Abstract
Post-translational modifications of proteins can have a major role in disease initiation and progression. Incredible efforts have recently been made to study the regulation of glycoproteins for disease prognosis and diagnosis. It is essential to elucidate glycans and intact glycoproteins to understand the role of glycosylation in diseases. Sialylated N-glycans play crucial roles in physiological and pathological processes; however, it is laborious to study sialylated glycoproteins due to the labile nature of sialic acid residues. In this study, an integrated platform is developed for the analysis of intact glycoproteins and glycans using a chemoenzymatic approach for immobilization and derivatization of sialic acids. N-Glycans, deglycosylated proteins, and intact glycoproteins from heart tissues of wild type (WT) and transverse aortic constriction (TAC) mouse models were analyzed. We identified 291 unique glycopeptides from 195 glycoproteins; the comparative studies between WT and TAC mice indicate the overexpression of extracellular proteins for heart matrix remodeling and the down-regulation of proteins associated with energy metabolism in cardiac hypertrophy. The integrated platform is a powerful tool for the analysis of glycans and glycoproteins in the discovery of potential cardiac hypertrophy biomarkers.
Graphical abstract
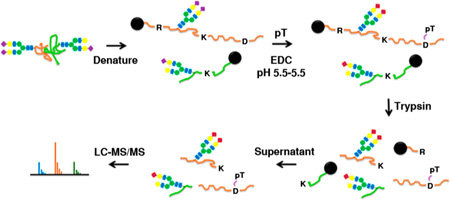
Glycosylation is an important protein modification which modulates a variety of biological functions.1 It is therefore important to investigate glycoproteins and quantitate their relative abundance from a complex biological specimen to understand their roles in health and disease. Glycoproteins that anchor carbohydrates on their asparagine residues (N-X-S/T, where X is any amino acid except P) are highly decorated on the cell membrane or they are secreted to the extracellular space. Tissue glycoproteins carry tissue-specific information that can be used as a gauge to survey the status of diseased tissues. While determination of proteins has been reported routinely, glycoproteomic analysis of N-linked glycan (or N-glycan) structures faces enormous challenges due to their diversity arising from complex non-template-based biosynthesis.2,3 A high-throughput platform may be beneficial for studying complex glycans and glycoproteins in biological specimens.
The identification of protein glycosylation relies on various capture strategies for specific enrichment of glycans or glycopeptides. By oxidation of glycans that remain conjugated to proteins, glycoproteins are chemically immobilized on a solid support while other molecules remain in solution.4 This method is highly specific for the enrichment of N-linked glycopeptides. Yet, the structures are altered due to oxidation on cis-diol groups of N-glycans. To identify intact glycopeptides, glycoproteins are directly digested with trypsin in combination with different enrichment practices such as lectins,5 boronic acid functionalized porous silica,6 or titanium dioxide.7 In addition, the complex biological specimen likely consists of labile residues of sialic acids, which can be partially or completely lost during the sample preparation or mass spectrometry (MS).8 To mitigate loss of sialic acids for accurate quantitative analysis, derivatization is often required during sample preparation.8–10 Chemical modifications such as carbodiimide or esterification can efficiently label sialic acids;11,12 however, reaction in solution creates challenges for sample cleanup by virtue of the similar properties between chemical compounds and labeled glycans. Sialic acid esterification on solid phase using hydrazine chemistry can be accomplished; yet, hydrazone hydrolysis in buffer triggers sample loss.13
We recently developed a technique that immobilizes proteins on a solid support for derivatization of sialic acids before release of N-glycans.14,15 The aldehyde groups on resin capture primary amines of proteins via a reductive amination reaction. The resin is designed with submicrometer-sized pores that remarkably increase the density of amine-reactive groups, thereby maximizing the efficiency of protein immobilization. Derivatization of carboxylic acid residues on glycoproteins becomes expedient because reagents can be removed through washing, ultimately ameliorating overall yield without sample loss. Likewise, sialic acids can be chemically modified so that they are stable in MS analysis by electrospray ionization (ESI) or matrix-assisted laser desorption/ionization (MALDI).15 The derivatization of glycans can not only enhance their ionization, but also appreciably improve the signal of their MSn ions upon fragmentation.10 Since derivatization utilizes carboxylic acid groups by carbodiimide coupling, the C-termini as well as aspartic acid (Asp) or glutamic acid (Glu) residues are also modified; thus, they can be employed to label the proteins using stable isotope tags for quantitative proteomic analysis. We used this approach for extraction of N-glycans in serum15 and the quantitative analysis of sialic acid using isotope labeling.14 However, proteins and glycoproteins on resin have not been utilized for global proteomics and intact glycoproteomics.
In this study, we established an integrated platform for analysis of N-glycans, global proteins, and intact glycoproteins using MS. The platform is entitled integrated glycoprotein immobilization for glycoprotein and glycan analysis (iGIG). Analysis of intact glycopeptides requires information on glycan composition and amino acid sequence. To establish glycan and peptide database, we first immobilized glycoproteins on resin for release of N-glycans after sialic acid stabilization; the deglycosylated proteins were further digested with trypsin. For analysis of intact glycopeptides, the immobilized glycoproteins were directly digested using trypsin after sialic acid modification without release of N-glycans. The intact glycopeptides were analyzed by liquid chromatography–electrospray ionization tandem mass spectrometry (LC–ESI-MS/MS), and the resulting spectra were searched against a matching database of N-glycans and deglycopeptides. To quantitate the relative abundance in different samples, glycopeptides were labeled using p-toluidine (pT) and iTRAQ (isobaric tags for relative and absolute quantitation). The iGIG method was applied for quantitative analysis of N-glycans, proteins, and glycoproteins from hypertrophic mouse hearts.
EXPERIMENTAL PROCEDURES
Mouse Transverse Aortic Constriction Model
Cardiac hypertrophy induced by pressure overload was produced by constricting the transverse aorta as described by Takimoto et al.16 Male mice (age 12 weeks, 25–30 g) were subjected to transverse aortic constriction (TAC). Briefly, mice were anesthetized and placed supine on a 37 °C heating pad. An incision was made at the suprasternal notch, and a 7.0 silk suture was passed under the aorta. Then a needle of 27-gauge was placed next to the aortic arch, the suture was tied around the needle and the aorta, and the needle was removed. The chest was closed, and the animal was allowed to recover. Sham-operated mice [wild type (WT)] underwent the same procedure without aortic constriction. Four weeks after TAC surgery, echocardiographic analysis was conducted to confirm the overload in TAC mice.16 Mice were sacrificed by cervical dislocation. Hearts were removed, weighed, and flash-frozen until protein extraction. In this work, we collected tissues from six control and six TAC mice. The protocol was approved by the Johns Hopkins Medical Institutions Animal Care and Use Committee.
