Abstract
Metabolic syndrome (MetS) and its components are highly predictive of cardiovascular diseases. The primary aim of this systematic review and meta-analysis was to assess the prevalence of MetS and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder, comparing subjects with different disorders and taking into account demographic variables and psychotropic medication use. The secondary aim was to compare the MetS prevalence in persons with any of the selected disorders versus matched general population controls. The pooled MetS prevalence in people with severe mental illness was 32.6% (95% CI: 30.8%-34.4%; N = 198; n = 52,678). Relative risk meta-analyses established that there was no significant difference in MetS prevalence in studies directly comparing schizophrenia versus bipolar disorder, and in those directly comparing bipolar disorder versus major depressive disorder. Only two studies directly compared people with schizophrenia and major depressive disorder, precluding meta-analytic calculations. Older age and a higher body mass index were significant moderators in the final demographic regression model (z = −3.6, p = 0.0003, r2 = 0.19). People treated with all individual antipsychotic medications had a significantly (p<0.001) higher MetS risk compared to antipsychotic-naïve participants. MetS risk was significantly higher with clozapine and olanzapine (except vs. clozapine) than other antipsychotics, and significantly lower with aripiprazole than other antipsychotics (except vs. amisulpride). Compared with matched general population controls, people with severe mental illness had a significantly increased risk for MetS (RR = 1.58; 95% CI: 1.35-1.86; p<0.001) and all its components, except for hypertension (p = 0.07). These data suggest that the risk for MetS is similarly elevated in the diagnostic subgroups of severe mental illness. Routine screening and multidisciplinary management of medical and behavioral conditions is needed in these patients. Risks of individual antipsychotics should be considered when making treatment choices.
Keywords: Metabolic syndrome, severe mental illness, schizophrenia, bipolar disorder, major depressive disorder, antipsychotics
People with severe mental illness (SMI), including schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder (MDD), experience a two-three times higher mortality rate than the general population (1,2). This mortality gap translates into a 10-20 year shortened life expectancy (3,4) and appears to be widening (5). About 60% of the excess mortality observed in SMI is due to physical comorbidities, predominantly cardiovascular diseases (CVD) (6). Factors predisposing people with SMI to CVD include antipsychotic medication and unhealthy lifestyles (7) as well as their reduced likelihood to receive standard levels of medical care (8–12).
To assist clinicians in identifying and treating patients at an increased risk of CVD, the concept of metabolic syndrome (MetS) has been introduced. MetS is defined by a combination of central obesity, high blood pressure, low high-density lipoprotein (HDL) cholesterol, elevated triglycerides and hyperglycaemia. In the general population, these clustered risk factors have been associated with the development of CVD and excess mortality (13–15). Current definitions (16–19) for MetS are aimed at being easy to use in clinical settings and share similar diagnostic thresholds (20). However, the role of abdominal obesity is central to the MetS definition of the International Diabetes Federation (IDF) (18), with provision of ethnic specific thresholds for waist circumference, while central obesity is not a mandatory criterion in the MetS definition of the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) (16,17). As a prevalent condition and predictor of CVD across racial, gender and age groups, MetS provides the opportunity to identify high-risk populations and prevent the progression of some major causes of morbidity and mortality (20).
Previous meta-analyses (21–24) documented that people with SMI have an increased risk for developing MetS compared with the general population. A brief meta-analytic report comparing MetS frequencies in patients with schizophrenia and bipolar disorder found that these populations are at similar risk (25). However, these findings should be interpreted with caution, since comparisons were performed at study level and not limited to studies directly comparing the two populations, and patient samples were not matched for age and illness duration (26). Meta-analytic comparisons of schizophrenia and related psychotic disorders or bipolar disorder with major depressive disorder are currently lacking. In the same way, meta-analytic data including all major diagnostic SMI subgroups (i.e., schizophrenia and related psychotic disorders versus bipolar disorder versus major depressive disorder) are absent in the literature.
Large-scale pooled analyses in the SMI population are highly relevant, as they enable investigation of risk factors across large numbers of studies and participants, dissecting risk factors for MetS associated with SMI from those independent of it. Pooling data across major diagnostic categories allows for investigation of the effect of demographic variables (age, illness duration, gender, setting, geographical region) and treatments (particularly mood stabilizers and antipsychotics, as well as polypharmacotherapy versus monotherapy). If risk stratification is observed, this could potentially help guide clinicians in monitoring and treatment.
We conducted a systematic review and meta-analysis to assess pooled prevalences of MetS and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder, selecting studies directly comparing subjects with different disorders and taking into account demographic variables and medication use. Our secondary aim was to compare the MetS prevalence in persons with any of the selected disorders versus matched general population controls.
METHODS
Inclusion and exclusion criteria
This systematic review was conducted in accordance with the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines (27) and in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standard (28). We included observational studies (cross-sectional, retrospective and prospective studies) in adults that fulfilled the following criteria: a) a diagnosis of schizophrenia or a related psychotic disorder, bipolar disorder or major depressive disorder according to the DSM-IV or ICD-10, irrespective of clinical setting (inpatient, outpatient or mixed); and b) a MetS diagnosis according to non-modified ATP-III (16), ATP-III-A (17), IDF (18) or World Health Organization (19) standards. For a randomized control trial, we extracted the variables of interest at baseline. There were no language or time restrictions.
For estimation of the prevalence of MetS, we excluded studies with: a) non-standardized diagnoses, b) non-standardized definitions of MetS, c) insufficient data for extraction of MetS frequencies, d) restriction to patients at risk for or without cardiovascular diseases, and e) restriction to children and/or adolescents. In the case of multiple publications from the same study, only the most recent paper or the article with the largest sample was included. When required, we contacted the primary/corresponding authors of potential studies to confirm eligibility, or to acquire the variables of interest if they were not available in the publication.
Search criteria, study selection and critical appraisal
Two independent authors (DV, BS) searched MEDLINE, PsycARTICLES, EMBASE and CINAHL from database inception to January 1, 2015. Key words used were “metabolic syndrome” AND “severe mental illness” OR “schizophrenia” OR “bipolar disorder” OR “depression” OR “depressive disorder” in the title, abstract or index term fields. Manual searches were also conducted using the reference lists from recovered articles and recent meta-analyses (21–24).
