Summary
While the mainstay of periodontal assessment is clinical probing, radiographic assessment is also commonly employed and has the potential to provide facile quantitative information on the status of tooth-supporting bone. This article provides a brief review of standard methods to assess periodontal structures, including basic tenants of radiograph acquisition, assessment of alveolar crest levels, and typical patterns of bone loss seen in periodontal patients. Studies of the use of computer technology to objectively assess loss of alveolar crest from standardized and non-standardized radiographs are reviewed. Several recent developments in computer-assisted quantitation of alveolar crest height are described. Although probing measurements continue to be viewed as more practical than radiographic measurements, radiographic assessment can be made quantitative and likely easier and more precise than probing for routine assessment of periodontal disease activity.
Keywords: Radiologic Assessment, Alveolar Crest Height, Periodontal Disease, Periodontal Examination, Radiographs
Introduction
Periodontitis, which is caused by inflammatory processes initiated by bacteria that colonize as oral biofilms (dental plaque) on teeth, results in tissue destruction that manifests as gingival pockets, periodontal ligament destruction and tooth-supporting alveolar bone loss [1]. A variety of methods are used to evaluate the periodontium in order to assess periodontal status. These methods include visual description of the gingival tissues, periodontal probing and radiographic assessment of underlying bone.
Tissue destruction resulting from periodontitis has been found to follow an irregular time course, and may occur as episodic “bursts” [2] [3]. The disease can remain stable and then rapidly progress, often at individual tooth sites. For some individuals, tissue destruction can be minimal, even in the presence of poor bacterial biofilm control, while for others, tissue destruction can be extensive and result in tooth loss. The cause for this difference in susceptibility may be related to systemic, environmental or genetic risk factors (e. g., smoking, diabetes mellitus, HIV, stress, etc.) [4].
Clinical examination, including periodontal probing and assessment of gingival inflammation (bleeding on probing), is the mainstay of periodontal diagnosis and disease assessment. A variety of clinical assessment indices can be used, each with its own advantages and limitations. These include the periodontal index (PI), periodontal disease index (PDI), gingival index and sulcus bleeding index [1]. The PI is an index designed to give more attention to periodontal tissue destruction compared to gingival inflammatory status, and it follows a scoring system ranging from 0 to 8, where a 0 score reflects the absence of both gingival inflammation and supporting tissue destruction, and a score of 8 reflects advanced periodontal destruction with tooth mobility and/or migration. On the other hand , PDI is an index designed to measure the periodontal status of six preselected teeth, known as the “Ramfjord teeth”, which are the maxillary right first molar, maxillary left central incisor, maxillary left first premolar, mandibular left first molar, mandibular right central incisor and mandibular right first premolar.
The gingival index system provides an overall assessment of gingival inflammatory status that can be used clinically to compare the efficiency of phase I treatment and to compare results before and after surgical therapy, as well as establishing good inter-examiner and intra-examiner calibration between dentists [1] [5]. The sulcus bleeding index system is designed to give reproducible assessment of the gingival status, which is characterized by early detection of inflammatory changes at the base of the pocket or gingival crevice that is not easily detectable or visible with ordinary clinical examination. In addition, this index can be used to motivate the patient to perform better oral hygiene measures, since gingival bleeding in an early sign of disease development [1].
Comprehensive clinical periodontal examination requires periodontal charting of all teeth. It is known, however, that probing is not precise since a good deal of variation is inherent to the procedure [1] [5]. In order to provide periodontal care that provides timely intervention to prevent progression of disease, there is need for a more precise method to monitor tissue destruction.
2. Radiographic assessment of alveolar crest level
In most cases, clinical examination is supplemented with radiographic assessment. Radiographic examination and assessment of alveolar crest levels around individual teeth is a useful diagnostic adjunct to clinical periodontal examination. In general, radiographic examination allows for the accurate evaluation of crestal-bone architecture, crown-root ratios, the presence of vertical or horizontal bone defects, furcation involvements, and the overall morphology of bone. Ideal radiographic imaging should satisfy the following criteria in order to achieve the most accurate diagnosis [6] [5]:
The radiograph should record the complete area of interest, to allow assessment of alveolar crest height and the presence of furcation involvement or vertical bone defects.
Radiographic distortion should be minimized by use of proper x-ray projection geometry.
Radiographs should have the optimal contrast and density.
