Abstract
Objective
Characterize the onset and timing of cognitive decline in PD from the first recognizable stage of cognitively symptomatic PD - mild cognitive impairment (PD-MCI) - to PD dementia (PDD). Thirty-nine participants progressed from PD to PDD and 25 remained cognitively normal.
Method
Bayesian estimated disease-state models described the onset of an individual’s cognitive decline across 12 subtests with a changepoint.
Results
Subtests measuring working memory, visual imagery, and crystalized memory changed significantly 3–5 years before their first non-zero CDR and progressively worsened from PD to PD-MCI to PDD. Crystalized memory deficits were the hallmark feature of imminent conversion of cognitive status. Episodic memory tasks were not sensitive to onset of PD-MCI. For cognitively intact PD, all 12 subtests showed modest linear decline without evidence of a changepoint.
Conclusions
Longitudinal disease-state models support a prodromal dementia stage (PD-MCI) marked by early declines in working memory and visuospatial processing beginning 5 years prior to clinical diagnosis of PDD. Cognitive declines in PD affect motor ability (bradykinesia), working memory and processing speed (bradyphrenia) resulting in PD-MCI where visuospatial imagery and memory retrieval deficits manifest before eventual development of overt dementia. Tests of episodic memory may not be sufficient to detect and quantify cognitive decline in PD.
Keywords: Alzheimer’s disease, Parkinson’s disease with dementia, Parkinson’s disease/Parkinsonism, Longitudinal, Piecewise regression, Bayes Theorem
INTRODUCTION
Clinical research in Parkinson’s disease (PD) has demonstrated that a majority of the patient population with PD will develop an associated dementia marked by declines in episodic and working memory, visuospatial processing, executive functioning, and functional abilities.1–13 Building on existing consensus criteria14–19 a taskforce sponsored by Movement Disorders Society20,21 has established diagnostic criteria for an early stage cognitive impairment associated with PD (PDD) coined Mild Cognitive Impairment (PD-MCI). Analogous to criteria for MCI in Alzheimer disease, PD-MCI connotes the earliest clinically recognizable stage of PDD - a prodrome of the dementia state where symptoms first appear eventually worsening over time.22 Identification of a prodromal phase is important so that interventions can be administered at a time when they are most effective, before the disease progresses and damage becomes widespread.23 The establishment of the task force and potential standardization of the PD-MCI has initiated debate about the composition of this prodromal stage. For instance, are there specific domains that are early milestones in the progression of the dementia? What are the cognitive and functional domains that clinicians need to assess to detect the dementia as its symptoms first appear? What extent of functional impairment is needed to distinguish PD-MCI from PDD? Since criteria for PD-MCI were published a discussion has developed regarding subtest sensitivities,24,25,26 cutpoints that should be applied,25,27,26 and whether or not there exists a single domain PD-MCI.24 The burgeoning literature suggests that extensive psychometric work will need to be conducted by many centers.
In a well-controlled longitudinal study, we previously demonstrated that cognitive ability declined in PD several years prior to detection of any overt neurocognitive or functional symptoms.12 A visuospatial composite score based on factor analysis was the most potent harbinger of a subsequent dementia diagnosis, occurring about 3 years prior on average. However a similar decline on the visuospatial factor was also witnessed in Alzheimer’s disease28,29 (although not as pronounced or as early) and declining scores were also prominent in PD without dementia12 (PDND). Visuospatial processing ability was a sensitive indicator but lacked specificity. The previous study used factor scores that were derived from large patient samples with and without Alzheimer’s dementia, applied to patients with PD. The result was an accurate test of PD cognitive performance relative to Alzheimer’s disease-specific indices, not specific to PD cognitive decline.
Since our first report we have developed more sensitive analytic techniques to measure disease transition that index profiles of neuropsychological performance of patients transitioning from cognitive intact PD to cognitive symptomatic PD, permitting characterization of a PD-MCI stage. In this study, we applied a novel analytic perspective on the diagnostic canonization of PD-MCI using a Bayesian estimated disease-state model (multiphase random changepoint model; MRC) 30–32 to determine the onset of decline on an individual-by-individual basis yielding precise estimates of disease onset. Disease state models describe the longitudinal trajectory of scores with a changepoint. Before the changepoint, cognition tends to be relatively stable. After the changepoint, cognitive begins an accelerating decline. Thus the longitudinal trajectories of cognitively normal individuals who eventually develop dementia start shallow and then decline precipitously. The placement of the changepoint marks the onset of a disease process. By using individual specific changepoints we are better able to capture disease progression by allowing the individual’s performance to vary over time due to multiple disease processes. These models are flexible enough to estimate the state of disease progression on an individual-by-individual basis. This report extends our previous work28,29 that compared the declining cognitive trajectories in PD to AD that showed PDD follows similar overall trajectories but starts in a different mix of subtests than does AD. Here we examine cognitive profiles of PD patients followed for more than 7 years on average to characterize the onset and timing of declining cognitive performance. We hypothesized that cognitive decline from PD to PDD passes through a definable phase of mild cognitive impairment (PD-MCI) with the greatest decline in visuospatial, working memory and perceptual speed domains and that examination of specific subtests with a model based on individual differences for disease onset would reveal earlier deficits.