After the removal of duplicates, the reviewers screened the titles and abstracts of all potentially eligible articles. Both authors applied the eligibility criteria, and a list of full text articles was developed through consensus. The reviewers then considered the full texts of these articles and the final list of included articles was reached through consensus. A third reviewer (AJM) was available for mediation throughout this process.
Methodological appraisal was performed according to PRISMA standards (28), including evaluation of bias (confounding, overlapping data, publication bias). Publication bias was tested using the Egger’s regression method (29) and Begg-Mazumdar test (30), with a p value <0.05 suggesting the presence of bias.
Statistical analyses
We pooled individual study data using the DerSimonian-Laird proportion method with StatsDirect (31). The trim-and-fill approach (32) was used to adjust the overall estimate for funnel plot asymmetry. Due to anticipated heterogeneity, a random effects meta-analysis was employed. Heterogeneity was measured with the Q statistic, yielding a chi-square p value, with p<0.05 indicating significant heterogeneity of the pooled results. We calculated the relative risk (RR) to investigate the prevalence of MetS and its components within and across SMI subgroups, the latter only in those studies directly comparing diagnostic subgroups. Moreover, we compared the prevalence of MetS between people with schizophrenia, bipolar disorder and major depressive disorder versus general population control groups that were matched on age and sex, also only using data from studies in which they were directly compared. In both analyses, only comparisons of specific SMI groups or an SMI group with a matched general population group were included that had been performed within the same study, in order to minimize variability of MetS frequencies due to different sampling and assessment procedures.
In order to increase homogeneity of compared samples and eliminate smaller studies with less precise point estimates, we also conducted sensitivity analyses, restricting the sample to large, population-based studies. Furthermore, in the entire dataset, we conducted subgroup analyses (including χ2 tests, t tests, odds ratios) to investigate differences between the three main diagnostic subgroups and between first episode and multi-episode illness, gender differences, and differences across medication regimes (antipsychotics, antidepressants, mood stabilizers, monotherapy versus polypharmacotherapy) and geographical regions. In order to reduce heterogeneity, we did not calculate diagnostic and gender differences across studies, but pooled only data of studies that compared these differences on a patient level.
Further, we conducted meta-regression analyses to investigate potential moderators (age, percentage of males, illness duration, body mass index, smoking rates) with Comprehensive Meta Analysis (version 3). Finally, since patients with a first episode of schizophrenia and those with chronic schizophrenia differ significantly in age, and since older age is a significant moderator of higher MetS rates, we also conducted a multivariable meta-regression analysis, adding both first versus multi-episode schizophrenia and age as variables into the analysis.
RESULTS
Search results and included participants
Our search yielded 429 publications, of which 198 met inclusion criteria (Figure 1). The list of included and excluded studies (with reasons) is available upon request. The final sample comprised 52,678 unique persons with SMI. Sample sizes ranged from 14 to 3,568 participants, with a mean sample size of 264. Mean age was 41.3 years (range 22.2-73.2), and mean illness duration was 12.4 years. Fifty-seven studies (n = 12,560) reported smoking frequencies, and half of the included participants (50.4%, 95% CI: 46.7%-54.0%, Q = 1192.0, p<0.001) smoked. The mean body mass index of the sample was 27.3 (SD = 2.7).
Figure 1.
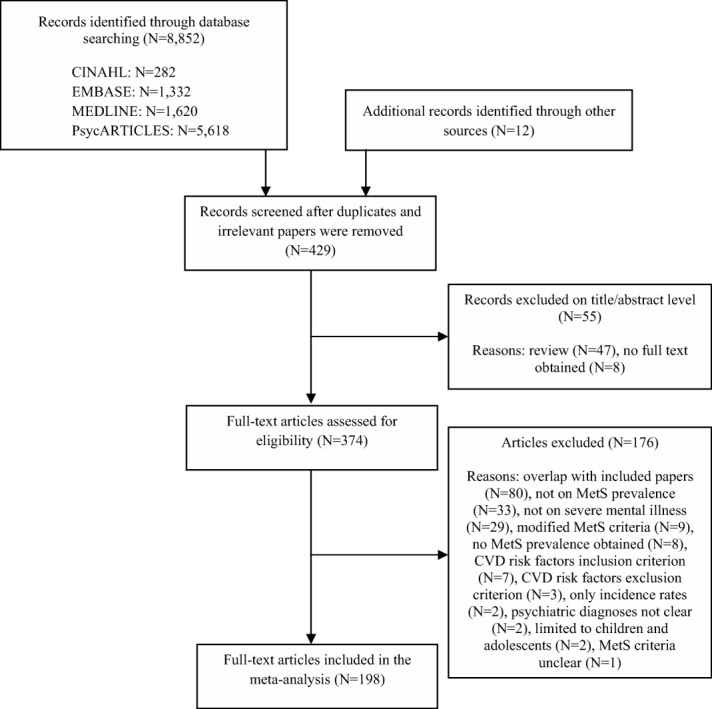
Flow diagram for the search strategy. MetS – metabolic syndrome, CVD – cardiovascular disease
Prevalence of metabolic syndrome and its components
The estimated weighted mean prevalence of MetS was 32.6% (95% CI: 30.8%-34.4%, Q = 3696, p<0.001, n=52,678). The Begg-Mazumdar (Kendall’s tau b = 0.15, p=0.0015) and Egger test (bias = 1.46, 95% CI: 0.15-2.77, p=0.0292) indicated some publication bias. The trim-and-fill method demonstrated that adjusting for publication bias had little effect on the pooled MetS estimate, which was virtually identical (32.5%, 95% CI: 30.8%-34.2%, Q = 2991, p<0.01, n = 52,678). Restricting the analysis to population-based studies (N = 29, n = 18,594), the overall weighted mean prevalence of MetS was 35.9% (95% CI: 31.8%-40.0%, Q = 934.8, p<0.001).
