Abstract
Obsessive-compulsive disorder (OCD) often co-occurs with anorexia nervosa (AN), a comorbid profile that complicates the clinical management of both conditions. This population-based study aimed to examine patterns of comorbidity, longitudinal risks, shared familial risks and shared genetic factors between OCD and AN at the population level. Participants were individuals with a diagnosis of OCD (N=19,814) or AN (N=8,462) in the Swedish National Patient Register between January 1992 and December 2009; their first-, second- and third-degree relatives; and population-matched (1:10 ratio) unaffected comparison individuals and their relatives. Female twins from the population-based Swedish Twin Register (N=8,550) were also included. Females with OCD had a 16-fold increased risk of having a comorbid diagnosis of AN, whereas males with OCD had a 37-fold increased risk. Longitudinal analyses showed that individuals first diagnosed with OCD had an increased risk for a later diagnosis of AN (risk ratio, RR=3.6), whereas individuals first diagnosed with AN had an even greater risk for a later diagnosis of OCD (RR=9.6). These longitudinal risks were about twice as high for males than for females. First- and second-degree relatives of probands with OCD had an increased risk for AN, and the magnitude of this risk tended to increase with the degree of genetic relatedness. Bivariate twin models revealed a moderate but significant degree of genetic overlap between self-reported OCD and AN diagnoses (ra=0.52, 95% CI: 0.26-0.81), but most of the genetic variance was disorder-specific. The moderately high genetic correlation supports the idea that this frequently observed comorbid pattern is at least in part due to shared genetic factors, though disorder-specific factors are more important. These results have implications for current gene-searching efforts and for clinical practice.
Keywords: Obsessive-compulsive disorder, anorexia nervosa, eating disorders, genetic epidemiology, comorbidity, shared genetic factors
An association between obsessive-compulsive disorder (OCD) and eating disorders, particularly anorexia nervosa (AN), has long been observed. In clinical academic settings, these conditions co-occur far more frequently than would be expected by chance, with lifetime prevalence estimates of OCD ranging from 9.5 to 62% in patients with eating disorders (1,2). Similarly, the estimated lifetime prevalence of eating disorders in OCD samples ranges between 11 and 42% (3–5). OCD may, in fact, precede the onset of eating disorders in as many as a quarter of cases, though the relevant studies, with few exceptions (5), have been retrospective (1,2,6,7).
Clinically, the comorbidity between OCD and eating disorders poses special challenges. For example, due to the cognitive effects of starvation, very low-weight patients with AN have difficulty engaging in, and benefiting from, cognitive behavioral therapy for OCD.
Given these clinical considerations, there is a need to better understand the nature of the association between OCD and AN. One possibility is that OCD shares familial risk factors with AN. Controlled family studies have indicated elevated rates of OCD in relatives of patients with eating disorders, particularly restricting-type AN (8,9). On the other hand, family studies have not observed elevated rates of eating disorders in relatives of OCD patients (10,11), although this could be related to the low population prevalence of eating disorders. Thus, it is currently unclear whether there is shared familial transmission between OCD and eating disorders (12).
In this study, we linked longitudinal national Swedish registers, including multigenerational families and twins, to shed new light on the nature of the relationships between OCD and AN. We first examined the comorbidity patterns between OCD and AN at the population level. We next employed longitudinal analyses to examine the sequential risk of AN in individuals first diagnosed with OCD, and the sequential risk of OCD in patients first diagnosed with AN. Next, we investigated the risk of AN in relatives of individuals with OCD who did not have a lifetime diagnosis of AN, compared with the risk in relatives of individuals without a diagnosis of OCD or AN, stratified by degree of genetic relatedness to the probands. Finally, we conducted a bivariate twin analysis of self-reported OCD and AN diagnoses in a large population-based female twin sample. Our multi-method approach controls for many of the disease-related confounding factors that can create spurious associations between disorders.