METHODS
Participants
Archival data were examined two groups of volunteers enrolled in a longitudinal study of healthy aging and dementia at the Knight Alzheimer Disease Center at Washington University. The first group remained cognitive intact in all assessments (PDND, n=25). The second group developed PDD (n=39). All participants were recruited either from (1) a project to examine clinical outcomes in PD8 or (2) were originally enrolled as nondemented controls and later developed PD. For this later group, only those times-of-assessment where PD was diagnosed were included. Thus PD patients reported here are a subset of a larger clinical cohort reported previously1,2,4,5,12 with the important distinction of having been assessed at least 3 times so changepoint models have sufficient repeated measures data to be estimated. Thus all PD participants enrolled without cognitive impairment (i.e., CDR 0); and there were sufficient number of repeated measures to estimate MRC change points. Disorders that can result in other types of dementia (e.g., cerebrovascular disease, frontal lobe syndromes) were excluded. Medication of PD symptoms was managed outside of the current study, but by study end all PD participants (PDD and PDND) were placed on dopaminergic agonists. All participants spoke English and lived in the greater St. Louis metropolitan area. The Washington University Human Studies Committee approved all procedures.
Clinical evaluation
Detailed information about the clinical assessment and the diagnostic criteria for PD was based on United Kingdom PD criteria.33 The diagnosis of PDD was based on DSM-IV criteria and a Clinical Dementia Rating (CDR34) ≥ 0.5, suggesting earliest recognizable stage of cognitive impairment. None of the PDND participants received a diagnosis of dementia or a CDR > 0 at any time of assessment.
Standardized assessments of PD such as Hoehn and Yahr35 stages or Unified Parkinson Disease Rating Scale (UPDRS) motor scores36 were not available for all cases. Therefore, in order to control for bradykinesia, we used Crossing Off37, a psychometric assessment of simple motor speed, as a gross index of motor slowing across all groups. Although not diagnostic of PD, Crossing Off accurately reflects motor slowing associated with PD (Table 1) and was used here as a psychometric (not clinical) control variable.
Table 1.
Demographic Means at the first measurement time (SD)
| Stable (n=25) | Progressed (n=39) | |
|---|---|---|
| Age (yrs) | 69.3 (7.4) | 71.6 (7.5) * |
| Education (yrs) | 13.6 (2.7) | 15.4 (3.2) † |
| Gender (% men) | 80 | 74.4 * |
| Crossing Off a (Simple Motor Speed) | 141.8 (30.1) | 142.5 (33.1) |
| Cognitive Battery | ||
| Logical Memory | 7.2 (2.8) | 7.0 (2.6) |
| Information | 21.2 (4.7) | 20.4 (3.9) |
| Boston Naming | 54.3 (4.9) | 55.2 (3.3) |
| Associate Learning | 12.9 (3.2) | 13.0 (3.9) |
| Trailmaking A (sec)b | 51.9 (23.2) | 59.6 (28.9) |
| Block Design | 29.7 (9.0) | 28.2 (8.9) |
| Benton (copy) | 5.6 (1.3) | 5.5 (1.8) |
| Digit Symbol | 41.0 (10.9) | 38.8 (11.0) |
| Mental Control | 7.2 (1.6) | 7.3 (1.7) |
| Digits Forward | 6.8 (1.2) | 6.8 (1.2) |
| Digits Backward | 4.9 (1.4) | 4.6 (1.3) |
| Letter Fluency (S & P) | 26.5 (9.9) | 28.1 (10.7) |
Note: All cognitive tests equivalent at baseline testing
The 2 patient groups differ at p < .05; degrees of freedom (df) vary slightly due to missing data (median df = 63; no difference > 3)
Trend for difference (t =1.84; p = .07)
Score is the reciprocal of the number of seconds to complete multiplied by 100; higher scores indicate better performance
Higher scores indicate poorer performance
Neuropsychological Assessment
This psychometric battery assessed a broad spectrum of abilities (i.e., semantic memory, episodic memory, working memory, and visuospatial ability) across multiple cognitive domains and previously described in detail29. It was administered annually to all participants approximately 2 weeks after clinical evaluation. Psychometricians were not informed of the results of the clinical evaluation, nor did clinicians use the psychometric data to determine diagnosis or CDR. We used a psychometric strategy that put all patients’ test scores on a common (comparable) metric relative to their first time-of-assessment. All scores were standardized (M=0, SD=1) to the patients’ first time of assessment (best performance available) and Trailmaking A was reversed scored so that a high score on all variables indicated good performance. These standard scores were also residualized for age, education, and motor speed (Crossing-Off37) thus controlling for these common covariates. Subtests included: Digit Symbol Substitution,38 Block Design, 38 Benton Visual Retention Test – Copy (BVRT-Copy), 39 Letter Fluency (S&P),40 Mental Control, 38 Digit Span Forward & Backward, 41 Information,38 Paired Associate Learning,38 Boston Naming,42 and Logical Memory.38
Multiphase Random Changepoint (MRC) Model
We applied a Bayesian estimated MRC model to the longitudinal summary scores of cognitive abilities. This longitudinal model is a 2-segmented continuous regression that captured the trajectory of a clinical index before and after a disease-state change has occurred. MRC used a subject specific changepoint, thus allowing for individual differences in how dementia onsets.43 Estimation was achieved using MCMC to sample from the posterior distributions; repeated samples from the conditional distributions drawn for each parameter using the Gibbs sampling algorithm in JAGS44 and the R statistical platform.45 Simulation studies show that these Bayesian estimation procedures yield highly reliable fixed-effects and Level-1 variance parameters in the MRC model.46 We used the Bayesian estimates of the MRC posterior means for intercepts, slopes, and change-points of pre and post-clinical PDD stages to characterize individual trajectories of scores relative to disease activity (Figure 1). Estimates of longitudinal change were not based on time of diagnosis or absolute time of assessment, but rather time of assessment relative to disease activity. Groups were combined and all raw data aligned at the first non-zero CDR for PDD and at the last available measurement for PDND. Credible intervals (Bayesian equivalent of a confidence interval) for the pre and post slopes served as criteria to accept/reject the changepoint estimate as significant and thus clinically meaningful. Only when distributions’ of the slope (β1) in the first phase and the slope in the second phase (β2) did not contain one another (i.e., fell outside of each other’s 95% credible intervals) was a changepoint considered to be significant.
