Abstract
Cancer immunotherapy (CIT) initiates or enhances the host immune response against cancer. Following decades of development, patients with previously few therapeutic options may now benefit from CIT. Although the quantitative clinical pharmacology (qCP) of previous classes of anticancer drugs has matured during this time, application to CIT may not be straightforward since CIT acts via the immune system. Here we discuss where qCP approaches might best borrow or start anew for CIT.
The maturation of cancer immunotherapy (CIT) from initial application to recent clinical success has spanned over a century.1 During this time, surgery and other classes of anticancer drugs have become standards of care. Cytotoxics, an early class of anticancer drugs, remain a standard of care for many malignancies, but indiscriminate action on both tumor and nontumor cells leads to considerable toxicity and consequently maximally tolerated dose (MTD)-based dose selection. Targeted agents promise more specific action but, given the highly mutagenic environment of the tumor, patients often acquire resistance. Research has shown that tumors can facilitate their growth by expressing factors that co-opt and suppress immune-surveillance mechanisms. Immunotherapy aims to initiate or augment the highly adaptable immune response towards cancer, providing durable responses that might be more robust to mutagenesis. Indeed, modulation of targets such as programmed death receptor have recently shown significant utility in cancer treatment.2,3 The cancer immunotherapy cycle3 partitions events in anticancer immunity into conceptual steps, starting with release of cancer cell antigens and ultimately proceeding to cancer cell kill and subsequent return to antigen release for reinitiation of the cycle. Each of these steps represents a point of possible therapeutic intervention for CIT, as outlined elsewhere.3 Indeed, interplay between these steps affords a vast opportunity for combination therapies, including addition to chemotherapy.
Clinical realization of CIT has been slowed by several factors, spanning the incomplete understanding of antitumor immune response to questions pertaining to dosing1; informing dosing relies on our understanding of the underlying pharmacokinetic/pharmacodynamic (PK/PD) relationship. Our knowledge regarding PK/PD relationships in oncology is based predominantly on cytotoxic and targeted agents that act directly on the tumor cells, whereas CIT acts on cancer–immune system interactions.3 This difference in the mechanism of action (MOA) would be expected to have corresponding consequences on the underlying PK/PD relationship. Below, we outline five related areas that invite the question of what approaches we may borrow from previous classes of anticancer drugs versus what new approaches we should consider for quantitative clinical pharmacology (qCP) in CIT: 1) Clinical Translation; 2) Accelerated Clinical Development; 3) Recommended Phase II/Phase III Dose (RPIID/RPIIID); 4) Model-Informed Drug Development (MIDD); 5) Combinations.
CLINICAL TRANSLATION
Clinical translation of mouse cancer data is challenging; the tumor and immunological characteristics of the mouse model and the metrics derived from mouse experiments affect translatability. For CIT, translatability is particularly sensitive to the interaction of the mouse immune system with the tumor. Further, tumor size response patterns for CIT may not resemble those observed for chemotherapy. Relative to chemotherapy-like responses, which can develop over weeks, clinical responses following CIT can be delayed and occur over months. The clinical CIT responses may also occur after pseudoprogression either as a result of delayed antitumor activity or influx of immune cells to the lesion. Accordingly, Pennock et al.4 describe four patterns of response for ipilimumab (Yervoy): response in baseline lesions, gradual decline in tumor burden, response following increase in tumor burden, and response in index lesion with new lesions that subsequently regressed.
Mathematical models of varying complexity have been proposed to capture tumor size dynamics in mice following CIT. One illustrative and relatively parsimonious model5 maintains several features of the tumor growth inhibition model used by Simeoni et al.6 for previous classes of anticancer agents. Tumor size was represented as a competition of tumor growth and kill rates, but with drug effect incorporated into the tumor kill rate. Additional provisions were required to capture tumor growth dynamics following administration of a tumor vaccine. First, a transit compartment allowed for the hallmark time delay prior to CIT action on tumor size. Second, a mixture model allowed for two populations of animals, exhibiting transient and durable drug responses, respectively. With this, researchers were able to adapt previous models to capture complex dynamics observed with CIT in mice. Future translation may benefit from mechanistic or systems-based models that leverage preclinical measurements in immune-competent models to derive model structures and parameters that can be adapted to the human context.