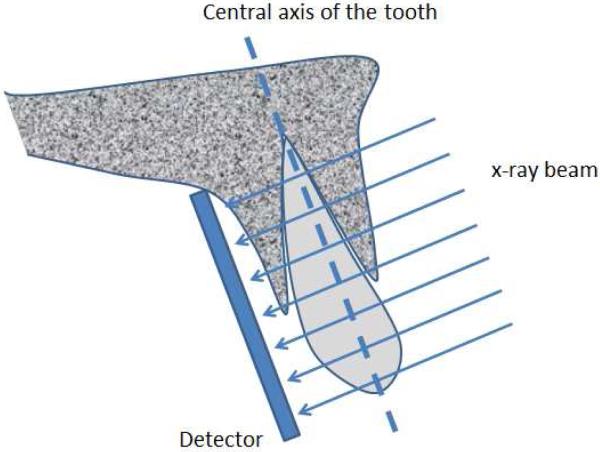
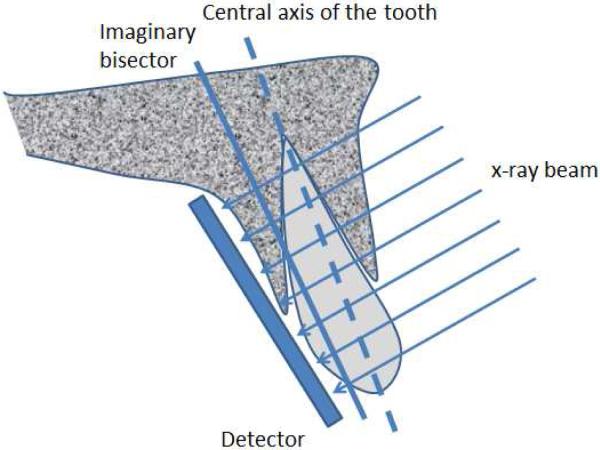
Periapical or bitewing radiographs are the most common types of radiographs used for evaluation of periodontal bone loss. Periapical radiographs utilize two different types of projection techniques [6]; the parallel-projection technique (or right angle-long cone technique), or the bisecting angle technique. The goal of the parallel-projection technique is to position the X-ray film parallel to the tooth so that the beam hits both the tooth and the X-ray film at right angles (see Figure 1), resulting in minimal geometric distortion. The bisecting-angle technique (See Figure 2) is best described by Cieszynski's rule of isometry, which states “that two triangles are equal when they share one complete side and 2 equal angles”. The clinical application of this is achieved by placing the film as close as possible to the lingual surface of the teeth, as the plane of the film and the long axis of the teeth will form an angle with its apex at the point where the tooth and film come in contact. An imaginary line is formed which bisects the triangle, and the X-ray beam is directed along this line and called the bisector. This results in two triangles with equal angles and a common side. When this technique is performed accurately, the resulting tooth image will have a length equal to the tooth itself, which is an advantage compared to the parallel technique. However, the bisected-angle technique has two limitations. The first regards multi-rooted teeth, where the central bisecting beam must be angled differently for each root. The second limitation is related to the alveolar ridge, which is usually projected more coronally than its true position when compared to the parallel-projection technique. In general, periapical radiographs are more useful when compared to bitewing radiographs in that the periapical film can be used to accurately assess important anatomical structures, such as the mandibular nerve, the mental foramen, and the floor of the maxillary sinus. In addition, the periapical film can also be used to evaluate the presence or absence of any periapical pathology [7].
Figure 1.
Illustration of the paralleling technique. The film is placed so that its surface is parallel to the tooth axis, both of which are perpendicular to the x-ray beam.
Figure 2.
Illustration of the bisecting-angle technique. The film is placed as close as possible to the lingual surface of the tooth, the x-ray beam is perpendicular to the imaginary bisector plane.
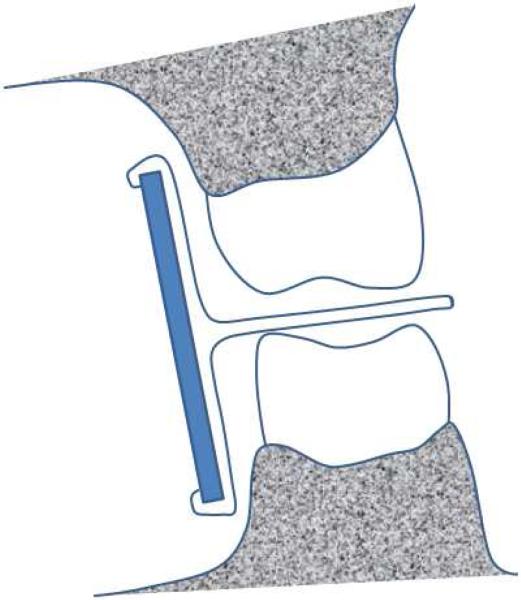
Bitewing radiographs differ from periapical radiographs in that they are usually limited to capturing the image of the crowns of both maxillary and mandibular posterior teeth along with the alveolar crest in the same radiographic film [6]. Bitewings are commonly used in general practice for dental caries detection as well as for evaluation of alveolar crest height around teeth. The horizontal-bitewing technique uses the X-ray beam aligned between the teeth and penetrates through the contact areas while at the same time being parallel to the occlusal plane (Figure 3a). The ideal bitewing radiograph provides a clear view of the mandibular and maxillary alveolar bone and teeth with minimal overlap between the teeth. Vertical bitewings differ from the horizontal bitewing in that the radiographic film has greater vertical dimension to allow for more complete evaluation of the alveolar bone in patients expected to have moderate to severe periodontal bone loss (see Figure 3b). Precise detection of proximal caries is considered an advantage of the horizontal bitewing technique while the unobstructed view of the alveolar bone is the advantage of the vertical bitewing technique, which makes the later a more valuable diagnostic tool for periodontal disease assessment [6].
Figure 3.
(a) Illustration of the horizontal-bitewing geometry. Images are obtained of both the maxillar and mandibular teeth, however, less bone is imaged than in the vertical-bitewing technique. (b) An example of a bitewing radiograph.
Rapid developments in computer technology have allowed for the development of digital radiographic imaging procedures, which minimize processing errors inherent to conventional (analog) radiographic techniques. Among these technological innovations is digital subtraction radiography, which is based on subtraction of two images of the same object, recorded at different times [6] [5] [1]. This technique is useful in detection of changes in tissue density between baseline and follow-up examinations. In general, the radiographic changes are either reflected as bright or dark areas. A bright area indicates gain in tissue density (mostly bone), while a darker area indicates tissue loss. The main disadvantage of subtraction radiography is that the geometry of the baseline image must be exactly reproduced in follow-up images or differences in geometry appropriately corrected, otherwise obfuscating artifacts appear in the image [6].
Another recent advance in diagnostic imaging is Computer-Assisted Densitometric Image Analysis System (CADIA) [8]. CADIA uses digitized radiographic information to measure the light transmitted through the radiograph, which is then converted into a gray-scale image that is then transferred into the computer where mathematic manipulations of the images take place that can be displayed, manipulated, and interpreted. This technique can also be used to assess alveolar bone density over time, similar to subtraction radiography. CADIA is more sensitive, accurate, and reproducible when compared to subtraction radiography. CADIA has been used in longitudinal clinical studies to follow the progression of periodontal bone loss and clinical attachment loss, and is shown to be more reliable compared to conventional radiographic techniques [1].
