Abstract
The brain possesses two intricate mechanisms that fulfill its continuous metabolic needs: cerebral autoregulation, which ensures constant cerebral blood flow over a wide range of arterial pressures and functional hyperemia, which ensures rapid delivery of oxygen and glucose to active neurons. Over the past decade, a number of important studies have identified astrocytes as key intermediaries in neurovascular coupling (NVC), the mechanism by which active neurons signal blood vessels to change their diameter. Activity-dependent increases in astrocytic Ca2+ activity are thought to contribute to the release of vasoactive substances that facilitate arteriole vasodilation. A number of vasoactive signals have been identified and their role on vessel caliber assessed both in vitro and in vivo. In this review, we discuss mechanisms implicating astrocytes in NVC-mediated vascular responses, limitations encountered as a result of the challenges in maintaining all the constituents of the neurovascular unit intact and deliberate current controversial findings disputing a main role for astrocytes in NVC. Finally, we briefly discuss the potential role of pericytes and microglia in NVC-mediated processes.
INTRODUCTION
Given limited energy reserves, the brain possesses two intricate mechanisms that fulfill its continuous metabolic needs: cerebral autoregulation (CA) which ensures constant cerebral blood flow (CBF) over a wide range of arterial pressures (60–150 mmHg) and functional hyperemia (FH) which ensures rapid delivery of oxygen and glucose to active neurons (Iadecola and Nedergaard, 2007). Both of these signaling pathways are orchestrated by mechanisms intrinsic to the cerebral vasculature (e.g. myogenic responses and endothelial-mediated signaling) as well as via cell-to-cell communication modalities among vascular and non-vascular cells. The cellular processes underlying cell-to-cell communication leading to changes in vascular diameter are referred to as neurovascular coupling (NVC). The focus on this review is to highlight the role of astrocytes in NVC-mediated signaling, without de-emphasizing the importance of direct neuronal-to-vessel communication (Yang et al., 2000, Hamel, 2004, Drake and Iadecola, 2007, Attwell et al., 2010). In addition, we also discuss current controversies in the field addressing a relevant role for astrocytes during in vivo neuronal stimulation.
According to our current understanding of brain microanatomy, the neurovascular unit (NVU) is comprised of vascular cells (endothelial cells, pericytes and vascular smooth muscle cells (VSMCs)), neuronal terminals or varicosities and astrocytes. Additionally, we include microglia cells in the NVU as these cells play an important role in surveilling neuronal function and blood vessels thereby contributing to brain homeostasis. The orchestrated communication between these various cell types contributes to vascular-related processes such as angiogenesis and blood brain barrier (BBB) formation/maintenance as well as the regulation of CBF. A number of structural components facilitate cell-to-cell interactions within the NVU including, gap junctions (Simard et al., 2003, Figueroa and Duling, 2009), anchoring proteins (e.g. integrins (del Zoppo and Milner)) and specialized ion channels [e.g. aquaporin 4, K+ channels, transient receptor potential (TRP) channels (Brayden et al., 2008)] expressed on cell-cell interface membranes.
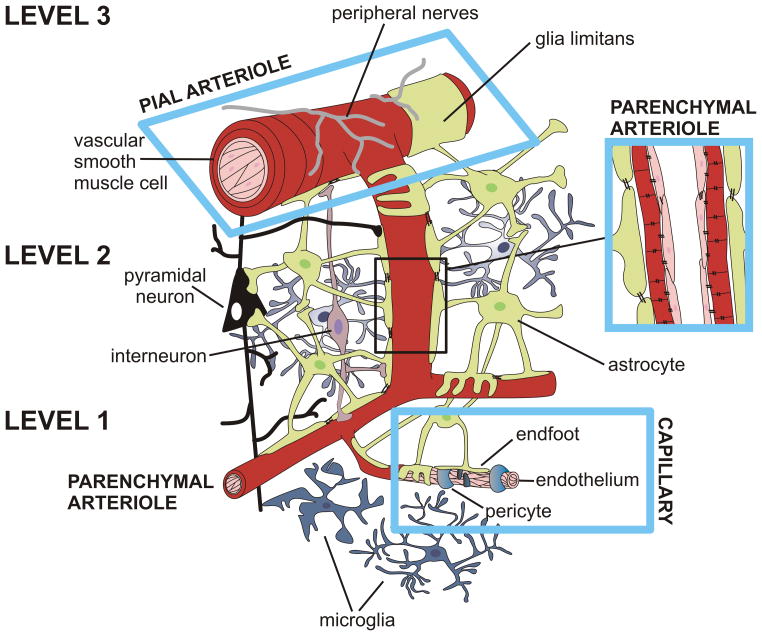
It is of critical importance to emphasize that brain perfusion takes place through the integration of information at various control points. A simplified illustration of these control points, represented as levels 1–3, is shown in Fig 1. Since a major goal in the field is to understand how neuronal activity initiates the delivery of oxygen and glucose to the neuropil, we will start our description at level 1, the capillary level. At this site, the main constituents of the NVU include the capillary constituents (endothelial cells and pericytes) as well as astrocytes and neurons. Given that neurons lie in close proximity to capillaries (8–20 μm) (Schlageter et al., 1999), level 1 could be considered the initiation site for NVC-mediated responses. While the endothelium continues throughout all