Sixty-five studies reported on obesity frequency defined as waist circumference >102 cm in males and >88 cm in females (ATP-III or ATP-III-A), while 14 studies reported the obesity frequency following the ethnicity-specific IDF criteria. Overall, the proportion of patients with abdominal obesity was 50.3% by the ATP definitions (n = 20,210; 95% CI: 46.9%-53.7%, Q = 1.6, p<0.001) and 63.2% according to IDF (n = 3,789; 95% CI: 53.6%-72.3%, Q = 480.9, p<0.001). In studies reporting on hyperglycaemia, the frequency was 18.8% (N = 56, n = 17,508; 95% CI: 16.6%-21.2%, Q = 906.9, p<0.001) when the threshold was ≥110 mg/dl (ATP-III), while it was 23.0% (N = 28, n = 8,205; 95% CI: 17.3%-29.2%, Q = 1.1, p<0.001) when the threshold was ≥100 mg/dl (ATP-III-A and IDF). Hypertriglyceridemia was present in 36.2% (N = 87, n = 26,577; 95% CI: 33.1%-39.3%, Q = 2.7, p<0.001). Low HDL cholesterol was present in 39.1% (N = 86, n = 26,193; 95% CI: 36.4%-41.9%, Q = 1.9, p<0.001). Hypertension (ATP-III, ATP-III-A and IDF) was present in 39.3% (N = 88, n = 27,441; 95% CI: 36.1%-42.5%, Q = 2.7, p<0.001).
Subgroup analyses and predictors of metabolic syndrome
Diagnostic subgroups
The pooled MetS prevalence was 33.4% (95% CI: 30.8%-36.0%, Q = 1955.0, p<0.001) in people with schizophrenia (N = 93, n = 29,596), and 34.6% (95% CI: 29.3%-40.0%, Q = 110.2, p<0.001) in those with related psychotic disorders (N = 13, n = 2,850). Similar pooled MetS prevalences were observed in patients with bipolar disorder (31.7%, 95% CI: 27.3%-36.3%, Q = 843.5, p<0.001; N = 33, n = 5,827) and major depressive disorder (31.3%, 95% CI: 27.3%-35.5%, Q = 142.7, p<0.001; N = 19, n = 5,415). In population-based studies, the pooled prevalence of MetS was 38.9% (95% CI: 34.6%-43.4%, Q = 458.1, p<0.001; N = 20, n = 12,770) for schizophrenia and 22.7% (95% CI: 20.4%-25.1%, Q = 2.28, p = 0.31; N = 3, n = 1,503) for major depressive disorder. There were insufficient data for bipolar disorder.
The releative risk of MetS versus age- and gender-matched healthy controls was 1.87 in schizophrenia and related psychotic disorders (95% CI: 1.53-2.29; p<0.001, Q = 18.3, p = 0.03; N = 11, n = 1,413), 1.58 in bipolar disorder (95% CI: 1.24-2.03; p<0.001, Q = 6.6, p = 0.25; N = 6, n = 1,125) and 1.57 in major depressive disorder (95% CI: 1.38-1.79, p<0.001, Q = 19.0, p = 0.26; N = 17, n = 5,267).
Relative risk meta-analyses established that there was no significant difference in MetS in studies directly comparing schizophrenia (39.2%, 95% CI: 30.5%-48.3%; n = 2,338) versus bipolar disorder (35.5%, 95% CI: 27.0-44.3%; n = 2,077) (N = 10, RR = 0.92, 95% CI: 0.79%-1.06%; χ2 = 1.33, p = 0.24; Q = 21.3, p<0.011). Similarly, there were no differences in the study directly comparing bipolar disorder (29.2%, 95% CI: 14.5%-46.2%; n = 137) versus major depressive disorder (34.0%, 95% CI: 19.4%-50.3%; n = 176) (N = 4; RR = 0.87, 95% CI: 0.48- 1.55; χ2 = 0.21, p = 0.64; Q = 7.73, p = 0.0518). Only two studies directly compared MetS in people with schizophrenia and major depressive disorder, precluding meta-analytic calculations.
Comparing MetS in first versus multi-episode patients within illness subgroups, first episode psychosis patients (13.7%, 95% CI: 10.4%-16.9%, Q = 8.659, p = 0.034; N = 4, n = 424) had a significantly lower MetS risk than those with multi-episode schizophrenia (34.2%, 95% CI: 30.8%-36.0%, Q = 1,955, p<0.001; N = 105, n = 29,596) (z = −8.9, p<0.001). In order to assess if the difference in MetS rates remained significant when age was entered into the analyses, we conducted a multivariable meta-regression analysis. Within this, we pooled the prevalence of MetS in first and multi-episode schizophrenia and found that, although mean age predicted MetS prevalence (coefficient = 0.0296; 95% CI: 0.013 to 0.0463, z = 3.49, p = 0.005), first episode was also a unique predictor of lower MetS (coefficient = −0.7517; 95% CI: −1.4877 to −0.0157; z = −2, p = 0.04; r2 = 0.24). There were no data in first-episode bipolar disorder or major depressive disorder patients, precluding a comparison with multi-episode patients.
Demographic variables
A relative risk meta-analysis across 64 studies directly comparing MetS frequencies in male (33.5%, 95% CI: 30.0%-36.7%, Q = 814, p<0.001; n = 10,798) versus female (33.4%, 95% CI: 31.5%-38.4%, Q = 615, p<0.001; n = 8,027) participants with SMI found no gender differences (RR = 0.94; 95% CI: 0.85-1.02; χ2 = 2.06, p = 0.15; Q = 232.0, p<0.011).
Separate meta-regression analyses revealed that higher MetS frequencies were moderated by older age (coefficient = 0.0278; 95% CI: 0.0178-0.0379, z = 5.5, p<0.0001), longer illness duration (coefficient = 0.0339; 95% CI: 0.0115-0.0564, z = 2.96, p = 0.003) and higher body mass index (coefficient = 0.1537; 95% CI: 0.095-0.2123, z = 5.14, p<0.0001), but not by smoking status (p = 0.49). When all significant predictors were entered in one meta-regression model, body mass index (coefficient = 0.142, 95% CI: 0.0438-0.2405, z = 2.83, p = 0.004) and age (coefficient = 0.0556, 95% CI: 0.0025-0.1087, z = 2.05, p = 0.04) remained significant predictors, whilst illness duration did not (p = 0.19). Overall, the final model was a significant predictor of the variance in MetS (z = −3.6, p = 0.0003; r2 = 0.19).
