Abstract
Objective:
To compare the cancer risk of cladribine and other disease-modifying drugs (DMDs) in trials of people with relapsing multiple sclerosis (pwRMS).
Methods:
Meta-analysis of phase III trials of licensed DMDs for pwRMS and a phase III trial of cladribine (CLARITY). Cancer rates were compared using Fisher exact test.
Results:
Eleven trials were included. Investigated treatments included cladribine, dimethyl fumarate, fingolimod, teriflunomide, natalizumab, alemtuzumab, and glatiramer acetate. The cancer rate in the CLARITY treatment group (0.34%) was not increased compared to all other treatment groups, whether including placebo-controlled trials only (0.6%, p = 0.4631) or all trials, i.e., including those with an active comparator arm (0.67%, p = 0.3669). No cancer was reported in the CLARITY placebo group, whereas the combined cancer rate of all other placebo groups was 1.19% (p = 0.0159). The cancer rate of zero in the CLARITY placebo group was also lower than that in the phase III trial of cladribine in people with clinically isolated syndrome (ORACLE MS, 2.91%, p = 0.0012). In fact, no difference was detected between cancer rates in the treatment groups of CLARITY (0.34%) and ORACLE MS (0.49%) (p = 0.6546).
Conclusions:
Our study does not support an increased cancer risk from cladribine in the doses used in CLARITY and ORACLE MS, which previously contributed to refusal of market authorization of cladribine in Europe. Longer-term follow-up is required to assess the safety profile of cladribine, as well as currently licensed DMDs, to definitively assess cancer risk.
Multiple sclerosis (MS) is characterized by focal inflammatory demyelination of the CNS.1 Evidence suggests an important role of autoreactive T and B lymphocytes in MS pathophysiology.1 Cladribine is a synthetic purine analogue cytotoxic to lymphocytes and, to a lesser degree, monocytes and hematopoietic cells.2 As a result, cladribine induces a dose-dependent reduction of T and B cells lasting months to years. This lymphopenia is thought to underlie its therapeutic activity in people with relapsing MS (pwRMS). Evidence from a large phase III trial (CLARITY) in pwRMS suggests cladribine is a highly effective disease-modifying drug (DMD), with 45% of patients showing no evidence of disease activity after 96 weeks, following 2 courses of cladribine.3,4 This efficacy is comparable to the most effective DMDs licensed for pwRMS, including the monoclonal CD52-specific antibody alemtuzumab (Lemtrada).5,6 Like cladribine, alemtuzumab is an induction treatment given in 2 or more annual treatment cycles. However, oral cladribine was refused a license by the European Medicines Agency in 2013. A suspected increase in cancer risk was a key reason for this refusal.7 However, the small number of cancers observed during CLARITY is not in itself sufficient to assess cancer risk. We performed a meta-analysis comparing the cancer risk of cladribine in CLARITY with other DMDs used for pwRMS.
METHODS
Literature search.
The terms “phase III” and “multiple sclerosis” were used for a PubMed search with no time restriction in March 2014. References of identified articles were searched for further sources. Studies were eligible for inclusion if they (1) were phase III trials of DMDs in pwRMS and (2) reported the rate of neoplasms and cancers observed. Conference abstracts were excluded as were studies investigating only an acute dose effect or follow-up studies of previously published phase III trials.
Statistical analysis.
The proportion of pwRMS who developed cancer was extracted from each study. In trials where multiple doses were tested, the sum total of cancers in all treatment arms was used. Given the relatively short and very similar duration of analyzed studies, the proportion of pwRMS in whom cancer occurred was used as an estimate of the “rate” of cancer. The 95% confidence interval of cancer rates was calculated using the modified Wald method.8 Two-tailed Fisher exact test was used to compare cancer rates. The cancer rate in the treatment group of CLARITY was first compared to the combined cancer rate of all treatment groups of placebo-controlled phase III trials of other DMDs. The cancer rate in the placebo group of CLARITY was then compared to the combined cancer rate of the placebo groups of all other phase III trials. Study heterogeneity was calculated using χ2 test and publication bias was assessed using a funnel plot. We also calculated the annualized cancer rate. Significance was set at p < 0.05. Statistical analysis was performed using GraphPad Prism, RevMan, and STATA 13.1 (StataCorp, College Station, TX).
