Abstract
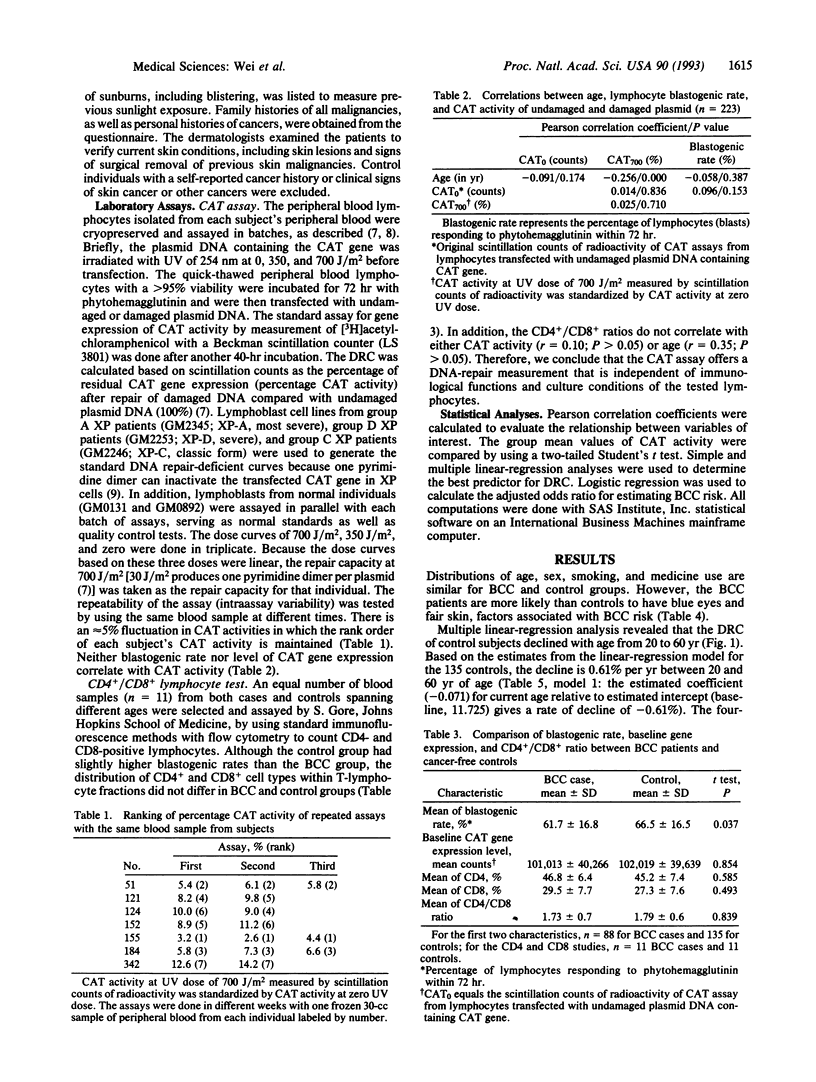
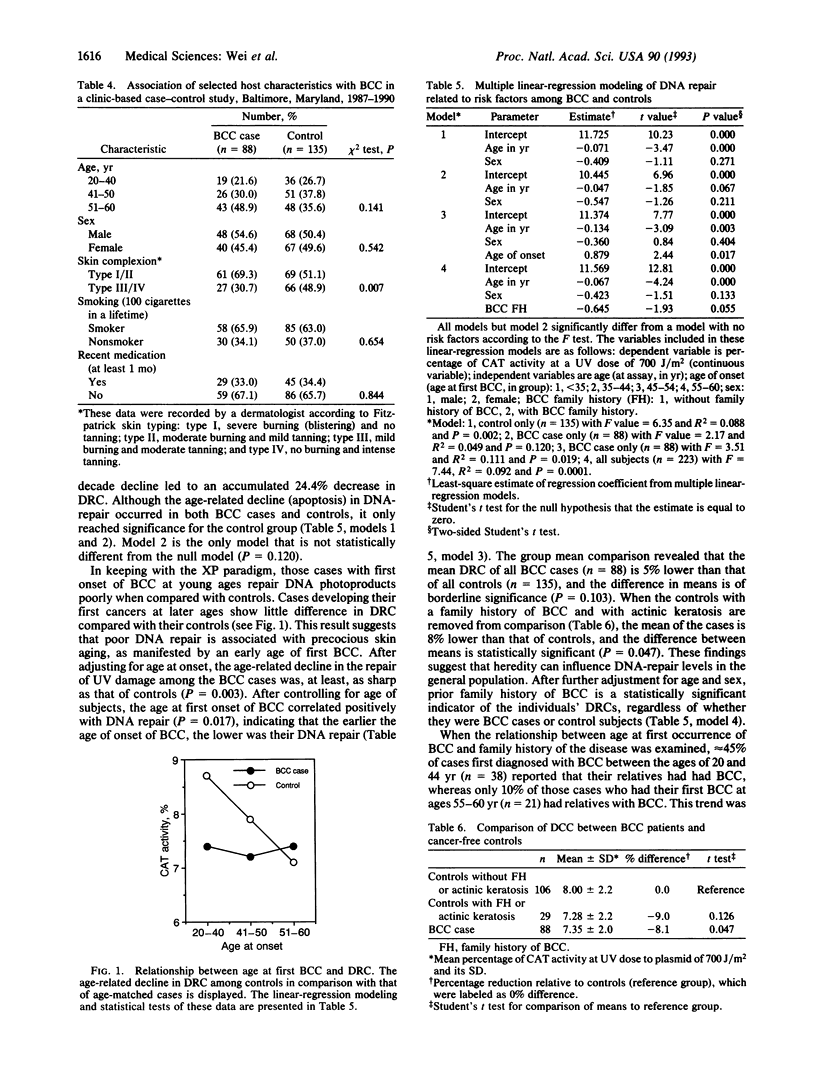
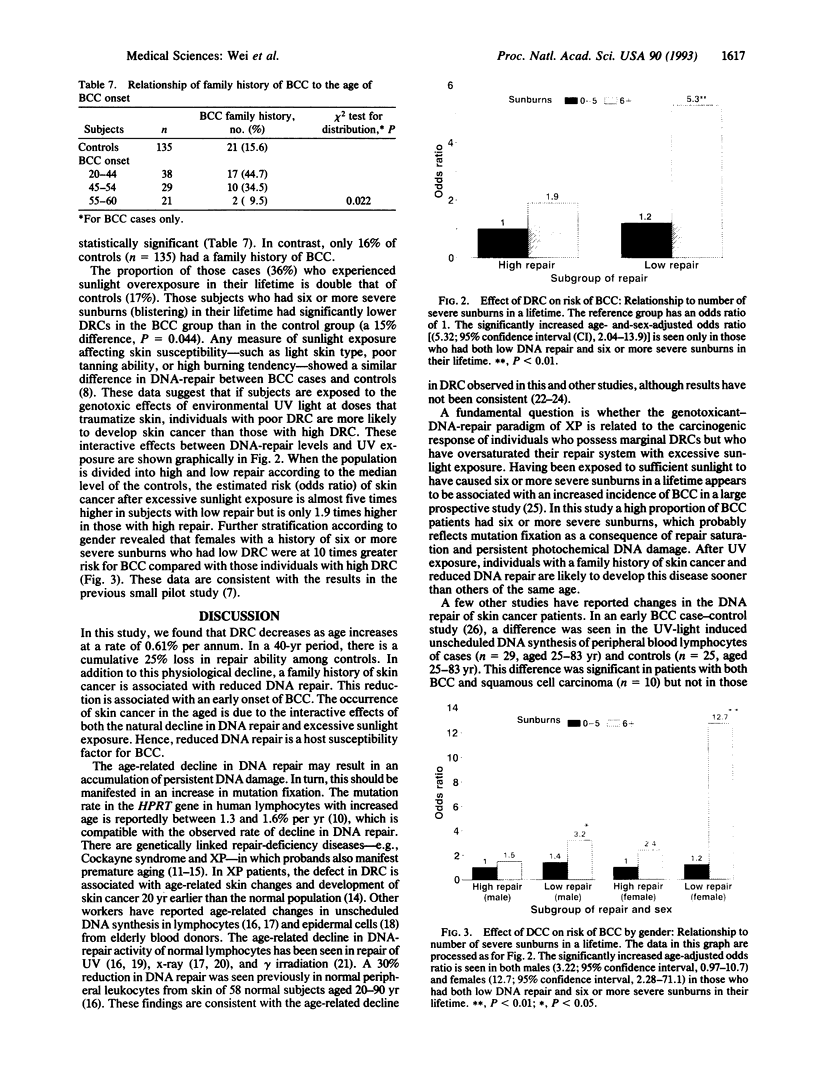
This molecular epidemiology study examines the DNA-repair capacities (DRCs) of basal cell carcinoma (BCC) skin cancer patients (88) and their controls (135) by using a plasmid/host-cell reactivation assay. In this assay UV-damaged expression vector plasmid is transfected into peripheral blood T lymphocytes from the subjects. The host-cellular repair enzymes repair the photochemical damage in the plasmid, and 40 hr later the plasmid-encoded reporter chloramphenicol acetyltransferase is measured. An age-related decline in this DRC, amounting to approximately 0.61% per yr occurred in the controls from 20 to 60 yr of age. Reduced DRC was a particularly important risk factor for young individuals with BCC and for those individuals with a family history of skin cancer. Young individuals with BCC repaired DNA damage poorly when compared with controls. As the BCC patients aged, however, differences between cases and controls gradually disappeared. The normal decline in DNA repair with increased age may account for the increased risk of skin cancer that begins in middle age, suggesting that the occurrence of skin cancer in the young may represent precocious aging. Patients with reduced DRCs and overexposure to sunlight had an estimated risk of BCC > 5-fold greater than the control group. Such a risk was even greater (10-fold) in female subjects.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcalay J., Freeman S. E., Goldberg L. H., Wolf J. E. Excision repair of pyrimidine dimers induced by simulated solar radiation in the skin of patients with basal cell carcinoma. J Invest Dermatol. 1990 Nov;95(5):506–509. doi: 10.1111/1523-1747.ep12504707. [DOI] [PubMed] [Google Scholar]