ACCELERATED CLINICAL DEVELOPMENT
The traditional “learn–confirm” paradigm is predicated upon learning gained in phase I/II evaluations in healthy volunteers and targeted patients and confirming in late-stage trials providing a continuum for MIDD. For CIT, development is likely to deviate from this paradigm (Figure 1). Promising data obtained in phase I trials can drive accelerated development plans for quick access to new drugs. In the case of the anti-PD-1 monoclonal antibody pembrolizumab (Keytruda), accelerated approval was granted in unresectable or metastatic melanoma based on a phase Ib clinical trial, KEYNOTE-001.7 This accelerated development necessitates a reconsideration of how qCP can best inform clinical development. Arguably, a fit-for-purpose approach emphasizing speed and agility may be best suited, given the imperative to deliver new treatment options quickly. Likewise, this may be an opportunity for prospective modeling using qCP approaches that are informed independently of the dataset that would otherwise come from a more traditional drug development. Models capturing the formation and depletion of immune cells “quantify the system”8 and provide supportive guidance for the otherwise somewhat empiric clinical approaches. Regardless of approach, implementation of a robust qCP strategy prior to human studies that is both robust to this accelerated development paradigm and flexible to changes to the development plan would maximize chances for impact.
Figure 1.
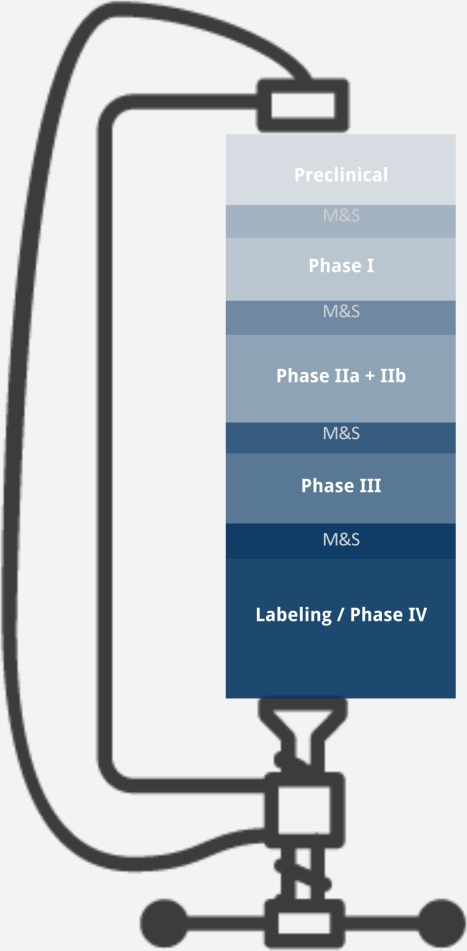
The idealized learn–confirm cycle assumes multiple phases of development for a New Molecular Entity, which due to considerable timeline pressures may not be entirely representative of CIT clinical development. qCP approaches based on the traditional learn–confirm cycle require adaptation for use in CIT.
RPIID/RPIIID
The RPIID/RPIIID selection for CIT should represent a similar challenge as with other classes of drugs with the putative therapeutic window defined by 1) the intended effect of redirecting the host immune system against the tumor and 2) adverse event profiles. However, often both dose-tolerability and -response relationships have proven elusive for CIT.1 The MTD was not defined for ipilimumab or any of the anti-PD-L1 or -PD-1 molecules currently under development. Accordingly, the historically significant MTD-based RPIID/RPIIID is not necessarily applicable to CIT. In lieu of an MTD-based rationale, determining the RPIID/RPIIID upon a putative therapeutic window is nonobvious and subject to regulatory scrutiny. For example, for ipilimumab a postmarketing requirement was issued to compare the efficacy and safety at the 3 mg/kg (the approved dose) and 10 mg/kg dose levels in melanoma patients. The optimal dosing duration for CIT is likewise unclear; following a sufficient number of doses a patient may theoretically derive therapeutic benefit in the absence of continued dosing, as is observed following just four cycles of ipilimumab therapy. Current recommended dosing for both pembrolizumab and nivolumab (Opdivo) in melanoma is to disease progression or unacceptable toxicity rather than a particular number of cycles. It is unclear if this shall remain the default or the exception either for new CIT candidates or for new combinations and indications for a given CIT.
MIDD
The state of MIDD is evolving for CIT, with some methods common to those for non-CIT drugs and others that may eventually become specific to CIT. Relationships between early tumor assessments and overall survival (OS) have been established for non-CIT drugs; extrapolation of similar methods for nivolumab and ipilimumab suggests an association between week 8 tumor size and OS in melanoma and nonsmall-cell lung cancer, respectively, and potential utility for dose selection in pivotal trials.9 As mentioned previously, the Simeoni-like model has been adapted to capture differences in response in the translational space with a mixture model to capture the potency; in the clinical space, the heterogeneous response for pembrolizumab was again captured using a mixture model.10 These analyses leverage historical model-based approaches that were informed with cytotoxics and targeted agents and represent a pragmatic option for application to CIT. However, events occurring between effect of CIT on the immune system and eventual effect on tumor size are ultimately included as empirical provisions (i.e., transit compartments and mixture models) in these approaches, possibly limiting the broader utility of the mathematical model, especially for questions pertaining to duration of effect in the absence of circulating CIT. In the absence of biomarker information, robust parameterization of a more mechanistic model with explicit steps prior to the effect on tumor size may prove problematic. The increasing database of biomarker information in CIT may provide the foundation for the next generation of CIT models, including those that are more amenable to questions of optimal dosing duration.