Analysis was similarly done using the cancer rates reported in a recently completed phase III trial on the effect of oral cladribine on time to conversion to clinically definite MS in patients with a first demyelinating event (ORACLE MS).9
Furthermore, meta-analysis pooling using a random-effects model was performed based on risk difference to assess any excess risk from cladribine. Risk difference was selected as a measure rather than the more commonly used measures of relative risk or relative odds because when incidence rates are low and the comparator arm has zero cases (as in CLARITY), these latter measures are not reliably estimated.10
RESULTS
Including CLARITY, a total of 11 phase III trials in pwRMS were eligible for inclusion in the analysis.3,5,6,11–18 DMDs investigated in these studies were cladribine, dimethyl fumarate, fingolimod, teriflunomide, natalizumab, alemtuzumab, and glatiramer acetate. The CombiRx trial (interferon [IFN]-β-1a plus glatiramer acetate) was excluded because malignant and nonmalignant neoplasms were not reported separately.19 Seven of the included studies compared DMD with placebo; 4 compared DMD with IFN-β-1a. One trial compared natalizumab as an add-on to IFN-β-1a with placebo added to IFN-β-1a. Study characteristics, references, and annualized cancer rate of DMDs included in the analysis are provided in table e-1 at Neurology.org/nn.
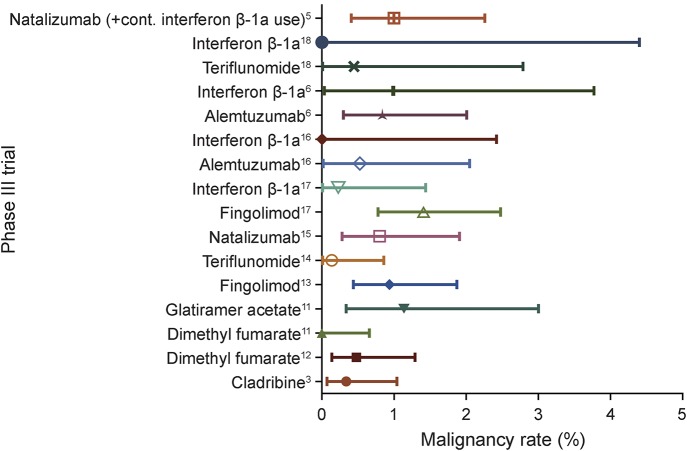
The cancer rate in the CLARITY treatment group was 0.34% and thus not different from all other treatment groups of placebo-controlled trials (0.6%, p = 0.4631). Among these treatment groups, the cancer rate in CLARITY was the third lowest observed (figure 1). Among placebo groups, the cancer rate of zero in CLARITY was the lowest observed and significantly lower than the combined cancer rate of all other placebo groups (1.19%, p = 0.0159; figure 2).
Figure 1. Malignancy rates in treatment groups of placebo-controlled trials.
Forest plot of malignancy rates in treatment groups from phase III trials with a placebo group.
Figure 2. Malignancy rates in placebo groups of trials.
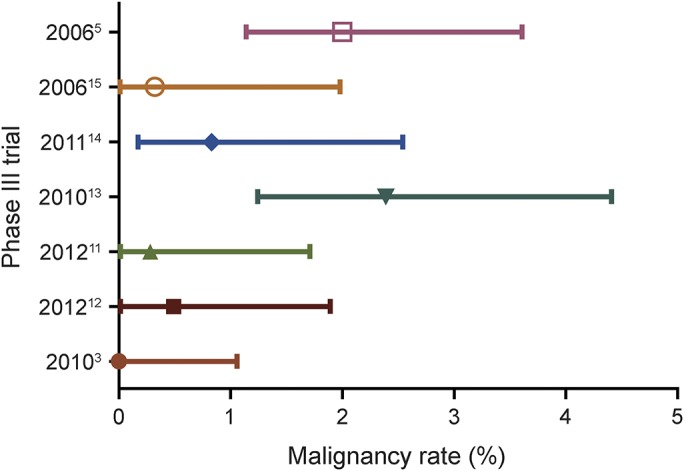
Forest plot of malignancy rates in placebo groups of phase III trials.