As used in most clinical settings, radiographic examination of periodontal disease does not permit accurate detection of minor destruction of alveolar bone. Rather, radiographs demonstrate bone loss only after a significant amount of bone destruction has already occurred [9]. It has been reported that the difference between clinical alveolar crest height and radiographic crest height can range from 0 mm to 1.6 mm, with the radiographic measurements very much affected by radiographic beam angulation [10].
Periodontal disease shows radiographic changes that usually starts as a “fuzziness” or discontinuity of the lamina dura on the mesial and distal aspects of the interdental septa [1] [11]. This will then transform into a wedge-shaped radiolucent area in the interdental septa. Afterwards, bone destruction will appear to extend across the interdental septa and finally as a marked reduction in the height of interdental septum, resulting in a periodontal bone defect. However, there are several anatomical factors that can affect the morphology of periodontal bone defects including [11] [1] (Box 1). Additional factors that can complicate interpretation of the morphology of periodontal bone include the presence of exostoses, trauma from occlusion, buttressing bone formation, and food impaction [11] [1].
Data from Manson, J. and K. Nicholson, The distribution of bone defects in chronic periodontitis. Journal of periodontology, 1974. 45(2): p. 88; and Newman, M.G., H.H. Takei, and F.A. Carranza, Carranza's Clinical Periodontology. 2006.
There are several patterns that develop regarding the distribution and classification of bone loss in periodontal disease patients [11] [1]. Horizontal bone loss is the most common pattern of bone loss in chronic periodontitis [11]. Generally, the overall bone height is reduced while the margins of the alveolar crest are perpendicular to the tooth surface. Horizontal bone loss usually affects the facial and lingual plates of the interdental septa of a group of teeth or even all teeth in the mouth. In contrast, osseous defects are common in periodontal-disease patients. These can be detected on radiographs or more precisely evaluated during surgical procedures in order to know their exact morphology and extent, and to plan suitable treatment approaches for each type of defect. The most common osseous defects are vertical defects, defined as bone loss occurring in a vertical or oblique direction resulting in a hollowed-out trough within the bone alongside a root surface, while the base of the defect is located apical to the surrounding bone. These types of defects usually occur as intrabony (infrabony) defects. These unique defects are often classified according to the number of the osseous walls involved in the destructive process. So, for example, one-wall defects, two-wall defects and three-wall defects can be described. However when the number of osseous walls differs between the apical and the occlusal portion of the defect, the defect is termed combined-osseous defect. Vertical defects are more likely to occur on the mesial and distal aspects of teeth, and the molar teeth are more commonly affected (the second and third maxillary and mandibular molars), especially in the case of three-wall defects (also referred to intrabony (infrabony) defects). One-wall defects, also termed hemiseptal defects, offer great challenge to radiographic assessment because they can be obscured by radiopaque facial, palatal and lingual plates of bone. In some cases, the only diagnostic option is surgical exploration to appropriately assess and manage such defects.
Reverse architecture is more commonly observed in the maxilla compared to the mandible. This occurs as a result of the loss of interdental bone of the facial, lingual and palatal plates, while radicular bone is preserved. Thus, bone heights appear reversed from the normal architecture of alveolar bone.
Osseous craters appear as continuous concavities of bone that affect multiple surfaces of teeth, for example interdental, facial, lingual, and/or palatal bone. These defects constitute more than one third of all defects and are most commonly found to affect the posterior teeth[1] [5] [11]. These types of defects tend to affect posterior teeth because bone around these teeth tends to have a flat interdental bone configuration when compared to anterior segments of teeth.
Furcation involvements are defined as the loss of bone in the bifurcation or trifurcation area of multi-rooted teeth. Furcation involvements are classified into four grades according to the amount of bone loss and tissue destruction (Grade I - incipient bone loss; Grade II - partial bone loss (or so called CUL-DE-SAC defects); Grade III - total loss of radicular bone with a through-and-through opening of the furcation, but without gingival recession; Grade IV - similar to Grade III but with gingival recession, which makes the furcation clinically visible). The radiographic assessment of furcation involvement is helpful and useful in evaluation of Grade III and IV lesions. But, the angulation of the x-ray beam might result in the overlap of neighboring radiopaque structures to obscure this assessment.
Several other patterns of alveolar bone loss are less commonly encountered. A marginal gutter is defined as a shallow linear defect between the margin of a radicular cortical plate or interdental crest and along the length of one or more root surfaces. A dehiscence usually occurs in the form of a U-shaped or angular defect on the facial or lingual alveolar plates and also involving the marginal crest of bone of the tooth. A fenestration occurs in the form of circumscribed well-demarcated defect on the lingual or facial plate without the involvement of marginal crest of bone.
Radiographic measurement of alveolar crest height
A simple, inexpensive and quantitative technique for monitoring changes in periodontal support would enable the dentist to monitor periodontal disease activity. Such a technique could be used for monitoring patients in periodontal maintenance to flag those in need of more aggressive intervention. In most cases, assessment of periodontal disease status is now performed using periodontal probing (with clinical attachment-loss measurements made in relation to a fixed anatomical landmark, usually the CEJ) and/or alveolar bone levels from intraoral radiographs. Both of these techniques are vulnerable to errors [12, 13, 14]. While periodontal probing remains the gold standard for periodontal assessment, it has several limitations regarding reproducibility and sensitivity, depending on the degree of edema and probing technique (for example, probing force, angle of the probe, size of the probe and probe calibration [that can differ from one brand to another]), making the detection of small changes difficult [15]. Probing force is considered to be the most important factor affecting the precision, reproducibility and consistency of the probing technique. The use of controlled force pressure probes of up to 30 g within the junctional epithelium, and of 50 g for osseous defects, is required for the accurate use of probes [16] [17]. The National Institute of Dental and Craniofacial Research (NIDCR) has proposed the development of standardized computerized physical measurement techniques for the assessment of periodontal disease with eight criteria (Box 2) [1].