- Ananthaswamy H. N., Price J. E., Goldberg L. H., Bales E. S. Detection and identification of activated oncogenes in human skin cancers occurring on sun-exposed body sites. Cancer Res. 1988 Jun 15;48(12):3341–3346. [PubMed] [Google Scholar]
- Andrews A. D., Barrett S. F., Yoder F. W., Robbins J. H. Cockayne's syndrome fibroblasts have increased sensitivity to ultraviolet light but normal rates of unscheduled DNA synthesis. J Invest Dermatol. 1978 May;70(5):237–239. doi: 10.1111/1523-1747.ep12541383. [DOI] [PubMed] [Google Scholar]
- Athas W. F., Hedayati M. A., Matanoski G. M., Farmer E. R., Grossman L. Development and field-test validation of an assay for DNA repair in circulating human lymphocytes. Cancer Res. 1991 Nov 1;51(21):5786–5793. [PubMed] [Google Scholar]
- Brash D. E., Rudolph J. A., Simon J. A., Lin A., McKenna G. J., Baden H. P., Halperin A. J., Pontén J. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10124–10128. doi: 10.1073/pnas.88.22.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver J. E. Defective repair replication of DNA in xeroderma pigmentosum. Nature. 1968 May 18;218(5142):652–656. doi: 10.1038/218652a0. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. Xeroderma pigmentosum: a human disease in which an initial stage of DNA repair is defective. Proc Natl Acad Sci U S A. 1969 Jun;63(2):428–435. doi: 10.1073/pnas.63.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J., Green M. H., James S. E., Henderson L., Cole H. A further assessment of factors influencing measurements of thioguanine-resistant mutant frequency in circulating T-lymphocytes. Mutat Res. 1988 Mar;204(3):493–507. doi: 10.1016/0165-1218(88)90044-4. [DOI] [PubMed] [Google Scholar]
- Harris G., Holmes A., Sabovljev S. A., Cramp W. A., Hedges M., Hornsey S., Hornsey J. M., Bennett G. C. Sensitivity to X-irradiation of peripheral blood lymphocytes from ageing donors. Int J Radiat Biol Relat Stud Phys Chem Med. 1986 Oct;50(4):685–694. doi: 10.1080/09553008614551091. [DOI] [PubMed] [Google Scholar]