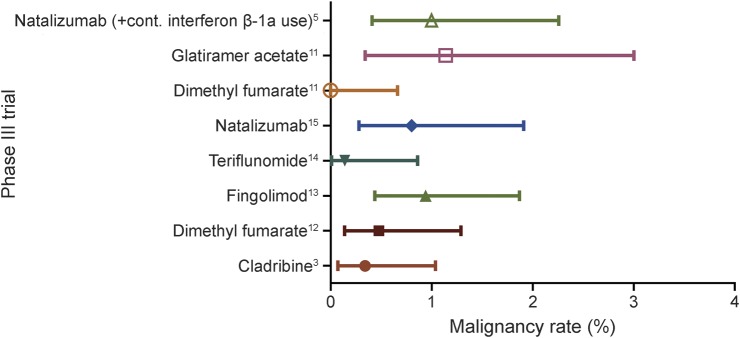
Comparing all trials (i.e., placebo-controlled and active comparator IFN-β-1a–controlled), no difference was detected in the cancer rate of CLARITY treatment arms vs all other trial arms (0.67%, p = 0.3669; figure 3). No difference in cancer rate was detected between treatment arms of CLARITY and ORACLE MS (0.34% vs 0.49%, p = 0.6546); both trials used identical doses of cladribine. Comparison of the total neoplasm rate (including both malignant and nonmalignant neoplasms) in CLARITY treatment arms (1.13%) vs ORACLE MS treatment groups (0.98%) did not reveal any difference (p = 1). The CLARITY placebo group had a lower neoplasm rate (0%) than the ORACLE MS placebo group (2.91%, p = 0.0012). No malignancies were reported in the placebo group of either trial.
Figure 3. Malignancy rates in treatment groups of trials.
Forest plot of malignancy rates in treatment groups of all phase III trials.
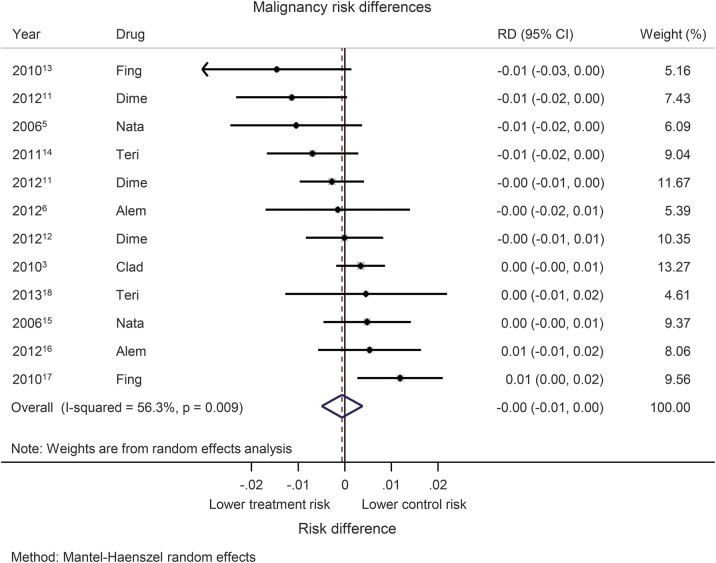
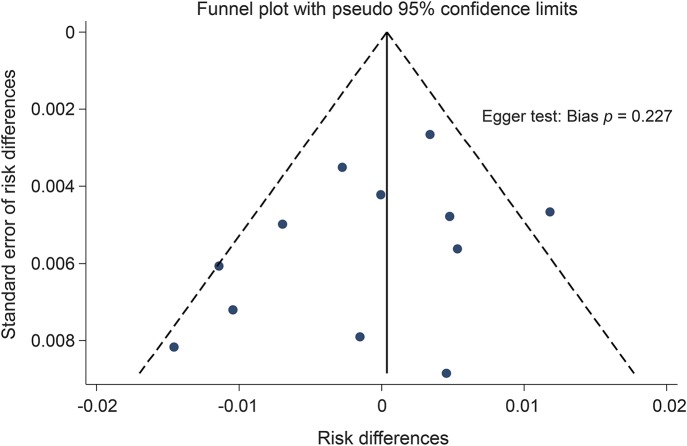
Random-effects pooling based on risk difference indicated a cancer risk profile from the CLARITY trial comparable to that of other DMDs (cladribine had a lower risk difference compared to 4 other trials; figure 4). We further noted that the CLARITY trial did not indicate a higher risk difference than the other “induction” DMD, alemtuzumab, in previously untreated pwRMS in the Cohen 2012 trial. Given the heterogeneity of treatments, significant heterogeneity between trials was detected (I2 = 56.3%, p = 0.009). Potential publication bias is demonstrated using a funnel plot (figure 5).
