Abstract
Growth-restricted fetuses with absent or reversed end-diastolic velocities in the umbilical artery are at substantially increased risk for adverse perinatal and long-term outcome, even in comparison to growth-restricted fetuses with preserved end-diastolic velocities. Translational studies show that this Doppler velocimetry correlates with fetoplacental blood flow, with absent or reversed end-diastolic velocities signifying abnormally elevated resistance within the placental vasculature. The fetoplacental vasculature is unique in that it is not subject to autonomic regulation, unlike other vascular beds. Instead, humoral mediators, many of which are synthesized by local endothelial cells, regulate placental vascular resistance. Existing data demonstrate that in growth-restricted pregnancies complicated by absent or reversed umbilical artery end-diastolic velocities, an imbalance in production of these vasoactive substances occurs, favoring vasoconstriction. Morphologically, placentas from these pregnancies also demonstrate impaired angiogenesis, whereby vessels within the terminal villi are sparsely branched, abnormally thin, and elongated. This structural deviation from normal placental angiogenesis restricts blood flow and further contributes to elevated fetoplacental vascular resistance. Although considerable work has been done in the field of fetoplacental vascular development and function, much remains unknown about the mechanisms underlying impaired development and function of the human fetoplacental vasculature, especially in the context of severe FGR with absent or reversed umbilical artery end-diastolic velocities. Fetoplacental endothelial cells are key regulators of angiogenesis and vasomotor tone. A thorough understanding of their role in placental vascular biology carries the significant potential of discovering clinically relevant and innovative approaches to prevention and treatment of fetal growth restriction with compromised umbilical artery end-diastolic velocities.
Key words/phrases: Fetal growth restriction, Umbilical artery Doppler, Fetoplacental endothelium
Introduction
Fetal growth restriction (FGR) confers substantial risk for adverse perinatal outcomes, including stillbirth, neonatal death, and complications related to prematurity. Beyond the perinatal period, children who were growth-restricted in utero remain at higher risk for neurocognitive delay, and they are more likely to develop obesity, metabolic syndrome, and cardiovascular disease later in life.1–6 While current obstetric management paradigms may be able to time delivery in order to avert stillbirth in this population, long-term outcomes remain unchanged.7–9
Although abnormalities in the maternal circulation can certainly contribute to the pathophysiology of FGR, the fetoplacental vasculature also plays a critical role in normal development, as studies clearly indicate that growth-restricted fetuses with absent or reversed end-diastolic umbilical artery velocities (AEDV/REDV) suffer even worse outcomes than fetuses with FGR and preserved end-diastolic velocities.5,10–16 As current clinical interventions have not been shown to improve outcome in FGR fetuses with AEDV/REDV, a thorough understanding of the fetoplacental vasculature, including its unique development and functional regulation, has the potential to open up new avenues of prevention and/or treatment.
Umbilical artery Doppler assessment in FGR
Both the Society for Maternal-Fetal Medicine and the American Congress of Obstetricians and Gynecologists endorse umbilical artery Doppler assessment in high-risk pregnancies with suspected FGR.17,18 This recommendation is based upon a body of fundamental translational and clinical studies.
In an early, key ovine study, embolizing the fetoplacental cotyledons along the umbilical arteries resulted in higher placental vascular resistance as measured by radioactive microsphere count.19 This, in turn, led directly to decreased umbilical artery end-diastolic velocities and higher peak systolic/diastolic (S/D) ratios.19 Both these findings confirmed that velocity waveforms within the umbilical artery reflect placental vascular resistance.
Fetoplacental vascular resistance, as assessed by the umbilical S/D ratio as one example, normally decreases as gestation progresses.20–22 In growth-restricted fetuses, however, umbilical artery end-diastolic velocities were frequently lower than expected for gestational age.21,23,24 The elevated resistance represented by these low velocities correlated with placental structural and histopathologic abnormalities, as well as with adverse pregnancy outcomes.25–29
Subsequently, umbilical artery Doppler velocimetry underwent rigorous clinical testing. In general, the majority of these trials demonstrated a lack of benefit in the low-risk obstetric population.31–35 In contrast, when limited to women at risk for a potentially compromised fetus (i.e. “high-risk”), most trials found that umbilical artery Doppler testing offered some degree of value, with outcomes ranging from fewer emergency deliveries to less death and serious neonatal morbidities.36–40 A key meta-analysis, as well as a recent Cochrane review, concluded that using umbilical artery Doppler in high-risk pregnancies reduced the risk of perinatal death by 29 to 38 percent, without increasing interventions like iatrogenic preterm delivery.30,41 These findings solidified the role of umbilical artery Doppler ultrasonography in high-risk pregnancies. Despite the reduction in risk of perinatal death with use of umbilical artery Doppler velocimetry, however, existing studies demonstrate that overall survival and long-term outcomes are unchanged.7–9 Thus, an in-depth understanding of the fetoplacental vasculature is needed if preventions and treatments mitigating this end-stage process are to be found.
The fetoplacental vasculature in FGR
Maternal hypoperfusion of the placenta is a common cause of FGR.42,43 However, the fetoplacental vasculature is also an important component of placental perfusion and hence vital to fetal growth. This is demonstrated by a cohort of 34 growth-restricted fetuses; while all demonstrated abnormally low umbilical artery diastolic flow velocities, 21 of these pregnancies were found to have normal uterine artery Dopplers.44 Thus, fetal growth abnormalities and abnormal umbilical artery Doppler velocimetry can occur even in the presence of normal maternal uteroplacental blood flow..44
Common placental pathologic findings in FGR include a small placenta, avascular terminal villi, fibrinoid necrosis, and multiple villous infarcts.45–47 However, additional pathologic features often then diverge, depending upon whether umbilical artery end-diastolic velocity is absent/reversed or preserved. Placentas from pregnancies complicated by FGR with AEDV/REDV are significantly more likely to have marginal cord insertions when compared to those from growth-restricted pregnancies with preserved diastolic velocities, even those with elevated S/D ratios.48 The stem villous vessels from placentas complicated by FGR with AEDV/REDV demonstrate luminal obliteration and concentric intimal and medial wall thickening, and the percentage of abnormal vessels directly correlate with fetoplacental vascular resistance.27,49
FGR placentas also differ at the microvascular level depending on whether end-diastolic velocities are present or absent. For example, those with preserved end-diastolic velocities have normal or more highly branched capillary beds.26,50 In contrast, branches of mature intermediate villi are largely absent in FGR placentas with AEDV/REDV, and terminal capillaries appear thin and elongated.27,51–53 This decrease in peripheral villous vasculature contributes to elevated fetoplacental vascular resistance.27,52
Fetoplacental endothelium
Understanding the mechanisms that underlie these placental pathologic findings lies, at least in part, in the fetoplacental endothelium. Throughout the body, the endothelium plays a key role in vascular physiology by regulating vasomotor tone, balancing pro- and anticoagulant activity, tempering inflammatory mediators, modulating cellular and nutrient trafficking, and driving angiogenesis.54 Endothelial cells are also subject to their local environment, leading to tissue-specific phenotypes. This heterogeneity is a key feature of the endothelium.55–59
The endothelium in the human placenta reaches approximately 550 km in length and occupies 15 square meters at term gestation.60 Despite being found in continuity and within the same organ, the endothelium in the umbilical cord and in the placental vasculature can exhibit significant phenotypic diversity. Within the umbilical cord alone, endothelium from the human umbilical artery differs from that within the umbilical vein. For example, after culturing separately isolated endothelial cells from the umbilical vein and umbilical artery and further subjecting them to shear stress, the expression of endothelin-1, a vasoconstrictor, is significantly lower in human umbilical vein endothelial cells than in umbilical artery endothelial cells.61 This may be one contributing mechanism that allows the umbilical vein to maintain proper patency in its setting of high flow.61