Protein Immobilization and Derivatization
The flowchart is illustrated in Figure 1. Proteins were extracted from mouse heart tissues with freshly added protease inhibitor in RIPA buffer (1× PBS, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 2 mM EDTA, and 50 mM NaF). The protein extract was centrifuged at 16 000g for 30 min; the supernatant was exchanged by 1× PBS buffer using desalting column (Zeba; Life Technologies). Protein concentration was estimated by Nano-drop (Thermo Scientific). Same amounts of proteins (TAC vs WT) were conjugated on beads (Aminolink resin) by glycoprotein immobilization for glycan extract (GIG).15 Amounts of 4 mg of total proteins per sample were immobilized on beads via reductive amidation using 500 µL of 1× PBS buffer. After blocking the active aldehyde sites on beads (50 mM NaCNBH3 in 1 M Tris–HCl; 30 min), pT was added for derivatization of sialic acid at room temperature for 4 h with mixing (42.8 mg of pT in 400 µL of 1 M HCl, 40 µL of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC), and 25 µL of concentrated HCl).15 The beads were washed with 10% formic acid, 10% ACN (0.1% TFA), 1 M NaCl, and DI water (500 µL; 3×).10,15
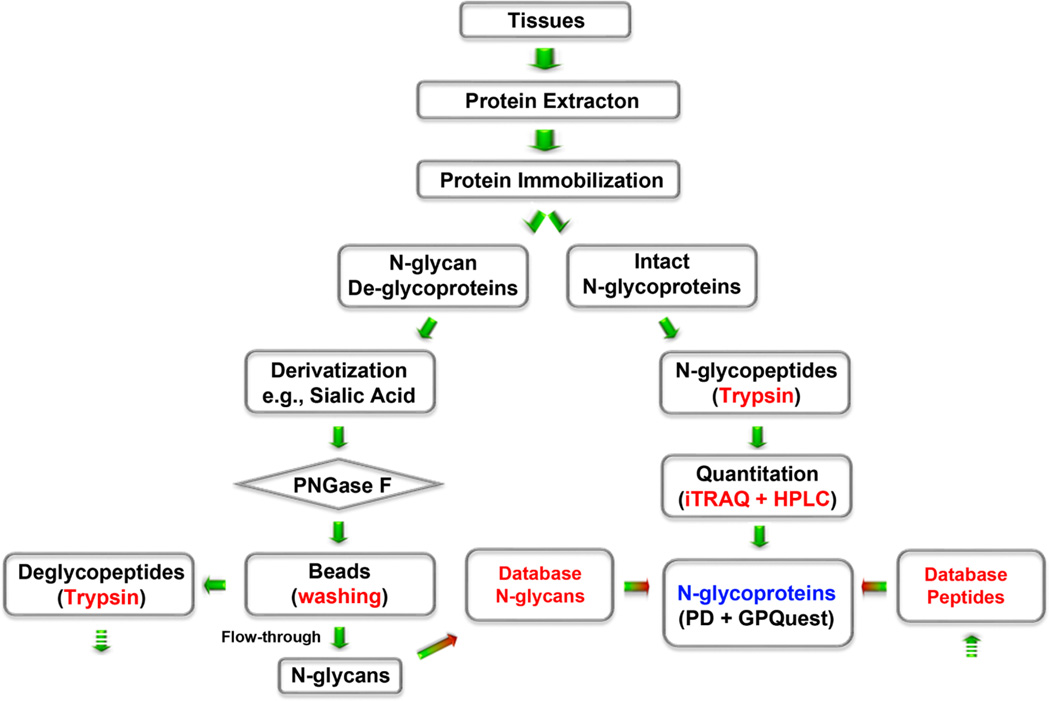
Figure 1.
Schematic flowchart for analysis of glycoproteins and glycans using an integrated glycoprotein immobilization for glycopeptide and glycan (iGIG). Tissue is sonicated in lysis buffer (RIPA) for protein extraction. Aminolink resin is functionalized with aldehydes for conjugation of the extracted proteins via reductive amination. Sialic acids and acidic amino acids are derivatized via carbodiimide coupling. One-quarter of the proteins are used for identification of N-glycans and deglycopeptides; three-quarters of the proteins are directly digested for analysis of intact glycopeptides. N-Glycans are cleaved off the resin using PNGase F, and the remaining deglycoproteins are digested with trypsin. The intact glycopeptides are quantitated using iTRAQ and fractioned for LC–ESI-MS/MS. N-Glycopeptides are searched by precursor match using a database of N-glycans and deglycopeptides.
Release of N-Glycans and Deglycopeptides from Beads
N-Glycans were cleaved from the immobilized glycoproteins (one-quarter of the beads ≅ 1 mg of proteins) by 4 µL of PNGase F with 40 µL of 500 mM sodium phosphate (pH 7.5) in 356 µL of DI water followed by incubation at 37 °C for 2 h (Figure 1). N-Glycans were eluted with 500 µL of 80% ACN in 0.1% TFA (2×) after Carbograph cleanup.17 Samples were dried in a SpeedVac (Savant SPD; Thermo Scientific) for MALDI-MS analysis (Shimadzu Resonance Axima). Beads were washed subsequently with 10% ACN and DI water (500 µL; 3×) before reduction with 10 mM TCEP (tris(2-carboxyethyl)phosphine hydrochloride) in 8 M urea/0.2 M NH4HCO3 (pH 8.0) (500 µL) (1 h at 37 °C), followed by alkylation (50 µL of 200 mM iodoacetamide; 30 min). The beads were centrifuged, and proteins on beads were digested with 50 µL of trypsin (0.5 µg/µL) in 450 µL of 2 M urea/0.2 M NH4HCO3 overnight at 37 °C with mixing. Peptides were purified by C18 cartridge (Waters Corporation; Milford, MA) and eluted with 500 µL of 60% ACN (0.1% TFA) twice.
To quantitate N-glycans and proteins in TAC compared to WT, the proteins extracted from six mice (WT or TAC) were pooled to generate two replicates (4 mg × 2). Each replicate consists of 1.33 mg of protein from individual heart tissue. N-Glycans from WT and TAC were analyzed by MALDI-MS; deglycoproteins were further digested with trypsin. For quantitation, 4-plex iTRAQ (Sciex; Framingham, MA) was applied to label peptides using protocols described in a previous study.18 The iTRAQ-labeled peptides were purified by C18 and fractionated by basic reversed-phase liquid chromatography (RPLC) for LC–ESI-MS/MS analysis.
Intact Glycopeptides
The immobilized glycoproteins (three-quarter of the beads ≅ 3 mg; Figure 1) were directly digested using 150 µL of trypsin in 350 µL of 2 M urea/0.2 M NH4HCO3 after reduction (TCEP) and alkylation (IAA) and incubated at 37 °C overnight with mixing. The released glycopeptides were labeled by 4-plex iTRAQ (two WTs and two TACs) after C-18 cleanup. The iTRAQ-labeled glycopeptides (50 µg) were also separated into 24 fractions by basic RPLC for LC–MS analysis.
Peptide LC–MS
Each peptide fraction (~1 µg) was further separated using a Dionex Ultimate 3000 RSLC nano system with a 75 µm × 50 cm Acclaim PepMap100 RSLC column protected by a 2 cm guarding column (Thermo Scientific). Deglycopeptide MS/MS spectra were directly searched using the SEQUEST search engine [Thermo Proteome Discoverer 1.4.0.288 (PD)] against the NCBI Mus musculus database (306977; download Aug 2014). MS/MS spectra of intact glycopeptides were matched to the spectra of N-glycans and deglycopeptides using recently designed GPQuest software.19
Peptide Search in Proteome Discoverer
Carbamidomethylations of cysteine residues were set as fixed modification; oxidation of methionine and deamidation of asparagine were set as variable modifications. pT on C-termini, aspartic acids, and glutamic acids was set as variable modifications. The mass tolerance of the precursor is 10 ppm, while the mass tolerance of fragment ions is 20 ppm.