Figure 4. Malignancy risk differences.
Forest plot of malignancy risk differences (RDs) using Mantel-Haenszel pooling. Alem = alemtuzumab; CI = confidence interval; Clad = cladribine; Dime = dimethyl fumarate; Fing = fingolimod; Nata = natalizumab; Teri = teriflunomide.
Figure 5. Risk differences and Egger test assessment.
Funnel plot for the risk differences and Egger test assessment of publication bias.
DISCUSSION
The main findings of this study are that (1) the rate of cancer was similar between the only phase III trial of cladribine and all other phase III trials of DMDs in pwRMS, and (2) a significant difference emerged when comparing the placebo groups of CLARITY (no cancers) with the placebo arms of all other phase III trials included in this study. These findings were corroborated by a cancer rate difference in CLARITY that was comparable to that of all other phase III trials for DMDs for pwRMS.
Focusing on cancers observed in pwRMS treated with cladribine in CLARITY (and ORACLE MS) might lead to the suspicion that cladribine plays a causative role, given the fact that 3 pwRMS on cladribine developed cancer whereas none on placebo did. However, comparison with other DMDs shows that the number of malignancies in CLARITY was not increased. Indeed, the cancer rate in CLARITY was among the lowest observed for any treatment group, suggesting that the cancers developed on cladribine are a reflection of the background risk in the population. Moreover, the short latency between initiation of treatment and cancer diagnosis in CLARITY (≤18 months) renders a causal relationship unlikely. We also note that in contrast to the trials of alemtuzumab, cladribine was compared with placebo (rather than an active comparator). It is impossible to conclude whether comparison with placebo exaggerates the relative excess risk.
Investigations of the general risk of cancer among pwRMS in large cohorts reported conflicting results. In a Danish study using population-based registers of MS and cancer, there was no evidence of an overall increased risk of cancer after a diagnosis of MS; however, they did report a small excess risk of breast cancer among women.20 A recent systematic review of the incidence and prevalence of cancer in MS reported substantial variation in reported estimates between studies. Overall, the risk of “any” cancer was most commonly reported to be lower in pwRMS compared to the general population. Cancer risk appeared to be higher for malignant brain tumors and for cancers affecting the urinary tract system. However, potential surveillance bias and variation of age and sex of pwRMS across studies stand in the way of more definitive conclusions.21
The data from our meta-analysis of DMDs in pwRMS are supported by the long-term outcome of people with leukemia treated with cladribine. In a 20-year follow-up report of 88 patients diagnosed with hairy cell leukemia before the age of 40, no increase in the incidence of secondary malignancies was detected.22 According to the preliminary report of the 96-week phase II “oral cladribine as add-on to IFN-β therapy in patients with active multiple sclerosis” (ONWARD) trial, in which oral cladribine (dose 3.5 mg/kg) was compared vs placebo as an add-on to interferon-beta treatment, 1 of 124 patients, a 57-year-old woman with a 25-year smoking history, developed squamous cell carcinoma about 2 years following first cladribine exposure.23 Cladribine administered as an injection or infusion has previously been investigated in placebo-controlled phase II trials of varying dosages, with results demonstrating reduced disease activity (clinically and on MRI) with no reported cancer signal.24
Limitations of our study include the inevitable variation between trials in terms of sample size, inclusion criteria, follow-up period, and dosing regimens; the fact that not all phase III trials for approved DMDs in pwRMS reported data on cancer rates; and the fact that all cancers were considered together, i.e., regardless of affected organ(s). Furthermore, CLARITY was 2 months shorter than most of the other trials included, which lasted 2 years. Mathematically, a slightly lower number of cancers would therefore be expected in CLARITY due to the shorter observation period. However, there is no indication that this difference had any material impact on the results of our meta-analysis.
We also acknowledge that in a randomized trial such as CLARITY, if the cancer rate is truly “low,” it should also have been low relative to the placebo group in its own trial. However, the apparently high risk in CLARITY relative to placebo may be due to the use of the risk ratio rather than the risk difference; the former is inappropriate in this context because of the zero cases in the CLARITY placebo group.10 Our meta-analysis of risk differences, which does not require arbitrary substitutions for the zeros, suggests that the risk difference for several other comparable drugs is higher than that in CLARITY.