Pooled MetS prevalences per geographical region and country (if N≥5) can be found in Table1. The MetS prevalence was significantly higher in Australia and New Zealand compared with all other regions (p<0.001). Pooled MetS prevalences per country ranged from 25.4% (95% CI: 18.5%-32.9%) in Brazil to 50.2% (95% CI: 32.9%-67.4%) in Australia.
Table 1.
Geographical differences in pooled metabolic syndrome (MetS) prevalence
| Region | No. studies | Pooled MetS prevalence | Cochran Q |
|---|---|---|---|
| Australia and New Zealand* | 6 | 50.2% (95% CI: 35.3%-65.0%) | 73.8, p<0.001 |
| Middle-East | 6 | 35.3% (95% CI: 31.3%-39.5%) | 1287.6, p<0.001 |
| North-America | 46 | 32.4% (95% CI: 24.7%-40.8%) | 38.0, p<0.001 |
| Europe | 81 | 32.0% (95% CI: 29.4%-34.7%) | 1226.4, p<0.001 |
| Asia | 50 | 31.0% (95% CI: 27.7%-34.4%) | 691.3, p<0.001 |
| South-America | 10 | 25.8% (95% CI: 20.7%-31.3%) | 42.3, p<0.001 |
| Country | No. studies | Pooled MetS prevalence | Cochran Q |
|---|---|---|---|
| Australia | 5 | 50.2% (95% CI: 32.9%-67.4%) | 72.7, p<0.001 |
| South Korea | 7 | 38.9% (95% CI: 30.8%-47.3%) | 103.3, p<0.001 |
| The Netherlands | 11 | 36.5% (95% CI: 29.0%-44.4%) | 167.3, p<0.001 |
| USA | 38 | 36.4% (95% CI: 32.0%-40.9%) | 1217.8, p<0.001 |
| Croatia | 7 | 33.1% (95% CI: 24.6%-42.3%) | 39.1, p<0.001 |
| Spain | 12 | 31.0% (95% CI: 24.5%-37.9%) | 210.3, p<0.001 |
| Finland | 5 | 30.4% (95% CI: 21.8%-39.8%) | 17.9, p<0.001 |
| Taiwan | 13 | 29.8% (95% CI: 24.7%-35.1%) | 124.1, p<0.001 |
| Germany | 6 | 28.7% (95% CI: 19.2%-39.2%) | 62.8, p<0.001 |
| Canada | 5 | 27.4% (95% CI: 17.3%-38.7%) | 44.2, p<0.001 |
| India | 16 | 26.3% (95% CI: 19.0%-34.3%) | 193.0, p<0.001 |
| Brazil | 8 | 25.4% (95% CI: 18.5%-32.9%) | 39.4, p<0.001 |
Significantly higher than in other regions, p<0.01
Medication use
Data from five studies demonstrated a trend for lower pooled MetS prevalence in participants receiving monotherapy (30.4%, 95% CI 25.4%-35.5%, Q = 15.2, p = 0.004; n = 1,364) versus polytherapy (35.2%, 95% CI: 23.8%-47.5%, Q = 18.8, p = 0.008; n = 313) (RR = 0.81; 95% CI: 0.66-1.01; χ2 = 3.41, p = 0.065; Q = 5.87, p = 0.21).
Forty-eight papers including 147 analyses reported on antipsychotics (monotherapy and N≥5). The prevalence of MetS was lowest in antipsychotic-naïve participants (10.2%, 95% CI: 6.8%-14.3%). Among those receiving antipsychotics, participants taking aripiprazole had the lowest MetS prevalence (19.4%, 95% CI:8.0%-34.2%; N = 6), whilst those taking clozapine had the highest (47.2%, 95% CI: 42.0%-52.6%; N = 30). Patients treated with amisulpride, typical antipsychotics, risperidone, olanzapine and quetiapine had MetS frequencies of 22.8% (95% CI: 7.6%-43.2%; N = 5), 28.0% (95% CI: 19.8%-37.2%; N = 15), 30.7% (95% CI: 23.7%-38.1%; N = 20), 36.2% (95% CI: 31.8%-40.9%; N = 26) and 37.3% (95% CI: 27.4-47.8%; N = 11), respectively.
An overview of the odds ratios comparing individual medications (if monotherapy and N≥5) with each other (at study level) is presented in Table2. Patients treated with all individual antipsychotic medications had significantly (p<0.001) higher MetS risk compared to antipsychotic-naïve participants. Those treated with clozapine consistently had significantly (p<0.001) higher MetS prevalence than those treated with any other individual antipsychotic medication. Those treated with olanzapine had significantly higher MetS prevalence than those treated with amisulpride (p<0.05), aripiprazole (p<0.001), risperidone (p<0.01) and typical antipsychotic medications (p<0.05). Those treated with aripiprazole had significantly lower odds of MetS compared to other antipsychotic medications (except vs. amisulpride). There were insufficient data to compare the MetS prevalence between antipsychotic-naïve persons and those treated with specific antidepressants or mood stabilizers in similar populations.
Table 2.