METHODS
National registers
Following approval from the Regional Ethics Board in Stockholm, we linked three Swedish national registers, using the individual personal identification numbers assigned at birth or, for resident immigrants, upon arrival to the country. The Total Population Register contains demographic data on all individuals registered as Swedish inhabitants since 1968, and is extended by the Multi-Generation Register, which contains information about the identity of biological parents of all individuals born in Sweden since 1932 and individuals living in Sweden since 1961. The Swedish National Patient Register (13) covers psychiatric inpatient care since 1969 and psychiatric outpatient care since 2001.
Definition and validity of ICD codes for OCD and AN
OCD probands were defined as individuals identified in the National Patient Register with at least one ICD-10 diagnosis of OCD (F42). The ICD-10 codes for OCD were validated by obtaining a random sample of patient records (N=68) from three Swedish counties. Each file was reviewed and blindly rated by two independent psychiatrists. The ICD-10 codes had excellent validity, with a positive predictive value of 91% (rater 1) and 96% (rater 2). The inter-rater agreement was outstanding (kappa=0.98, p<0.001) (14).
AN probands were defined as individuals identified in the National Patient Register with at least one ICD-10 diagnosis of AN (F50.0 or F50.1). The ICD-10 codes for AN were validated by comparing the eating disorder diagnoses in the National Patient Register to the diagnoses in two specialized quality registers: the Riksät-National Quality Register for Specialized Treatment for Eating Disorders and the Stepwise-regional Quality Assurance System for Eating Disorders (15). This yielded a positive predictive value of 83% and a negative predictive value of 73%.
Twin data
Twins were recruited from the population-based STAGE (Screening Twin Adults: Genes and Environment) study, based on all twins from the Swedish Twin Registry born from 1959 to 1985 (16). The STAGE target population included approximately 43,000 eligible twins. In 2005-2006, twins were invited by mail to participate in the study; nearly 25,000 individuals responded to the questionnaire, which covered common complex diseases. Twins could also opt to complete a phone interview with a trained interviewer using a computer-based data collection method.
Self-reported OCD was assessed using a single item: “Do you have/have you ever had OCD?” Response options were “yes”, “no”, and “don’t know/refuse”. AN was assessed using an expanded, on-line Structured Clinical Interview for DSM-IV (SCID)-based instrument. Study criteria for AN were lifetime lowest illness-related body mass index < 18.55, at least slightly afraid of gaining weight or becoming fat while at low weight, and feeling at least slightly fat while at low weight. Participants were coded “1” if all criteria were present, “0” if fewer than all criteria were present, and “missing” if a diagnosis could not be made. Because there were too few men (N=10) with a diagnosis of AN, only women from monozygotic and same sex dizygotic pairs were included in the twin analyses (17).
STAGE was approved by the Regional Ethics Board, and participants provided informed consent by responding to the questionnaire or verbally over the telephone before participation. This study was also approved by the Biomedical Institutional Review Board at the University of North Carolina.
Statistical analyses
In the population analyses, we first examined the risk for AN in individuals with OCD, compared with individuals without OCD at the time of the first diagnosis of the probands. For each individual with OCD, 10 comparison individuals matched by birth year, sex, and county of residence were randomly selected from the general population. Comparison individuals had to be alive, living in Sweden, and not diagnosed with OCD at the date of the first OCD diagnosis of the proband.
In longitudinal analyses, we estimated the risk that individuals with OCD would receive a later diagnosis of AN during the follow-up period, compared with individuals without an OCD diagnosis. Conversely, we examined the risk that individuals first diagnosed with AN would later receive a diagnosis of OCD during the follow-up period, compared with individuals without an AN diagnosis. We also calculated the median number of years (plus interquartile range) between the first diagnosis (e.g., OCD) and the subsequent diagnosis (e.g., AN).