- Hunter D. J., Colditz G. A., Stampfer M. J., Rosner B., Willett W. C., Speizer F. E. Risk factors for basal cell carcinoma in a prospective cohort of women. Ann Epidemiol. 1990 Oct;1(1):13–23. doi: 10.1016/1047-2797(90)90015-k. [DOI] [PubMed] [Google Scholar]
- Hübscher U., Kuenzle C. C., Spadari S. Variation of DNA polymerases-alpha, -beta. and -gamma during perinatal tissue growth and differentiation. Nucleic Acids Res. 1977 Aug;4(8):2917–2929. doi: 10.1093/nar/4.8.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs E., Weber W., Müller H. Age-related variation in the DNA-repair synthesis after UV-C irradiation in unstimulated lymphocytes of healthy blood donors. Mutat Res. 1984 May-Jun;131(5-6):231–237. doi: 10.1016/0167-8817(84)90030-0. [DOI] [PubMed] [Google Scholar]
- Kraemer K. H., Lee M. M., Scotto J. Xeroderma pigmentosum. Cutaneous, ocular, and neurologic abnormalities in 830 published cases. Arch Dermatol. 1987 Feb;123(2):241–250. doi: 10.1001/archderm.123.2.241. [DOI] [PubMed] [Google Scholar]
- Kutlaca R., Seshadri R., Morley A. A. Effect of age on sensitivity of human lymphocytes to radiation. A brief note. Mech Ageing Dev. 1982 Jun;19(2):97–101. doi: 10.1016/0047-6374(82)90001-x. [DOI] [PubMed] [Google Scholar]
- Lambert B., Ringborg U., Skoog L. Age-related decrease of ultraviolet light-induced DNA repair synthesis in human peripheral leukocytes. Cancer Res. 1979 Jul;39(7 Pt 1):2792–2795. [PubMed] [Google Scholar]
- Licastro F., Franceschi C., Chiricolo M., Battelli M. G., Tabacchi P., Cenci M., Barboni F., Pallenzona D. DNA repair after gamma radiation and superoxide dismutase activity in lymphocytes from subjects of far advanced age. Carcinogenesis. 1982;3(1):45–48. doi: 10.1093/carcin/3.1.45. [DOI] [PubMed] [Google Scholar]
- Munch-Petersen B., Frentz G., Squire B., Wallevik K., Horn C. C., Reymann F., Faber M. Abnormal lymphocyte response to u.v. radiation in multiple skin cancer. Carcinogenesis. 1985 Jun;6(6):843–845. doi: 10.1093/carcin/6.6.843. [DOI] [PubMed] [Google Scholar]