Odds ratios for metabolic syndrome risk for individual antipsychotic medications (if monotherapy and N≥5)
| Medication | Antipsychotic-naïve | Amisulpride | Aripiprazole | Clozapine | Olanzapine | Quetiapine | Risperidone |
|---|---|---|---|---|---|---|---|
| Amisulpride | 3.86*** (↑) (2.54-5.84) N = 15; n = 999 | / | / | / | / | / | / |
| Aripiprazole | 3.25*** (↑) (2.36-4.49) N = 16; n = 1,319 | 0.84 (↔) (0.57-1.25) N = 11; n = 692 | / | / | / | / | / |
| Clozapine | 7.81*** (↑) (6.02-10.22) N = 22; n = 2,398 | 2.02*** (↑) (1.45-2.83) N = 17; n = 1,177 | 2.40*** (↑) (1.91-3.03) N = 18; n = 2,091 | / | / | / | / |
| Olanzapine | 5.87*** (↑) (4.53-7.67) N = 22; n = 2,633 | 1.52* (↑) (1.08-2.16) N = 15; n = 2,006 | 1.81*** (↑) (1.44-2.27) N = 16; n = 2,326 | 0.75*** (↓) (0.65-0.86) N = 22; n = 3,405 | / | / | / |
| Quetiapine | 5.14*** (↑) (3.75-7.07) N = 21; n = 1,266 | 1.33 (↔) (0.90-1.97) N = 16; n = 639 | 1.58*** (↑) (1.19-2.11) N = 17; n = 959 | 0.66*** (↓) (0.53-0.82) N = 23; n = 2,038 | 0.88 (↔) (0.70-1.09) N = 22; n = 2,273 | / | / |
| Risperidone | 4.57*** (↑) (3.48-6.03) N = 30; n = 2,025 | 1.18 (↔) (0.83-1.69) N = 25; n = 1398 | 1.40*** (↑) (1.10-1.79) N = 26; n = 1,718 | 0.58*** (↓) (0.50-0.68) N = 32; n = 2,797 | 0.78** (↓) (0.66-0.91) N = 30; n = 3,032 | 0.89 (↔) (0.70-1.12) N = 31; n = 1,665 | / |
| Typical antipsychotics | 4.97*** (↑) (3.83-6.51) N = 17; n = 2,525 | 1.28 (↔) (0.91-1.83) N = 12; n = 1,898 | 1.53*** (↑) (1.23-1.91) N = 13; n =2,218 | 0.64*** (↓) (0.55-0.73) N = 19; n = 3,297 | 0.85* (↓) (0.74-0.97) N = 17; n = 3,532 | 0.97 (↔) (0.77-1.21) N = 18; n = 2,165 | 1.09 (↔) (0.93-1.28) N = 27; n = 2,924 |
Two-sided p<0.05
two-sided p<0.01
two-sided p<0.001
↑ = higher risk, ↓ = lower risk, ↔ = no significant risk difference
Risk of metabolic syndrome and its components in persons with various disorders compared with general population controls
Thirty studies also provided data on MetS prevalence in healthy control subjects. In a pooled relative risk meta-analysis, persons with SMIs (n = 6,610; 29.2%, 95% CI: 25.9%-32.6%; Q = 230, p<0.001), compared with general population controls (n = 101,223; 18.1%, 95% CI: 15.8%-20.5%, Q = 230, p<0.001), had significantly increased risk of MetS (RR = 1.58, 95% CI: 1.35-1.86, p<0.001; Q = 62, p = 0.003).
People with severe mental illness had significantly increased risk for abdominal obesity (N = 18; RR = 1.43, 95% CI: 1.23-1.66, p<0.001; Q = 198.8, p<0.001), low HDL cholesterol (N = 19; RR = 1.33, 95% CI: 1.15-1.54, p<0.001; Q = 114.7, p<0.001), hypertriglyceridemia (N = 19; RR = 1.49, 95% CI: 1.28-1.73, p<0.001; Q = 91.2, p<0.001), and hyperglycaemia (N = 20; RR = 1.51, 95% CI: 1.24-1.84, p<0.001; Q = 94.4, p<0.001), with a statistical trend for hypertension (N = 12; RR = 1.12, 95% CI: 0.99-1.28, p = 0.07; Q = 127.1, p<0.001).
DISCUSSION
To our knowledge, this is the first meta-analysis of MetS and its components including and comparing data from the main SMIs: schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder. Approximately one third, 32.6% (95% CI: 30.8%-34.4%), of this population had MetS and the relative risk was 1.58 times higher than in the respective general population. MetS prevalences were consistently elevated for each of the three diagnostic subgroups compared to the general population, and comparative meta-analyses found no significant differences across schizophrenia, bipolar disorder and major depressive disorder. Importantly, we also showed for the first time on a large meta-analytic scale that MetS risk differs significantly across commonly used antipsychotic medications.
Knowledge of factors associated with the highest MetS risk can help identify individuals at greatest need for intensive monitoring and intervention. Consistent with population studies (33,34), we found no significant difference between men and women. Our results confirm earlier meta-analyses (22,35) in that MetS prevalence was higher in individuals with multi-episode schizophrenia compared with persons in their first episode. The current meta-analysis adds to the literature that a first episode diagnosis is even a unique predictor of lower MetS prevalence independent of mean age.
Also in line with data in the general population (36) and earlier work in people with schizophrenia (23), increasing age was a key predictor of MetS. When age and illness duration were entered into the same model, age was a more important determinant of MetS. However, this may also be due to the limited data available for illness duration compared to age data. Since age is a relevant risk factor for MetS in the general population too, the relative MetS risk compared to the general population is greatest in younger people with SMI and those treated with antipsychotics (37,38). Considering the current meta-analytic data, it appears that a cumulative long-term effect of poor health behaviors and psychotropic medication use places people with SMI at the greatest risk for cardiometabolic disorders, more so than psychiatric diagnosis per se.
Our data suggest that patients receiving all individual antipsychotic medications are at higher MetS risk when compared to those who are antipsychotic-naïve. In line with the available literature (11,32,39–41), MetS risk was significantly higher with clozapine, followed by olanzapine. Moreover, MetS risk was significantly lower with aripiprazole than with each other antipsychotic for which data were available, including pooled typical antipsychotics, with the only exception of amisulpride. The lowest MetS prevalence for aripiprazole is noteworthy, as antipsychotics with lower cardiometabolic risk profiles in short-term studies are often prescribed for higher risk patients in clinical care, which may lead to a not reduced or even increased cardiometabolic risk in naturalistic settings (42).