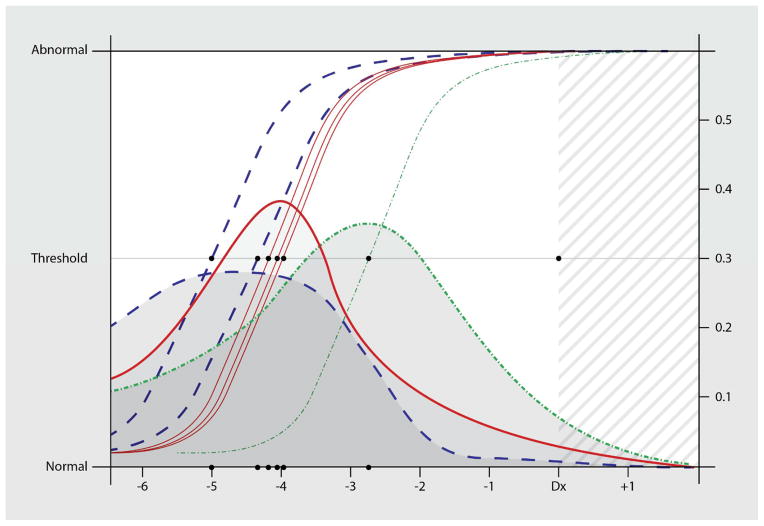
Figure 1.
Logistic curves (left-side Y-axis scale) represent the predicted clinical thresholds for decline on each of the subtests sensitive to disease onset. These thresholds are the models’ average departure from the stable state to the active disease state in participants who eventually develop PDD. They are derived from the probability densities (right-side Y-axis scale) which represent the cumulative distributions of these models’ estimated time-of- onset for the active disease state (changepoint). In this figure we have convolved the subtests’ distributions to indicate the 3 proposed epochs. These epochs are based on our previous longitudinal factor analysis using this sample. Standard errors reflect the sensitivity/specificity of the subtests to detect change (slope of the logistic curves measured at the threshold).
-
(a)Digit Span - Backwards Subtest of the WMS
-
(b)Mental Control Subtest of the WMS
-
(c)Digit Symbol Substitution Subtest of the WMS
-
(d)Block Design Subtest of the WAIS
-
(e)Benton Visual Retention Test – Copy
-
(f)Information Subtest of the WMS
- α, β1, β2 ~ N (0,1000)
- δi ~ N(μi, τi)
- μi ~ N (0,1000) < 0
- τi ~ Gamma (0.001,0.001)
RESULTS
Demographic information (Table 1) and performance on neuropsychological testing for PDND (n = 25) and PDD (n = 39) groups from their first assessment (all cognitively normal; CDR 0) indicates that the PDD group (mean age= 71.5±7.5 yrs) was older at baseline than the PDND group (mean age= 69.3±7.4 yrs). The PDD group performed significantly worse on all cognitive subtests at their first assessment (all t > 2.29, p < .05). The 2 groups were statically equivalent for level of education (t = 1.84, p = .07); however, PDD trended higher. This trend is likely an artifact of the sampling process. The original memory and aging program where this patient population was drawn is highly educated (Range 12 to 24 years), seeks out medical care, and volunteers. Thus they are not typical of epidemiological sampled populations. Both the PDND and PDD groups were predominantly male (75%). The average number of years for participant follow up was 6.4 (range = 4–19) in the PDND group and 7.2 years (range = 4–14) in the PDD group.