Estrogen receptors are expressed in a wide variety of tissue including endothelial cells and vascular smooth muscle cells.62,63 With respect to vascular physiology, estrogen receptors regulate expression of multiple vasodilator and vasoconstrictor proteins, and whether a vessel constricts or dilates in response to estrogen appears to be dependent on estrogen receptor profile and tissue specificity.62–68 Expression of genes related to estrogen biology can also vary within the fetoplacental endothelium. For example, estrogen receptor-β is expressed in higher quantities in human umbilical artery endothelial cells than human umbilical vein endothelial cells, as is 17 beta-hydroxysteroid dehydrogenase type 2, a gene that encodes an enzyme that converts estradiol into its less biologically active form estrone.69,70 Although the physiologic implications of these specific findings on normal placental vascular biology remain incompletely understood, estrogen receptor-β expression is higher within fetoplacental endothelium from FGR placentas with AEDV/REDV compared to gestational age-matched, appropriately grown control subjects.71 This higher estrogen receptor-β expression results in up-regulation of cyclooxygenase-2 (COX-2) expression and activity and down-regulation of vasodilator gene expression. These changes in gene expression of key enzymes shift the vascular prostanoid profile derived from endothelial cells toward production of vasoconstrictive mediators.71,72
Within chorionic plate and stem villous vessels, microarray data also demonstrate differences in gene expression between arterial and venous endothelial cells. Compared to placental arterial endothelial cells, placental venous endothelial cells more strongly express genes associated with transport activity and lipid metabolism, suggesting that venous endothelial cells may have a phenotype that allows for enhanced nutrient transport to the fetus.74 In contrast, most of the genes in placental arterial endothelial cells are associated with signal transduction and other molecular pathways including vascular endothelial growth factor A (VEGF) signaling.74 VEGF stimulates angiogenesis and stabilizes newly formed vessels.75–78 Thus, the fact that placental arterial endothelial cells express more genes related to VEGF signaling than placental venous ones do suggests that placental arterial endothelial cells play a more important role in forming the fetoplacental vasculature.
Epigenetic regulation also plays a role in placental endothelial cell diversity. For example, genome-wide methylation studies have found that venous endothelial cells in the chorionic plate demonstrated higher degrees of hypomethylation than arterial endothelial cells in the same location..79 For example, increased promoter methylation was found within the proximal promoter region of the endothelial nitric oxide synthase gene in placental arterial endothelial cells compared to venous endothelial cells, resulting in higher nitric oxide synthase expression by placental venous endothelial cells.79,80 While the implications of these findings are not yet clear, it is possible that higher nitric oxide within the placental venous circulation contributes to maintenance of proper blood flow to the fetus. Interestingly, umbilical arterial endothelial cells from FGR pregnancies demonstrate decreased methylation at these same promoter sites compared to umbilical arterial endothelial cells from normal pregnancies, suggesting that stable alterations in gene expression potential that arise during development may affect fetoplacental vascular function.80
Further demonstrating the heterogeneity of endothelial cells within the placenta, placental microvascular endothelial cells differ from macrovascular umbilical vein endothelial cells. Functionally, placental microvascular endothelial cells secrete more prostanoids including 6- keto prostaglandin F1α (stable metabolite of the vasodilator prostacyclin) and thromboxane B2 (stable metabolite of the vasoconstrictor thromboxane A2) than do umbilical vein endothelial cells.59 Placental microvascular endothelial cells also proliferate in greater quantities than umbilical vein endothelial cells in response to VEGF.59 Thus, some investigators have suggested that human umbilical vein endothelial cells, the most commonly used endothelial cell type for experiments, may not always be the best model to study the biology of placental endothelial cells.59,81 Instead, endothelial cells isolated from a particular part of the placenta (e.g. arterial vs. venous and macrovascular vs. microvascular) that is most applicable to the specific area of study might serve as a better model of investigation.
Fetoplacental endothelial cells and mediation of vascular function
In most vascular beds, small arterioles contribute to most of the vascular resistance via autonomic and humoral influences.54,82 However, placental chorionic plate and stem villous vessels, which are similar in size to these arterioles, uniquely lack innervation.83 Instead, their vasomotor tone is solely controlled by locally produced vasoactive mediators, most of which are endothelially-derived.84,85 These placental vessels also respond differently to humoral factors than vessels in other vascular beds. For example, the placental vasculature is the only vascular bed that has been reported to constrict rather than dilate in response to prostaglandin E2.86 It also demonstrates blunted responses to other vascular mediators including acetylcholine, bradykinin, and angiotensin II.87–89
Fetoplacental endothelial cells are essential for vasoactive mediator responses such as nitric oxide-dependent vasodilatation and endothelin-1-mediated vasoconstriction within stem villous vessels, substantiating an endothelial role in control of fetoplacental vascular function.83,90 In a study comparing concentrations of vasoactive mediators in cordocentesis specimens between gestational age-matched, appropriately grown fetuses and FGR fetuses (with 60 percent of these fetuses with AEDV/REDV and the other 40 percent with an umbilical artery S/D ratio of > 95th percentile for gestational age), investigators found that endothelin-1 concentrations were significantly higher, while 6-keto prostaglandin F1α levels were lower in the FGR population in comparison to the controls.91 This suggests that circulating levels of vasoconstrictors are increased and vasodilators are decreased in FGR fetuses. Similarly, others have also confirmed that 6-keto prostaglandin F1α is synthesized in lower quantities within the umbilical artery in the setting of FGR.86,92,93 The exact mechanisms that underlie these changes in endothelial cell-derived vasoactive mediators have not been fully elucidated. Existing literature, however, has demonstrated that in addition to changes in endothelial estrogen receptor-β leading to alterations in vascular prostanoid production as previously described, fetal COX-2 gene polymorphisms that correlate to decreased COX-2 gene expression were associated with more placental malperfusion and FGR.94
The role that nitric oxide regulation plays in FGR remains uncertain. Suggesting decreased nitric-oxide-mediated vasodilation, the umbilical artery of FGR pregnancies has been found to exhibit less nitric oxide synthase protein expression than controls in an ovine model; 95 similarly, transport of L-arginine, the precursor to nitric oxide, is down-regulated in human umbilical vein endothelial cells of pregnancies complicated by FGR.96 In contrast, other studies have found that nitric oxide synthesis in FGR pregnancies is unaffected or actually upregulated, perhaps representing compensation for the vascular derangements of FGR.97–100 T
In addition to humoral mediators, potassium (K+) channel expression is a major component of endothelial interaction with smooth muscle.101 Although the specifics of all the various K+ channels are beyond the scope of this review, K+ channels generally have the ability to form pores and to influence cell membrane potential, thereby helping regulate vascular smooth muscle tone. Fetoplacental endothelial cells express functional K+ channels, which play a role in controlling placental vascular resistance.102 For example, blocking K+ channels within chorionic plate arteries and veins of FGR pregnancies increases basal tone.103
Angiogenesis of the fetoplacental vasculature
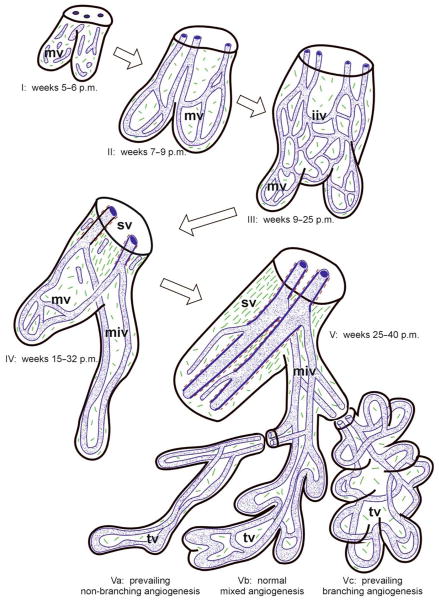
In addition to endothelial cell-mediated regulation of vasomotor tone, the anatomic configuration of the villous vasculature is also critical to fetoplacental blood flow. Vasculogenesis, the de novo formation of blood vessels, normally occurs within the human placenta by approximately 6 weeks gestation, resulting in formation of tertiary villi (Figure 1; I).104 As pregnancy progresses, these tertiary villi continue to differentiate and expand into immature intermediate villi and stem villi (Figure 1; II–IV). Concomitantly, there is a gradual increase in angiogenesis, whereby new blood vessels form from pre-existing vessels.105–107 However, the rate of angiogenesis significantly accelerates starting at around 25 weeks gestation, leading to exponential increases in the total length of the villous vascular tree, continuing until 40 weeks gestation (Figure 1; V).60,108 This sustained angiogenesis of the fetoplacental vasculature is a key reason for the normal, progressive increase in umbilical artery end-diastolic velocities that occurs as gestation advances.