We used the multigenerational family design to examine the possible etiological overlap between OCD and AN. Specifically, the risk of AN in relatives of individuals with OCD who did not have a lifetime diagnosis of AN was compared with the risk in relatives of individuals without a diagnosis of OCD or AN. For each proband-relative pair, 10 randomly selected unexposed-relative pairs were matched by birth year and sex, and these individuals had to be alive, living in Sweden, and without a diagnosis of OCD at the time of the first diagnosis of the proband. This method reduces the potential bias introduced by individuals in the population registers entering the study at different times (left truncation). OCD-affected relatives of individuals with OCD were excluded, in order to be sure that we studied independent transmission of the conditions. Shared familial (genetic and environmental) risk factors are assumed when individuals with the index disorder (i.e., OCD) have relatives with the other disorder (i.e., AN) but not the index disorder (18). First-, second- and third-degree relatives were analyzed separately to examine the extent to which the familial associations were influenced by genetic and shared environmental factors.
To estimate the concurrent and sequential risks of AN in individuals with OCD (and vice versa), we calculated risk ratios (RR) and 95% confidence intervals (CI) using conditional logistic regression. When assessing risks within families, CI were obtained with a robust sandwich estimator function to adjust for the correlated data structure. All analyses were conducted in SAS version 9.4 (SAS Institute, Cary, NC, USA).
Twin analyses were done using the Mx program (http://www.vcu.edu/mx/). The classic twin study evaluates the proportion of phenotypic variation attributable to genetic variation among individuals (heritability) and what proportions are due to common and unique environmental factors. Specifically, it estimates the proportion of variance due to: a) additive genetic effects (representing the cumulative impact of several genes, i.e., heritability, a2); b) common environmental effects (environmental influences to which both members of a twin pair are exposed regardless of zygosity, c2); and c) unique environmental effects (environmental effects impacting one twin but not the other) and measurement error (e2). Thus, the sum of a2 + c2 + e2=1 (total variance).
An extension of this twin model, a bivariate structural equation model using Cholesky’s decomposition, was fitted to the data. We applied a reduced model including estimates for two sources of variation (additive genetic effects and unique environmental effects, AE model) for OCD and for AN, and correlations indicating the proportion of variance that the two traits share due to genetic (ra) and unique environmental (re) factors. Model selection was based on the best fitting models for both OCD and AN published elsewhere (19,20) (there was no loss of fit of the AE model compared with the full model). We applied the raw ordinal data option in Mx, which allows data from both complete and incomplete twin pairs to be analyzed. We report parameter estimates with their 95% CI.
RESULTS
Comorbidity
We identified 19,814 individuals ever diagnosed with OCD (43.5% males) and 8,462 individuals ever diagnosed with AN (6.4% males). Individuals with OCD had a 17 times higher risk of having a comorbid diagnosis of AN. Although males with OCD had a lower absolute risk of AN (0.6%) compared to females with OCD (4.8%), the relative risk was significantly higher for male than female OCD probands (Table1).
Table 1.
Risk of anorexia nervosa in individuals with OCD, compared with matched comparison individuals without OCD from the general population
| OCD probands (N=19,512)a | Matched comparison individuals (N=195,120) | RR (95% CI) | |
|---|---|---|---|
| Females + males | 572 (2.9%) | 368 (0.2%) | 16.9 (14.8-19.4) |
| Females | 524 (4.8%) | 355 (0.3%) | 16.1 (14.0-18.5) |
| Males | 48 (0.6%) | 13 (0.02%) | 36.9 (20.0-68.1) |
OCD – obsessive-compulsive disorder, RR – risk ratio, CI – confidence interval
302 patients with OCD could not be assigned comparison individuals per the matching criteria
Longitudinal analyses
Individuals first diagnosed with OCD had a 4-fold higher risk of receiving a later diagnosis of AN during the follow-up period, compared with individuals without OCD (Figure 1). The median time between the first diagnosis of OCD and the subsequent first diagnosis of AN was 2.2 years (interquartile range, IR=2.8). These risks were approximately double for male than female OCD patients (Table2).