This MRC estimated subject-specific intercepts, preclinical slopes, changepoints, and post-changepoint slopes for both the stable and progressed groups together (Table 2). Of the 12 subtests examined, 6 showed meaningful prodromal changepoints. For the PDD group, all 6 changepoints reached a threshold for significance, occurring 2.8 to 5.2 years before the first non-zero CDR. Subtests included Digit Span Backward, Block Design, BVRT-Copy, Mental Control, Digit Symbol, and Information. In contrast, all 6 of these subtests showed linear decline in the PDND group because all slopes (first and second phase) fell within each other’s credible intervals.
Table 2.
Estimated changepoints (standard error) for each subtest by patient group
| Parkinson’s Dementia | |||||
|---|---|---|---|---|---|
| Intercept (α) | Slope in Pre(β1) | Slope in Post (β2) | Predicted Change(δ) Relative to Diagnosis | CDR Sum of Boxes at Time of Assessment | |
| Epoch I – Working memory | |||||
| Digit Span - Backwards* | 0.35 (0.15) | 0.01 (0.02) | −0.09 (0.02) | −5.15 (1.79) | 3 |
| Mental Control* | 0.11 (0.14) | 0.02 (0.02) | −0.09 (0.03) | −4.45 (2.06) | 4 |
| Epoch II – Visuospatial | |||||
| Digit Symbol* | −0.57 (0.22) | −0.05 (0.03) | −0.15 (0.02) | −4.33 (1.31) | 4 |
| Block Design* | 0.17 (0.14) | 0.01 (0.02) | −0.16 (0.02) | −4.32 (1.13) | 4 |
| BVRT - Copy* | 0.02 (0.20) | 0.00 (0.03) | −0.14 (0.03) | −4.15 (1.67) | 4 |
| Epoch III – Semantic Memory | |||||
| Information* | 0.17 (0.09) | 0.04 (0.01) | −0.07 (0.02) | −2.83 (1.31) | 6 |
| Non-Significant Subtests | |||||
| Logical Memory | −0.05 (0.15) | 0.08 (0.05) | −0.04 (0.02) | −6.65 (1.91) | |
| Associate Learning | 0.07 (0.16) | 0.03 (0.02) | −0.06 (0.02) | −4.99 (1.97) | |
| Boston Naming | 0.35 (0.23) | −0.03 (0.05) | −0.07 (0.02) | −5.71 (2.85) | |
| Trailmaking A | 50.52 (8.22) | 1.07 (1.58) | 2.28 (0.70) | −5.82 (2.85) | |
| Digits Forward | 0.43 (0.15) | 0.00 (0.04) | −0.06 (0.02) | −4.87 (2.67) | |
| Word Fluency | 0.04 (0.39) | −0.12 (0.04) | −0.08 (0.03) | −3.86 (3.25) | |
| Parkinson’s No Dementia | |||||
| Intercept (α) | Slope in Pre(B1) | Slope in Post (B2) | Predicted Change(δ ) Relative to Last Assessment | ||
| Digit Span - Backwards | −0.06 (0.16) | −0.07 (0.02) | −0.27 (98.17) | −0.63 (0.96) | |
| Mental Control | −0.28 (0.15) | −0.07 (0.02) | −0.27 (98.38) | −0.59 (0.78) | |
| Digit Symbol | −1.29 (0.22) | −0.19 (0.02) | 0.23 (98.20) | −0.61 (0.83) | |
| Block Design | −0.42 (0.13) | −0.08 (0.02) | −0.38 (98.83) | −0.55 (0.59) | |
| BVRT-Copy | 5.05 (0.22) | −0.12 (0.03) | 2.09 (93.65) | −0.72 (0.90) | |
| Information | −0.16 (0.16) | −0.06 (0.02) | 0.15 (98.82) | −0.62 (0.93) | |
| Logical Memory | −0.22 (0.12) | −0.02 (0.02) | −0.27 (99.27) | −0.55 (0.58) | |
| Associate Learning | −0.32 (0.15) | −0.05 (0.021) | −0.41 (98.84) | −0.56 (0.61) | |
| Boston Naming | −0.25 (0.14) | −0.05 (0.02) | 0.44 (98.84) | −0.56 (0.59) | |
| Trailmaking A | 58.67 (5.61) | 1.78 (0.65) | −0.68 (85.48) | −1.38 (2.00) | |
| Digits Forward | −0.06 (0.12) | −0.08 (0.02) | 0.44 (98.81) | −0.55 (0.58) | |
| Word Fluency | −0.54 (0.16) | −0.09 (0.02) | 0.39 (98.21) | −0.59 (0.83) | |
Indicates a significant difference between Phase 1 and Phase 2
During the progression of the PDD group, a domain-by-domain onset of declining performance in 6 cognitive tests marking a distinct prodromal phase. First were declines in Fluid Intelligence tasks demanding Working Memory and Visuospatial Processing ability. These declines persisted when controlling for simple motor speed (Crossing-Off37). The PDD group then declined on a prototypical crystallized intelligence task – the Information subtest of the WAIS.38 This pattern of decline was similar but not identical to our previous findings from a longitudinal factor analysis of PD with and without dementia. The previous research created composite scores of multiple subtests to index change and found that Visuospatial Processing ability declined 2-years on average before Working Memory declined (both 1–3 years before diagnosis). In contrast here we examined the change profiles of individual subtests (some of which were not sensitive to PD and its progression to PDD) and found that Working Memory tasks preceded Visuospatial tasks. However, when the overlap in credible intervals is examined, the 2-domains are statistically indistinguishable suggesting a continuous disease process that affects both Working Memory and Visuospatial Processing. Note that what is absent in these analyses is a definable changepoint in any of the traditional tasks for episodic memory – Logical Memory and Paired Associate Learning. Inspection of these changepoints and slopes reveal that subtests are declining linearly and are not specific to any preclinical epoch, nor changing till very near the eventual PDD diagnosis. This suggests that classic tests of episodic memory may not be sufficient to detect and quantify cognitive decline in PD. In contrast these data indicate that a decline in accessing crystalized information (Information subtest) is a harbinger of diagnosis, and may represent the accumulation of Lewy-related pathology (Lewy bodies and/or Lewy neurites) in cortical and subcortical regions responsible for memory retrieval and mental flexibility. As bradyphrenia is a common symptom associated with PD, a test such as Information could be used to predict which PD patient is likely to go on to MCI and subsequent dementia.
