Abstract
Recent attention has focused on fructose as having a unique role in the pathogenesis cardiometabolic diseases. However since we rarely consume fructose in isolation, the major source of fructose in the diet comes from fructose-containing sugars, sucrose and high fructose corn syrup, in sugar sweetened beverages. Intake of these beverages has been consistently linked to increased risk of obesity, type 2 diabetes and cardiovascular disease in various populations. Putative underlying mechanisms include incomplete compensation for liquid calories, adverse glycemic effects and increased hepatic metabolism of fructose leading to de novo lipogenesis, production of uric acid and accumulation of visceral and ectopic fat. In this review we summarize the epidemiological and clinical trial evidence evaluating added sugars especially sugar-sweetened beverages, and risk of obesity, diabetes and cardiovascular disease addressing potential biological mechanisms with an emphasis on fructose physiology. We also discuss strategies to reduce intake of fructose-containing beverages.
Keywords: cardiometabolic diseases, fructose, obesity, diabetes
Introduction
The adverse health effects of sugar have long been a matter of much public and scientific interest. For decades, it has been thought that a high intake of sugar is associated with the development of obesity, type 2 diabetes and cardiovascular disease. Given the distinct metabolic fates that differentiate fructose from glucose, recent attention has focused on fructose as having a unique role in the etiology of these conditions. Fructose is found in sucrose or common table sugar, which is a disaccharide composed of one glucose molecule and one fructose molecule linked via an α1–4 glycoside bond, and is obtained from either sugar cane or beets. Fructose and glucose are also both found as naturally occurring monosaccharides that exists in fruit, honey and some vegetables. Sweeteners, such as high fructose corn syrup (HFCS), produced from corn starch through industrial processing contain free fructose and free glucose in relatively equal proportions and have progressively replaced use of sugar in the US since its appearance in the market in the late 1960’s primarily due to its low cost. The most common forms of HFCS contain either 42 % (HFCS-42) or 55 % (HFCS-55) fructose, along with glucose and water. HFCS-55 has the sweetness equivalent of sucrose and is widely used to flavor carbonated soft drinks. HFCS-42 is somewhat less sweet and is mainly used in processed foods including canned foods (e.g., soups, fruits), cereals, baked goods, desserts, sweetened dairy products, condiments, fruit-flavored noncarbonated beverages, candies, and many fast food items.
Based on national survey data from the US, mean intake of total fructose as a percentage of total energy increased from 8.1% in 1978 to 9.1% in 2004, with greater increases observed in adolescents and young adults (1). It is important to note that this increase was due to increases in fructose from sugars and sweeteners and not from naturally occurring fructose in fruit. With the exception of 1–3 year olds, the estimated intake of naturally occurring fructose decreased from 11–16 grams /day in 1978 to 7–9 grams/day in 2004 for all age groups, representing an overall decrease of 3–7 grams/day (1).
Since we rarely consume fructose in isolation, the major source of fructose in the diet comes from fructose-containing sugars (sucrose and HFCS) largely in the form of added sugar i.e. those sugars, which are added to foods and beverages during processing and preparation. As a result, glucose intake tends to co-vary with fructose intake, and epidemiologic studies cannot differentiate between the effects of fructose per se and those specifically attributable to glucose.
Time-trend data over the past 3–4 decades have shown a close parallel between the rise in added sugar intake and the obesity and diabetes epidemics in the US (2). Largely driving these trends has been the dramatic increase in the consumption of sugar sweetened beverages (SSBs), which are the single greatest source of calories and added sugars in the US diet, accounting for nearly half of all added sugar intake (3) (Table 1). One 360-mL can of regular soda contains about 35 g of sugar (140 calories) or 7% of total calories (based on 2000 kcal/d.) (4). In the US, SSBs are primarily sweetened with HFCS while in Europe sucrose is the predominant sweetener.
Table 1.
Mean intake of added sugars and percentage contribution of various foods among US population, by age, National Health and Nutrition Examination Survey 2005–06
| All persons | 2–18 years | 19+ years | ||
|---|---|---|---|---|
| Sample size | 8,272 | 3,553 | 4,719 | |
| Mean intake of added sugars (tsp.) | 21 | 23 | 20 | |
| Rank* | Food Group | |||
| 1 | Soda/energy/sports drinks | 35.7 | 31.8 | 37.1 |
| 2 | Grain-based desserts | 12.9 | 10.9 | 13.7 |
| 3 | Fruit drinks | 10.5 | 15.0 | 8.9 |
| 4 | Dairy desserts | 6.6 | 7.9 | 6.1 |
| 5 | Candy | 6.1 | 6.8 | 5.8 |
| 6 | Ready-to-eat cereals | 3.8 | 6.4 | 2.9 |
| 7 | Sugars/honey | 3.5 | 1.4 | 4.2 |
| 8 | Tea | 3.5 | 2.1 | 4.0 |
| 9 | Yeast breads | 2.1 | 1.9 | 2.2 |
| 10 | Syrups/toppings | 1.9 | 2.8 | 1.5 |
Rank for all persons only. Columns for other age groups are ordered by this ranking. From: http://riskfactor.cancer.gov/diet/foodsources/added_sugars
Consumption of SSBs thus accounts for the majority of total fructose intake in the diet, either from sucrose or HFCS and in this regard relations between SSB and cardiometabolic diseases reflect potential effects of fructose and glucose or unique metabolic effects of fructose alone in epidemiologic studies (Textbox 1).
Textbox 1. Key points regarding fructose, high fructose corn syrup and sugar sweetened beverages.
Fructose
Fructose is found in: sucrose, a disaccharide composed of one glucose molecule and one fructose molecule; high fructose corn syrup (HFCS), containing relatively equal amounts of glucose; and fruit, honey and some vegetables as a naturally occurring monosaccharide.
The major source of fructose in the diet come from fructose-containing sugars (sucrose and HFCS) that are added to foods and beverages and contain relatively equal amounts of glucose.
Thus intakes of glucose and fructose co-vary and epidemiologic studies cannot differentiate between their effects.
High fructose corn syrup
HFCS is produced from corn starch through industrial processing. The most common forms contain 42% or 55% fructose along with glucose and water.
Use of HFCS has progressively replaced use of sugar in the US due to its low cost.
HFCS is the primary sweetener used in sugar sweetened beverages (SSBs) in the United States and in many processed foods.