- Nette E. G., Xi Y. P., Sun Y. K., Andrews A. D., King D. W. A correlation between aging and DNA repair in human epidermal cells. Mech Ageing Dev. 1984 Mar;24(3):283–292. doi: 10.1016/0047-6374(84)90114-3. [DOI] [PubMed] [Google Scholar]
- Protić-Sabljić M., Kraemer K. H. One pyrimidine dimer inactivates expression of a transfected gene in xeroderma pigmentosum cells. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6622–6626. doi: 10.1073/pnas.82.19.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M., Emmons L. R., Häner M., Müller H. J., Boyle J. M. Age-related decrease in an early step of DNA-repair of normal human lymphocytes exposed to ultraviolet-irradiation. Exp Cell Res. 1989 Jan;180(1):171–177. doi: 10.1016/0014-4827(89)90221-8. [DOI] [PubMed] [Google Scholar]
- Setlow R. B. DNA repair, aging, and cancer. Natl Cancer Inst Monogr. 1982;60:249–255. [PubMed] [Google Scholar]
- Setlow R. B., Regan J. D., German J., Carrier W. L. Evidence that xeroderma pigmentosum cells do not perform the first step in the repair of ultraviolet damage to their DNA. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1035–1041. doi: 10.1073/pnas.64.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. P., Danner D. B., Tice R. R., Brant L., Schneider E. L. DNA damage and repair with age in individual human lymphocytes. Mutat Res. 1990 May-Jul;237(3-4):123–130. doi: 10.1016/0921-8734(90)90018-m. [DOI] [PubMed] [Google Scholar]
- Turner D. R., Griffith V. C., Morley A. A. Ageing in vivo does not alter the kinetics of DNA strand break repair. Mech Ageing Dev. 1982 Aug;19(4):325–331. doi: 10.1016/0047-6374(82)90016-1. [DOI] [PubMed] [Google Scholar]
- Wade M. H., Chu E. H. Effects of DNA damaging agents on cultured fibroblasts derived from patients with Cockayne syndrome. Mutat Res. 1979 Jan;59(1):49–60. doi: 10.1016/0027-5107(79)90194-5. [DOI] [PubMed] [Google Scholar]