Discussion
Our analyses allow us to characterize the longitudinal transition in cognitive decline from PD to PDD. We found 6 of 12 tasks revealed significant changepoints during the longitudinal transition that many represent the PD-MCI stage. An initial phase showed very shallow or no decline later followed by a cognitively symptomatic state marked by a significant acceleration of declining test performance. In contrast there was no indication of cognitive change in any of these 12 subtests for the PDND sample. This is strong evidence for the existence of a transitional period of PD-MCI before overt PDD where early decline in visuospatial processing and working memory are commonly seen in PD followed by deficits in crystalized memory before the eventual transition to dementia. Further, these changes are occurring up to 5 years prior to diagnosis of cognitively symptomatic PD.
The onset and time course of these data also show that PD-MCI is made up of 3 disease-specific epochs, the first beginning approximately 5 years prior to clinical diagnosis of PDD. Transition of PD to PD-MCI first begins with mild deficits in Working Memory (Digit Span – Backward, Mental Control,38). This is consistent with clinical observation and patient reports of slowed thinking and processing (bradyphrenia).47,48 In the next 1–2 years, individuals transitioning to PD-MCI experience difficulties in visual imagery in problem solving. The visuospatial deficits appear to be focal and severe because they are affecting a wide range of tests on this cognitive battery in a very short epoch of disease progression (Block Design,38 Benton Visual Retention,39 and Digit Symbol Substitution38). In our previous report longitudinal indices of visuospatial ability were the strongest indicators of change in disease status in this battery of tests. The combined deficits of working memory and visuospatial abilities make up a core feature of PD-MCI and is discernable in the longitudinal decline described here using disease state models. Finally, PD-MCI culminates in deficits in retrieval from long term memory changing about 3 years prior to overt PDD. From this pattern, we posit that cognitive change in PD proceeds through problems with mental flexibility followed by visual-spatial imagery followed by retrieval of well-learned knowledge finally followed by the overt dementia state with trouble learning new information (i.e. episodic memory deficits) becoming a prominent feature and suggests a continuous, rather than dichotomous process.
As a function of time, progressive declines in cognitive, motor, function, mood and behavior may cloud the ability to tease out individual and cumulative cognitive domains affected in PD.8 This is consistent with the findings of a data reduction study24 that found cognitive testing of PDD resulted in a complex profile of domains affected and other studies that find complex profiles of poor performance in PD-MCI25–27 By result the cumulative effect of domain-by-domain cognitive declines becomes a marker of the time in PDD-specific cognitive decline. Moreover these declines are accumulating quickly (within 1–2 years) and early changes in CDR staging (see Sum of Boxes in Table 2) need to trigger for more intensive testing. Thus staging may only be possible if repeated cognitive measures are applied within months and not years of each other. A very early Working Memory-only decrement precedes Working Memory + Visuospatial decrement which precedes the cumulative Working Memory + Visuospatial + Long Term Memory decrement that heralds clinical diagnosis of overt PDD. Use of an episodic memory task (e.g. list learning, paragraph recall, or associate learning) will likely miss this early decline and falsely classify the individual as cognitively intact when in fact cognitive impairment is proceeding on a downward course.
MRC and related disease state models provide us a powerful subject-specific temporal segmentation of the pre and post-clinical stages of a progressive disease. In the current report they have allowed us to probe theories of disease onset and the relative time course of multiple symptom domains49,50 and to understand the phenotype of early stages of cognitively symptomatic PD. MRC provide a strong alternative to modelling primary progressive disease because they do not rely on normative based performance indices that obscure the progressive course of disease. Further, frequent co-morbidities in this population may impact cognitive and functional abilities in ways that cannot be taken into account using normative data for age, gender and education. When disease-related changes are modeled at the level of the individual, resultant indices reveal a pattern of symptom onset, frequency, severity, and duration that describe the progression of no impairment to MCI to dementia in PD. The application of subtest cutscores may be useful as threshold markers for each cognitive domain sampled; however, the overall profile of decline best characterizes PDD.