Figure 1.
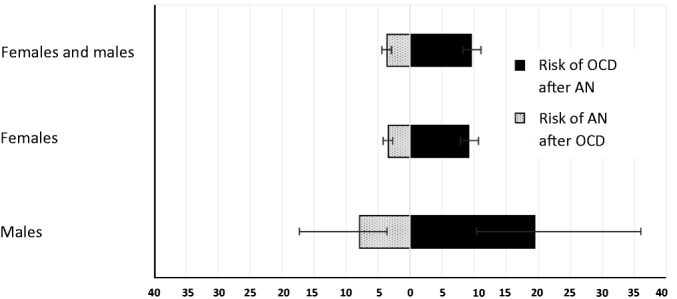
Sequential risks of receiving a diagnosis of obsessive-compulsive disorder (OCD) after having received an initial diagnosis of anorexia nervosa (AN) (right side, in black), and vice versa (left side, in grey), by proband gender. Values represent risk ratios and confidence intervals
Table 2.
Longitudinal risk of receiving a later diagnosis of anorexia nervosa during the follow-up period in probands with an initial diagnosis of OCD compared with individuals without OCD
| Initial diagnosis of OCD | |||
|---|---|---|---|
| Present (N=19,069)a | Absent (N=190,690) | RR (95% CI) | |
| Females + males | 129 (0.7%) | 366 (0.2%) | 3.6 (2.9-4.4) |
| Females | 118 (1.1%) | 352 (0.3%) | 3.4 (2.7-4.2) |
| Males | 11 (0.1%) | 94 (0.02%) | 7.9 (3.6-17.3) |
OCD – obsessive-compulsive disorder, RR – risk ratio, CI – confidence interval
OCD patients with patients with a prior diagnosis of anorexia nervosa where excluded from the analyses
Conversely, individuals first diagnosed with AN had a 10-time higher risk of receiving a later diagnosis of OCD during the follow-up period, compared with individuals without AN (Figure 1). The median time between diagnoses was 2.4 years (IR=3.0). These risks were significant for both female and male AN patients, though the magnitude of the risk was more than doubled in males (Table3).
Table 3.
Longitudinal risk of receiving a later diagnosis of OCD during the follow-up period in probands with an initial diagnosis of anorexia nervosa compared with individuals without anorexia nervosa
| Initial diagnosis of anorexia nervosa | |||
|---|---|---|---|
| Present (N=8,192)a | Absent (N=81,920) | RR (95% CI) | |
| Females + males | 369 (4.5%) | 403 (0.5%) | 9.6 (8.3-11.1) |
| Females | 339 (4.4%) | 386 (0.5%) | 9.2 (7.9-10.7) |
| Males | 30 (6.0%) | 17 (0.3%) | 19.4 (10.4-36.1) |
OCD – obsessive-compulsive disorder, RR – risk ratio, CI – confidence interval
Anorexia nervosa patients with a prior diagnosis of OCD were excluded from the analyses
Family analyses
When the proband had OCD (but not AN), his/her OCD-unaffected first-, second- and third-degree relatives had an increased risk for AN. This was statistically significant for first-degree relatives (both female and male) and second-degree relatives (female only), and at a trend level for third-degree relatives (Table4). The magnitude of this risk tended to increase as genetic proximity increased, though the confidence intervals overlapped.
Table 4.