Examples of physical measurement techniques are the Florida Probe, the Interprobe, the Periprobe, the Foster-Miller probe, and Toronto-Automated probe systems. The Florida Probe system has been modified to use the CEJ as a reference point instead of the occlusal surface [18-21]. Moreover, the Florida Probe system has been found to provide relatively reproducible measurements, with a mean standard deviation of 0.3 mm, which is superior to conventional manual probes, which show a standard deviation of 0.83 mm [22]. However, computerized automated physical measurement systems tend to underestimate deep pocket depths, which is considered to be their major drawback. Relatively few longitudinal clinical studies with long-term follow up have been reported to evaluate these systems [18-21].
Data from Newman, M.G., H.H. Takei, and F.A. Carranza, Carranza's Clinical Periodontology. 2006.
Radiographic techniques for measuring longitudinal changes in alveolar bone levels also have several shortcomings. A review of the literature to generate guidelines to improve the reliability of radiographic measurement found that the factors affecting image formation is of prime importance to develop a clinical monitoring system capable of detecting small changes in alveolar bone from serial radiographic films [23]. Two models were found to predict the length of time needed to detect marginal crestal bone loss at rate of 0.1 mm/year. The first model assumes a CEJ-crest measurement error of +/− 0.3 mm, while the second assumed +/− 0.9 mm. The first model estimated that it would take 7 to 13 years for the system to detect crestal bone loss of 1.0 mm caused by actual loss of 0.7 to 1.3 mm, while the second model estimated that 1.0 mm of crestal bone loss would take between 1 to 19 years to be detected, caused by actual bone loss between 0.1 to 1.9 mm.
The suggested guidelines for radiographic reliability and measurements include the following requirements [23]:
Repositionable stentless film holder to standardize the radiation geometry
Very accurate reproducible measuring technique and landmarks
Automatic computer-based measuring system
A number of studies have been published that describe computerized approaches for assessment of alveolar crest height to assess periodontal disease progression. Early on, attempts were made to standardize radiographic technique by the addition of a modified alignment system that used a reference pin in the bite block that facilitated the repositioning of the film for subsequent exposures and the preservation of geometry [24]. The measurement of alveolar bone height was then performed using a “side-by-side technique” [25], where baseline and longitudinal radiographs are displayed on a computer monitor next to each other. The baseline radiograph is captured digitally and displayed with contrast and brightness settings chosen to optimize the visibility of structures of interest. Then the longitudinal radiograph is placed in a holder capable of translation and rotation. The longitudinal radiograph is captured and displayed. This radiograph is rotated and translated in the holder until its orientation is comparable to that of the baseline radiograph; then, its contrast and brightness are adjusted to match that of the baseline radiograph. This study showed smaller measurement differences and greater geometric accuracy than a conventional system, indicating that this approach could facilitate detection of small changes in crestal bone [24]. Later, a computerized measurement system for the analysis of non-standardized serial radiographs was developed [25]. It was shown that a difference of 0.87 mm between the CEJ and the alveolar crest measurement is required for a significant loss in crestal bone height to be observed [25]. Thus, changes in crestal bone height of less than 1 mm can be detected using bitewing radiographs, suggesting this could be a useful diagnostic tool for monitoring periodontal disease activity.
The precision of computerized measurements of marginal alveolar bone height from bite-wing radiographs has also been reported [26]. Using a digitization technique, previously shown to be useful for assessment of bone height in periapical radiographs in adults, marginal alveolar bone height on 432 sites in non-standardized bite-wing radiographs in patients with intact CEJs was assessed. The mean difference between repeated readings of the same site was 0.09 mm with a confidence limit of 99.9 %. Thus, digital techniques may be reliable for measurements of alveolar bone height from non-standardized bite-wing radiographs.
Radiographic bone heights measured using the side-by-side technique for pairs of digitized images have been compared with probing attachment level measured using an electronic probing system (Florida probe) [13]. Both measurements were made at baseline and after 1 year, with 13% loss found using the radiographic technique and 9.6% attachment loss measured by the Florida probe. The concordance in radiographic and attachment-level changes in 82% of the sites indicates that both techniques hold diagnostic value for assessment of disease progression. Further studies investigated the reproducibility of bone height measurement on serial radiographs using three different alignment systems; the results varied with the precision of each system [27].
The correlation of histometric measurements of bone level with radiographic and probing techniques has been assessed [28]. A difference of 0.14 mm between bone probing depths and histometric bone measurements (correlation coefficient 0.90) was reported, while the difference between radiographic bone level and the histometric bone measurement was 0.6 mm (correlation coefficient 0.73). It was concluded that probing may be a more reliable measure for detecting the actual bone level after periodontal regenerative treatment. It was also suggested that changes in clinical attachment and radiographic bone level progress somewhat independently; short term, the different measures may not follow the same course, but long term the differences become minimal. However, for the purpose of longitudinal monitoring of response to therapy, or cross-sectional studies, both techniques can be used [14].
Evaluation of radiographic assessment of periodontal endosseous defects was compared using periapical and panoramic radiographs against surgical measurements[29]. The results showed that the detection of periodontal bone defects from radiographs was low and that defect evaluation of periapical radiographs was affected by defect depth and buccolingual width, the number of osseous walls and the relative location in the jaw. Furthermore, osseous defects of a small width and depth were the most difficult to detect radiographically, and detection of osseous defects was more successful in periapical radiographs than in panoramic radiographs. The difference in the accuracy of osseous defect detection between periapical and panoramic radiographs depends on the defect location and dimensions.