These findings have several limitations. We would have preferred to use a more sensitive test of motor slowing than Crossing Off as a statistical control in our analyses. Motor slowing was likely affecting the progressed group’s subtest performance across the battery at a disproportionately higher rate. Better measures (e.g., UPDRS-3 motor scores) would likely yield a better estimation of the effect of motor slowing on cognition. In spite of its limitations, Crossing Off provided a useful measure of motor speed with relatively little floor effect. This particular neuropsychological battery lacked more sensitive measures of executive functioning and working memory. The addition of other executive tests (i.e. Wisconsin Card Sort) 51 and selective attention or inhibition (i.e. Stroop)52 may increase sensitivity to detect hypothesized decrements in executive functioning in PD-MCI and PDD not examined in this study.
The next generation of test strategies needs to explore the component processes of PDD decline across multiple domains. Now that cognitive domains sensitive to PDD-related decline have been established, next generation testing needs to apply multiples of these types of tests to understand the nature of the domain-specific declines and gain better sensitivity and specificity for early PDD declines and test explicitly the hypothesis that cognition declines cumulatively in PDD. It also seems critical to understand in PDD as visuospatial dominant disability as found here and elsewhere.53–56 For instance, the visuospatial subtests used here are often thought of as tests of executive function in classical testing theory (e.g., Digit Symbol and Trailmaking-B) but most of them are also speeded. From a components processes approach these neuropsychological measures are multimodal made up visuospatial processing (common to all), executive functioning, and speed-of-processing. Our data supports the evolving hypothesis that PD is frequently a dementing illness that has at its core a long phase of extrapyramidal motor impairment (bradykinesia) followed by decrements in working memory and processing speed (bradyphrenia) passing through a PD-MCI stage of visuospatial imagery and memory retrieval deficits before the development of overt dementia.
Acknowledgments
Data collection was supported by National Institute on Aging grants P01 AG03991 and P50 AG05681 (JCM). Data analyses was supported by grants from the National Institute on Aging R01 AG040211, Michael J Fox Foundation, and Morris and Alma Schapiro Fund (JEG)
Footnotes
Disclosure: The authors report no conflicts of interest.
- Research project: A. Conception, B. Organization, C. Execution;
- Statistical Analysis: A. Design, B. Execution, C. Review and Critique;
- Manuscript: A. Writing of the first draft, B. Review and Critique.
| David K. Johnson: | 1A 1B 1C 2A 2C 3A 3B |
| Zachary Langford: | 1A 2A 2C 3A |
| Mauricio Garnier-Villarreal: | 1C 2B 2C |
| John C. Morris: | 2C 3B |
| James E. Galvin: | 1A 1B 1C 2C 3A 3B |
References
- 1.Goldman WP, Baty JD, Buckles VD, Sahrmann S, Morris JC. Cognitive and motor functioning in Parkinson disease: subjects with and without questionable dementia. Arch Neurol. 1998;55(5):674–680. doi: 10.1001/archneur.55.5.674. [DOI] [PubMed] [Google Scholar]
- 2.Smith MC, Goldman WP, Janer KW, Baty JD, Morris JC. Cognitive speed in nondemented Parkinson’s disease. Journal of the International Neuropsychological Society : JINS. 1998;4(6):584–592. doi: 10.1017/s1355617798466074. [DOI] [PubMed] [Google Scholar]
- 3.Levy G, Jacobs DM, Tang MX, et al. Memory and executive function impairment predict dementia in Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2002;17(6):1221–1226. doi: 10.1002/mds.10280. [DOI] [PubMed] [Google Scholar]
- 4.Galvin JE. Cognitive change in Parkinson disease. Alzheimer Dis Assoc Disord. 2006;20(4):302–310. doi: 10.1097/01.wad.0000213858.27731.f8. [DOI] [PubMed] [Google Scholar]
- 5.Galvin JE, Pollack J, Morris JC. Clinical phenotype of Parkinson disease dementia. Neurology. 2006;67(9):1605–1611. doi: 10.1212/01.wnl.0000242630.52203.8f. [DOI] [PubMed] [Google Scholar]
- 6.Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Movement disorders : official journal of the Movement Disorder Society. 2008;23(6):837–844. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- 7.Muslimovic D, Post B, Speelman JD, Schmand B. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology. 2005;65(8):1239–1245. doi: 10.1212/01.wnl.0000180516.69442.95. [DOI] [PubMed] [Google Scholar]
- 8.Muslimovic D, Schmand B, Speelman JD, de Haan RJ. Course of cognitive decline in Parkinson’s disease: a meta-analysis. Journal of the International Neuropsychological Society : JINS. 2007;13(6):920–932. doi: 10.1017/S1355617707071160. [DOI] [PubMed] [Google Scholar]
- 9.Muslimovic D, Post B, Speelman JD, De Haan RJ, Schmand B. Cognitive decline in Parkinson’s disease: a prospective longitudinal study. Journal of the International Neuropsychological Society : JINS. 2009;15(3):426–437. doi: 10.1017/S1355617709090614. [DOI] [PubMed] [Google Scholar]