Our data suggest that although current evidence cannot rule out an increased risk of cancer on cladribine, it also cannot confirm that such a risk exists. A more definitive assessment of cancer risk will only be feasible through long-term follow-up,25 be it of people treated with cladribine (“Prospective observational long-term safety registry of multiple sclerosis patients who have participated in cladribine clinical trials” [PREMIERE]; ClinicalTrials.gov NCT01013350) or other DMDs. The results of our meta-analysis should encourage further investigation into the potential use of cladribine as a DMD for pwRMS.
Supplementary Material
ACKNOWLEDGMENT
The authors are grateful to Barts Charity for funding to cover publication costs.
GLOSSARY
- DMD
disease-modifying drug
- IFN
interferon
- MS
multiple sclerosis
- pwRMS
people with relapsing MS
Footnotes
Supplemental data at Neurology.org/nn
AUTHOR CONTRIBUTIONS
Study concept and design: K.S., D.B. Acquisition and analysis of data: J.P., G.D., D.R.A. Interpretation of data: J.P., G.D., D.R.A., S.P., B.P.T., M.M., G.J., D.B., J.C., K.S. Drafting of the manuscript: J.P. Critical revision of the manuscript for important intellectual content: G.D., D.R.A., S.P., B.P.T., M.M., G.J., D.B., J.C., K.S. Study supervision: K.S.
STUDY FUNDING
K.S. has been supported by a Higher Education Funding Council for England (HEFCE) Clinical Senior Lectureship.
DISCLOSURE
J. Pakpoor reports no disclosures. G. Disanto is on the editorial board for BMC Neurology. D.R. Altmann consulted for Merck & Co and received research support from Multiple Sclerosis Society of Great Britain and Northern Ireland. S. Pavitt reports no disclosures. B.P. Turner is on the scientific advisory board for Biogen, Novartis, and Sanofi Aventis. M. Marta received travel funding from Biogen and Abbott Laboratories and received research support from Merck-Serono. G. Juliusson was on the scientific advisory board for Merck-Serono. D. Baker has a patent filed concerning the treatment of multiple sclerosis with cladribine and other compounds; has consulted for Canbex Therapeutics; received research support from Genzyme Sanofi, National Multiple Sclerosis Society, and academic entities; has stock options from Canbex Therapeutics; and provided legal consult for Teva. J. Chataway is on the scientific advisory board for Novartis; received travel funding and/or speaker honoraria from Novartis, Teva, and Sanofi; and received research support from Novartis, UK MRC, and UK National Institute of Health Research (NIHR) University College London Hospitals/UCL Biomedical Research Centres funding scheme. K. Schmierer is a principal investigator of trials sponsored by Novartis, Roche, and Teva. He has received speaking honoraria from, and served on advisory boards for, Biogen, Novartis, Teva, Merck-Serono, and Merck Inc; and received research support from Novartis, the Multiple Sclerosis Society of Great Britain & Northern Ireland, the National MS Society, the Royal College of Radiologists, and Barts Charity. Go to Neurology.org/nn for full disclosure forms.
REFERENCES
- 1.Compston A, Coles A. Multiple sclerosis. Lancet 2008;372:1502–1517. [DOI] [PubMed] [Google Scholar]
- 2.Carson DA, Wasson DB, Taetle R, Yu A. Specific toxicity of 2-chlorodeoxyadenosine toward resting and proliferating human lymphocytes. Blood 1983;62:737–743. [PubMed] [Google Scholar]
- 3.Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med 2010;362:416–426. [DOI] [PubMed] [Google Scholar]
- 4.Giovannoni G, Cook S, Rammohan K, et al. Sustained disease-activity-free status in patients with relapsing-remitting multiple sclerosis treated with cladribine tablets in the CLARITY study: a post-hoc and subgroup analysis. Lancet Neurol 2011;10:329–337. [DOI] [PubMed] [Google Scholar]
- 5.Rudick RA, Stuart WH, Calabresi PA, et al. ; SENTINEL Investigators. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med 2006;354:911–923. [DOI] [PubMed] [Google Scholar]
- 6.Coles AJ, Twyman CL, Arnold DL, et al. ; CARE-MS II investigators. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 2012;380:1829–1839. [DOI] [PubMed] [Google Scholar]
- 7.European Medicines Agency. Refusal of the marketing authorization for Movectro (cladribine): outcome of re-examination. Published January 20, 2011. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_-_Initial_authorisation/human/001197/WC500101072.pdf. Accessed July 22, 2015.