Our meta-analysis also highlighted geographical differences in MetS, which indicates the possible influence of lifestyle and other environmental factors with or without genetic risk differences. This finding may, however, be somewhat affected by different MetS criteria, with IDF criteria, which are often used in Australian studies, being associated with the highest MetS prevalences. Nevertheless, people with SMI are more likely than the general population to have unhealthy lifestyle behaviors, such as being sedentary (43), smoking (44) and having diets that are high in saturated fats and refined sugars, while low in fruit and vegetables (45), placing them at higher risk for MetS and CVD than the general population. Thus, screening for and trying to minimize risk factors (including adverse lifestyle factors and antipsychotic medication choice and use) should be a key priority in the multidisciplinary treatment of people with SMI (46–49).
Whilst this is the most comprehensive and thorough meta-analysis of MetS in people with SMI conducted to date, we acknowledge some limitations that are largely related to the primary data. First, there was considerable methodological heterogeneity across studies. Second, because our study findings were based on cross-sectional rather than longitudinal data, directionality of the association between medication use and observed metabolic parameters cannot be deduced with certainty; that is, it is possible that those with inherently higher metabolic risk factors may be more likely to receive antipsychotics. Third, variables such as clinical subtypes of major depressive and bipolar disorder and concomitant or previous use of antidepressants and mood stabilizers were not reported or were insufficiently reported or controlled for in most available studies. Fourth, a threat to the validity of any meta-analysis is publication bias and heterogeneity, which we encountered in several of our analyses. However, although the main findings were heterogeneous, they were also highly robust and not influenced by publication bias, being virtually unaltered after applying the trim-and-fill method. Fifth, there were inadequate data on ethnic distribution and lifestyle behaviors, precluding meta-analytic assessment of these factors as moderating or mediating variables. Despite the above-mentioned caveats, this is the largest study of MetS risk and its moderators in people with SMI, and the first meta-analysis pooling and comparing all available data across patients with schizophrenia, bipolar disorder and major depressive disorder, comparing MetS risk across different antipsychotics and comparing the pooled risk of the three main SMI categories as well as the individual diagnostic groups with concurrently assessed, matched general population control groups.
Since antipsychotic medications are increasingly used as frontline treatments for bipolar disorder (50) and major depressive disorder (51), research on the underlying mechanisms for the development of metabolic abnormalities after pharmacotherapy initiation is urgently needed. Future studies should also examine whether different clinical subtypes of depression (i.e., melancholic or atypical) and bipolar disorder (e.g., type 1 or 2, mixed, cyclothymic), different mood states (manic, depressive or euthymic), or different antidepressant or mood stabilizers significantly modulate MetS risk. For example, previous studies (52) found that some antidepressants may, in some circumstances, reduce hyperglycaemia, normalize glucose homeostasis and also increase insulin sensitivity, whereas others, including tricyclic antidepressants, may exacerbate glycaemic dysfunction or have little effect on glucose homeostasis (53,54). Further, persons with atypical depression have significantly higher levels of inflammatory markers, body mass index, waist circumference and triglycerides, and lower HDL cholesterol than those with melancholic depression (55).
The pathophysiology underlying the association between SMI and MetS is complex and not well understood, requiring further investigation. Emerging evidence (56–59) suggests that they share pathophysiological features, including hypothalamic-pituitary-adrenal and mitochondrial dysfunction, neuro-inflammation, common genetic links and epigenetic interactions. Future research should comprehensively assess MetS risk factors and evaluate the optimal monitoring regimen and interventions. Moreover, long-term follow-up is required to accurately document the emergence of more distal outcomes, such as diabetes, ischemic heart disease, medical costs, and premature mortality (58).
Acknowledgments
The authors are very grateful to the following researchers for sending additional data: K. Blank (Hartford Hospital, Hartford, CT, USA); R.K. Chadda (All India Institute of Medical Sciences, Ansari Nagar, New Delhi, India); R. Chengappa (University of Pittsburgh, School of Medicine, Pittsburgh, PA, USA); H.-W. Chiu (Taipei Medical University, Taipei, Taiwan); D. Cohen (Geestelijke Gezondheidszorg Noord - Holland Noord, The Netherlands); T. Cohn (University of Toronto, Toronto, Canada); J. Crilly and J.S. Lamberti (University of Rochester Medical Center, Rochester, NY, USA); V. Ellingrod (University of Michigan College of Pharmacy, Ann Arbor, MI, USA); S. Grover (Postgraduate Institute of Medical Education and Research, Chandigarh, India); T. Heiskanen and H. Koponen (Kuopio University Hospital, Kuopio, Finland); P. Ifteni (Transilvania University, Brasov, Romania); F. Lamers (VU University Medical Centre Amsterdam, Amsterdam, The Netherlands); G.J. L’Italien (Yale University School of Medicine, New Haven, CT, USA); P. Mackin (Newcastle University, Newcastle upon Tyne, UK); J. Meyer (University of California, San Diego, CA, USA); H. Mulder (Utrecht University, Utrecht and Wilhelmina Hospital, Assen, The Netherlands); J.K. Patel (University of Massachusetts Medical School, Worcester, MA, USA); T. Sanchez-Araña Moreno (Hospital de la Merced, Osuna, Spain); K. Taxis (University of Groningen, Groningen, The Netherlands); P.J. Teixeira (Instituto de Previdência dos Servidores do Estado de Minas Gerais, Belo Horizonte, Brazil); S. Tirupati (James Fletcher Hospital, Newcastle, New South Wales, Australia); B. Vuksan (University Hospital Centre Zagreb, Zagreb, Croatia).