- 10.Kandiah N, Narasimhalu K, Lau PN, Seah SH, Au WL, Tan LC. Cognitive decline in early Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2009;24(4):605–608. doi: 10.1002/mds.22384. [DOI] [PubMed] [Google Scholar]
- 11.Reid WG, Hely MA, Morris JG, Loy C, Halliday GM. Dementia in Parkinson’s disease: a 20-year neuropsychological study (Sydney Multicentre Study) Journal of neurology, neurosurgery, and psychiatry. 2011;82(9):1033–1037. doi: 10.1136/jnnp.2010.232678. [DOI] [PubMed] [Google Scholar]
- 12.Johnson DK, Galvin JE. Longitudinal changes in cognition deficits in Parkinson’s disease with and without dementia. Dementia and Geriatric Cognitive Disorders. 2011;31(2):98–108. doi: 10.1159/000323570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Broeders M, de Bie RM, Velseboer DC, Speelman JD, Muslimovic D, Schmand B. Evolution of mild cognitive impairment in Parkinson disease. Neurology. 2013;81(4):346–352. doi: 10.1212/WNL.0b013e31829c5c86. [DOI] [PubMed] [Google Scholar]
- 14.Lippa CF, Duda JE, Grossman M, et al. DLB and PDD boundary issues: diagnosis, treatment, molecular pathology, and biomarkers. Neurology. 2007;68(11):812–819. doi: 10.1212/01.wnl.0000256715.13907.d3. [DOI] [PubMed] [Google Scholar]
- 15.Dubois B. Is PD-MCI a useful concept? Movement disorders : official journal of the Movement Disorder Society. 2007;22(9):1215–1216. doi: 10.1002/mds.21566. [DOI] [PubMed] [Google Scholar]
- 16.Dubois B, Burn D, Goetz C, et al. Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Movement disorders : official journal of the Movement Disorder Society. 2007;22(16):2314–2324. doi: 10.1002/mds.21844. [DOI] [PubMed] [Google Scholar]
- 17.Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2007;22(12):1689–1707. doi: 10.1002/mds.21507. quiz 1837. [DOI] [PubMed] [Google Scholar]
- 18.Kulisevsky J, Pagonabarraga J. Cognitive impairment in Parkinson’s disease: tools for diagnosis and assessment. Movement disorders : official journal of the Movement Disorder Society. 2009;24(8):1103–1110. doi: 10.1002/mds.22506. [DOI] [PubMed] [Google Scholar]
- 19.Marshall VL, Reininger CB, Marquardt M, et al. Parkinson’s disease is overdiagnosed clinically at baseline in diagnostically uncertain cases: a 3-year European multicenter study with repeat [123I]FP-CIT SPECT. Movement disorders : official journal of the Movement Disorder Society. 2009;24(4):500–508. doi: 10.1002/mds.22108. [DOI] [PubMed] [Google Scholar]
- 20.Litvan I, Goldman JG, Troster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Movement disorders : official journal of the Movement Disorder Society. 2012;27(3):349–356. doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geurtsen GJ, Hoogland J, Goldman JG, et al. Parkinson’s disease mild cognitive impairment: application and validation of the criteria. Journal of Parkinson’s disease. 2014;4(2):131–137. doi: 10.3233/JPD-130304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58(12):1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 23.Price JL, Ko AI, Wade MJ, Tsou SK, McKeel DW, Morris JC. Neuron Number in the Entorhinal Cortex and CA1 in Preclinical Alzheimer Disease. Arch Neurol. 2001;58(9):1395–1402. doi: 10.1001/archneur.58.9.1395. [DOI] [PubMed] [Google Scholar]
- 24.Cholerton BA, Zabetian CP, Wan JY, et al. Evaluation of mild cognitive impairment subtypes in Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2014;29(6):756–764. doi: 10.1002/mds.25875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biundo R, Weis L, Facchini S, et al. Cognitive profiling of Parkinson disease patients with mild cognitive impairment and dementia. Parkinsonism & related disorders. 2014;20(4):394–399. doi: 10.1016/j.parkreldis.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Barton BR, Bernard B, Czernecki V, et al. Comparison of the Movement Disorder Society Parkinson’s disease dementia criteria with neuropsychological testing. Movement disorders : official journal of the Movement Disorder Society. 2014 doi: 10.1002/mds.25902. [DOI] [PubMed] [Google Scholar]
- 27.Goldman JG, Holden S, Bernard B, Ouyang B, Goetz CG, Stebbins GT. Defining optimal cutoff scores for cognitive impairment using Movement Disorder Society Task Force criteria for mild cognitive impairment in Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2013;28(14):1972–1979. doi: 10.1002/mds.25655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson DK, Storandt M, Morris JC, Galvin JE. Longitudinal study of the transition from healthy aging to Alzheimer disease. Arch Neurol. 2009;66(10):1254–1259. doi: 10.1001/archneurol.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson DK, Storandt M, Morris JC, Langford ZD, Galvin JE. Cognitive profiles in dementia: Alzheimer disease vs nondemented aging. Neurology. 2008;71(22):1783–1789. doi: 10.1212/01.wnl.0000335972.35970.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein JP, Klotz JH, Grever MR. A biological marker model for predicting disease transitions. Biometrics. 1984;40(4):927–936. [PubMed] [Google Scholar]