RESULTS AND DISCUSSION
Overview of the iGIG Platform
iGIG consists of analysis of glycans, deglycopeptides, intact glycopeptides, and spectra search (Figure 1). The immobilized glycoproteins and their glycans can be chemically or enzymatically derivatized without sample loss. Common analytical challenges are eliminated by iGIG for removal of reagents compared to traditional chromatographic purification, i.e., pT and EDC. Using iGIG, reagents or chemical compounds are completely washed off beads by extensive rinsing using a variety of solutions. N-Glycans are specifically cleaved off beads by PNGase F; subsequently, the deglycoproteins remaining on beads are digested with trypsin for peptide analysis. A database is then established from the MS-based results of N-glycan and deglycopeptide analysis. Meanwhile, the intact glycoproteins are digested with trypsin without release of N-glycan. iGIG is a powerful approach for the quantitative identification of deglycopeptides and intact glycopeptides with sialic acid and isobaric labeling. It is therefore a high-throughput method for analysis of N-glycoproteins in complex biological specimens.
Protein Conjugation and Digestion Using iGIG
It is ideal to only conjugate protein N-termini to aldehyde groups on beads. Proteins usually contain primary amines at N-termini and lysine residues (K). Lysines are basic amino acids with a pI at 9.74; thus, they may be protonated at pH below their pI.20 Slightly different from our previous protocol, reductive amination was performed at a pH less than 9.0, e.g., 7.4 in 1× PBS. Under such conditions, lysine residues are most protonated so that reductive amination on lysine could be minimized. To determine whether the immobilized protein on beads can be digested by trypsin, we compared digestion of fetuin in solution versus iGIG. Seven of the most abundant fetuin peptides are identified from both methods (Supporting Information Table 1). (Note: no carbodiimide coupling was conducted for in-solution digestion due to the protein precipitation in this condition.) Amidation could occur at any carboxylic acid; consequently, acidic peptides, i.e., Glu (E) and Asp (D), are labeled by pT along with sialic acid residues. Because of the hydrophobicity of pT, the modified peptides have a prolonged retention time on C18 compared to their unmodified counterparts.
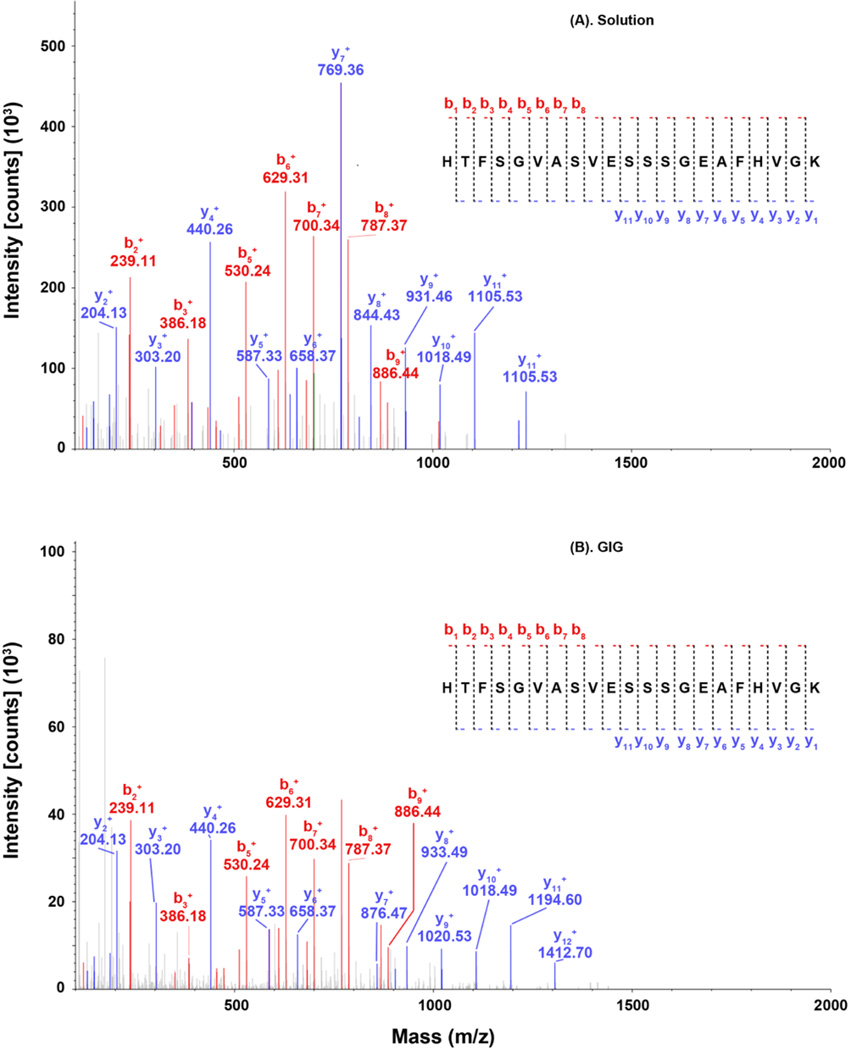
Fetuin peptides show minimally missed cleavage despite chemical modifications, indicating efficiently tryptic digestion on beads. Tandem MS spectra in Figure 2 depict the mass fingerprint of HTFSGVASVESSSGEAFHVGK. In solution, we observed 8 b-ions and 11 y-ions (Figure 2A); in iGIG, all eight b-ions and y1–y6 ions have the same m/z, while ions from y7 to y11 are shifted by the mass of one pT (Figure 2B). This data indicates that tryptic digestion is negligibly affected by protein coupling on beads. The N-glycans were released before trypsin digestion (iGIG); thus, N-glycosites can be determined by deamidation on Asn residues within the N-X-S/T motif, e.g., LCPDCPLLAPLnDSR. Although iGIG could miss glycopeptides that are located between the protein N-terminus and the first lysine or arginine, our result provides evidence that iGIG can be employed for analysis of N-glycosites in addition to N-glycans.
Figure 2.
MS/MS spectra of the abundant peptides from bovine fetuin serum. Fetuin peptides are digested using trypsin via (A) in solution and (B) iGIG. Peptides that contain E or D, such as HTFSGVASVESSSGEAFHVGK, can be modified by p-toluidine on iGIG; thereby, the retention time differs between the native (42.27 min) and the modified peptide (65.68 min).
Profiling of N-Glycans of Hypertrophic Heart
As described previously, a hypertrophic heart can be constructed by TAC. It is noteworthy to investigate protein glycosylation in overload-induced hypertrophy since studies have shown that the cellular concentrations of nucleotide donors for glycosyltransferases, e.g., UDP-GlcNAc, became elevated in response to pressure overload.21,22 Recent studies suggested that sialylated glycans were up-regulated during the progression of cardiac hypertrophy.23 To profile and differentiate N-glycans in TAC, iGIG was first applied to analyze N-glycans in the pathological left ventricle of hypertrophic heart (LVH). To estimate the quantity of N-glycans, we spiked 1 µL of 25 µM DP7 (maltoheptaose) to the samples and analyzed them by MALDI-MS/MS.
