and NSAID use (β: 3.27; 95% CI: 1.23, 5.31) (Table 4).
Physical function
Children with polyarticular RF positive, polyarticular RF negative, and ERA reported the highest prevalence of decreased physical function. Polyarticular RF positive, ERA and undifferentiated JIA reported moderate and severe impairment in function more commonly than other JIA categories (p<0.01). In multivariate analysis factors associated with decreased function in all patients with JIA included ERA (β: 0.15; 95% CI: 0.06, 0.24), undifferentiated JIA (β: 0.15; 95% CI: 0.02, 0.28), physician global disease assessment (β: 0.06; 95% CI: 0.04, 0.07), female sex (β: 0.10; 95% CI: 0.03, 0.18), active joint count (β: 0.01; 95% CI: 0.01, 0.02), and NSAID use (β: 0.07; 95% CI: 0.03, 0.11) (Table 2). There was no significant interaction between biologic and DMARD exposure (p=0.66) and no significant association of biologic exposure and function.
ERA and undifferentiated JIA reported higher intensity of decreased physical function than other JIA categories. In multivariate analysis of children with ERA, significant factors associated with decreased physical function included: female sex (β: 0.28; 95% CI: 0.10, 0.45), tender enthesis count (β: 0.06; 95% CI: 0.04, 0.08), and systemic glucocorticoid use (β: 0.16; 95% CI: 0.01, 0.31) (Table 3). For children with undifferentiated JIA, significant factors associated with decreased function were: physician global disease activity assessment (β: 0.05; 95% CI: 0.01, 0.10), female sex (β: 0.31; 95% CI: 0.05, 0.57), active joint count (β: 0.09; 95% CI: 0.03, 0.14), and NSAID use (β: 0.22; 95% CI: 0.07, 0.37) (Table 4).
Correlation of physician rating with PROs
The Spearman correlation for physician global disease activity assessment with parent/patient reported pain, quality of life, physical function, and health status were moderate (0.53, 0.40, 0.43, and 0.53, respectively with all p<0.01).
Discussion
This is one of the largest studies to evaluate pain, quality of life, and function over time across JIA categories. We found statistically significant differences in PROs across JIA categories, notably children with ERA and undifferentiated JIA consistently reported the poorest PROs. Children with ERA and undifferentiated JIA had significantly more pain, worse quality of life, and poorer function than children in the other JIA categories. Physician overall disease activity assessment only moderately mirrored PROs. Interestingly, in this cohort, physicians gave the highest disease activity scores to children with rheumatoid factor negative polyarticular disease, psoriatic arthritis, and ERA. This discordance highlights that disease manifestations and the relevance of PROs to overall disease activity assessment are contemplated and valued differently by patients and their parents and physicians.
These results need to be interpreted in light of several limitations. This is a retrospective study so data collection was not complete as it would be with a prospective trial. While PROs at our institution are routinely collected as part of clinical care there was some missing data. However, less than 25% of PROs and less than 15% of physician reported outcomes were missing in our data set. Spectrum and/or selection bias are possible as our results are from a single-center tertiary care medical center. However, our rheumatologists treat patients with wide variation in symptoms and severity of disease, and thus our patients should represent the full breath of cases that present across other institutions.
There are several interesting findings from this study that warrant further discussion. First, the children that had poorer PROs (pain, quality of life, and function) were those in the JIA categories under the broader umbrella term juvenile spondyloarthritis (SpA). The term SpA primarily encompasses the ERA, psoriatic, and undifferentiated JIA categories but also includes juvenile ankylosing spondylitis, reactive arthritis, and inflammatory bowel disease associated arthritis. Distinct features of SpA in comparison to other JIA categories include enthesitis, axial disease, skin, and nail findings. Interestingly, some of these unique attributes were significant or trended towards being significantly associated with pain in the restricted analyses of PROs for these categories. While not statistically significant, psoriatic arthritis also trended to having increased pain and decreased quality of life. These results suggest that some of our treatment algorithms or medications themselves may not be as effective for these disease manifestations and specifically despite advent of newer pharmacotherapies patients with ERA continue to report worse PROs (12). Indeed, several studies in adults with SpA have shown that enthesitis and axial disease may not respond as well as arthritis to traditional DMARD and/or biologic therapy (24, 25).