Figure 1. Fetoplacental villous development throughout pregnancy.
(I) In post-menstrual (p.m.) weeks 5–6, fetal capillary segments are formed by vasculogenesis within mesenchymal villi (mv). (II) These fuse to form a simple capillary bed in weeks 7–8. (III) Between 9–25 weeks, this capillary bed expands by angiogenesis as mesenchymal villi develop into immature intermediate villi (iiv). (IV) Immature intermediate villi become transformed into stem villi (sv), while peripheral mesenchymal villi are transformed into mature intermediate villi (miv) between weeks 15–32. Concomitantly, centrally located capillaries develop into stem villous vessels, and the peripheral vasculature elongates. (V) In the last half of pregnancy, there is continued angiogenesis as terminal villi (tv) develop, resulting in the villous morphology demonstrated in Vb. In placentas from FGR pregnancies with preserved end-diastolic velocities, villi either resemble that illustrated in Vb or Vc, whereas FGR pregnancies complicated by AEDV/REDV have villi similar to that depicted in Va. (Blue: Endothelial tubes; Brown: Vascular smooth muscle cells; Green: Collagen fibers). From: Benirschke K, Burton GJ, Baergen RN. Architecture of normal villous trees. In: Pathology of the human placenta. New York, NY: Springer-Verlag Berlin Heidelberg; 2012: 122. With permission from Dr. Kurt Benirschke, Dr. Graham Burton, and Dr. Rebecca Baergen in addition to Springer Science + Business Media.
As mentioned previously, villous capillary density is either normal or even increased in FGR placentas with preserved end-diastolic velocities, even if the S/D ratio is greater than the 95th percentile for gestational age.51–53 In contrast, pregnancies complicated by FGR with AEDV/REDV have sparsely branched and abnormally thin capillaries, leading to fewer capillary loops.51–53,109,110 This decreased branching results in lower volume density, which in turn provides a structural basis for elevated fetoplacental vascular resistance.111
The mechanisms underlying this impaired angiogenesis in human fetoplacental vasculature remain incompletely elucidated, but endothelial cells are essential in the process of angiogenesis. They initiate the process by increasing vascular permeability and degradation of the endothelial cell basement membrane.112 Endothelial cells then proliferate and migrate, contact each other, form lumen, and finally, recruit pericytes and other cell types to stabilize the newly formed vessel.112,113
Various endothelial cell-derived angiogenic and anti-angiogenic factors are important for this process to occur. These include VEGF, soluble fms-like tyrosine kinase-1 (sFLT1), placental growth factor (PlGF), and fibroblast growth factor 2 (FGF2). While derangements in maternal serum levels of these factors have been implicated in disorders of placentation such as FGR, there is growing evidence that they also play a role in development of the fetoplacental vasculature.114–122 Compared to controls, endothelial cells isolated from placentas of pregnancies complicated by FGR with AEDV/REDV show evidence of impaired angiogenesis, as manifested by deficient tube formation (i.e. formation of capillary-like structures in vitro).123 One mechanism that contributes to this deficient angiogenic potential in endothelial cells derived from FGR with AEDV/REDV is abnormal regulation of VEGF expression.123 Imbalances between these angiogenic and antioangiogenic factors have also been found within fetal blood. For example, umbilical vein sFLT1 and FGF2 levels are increased in FGR pregnancies, while PlGF concentrations are decreased.124 Additionally, there was a positive correlation between umbilical vein sFLT1 and umbilical artery pulsatility index in FGR fetuses, while PlGF was negatively correlated.118 In contrast, these factors within the umbilical vein had no correlation to uterine artery pulsatility index, suggesting that the balance between angiogenic and antiangiogenic factors within the fetal circulation may play a direct role in fetoplacental angiogenesis.118
Conclusion
Normal intrauterine growth is dependent upon not just the maternal environment, but also the fetal component of placental perfusion. Impaired fetoplacental blood flow clinically manifests as absent or reversed end-diastolic velocities in the umbilical artery; in the setting of FGR, this Doppler finding portends a significantly elevated risk for adverse pregnancy outcome.
Translational studies have demonstrated clear correlation between Doppler velocimetry and fetoplacental perfusion, with absent or reversed end-diastolic velocities denoting abnormally elevated resistance within the placental vascular tree. The physiology of this flow impediment is twofold, comprised of both functional and structural etiologies.
The fetoplacental vasculature is unique in its lack of innervation, singular in being independent from the autonomic regulation to which other vascular beds are subject. Instead, existing data suggest that resistance within this system is regulated largely by humoral mediators from local endothelial cells. In pregnancies complicated by FGR with AEDV/REDV, an imbalance in these mediators yields a humoral milieu favoring vasoconstriction. Inhibited and impaired angiogenesis further contribute to placental vascular resistance in this population, creating structural changes that restrict blood flow.
Significant work has been done in the field of fetoplacental vascular formation and function. However, in the current absence of effective clinical management strategies for cases of FGR with AEDV/REDV, continued investigation into the physiological, cellular, molecular, and epigenetic regulation of the fetoplacental vasculature and endothelium are needed. This knowledge is essential if either preventive or therapeutic approaches that will truly improve perinatal and long-term outcomes are to be discovered.
Acknowledgments
Funding source: National Institutes of Health (HL119846), American Association of Obstetricians and Gynecologists Bridge Award
Parts of this work were supported by the National Institutes of Health (HL119846) and the American Association of Obstetricians and Gynecologists Foundation Bridge Fund Award.
I am indebted to Wayne Soong for his in-depth and thorough efforts at editing this review.