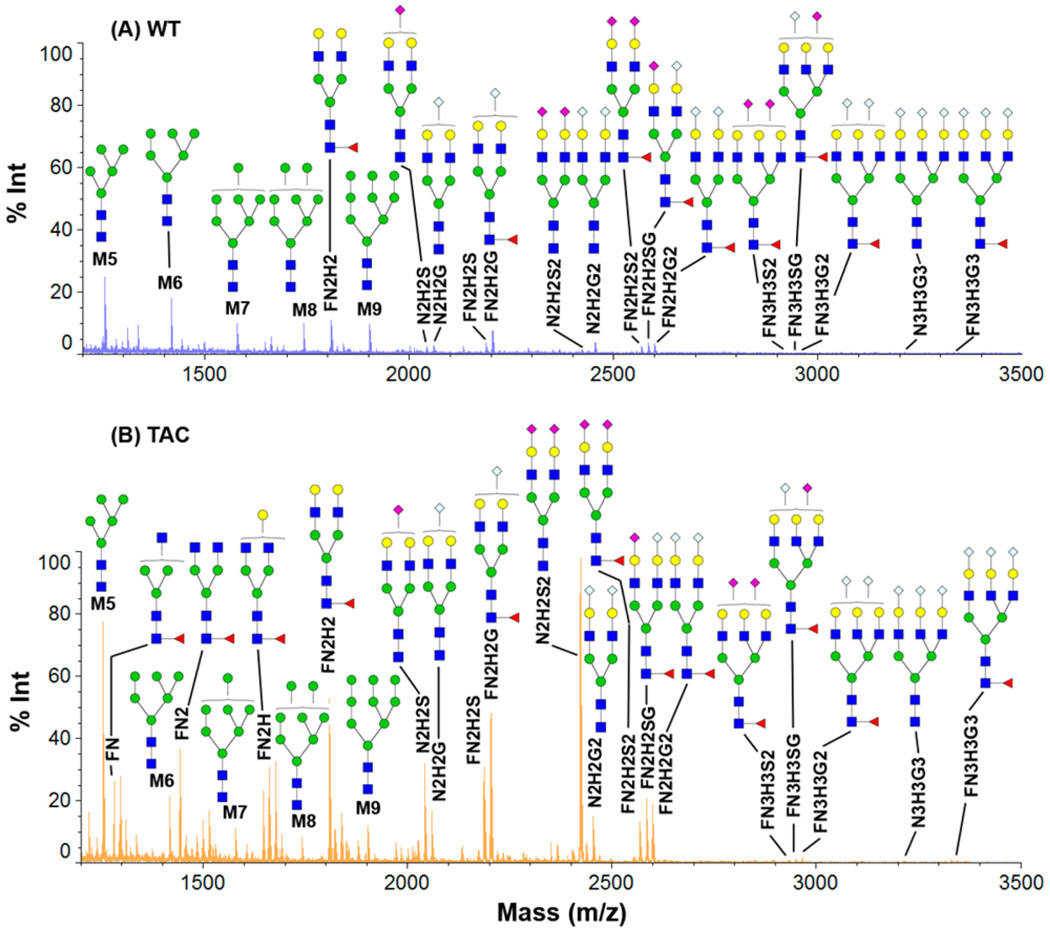
Global N-glycans are up-regulated in TAC tissues compared to normal tissue. Figure 3 shows the relative abundance of N-glycans in WT (Figure 3A) and hypertrophic heart (Figure 3B) (quantitative comparison based on peak area is given in the Supporting Information). Consistently, five high oligomannoses (Man5–Man9) have the same order of magnitude increase in their relative abundance, except Man5, which is upregulated by ~3-fold in TAC compared to WT. This observation is in agreement with previous reports that oligomannoses were prominently up-regulated in heart failure.24 We observed significant overexpression of fucosylated and sialylated N-glycans in TAC. Particularly, the complex N-glycans that are fucosylated are significantly up-regulated. In TAC tissues, the fucosylated N-glycans, e.g., FN, FN2, FN2H, and FN2H2, are the dominant species in the lower molecular range, while these glycans were much less or negligibly expressed in cardiac hypertrophy. For large molecules (m/z > 2000 Da), the sialylated N-glycans, particularly Neu5Ac, are substantially increased in TAC tissues. In normal tissue, the most abundant sialic acids, such as FN2H2G or N2H2G2, are approximately at the same level as Man7 or Man9; other sialic acids have much lower concentrations, e.g., FN2H2S2, FN2H2SG, and FN2H2G2. Hypertrophic cardiomyopathy (HCM) tissue has a high abundance of complex N-glycans in which the majority is coincidently fucosylated, e.g., FN2H2S2, FN2H2SG, and FN2H2G2. Interestingly, we found considerable increase of N2H2S2 in TAC compared to the WT mice. The list of N-glycans in the Supporting Information was used for MS1 spectra match.
Figure 3.
MALDI-TOF-MS profiles of the extracted N-glycans using glycoprotein immobilization for glycan extraction (GIG). (A) WT and (B) transverse aortic constriction (TAC) of mice heart tissues using iGIG. An amount of 1 µL out of 40 µL of glycan extraction is analyzed using a Shimadzu Axima Resonance MALDI-MS.
Analysis of Deglycopeptides
Proteins and deglycoproteins were digested with trypsin on resin for identification, and they were isobarically labeled for quantitation using ESI-MS/MS. Quantitative analysis of proteins in TAC versus WT is given in the Supporting Information. We identified 3593 unique proteins from 15 148 unique peptides. A total of 142 proteins are up-regulated at least 1.5-fold in TAC tissue compared to WT, accounting for 3.95% of the total proteins given in the Supporting Information; 19 proteins are down-regulated (0.67-fold as cutoff) in TAC tissue compared to WT, or 0.53% among total proteins in given the Supporting Information. We categorized the identified proteins with regards to cellular component and biological processes, and the results are summarized in the Supporting Information.
The regulated proteins are found in a variety of cellular components. Among 3593 unique proteins, 878 proteins are cytoplasmic. Quantitative analysis of proteins show that 68 proteins are highly up-regulated in TAC, including Lama4, Lama5, Adam17, Insr, Myh7, Sgcb, Nrap, and Hp. Eighty-one proteins are localized to the extracellular matrix (ECM), in which several glycoproteins (Col12a1, Acta1, Emilin1, Itgb1, Itgb2, Mfap4, Postn, Fn1, and Lgals3bp) are overexpressed in TAC. The regulation of protein expression in TAC could provide insight on analysis of signaling pathways in cardiac hypertrophy. However, since all glycoproteins have been deglycosylated on beads by PNGase F, the specific glycosite and intact glycopeptide information is lost. To fulfill those goals, we further investigated the intact glycopeptides, i.e., peptides with preserved N-glycans.