References
- 1.Osborn DPJ, Levy G, Nazareth I, et al. Relative risk of cardiovascular and cancer mortality in people with severe mental illness from the United Kingdom’s General Practice Research Database. Arch Gen Psychiatry. 2007;64:242–49. doi: 10.1001/archpsyc.64.2.242. [DOI] [PubMed] [Google Scholar]
- 2.Reininghaus U, Dutta R, Dazzan P, et al. Mortality in schizophrenia and other psychoses: a 10-year follow-up of the ÆSOP first-episode cohort. Schizophr Bull. 2015;41:664–73. doi: 10.1093/schbul/sbu138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang CK, Hayes RD, Perera G, et al. Life expectancy at birth for people with serious mental illness from a secondary mental health care case register in London, UK. PLoS One. 2011;6:e19590. doi: 10.1371/journal.pone.0019590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawrence D, Hancock KJ, Kisely S. The gap in life expectancy from preventable physical illness in psychiatric patients in Western Australia: retrospective analysis of population based registers. BMJ. 2013;346:f2539. doi: 10.1136/bmj.f2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia. Arch Gen Psychiatry. 2007;64:1123–31. doi: 10.1001/archpsyc.64.10.1123. [DOI] [PubMed] [Google Scholar]
- 6.Hoang U, Goldacre MJ, Stewart R. Avoidable mortality in people with schizophrenia or bipolar disorder in England. Acta Psychiatr Scand. 2013;127:195–201. doi: 10.1111/acps.12045. [DOI] [PubMed] [Google Scholar]
- 7.De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10:52–77. doi: 10.1002/j.2051-5545.2011.tb00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell AJ, Lord O. Do deficits in cardiac care influence high mortality rates in schizophrenia? A systematic review and pooled analysis. J Psychopharmacol. 2010;24(Suppl. 4):69–80. doi: 10.1177/1359786810382056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell AJ, Lord O, Malone D. Differences in the prescribing of medication for physical disorders in individuals with v. without mental illness: meta-analysis. Br J Psychiatry. 2012;201:435–43. doi: 10.1192/bjp.bp.111.094532. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell AJ, Malone D, Doebbeling CC. Quality of medical care for people with and without comorbid mental illness and substance misuse: systematic review of comparative studies. Br J Psychiatry. 2009;194:491–9. doi: 10.1192/bjp.bp.107.045732. [DOI] [PubMed] [Google Scholar]
- 11.De Hert M, Vancampfort D, Correll CU, et al. Guidelines for screening and monitoring of cardiometabolic risk in schizophrenia: systematic evaluation. Br J Psychiatry. 2011;199:99–105. doi: 10.1192/bjp.bp.110.084665. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell AJ, Delaffon V, Vancampfort D, et al. Guideline concordant monitoring of metabolic risk in people treated with antipsychotic medication: systematic review and meta-analysis of screening practices. Psychol Med. 2012;42:125–47. doi: 10.1017/S003329171100105X. [DOI] [PubMed] [Google Scholar]
- 13.Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403–14. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 14.Galassi A, Reynolds K, He J. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med. 2006;119:812–9. doi: 10.1016/j.amjmed.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 15.Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113–32. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 16.Expert Panel on Detection and Evaluation of High Blood Cholesterol in Adults. Executive summary of the third report of the expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 17.Grundy SM, Cleeman JI, Daniels RS, et al. Diagnosis and management of the metabolic syndrome: an American Heart/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 18.Alberti KG, Zimmet P, Shaw P. The metabolic syndrome, a new worldwide definition. A consensus statement from the International Diabetes Federation. Diab Med. 2006;23:469–80. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Geneva: World Health Organization; 1999. [Google Scholar]
- 20.Alberti KG, Eckel RH, Grundy SM, et al. A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell AJ, Vancampfort D, Sweers K, et al. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders – a systematic review and meta-analysis. Schizophr Bull. 2013;39:306–18. doi: 10.1093/schbul/sbr148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell AJ, Vancampfort D, De Herdt A, et al. Is the prevalence of metabolic syndrome and metabolic abnormalities increased in early schizophrenia? A comparative meta-analysis of first episode, untreated and treated patients. Schizophr Bull. 2013;39:295–305. doi: 10.1093/schbul/sbs082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vancampfort D, Vansteelandt K, Correll CU, et al. Metabolic syndrome and metabolic abnormalities in bipolar disorder: a meta-analysis of prevalence rates and moderators. Am J Psychiatry. 2013;170:265–74. doi: 10.1176/appi.ajp.2012.12050620. [DOI] [PubMed] [Google Scholar]
- 24.Vancampfort D, Correll CU, Wampers M, et al. Metabolic syndrome and metabolic abnormalities in patients with depression: a meta-analysis of prevalence rates and moderators. Psychol Med. 2014;94:2017–28. doi: 10.1017/S0033291713002778. [DOI] [PubMed] [Google Scholar]
- 25.Bartoli F, Carrà G, Crocamo C, et al. Bipolar disorder, schizophrenia, and metabolic syndrome. Am J Psychiatry. 2013;170:927–8. doi: 10.1176/appi.ajp.2013.13040447. [DOI] [PubMed] [Google Scholar]
- 26.Vancampfort D, Mitchell AJ, Correll CU, et al. Response to Bartoli et al. Am J Psychiatry. 2013;170:928–9. doi: 10.1176/appi.ajp.2013.13040447r. [DOI] [PubMed] [Google Scholar]
- 27.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 28.Moher D, Liberati A, Tetzlaff J, et al. The PRISMA 660 Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egger M, Davey SG, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 31.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 32.Duvall S, Tweedie R. A non-parametric ‘trim and fill’ method for assessing publication bias in meta-analysis. J Am Stat Assoc. 2000;95:89–98. [Google Scholar]