COMBINATIONS
The cancer immunotherapy cycle3 illustrates how combination treatment is an especially promising avenue for CIT. Rational targeting of multiple nodes promises greater therapeutic benefit to patients than with any single node. Biomarkers may help define patient populations that are most likely to benefit from monotherapy versus other approaches, including combination.3 Recent experience with nivolumab and ipilimumab suggests, however, that there is a possibility for tolerability concerns as well.2 Accordingly, selection of the appropriate dose and schedule of CIT when given in combination represents yet another consideration to realize the full therapeutic potential. Leveraging available literature models for marketed cytotoxics and targeted therapies with nascent models specifically developed for CIT may represent an avenue to inform optimal CIT combinations. MIDD could then greatly help facilitate trial design and optimal dose/schedule selection.
In conclusion, our understanding of the qCP of CIT is increasing, and our tools are in a state of rapid evolution for MIDD. Model development for CIT is proceeding following a much longer history of model-based evaluations of the cytotoxics and targeted therapies that may be combined with CIT. Although challenging, the immense promise of MIDD to probe multiple schedules and doses in silico provides the motivation to press forward and possibly unleash the full promise suggested by the cancer immunity cycle.
Acknowledgments
We thank Dr Bert Lum for helpful comments in the preparation and refinement of this Perspective. This article represents the output of discussions in which all authors were equal contributors.
Author Contributions: M.S., D.J.C., C.-C.L., J.W., B.R., S.R., J.J., J.X., J.-E.C., Z.-X.X., P.N.M., J.D.D., and A.P. wrote the article.
Conflict of Interest/Disclosure: J.D., J.J., C.-C.L., S.R., J.X., and M.S. are or were employees of Genentech, a member of the Roche Group, at the time of authorship and are shareholders of R.A.P., J.-E.C., D.C., J.W., B.R., Z.-X.X., and P.M. are employees and shareholders of Roche.
References
- Lesterhuis WJ, Haanen JB. Punt CJ. Cancer immunotherapy—revisited. Nat. Rev. Drug Discov. 2011;10(8):591–600. doi: 10.1038/nrd3500. [DOI] [PubMed] [Google Scholar]
- Wolchok JD, et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 2013;369(2):122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DS. Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Pennock GK, Waterfield W. Wolchok JD. Patient responses to ipilimumab, a novel immunopotentiator for metastatic melanoma: how different are these from conventional treatment responses? Am. J Clin Oncol. 2012;35(6):606–611. doi: 10.1097/COC.0b013e318209cda9. [DOI] [PubMed] [Google Scholar]
- Parra-Guillen ZP, Berraondo P, Grenier E, Ribba B. Troconiz IF. Mathematical model approach to describe tumour response in mice after vaccine administration and its applicability to immune-stimulatory cytokine-based strategies. AAPS J. 2013;15(3):797–807. doi: 10.1208/s12248-013-9483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeoni M, et al. Predictive pharmacokinetic-pharmacodynamic modeling of tumor growth kinetics in xenograft models after administration of anticancer agents. Cancer Res. 2004;64(3):1094–1101. doi: 10.1158/0008-5472.can-03-2524. [DOI] [PubMed] [Google Scholar]
- Berman D, et al. The development of immunomodulatory monoclonal antibodies as a new therapeutic modality for cancer: The Bristol-Myers Squibb experience. Pharmacol. Ther. 2015;148:132–153. doi: 10.1016/j.pharmthera.2014.11.017. [DOI] [PubMed] [Google Scholar]
- Palsson S, et al. The development of a fully-integrated immune response model (FIRM) simulator of the immune response through integration of multiple subset models. BMC Syst. Biol. 2013;7:95. doi: 10.1186/1752-0509-7-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng YA, Gupta M, Masson E. Roy A. 2014. & Association between immune checkpoint inhibitor-induced tumor shrinkage (TS) and overall survival (OS) in advanced melanoma and non-small cell lung cancer (NSCLC). American Society of Clinical Oncology 2014 Annual Meeting; Chicago, IL ( )
- Elassaiss-Schaap JL, et al. Modeling of Tumor Size Reduction Patterns in Advanced Melanoma Treated With Pembrolizumab, a Potent Antibody Against PD-1. Alicante, Spain: PAGE; 2014. [Google Scholar]