Intact Glycopeptides Identified by GPQuest
Intact glycopeptides were analyzed by using recently developed software, GPQuest,19 in combination with a database of N-glycans and deglycopeptides (Supporting Information). GPQuest is MATLab-based software for intact glycopeptide analysis. It uses precursor mass matching (PMM) with or without iTRAQ as search algorithms. Modifications of peptides or glycans are integrated in the software. False discovery rate (FDR) is also incorporated to improve identification accuracy. Glycan and peptide databases are required to identify intact glycopeptides. The N-glycan database, determined by MALDI-MS/MS data, consists of 68 N-glycans; the peptide database, quantitatively identified by ESI-MS/MS, uses 15 148 unique peptide sequences. In addition, 10 oxonium ions have been selected, in which 204.08 Da is set as “essential oxonium ion” and at least two oxonium ions are matched in the spectra. Top 10 peaks are selected for matching b- and y-ions with one and/or two charges.
Glycopeptides and Glycoproteins in Cardiac Hypertrophy
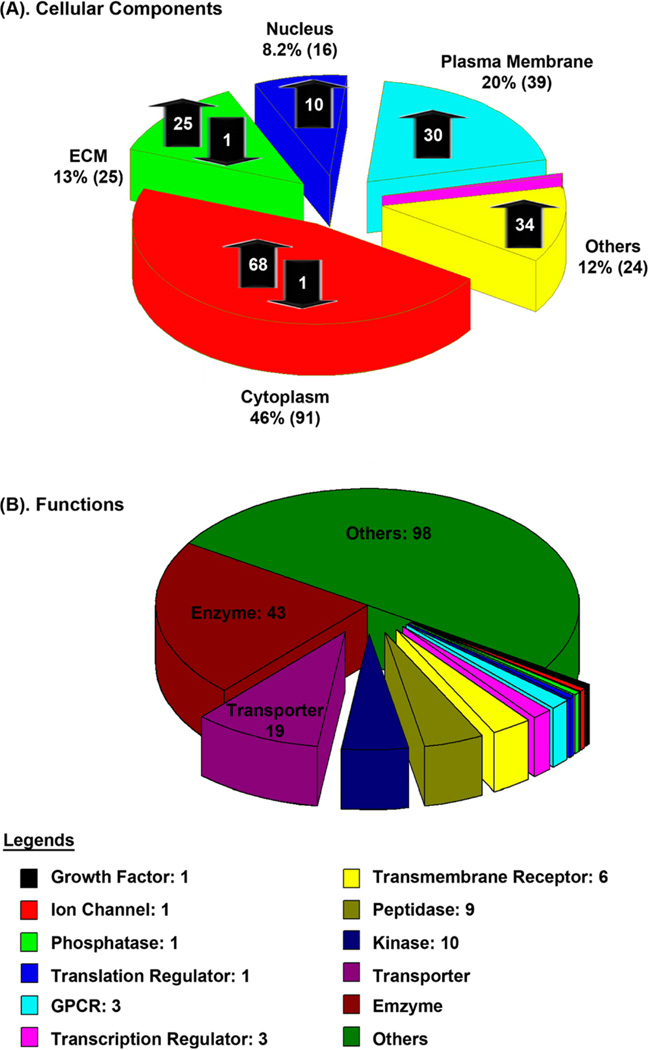
We identified 291 unique glycopeptides from 195 unique glycoproteins. As shown in Figure 4A, these glycoproteins are located at different cellular components, including cytoplasm (91; 68 up, 1 down, 22 no change), extracellular matrix (25; 21 up, 1 down), nucleus (16; 10 up), plasma membrane (39; 30 up), mitochondrion (1), and others (24; 18 up). Among them, 97 glycoproteins are classified according to their roles in signaling pathways, including enzyme (43; 34 up and 1 down), GPCR (3 up), growth factor (1 up), ion channel (1 up), kinase (10; 7 up), peptidase (9; 6 up), phosphatase (1), transcription regulator (3), translation regulator (1 up), transmembrane receptor (6 up), and transporter (19; 13 up and 1 down) (Figure 4B).
Figure 4.
Regulation of glycoproteins in hypertrophic heart and their functions. Quantitative analysis is performed using iGIG and iTRAQ-LC–MS/MS.
We compared the fold-change of deglycopeptides and glycopeptides in TAC (Supporting Information); the comparative results are given in Supporting Information Table 8. A total of 26 unique glycoproteins are found highly regulated in TAC (>1.5-fold). Several findings are manifest: (a) 31 out of 60 (52%) glycopeptides consist of sialic acids. Derivatization is indispensable for identification and quantitation; (b) intact glycopeptides are differently regulated in contrast to deglycopeptides; (c) all glycopeptides have an NXS or NXT motif. To confirm whether the N-glycosite of the identified peptide has an N-glycosite, we used a Uniprot database (M. musculus) to match known N-linked glycosites; (d) the same glycosylated peptide could possess different glycoforms; for example, RF[89]NGSVSFFR of Mfap4 has six glycans; (e) quantified intact glycopeptides indicate changes of peptide substrate and glycan, while deglycosylated peptides only reveal the overall change of the same peptide; (f) potential glycosites are detected, such as TNAANLSEAK of Nrap. This protein has been reported as a potential glycoprotein marker in human plasma, even though Uniprot does not specify whether it has any glycosites.25
DIFFERENTIAL PROTEIN EXPRESSION IN CARDIAC HYPERTROPHY
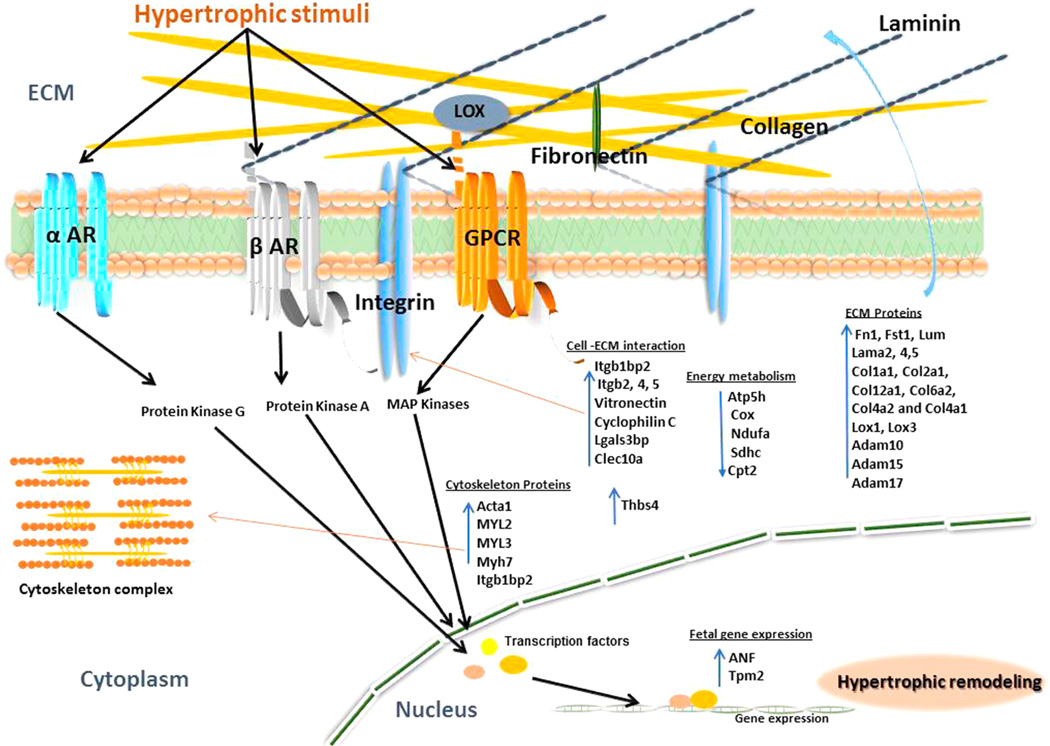
Hypertrophic cardiomyopathy is a primary disease of the myocardium and a genetic cardiac disease caused by a variety of mutations in genes such as those encoding sarcomeric proteins.26 It is a pathological hypertrophy of heart as a result of enlarged cardiomyocyte size, heart weight, and fibrosis. It also engenders contractile dysfunction and heart failure. HCM is most often diagnosed during infancy or adolescence and is the second most common form of heart muscle disease.27 Studies over the last decades revealed that mutations of sarcomere genes may contribute to cardiac hypertrophy.28–30 Quantitative analysis of proteins and their glycosylation could be beneficial for the molecular level of understanding on the pathological mechanisms in HCM. On the basis of global and glycoprotein analysis, we proposed pathways to elucidate myocardial protein abnormalities in cardiac hypertrophy (Figure 5). Briefly, extracellular matrix proteins are overexpressed upon pressure overload. These proteins aggregate in the extracellular matrix, ultimately leading to fibrosis. Consequently, pressure overload activates various membrane protein receptors which transduce downstream signals, followed by activation of secondary messengers in the cytoplasm. The signal is further transduced by nuclear factors to control the expression of genes involved in pathological hypertrophy.