Second, our results are in accordance with prior studies that suggest altered pain perception in adults and children with SpA (26). Recent work has shown that children with ERA have lower thresholds of pain at the enthesis than healthy children (10). This altered perception occurred at enthesis with and without underlying ultrasound-confirmed tendinopathy or increased Doppler blood flow. However, it is unknown if these altered pain perceptions are unique to SpA or whether they exist across all JIA categories (27, 28). Although children with JIA can have more pain than healthy children, it is unknown if this is primarily from their disease or secondary to psychological factors and deconditioning from having a chronic disease (29).
Third, while we included as many clinical variables as available in our medical charts, our models could not perfectly predict the outcomes of pain, quality of life, and physical function. This highlights that these constructs are very complex and despite having access to qualitative and quantitative variables, there are still other unidentified factors that likely contribute to pain, quality of life, and physical function. The presence of fatigue and amplified musculoskeletal pain have been reported to influence PROs (30, 31), and this was not systematically recorded in our cohort. Another study suggests that patients place emphasis on being like their peers without a chronic condition and emphasize the psychosocial aspect of living with JIA (32), and these domains were not fully captured by our PROs. Similarly, we do not have measures of baseline physical activity, school performance, comorbid conditions, or psychological diagnosis which may also influence PROs.
In summary, our findings highlight that there are significant differences in PROs across JIA categories. Those children with ERA and undifferentiated JIA had poorer outcomes in all PROs assessed. Additionally, despite the poorer PROs, physician assessments of disease activity in these children were only moderately correlated. Our results highlight the importance of assessing both physician and patient reported outcomes as part of both routine care and clinical studies. Further work is needed to address ways to improve PROs in children with JIA, with a special focus on children with ERA and undifferentiated JIA.
Acknowledgments
Sources of support: This work was supported by the Rheumatology Research Foundation, CTSA grants NCRR UL1RR024134 and NCATS UL1TR000003, and The National Institute of Child Health & Human Development Clinical Pharmacoepidemiology Training Grant 5T32HD645674. Dr. Weiss’ work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases grant 1K23AR05974901A1.
The authors would like to thank The Children’s Hospital of Philadelphia Center for Biomedical Informatics, Andrew Klink, and Keshia Maughn for helping prepare analytic data sets.
References
- 1.Khanna D, Krishnan E, Dewitt EM, Khanna PP, Spiegel B, Hays RD. The future of measuring patient-reported outcomes in rheumatology: Patient-Reported Outcomes Measurement Information System (PROMIS) Arthritis Care Res. 2011;63(Suppl 11):S486–90. doi: 10.1002/acr.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia-Munitis P, Bandeira M, Pistorio A, Magni-Manzoni S, Ruperto N, Schivo A, et al. Level of agreement between children, parents, and physicians in rating pain intensity in juvenile idiopathic arthritis. Arthritis Rheum. 2006;55:177–83. doi: 10.1002/art.21840. [DOI] [PubMed] [Google Scholar]