Two forms of radiographic analyses, linear measurements and CADIA, were compared to assess post-surgical bone fill as measured by surgical reentry [29]. It was found that linear radiographic measurements significantly underestimated post-treatment bone fill compared to re-entry measures, while the CADIA method provided the highest accuracy. Hildebolt and colleagues assessed the reliability of linear alveolar bone-loss measurements of mandibular posterior teeth using digitized bite-wing radiographs [31]. Linear measurements between the CEJ and the alveolar crest at 6 different sites per tooth were performed by three observers. It was shown that a difference in alveolar height of 0.3 mm can be detected based on digital radiograph measurements. In a subsequent study, they developed a computer-based pattern recognition system for the purpose of objective classification of periodontal disease using intraoral periapical and bitewing radiographs [32]. The system allows adjustments of the contrast and brightness level, linear measurement of bone loss and linear crown height. This pattern recognition system was able to correctly grade periodontal disease 78% to 91% of the time.
Hou, et al., evaluated the consistency and reliability of periodontal bone level measurements using a digital scanning radiographic image analysis system [33]. Radiographic measurements were done on standardized periapical radiographs obtained using paralleling technique and were recorded by two examiners based on intra and inter-examiners’ data. In assessing the consistency and reliability for each group, they found inter- and intra-examiner reliability coefficients ranging from 0.986 to 0.995 (p<0.001). These results are similar to those obtained by Machtei, et al., [34] who compared longitudinal measurements of pocket depths, attachment level, and alveolar crest height assessment during periodontal treatment vs. non-treatment. Evaluations of 108 treated and 79 non-treated patients showed change in alveolar crest height to be minimal for the treated group, while non-treated subjects showed greater bone loss over time. However, Eickholz and Hausmann [35] found that radiographic assessment underestimated bone loss (1.41 ± 2.58 mm) when compared to surgical measurements in a study of 22 patients. Thus, while radiographic measurements of alveolar bone loss is relatively precise, the accuracy of these measurements must be taken into account in management of periodontal disease.
Currently, standards of care in dental practice involve use of periodontal probing for the diagnosis of periodontal disease. However, the literature has consistently shown that manual methods of measurement of alveolar crestal bone height in radiographs have a precision of approximately 0.5 mm or better [13, 23, 25, 26, 36-42]. Merchant [43] concluded that “Periodontitis assessed as mean alveolar bone loss or the prevalence of disease based on alveolar bone loss can be accurately and reliably evaluated from non-standardized radiographs.” The literature indicates that determination of accurate and precise crest heights from clinically acquired dental radiographs is possible and recommended. Until now, no one has stepped forward to provide a system which provides periodontists and general practitioners with this capability.
Looking toward the future of radiographic assessment of periodontal disease
While assessment of supporting bone can be appreciated by open flap (histometric) measurements, such an approach is not practical for routine assessment since it is invasive and often not possible in most cases. Although probing measurements continue to be viewed as more accurate than radiographic measurements, radiographic assessment can be made quantitative and likely made easier and more precise than probing. Unfortunately, appropriate image analysis requires more manipulation of the images than is currently available from most digital imaging systems.
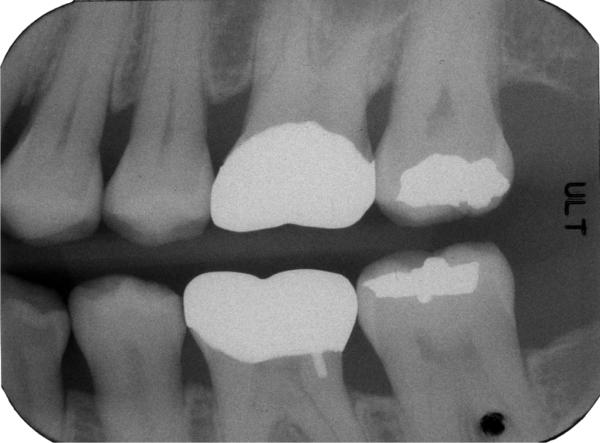
Over the past several years, digital acquisition devices (photostimulable phosphor plates [PSPs]) or direct digital capture are being used more frequently in dental imaging. While digital detectors do not have the physical flexibility of film in terms of positioning the detector in the mouth and thus tend to reduce patient comfort, they do provide the ability for digital archiving of images, for adjusting the brightness and contrast to correct for incorrect exposures allowing better visualization of anatomical features, and for some measurements, including alveolar crest height. However, the potential for the use of digital imaging to contribute to periodontal assessment has yet to be realized, even though digital analysis of intraoral radiographs is not new, as discussed above. Indeed, the vast majority of radiographic analysis by dentists remains qualitative, e.g., simply “eyeballing” radiographs. Vendors are providing measurement tools with their image presentation software (Figure 4). Currently, manual indications or measurements are time consuming and can introduce some level of error arising primarily from projection geometry differences [13, 44].
Figure 4.
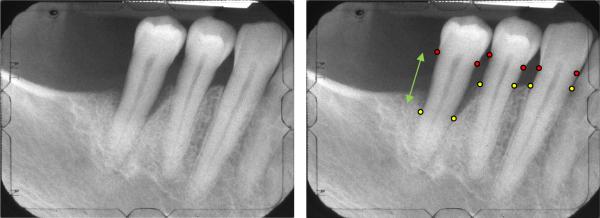
(a)Radiograph of the right posterior jaw with the cemento-enamel junction (CEJ) and the base of defect (BD) indicated by the red and yellow markers, respectively. The CEJ can be identified as the interface of the higher contrast enamel and the lower contrast dentin. The BD can be identified as the interface between the lower density of the bone-free or bone-reduced regions and the alveolar bone. Bone height or crest height (green arrow) is taken as the distance between the CEJ and BD.
However, a number of digital analysis systems have been proposed which have been shown to provide improved precision and accuracy. Hausmann has shown using his side-by-side technique [25] that adjustment of orientation, contrast and brightness improves the accuracy and precision of radiographic change measurements. Subtraction techniques [1, 5, 6] after image registration [45, 46] may not only facilitate change measurement but perhaps more importantly subtle change detection.