levels, other constituents of the NVU change upstream of level 1. At level 2, a single layer of VSMCs now forms parenchymal arterioles, which are surrounded by some pericytes and processes from both astrocytes and neurons. Moving upstream to level 3, the endothelium is surrounded by multiple layers of VSMCs making up pial arterioles; a layer of specialized astrocyte processes termed the glia limitans and perivascular nerve endings are also evident. From this illustration, it is clear that the components of the NVU are not homogeneous across the cerebrovascular tree. At various levels, not only are the neurovascular innervations different but the cellular constituents (e.g. ion channel expression) are also region specific. For example, small and intermediate conductance Ca2+ activated K+ channels are differentially expressed in pial vs. parenchymal arterioles (Cipolla et al., 2009). Consequently, studying the function of these levels in isolation requires careful consideration because experiments conducted in different levels often yield confounding results.
Figure 1.
Representative illustration of the constituents of the neurovascular unit (NVU) from the capillary level to upstream pial arterioles. The diagram illustrates various control points across the cerebrovascular tree, involved in the integration of information that results in the coordinated delivery of oxygen and glucose to the brain under basal conditions and in response to increased metabolic demands. A simplified illustration of these control points, represented as levels 1–3, is shown in Fig 1. At the capillary level (level 1), the main constituents of the NVU include: endothelial cells, pericytes, astrocytes and neurons; level 1 represents the initiation site for neurovascular coupling-mediated responses. Of note, the endothelium continues throughout all levels (1–3). At level 2, the main constituents of the NVU include: a single layer of vascular smooth muscle cells (VSMCs) making up parenchymal arterioles, pericytes (not illustrated) and processes from both astrocytes and neurons. At level 3, the endothelium is surrounded by multiple layers of VSMCs making up pial arterioles, specialized astrocyte processes making up the glia limitans, and perivascular nerve endings originating from peripheral ganglia.
As shown in Fig 1, both extracerebral and intracerebral arteries/arterioles constitute the cerebral circulation. Branches of large extracerebral arteries give rise to pial arterioles which surround the surface of the brain. As these vessels divide further, they give rise to penetrating or parenchymal arterioles which enter the brain parenchyma and continue to divide giving rise to capillary networks and then venules (Edvinsson and MacKenzie, 2002). Neurovascular control of extracerebral arteries is well characterized, as described in these noted reviews (Bleys and Cowen, 2001, Gulbenkian et al., 2001, Hamel, 2006). On the other hand, the neurovascular control of parenchymal arterioles is primarily mediated by local interneurons and neuronal terminals from subcortical origins (e.g. basal forebrain, raphe and locus coeruleus) (Iadecola, 1998, Gulbenkian et al., 2001, Hamel, 2006) as well as glial cells (Goadsby and Edvinson, 2002). In the following sections we discuss current evidence for NVC-mediated responses and, whenever possible, define whether the observations were made in vitro or in vivo depending on the approach used.
Neurovascular coupling and astrocytes
A number of studies support the idea, but not without controversy, that astrocytes participate in the regulation of cerebrovascular tone (Gordon et al., 2007, Iadecola and Nedergaard, 2007, Jakovcevic and Harder, 2007, Attwell et al., 2010, Carmignoto and Gomez-Gonzalo, 2010). While it is well established that astrocytes contribute to cerebrovascular vasodilation during NVC, astrocytic signaling has also been associated with cerebrovascular vasoconstriction raising additional questions about the complex communication modalities within the neurovascular unit (NVU) and the precise pathways defining NVC-mediated vasodilatory vs. vasoconstrictive responses. In the next section, we discuss known astrocyte-mediated NVC signaling pathways and highlight current data disputing a major role for astrocytes in FH.