Figure 5.
Schematic of myocardial protein abnormalities in hypertrophic heart. The diagram depicts how mechanical activation of various hypertrophic stimuli via different receptors activates various downstream signaling cascades leading to hypertrophy. Components of the β-adrenergic (β-AR), α-adrenergic (α-AR), and G protein coupled receptor (GPCR) pathways activate protein kinases (PKA, PKG) and MAP kinases. These pathways are involved in inducing fetal proteins (ANP, Tpm2) expression, overexpression, and modification of extracellular matrix proteins (laminin, collagen, fibronectin, LOX), regulatory thin-filament proteins (Acta1, MYLs), and profibrotic pathways which impact myofilament stiffness and contractile function in hypertrophic cardiomyopathy.
Remodeling of ECM Proteins
Matrix remodeling is a key factor that governs the physiological process during hypertrophic progression. The ECM provides physical support to the tissue and plays an important role in the regulation of cellular function. Accumulation of ECM proteins leads to the formation of fibrotic lesions. We found that TAC mice exhibit excess myocardial fibrosis (Figure 5), a well-known cause of diastolic dysfunction and diastolic heart failure.31 For example, the extracellular glycoproteins including periostin (Postn) and laminin (Lama2) are substantially up-regulated in TAC mice. Likewise, the dynamic cytoskeletal remodeling also occurs in response to pressure overload. Our data confirms that the expression of several actin binding proteins and other cytoskeletal glycoproteins is highly up-regulated in the TAC tissues, which includes α-actin (Acta1), fibronectin 1 (Fn1), skeletal muscle LIM protein, and capping protein (actin filament). These observations are consistent with the previous discoveries.32–34 TAC mice are also associated with elevated levels of fibrillar collagens (Col1a1, Col2a1, and Col12a1) and cell binding collagens (Col6a2, Col4a2, and Col4a1). Together with collagens, we found an up-regulation of proteins that stabilizes collagen fibers, e.g., Lox1 and 3 (lysyl oxidase 1 and 3). Lox mediates cross-linking of collagen fibrils, leading to the formation of stiff collagen.35 Evidence from both experimental and clinical studies exhibits that excess Lox is associated with increased collagen cross-linking and stiffness. The up-regulation of Lox indicates increased cross-linking and stiffness of collagen, resulting in an underperforming heart.
Regulation of Protein Receptors
G protein coupled receptors and adrenergic receptors play crucial roles in pressure overload induced hypertrophic signaling.36 Integrins are cell-surface receptors that mediate the cell–ECM adhesion and convert extracellular biomechanical stress into intracellular biochemical signals.37 Integrins can interact with mechanical stimuli to regulate expression of tissue myosin heavy chain (Myh).38 For example, the myosin heavy polypeptide 7 (Myh7) is significantly increased in TAC (Supporting Information Table 7) and it is associated with the proliferation rate of fibroblasts which is attributed to an increase in left ventricular muscle mass.39 The protein level of integrins increases during the compensatory phase of cardiac response to pressure overload. Particularly, Itgb1bp2 is known to interact with melusin, a muscle-specific protein, to prevent cardiac failure in response to mechanical stimuli.40 A receptor for intercellular adhesion molecules (ICAMs), Itgb2 is known to contribute to skeletal muscle hypertrophy in mice.41 The levels of vitronectin and galectin-3-binding protein (Lgals3bp) were also found to be up-regulated in TAC tissues. Lgals3bp also promotes intergrin-mediated cell adhesion in cardiac hypertrophy.42
Activation of Kinases in TAC
Angiotensin II (AngII) is a well-known agonist of cardiac hypertrophy. It is known to stimulate epidermal growth factor receptor (EGFR) activation and vascular smooth muscle cell hypertrophy. It was found that AngII can induce tyrosine phosphorylation of insulin receptor substrates (Insr), and it ultimately activates insulin signaling pathways.43,44 Insulin receptor coordinates the regulation of cardiac heart size, metabolism, and contractility. Our data shows substantial increase of insulin receptor substrate (Supporting Information Table 7), indicating regulation of insulin pathway in hypertrophic heart.
Transcription Factor
Several transcription factors were found to be up-regulated in TAC, e.g., cardiac ankyrin repeat domain 1 (Ankrd1), transcription elongation factor (Supt5), transcription intermediary factor 1-β (Trim28), and Tudor domain-containing protein 3 (Tdrd3). Ankrd1 mutations were observed in HCM45 and play an enigmatic role in heart development and disease. Consistent with previous findings, we found an overexpression of fetal protein-atrial natriuretic factor (ANF) in hypertrophic heart, which is a potential biomarker for early detection of hypertrophic heart.
Energy Metabolism
Metabolic dysregulation in the heart occurs in response to chronic metabolic and hemodynamic stress causing contractile dysfunction. In response to a prolonged pressure or volume overload, alterations occur in myocardial fatty acid, glucose, and glycogen metabolism.46 This is associated with down-regulation of fatty acid oxidation, TCA cycle, and oxidative phosphorylation proteins (Figure 5). For example, Atp5h, Cox, Ndufa, Sdhc, and Cpt2 proteins were found to be down-regulated in the TAC hearts. These findings are consistent with the earlier observations of heart failure or cardiomyopathy.47
CONCLUSION
This study describes an integrated platform (iGIG) for the analysis of N-glycans and intact N-glycoproteins from hypertrophic heart. Glycopeptide derivatization has been demonstrated on the immobilized glycoproteins. iGIG captures N-termini of proteins and successfully digests peptides on solid phase. N-Glycans and deglycopeptides are quantitatively identified by enzymatic digestion and mass spectrometry, and the intact glycopeptides are further quantitatively analyzed by spectrum precursor matching.