- 31.Kiuchi AS, Hartigan JA, Holford TR, Rubinstein P, Stevens CE. Change points in the series of T4 counts prior to AIDS. Biometrics. 1995;51(1):236–248. [PubMed] [Google Scholar]
- 32.Slate EH, Turnbull BW. Statistical models for longitudinal biomarkers of disease onset. Statistics in Medicine. 2000;19(4):617–637. doi: 10.1002/(sici)1097-0258(20000229)19:4<617::aid-sim360>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 33.Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson’s disease: A clinicopathologic study. Neurology. 1992;42:1142–1146. doi: 10.1212/wnl.42.6.1142. [DOI] [PubMed] [Google Scholar]
- 34.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412b–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 35.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 36.Fahn S, Elton RL, Fahn S, Marsden CD, Goldstein M, Calne DB. Recent Developments in Parkinson’s Disease. Florham Park, NJ: Macmillan Healthcare Information; 1987. Unified Parkinson’s Disease Rating Scale; pp. 153–163. [Google Scholar]
- 37.Botwinick J, Storandt M. Speed functions, vocabulary ability, and age. Percept Mot Skills. 1973;36:1123–1128. doi: 10.2466/pms.1973.36.3c.1123. [DOI] [PubMed] [Google Scholar]
- 38.Wechsler D. Adminstration and scoring manual: Wechsler Adult Intelligence Scale. San Antonio, TX: Harcourt Brace; 1997. [Google Scholar]
- 39.Benton AL. The revised visual retention test: Clinical and experimental applications. New York: Psychological Corporation; 1963. [Google Scholar]
- 40.Thurstone LL, Thurstone LG. Examiner Manual for the SRA Primary Mental Abilities Test. Chicago: Science Research Associates; 1949. [Google Scholar]
- 41.Wechsler D. Administration and scoring manual: Wechsler Memory Scale. San Antonio, TX: Harcourt Brace; 1997. [Google Scholar]
- 42.Goodglass H, Kaplan E. Boston Naming Test scoring booklet. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 43.Cudeck R, Klebe KJ. Multiphase mixed-effects models for repeated measures data. Psychol Methods. 2002;7(1):41–63. doi: 10.1037/1082-989x.7.1.41. [DOI] [PubMed] [Google Scholar]
- 44.Plummer M, Plummer M. Just Another Gibbs Sampler: A program for analysis of Bayesian graphical models using Gibbs sampling. 2003. [Google Scholar]
- 45.R: A language and environment for statistical computing [computer program] Vienna, Austria: R Foundation for Statistical Computing; 2012. [Google Scholar]
- 46.Wang LJ, McArdle JJ. A simulation study comparison of Bayesian estimation with conventional methods for estimating unknown change points. Structural Equation Modeling-A Multidisciplinary Journal. 2008;15(1):52–74. [Google Scholar]
- 47.Duncombe ME, Bradshaw JL, Iansek R, Phillips JG. Parkinsonian patients without dementia or depression do not suffer from bradyphrenia as indexed by performance in mental rotation tasks with and without advance information. Neuropsychologia. 1994;32(11):1383–1396. doi: 10.1016/0028-3932(94)00071-9. [DOI] [PubMed] [Google Scholar]
- 48.Pate DS, Margolin DI. Cognitive slowing in Parkinson’s and Alzheimer’s patients: distinguishing bradyphrenia from dementia. Neurology. 1994;44(4):669–674. doi: 10.1212/wnl.44.4.669. [DOI] [PubMed] [Google Scholar]
- 49.Jack CR, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Troncoso JC, Cataldo M, Nixon RA, et al. Neuropathology of preclinical and clinical late-onset Alzheimer’s disease. Annals of Neurology. 1998;43:673–676. doi: 10.1002/ana.410430519. [DOI] [PubMed] [Google Scholar]
- 51.Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtis G. Wisconsin Card Sorting Test Manual: revised and expanded. Odessa, FL: Psychological Assessment Resources; 1993. [Google Scholar]
- 52.Stroop J. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- 53.Marinelli L, Crupi D, Di Rocco A, et al. Learning and consolidation of visuo-motor adaptation in Parkinson’s disease. Parkinsonism & related disorders. 2009;15(1):6–11. doi: 10.1016/j.parkreldis.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marinelli L, Perfetti B, Moisello C, et al. Increased reaction time predicts visual learning deficits in Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2010;25(10):1498–1501. doi: 10.1002/mds.23156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stewart T, Liu C, Ginghina C, et al. Cerebrospinal fluid alpha-synuclein predicts cognitive decline in Parkinson disease progression in the DATATOP cohort. The American journal of pathology. 2014;184(4):966–975. doi: 10.1016/j.ajpath.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yarnall AJ, Breen DP, Duncan GW, et al. Characterizing mild cognitive impairment in incident Parkinson disease: the ICICLE-PD study. Neurology. 2014;82(4):308–316. doi: 10.1212/WNL.0000000000000066. [DOI] [PMC free article] [PubMed] [Google Scholar]