From an anatomical perspective, astrocytes are ideally positioned to conduct information in a bidirectional manner between neurons and blood vessels. A single astrocyte contacts thousands of synapses (Bushong et al., 2002), and specialized astrocyte processes, termed “endfeet”, cover about 99% of the abluminal surface of cerebral vessels (Kacem et al., 1998, Simard and Nedergaard, 2004, Mathiisen et al., 2010, McCaslin et al., 2011). The fact that astrocytes form non-overlapping domains (Bushong et al., 2002, Ogata and Kosaka, 2002) also suggests that a single astrocyte may be capable of executing multiple functions such as modulating synaptic transmission, mediating NVC, and regulating ionic homeostasis to name a few (Zorec et al., 2012). Astrocytes are also coupled via gap junctions (Giaume and Liu, 2012) which establish an electrical syncytium that allows localized signals to be spread across broad regions. This structural coupling provides an efficient means to spread and amplify signals across neural networks (Theis et al., 2005, Giaume et al.). Moreover, gap junctions also provide a route for information to be conducted to the upstream pial circulation (Figueroa and Duling, 2009). To this end, Xu et al., (2008) demonstrated that astrocytes mediate upstream vasodilation of pial arterioles following sciatic nerve stimulation via a purinergic signaling mechanism.
These unique astrocytic properties raise the question as to whether specialized astrocytic subpopulations exist (e.g. astrocytes involved in detecting signals/information around the synapse/blood vessel vs. those spreading information across neuronal networks). If this were the case, astrocytes involved in NVC may comprise a small subpopulation of cells, thus representing a potential challenge when detecting their activation and addressing their role in NVC-mediated vascular responses. To date, the few studies reporting the percentage of responding astrocytes during NVC-mediated responses have discrepancies with regards to the onset of the Ca2+ response relative to that of the stimulus or vasodilatory response. Winship et al., 2007 reported that ~5% of astrocytes respond to limb stimulation with a corresponding time to peak response of 0.5 seconds. While a similar percentage of responding astrocytes was reported by Nizar et al., 2013 (~7%) following electrical forepaw stimulation, the Ca2+ response onset was significantly delayed from that of the vasodilation onset (3.6±1.2 seconds vs. 0.7±0.4 sec, respectively). Using thalamocortical stimulation, Lind et al., 2013 reported both a rapid (98 msec) Ca2+ response onset in ~66% of astrocytic somas and a slower response (~ 5 sec after stimulation) in ~8% of astrocytes. Finally, using brain slices, Otsu et al., 2015 reported that the percentage of responsive astrocytes was dependent upon stimulation intensity with ~14% of astrocytes responding to single-pulse stimulation of olfactory sensory neuron axons and 70% of astrocytes responding to single-pulse stimulation in the presence of glutamate transporter antagonists to increase glutamate spillover. Indeed, these studies highlight the necessity of further research dedicated to elucidating whether functionally distinct astrocytic subpopulations do, in fact, exist.
Mechanisms underlying astrocyte-mediated vascular responses
During FH, increases in neuronal activity stimulate multiple pathways, some which involve direct neuronal signaling to vessels (Cauli and Hamel, 2010) and others that involve astrocytes as intermediaries (Attwell et al., 2010). Regardless of the specific pathway triggered, increased neuronal activity results in a rapid vasodilatory response and the spatiotemporally restricted delivery of glucose and oxygen to areas with increased metabolic demands in vivo (Fig. 2). However, in vitro studies have been notorious for failing to demonstrate that astrocytes respond within the same temporal constraints as those expected during the in vivo hyperemic response. While this may result from some of the limitations encountered with in vitro approaches (e.g. lack of pressurized vessels, subphysiological temperatures and differing O2 gradients (for review see (Filosa, 2010)), the fact that some in vivo studies observe significantly delayed astrocyte responses relative to the onset of the vasodilatory response raises the concern as to whether astrocytes are indeed intermediaries in NVC.
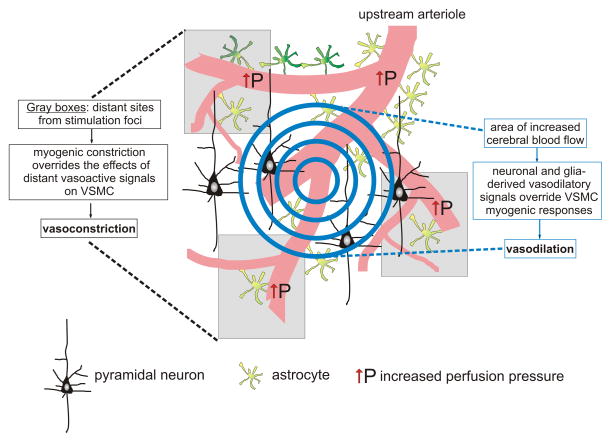
Figure 2.