Analysis of N-glycans and proteins in the pathological left ventricular hypertrophic heart in response to transverse aortic constriction exhibits substantial overexpression of global N-glycans and proteins associated with matrix remodeling and fibrosis. N-Glycans that are sialylated and/or fucosylated are highly up-regulated in TAC tissues; ECM and associated proteins are overexpressed in TAC. Fetal proteins are reactivated and play a critical role in controlling calcium regulated sarcomeric contraction through their interactions with actin and the troponin complex. Atrial natriuretic factor is up-regulated in TAC tissues and it is a potential biomarker for early detection of cardiac hypertrophy. Conversely, many mitochondrial proteins that are associated with TCA cycle and oxidative phosphorylation are down-regulated in TAC. The regulated glycoproteins and proteins have been found to be associated with hypertrophic cardiomyopathy in the structural network of the myocyte.
The identification of intact glycopeptides could be further improved by HPLC separation of N-glycans, pre-enrichment of glycoproteins, and different fragmentation methods. The glycopeptide matching is based on N-glycan and peptide databases; therefore, increased identification of N-glycans can be achieved by chipLC separation.48 HCD fragmentation generates good signals for glycosidic fragments, but peptides fragmented by HCD have weak signals. Electron-transfer dissociation (ETD) fragmentation should yield better fragments for both glycans and peptides.49 In addition, enrichment of glycoproteins using lectin could enhance the detection of low abundance of glycopeptides to significantly improve their identification.50
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Dr. Punit Shah for help on MS, Ms. Shadi Toghi Eshghi for help on GPQuest, and Dr. Stefani Thomas for assistance in manuscript preparation. This work was supported in part by the National Institutes of Health under grants and contracts of the National Cancer Institute, Clinical Proteomics Tumor Analysis Consortium (U24CA160036), the Early Detection Research Network (EDRN, U01CA152813 and U24CA115102), and R01CA112314, the National Heart Lung Blood Institute, Programs Excellence in Glycosciences (P01HL107153), and the NHLBI Proteomic Center (N01-HV-00240).
Footnotes
ASSOCIATED CONTENT
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.analchem.5b01663.
Experimental procedures, list of tables for Supporting Information, Supporting Information Tables 1 and 8, and Supporting Information Figure 1 (PDF)
Supporting Information Table 2: Quantitation of N-glycans extracted from mice tissues (XLSX)
Supporting Information Table 3: Quantitative analysis of global proteins in the pathological left ventricular hypertrophic (LVH) heart in response to transverse aortic constriction (TAC) (XLSX)
Supporting Information Table 4: Up-regulated proteins in the pathological LVH heart in response to TAC (XLSX)
Supporting Information Table 5: Down-regulated proteins in the pathological LVH heart in response to TAC (XLSX)
Supporting Information Table 6: N-Glycan database identified from mouse heart tissue by GIG (XLSX)
Supporting Information Table 7: Deglycosylated peptide database identified from mouse heart tissue by GIG (XLSX)
Supporting Information Table 8: Intact glycopeptides identified using iGIG (XLSX)
The authors declare no competing financial interest.
REFERENCES
- 1.Taylor ME, Drickamer K. Introduction to Glycobiology. 3rd ed. Oxford, U.K.: Oxford University Press; 2011. [Google Scholar]
- 2.Haslam SM, North SJ, Dell A. Curr. Opin. Struct. Biol. 2006;16:584–591. doi: 10.1016/j.sbi.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Raman R, Raguram S, Venkataraman G, Paulson JC, Sasisekharan R. Nat. Methods. 2005;2:817–824. doi: 10.1038/nmeth807. [DOI] [PubMed] [Google Scholar]
- 4.Zhang H, Li X-j, Martin DB, Aebersold R. Nat. Biotechnol. 2003;21:660–666. doi: 10.1038/nbt827. [DOI] [PubMed] [Google Scholar]
- 5.Madera M, Mechref Y, Novotny MV. Anal. Chem. 2005;77:4081–4090. doi: 10.1021/ac050222l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu Y, Wu Z, Zhang L, Lu H, Yang P, Webley PA, Zhao D. Anal. Chem. 2009;81:503–508. doi: 10.1021/ac801912t. [DOI] [PubMed] [Google Scholar]
- 7.Palmisano G, Lendal SE, Engholm-Keller K, Leth-Larsen R, Parker BL, Larsen MR. Nat. Protoc. 2010;5:1974–1982. doi: 10.1038/nprot.2010.167. [DOI] [PubMed] [Google Scholar]
- 8.Sekiya S, Wada Y, Tanaka K. Anal. Chem. 2005;77:4962–4968. doi: 10.1021/ac050287o. [DOI] [PubMed] [Google Scholar]
- 9.Powell AK, Harvey DJ. Rapid Commun. Mass Spectrom. 1996;10:1027–1032. doi: 10.1002/(SICI)1097-0231(19960715)10:9<1027::AID-RCM634>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 10.Yang S, Zhang H. Curr. Protoc. Chem. Biol. 2014;6:191–201. doi: 10.1002/9780470559277.ch140085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toyoda M, Ito H, Matsuno Y-k, Narimatsu H, Kameyama A. Anal. Chem. 2008;80:5211–5218. doi: 10.1021/ac800457a. [DOI] [PubMed] [Google Scholar]
- 12.Powell AK, Harvey DJ. Rapid Commun. Mass Spectrom. 1996;10:1027–1032. doi: 10.1002/(SICI)1097-0231(19960715)10:9<1027::AID-RCM634>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 13.Miura Y, Shinohara Y, Furukawa Ji, Nagahori N, Nishimura SI. Chem. —Eur. J. 2007;13:4797–4804. doi: 10.1002/chem.200601872. [DOI] [PubMed] [Google Scholar]