Sugar sweetened beverages
SSBs include soft drinks, fruit drinks and energy drinks, which are sweetened by HFCS or sucrose that are added to the beverages by manufacturers, establishments or individuals.
SSBs are the greatest source of fructose- containing sugars in the diet and thus account for the majority of total fructose intake.
Relations between SSB and cardiometabolic diseases reflect potential effects of fructose and glucose or unique metabolic effects of fructose alone in epidemiologic studies.
Although consumption of SSBs and added sugar appear to have decreased modestly in the past decade(4), data from NHANES show that half of the US population consumes SSBs on a given day with 1 in 4 obtaining at least 200 calories from these beverages and 5% obtaining at least 567 calories- equivalent to 4 cans of soda (5). These values exceed American Heart Association recommendations for no more than 100 –150 kcal/d from all added sugar for most adults as well as recommendations from the World Health Organization and the 2015 Dietary Guidelines Advisory Committee to limit intake of added sugars to no more than 10% of energy.
Over the past decade, a large body of evidence has accumulated, which shows a strong association between SSBs and obesity and related chronic diseases (6–8). For this reason and because they provide “empty” calories and almost no nutritional value, SSBs have been identified as a suitable target for public health interventions. However, controversy remains whether the associations are causal, if glucose or fructose moieties of sugars differentially impact cardiometabolic risk and regarding the type of public action that should be taken on the basis of existing evidence. In this review, we provide a brief overview of fructose metabolism and summarize the epidemiological evidence evaluating the relationship between fructose, obesity, diabetes and cardiovascular risk in adults, focusing on fructose-containing beverages or SSBs, since they are the most abundant and well-characterized source of fructose in the diet. We also discuss biological mechanisms underlying these associations with an emphasis on the role of fructose. Finally we discuss healthier alternatives to SSBs, and strategies to reduce SSB intake.
Fructose Metabolism
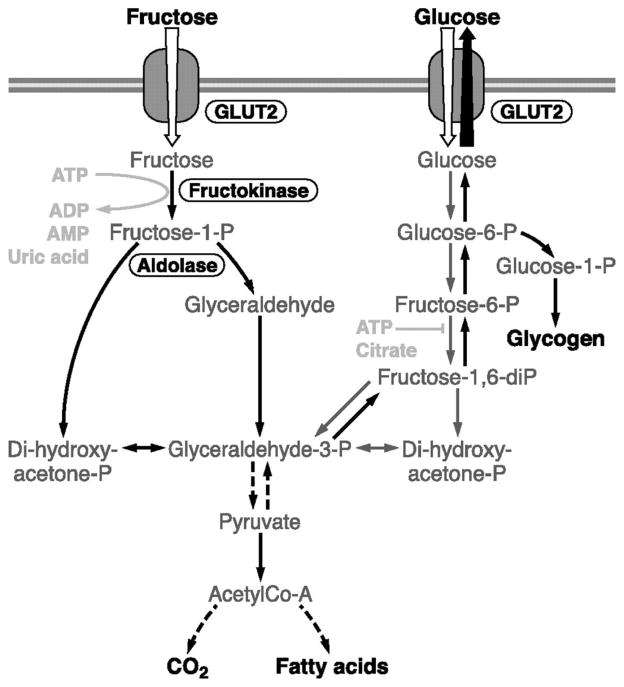
Fructose metabolism differs from that of glucose in two major ways. First, there is nearly complete hepatic extraction of fructose and second, as shown in Figure 1, there are different enzymatic reactions in the initial steps of the metabolism of fructose and glucose. Fructose is absorbed from the gut into the portal vein and is metabolized in the liver where it is converted into fructose-1-phosphate by the enzyme fructokinase. Fructose-1-phosphate is then split into two 3-carbon molecules, namely glyceraldehyde and dihydroxyacetone phosphate (DHAP) by aldolase. Glyceraldehyde is further converted into glyceraldehyde-3-phosphate, which along with DHAP can then enter various metabolic pathways to form “classical” energy substrates such as glucose, glycogen, lactate and fatty acids. Because these processes are not dependent on insulin, fructose is metabolized without requiring insulin secretion and without increasing plasma glucose.
Figure 1. Fructose metabolism in liver cells.
Fructose metabolism (grey arrows) differs from glucose (black arrows) due to 1) a nearly complete hepatic extraction and 2) different enzyme and reactions for its initial metabolic steps. Fructose taken up by the liver can be oxidized to CO2 and then converted into lactate and glucose; glucose and lactate are subsequently either released into the circulation for extrahepatic metabolism or converted into hepatic glycogen or fat. The massive uptake and phosphorylation of fructose in the liver can lead to a large degradation of ATP to AMP and uric acid. From: Physiol Rev 90: 23–46, 2010; doi:10.1152/physrev.00019.2009.
Of particular note, unlike glucose, fructose can bypass the main rate limiting step of glycolysis at the level of phosphofructokinase, allowing it to act as a substrate for hepatic de novo lipogenesis and production of lipids. Thus intake of fructose in high amounts can promote triglyceride synthesis from unchecked pathways. The actual amount of fructose needed to increase blood triglyceride levels is debated (9). Significant increases in postprandial triglycerides have been shown in response to consumption of 25% of energy from fructose and HFCS, but not glucose (10). Recent data has also shown that consuming HFCS sweetened beverages containing 10% – 25% of energy produced significant linear increases in postprandial triglycerides, suggesting a dose-response relationship between fructose consumption and increases in triglycerides (11). Since added sugar intake in the US constitutes about 14.9% of energy with 71% of the population consuming ≥ 10 % energy from added sugar (4), these effects of fructose are relevant to usual consumption patterns.
The massive uptake and phosphorylation of fructose in the liver can also deplete intracellular ATP leading to an increase in uric acid production, which has been shown to induce metabolic complications. These differences in hepatic metabolism can theoretically lead to a variety of different short- and long-term cardiometabolic effects of fructose compared with glucose.