- 3.Sztajnbok F, Coronel-Martinez DL, Diaz-Maldonado A, Novarini C, Pistorio A, Viola S, et al. Discordance between physician’s and parent’s global assessments in juvenile idiopathic arthritis. Rheumatology. 2007;46:141–5. doi: 10.1093/rheumatology/kel201. [DOI] [PubMed] [Google Scholar]
- 4.Consolaro A, Vitale R, Pistorio A, Lattanzi B, Ruperto N, Malattia C, et al. Physicians’ and parents’ ratings of inactive disease are frequently discordant in juvenile idiopathic arthritis. J Rheumatol. 2007;34:1773–6. [PubMed] [Google Scholar]
- 5.Luca NJ, Feldman BM. Health outcomes of pediatric rheumatic diseases. Best Pract Res Clin Rheumatol. 2014;28:331–50. doi: 10.1016/j.berh.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390–2. [PubMed] [Google Scholar]
- 7.Beukelman T, Patkar NM, Saag KG, Tolleson-Rinehart S, Cron RQ, DeWitt EM, et al. 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features. Arthritis Care Res. 2011;63:465–82. doi: 10.1002/acr.20460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tynjala P, Vahasalo P, Tarkiainen M, Kroger L, Aalto K, Malin M, et al. Aggressive combination drug therapy in very early polyarticular juvenile idiopathic arthritis (ACUTE-JIA): a multicentre randomised open-label clinical trial. Ann Rheum Dis. 2011;70:1605–12. doi: 10.1136/ard.2010.143347. [DOI] [PubMed] [Google Scholar]
- 9.Wallace CA, Giannini EH, Spalding SJ, Hashkes PJ, O’Neil KM, Zeft AS, et al. Trial of early aggressive therapy in polyarticular juvenile idiopathic arthritis. Arthritis Rheum. 2012;64:2012–21. doi: 10.1002/art.34343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiss PF, Beukelman T, Schanberg LE, Kimura Y, Colbert RA. Enthesitis-related arthritis is associated with higher pain intensity and poorer health status in comparison with other categories of juvenile idiopathic arthritis: the Childhood Arthritis and Rheumatology Research Alliance Registry. J Rheumatol. 2012;39:2341–51. doi: 10.3899/jrheum.120642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boiu S, Marniga E, Bader-Meunier B, Mouy R, Compeyrot-Lacassagne S, Quartier P, et al. Functional status in severe juvenile idiopathic arthritis in the biologic treatment era: an assessment in a French paediatric rheumatology referral centre. Rheumatology. 2012;51:1285–92. doi: 10.1093/rheumatology/kes004. [DOI] [PubMed] [Google Scholar]
- 12.Flato B, Hoffmann-Vold AM, Reiff A, Forre O, Lien G, Vinje O. Long-term outcome and prognostic factors in enthesitis-related arthritis: a case-control study. Arthritis Rheum. 2006;54:3573–82. doi: 10.1002/art.22181. [DOI] [PubMed] [Google Scholar]
- 13.Seid M, Opipari L, Huang B, Brunner HI, Lovell DJ. Disease control and health-related quality of life in juvenile idiopathic arthritis. Arthritis Rheum. 2009;61:393–9. doi: 10.1002/art.24477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Behrens EM, Beukelman T, Cron RQ. Juvenile idiopathic arthritis classification criteria: loopholes and diagnosis software [letter] J Rheumatol. 2007;34:234. [PubMed] [Google Scholar]
- 15.Price DD, Bush FM, Long S, Harkins SW. A comparison of pain measurement characteristics of mechanical visual analogue and simple numerical rating scales. Pain. 1994;56:217–26. doi: 10.1016/0304-3959(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 16.Carlsson AM. Assessment of chronic pain. I. Aspects of the reliability and validity of the visual analogue scale. Pain. 1983;16:87–101. doi: 10.1016/0304-3959(83)90088-X. [DOI] [PubMed] [Google Scholar]
- 17.Bijur PE, Latimer CT, Gallagher EJ. Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Acad Emerg Med. 2003;10:390–2. doi: 10.1111/j.1553-2712.2003.tb01355.x. [DOI] [PubMed] [Google Scholar]