Footnotes
Disclosures: The author reports no conflict of interest
Wayne Soong, MD, MSCI, FCCP, VA Eastern Colorado Healthcare System, Compensation: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lackman F, Capewell V, Richardson B, daSilva O, Gagnon R. The risks of spontaneous preterm delivery and perinatal mortality in relation to size at birth according to fetal versus neonatal growth standards. American journal of obstetrics and gynecology. 2001;184(5):946–953. doi: 10.1067/mob.2001.111719. [DOI] [PubMed] [Google Scholar]
- 2.Helderman JB, O’Shea TM, Kuban KC, et al. Antenatal antecedents of cognitive impairment at 24 months in extremely low gestational age newborns. Pediatrics. 2012;129(3):494–502. doi: 10.1542/peds.2011-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arcangeli T, Thilaganathan B, Hooper R, Khan KS, Bhide A. Neurodevelopmental delay in small babies at term: a systematic review. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2012;40(3):267–275. doi: 10.1002/uog.11112. [DOI] [PubMed] [Google Scholar]
- 4.Chan PY, Morris JM, Leslie GI, Kelly PJ, Gallery ED. The long-term effects of prematurity and intrauterine growth restriction on cardiovascular, renal, and metabolic function. International journal of pediatrics. 2010;2010:280402. doi: 10.1155/2010/280402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bjarnegard N, Morsing E, Cinthio M, Lanne T, Brodszki J. Cardiovascular function in adulthood following intrauterine growth restriction with abnormal fetal blood flow. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2013;41(2):177–184. doi: 10.1002/uog.12314. [DOI] [PubMed] [Google Scholar]
- 6.Geva R, Eshel R, Leitner Y, Valevski AF, Harel S. Neuropsychological outcome of children with intrauterine growth restriction: a 9-year prospective study. Pediatrics. 2006;118(1):91–100. doi: 10.1542/peds.2005-2343. [DOI] [PubMed] [Google Scholar]
- 7.Walker DM, Marlow N, Upstone L, et al. The Growth Restriction Intervention Trial: long-term outcomes in a randomized trial of timing of delivery in fetal growth restriction. American journal of obstetrics and gynecology. 2011;204(1):34e31–39. doi: 10.1016/j.ajog.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 8.GRIT Study Group. A randomised trial of timed delivery for the compromised preterm fetus: short term outcomes and Bayesian interpretation. BJOG : an international journal of obstetrics and gynaecology. 2003;110(1):27–32. doi: 10.1046/j.1471-0528.2003.02014.x. [DOI] [PubMed] [Google Scholar]
- 9.Thornton JG, Hornbuckle J, Vail A, Spiegelhalter DJ, Levene M group Gs. Infant wellbeing at 2 years of age in the Growth Restriction Intervention Trial (GRIT): multicentred randomised controlled trial. Lancet. 2004;364(9433):513–520. doi: 10.1016/S0140-6736(04)16809-8. [DOI] [PubMed] [Google Scholar]
- 10.Baschat AA, Gembruch U, Reiss I, Gortner L, Weiner CP, Harman CR. Relationship between arterial and venous Doppler and perinatal outcome in fetal growth restriction. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2000;16(5):407–413. doi: 10.1046/j.1469-0705.2000.00284.x. [DOI] [PubMed] [Google Scholar]
- 11.Eger SH, Sommerfelt K, Kiserud T, Markestad T. Foetal umbilical artery Doppler in small preterms: (IQ) neurocognitive outcome at 5 years of age. Acta paediatrica. 2013;102(4):403–409. doi: 10.1111/apa.12164. [DOI] [PubMed] [Google Scholar]
- 12.Flood K, Unterscheider J, Daly S, et al. The role of brain sparing in the prediction of adverse outcomes in intrauterine growth restriction: results of the multicenter PORTO Study. American journal of obstetrics and gynecology. 2014;211(3):288.e281–285. doi: 10.1016/j.ajog.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez JM, Stamilio DM, Ural S, Macones GA, Odibo AO. Relationship between abnormal fetal testing and adverse perinatal outcomes in intrauterine growth restriction. American journal of obstetrics and gynecology. 2007;196(5):e48–51. doi: 10.1016/j.ajog.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 14.McCowan LM, Erskine LA, Ritchie K. Umbilical artery Doppler blood flow studies in the preterm, small for gestational age fetus. American journal of obstetrics and gynecology. 1987;156(3):655–659. doi: 10.1016/0002-9378(87)90071-8. [DOI] [PubMed] [Google Scholar]
- 15.Crimmins S, Desai A, Block-Abraham D, Berg C, Gembruch U, Baschat AA. A comparison of Doppler and biophysical findings between liveborn and stillborn growth-restricted fetuses. American journal of obstetrics and gynecology. 2014;211(6):669.e661–610. doi: 10.1016/j.ajog.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 16.Brar HS, Platt LD. Reverse end-diastolic flow velocity on umbilical artery velocimetry in high-risk pregnancies: an ominous finding with adverse pregnancy outcome. American journal of obstetrics and gynecology. 1988;159(3):559–561. doi: 10.1016/s0002-9378(88)80007-3. [DOI] [PubMed] [Google Scholar]
- 17.Fetal Growth Restriction. Practice Bulletin No. 134. American Congress of Obstetricians and Gynecologists. Obstet Gynecol. 2013;121:1122–1133. doi: 10.1097/01.AOG.0000429658.85846.f9. [DOI] [PubMed] [Google Scholar]
- 18.Society for Maternal-Fetal Medicine Publications C. Berkley E, Chauhan SP, Abuhamad A. Doppler assessment of the fetus with intrauterine growth restriction. American journal of obstetrics and gynecology. 2012;206(4):300–308. doi: 10.1016/j.ajog.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 19.Trudinger BJ, Stevens D, Connelly A, et al. Umbilical artery flow velocity waveforms and placental resistance: the effects of embolization of the umbilical circulation. American journal of obstetrics and gynecology. 1987;157(6):1443–1448. doi: 10.1016/s0002-9378(87)80241-7. [DOI] [PubMed] [Google Scholar]
- 20.Schulman H, Fleischer A, Stern W, Farmakides G, Jagani N, Blattner P. Umbilical velocity wave ratios in human pregnancy. American journal of obstetrics and gynecology. 1984;148(7):985–990. doi: 10.1016/0002-9378(84)90541-6. [DOI] [PubMed] [Google Scholar]
- 21.Fleischer A, Schulman H, Farmakides G, Bracero L, Blattner P, Randolph G. Umbilical artery velocity waveforms and intrauterine growth retardation. American journal of obstetrics and gynecology. 1985;151(4):502–505. doi: 10.1016/0002-9378(85)90278-9. [DOI] [PubMed] [Google Scholar]
- 22.Sutton MS, Theard MA, Bhatia SJ, Plappert T, Saltzman DH, Doubilet P. Changes in placental blood flow in the normal human fetus with gestational age. Pediatric research. 1990;28(4):383–387. doi: 10.1203/00006450-199010000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Groenenberg IA, Wladimiroff JW, Hop WC. Fetal cardiac and peripheral arterial flow velocity waveforms in intrauterine growth retardation. Circulation. 1989;80(6):1711–1717. doi: 10.1161/01.cir.80.6.1711. [DOI] [PubMed] [Google Scholar]