Added sugars and SSBs in relation to obesity, diabetes and cardiovascular risk
SSBs are a major source of added sugars (including both fructose and glucose) in the US diets. Numerous epidemiologic studies have evaluated the relationship between consumption of SSBs and the development of obesity and related cardiometabolic conditions in adults. Cross-sectional and ecologic studies are not able to establish temporality and infer causality, thus evidence from these designs are not discussed in this review. Rather, we consider carefully conducted and analyzed prospective cohort studies, which are considered the strongest non-randomized study design, able to capture long-term diet and disease relationships. All of these studies adjusted their analyses for potential confounding by various diet and lifestyle factors, however residual confounding by unmeasured or imperfectly measured factors may still exist. Higher SSB or sugar intake could be a marker of a globally unhealthy diet. Therefore incomplete adjustment for various lifestyle factors could lead to an overestimation of associations. Although RCT’s with hard clinical outcomes are regarded as the highest grade of evidence in epidemiology they are not the most appropriate or feasible study design to evaluate longitudinal effects of diet on disease. However RCT’s of intermediate outcomes can provide important mechanistic insight. Most of the studies that we consider defined SSBs to include carbonated and noncarbonated soft drinks and sweetened fruit drinks that contain caloric sweeteners however slight differences in definitions are expected due to heterogeneity in assessment methods. Such differences are unlikely to impact levels of fructose consumption.
Obesity: Observational Studies
The majority (3,6,7) but not all (12) of systematic reviews have reported positive associations between SSB and weight gain or risk of overweight or obesity. We recently conducted a comprehensive systematic review and meta-analysis of cohort studies and randomized controlled trials of SSBs and weight gain in children and adults (7). Based on 7 cohort studies in adults, with 174,252 participants, a one-serving per day increase in SSBs was associated with an additional weight gain of 0.12 kg over 1 year. Although this estimate seems modest, adult weight gain in the general population is a gradual process, occurring over decades and averaging about 1 pound (0.45 kg) per year. Thus, eliminating SSBs from the diet could be an effective way to prevent age- related weight gain.
The association between SSBs and obesity is strengthened by our analysis of gene-SSB interactions, which examined whether consumption of SSBs can modify the genetic risk of obesity, using a genetic predisposition score based on 32 obesity genes identified from genome-wide association studies (13). Based on data from 3 large cohorts we found that individuals who consumed one or more servings of SSBs per day had genetic effects on BMI and obesity risk that were approximately twice as large as those who consumed less than one serving per month. These data suggest that regular consumers of SSBs may be more susceptible to genetic effects on obesity, implying that a genetic predisposition to obesity can be partly offset by healthier beverage choices.
Obesity: RCTs
Compared to observational studies, evidence from RCTs is limited, and the majority of trials evaluate short-term effects of specific interventions on weight change rather than long-term patterns. In our recent meta-analysis of 5 trials including 292 adults, we found that adding SSBs to the diet significantly increased body weight (7). Similarly, another meta-analysis of 7 RCTs found a significant dose-dependent increase in body weight when SSB’s were added to participants’ diets (12). However, in their meta-analysis of another 8 trials aiming to reduce SSB consumption (for prevention of weight gain), there was no overall effect on BMI, but a significant benefit was observed among individuals who were initially overweight (12). This meta-analysis included two large and rigorously conducted RCT’s in children and adolescents (14,15), which have overcome many of the limitations of previous trials such as small sample sizes, short duration, lack of blinding and poor compliance. Although the trial by Ebbeling et al. found a significant benefit of reducing SSBs on BMI in the first year of the trial during active intervention, it did not find a significant between-group difference after an additional 1 year of follow-up without active intervention (14). This finding actually supports rather than refutes a benefit of reducing SSB consumption on adolescent obesity suggesting that to achieve long-term benefits, the intervention needs to be sustained over time. These studies provide strong evidence that decreasing consumption of SSBs significantly reduces weight gain and obesity in this age-group.
Type 2 Diabetes
A growing body of evidence indicates that SSB consumption is associated with increased risk of diabetes through effects on adiposity and independently through other metabolic effects. Although experimental evidence from RCTs is lacking due to high cost and other feasibility considerations, findings from prospective cohort studies have shown a relatively strong and consistent association in well-powered studies. We conducted a meta-analysis of 8 prospective cohort studies evaluating SSB intake and risk of diabetes (8). Based on 310,819 participants and 15,043 cases, individuals in the highest category of SSB intake (usually 1–2 servings per day) had a 26% greater risk of developing diabetes compared to those in the lowest category (none or less than one per month). For this analysis we selected estimates that did not adjust for potential intermediates in the etiologic chain such as total energy intake and BMI. A similar association was found in a sub-cohort of 15,374 participants and 11,684 incident cases from the European Prospective Investigation into Cancer and Nutrition (EPIC) study (16) where a one serving per day increase in SSBs was associated with a 22% increased risk of diabetes. A recent meta-analysis of 17 cohort studies found that a one serving per day increase in SSBs was associated with an 18% increased risk of diabetes. Adjusting for BMI reduced this estimate to 13%. Given the similar estimates from studies in the US where HFSC is the primary sweetener and Europe where sucrose is used, there does not appear to be any appreciable difference regarding the impact of sweetener type on risk of diabetes. However, food sources of fructose may make a difference in metabolic effects. Some studies have shown beneficial effects of whole fruit consumption on risk of diabetes, whereas higher consumption of fruit juices was associated with increased risk (17). These results indicate that the liquid vs. solid forms of calories from sugars may impact metabolic diseases differently. Fructose in beverages is absorbed more quickly than fructose in whole foods such as fruit and vegetables, which are absorbed more slowly due to their fiber content and slow digestion. The rapid absorption of liquid fructose increases the rate of hepatic extraction of fructose, de novo lipogenesis and production of lipids.
Cardiovascular Risk
There is increasing evidence that higher SSB consumption increases cardiovascular risk by contributing to the development of hypertension, dyslipidemia, inflammation, coronary heart disease and stroke. In over 88,000 women in the NHS followed for 24 years, we found that those who consumed ≥ 2 servings per day of SSBs had a 35% greater risk of CHD (non-fatal myocardial infarction or fatal CHD) compared with infrequent consumers (18). Additional adjustment for potential mediating factors (including BMI, total energy intake and incident diabetes) attenuated the association, but it remained statistically significant, suggesting that the effect of SSBs may not be entirely mediated by these factors. Similar results were found in the HPFS among 42,883 men (19). In this study, intake of SSBs was also significantly associated with increased plasma concentrations of inflammatory cytokines (19).