In general, longitudinal assessment of alveolar crest height by radiographic analysis may require use of standardized geometry to minimize angulation differences that introduce errors in the measurements [36, 44]. Rotations about the jaw axis introduce foreshortening of the tooth and the measurements of bone height as well as introducing false contours that can be take as horizontal defects. Rotations about the vertical axis can introduce double lateral edges and reduce the contrast of the CEJ. Foreshortening can be overcome using alignment and rescaling techniques based on concepts used in the Schei ruler method [47]. The false contouring can be identified if the foreshortening is first recognized via the alignment results. Identification of double lateral edges can also be identified by aligning images of the same tooth taken at different angles and/or times. With the image having higher contrast CEJ providing “guidance” on the location of the CEJ in the angulated image, the effects of the double lateral edge or reduced contrast can be mimimized.
Such effects can be substantially reduced (and the results of the analyses described can be improved) if a film positioning device is used, as has been shown using the Rinn positioning device that improves not only the quality of the images but the reproducibility of the geometry of the acquisition [27]. Also, as has been discussed by Jeffcoat [36] and Byrd [44] the issues which arise from the placement of the imaging plate in the mouth can also be addressed using similar approaches. More automated, accurate, geometry correcting analyses of digital dental radiographs should facilitate monitoring of alveolar bone levels around teeth and implants.
However, there are two additional issues that may limit progress and use of more complete digital analysis in periodontics. The first is the acceptance and use of digital imaging technology itself. Here, patient comfort remains an issue as film is more pliable than the PSP devices and the direct capture devices. We expect that the technology will continue to advance to improve patient comfort. The second issue is the impact of these analyses on clinical work flow. Ultimately, artificial intelligence principles will allow application of algorithms that will require minimal user input. Ideally, fully automated techniques would be developed where teeth, surrounding bone levels, and background are automatically identified, adjustments are made, and the results are available for quantitative inspection within seconds. However, before that some user input will be required as full automation is daunting. Indication of the tooth or teeth of interest, perhaps some points along the tooth or in the bone to guide the subsequent analyses, will be required initially (see Figure 4). But the user-interface will need to facilitate these indications while minimizing the number of mouse clicks (our experience is that none is ideal, 1-5 is tolerable, over 10 is unacceptable). An additional consideration is the use of hygenists to provide the necessary input, with the dentist interpreting the results.
Intraoral radiographs are indispensible for assessment of periodontal disease status. Systems are now under development that will enable the practitioner to easily and conveniently measure alveolar bone levels of individual patients for longitudinal assessment of disease activity. Such systems will provide more objective criteria to properly manage patients for the prevention of the initiation and/or progression of inflammatory periodontal diseases.
Key Points.
While the mainstay of periodontal assessment is clinical probing, radiographic assessment is also commonly employed and has the potential to provide facile quantitative information on the status of tooth-supporting bone.
Although probing measurements continue to be viewed as more practical than radiographic measurements, radiographic assessment can be made quantitative and likely easier and more precise than probing for routine assessment of periodontal disease activity.
Intraoral radiographs are indispensible for assessment of periodontal disease status.
Systems are now under development that will enable the practitioner to easily and conveniently measure alveolar bone levels of individual patients for longitudinal assessment of disease activity. Such systems will provide more objective criteria to properly manage patients for the prevention of the initiation and/or progression of inflammatory periodontal diseases.
Box 1 – Factors affecting radiographic appearance of periodontal defects.
The thickness, width, and angulation of the alveolar crest.
Thickness of lingual and facial alveolar bone plates.
Presence of fenestration and dehiscence.
Tooth alignment in the jaw.
Root and root trunk anatomy.
Root position within the alveolar process.
Proximity with another tooth surface.
Box 2 - Standards for computerized physical measurement techniques having a precision of 0.1 mm.
Range of 10 mm
Constant and standardized probing force
Acceptable reach and easy access to any tooth and any location around the tooth
Guidance system to ensure proper angulation
Complete sterilization of all parts entering the mouth
Noninvasive, light weight, and easy to use
Biocompatible and safe without the risk of electrical shock to the patient
Direct electronic reading in a form of digital output
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Newman MG, Takei HH, Carranza FA. Carranza's Clinical Periodontology. 2006 [Google Scholar]