- 24.Reuwer PJ, Bruinse HW, Stoutenbeek P, Haspels AA. Doppler assessment of the fetoplacental circulation in normal and growth-retarded fetuses. European journal of obstetrics, gynecology, and reproductive biology. 1984;18(4):199–205. doi: 10.1016/0028-2243(84)90117-5. [DOI] [PubMed] [Google Scholar]
- 25.Giles WB, Trudinger BJ, Baird PJ. Fetal umbilical artery flow velocity waveforms and placental resistance: pathological correlation. British journal of obstetrics and gynaecology. 1985;92(1):31–38. doi: 10.1111/j.1471-0528.1985.tb01045.x. [DOI] [PubMed] [Google Scholar]
- 26.Hitschold T, Weiss E, Beck T, Hunterfering H, Berle P. Low target birth weight or growth retardation? Umbilical Doppler flow velocity waveforms and histometric analysis of fetoplacental vascular tree. American journal of obstetrics and gynecology. 1993;168(4):1260–1264. doi: 10.1016/0002-9378(93)90377-u. [DOI] [PubMed] [Google Scholar]
- 27.Salafia CM, Pezzullo JC, Minior VK, Divon MY. Placental pathology of absent and reversed end-diastolic flow in growth-restricted fetuses. Obstet Gynecol. 1997;90(5):830–836. doi: 10.1016/S0029-7844(97)00473-0. [DOI] [PubMed] [Google Scholar]
- 28.Trudinger BJ, Cook CM, Giles WB, et al. Fetal umbilical artery velocity waveforms and subsequent neonatal outcome. British journal of obstetrics and gynaecology. 1991;98(4):378–384. doi: 10.1111/j.1471-0528.1991.tb13428.x. [DOI] [PubMed] [Google Scholar]
- 29.Trudinger BJ, Giles WB, Cook CM, Bombardieri J, Collins L. Fetal umbilical artery flow velocity waveforms and placental resistance: clinical significance. British journal of obstetrics and gynaecology. 1985;92(1):23–30. doi: 10.1111/j.1471-0528.1985.tb01044.x. [DOI] [PubMed] [Google Scholar]
- 30.Alfirevic Z, Neilson JP. Doppler ultrasonography in high-risk pregnancies: systematic review with meta-analysis. American journal of obstetrics and gynecology. 1995;172(5):1379–1387. doi: 10.1016/0002-9378(95)90466-2. [DOI] [PubMed] [Google Scholar]
- 31.Omtzigt AM, Reuwer PJ, Bruinse HW. A randomized controlled trial on the clinical value of umbilical Doppler velocimetry in antenatal care. American journal of obstetrics and gynecology. 1994;170(2):625–634. doi: 10.1016/s0002-9378(94)70240-3. [DOI] [PubMed] [Google Scholar]
- 32.Davies JA, Gallivan S, Spencer JA. Randomised controlled trial of Doppler ultrasound screening of placental perfusion during pregnancy. Lancet. 1992;340(8831):1299–1303. [PubMed] [Google Scholar]
- 33.Mason GC, Lilford RJ, Porter J, Nelson E, Tyrell S. Randomised comparison of routine versus highly selective use of Doppler ultrasound in low risk pregnancies. British journal of obstetrics and gynaecology. 1993;100(2):130–133. doi: 10.1111/j.1471-0528.1993.tb15207.x. [DOI] [PubMed] [Google Scholar]
- 34.Whittle MJ, Hanretty KP, Primrose MH, Neilson JP. Screening for the compromised fetus: a randomized trial of umbilical artery velocimetry in unselected pregnancies. American journal of obstetrics and gynecology. 1994;170(2):555–559. doi: 10.1016/s0002-9378(94)70226-8. [DOI] [PubMed] [Google Scholar]
- 35.Newnham JP, Evans SF, Michael CA, Stanley FJ, Landau LI. Effects of frequent ultrasound during pregnancy: a randomised controlled trial. Lancet. 1993;342(8876):887–891. doi: 10.1016/0140-6736(93)91944-h. [DOI] [PubMed] [Google Scholar]
- 36.Newnham JP, O’Dea MR, Reid KP, Diepeveen DA. Doppler flow velocity waveform analysis in high risk pregnancies: a randomized controlled trial. British journal of obstetrics and gynaecology. 1991;98(10):956–963. doi: 10.1111/j.1471-0528.1991.tb15332.x. [DOI] [PubMed] [Google Scholar]
- 37.Trudinger BJ, Cook CM, Giles WB, Connelly A, Thompson RS. Umbilical artery flow velocity waveforms in high-risk pregnancy. Randomised controlled trial. Lancet. 1987;1(8526):188–190. doi: 10.1016/s0140-6736(87)90003-1. [DOI] [PubMed] [Google Scholar]
- 38.Tyrrell SN, Lilford RJ, Macdonald HN, Nelson EJ, Porter J, Gupta JK. Randomized comparison of routine vs highly selective use of Doppler ultrasound and biophysical scoring to investigate high risk pregnancies. British journal of obstetrics and gynaecology. 1990;97(10):909–916. doi: 10.1111/j.1471-0528.1990.tb02446.x. [DOI] [PubMed] [Google Scholar]
- 39.Almstrom H, Axelsson O, Cnattingius S, et al. Comparison of umbilical-artery velocimetry and cardiotocography for surveillance of small-for-gestational-age fetuses. Lancet. 1992;340(8825):936–940. doi: 10.1016/0140-6736(92)92818-z. [DOI] [PubMed] [Google Scholar]
- 40.Pattinson RC, Norman K, Odendaal HJ. The role of Doppler velocimetry in the management of high risk pregnancies. British journal of obstetrics and gynaecology. 1994;101(2):114–120. doi: 10.1111/j.1471-0528.1994.tb13075.x. [DOI] [PubMed] [Google Scholar]
- 41.Alfirevic Z, Stampalija T, Gyte GM. Fetal and umbilical Doppler ultrasound in high-risk pregnancies. The Cochrane database of systematic reviews. 2013;11:CD007529. doi: 10.1002/14651858.CD007529.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brosens I, Dixon HG, Robertson WB. Fetal growth retardation and the arteries of the placental bed. British journal of obstetrics and gynaecology. 1977;84(9):656–663. doi: 10.1111/j.1471-0528.1977.tb12676.x. [DOI] [PubMed] [Google Scholar]
- 43.Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. American journal of obstetrics and gynecology. 2011;204(3):193–201. doi: 10.1016/j.ajog.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trudinger BJ, Giles WB, Cook CM. Flow velocity waveforms in the maternal uteroplacental and fetal umbilical placental circulations. American journal of obstetrics and gynecology. 1985;152(2):155–163. doi: 10.1016/s0002-9378(85)80016-8. [DOI] [PubMed] [Google Scholar]
- 45.Rayburn W, Sander C, Compton A. Histologic examination of the placenta in the growth-retarded fetus. American journal of perinatology. 1989;6(1):58–61. doi: 10.1055/s-2007-999546. [DOI] [PubMed] [Google Scholar]
- 46.Salafia CM, Minior VK, Pezzullo JC, Popek EJ, Rosenkrantz TS, Vintzileos AM. Intrauterine growth restriction in infants of less than thirty-two weeks’ gestation: associated placental pathologic features. American journal of obstetrics and gynecology. 1995;173(4):1049–1057. doi: 10.1016/0002-9378(95)91325-4. [DOI] [PubMed] [Google Scholar]
- 47.Salafia CM, Vogel CA, Bantham KF, Vintzileos AM, Pezzullo J, Silberman L. Preterm delivery: correlations of fetal growth and placental pathology. American journal of perinatology. 1992;9(3):190–193. doi: 10.1055/s-2007-999318. [DOI] [PubMed] [Google Scholar]
- 48.Nordenvall M, Ullberg U, Laurin J, Lingman G, Sandstedt B, Ulmsten U. Placental morphology in relation to umbilical artery blood velocity waveforms. European journal of obstetrics, gynecology, and reproductive biology. 1991;40(3):179–190. doi: 10.1016/0028-2243(91)90115-2. [DOI] [PubMed] [Google Scholar]