Recent evidence has also emerged linking intake of SSBs to increased risk of stroke. Among 84,085 women and 43,371 men in the Harvard cohorts followed for 28 and 22 years respectively, ≥ 1 serving of SSB per day was associated with 16% increased risk of total stroke compared with none in multivariable adjusted models including BMI (20). This association was attenuated and no longer statistically significant after adjusting for hypertension and diabetes, suggesting that these factors may be mediators. In the multi-ethnic cohort of 2,564 residents in Northern Manhattan followed for a mean of 10 years, daily soft drink consumption was associated with an increased risk of vascular events only in participants free of obesity, diabetes and metabolic syndrome at baseline and adjusted for a number of factors including BMI and hypertension (21). A Japanese cohort of 39,786 men and women followed for 18 years found significant positive associations between SSB intake and total and ischemic stroke in women but not in men in models adjusted for hypertension and diabetes (22). Adjustment for BMI and total energy intake had little effect on estimates, suggesting that these factors are not major mediators.
Intake of both added sugar and SSBs was associated with an increased risk for CVD mortality in an analysis of NHANES III Linked Morality cohort data (4). After a median of 14.6 years of follow-up, added sugar intake was associated with a 2-fold greater risk of CVD death comparing extreme quintiles of intake. In contrast, an analysis from the NIH-AARP Diet and Health Study; a prospective cohort of older US adults, found that intake of total fructose but not of added sugar was associated with a modest increase in risk of all-cause mortality in men and women (23). However, total sugars from beverages, including added sugar were positively associated with risk of all-cause, CVD and other-cause mortality in women while only fructose from beverages was positively associated with risk of all-cause and CVD mortality in men. The authors suggest that the differential associations by sex may be due to hormonal and biological differences or different levels of dietary misreporting, but these results may be also due to chance.
Fructose, gout, and other metabolic conditions
Regular consumption of SSBs has been associated with hyperuricemia as well as with gout, which is a common form of inflammatory arthritis arising from deposition of uric acid in articular cartilage, in two large cohorts (24,25). In particular, higher consumption of both total and added fructose from SSBs was associated with increased risk of gout in a dose-response manner. Gout and hyperuricaemia have been associated with hypertension, diabetes, metabolic syndrome, kidney disease and CVD (26). Consumption of SSB’s, has also been associated with development of albuminuria; a marker of early kidney damage, formation of kidney stones and increased risk of chronic kidney disease (27,28). In the NHS II, sucrose consumption was associated with an increased risk of kidney stones (29). Observational studies have also found that a higher intake of sucrose and fructose is associated with a higher frequency of gallstones (30). Individuals with either kidney disease or gallstones have been shown to have elevated cardiovascular risk (31,32). The studies discussed in this section reported estimates that were adjusted for BMI, suggesting that these associations are not completely dependent on body weight.
RCTs of fructose, SSBs, and cardiovascular risk markers
Data from short-term trials and experimental studies of intermediate outcomes also provide important evidence linking fructose-containing beverages with diabetes and cardiovascular risk and support findings from observational studies. Recently, Stanhope and colleagues showed that consuming beverages containing 10%, 17.5%, or 25% of energy requirements from HFCS produced significant linear dose-response increases in postprandial triglycerides, fasting LDL cholesterol and 24-hour mean uric acid concentrations in a 2-week parallel-arm, nonrandomized double blinded intervention study (11). Raben et al. found that a sucrose-rich diet consumed for 10 weeks resulted in significant elevations of postprandial glycemia, insulinemia, and lipidemia compared to a diet rich in artificial sweeteners in overweight healthy subjects (33). A randomized crossover trial among normal weight healthy men found that after 3 weeks, SSBs consumed in small to moderate quantities (600 mL SSB/day containing 40–80 g of sugar) significantly impaired glucose and lipid metabolism and promoted inflammation (34). Of note, LDL particle size was reduced for high fructose and high sucrose SSBs. A 10-week intervention comparing effects of sucrose and artificially sweetened food/beverages on markers of inflammation found that serum levels of haptoglobin, transferrin, and CRP were elevated in the sucrose group compared to the sweetener group (35).
Biological mechanisms: liquid calories and unique metabolic effects of fructose
The prevailing mechanisms linking SSBs intake to weight gain are decreased satiety and an incomplete compensatory reduction in energy intake at subsequent meals following ingestion of liquid calories (6). A typical 12 oz. (360 mL) serving of soda contains on average 140–150 calories and 35 to 37.5 grams of sugar. If these calories are added to the typical diet without compensation for the additional calories, 1 can of soda per day, could in theory lead to a weight gain of 5 pounds in one year. Short-term feeding studies comparing SSBs to artificially sweetened beverages in relation to energy intake (36) and weight change (33,36–39) illustrate this point. Some limited evidence supporting incomplete compensation for liquid calories has also been provided by studies showing greater energy intake after isocaloric consumption of beverages compared to solid food (40,41). These studies argue that sugar or HFCS in liquid beverages may not suppress intake of solid foods to the level needed to maintain energy balance, however the mechanisms responsible for this response is largely unknown.
SSBs may contribute to the development of diabetes and cardiovascular risk in part through caloric effects and the ability to induce weight gain, but also independently through non-calorically related metabolic effects of constituent sugars (Central Illustration). Consumption of SSBs has been shown to induce rapid spikes in blood glucose and insulin levels (33), which in combination with the large volumes consumed contribute to a high dietary glycemic load (GL). High GL diets are thought to stimulate appetite and promote weight gain due to the higher postprandial insulin response following ingestion of a high GL meal (42) and have been shown to promote hyperinsulinemia and insulin resistance (42). High GL diets have also been shown to exacerbate inflammatory biomarkers such as C-reactive protein (43) and have been associated with increased risk of diabetes (44) and CHD (18,19). SSBs may affect risk of CHD through effects on inflammation (45), which influences atherosclerosis, plaque stability and thrombosis (46). Intake of SSBs could stimulate an inflammatory response through hyperglycemia, which can activate the electron transport chain to produce superoxide radicals (47).
Some evidence suggests that consuming fructose from SSBs as a constituent of sucrose and in slightly higher amounts HFCS and from fruit juices may exert additional adverse cardiometabolic effects. Fructose alone is poorly absorbed but is enhanced by glucose in the gut, thus accounting for the rapid and complete absorption of both fructose and glucose when ingested as sucrose or HFCS. As previously described, fructose is preferentially metabolized to lipid in the liver, and can lead to increased hepatic de novo lipogenesis, atherogenic dyslipidemia and insulin resistance. The increase in hepatic lipid content promotes production and secretion of VLDL leading to increased concentrations of postprandial triglyceride. Consumption of fructose-containing sugars has been associated with production of small dense LDL-cholesterol, which may be due to increased levels of VLDL induced lipoprotein remodeling, mediated by cholesteryl ester transfer protein and hepatic lipase (9,11).