- 2.Haffajee AD, Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontology 2000. 1994;5(1):78–111. doi: 10.1111/j.1600-0757.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 3.Socransky SS, Haffajee AD. The Bacterial Etiology of Destructive Periodontal Disease: Current Concepts*. Journal of periodontology. 1992;63(4s):322–331. doi: 10.1902/jop.1992.63.4s.322. [DOI] [PubMed] [Google Scholar]
- 4.Khocht A, Albandar JM. Aggressive forms of periodontitis secondary to systemic disorders. Periodontology 2000. 2014;65(1):134–148. doi: 10.1111/prd.12015. [DOI] [PubMed] [Google Scholar]
- 5.Rose LF. Periodontics: medicine, surgery, and implants. Mosby; 2004. [Google Scholar]
- 6.White SC, Pharoah MJ. Oral radiology: principles and interpretation. Elsevier Health Sciences; 2013. [Google Scholar]
- 7.White SC, Pharoah MJ. Oral radiology: principles and interpretation. Elsevier Health Sciences; 2014. [Google Scholar]
- 8.Brägger U, Pasquali L, Rylander H, Carnes D, Kornman KS. Computer-assisted densitometric image analysis in periodontal radiography. A methodological study. J Clin Periodontol. 1988;15(1):27–37. doi: 10.1111/j.1600-051x.1988.tb01551.x. [DOI] [PubMed] [Google Scholar]
- 9.Ramadan A-BE, Mitchell DF. A roentgenographic study of experimental bone destruction. Oral Surgery, Oral Medicine, Oral Pathology. 1962;15(8):934–943. doi: 10.1016/0030-4220(62)90087-7. [DOI] [PubMed] [Google Scholar]
- 10.Regan J, Mitchell D. Roentgenographic and dissection measurements of alveolar crest height. Journal of the American Dental Association (1939) 1963;66:356–359. doi: 10.14219/jada.archive.1963.0106. [DOI] [PubMed] [Google Scholar]
- 11.Manson J, Nicholson K. The distribution of bone defects in chronic periodontitis. Journal of periodontology. 1974;45(2):88. doi: 10.1902/jop.1974.45.2.88. [DOI] [PubMed] [Google Scholar]
- 12.Jeffcoat MK, Reddy MS. Advances in measurements of periodontal bone and attachment loss. 2004 doi: 10.1159/000061636. [DOI] [PubMed] [Google Scholar]
- 13.Hausmann E, Allen K, Norderyd J, Ren W, Shibly O, Machtei E. Studies on the relationship between changes in radiographic bone height and probing attachment. J Clin Periodontol. 1994;21(2):128–32. doi: 10.1111/j.1600-051x.1994.tb00290.x. [DOI] [PubMed] [Google Scholar]
- 14.Machtei EE, Hausmann E, Grossi SG, Dunford R, Genco RJ. The relationship between radiographic and clinical changes in the periodontium. J Periodontal Res. 1997;32(8):661–6. doi: 10.1111/j.1600-0765.1997.tb00576.x. [DOI] [PubMed] [Google Scholar]
- 15.Listgarten M, Mao R, Robinson P. Periodontal probing and the relationship of the probe tip to periodontal tissues. Journal of Periodontology. 1976;47(9):511–513. doi: 10.1902/jop.1976.47.9.511. [DOI] [PubMed] [Google Scholar]
- 16.Armitage GC, Svanberc GK, Löe H. Microscopic evaluation of clinical measurements of connective tissue attachment levels. Journal of Clinical Periodontology. 1977;4(3):173–190. doi: 10.1111/j.1600-051x.1977.tb02271.x. [DOI] [PubMed] [Google Scholar]
- 17.Kalkwarf KL, Kaldahl WB, Patil KD. Comparison of Manual and Pressure-Controlled Periodontal Probing*. Journal of periodontology. 1986;57(8):467–471. doi: 10.1902/jop.1986.57.8.467. [DOI] [PubMed] [Google Scholar]
- 18.Alves Rde V, Machion L, Andia DC, Casati MZ, Sallum AW, Sallum EA. Reproducibility of clinical attachment level and probing depth of a manual probe and a computerized electronic probe. J Int Acad Periodontol. 2005;7(1):27–30. [PubMed] [Google Scholar]
- 19.Christensen MM, Joss A, Lang NP. Reproducibility of automated periodontal probing around teeth and osseointegrated oral implants. Clin Oral Implants Res. 1997;8(6):455–64. doi: 10.1034/j.1600-0501.1997.080603.x. [DOI] [PubMed] [Google Scholar]
- 20.Wang SF, Leknes KN, Zimmerman GJ, Sigurdsson TJ, Wikesjö UM, Selvig KA. Intra -and inter-examiner reproducibility in constant force probing. J Clin Periodontol. 1995;22(12):918–22. doi: 10.1111/j.1600-051x.1995.tb01795.x. [DOI] [PubMed] [Google Scholar]
- 21.Wang SF, Leknes KN, Zimmerman GJ, Sigurdsson TJ, Wikesjö UM, Selvig KA. Reproducibility of periodontal probing using a conventional manual and an automated force-controlled electronic probe. J Periodontol. 1995;66(1):38–46. doi: 10.1902/jop.1995.66.1.38. [DOI] [PubMed] [Google Scholar]
- 22.Haffajee A, Socransky S, Goodson J. Comparison of different data analyses for detecting changes in attachment level. Journal of Clinical Periodontology. 1983;10(3):298–310. doi: 10.1111/j.1600-051x.1983.tb01278.x. [DOI] [PubMed] [Google Scholar]
- 23.Benn DK. A review of the reliability of radiographic measurements in estimating alveolar bone changes. Journal of clinical periodontology. 1990;17(1):14–21. doi: 10.1111/j.1600-051x.1990.tb01041.x. [DOI] [PubMed] [Google Scholar]
- 24.Carpio LC, Hausmann E, Dunford RG, Allen KM, Christersson LA. Evaluation of a simple modified radiographic alignment system for routine use. Journal of periodontology. 1994;65(1):62–67. doi: 10.1902/jop.1994.65.1.62. [DOI] [PubMed] [Google Scholar]
- 25.Hausmann E, Allen K, Carpio L, Christersson LA, Clerehugh V. Computerized methodology for detection of alveolar crestal bone loss from serial intraoral radiographs. Journal of periodontology. 1992;63(8):657–662. doi: 10.1902/jop.1992.63.8.657. [DOI] [PubMed] [Google Scholar]
- 26.Fredriksson M, Zimmerman M, Martinsson T. Precision of computerized measurement of marginal alveolar bone height from bite-wing radiographs. Swedish dental journal. 1988;13(4):163–167. [PubMed] [Google Scholar]