- 33.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–9. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 34.Park YW, Zhu S, Palaniappan L, et al. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med. 2003;163:427–36. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vancampfort D, Wampers M, Mitchell AJ, et al. A meta-analysis of cardio-metabolic abnormalities in drug naïve, first-episode and multi-episode patients with schizophrenia versus general population controls. World Psychiatry. 2013;12:240–50. doi: 10.1002/wps.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circ Res. 2012;110:1097–108. doi: 10.1161/CIRCRESAHA.111.246876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Hert M, van Winkel R, Van Eyck D, et al. Prevalence of diabetes, metabolic syndrome and metabolic abnormalities in schizophrenia over the course of the illness: a cross-sectional study. Clin Pract Epidemiol Ment Health. 2006;2:14. doi: 10.1186/1745-0179-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Hert M, Detraux J, van Winkel R, et al. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2011;8:114–26. doi: 10.1038/nrendo.2011.156. [DOI] [PubMed] [Google Scholar]
- 39.Hammerman A, Dreiher J, Klang SH, et al. Antipsychotics and diabetes: an age-related association. Ann Pharmacother. 2008;42:1316–22. doi: 10.1345/aph.1L015. [DOI] [PubMed] [Google Scholar]
- 40.Nielsen J, Skadhede S, Correll CU. Antipsychotics associated with the development of type 2 diabetes in antipsychotic-naïve schizophrenia patients. Neuropsychopharmacology. 2010;35:1997–2004. doi: 10.1038/npp.2010.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Correll CU, Robinson DG, Schooler NR, et al. Cardiometabolic risk in first episode schizophrenia-spectrum disorder patients: baseline results from the RAISE-ETP Study. JAMA Psychiatry. 2014;71:1350–63. doi: 10.1001/jamapsychiatry.2014.1314. [DOI] [PubMed] [Google Scholar]
- 42.Kessing LV, Thomsen AF, Mogensen UB, et al. Treatment with antipsychotics and the risk of diabetes in clinical practice. Br J Psychiatry. 2010;197:266–71. doi: 10.1192/bjp.bp.109.076935. [DOI] [PubMed] [Google Scholar]
- 43.Vancampfort D, Probst M, Knapen J, et al. Associations between sedentary behaviour and metabolic parameters in patients with schizophrenia. Psychiatry Res. 2012;200:73–8. doi: 10.1016/j.psychres.2012.03.046. [DOI] [PubMed] [Google Scholar]
- 44.Dickerson F, Stallings CR, Origoni AE, et al. Cigarette smoking among persons with schizophrenia or bipolar disorder in routine clinical settings, 1999-2011. Psychiatr Serv. 2013;64:44–50. doi: 10.1176/appi.ps.201200143. [DOI] [PubMed] [Google Scholar]
- 45.Bly MJ, Taylor SF, Dalack G, et al. Metabolic syndrome in bipolar disorder and schizophrenia: dietary and lifestyle factors compared to the general population. Bipolar Disord. 2014;16:277–88. doi: 10.1111/bdi.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Hert M, Dekker JM, Wood D, et al. Cardiovascular disease and diabetes in people with severe mental illness position statement from the European Psychiatric Association (EPA), supported by the European Association for the Study of Diabetes (EASD) and the European Society of Cardiology (ESC) Eur Psychiatry. 2009;24:412–24. doi: 10.1016/j.eurpsy.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 47.McIntyre RS, Alsuwaidan M, Goldstein BI, et al. The Canadian Network for Mood and Anxiety Treatments (CANMAT) task force recommendations for the management of patients with mood disorders and comorbid metabolic disorders. Ann Clin Psychiatry. 2012;24:69–81. [PubMed] [Google Scholar]
- 48.Vancampfort D, De Hert M, Skjerven LH, et al. International Organization of Physical Therapy in Mental Health consensus on physical activity within multidisciplinary rehabilitation programmes for minimising cardio-metabolic risk in patients with schizophrenia. Disabil Rehabil. 2012;34:1–12. doi: 10.3109/09638288.2011.587090. [DOI] [PubMed] [Google Scholar]
- 49.Gierisch JM, Nieuwsma JA, Bradford DW, et al. Pharmacologic and behavioral interventions to improve cardiovascular risk factors in adults with serious mental illness: a systematic review and meta-analysis. J Clin Psychiatry. 2014;75:424–40. doi: 10.4088/JCP.13r08558. [DOI] [PubMed] [Google Scholar]
- 50.Pillarella J, Higashi A, Alexander GC, et al. Trends in use of second-generation antipsychotics for treatment of bipolar disorder in the United States, 1998-2009. Psychiatr Serv. 2012;63:83–6. doi: 10.1176/appi.ps.201100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davidson JR. Major depressive disorder treatment guidelines in America and Europe. J Clin Psychiatry. 2010;71 doi: 10.4088/JCP.9058se1c.04gry. e04. [DOI] [PubMed] [Google Scholar]
- 52.Hennings JM, Schaaf L, Fulda S. Glucose metabolism and antidepressant medication. Curr Pharm Des. 2012;18:5900–19. doi: 10.2174/138161212803523662. [DOI] [PubMed] [Google Scholar]
- 53.Mojtabai R. Antidepressant use and glycemic control. Psychopharmacology. 2013;227:467–77. doi: 10.1007/s00213-013-2972-5. [DOI] [PubMed] [Google Scholar]
- 54.Correl CU, Detraux J, De Lepeleire J, et al. Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in patients with schizophrenia, depression and bipolar disorder. World Psychiatry. 2015;14:119–36. doi: 10.1002/wps.20204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lamers F, Vogelzangs N, Merikangas KR, et al. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol Psychiatry. 2013;18:692–9. doi: 10.1038/mp.2012.144. [DOI] [PubMed] [Google Scholar]
- 56.Nousen EK, Franco JG, Sullivan EL. Unraveling the mechanisms responsible for the comorbidity between metabolic syndrome and mental health disorders. Neuroendocrinology. 2013;98:254–66. doi: 10.1159/000355632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Manu P, Correll CU, Wampers M, et al. Markers of inflammation in schizophrenia: association vs. causation. World Psychiatry. 2014;13:189–92. doi: 10.1002/wps.20117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rethorst CD, Bernstein I, Trivedi MH. Inflammation, obesity, and metabolic syndrome in depression: analysis of the 2009-2010 National Health and Nutrition Examination Survey (NHANES) J Clin Psychiatry. 2014;75:1428–32. doi: 10.4088/JCP.14m09009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Correll CU, Joffe BI, Rosen LM, et al. Cardiovascular and cerebrovascular risk factors and events associated with second-generation antipsychotic compared to antidepressant use in a non-elderly adult sample: results from a claims-based inception cohort study. World Psychiatry. 2015;14:55–62. doi: 10.1002/wps.20187. [DOI] [PMC free article] [PubMed] [Google Scholar]