- 8.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 1934;26:404–413. [Google Scholar]
- 9.Leist TP, Comi G, Cree BA, et al. ; Oral Cladribine for Early MS (ORACLE MS) Study Group. Effect of oral cladribine on time to conversion to clinically definite multiple sclerosis in patients with a first demyelinating event (ORACLE MS): a phase 3 randomised trial. Lancet Neurol 2014;13:257–267. [DOI] [PubMed] [Google Scholar]
- 10.Lane PW, Meta-analysis of incidence of rare events. Stat Methods Med Res 2013;22:117–132. [DOI] [PubMed] [Google Scholar]
- 11.Fox RJ, Miller DH, Phillips JT, et al. ; CONFIRM Study Investigators. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 2012;367:1087–1097. [DOI] [PubMed] [Google Scholar]
- 12.Gold R, Kappos L, Arnold DL, et al. ; DEFINE Study Investigators. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012;367:1098–1107. [DOI] [PubMed] [Google Scholar]
- 13.Kappos L, Radue EW, O'Connor P, et al. ; FREEDOMS Study Group. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010;362:387–401. [DOI] [PubMed] [Google Scholar]
- 14.O'Connor P, Wolinsky JS, Confavreux C, et al. ; TEMSO Trial Group. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med 2011;365:1293–1303. [DOI] [PubMed] [Google Scholar]
- 15.Polman CH, O'Connor PW, Havrdova E, et al. ; AFFIRM Investigators. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006;354:899–910. [DOI] [PubMed] [Google Scholar]
- 16.Cohen JA, Coles AJ, Arnold DL, et al. ; CARE-MS I investigators. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet 2012;380:1819–1828. [DOI] [PubMed] [Google Scholar]
- 17.Cohen JA, Barkhof F, Comi G, et al. ; TRANSFORMS Study Group. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010;362:402–415. [DOI] [PubMed] [Google Scholar]
- 18.Vermersch P, Czlonkowska A, Grimaldi LM, et al. ; TENERE Trial Group. Teriflunomide versus subcutaneous interferon beta-1a in patients with relapsing multiple sclerosis: a randomised, controlled phase 3 trial. Mult Scler 2014;20:705–716. [DOI] [PubMed] [Google Scholar]
- 19.Lublin FD, Cofield SS, Cutter GR, et al. ; CombiRx Investigators. Randomized study combining interferon and glatiramer acetate in multiple sclerosis. Ann Neurol 2013;73:327–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nielsen NM, Rostgaard K, Rasmussen S, et al. Cancer risk among patients with multiple sclerosis: a population-based register study. Int J Cancer 2006;118:979–984. [DOI] [PubMed] [Google Scholar]
- 21.Marrie RA, Reider N, Cohen J, et al. A systematic review of the incidence and prevalence of cancer in multiple sclerosis. Mult Scler 2015;21:294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenberg JD, Burian C, Waalen J, Saven A. Clinical characteristics and long-term outcome of young hairy cell leukemia patients treated with cladribine: a single-institution series. Blood 2014;123:177–183. [DOI] [PubMed] [Google Scholar]
- 23.Montalban X, Cohen B, Leist T, et al. Oral cladribine as add on to IFN β therapy in patients with active multiple sclerosis: results from the phase II ONWARD study (P07.099). Neurology 2013;80:P07.099. [Google Scholar]
- 24.Rice GP, Filippi M, Comi G. Cladribine and progressive MS: clinical and MRI outcomes of a multicenter controlled trial. Cladribine MRI Study Group. Neurology 2000;54:1145–1155. [DOI] [PubMed] [Google Scholar]
- 25.El Kebir S. Prospective observational long-term safety registry of multiple sclerosis patients who have participated in cladribine clinical trials (PREMIERE). ClinicalTrials gov. 2013. Available at: http://www.clinicaltrials.gov/ct2/show/NCT01013350. Accessed September 19, 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.