Risk of anorexia nervosa in unaffected relatives of individuals with OCD (exposed), compared with relatives of individuals without OCD (unexposed)
| Risk of anorexia nervosa | |||
|---|---|---|---|
| Exposed | Unexposed | RR (95% CI) | |
| First-degree relatives | |||
| Females + males | 108 (0.2%) | 548 (0.1%) | 1.9 (1.6-2.4) |
| Females | 102 (0.3%) | 526 (0.2%) | 1.9 (1.6-2.4) |
| Males | 6 (0.02%) | 22 (0.01%) | 2.6 (1.1-6.2) |
| Second-degree relatives | |||
| Females + males | 68 (0.1%) | 536 (0.1%) | 1.3 (1.0-1.6) |
| Females | 65 (0.2%) | 503 (0.1%) | 1.3 (1.1-1.6) |
| Males | 3 (0.01%) | 33 (0.01%) | 0.9 (0.3-2.8) |
| Third-degree relatives | |||
| Females + males | 151 (0.2%) | 1,381 (0.2%) | 1.1 (0.9-1.3) |
| Females | 142 (0.4%) | 1,311 (0.3%) | 1.1 (0.9-1.3) |
| Males | 60 (0.2%) | 492 (0.1%) | 1.3 (0.7-2.5) |
OCD – obsessive-compulsive disorder, RR – risk ratio, CI – confidence interval
Significant RRs are highlighted in bold
Twin analyses
The final sample for twin modeling included 8,550 female twins: 1,724 monozygotic pairs with complete data, 177 monozygotic pairs with incomplete data, 1,170 dizygotic pairs with complete data, and 117 dizygotic pairs with incomplete data. In addition, there were 1,035 monozygotic and 1,139 dizygotic individuals without co-twin information. The mean age of these participants was 33.1 years (SD=7.6).
The modeling results for the twin analyses were: a2=66% (95% CI: 54%-76%) and e2=34% (95% CI: 24%-46%) for OCD; and a2=38% (95% CI: 20%-54%) and e2=62% (95% CI: 46%-80%) for AN. The correlation between additive genetic factors for OCD and AN was ra=.52 (95% CI:. 26 to. 81), while for unique environmental factors it was re=.11 (95% CI: −.18 to. 39). Figure 2 shows the percent of variance attributed to genetic and unique environmental influences that are specific to each disorder and that are shared by the disorders.
Figure 2.
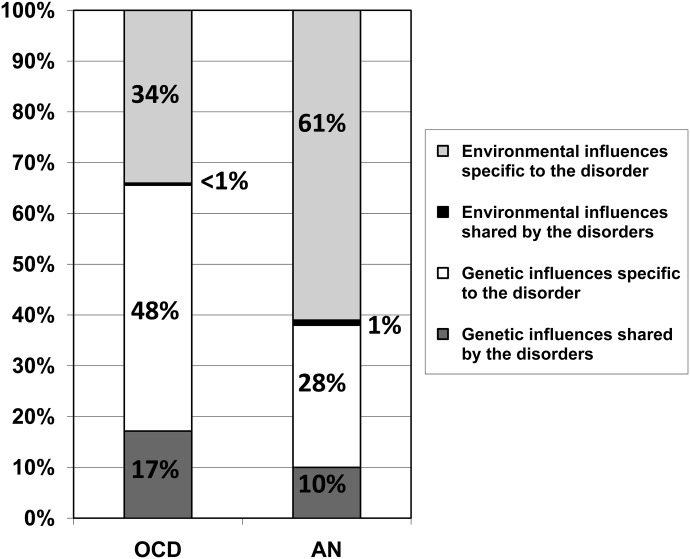
Proportion of the variance accounted for by common vs. disorder-specific genetic and environmental factors across obsessive-compulsive disorder (OCD) and anorexia nervosa (AN)
DISCUSSION
Our results extend previous reports by documenting that AN is far more common (17 times) in individuals with OCD than would be expected by chance. This was particularly true for male OCD patients, for whom the risk was increased by 37 times. In longitudinal analyses, we found that an initial diagnosis of OCD increased the risk of a later diagnosis of AN, and vice versa. Again, these longitudinal risks were substantially greater in males. The family analyses showed familial links between OCD and AN, and the bivariate twin analyses further confirmed a moderate degree of genetic overlap between these disorders. However, most of the genetic variance was disorder-specific.