- 18.Filocamo G, Schiappapietra B, Bertamino M, Pistorio A, Ruperto N, Magni-Manzoni S, et al. A new short and simple health-related quality of life measurement for paediatric rheumatic diseases: initial validation in juvenile idiopathic arthritis. Rheumatology. 2010;49:1272–80. doi: 10.1093/rheumatology/keq065. [DOI] [PubMed] [Google Scholar]
- 19.Weiss PF, Klink AJ, Faerber J, Feudtner C. The pediatric rheumatology quality of life scale: validation of the English version in a US cohort of juvenile idiopathic arthritis. Pediatr Rheumatol Online J. 2013;11:43. doi: 10.1186/1546-0096-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh G, Athreya BH, Fries JF, Goldsmith DP. Measurement of health status in children with juvenile rheumatoid arthritis. Arthritis Rheum. 1994;37:1761–9. doi: 10.1002/art.1780371209. [DOI] [PubMed] [Google Scholar]
- 21.Hirschfeld G, Zernikow B. Cut points for mild, moderate, and severe pain on the VAS for children and adolescents: what can be learned from 10 million ANOVAs? Pain. 2013;154:2626–32. doi: 10.1016/j.pain.2013.05.048. [DOI] [PubMed] [Google Scholar]
- 22.Dempster H, Porepa M, Young N, Feldman BM. The clinical meaning of functional outcome scores in children with juvenile arthritis. Arthritis Rheum. 2001;44:1768–74. doi: 10.1002/1529-0131(200108)44:8<1768::AID-ART312>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 23.Fleiss JL. The design and analysis of clinical experiments. New York: Wiley; 1986. [Google Scholar]
- 24.Molto A, Paternotte S, Claudepierre P, Breban M, Dougados M. Effectiveness of tumor necrosis factor alpha blockers in early axial spondyloarthritis: data from the DESIR cohort. Arthritis Rheumatol. 2014;66:1734–44. doi: 10.1002/art.38613. [DOI] [PubMed] [Google Scholar]
- 25.Spadaro A, Lubrano E, Marchesoni A, D’Angelo S, Ramonda R, Addimanda O, et al. Remission in ankylosing spondylitis treated with anti-TNF-alpha drugs: a national multicenter study. Rheumatology. 2013;52:1914–9. doi: 10.1093/rheumatology/ket249. [DOI] [PubMed] [Google Scholar]
- 26.Wu Q, Inman RD, Davis KD. Neuropathic pain in ankylosing spondylitis: a psychophysics and brain imaging study. Arthritis Rheum. 2013;65:1494–503. doi: 10.1002/art.37920. [DOI] [PubMed] [Google Scholar]
- 27.Leegaard A, Lomholt JJ, Thastum M, Herlin T. Decreased pain threshold in juvenile idiopathic arthritis: a cross-sectional study. J Rheumatol. 2013;40:1212–7. doi: 10.3899/jrheum.120793. [DOI] [PubMed] [Google Scholar]
- 28.Limenis E, Grosbein HA, Feldman BM. The relationship between physical activity levels and pain in children with juvenile idiopathic arthritis. J Rheumatol. 2014;41:345–51. doi: 10.3899/jrheum.130734. [DOI] [PubMed] [Google Scholar]
- 29.Bromberg MH, Schechter NL, Nurko S, Zempsky WT, Schanberg LE. Persistent pain in chronically ill children without detectable disease activity. Pain Manag. 2014;4:211–9. doi: 10.2217/pmt.14.6. [DOI] [PubMed] [Google Scholar]
- 30.Bromberg MH, Connelly M, Anthony KK, Gil KM, Schanberg LE. Self-reported pain and disease symptoms persist in juvenile idiopathic arthritis despite treatment advances: an electronic diary study. Arthritis Rheumatol. 2014;66:462–9. doi: 10.1002/art.38223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ringold S, Ward TM, Wallace CA. Disease activity and fatigue in juvenile idiopathic arthritis. Arthritis Care Res. 2013;65:391–7. doi: 10.1002/acr.21801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cartwright T, Fraser E, Edmunds S, Wilkinson N, Jacobs K. Journeys of adjustment: the experiences of adolescents living with juvenile idiopathic arthritis. Child Care Health Dev. 2014 doi: 10.1111/cch.12206. [DOI] [PubMed] [Google Scholar]