- 27.Hausmann E, Allen K. Reproducibility of Bone Height Measurements Made on Serial Radiographs*. Journal of periodontology. 1997;68(9):839–841. doi: 10.1902/jop.1997.68.9.839. [DOI] [PubMed] [Google Scholar]
- 28.Yun JH, Hwang SJ, Kim CS, Cho KS, Chai JK, Kim CK, Choi SH. The correlation between the bone probing, radiographic and histometric measurements of bone level after regenerative surgery. Journal of periodontal research. 2005;40(6):453–460. doi: 10.1111/j.1600-0765.2005.00825.x. [DOI] [PubMed] [Google Scholar]
- 29.Pepelassi EA, Tsiklakis K, Diamanti-Kipioti A. Radiographic detection and assessment of the periodontal endosseous defects. Journal of clinical periodontology. 2000;27(4):224–230. doi: 10.1034/j.1600-051x.2000.027004224.x. [DOI] [PubMed] [Google Scholar]
- 30.Toback GA, Brunsvold MA, Nummikoski PV, Masters LB, Mellonig JT, Cochran DL. The accuracy of radiographic methods in assessing the outcome of periodontal regenerative therapy. Journal of periodontology. 1999;70(12):1479–1489. doi: 10.1902/jop.1999.70.12.1479. [DOI] [PubMed] [Google Scholar]
- 31.Hildebolt C, Pilgram TK, Yokoyama-Crothers N, Fletcher G, Helbig JL, Bartlett TQ, Gravier M, Vannier MW, Shrout MK. Reliability of linear alveolar bone loss measurements of mandibular posterior teeth from digitized bitewing radiographs. Journal of clinical periodontology. 1998;25(11):850–856. doi: 10.1111/j.1600-051x.1998.tb02381.x. [DOI] [PubMed] [Google Scholar]
- 32.Hildebolt C, Vannier M. Automated Classification of Periodontal Disease Using Bitewing Radiographs*. Journal of periodontology. 1988;59(2):87–94. doi: 10.1902/jop.1988.59.2.87. [DOI] [PubMed] [Google Scholar]
- 33.Hou GL, Lin CH, Hung CC, Yang YS, Shieh TY, Lin IC, Tsai CC. The consistency and reliability of periodontal bone level measurements using digital scanning radiographic image analysis--a pilot study. Kaohsiung J Med Sci. 2000;16(11):566–573. [PubMed] [Google Scholar]
- 34.Machtei EE, Schmidt M, Hausmann E, Grossi S, Dunford R, Davies G, Chandler J, Genco RJ. Outcome variables in periodontal research: means and threshold-based site changes. J Periodontol. 2000;71(4):555–61. doi: 10.1902/jop.2000.71.4.555. [DOI] [PubMed] [Google Scholar]
- 35.Eickholz P, Hausmann E. Accuracy of radiographic assessment of interproximal bone loss in intrabony defects using linear measurements. Eur J Oral Sci. 2000;108(1):70–3. doi: 10.1034/j.1600-0722.2000.00729.x. [DOI] [PubMed] [Google Scholar]
- 36.Jeffcoat MK, Jeffcoat RL, Williams RC. A new method for the comparison of bone loss measurements on non-standardized radiographs. J Periodont Res. 1984;19(4):434–440. doi: 10.1111/j.1600-0765.1984.tb01017.x. [DOI] [PubMed] [Google Scholar]
- 37.Hausmann E, Allen K, Dunford R, Christersson L. A reliable computerized method to determine the level of the radiographic alveolar crest. J Periodontol Res. 1989;24(6):368–369. doi: 10.1111/j.1600-0765.1989.tb00884.x. [DOI] [PubMed] [Google Scholar]
- 38.Hausmann E, Allen K, Christersson L, Genco RJ. Effect of x-ray beam vertical angulation on radiographic alveolar crest level measurement. J Periodontal Res. 1989;24(1):8–19. doi: 10.1111/j.1600-0765.1989.tb00852.x. [DOI] [PubMed] [Google Scholar]
- 39.Hildebolt CF, Vannier MW, Shrout MK, Pilgram TK, Province M, Vahey EP, Rietz DW. Periodontal disease morbidity quantification. II. Validation of alveolar bone loss measurements and vertical defect diagnosis from digital bite-wing images. J Periodontol. 1990;61(10):623–32. doi: 10.1902/jop.1990.61.10.623. [DOI] [PubMed] [Google Scholar]
- 40.Verdonschot EH, Sanders AJ, Plasschaert AJ. Applicability of an image analysis system in alveolar bone loss measurement. J Clin Periodontol. 1991;18(1):30–6. doi: 10.1111/j.1600-051x.1991.tb01116.x. [DOI] [PubMed] [Google Scholar]
- 41.Wyatt CC, Bryant SR, Avivi-Arber L, Chaytor DV, Zarb GA. A computer-assisted measurement technique to assess bone proximal to oral implants on intraoral radiographs. Clin Oral Implants Res. 2001;12(3):225–9. doi: 10.1034/j.1600-0501.2001.012003225.x. [DOI] [PubMed] [Google Scholar]
- 42.Hildebolt CF, Couture R, Garcia NM, Dixon D, Miley DD, Shannon W, Mueller C, Langenwalter E, Spearie CA, Civitelli R. Alveolar bone measurement precision for phosphor-plate images. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(3):e96–107. doi: 10.1016/j.tripleo.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Merchant AT, Pitiphat W, Parker J, Joshipura K, Kellerman M, Douglass CW. Can nonstandardized bitewing radiographs be used to assess the presence of alveolar bone loss in epidemiologic studies? Community Dent Oral Epidemiol. 2004;32(4):271–6. doi: 10.1111/j.1600-0528.2004.00162.x. [DOI] [PubMed] [Google Scholar]
- 44.Byrd V, Mayfield-Donahoo T, Reddy MS, Jeffcoat MK. Semiautomated image registration for digital subtraction radiography. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85(4):473–8. doi: 10.1016/s1079-2104(98)90077-4. [DOI] [PubMed] [Google Scholar]
- 45.Zitová B, Flusser J. Image registration methods: a survey. Image and Vision Computing. 2003;21(11):977–1000. [Google Scholar]
- 46.Fitzpatrick JM, Hill DLG, Maurer CR. Image Registration, in “Handbook of Medical Imaging, Volume 2. Medical Image Processing and Analysis.” Eds: Milan Sonka; J Michael Fitzpatrick. SPIE Digital Library. 2000 [Google Scholar]
- 47.Bassiouny MA, Grant AA. The accuracy of the Schei ruler: a laboratory investigation. J Periodontol. 1975;46(12):748–52. doi: 10.1902/jop.1975.46.12.748. [DOI] [PubMed] [Google Scholar]