- 49.Fok RY, Pavlova Z, Benirschke K, Paul RH, Platt LD. The correlation of arterial lesions with umbilical artery Doppler velocimetry in the placentas of small-for-dates pregnancies. Obstet Gynecol. 1990;75(4):578–583. [PubMed] [Google Scholar]
- 50.Todros T, Sciarrone A, Piccoli E, Guiot C, Kaufmann P, Kingdom J. Umbilical Doppler waveforms and placental villous angiogenesis in pregnancies complicated by fetal growth restriction. Obstet Gynecol. 1999;93(4):499–503. doi: 10.1016/s0029-7844(98)00440-2. [DOI] [PubMed] [Google Scholar]
- 51.Jackson MR, Walsh AJ, Morrow RJ, Mullen JB, Lye SJ, Ritchie JW. Reduced placental villous tree elaboration in small-for-gestational-age pregnancies: relationship with umbilical artery Doppler waveforms. American journal of obstetrics and gynecology. 1995;172(2 Pt 1):518–525. doi: 10.1016/0002-9378(95)90566-9. [DOI] [PubMed] [Google Scholar]
- 52.Krebs C, Macara LM, Leiser R, Bowman AW, Greer IA, Kingdom JC. Intrauterine growth restriction with absent end-diastolic flow velocity in the umbilical artery is associated with maldevelopment of the placental terminal villous tree. American journal of obstetrics and gynecology. 1996;175(6):1534–1542. doi: 10.1016/s0002-9378(96)70103-5. [DOI] [PubMed] [Google Scholar]
- 53.Macara L, Kingdom JC, Kaufmann P, et al. Structural analysis of placental terminal villi from growth-restricted pregnancies with abnormal umbilical artery Doppler waveforms. Placenta. 1996;17(1):37–48. doi: 10.1016/s0143-4004(05)80642-3. [DOI] [PubMed] [Google Scholar]
- 54.Feletou M. The Endothelium. San Rafael, CA: Morgan & Claypool Life Sciences Publishers; 2011. [PubMed] [Google Scholar]
- 55.Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res. 2007;100(2):174–190. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- 56.Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007;100(2):158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 57.Craig LE, Spelman JP, Strandberg JD, Zink MC. Endothelial cells from diverse tissues exhibit differences in growth and morphology. Microvascular research. 1998;55(1):65–76. doi: 10.1006/mvre.1997.2045. [DOI] [PubMed] [Google Scholar]
- 58.Yano K, Gale D, Massberg S, et al. Phenotypic heterogeneity is an evolutionarily conserved feature of the endothelium. Blood. 2007;109(2):613–615. doi: 10.1182/blood-2006-05-026401. [DOI] [PubMed] [Google Scholar]
- 59.Lang I, Pabst MA, Hiden U, et al. Heterogeneity of microvascular endothelial cells isolated from human term placenta and macrovascular umbilical vein endothelial cells. European journal of cell biology. 2003;82(4):163–173. doi: 10.1078/0171-9335-00306. [DOI] [PubMed] [Google Scholar]
- 60.Burton GJ, Jauniaux E. Sonographic, stereological and Doppler flow velocimetric assessments of placental maturity. British journal of obstetrics and gynaecology. 1995;102(10):818–825. doi: 10.1111/j.1471-0528.1995.tb10849.x. [DOI] [PubMed] [Google Scholar]
- 61.Egorova AD, DeRuiter MC, de Boer HC, et al. Endothelial colony-forming cells show a mature transcriptional response to shear stress. In vitro cellular & developmental biology. Animal. 2012;48(1):21–29. doi: 10.1007/s11626-011-9470-z. [DOI] [PubMed] [Google Scholar]
- 62.Mendelsohn ME. Genomic and nongenomic effects of estrogen in the vasculature. The American journal of cardiology. 2002;90(1A):3F–6F. doi: 10.1016/s0002-9149(02)02418-9. [DOI] [PubMed] [Google Scholar]
- 63.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. The New England journal of medicine. 1999;340(23):1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 64.Gibson LL, Hahner L, Osborne-Lawrence S, et al. Molecular basis of estrogen-induced cyclooxygenase type 1 upregulation in endothelial cells. Circ Res. 2005;96(5):518–525. doi: 10.1161/01.RES.0000158967.96231.88. [DOI] [PubMed] [Google Scholar]
- 65.Li M, Kuo L, Stallone JN. Estrogen potentiates constrictor prostanoid function in female rat aorta by upregulation of cyclooxygenase-2 and thromboxane pathway expression. American journal of physiology. Heart and circulatory physiology. 2008;294(6):H2444–2455. doi: 10.1152/ajpheart.01121.2007. [DOI] [PubMed] [Google Scholar]
- 66.Mendelsohn ME. Mechanisms of estrogen action in the cardiovascular system. The Journal of steroid biochemistry and molecular biology. 2000;74(5):337–343. doi: 10.1016/s0960-0760(00)00110-2. [DOI] [PubMed] [Google Scholar]
- 67.Ospina JA, Krause DN, Duckles SP. 17beta-estradiol increases rat cerebrovascular prostacyclin synthesis by elevating cyclooxygenase-1 and prostacyclin synthase. Stroke; a journal of cerebral circulation. 2002;33(2):600–605. doi: 10.1161/hs0202.102732. [DOI] [PubMed] [Google Scholar]
- 68.Tamura M, Deb S, Sebastian S, Okamura K, Bulun SE. Estrogen up-regulates cyclooxygenase-2 via estrogen receptor in human uterine microvascular endothelial cells. Fertility and sterility. 2004;81(5):1351–1356. doi: 10.1016/j.fertnstert.2003.09.076. [DOI] [PubMed] [Google Scholar]
- 69.Simard M, Drolet R, Blomquist CH, Tremblay Y. Human type 2 17beta-hydroxysteroid dehydrogenase in umbilical vein and artery endothelial cells: differential inactivation of sex steroids according to the vessel type. Endocrine. 2011;40(2):203–211. doi: 10.1007/s12020-011-9519-5. [DOI] [PubMed] [Google Scholar]
- 70.Su EJ, Cheng YH, Chatterton RT, et al. Regulation of 17-beta hydroxysteroid dehydrogenase type 2 in human placental endothelial cells. Biology of reproduction. 2007;77(3):517–525. doi: 10.1095/biolreprod.106.059451. [DOI] [PubMed] [Google Scholar]
- 71.Su EJ, Ernst L, Abdallah N, et al. Estrogen receptor-beta and fetoplacental endothelial prostanoid biosynthesis: a link to clinically demonstrated fetal growth restriction. The Journal of clinical endocrinology and metabolism. 2011;96(10):E1558–1567. doi: 10.1210/jc.2011-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Su EJ, Lin ZH, Zeine R, et al. Estrogen receptor-beta mediates cyclooxygenase-2 expression and vascular prostanoid levels in human placental villous endothelial cells. American journal of obstetrics and gynecology. 2009;200(4):427.e421–428. doi: 10.1016/j.ajog.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 73.Corcoran JJ, Nicholson C, Sweeney M, et al. Human uterine and placental arteries exhibit tissue-specific acute responses to 17beta-estradiol and estrogen-receptor-specific agonists. Molecular human reproduction. 2014;20(5):433–441. doi: 10.1093/molehr/gat095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lang I, Schweizer A, Hiden U, et al. Human fetal placental endothelial cells have a mature arterial and a juvenile venous phenotype with adipogenic and osteogenic differentiation potential. Differentiation; research in biological diversity. 2008;76(10):1031–1043. doi: 10.1111/j.1432-0436.2008.00302.x. [DOI] [PubMed] [Google Scholar]