Compared to unaffected individuals, patients first diagnosed with OCD were approximately 4 times more likely to later develop AN, confirming largely retrospective reports from clinical samples (1,2,5–7) and suggesting that OCD is a risk factor for the development of AN. However, the possibility that subtle eating disorder symptoms were overlooked at initial assessment cannot be ruled out. A previous longitudinal study of pediatric OCD patients showed that those who developed an eating disorder were more likely to be female and to have a family history of an eating disorder (5). In that study, a total of 30% of those who developed an eating disorder at follow-up had eating disorder symptoms or food-related obsessions/compulsions at baseline. This suggests that the nature of OCD symptoms at presentation may assist in identifying individuals at highest risk for developing an eating disorder, and encourages eating disorders symptom screening in individuals seeking help for OCD.
Interestingly, the risk of receiving a diagnosis of OCD after an initial diagnosis of AN was much greater (approximately 10 times) than the risk of receiving a diagnosis of AN after an initial diagnosis of OCD (approximately 4 times). It may be that the diagnosis of AN, which often requires hospitalization, increases the surveillance and thus the detection of OCD. It is also possible that the progressive changes in cognitive function and neurobiology brought on by prolonged periods of starvation and low weight (21) may increase the risk of developing OCD. The relatively long gap between the two diagnoses (a median of over 2 years) is compatible with this interpretation. Although the possibility of misdiagnosis cannot be fully ruled out, our findings suggest that AN may be a more important risk factor for the development of OCD than previously recognized. Given the substantial challenges facing clinicians who manage OCD patients who are very underweight, early detection and management of incipient OCD symptoms in this population are warranted.
Our family analyses provided a rigorous, albeit not probative, test of the possible etiological link between OCD and AN. Indeed, AN was significantly more common in unaffected relatives of probands with OCD, compared with relatives of matched controls. Furthermore, the risks tended to be higher for first-degree relatives, compared to second- and third-degree relatives. Taken together, these findings suggest that shared genetic risk factors underlie the overlap between OCD and AN. This interpretation was further supported in separate bivariate twin analyses, revealing a moderate genetic correlation between self-reported OCD and AN (ra=.52) and minimal overlap in unique environmental influences (re=.11). Future cross-disorder analyses of genome-wide association data should provide confirmation of these analytic results (22).
Although our results are consistent with a genetic overlap between OCD and AN, disorder-specific genetic and environmental risk factors also seem to contribute to the etiology of each disorder. Our twin results suggest that the majority of genetic variance is disorder-specific and that non-shared environmental influences are largely unique to each condition. These findings may explain the obvious clinical differences between the two conditions (12). Identification of disorder-specific environmental risk factors and genome-wide investigations at cross-cutting dimensional levels will be important next steps.
The increased comorbidity and longitudinal risk in males is intriguing and, to our knowledge, previously unreported. Several interpretations are possible. First, males with AN in general may be less likely to seek treatment (23,24); however, those presenting with complex comorbidities, such as OCD, may be more likely to do so. This would result in an over-representation of males with both AN and OCD in the patient register. Another, not incompatible, explanation is that males could require a greater familial etiologic load to manifest the AN phenotype. Because, as we show in this study, AN and OCD share genetic factors, this would result in a greater comorbidity and sequential risk in males. Similar arguments have been employed to explain the striking male preponderance in autism spectrum disorder (25). Unfortunately, our study was underpowered to conduct twin analyses in males to shed further light on potential differences in heritability of AN between the genders.
Some limitations to our study should be considered when evaluating the results. First, both OCD and AN are under-represented in the Swedish National Patient Register, particularly OCD. This is largely due to the fact that OCD rarely requires hospitalization (outpatients were only included in the register from 2001) and that many sufferers do not seek treatment. Therefore, OCD patients with severe comorbidities (e.g., AN) may be more likely to be represented in the register, thus inflating the true comorbidity rates and longitudinal associations. Our family based analyses are less likely to be affected by this limitation, as the relatives of patients with OCD did not have a lifetime diagnosis of that disorder. It is still possible, however, that some relatives may have had OCD but did not seek treatment, or had sub-threshold symptoms. The twin analyses, conducted in a general population of twins, were largely unaffected by this limitation. Second, the diagnoses in STAGE were based on self-report and the OCD diagnosis was based on a single item. Finally, due to the low prevalence of AN in men, we were unable to examine possible gender differences in our bivariate twin models.