Hypothetical working model for the significance of astrocyte-mediated vasoconstriction. 1) Under resting conditions, basal vascular tone is set by the myogenic properties of the vascular smooth muscle cells (VSMCs) as well as a tonic vasoconstrictor influence from astrocytes (e.g. 20-HETE). 2) Increased neuronal activity (graded circles) causes the release of vasodilatory signals from both neurons and astrocytes resulting in a localized vasodilation, which rapidly overrides the myogenic constriction of the VSMC. 3) The rise in flow and pressure in distant arterioles however, favors vasoconstriction as the action of vasodilatory signals is now significantly diminished. We propose that the action of these opposing forces (vasodilation vs. vasoconstriction) contributes to the center surround phenomenon observed during functional hyperemia.
In vitro studies from cortical and hippocampal brain slices suggest glutamate as the main signal activating neurovascular responses (Fergus and Lee, 1997, Zonta et al., 2003a). The seminal work of Carmignoto’s group showed that neuronal stimulation triggers a metabotropic glutamate receptor (mGluR) dependent parenchymal arteriole vasodilatory response that is preceded by astrocytic Ca2+ activation (Zonta et al., 2003a). At the mechanistic level, the authors proposed prostaglandin E2 (PGE2), a downstream metabolite of arachidonic acid (AA), as the glial-derived vasodilatory signal (Zonta et al., 2003a). These results were supported by in vivo and in vitro data demonstrating that changes in intracellular Ca2+ in astrocytes resulted in the release of AA metabolites (Zonta et al., 2003a, Zonta et al., 2003b, Davis et al., 2004, Takano et al., 2006, Koehler et al., 2009, Carmignoto and Gomez-Gonzalo, 2010, Dabertrand et al., 2013). Additionally, astrocytic Ca2+ increases (Cornell-Bell et al., 1990, Aguado et al., 2002) contribute to the generation of other vasoactive signals including nitric oxide (NO) (Wiencken and Casagrande, 1999, Li et al., 2003, Chisari et al., 2004, Metea and Newman, 2006, de Labra et al., 2009, Carmignoto and Gomez-Gonzalo, 2010), epoxyeicosatrienoic acids (EETs) (Alkayed et al., 1996, Harder et al., 2002, Peng et al., 2002, Metea and Newman, 2006, Blanco et al., 2008, Koehler et al., 2009, Carmignoto and Gomez-Gonzalo, 2010, Liu et al., 2011b), glutamate, adenosine and ATP (Simard et al., 2003, Anderson et al., 2004, Shi et al., 2008, Koehler et al., 2009, Carmignoto and Gomez-Gonzalo, 2010, Kusano et al., 2010, Vetri et al., 2011), all of which are capable of modulating parenchymal arteriole vascular tone (Zonta et al., 2003a, Fellin and Carmignoto, 2004, Filosa et al., 2004).
While it was reasonable to appreciate astrocyte contributions to NVC-mediated vasodilations, the field was later confounded with evidence that increases in intracellular Ca2+ in astrocytes evoked parenchymal arteriole constriction (Mulligan and MacVicar, 2004). The physiological meaning of this response was challenged, as neuronal activity-evoked increases in astrocyte Ca2+ should favor vasodilation rather than vasoconstriction (Fig. 2). The same group, however, later reported that the polarity of the vascular response to astrocytic stimulation could be altered by the O2 concentration of the perfusate, which in turn alters the metabolic state of the tissue (i.e. extracellular lactate and adenosine) (Gordon et al., 2008). It was demonstrated that neuronal stimulation increased lactate, which inhibits PGE2 transporters at low PO2 levels; the resulting PGE2 buildup would subsequently favor vasodilation (Chan et al., 2002). On the other hand, when using supraphysiological PO2 levels (95% O2) (a common practice in the in vitro brain slice preparation), the resulting low lactate levels decreased extracellular PGE2 availability thus favoring vasoconstriction (Gordon et al., 2008). A number of additional in vitro studies provided further evidence for alternative mechanisms underlying astrocyte-evoked vasoconstrictions (Girouard et al., 2010, (Metea and Newman, 2006, Blanco et al., 2008). Interestingly, in vivo electrical forepaw stimulation or cortical spreading depression in the presence of hyperbaric conditions failed to show an O2-dependent vascular response (Lindauer et al., 2010). Consistent with these observations, in vitro studies performed in the retina also showed O2-dependent vascular responses, but, again, these responses were not observed in vivo (Mishra et al., 2011). These data suggest O2-dependent mechanisms may have a strong effect on the neurovascular response in isolated preparations, which investigate levels 1 & 2 from our working model (Fig 1). However, when the entire cerebral circulatory network (levels 1–3) is engaged, as occurs in vivo, the predominant response to neuronal stimulation is a vasodilation independent of O2 levels. It is possible, however, that the polarity of the vascular response could be altered by the direct effect of O2 on vasoactive signals and/or channels and receptors expressed on other cells within the NVU and not solely on the vascular network.