Fructose has also been shown to promote the accumulation of visceral adipose tissue and the deposition of ectopic fat (48,49). A 10-week study comparing beverages providing 25% of energy from fructose with a beverage providing 25% of energy from glucose showed that fructose-containing beverages increased de novo lipogenesis and visceral adiposity, promoted dyslipidemia and decreased insulin sensitivity compared to the glucose beverage (49). Another study compared daily intakes of 1 L per day of cola, diet cola, milk or water for 6 months and found that intake of cola increased liver fat visceral fat, muscle fat and triglycerides compared to the other beverages (50). Fructose is also the only sugar known to increase serum uric acid levels, which is associated with the development of gout (51). Hepatic uric acid production may also reduce endothelial nitric oxide, which may partly explain the association between SSB and CHD (52). Fructose has also been shown to stimulate transcription of inflammatory factors by activating nuclear factor-kB in mice, further supporting inflammation as a potential pathway between SSB and CHD (53). A recent meta-analysis found that fructose in isocaloric exchange with glucose increased total cholesterol, uric acid and postprandial triglycerides but had no adverse effect on other lipid parameters, insulin or markers of nonalcoholic fatty liver disease (NAFLD) and may be beneficial for body weight, blood pressure and glycemic control (54). As discussed by the authors, interpretation of these data are limited by the high dose range studied, negative comparators (glucose and starch), short follow-up and methodological limitations of the available trials (54).
Healthy-Alternatives to SSBs and Policy Strategies
Several beverages have been suggested as alternatives to SSBs including water, 100% fruit juice, coffee, tea and artificially sweetened drinks. Unlike SSBs, water does not contain liquid calories and for most people with access to safe drinking water, water is the optimal calorie-free beverage because it is affordable and accessible. We found that replacement of 1 serving per day of SSBs with one serving of water was associated with 0.49 kg less weight gain over each 4-year period (55). In the NHS II, substituting water for SSBs was also associated with a significantly lower risk of diabetes (56).
One hundred percent fruit juice could be perceived as a healthy alternative to SSBs, since juices contain some vitamins and other nutrients. However, fruit juices also contain a relatively high number of calories from natural sugars, with likely greater amounts of fructose. Previous cohort studies have found positive associations between consumption of fruit juice and greater weight gain (57) and diabetes (58), although some conflicting evidence exists (57,59), suggesting that further research exploring health effects of juice is warranted. Nonetheless, based on the current evidence it has been recommended that daily intake of fruit juices be limited to 4–6 oz.
Numerous prospective cohort studies have shown that regular consumption of coffee (decaffeinated or regular) and tea can have favorable effects on diabetes and cardiovascular disease risk (60,61) possibly because of their high polyphenol content. Thus coffee and tea are healthy alternatives to SSBs for individuals without contraindications provided that caloric sweeteners and creamers are used sparingly. In the NHS II, substituting one serving of SSBs with one cup of coffee daily was associated with a 17% lower risk of diabetes (62).
Artificially sweetened beverages may be a reasonable alternative to SSBs since they provide few to no calories, however little is known about the long-term health consequences of consuming artificial sweeteners. Some studies have reported positive associations between diet soda consumption and weight gain and risk of metabolic syndrome and diabetes (63,64). However, these findings may be due to reverse causation or residual confounding and short-term trials have reported modest benefits on weight with artificially sweetened beverages as a replacement for sugar sweetened beverages, but long-term data are lacking (7). On the other hand, some evidence suggests that the intense sweetness of artificial sweeteners may condition towards a greater preference for sweets and enhance appetite (6). Although consumption of artificially sweetened beverages is preferable to SSBs in the short term, further studies are needed to evaluate their long-term metabolic consequences.
In light of the evidence linking regular consumption of SSBs to obesity and related chronic diseases, national and international organizations have already called for reductions in intake of these beverages to help prevent obesity and improve overall health. The American Heart Association recommends no more than 100 –150 kcal/d from all added sugar and both the World Health Organization and 2015 Dietary Guidelines Advisory Committee recommend an upper limit of 10% of total energy from added sugar. Numerous other professional organizations also have specific recommendations for limiting intake of SSBs. In addition to strong and widespread public health recommendations, public policy interventions are needed to change consumption patterns because they can bring about rapid and effective changes in the food environment. Proposed changes to the nutrition facts label by the US food and Drug Administration include listing the amounts of added sugar in a product and the percent daily value (%DV) for added sugar. A combination of strategies across multiple levels, are thus needed to reduce intake of SSBs, as illustrated in textbox 2. Implementing and evaluating these types of strategies on changes in consumers’ purchasing and eating behaviors as well as health outcomes should be a high priority.
Textbox 2. Policy strategies to reduce consumption of SSBs.
Social marketing and public health campaigns are needed to raise awareness about the health effects of SSBs and added sugar and about healthy alternatives.
Governments should impose financial incentives such as taxation of SSBs of at 1east a 10% price increase, and implement limits for use of Supplemental Nutrition Assistance Program (SNAP) benefits for SSBs or subsidizing SNAP purchases of healthier foods, to encourage healthier beverages choices.
Regulations are needed to reduce exposure to marketing of unhealthy foods and beverages in the media and at sports events or other activities, particularly in relation to children.
Front of package labelling or other nutrition labeling strategies should be implemented to help guide consumers to make healthy food and beverage choices. These changes should be accompanied by concurrent public health awareness campaigns.
Policies should be put in place to reduce the availability of SSBs in the workplace, health care facilities, government institutions, and other public places and ensure access to safe water and healthy alternatives. Restrictions should also be put in place on large portion sizes.
Educational campaigns about the health risks associated with overconsumption of SSBs should be aimed at health care professionals and clinical populations.
National and international campaigns targeting obesity and chronic disease prevention should include the health risks associated with overconsumption of SSBs.
National and international dietary recommendations should include specific guidelines for healthy beverage consumption.