- 75.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97(6):512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 76.Gerber HP, McMurtrey A, Kowalski J, et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. The Journal of biological chemistry. 1998;273(46):30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 77.Kawasaki K, Smith RS, Jr, Hsieh CM, Sun J, Chao J, Liao JK. Activation of the phosphatidylinositol 3-kinase/protein kinase Akt pathway mediates nitric oxide-induced endothelial cell migration and angiogenesis. Molecular and cellular biology. 2003;23(16):5726–5737. doi: 10.1128/MCB.23.16.5726-5737.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liao WX, Feng L, Zheng J, Chen DB. Deciphering mechanisms controlling placental artery endothelial cell migration stimulated by vascular endothelial growth factor. Endocrinology. 2010;151(7):3432–3444. doi: 10.1210/en.2009-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Joo JE, Hiden U, Lassance L, et al. Variable promoter methylation contributes to differential expression of key genes in human placenta-derived venous and arterial endothelial cells. BMC genomics. 2013;14:475. doi: 10.1186/1471-2164-14-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Krause BJ, Carrasco-Wong I, Caniuguir A, Carvajal J, Farias M, Casanello P. Endothelial eNOS/arginase imbalance contributes to vascular dysfunction in IUGR umbilical and placental vessels. Placenta. 2013;34(1):20–28. doi: 10.1016/j.placenta.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 81.Lang I, Hoffmann C, Olip H, et al. Differential mitogenic responses of human macrovascular and microvascular endothelial cells to cytokines underline their phenotypic heterogeneity. Cell proliferation. 2001;34(3):143–155. doi: 10.1046/j.1365-2184.2001.00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Durand MJ, Gutterman DD. Diversity in mechanisms of endothelium-dependent vasodilation in health and disease. Microcirculation. 2013;20(3):239–247. doi: 10.1111/micc.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sabry S, Mondon F, Ferre F, Dinh-Xuan AT. In vitro contractile and relaxant responses of human resistance placental stem villi arteries of healthy parturients: role of endothelium. Fundamental & clinical pharmacology. 1995;9(1):46–51. doi: 10.1111/j.1472-8206.1995.tb00264.x. [DOI] [PubMed] [Google Scholar]
- 84.Poston L, McCarthy AL, Ritter JM. Control of vascular resistance in the maternal and feto-placental arterial beds. Pharmacology & therapeutics. 1995;65(2):215–239. doi: 10.1016/0163-7258(94)00064-a. [DOI] [PubMed] [Google Scholar]
- 85.Reilly FD, Russell PT. Neurohistochemical evidence supporting an absence of adrenergic and cholinergic innervation in the human placenta and umbilical cord. The Anatomical record. 1977;188(3):277–286. doi: 10.1002/ar.1091880302. [DOI] [PubMed] [Google Scholar]
- 86.Boura AL, Walters WA. Autacoids and the control of vascular tone in the human umbilical-placental circulation. Placenta. 1991;12(5):453–477. doi: 10.1016/0143-4004(91)90023-9. [DOI] [PubMed] [Google Scholar]
- 87.Allen J, Lauridsen V, Hansen V, Andersson KE, Forman A. Effects of indomethacin on human placental stem villous arteries. Gynecologic and obstetric investigation. 1989;27(3):118–121. doi: 10.1159/000293635. [DOI] [PubMed] [Google Scholar]
- 88.Mak KK, Gude NM, Walters WA, Boura AL. Effects of vasoactive autacoids on the human umbilical-fetal placental vasculature. British journal of obstetrics and gynaecology. 1984;91(2):99–106. doi: 10.1111/j.1471-0528.1984.tb05890.x. [DOI] [PubMed] [Google Scholar]
- 89.McCarthy AL, Woolfson RG, Evans BJ, Davies DR, Raju SK, Poston L. Functional characteristics of small placental arteries. American journal of obstetrics and gynecology. 1994;170(3):945–951. doi: 10.1016/s0002-9378(94)70311-6. [DOI] [PubMed] [Google Scholar]
- 90.Sabry S, Mondon F, Levy M, Ferre F, Dinh-Xuan AT. Endothelial modulation of vasoconstrictor responses to endothelin-1 in human placental stem villi small arteries. British journal of pharmacology. 1995;115(6):1038–1042. doi: 10.1111/j.1476-5381.1995.tb15915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rizzo G, Capponi A, Rinaldo D, Arduini D, Romanini C. Release of vasoactive agents during cordocentesis: differences between normally grown and growth-restricted fetuses. American journal of obstetrics and gynecology. 1996;175(3 Pt 1):563–570. doi: 10.1053/ob.1996.v175.a74253. [DOI] [PubMed] [Google Scholar]
- 92.Saldeen P, Olofsson P, Marsal K. Lack of association between Doppler velocimetry and synthesis of prostacyclin and thromboxane in umbilical cord vessels from growth retarded fetuses. Acta obstetricia et gynecologica Scandinavica. 1995;74(2):103–108. doi: 10.3109/00016349509008916. [DOI] [PubMed] [Google Scholar]
- 93.Stuart MJ, Clark DA, Sunderji SG, et al. Decrease prostacyclin production: a characteristic of chronic placental insufficiency syndromes. Lancet. 1981;1(8230):1126–1128. doi: 10.1016/s0140-6736(81)92298-4. [DOI] [PubMed] [Google Scholar]
- 94.Polydorides AD, Kalish RB, Witkin SS, Baergen RN. A fetal cyclooxygenase-2 gene polymorphism is associated with placental malperfusion. International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists. 2007;26(3):284–290. doi: 10.1097/01.pgp.0000236950.56785.a8. [DOI] [PubMed] [Google Scholar]
- 95.Arroyo JA, Anthony RV, Parker TA, Galan HL. Differential expression of placental and vascular endothelial nitric oxide synthase in an ovine model of fetal growth restriction. American journal of obstetrics and gynecology. 2006;195(3):771–777. doi: 10.1016/j.ajog.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 96.Casanello P, Krause B, Torres E, et al. Reduced l-arginine transport and nitric oxide synthesis in human umbilical vein endothelial cells from intrauterine growth restriction pregnancies is not further altered by hypoxia. Placenta. 2009;30(7):625–633. doi: 10.1016/j.placenta.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 97.Dyer JL, McMillen IC, Warnes KE, Morrison JL. No evidence for an enhanced role of endothelial nitric oxide in the maintenance of arterial blood pressure in the IUGR sheep fetus. Placenta. 2009;30(8):705–710. doi: 10.1016/j.placenta.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 98.Gardner DS, Fowden AL, Giussani DA. Adverse intrauterine conditions diminish the fetal defense against acute hypoxia by increasing nitric oxide activity. Circulation. 2002;106(17):2278–2283. doi: 10.1161/01.cir.0000033827.48974.c8. [DOI] [PubMed] [Google Scholar]
- 99.Gardner DS, Giussani DA. Enhanced umbilical blood flow during acute hypoxemia after chronic umbilical cord compression: a role for nitric oxide. Circulation. 2003;108(3):331–335. doi: 10.1161/01.CIR.0000080323.40820.A1. [DOI] [PubMed] [Google Scholar]
- 100.Kulandavelu S, Whiteley KJ, Bainbridge SA, Qu D, Adamson SL. Endothelial NO synthase augments fetoplacental blood flow, placental vascularization, and fetal growth in mice. Hypertension. 2013;61(1):259–266. doi: 10.1161/HYPERTENSIONAHA.112.201996. [DOI] [PubMed] [Google Scholar]