To conclude, the high comorbidity, sequential risk, and shared familial risks between OCD and AN suggest partially shared genetic etiological mechanisms between these disabling mental disorders, although the majority of the genetic variance was unique to each disorder. Clinicians should be aware that having one disorder might increase the risk of developing the other, even several years after the initial diagnosis. Our results underscore the importance of screening for the other disorder or nascent symptoms at clinical presentation and throughout treatment. Research into the optimal management of these complex comorbidities is warranted.
Acknowledgments
This study was supported by the Swedish Council for Working Life and Social Research, the Swedish Research Council, and the Anorexia Nervosa Genetics Initiative of the Klarman Family Foundation.
References
- 1.Godart NT, Flament MF, Perdereau F, et al. Comorbidity between eating disorders and anxiety disorders: a review. Int J Eat Disord. 2002;32:253–70. doi: 10.1002/eat.10096. [DOI] [PubMed] [Google Scholar]
- 2.Kaye WH, Bulik CM, Thornton L, et al. Comorbidity of anxiety disorders with anorexia and bulimia nervosa. Am J Psychiatry. 2004;161:2215–21. doi: 10.1176/appi.ajp.161.12.2215. [DOI] [PubMed] [Google Scholar]
- 3.Rubenstein CS, Pigott TA, L’Heureux F, et al. A preliminary investigation of the lifetime prevalence of anorexia and bulimia nervosa in patients with obsessive compulsive disorder. J Clin Psychiatry. 1992;53:309–14. [PubMed] [Google Scholar]
- 4.Rasmussen SA, Eisen JL. The epidemiology and differential diagnosis of obsessive compulsive disorder. J Clin Psychiatry. 1994;55(10):5–10. Suppl. [PubMed] [Google Scholar]
- 5.Micali N, Hilton K, Natatani E, et al. Is childhood OCD a risk factor for eating disorders later in life? A longitudinal study. Psychol Med. 2011;41:2507–13. doi: 10.1017/S003329171100078X. [DOI] [PubMed] [Google Scholar]
- 6.Bulik CM, Sullivan PF, Fear JL, et al. Eating disorders and antecedent anxiety disorders: a controlled study. Acta Psychiatr Scand. 1997;96:101–7. doi: 10.1111/j.1600-0447.1997.tb09913.x. [DOI] [PubMed] [Google Scholar]
- 7.Deep AL, Nagy LM, Weltzin TE, et al. Premorbid onset of psychopathology in long-term recovered anorexia nervosa. Int J Eat Disord. 1995;17:291–7. [PubMed] [Google Scholar]
- 8.Lilenfeld LR, Kaye WH, Greeno CG, et al. A controlled family study of anorexia nervosa and bulimia nervosa: psychiatric disorders in first-degree relatives and effects of proband comorbidity. Arch Gen Psychiatry. 1998;55:603–10. doi: 10.1001/archpsyc.55.7.603. [DOI] [PubMed] [Google Scholar]
- 9.Bellodi L, Cavallini MC, Bertelli S, et al. Morbidity risk for obsessive-compulsive spectrum disorders in first-degree relatives of patients with eating disorders. Am J Psychiatry. 2001;158:563–9. doi: 10.1176/appi.ajp.158.4.563. [DOI] [PubMed] [Google Scholar]
- 10.Bienvenu OJ, Samuels JF, Riddle MA, et al. The relationship of obsessive-compulsive disorder to possible spectrum disorders: results from a family study. Biol Psychiatry. 2000;48:287–93. doi: 10.1016/s0006-3223(00)00831-3. [DOI] [PubMed] [Google Scholar]