Conclusions
Intake of added sugar, predominantly sucrose and HFCS from SSBs has increased markedly in the US since the late 1960’s and constitutes the major source of fructose in the diet. In this regard, and since we rarely consume fructose in isolation, it is logical to gauge the potential cardiometabolic effects of fructose by evaluating associations with SSBs. Although consumption of SSBs has decreased moderately in recent years, intake levels remain high in the US population and are increasing rapidly in developing countries. Based on the available evidence from high-quality observational studies and experimental trials of risk markers we have the scientific basis to conclude that consumption of SSBs causes excess weight gain and is associated with increased risk of type 2 diabetes and cardiovascular disease and thus these beverages are unique dietary contributors to obesity and related chronic diseases. SSBs are thought to promote weight gain in part due to excess calories and incomplete compensation for liquid calories at subsequent meals. These beverages may also increase diabetes and cardiovascular risk independently through an adverse glycemic response and unique metabolic effects of fructose. Short-term mechanistic studies have shown that excess fructose ingestion can result in additional cardiometabolic effects due to increased hepatic de novo lipogenesis, accumulation of visceral adiposity and ectopic fat and production of uric acid.
Several public policy and regulatory strategies to reduce intake of SSBs are already in place or are being considered. Implementing and evaluating such policies are important areas for scientists and policymakers. Key areas that warrant future research include examining the effects of different sugars and sugar moieties on health outcomes over a broad range of doses, investigating the health effects of sugar consumed in solid form in comparison to liquid sugar and further elucidating the biological mechanism by which intake of liquid calories induces an incomplete compensatory intake of energy at subsequent meals. There is also a need for additional studies to examine the long-term health effects of consuming artificial sweeteners as a substitute for sugar. Lastly, more and higher quality RCT’s are needed to identify effective strategies to reduce SSB consumption at the individual and population level. Although reducing consumption of SSBs or added sugar alone is unlikely to solve the obesity epidemic entirely, limiting intake is one simple change that will have a measurable impact on weight control and prevention of cardiometabolic diseases.
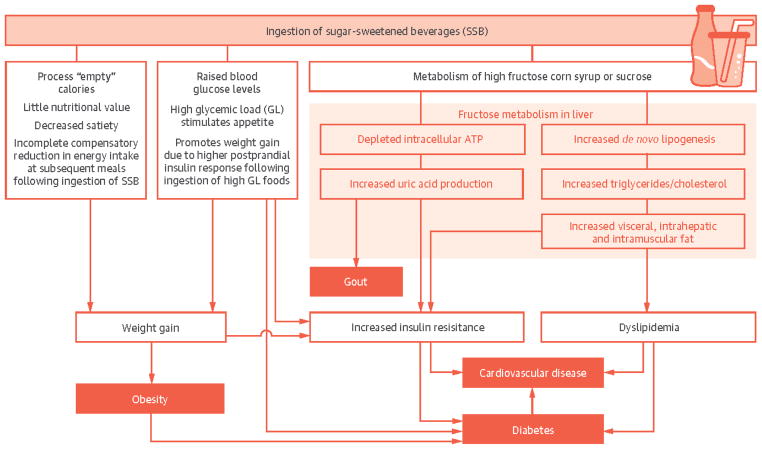
Figure 2. Central Illustration: Fructose and Cardiometabolic Health: Sugar-Sweetened Beverages.
Putative biological mechanisms linking excess sucrose and HFCS intake from sugar sweetened beverages to the development of obesity, gout, diabetes and cardiovascular disease. Excess calories, incomplete compensation for liquid calories and high glycemic load lead to obesity. Increased diabetes and cardiovascular disease risk also occur independent of weight through adverse glycemic effects and increased fructose metabolism in the liver. Excess fructose ingestion promotes hepatic uric acid production, de novo lipogenesis, accumulation of visceral adiposity and ecopic fat, which ultimately increase diabetes and cardiovascular disease risk. HFCS, high fructose corn syrup.
Acknowledgments
This research is supported by NIH grants P30 DK46200 and HL60712.
Abbreviations
- SSBs
sugar sweetened beverages
- BMI
body mass index (kg/m2)
- HFCS
high fructose corn syrup
- DHAP
dihydroxyacetone phosphate
- ATP
adenosine triphosphate
- NHS
Nurse’s Health Study
- HPFS
Health Professional’s Study
- NHANES
National Health and Nutrition Examination Survey
- VLDL
very low density lipoprotein
- CRP
C-reactive protein
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marriott BP, Cole N, Lee E. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J Nutr. 2009;139(6):1228S–35S. doi: 10.3945/jn.108.098277. [DOI] [PubMed] [Google Scholar]
- 2.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79(4):537–43. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- 3.Hu FB, Malik VS. Sugar-sweetened beverages and risk of obesity and type 2 diabetes: epidemiologic evidence. Physiol Behav. 2010;100(1):47–54. doi: 10.1016/j.physbeh.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Q, Zhang Z, Gregg EW, et al. Added Sugar Intake and Cardiovascular Diseases Mortality Among US Adults. JAMA Intern Med. 2014;174(4):516–524. doi: 10.1001/jamainternmed.2013.13563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogden CL, Kit BK, Carroll MD, Park S. Consumption of sugar drinks in the United States, 2005–2008. NCHS data brief. 2011;(71):1–8. [PubMed] [Google Scholar]
- 6.Malik VS, Popkin BM, Bray GA, et al. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation. 2010;121(11):1356–64. doi: 10.1161/CIRCULATIONAHA.109.876185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malik VS, Pan A, Willett WC, Hu FB. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am J Clin Nutr. 2013;98(4):1084–102. doi: 10.3945/ajcn.113.058362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malik VS, Popkin BM, Bray GA, et al. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care. 2010;33(11):2477–83. doi: 10.2337/dc10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michael I, Goran L, TaK-A L, editors. Dietary Sugars and Health. CRC Press, Taylor & Francis Group; Boca Raton, FL: 2015. [Google Scholar]