- 14.Shah P, Yang S, Sun S, Aiyetan P, Yarema KJ, Zhang H. Anal. Chem. 2013;85:3606–3613. doi: 10.1021/ac3033867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang S, Li Y, Shah P, Zhang H. Anal. Chem. 2013;85:5555–5561. doi: 10.1021/ac400761e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, Bedja D, Gabrielson KL, Wang Y, Kass DA. Nat. Med. 2005;11:214–222. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- 17.Yang S, Zhang H. Anal. Chem. 2012;84:2232–2238. doi: 10.1021/ac202769k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang S, Chen L, Sun S, Shah P, Yang W, Zhang B, Zhang Z, Chan D, Kass DA, van Eyk J, Zhang H. Proteomics. 2015;15:567–579. doi: 10.1002/pmic.201400151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toghi Eshghi S, Shah P, Yang W, Li X, Zhang H. Anal. Chem. 2015;87:5181–5188. doi: 10.1021/acs.analchem.5b00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carey F, Giuliano R. Organic Chemistry. New York: McGraw-Hill Education; 2013. [Google Scholar]
- 21.Zachara NE. Am. J. Physiol. Heart Circ. Physiol. 2012;302:H1905–H1918. doi: 10.1152/ajpheart.00445.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuzwa SA, Macauley MS, Heinonen JE, Shan X, Dennis RJ, He Y, Whitworth GE, Stubbs KA, McEachern EJ, Davies GJ, Vocadlo DJ. Nat. Chem. Biol. 2008;4:483–490. doi: 10.1038/nchembio.96. [DOI] [PubMed] [Google Scholar]
- 23.Rong J, Han J, Dong L, Tan Y, Yang H, Feng L, Wang Q-W, Meng R, Zhao J, Wang S-Q, Chen XJ. Am. Chem. Soc. 2014;136:17468–17476. doi: 10.1021/ja508484c. [DOI] [PubMed] [Google Scholar]
- 24.Kiarash A, Kelly CE, Phinney BS, Valdivia HH, Abrams J, Cala SE. Cardiovasc. Res. 2004;63:264–272. doi: 10.1016/j.cardiores.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Shamsi KS, Pierce A, Ashton AS, Halade DG, Richardson A, Espinoza SE. J. Gerontol., Ser. A. 2012;67:853–864. doi: 10.1093/gerona/glr224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maron BJ. JAMA. 2002;287:1308–1320. doi: 10.1001/jama.287.10.1308. [DOI] [PubMed] [Google Scholar]
- 27.Maron BJ. Circulation. 2002;106:2419–2421. doi: 10.1161/01.cir.0000034170.83171.0b. [DOI] [PubMed] [Google Scholar]
- 28.Geisterfer-Lowrance AA, Kass S, Tanigawa G, Vosberg H-P, McKenna W, Seidman CE, Seidman J. Cell. 1990;62:999–1006. doi: 10.1016/0092-8674(90)90274-i. [DOI] [PubMed] [Google Scholar]
- 29.Thierfelder L, Watkins H, MacRae C, Lamas R, McKenna W, Vosberg H-P, Seldman J, Seidman CE. Cell. 1994;77:701–712. doi: 10.1016/0092-8674(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 30.Watkins H, McKenna WJ, Thierfelder L, Suk HJ, Anan R, O'Donoghue A, Spirito P, Matsumori A, Moravec CS, Seidman JG, Seidman CE. N. Engl. J. Med. 1995;332:1058–1065. doi: 10.1056/NEJM199504203321603. [DOI] [PubMed] [Google Scholar]
- 31.Martos R, Baugh J, Ledwidge M, O'Loughlin C, Conlon C, Patle A, Donnelly SC, McDonald K. Circulation. 2007;115:888–895. doi: 10.1161/CIRCULATIONAHA.106.638569. [DOI] [PubMed] [Google Scholar]
- 32.Van den Bosch B, Lindsey P, Van den Burg C, Van der Vlies S, Lips D, Van der Vusse G, Ayoubi T, Doevendans P, Smeets H. Genomics. 2006;88:480–488. doi: 10.1016/j.ygeno.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 33.Wagner RA, Tabibiazar R, Powers J, Bernstein D, Quertermous TJ. Mol. Cell. Cardiol. 2004;37:1159–1170. doi: 10.1016/j.yjmcc.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Zeidan A, Javadov S, Karmazyn M. Cardiovasc. Res. 2006;72:101–111. doi: 10.1016/j.cardiores.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 35.López B, González A, Hermida N, Valencia F, de Teresa E, Díez J. Am. J. Physiol-Heart C. 2010;299:H1–H9. doi: 10.1152/ajpheart.00335.2010. [DOI] [PubMed] [Google Scholar]
- 36.Molkentin JD, Dorn GW., II Annu. Rev. Physiol. 2001;63:391–426. doi: 10.1146/annurev.physiol.63.1.391. [DOI] [PubMed] [Google Scholar]
- 37.Hynes RO. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 38.Desai LP, Wu Y, Tepper RS, Gunst SJ. Am. J. Physiol. Lung Cell Mol. Physiol. 2011;301:L275–L284. doi: 10.1152/ajplung.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teekakirikul P, Eminaga S, Toka O, Alcalai R, Wang L, Wakimoto H, Nayor M, Konno T, Gorham JM, Wolf CM, Kim JB, Schmitt JP, Molkentin JD, Norris RA, Tager AM, Hoffman SR, Markwald RR, Seidman CE, Seidman JG. J. Clin. Invest. 2010;120:3520–3529. doi: 10.1172/JCI42028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brancaccio M, Fratta L, Notte A, Hirsch E, Poulet R, Guazzone S, De Acetis M, Vecchione C, Marino G, Altruda F, Silengo L, Tarone G, Lembo G. Nat. Med. 2002;9:68–75. doi: 10.1038/nm805. [DOI] [PubMed] [Google Scholar]
- 41.Pizza FX, Peterson JM, Baas JH, Koh TJ. J. Physiol. 2005;562:899–913. doi: 10.1113/jphysiol.2004.073965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jin ZG, Melaragno MG, Liao DF, Yan C, Haendeler J, Suh YA, Lambeth JD, Berk BC. Circ. Res. 2000;87:789–796. doi: 10.1161/01.res.87.9.789. [DOI] [PubMed] [Google Scholar]
- 43.Brink M, Chrast J, Price SR, Mitch WE, Delafontaine P. Hypertension. 1999;34:1053–1059. doi: 10.1161/01.hyp.34.5.1053. [DOI] [PubMed] [Google Scholar]
- 44.Belke DD, Betuing S, Tuttle MJ, Graveleau C, Young ME, Pham M, Zhang D, Cooksey RC, McClain DA, Litwin SE, Taegtmeyer H, Severson D, Kahn CR, Abel ED. J. Clin. Invest. 2002;109:629–639. doi: 10.1172/JCI13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arimura T, Bos JM, Sato A, Kubo T, Okamoto H, Nishi H, Harada H, Koga Y, Moulik M, Doi YL. J. Am. Coll. Cardiol. 2009;54:334–342. doi: 10.1016/j.jacc.2008.12.082. [DOI] [PubMed] [Google Scholar]
- 46.Sambandam N, Lopaschuk GD, Brownsey RW, Allard MF. Heart Failure Rev. 2002;7:161–173. doi: 10.1023/a:1015380609464. [DOI] [PubMed] [Google Scholar]
- 47.Hubal MJ, Reich KA, De Biase A, Bilbie C, Clarkson PM, Hoffman EP, Thompson PD. Muscle Nerve. 2011;44:393–401. doi: 10.1002/mus.22081. [DOI] [PubMed] [Google Scholar]
- 48.Yang S, Toghi Eshghi S, Chiu H, DeVoe DL, Zhang H. Anal. Chem. 2013;85:10117–10125. doi: 10.1021/ac4013013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh C, Zampronio CG, Creese AJ, Cooper HJ. J. Proteome Res. 2012;11:4517–4525. doi: 10.1021/pr300257c. [DOI] [PubMed] [Google Scholar]
- 50.Drake RR, Schwegler EE, Malik G, Diaz J, Block T, Mehta A, Semmes OJ. Mol. Cell. Proteomics. 2006;5:1957–1967. doi: 10.1074/mcp.M600176-MCP200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.