- 101.Jackson WF. Potassium channels in the peripheral microcirculation. Microcirculation. 2005;12(1):113–127. doi: 10.1080/10739680590896072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wareing M, Bai X, Seghier F, et al. Expression and function of potassium channels in the human placental vasculature. American journal of physiology. Regulatory, integrative and comparative physiology. 2006;291(2):R437–446. doi: 10.1152/ajpregu.00040.2006. [DOI] [PubMed] [Google Scholar]
- 103.Wareing M, Greenwood SL, Fyfe GK, Baker PN. Reactivity of human placental chorionic plate vessels from pregnancies complicated by intrauterine growth restriction (IUGR) Biology of reproduction. 2006;75(4):518–523. doi: 10.1095/biolreprod.106.051607. [DOI] [PubMed] [Google Scholar]
- 104.Demir R, Kaufmann P, Castellucci M, Erbengi T, Kotowski A. Fetal vasculogenesis and angiogenesis in human placental villi. Acta anatomica. 1989;136(3):190–203. doi: 10.1159/000146886. [DOI] [PubMed] [Google Scholar]
- 105.Jauniaux E, Burton GJ, Moscoso GJ, Hustin J. Development of the early human placenta: a morphometric study. Placenta. 1991;12(3):269–276. doi: 10.1016/0143-4004(91)90008-4. [DOI] [PubMed] [Google Scholar]
- 106.te Velde EA, Exalto N, Hesseling P, van der Linden HC. First trimester development of human chorionic villous vascularization studied with CD34 immunohistochemistry. Human reproduction. 1997;12(7):1577–1581. doi: 10.1093/humrep/12.7.1577. [DOI] [PubMed] [Google Scholar]
- 107.van Oppenraaij RH, Koning AH, Lisman BA, et al. Vasculogenesis and angiogenesis in the first trimester human placenta: an innovative 3D study using an immersive Virtual Reality system. Placenta. 2009;30(3):220–222. doi: 10.1016/j.placenta.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 108.Mayhew TM. Fetoplacental angiogenesis during gestation is biphasic, longitudinal and occurs by proliferation and remodelling of vascular endothelial cells. Placenta. 2002;23(10):742–750. doi: 10.1016/s0143-4004(02)90865-9. [DOI] [PubMed] [Google Scholar]
- 109.Mayhew TM, Ohadike C, Baker PN, Crocker IP, Mitchell C, Ong SS. Stereological investigation of placental morphology in pregnancies complicated by pre-eclampsia with and without intrauterine growth restriction. Placenta. 2003;24(2–3):219–226. doi: 10.1053/plac.2002.0900. [DOI] [PubMed] [Google Scholar]
- 110.Teasdale F. Idiopathic intrauterine growth retardation: histomorphometry of the human placenta. Placenta. 1984;5(1):83–92. doi: 10.1016/s0143-4004(84)80051-x. [DOI] [PubMed] [Google Scholar]
- 111.Mayhew TM, Charnock-Jones DS, Kaufmann P. Aspects of human fetoplacental vasculogenesis and angiogenesis. III. Changes in complicated pregnancies. Placenta. 2004;25(2–3):127–139. doi: 10.1016/j.placenta.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 112.Risau W. Mechanisms of angiogenesis. Nature. 1997;386(6626):671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 113.Charnock-Jones DS, Kaufmann P, Mayhew TM. Aspects of human fetoplacental vasculogenesis and angiogenesis. I. Molecular regulation. Placenta. 2004;25(2–3):103–113. doi: 10.1016/j.placenta.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 114.Bergen NE, Bouwland-Both MI, Steegers-Theunissen RP, et al. Early pregnancy maternal and fetal angiogenic factors and fetal and childhood growth: the Generation R Study. Human reproduction. 2015 doi: 10.1093/humrep/dev070. [DOI] [PubMed] [Google Scholar]
- 115.Borras D, Perales-Puchalt A, Ruiz Sacedon N, Perales A. Angiogenic growth factors in maternal and fetal serum in pregnancies complicated with intrauterine growth restriction. Journal of obstetrics and gynaecology : the journal of the Institute of Obstetrics and Gynaecology. 2014;34(3):218–220. doi: 10.3109/01443615.2013.834304. [DOI] [PubMed] [Google Scholar]
- 116.Cerdeira AS, Karumanchi SA. Angiogenic factors in preeclampsia and related disorders. Cold Spring Harbor perspectives in medicine. 2012;2(11) doi: 10.1101/cshperspect.a006585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nevo O, Many A, Xu J, et al. Placental expression of soluble fms-like tyrosine kinase 1 is increased in singletons and twin pregnancies with intrauterine growth restriction. The Journal of clinical endocrinology and metabolism. 2008;93(1):285–292. doi: 10.1210/jc.2007-1042. [DOI] [PubMed] [Google Scholar]
- 118.Schlembach D, Wallner W, Sengenberger R, et al. Angiogenic growth factor levels in maternal and fetal blood: correlation with Doppler ultrasound parameters in pregnancies complicated by pre-eclampsia and intrauterine growth restriction. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2007;29(4):407–413. doi: 10.1002/uog.3930. [DOI] [PubMed] [Google Scholar]
- 119.Taylor RN, Grimwood J, Taylor RS, McMaster MT, Fisher SJ, North RA. Longitudinal serum concentrations of placental growth factor: evidence for abnormal placental angiogenesis in pathologic pregnancies. American journal of obstetrics and gynecology. 2003;188(1):177–182. doi: 10.1067/mob.2003.111. [DOI] [PubMed] [Google Scholar]
- 120.Vatten LJ, Asvold BO, Eskild A. Angiogenic factors in maternal circulation and preeclampsia with or without fetal growth restriction. Acta obstetricia et gynecologica Scandinavica. 2012;91(12):1388–1394. doi: 10.1111/j.1600-0412.2012.01516.x. [DOI] [PubMed] [Google Scholar]
- 121.Crispi F, Dominguez C, Llurba E, Martin-Gallan P, Cabero L, Gratacos E. Placental angiogenic growth factors and uterine artery Doppler findings for characterization of different subsets in preeclampsia and in isolated intrauterine growth restriction. American journal of obstetrics and gynecology. 2006;195(1):201–207. doi: 10.1016/j.ajog.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 122.Savvidou MD, Yu CK, Harland LC, Hingorani AD, Nicolaides KH. Maternal serum concentration of soluble fms-like tyrosine kinase 1 and vascular endothelial growth factor in women with abnormal uterine artery Doppler and in those with fetal growth restriction. American journal of obstetrics and gynecology. 2006;195(6):1668–1673. doi: 10.1016/j.ajog.2006.03.065. [DOI] [PubMed] [Google Scholar]
- 123.Su EJ, Xin H, Yin P, et al. Impaired fetoplacental angiogenesis in growth-restricted fetuses with abnormal umbilical artery doppler velocimetry is mediated by aryl hydrocarbon receptor nuclear translocator (ARNT) The Journal of clinical endocrinology and metabolism. 2015;100(1):E30–40. doi: 10.1210/jc.2014-2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wallner W, Sengenberger R, Strick R, et al. Angiogenic growth factors in maternal and fetal serum in pregnancies complicated by intrauterine growth restriction. Clinical science. 2007;112(1):51–57. doi: 10.1042/CS20060161. [DOI] [PubMed] [Google Scholar]