- 11.Bienvenu OJ, Samuels JF, Wuyek LA, et al. Is obsessive-compulsive disorder an anxiety disorder, and what, if any, are spectrum conditions? A family study perspective. Psychol Med. 2012;42:1–13. doi: 10.1017/S0033291711000742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phillips KA, Kaye WH. Relationship of body dysmorphic disorder and eating disorders to obsessive-compulsive disorder. In: Hollander E, Zohar J, Sirovatka PJ, editors. Obsessive-compulsive spectrum disorders. Refining the research agenda for DSM-V. Arlington: American Psychiatric Association; 2011. pp. 33–56. [Google Scholar]
- 13.Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11(1):16. doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rück C, Larsson KJ, Lind K, et al. Validity and reliability of chronic tic disorder and obsessive-compulsive disorder diagnoses in the Swedish National Patients Register. BMJ Open. 2015;5:e007520. doi: 10.1136/bmjopen-2014-007520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birgegard A, Bjorck C, Clinton D. Quality assurance of specialised treatment of eating disorders using large-scale Internet-based collection systems: methods, results and lessons learned from designing the Stepwise database. Eur Eat Disord Rev. 2010;18:251–9. doi: 10.1002/erv.1003. [DOI] [PubMed] [Google Scholar]
- 16.Lichtenstein P, Sullivan PF, Cnattingius S, et al. The Swedish Twin Registry in the third millennium: an update. Twin Res Hum Genet. 2006;9:875–82. doi: 10.1375/183242706779462444. [DOI] [PubMed] [Google Scholar]
- 17.Pisetsky EM, Thornton LM, Lichtenstein P, et al. Suicide attempts in women with eating disorders. J Abnorm Psychol. 2013;122:1042–56. doi: 10.1037/a0034902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szatmari P, White J, Merikangas KR. The use of genetic epidemiology to guide classification in child and adult psychopathology. Int Rev Psychiatry. 2007;19:483–96. doi: 10.1080/09540260701563619. [DOI] [PubMed] [Google Scholar]
- 19.Bulik CM, Thornton LM, Root TL, et al. Understanding the relation between anorexia nervosa and bulimia nervosa in a Swedish national twin sample. Biol Psychiatry. 2010;67:71–7. doi: 10.1016/j.biopsych.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mataix-Cols D, Boman M, Monzani B, et al. Population-based, multigenerational family clustering study of obsessive-compulsive disorder. JAMA Psychiatry. 2013;70:709–17. doi: 10.1001/jamapsychiatry.2013.3. [DOI] [PubMed] [Google Scholar]
- 21.Katzman DK, Christensen B, Young AR, et al. Starving the brain: structural abnormalities and cognitive impairment in adolescents with anorexia nervosa. Semin Clin Neuropsychiatry. 2001;6:146–52. doi: 10.1053/scnp.2001.22263. [DOI] [PubMed] [Google Scholar]
- 22.Bulik-Sullivan BK, Loh PR, Finucane HK, et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47:291–5. doi: 10.1038/ng.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Dea JA, Abraham S. Eating and exercise disorders in young college men. J Am Coll Health. 2002;50:273–8. doi: 10.1080/07448480209603445. [DOI] [PubMed] [Google Scholar]
- 24.Olivardia R, Pope HG, Jr, Mangweth B, et al. Eating disorders in college men. Am J Psychiatry. 1995;152:1279–85. doi: 10.1176/ajp.152.9.1279. [DOI] [PubMed] [Google Scholar]
- 25.Robinson EB, Lichtenstein P, Anckarsater H, et al. Examining and interpreting the female protective effect against autistic behavior. Proc Natl Acad Sci USA. 2013;110:5258–62. doi: 10.1073/pnas.1211070110. [DOI] [PMC free article] [PubMed] [Google Scholar]