- 10.Stanhope KL, Bremer AA, Medici V, et al. Consumption of fructose and high fructose corn syrup increase postprandial triglycerides, LDL-cholesterol, and apolipoprotein-B in young men and women. J Clin Endocrinol Metab. 2011;96(10):E1596–605. doi: 10.1210/jc.2011-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stanhope KL, Medici V, Bremer AA, et al. A dose-response study of consuming high-fructose corn syrup-sweetened beverages on lipid/lipoprotein risk factors for cardiovascular disease in young adults. Am J Clin Nutr. 2015;101(6):1144–54. doi: 10.3945/ajcn.114.100461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaiser KA, Shikany JM, Keating KD, Allison DB. Will reducing sugar-sweetened beverage consumption reduce obesity? Evidence supporting conjecture is strong, but evidence when testing effect is weak. Obes Rev. 2013;14(8):620–33. doi: 10.1111/obr.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qi Q, Chu AY, Kang JH, et al. Sugar-sweetened beverages and genetic risk of obesity. New Engl J Med. 2012;367(15):1387–96. doi: 10.1056/NEJMoa1203039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ebbeling CB, Feldman HA, Chomitz VR, et al. A randomized trial of sugar-sweetened beverages and adolescent body weight. New Engl J Med. 2012;367(15):1407–16. doi: 10.1056/NEJMoa1203388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Ruyter JC, Olthof MR, Seidell JC, Katan MB. A trial of sugar-free or sugar-sweetened beverages and body weight in children. New Engl J Med. 2012;367(15):1397–406. doi: 10.1056/NEJMoa1203034. [DOI] [PubMed] [Google Scholar]
- 16.Consumption of sweet beverages and type 2 diabetes incidence in European adults: results from EPIC-InterAct. Diabetologia. 2013;56(7):1520–30. doi: 10.1007/s00125-013-2899-8. [DOI] [PubMed] [Google Scholar]
- 17.Muraki I, Imamura F, Manson JE, et al. Fruit consumption and risk of type 2 diabetes: results from three prospective longitudinal cohort studies. BMJ. 2013;347:f5001. doi: 10.1136/bmj.f5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fung TT, Malik V, Rexrode KM, Manson JE, Willett WC, Hu FB. Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr. 2009;89(4):1037–42. doi: 10.3945/ajcn.2008.27140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Koning L, Malik VS, Kellogg MD, Rimm EB, Willett WC, Hu FB. Sweetened beverage consumption, incident coronary heart disease, and biomarkers of risk in men. Circulation. 2012;125(14):1735–41. S1. doi: 10.1161/CIRCULATIONAHA.111.067017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernstein AM, de Koning L, Flint AJ, Rexrode KM, Willett WC. Soda consumption and the risk of stroke in men and women. Am J Clin Nutr. 2012;95(5):1190–9. doi: 10.3945/ajcn.111.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gardener H, Rundek T, Markert M, Wright CB, Elkind MS, Sacco RL. Diet soft drink consumption is associated with an increased risk of vascular events in the Northern Manhattan Study. J Gen Intern Med. 2012;27(9):1120–6. doi: 10.1007/s11606-011-1968-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eshak ES, Iso H, Kokubo Y, et al. Soft drink intake in relation to incident ischemic heart disease, stroke, and stroke subtypes in Japanese men and women: the Japan Public Health Centre-based study cohort I. Am J Clin Nutr. 2012;96(6):1390–7. doi: 10.3945/ajcn.112.037903. [DOI] [PubMed] [Google Scholar]
- 23.Tasevska N, Park Y, Jiao L, et al. Sugars and risk of mortality in the NIH-AARP Diet and Health Study. Am J Clin Nutr. 2014 doi: 10.3945/ajcn.113.069369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi HK, Curhan G. Soft drinks, fructose consumption, and the risk of gout in men: prospective cohort study. BMJ. 2008;336(7639):309–12. doi: 10.1136/bmj.39449.819271.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi HK, Willett W, Curhan G. Fructose-rich beverages and risk of gout in women. JAMA. 2010;304(20):2270–8. doi: 10.1001/jama.2010.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richette P, Bardin T. Gout. Lancet. 2010;375(9711):318–28. doi: 10.1016/S0140-6736(09)60883-7. [DOI] [PubMed] [Google Scholar]
- 27.Saldana TM, Basso O, Darden R, Sandler DP. Carbonated beverages and chronic kidney disease. Epidemiology. 2007;18(4):501–6. doi: 10.1097/EDE.0b013e3180646338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shoham DA, Durazo-Arvizu R, Kramer H, et al. Sugary soda consumption and albuminuria: results from the National Health and Nutrition Examination Survey, 1999–2004. PloS one. 2008;3(10):e3431. doi: 10.1371/journal.pone.0003431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curhan GC, Willett WC, Knight EL, Stampfer MJ. Dietary factors and the risk of incident kidney stones in younger women: Nurses’ Health Study II. Arch Intern Med. 2004;164(8):885–91. doi: 10.1001/archinte.164.8.885. [DOI] [PubMed] [Google Scholar]
- 30.Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. Dietary carbohydrates and glycaemic load and the incidence of symptomatic gall stone disease in men. Gut. 2005;54(6):823–8. doi: 10.1136/gut.2003.031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382(9889):339–52. doi: 10.1016/S0140-6736(13)60595-4. [DOI] [PubMed] [Google Scholar]
- 32.Wirth J, Giuseppe R, Wientzek A, et al. Presence of gallstones and the risk of cardiovascular diseases: The EPIC-Germany cohort study. Eur J Prev Cardiol. 2015;22(3):326–34. doi: 10.1177/2047487313512218. [DOI] [PubMed] [Google Scholar]
- 33.Raben A, Moller BK, Flint A, et al. Increased postprandial glycaemia, insulinemia, and lipidemia after 10 weeks’ sucrose-rich diet compared to an artificially sweetened diet: a randomised controlled trial. Food Nutr Res. 2011:55. doi: 10.3402/fnr.v55i0.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aeberli I, Gerber PA, Hochuli M, et al. Low to moderate sugar-sweetened beverage consumption impairs glucose and lipid metabolism and promotes inflammation in healthy young men: a randomized controlled trial. Am J Clin Nutr. 2011;94(2):479–85. doi: 10.3945/ajcn.111.013540. [DOI] [PubMed] [Google Scholar]
- 35.Sorensen LB, Raben A, Stender S, Astrup A. Effect of sucrose on inflammatory markers in overweight humans. Am J Clin Nutr. 2005;82(2):421–7. doi: 10.1093/ajcn.82.2.421. [DOI] [PubMed] [Google Scholar]
- 36.DellaValle DM, Roe LS, Rolls BJ. Does the consumption of caloric and non-caloric beverages with a meal affect energy intake? Appetite. 2005;44(2):187–93. doi: 10.1016/j.appet.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Raben A, Vasilaras TH, Moller AC, Astrup A. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr. 2002;76(4):721–9. doi: 10.1093/ajcn/76.4.721. [DOI] [PubMed] [Google Scholar]
- 38.Tordoff MG, Alleva AM. Effect of drinking soda sweetened with aspartame or high-fructose corn syrup on food intake and body weight. Am J Clin Nutr. 1990;51(6):963–9. doi: 10.1093/ajcn/51.6.963. [DOI] [PubMed] [Google Scholar]
- 39.Reid M, Hammersley R, Hill AJ, Skidmore P. Long-term dietary compensation for added sugar: effects of supplementary sucrose drinks over a 4-week period. Br J Nutr. 2007;97(1):193–203. doi: 10.1017/S0007114507252705. [DOI] [PubMed] [Google Scholar]
- 40.DiMeglio DP, Mattes RD. Liquid versus solid carbohydrate: effects on food intake and body weight. Int J Obes Relat Metab Disord. 2000;24(6):794–800. doi: 10.1038/sj.ijo.0801229. [DOI] [PubMed] [Google Scholar]
- 41.Pan A, Hu FB. Effects of carbohydrates on satiety: differences between liquid and solid food. Curr Opin Clin Nutr Metab Care. 2011;14(4):385–90. doi: 10.1097/MCO.0b013e328346df36. [DOI] [PubMed] [Google Scholar]
- 42.Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA. 2002;287(18):2414–23. doi: 10.1001/jama.287.18.2414. [DOI] [PubMed] [Google Scholar]
- 43.Liu S, Manson JE, Buring JE, et al. Relation between a diet with a high glycemic load and plasma concentrations of high-sensitivity C-reactive protein in middle-aged women. Am J Clin Nutr. 2002;75(3):492–8. doi: 10.1093/ajcn/75.3.492. [DOI] [PubMed] [Google Scholar]
- 44.Bhupathiraju SN, Tobias DK, Malik VS, et al. Glycemic index, glycemic load, and risk of type 2 diabetes: results from 3 large US cohorts and an updated meta-analysis. Am J Clin Nutr. 2014;100(1):218–32. doi: 10.3945/ajcn.113.079533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 46.Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. 2004;109(21 Suppl 1):II2–10. doi: 10.1161/01.CIR.0000129535.04194.38. [DOI] [PubMed] [Google Scholar]
- 47.Esposito K, Nappo F, Marfella R, et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106(16):2067–72. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- 48.Teff KL, Grudziak J, Townsend RR, et al. Endocrine and metabolic effects of consuming fructose- and glucose-sweetened beverages with meals in obese men and women: influence of insulin resistance on plasma triglyceride responses. J Clin Endocrinol Metab. 2009;94(5):1562–9. doi: 10.1210/jc.2008-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stanhope KL, Schwarz JM, Keim NL, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119(5):1322–34. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maersk M, Belza A, Stodkilde-Jorgensen H, et al. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. Am J Clin Nutr. 2012;95(2):283–9. doi: 10.3945/ajcn.111.022533. [DOI] [PubMed] [Google Scholar]
- 51.Johnson RJ, Segal MS, Sautin Y, et al. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr. 2007;86(4):899–906. doi: 10.1093/ajcn/86.4.899. [DOI] [PubMed] [Google Scholar]
- 52.Nakagawa T, Tuttle KR, Short RA, Johnson RJ. Hypothesis: fructose-induced hyperuricemia as a causal mechanism for the epidemic of the metabolic syndrome. Nat Clin Pract Nephrol. 2005;1(2):80–6. doi: 10.1038/ncpneph0019. [DOI] [PubMed] [Google Scholar]
- 53.Roglans N, Vila L, Farre M, et al. Impairment of hepatic Stat-3 activation and reduction of PPARalpha activity in fructose-fed rats. Hepatology. 2007;45(3):778–88. doi: 10.1002/hep.21499. [DOI] [PubMed] [Google Scholar]
- 54.Sievenpiper JL, de Souza RJ, Cozma AI, et al. Fructose vs., glucose and metabolism: do the metabolic differences matter? Curr Opin Lipidol. 2014;25(1):8–19. doi: 10.1097/MOL.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 55.Pan A, Malik VS, Hao T, et al. Changes in water and beverage intake and long-term weight changes: results from three prospective cohort studies. Int J Obes (Lond) 2013 doi: 10.1038/ijo.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pan A, Malik VS, Schulze MB, et al. Plain-water intake and risk of type 2 diabetes in young and middle-aged women. Am J Clin Nutr. 2012;95(6):1454–60. doi: 10.3945/ajcn.111.032698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schulze MB, Manson JE, Ludwig DS, et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292(8):927–34. doi: 10.1001/jama.292.8.927. [DOI] [PubMed] [Google Scholar]
- 58.Bazzano LA, Li TY, Joshipura KJ, Hu FB. Intake of fruit, vegetables, and fruit juices and risk of diabetes in women. Diabetes Care. 2008;31(7):1311–7. doi: 10.2337/dc08-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ghanim H, Mohanty P, Pathak R, et al. Orange juice or fructose intake does not induce oxidative and inflammatory response. Diabetes Care. 2007;30(6):1406–11. doi: 10.2337/dc06-1458. [DOI] [PubMed] [Google Scholar]
- 60.van Dam RM. Coffee consumption and risk of type 2 diabetes, cardiovascular diseases, and cancer. Appl Physiol Nutr Metab. 2008;33(6):1269–83. doi: 10.1139/H08-120. [DOI] [PubMed] [Google Scholar]
- 61.Bhupathiraju SN, Pan A, Malik VS, et al. Caffeinated and caffeine-free beverages and risk of type 2 diabetes. Am J Clin Nutr. 2013;97(1):155–66. doi: 10.3945/ajcn.112.048603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Koning L, Malik VS, Rimm EB, et al. Sugar-sweetened and artificially sweetened beverage consumption and risk of type 2 diabetes in men. Am J Clin Nutr. 2011;93(6):1321–7. doi: 10.3945/ajcn.110.007922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nettleton JA, Lutsey PL, Wang Y, et al. Diet soda intake and risk of incident metabolic syndrome and type 2 diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes Care. 2009;32(4):688–94. doi: 10.2337/dc08-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Circulation. 2008;117(6):754–61. doi: 10.1161/CIRCULATIONAHA.107.716159. [DOI] [PubMed] [Google Scholar]