Likewise, the polarity of the vascular response to neuronal stimulation is also affected upon alterations in NO availability. In pial arterioles, NO induces arteriole dilation via guanylyl cyclase activation and cGMP formation (Sobey and Faraci, 1997, Faraci and Sobey, 1999). In addition, NO can inhibit ω-hydroxylase activity reducing 20-HETE formation (Alonso-Galicia et al., 1999, Sun et al., 2000). In contrast to the vasodilatory effects of NO, Metea and Newman, 2006 showed that modification of NO availability elicits opposing vascular responses with vasoconstrictions being favored in the presences of increased NO availability (Metea and Newman, 2006). Similar observations were reported by a Rancillac et al., 2006 study that showed glutamate-mediated nNOS-derived NO evoked cerebellar microvessel vasoconstriction (Rancillac et al., 2006). Increased NO could favor vasoconstriction via inhibition of cytochrome P450 inhibition (Udosen et al., 2003) thus preventing formation of the vasodilatory metabolite EET (Metea and Newman, 2006). NO could also exert its effects by altering baseline vascular tone. Along these lines, NO synthase (NOS) inhibition has been shown to constrict parenchymal arterioles from hippocampal brain slices (Fergus et al., 1996). Alternatively, upon NO-induced decreases in vascular tone, mGluR-mediated responses will favor vasoconstriction as previously demonstrated by our lab (Blanco et al., 2008). The role of NO in NVC is further complicated by its heterogeneous function and production among various brain regions and cell types (Laranjinha et al., 2012). In the cerebellum, NO is suggested as the primary signal mediating NVC (Yang et al., 1999, Yang et al., 2000); this, however, is not the case in the cortex where NO is mainly regarded as a modulatory signal (Lindauer et al., 1999). In addition to brain region specificity and interactions with other vasoactive signals/pathways, complex NO mediated NVC responses could depend on the intensity of the neuronal stimulus. Following stimulation of the lateral geniculate nucleus in the visual thalamus of the cat de Labra et al. 2009 demonstrated that low intensity (contrast) stimulation evoked a rise in blood flow measured by oxyhaemoglobin (OxyHb) attributed to a small increase in NO likely derived from an eNOS source (vascular or astrocyte). On the other hand, heightened neuronal activation induced by increasing the intensity of the stimulus (high contrast) evoked greater changes in OxyHb associated with neuronal-derived NO (de Labra et al., 2009). The complex nature of NO actions in addition to its profound effect on the different cell types of the NVU cannot be overstated as it can significantly alter the responsiveness of VSMCs to vasoactive signals.
In addition to NO, the gaseous molecule carbon monoxide (CO) has also been shown to contribute to NVC-mediated vascular responses. Both astrocytes (Leffler et al., 2006, Parfenova et al., 2012) and vascular cells (Fiumana et al., 2003, Leffler et al., 2003) are sources of CO production. Studies conducted in pial arterioles have shown that glutamate-evoked signaling mediates CO release from astrocytes resulting in arteriole dilation (Fiumana et al., 2003). Multiple astrocytic glutamate receptors have been implicated in this process including NMDA and AMPA/kainite ionotropic glutamate receptors (Parfenova et al., 2012). The mechanism underlying CO-mediated vasodilation involves increased Ca2+ sparks in VSMCs and subsequent activation of large conductance Ca2+ activated K+ channels (BK), which evokes VSMC membrane hyperpolarization and vasodilation (Jaggar et al., 2002, Xi et al., 2011). To our knowledge, the role of CO on parenchymal arteriole tone has not been directly addressed; moreover, parenchymal arteriole VSMCs do not seem to have Ca2+ sparks suggesting that this vasodilatory mechanism may be unique to pial arterioles. Pial arterioles thus respond to astrocyte-derived CO following increases in neuronal activation, denoting yet another signal involved in astrocyte-to-vessel communication and an alternative mechanism (as opposed to gap junctions) by which information from deeper layers of the neuropil (e.g. level 1, Fig. 1) are relayed to upstream extracerebral/pial arterioles (e.g. level 3, Fig. 1). Pointing to the complexity of the mechanisms underlying NVC, it was demonstrated that CO and NO signaling mechanisms interact with each other. While NO stimulates CO production (Leffler et al., 2011), prolonged increases in CO augment vascular tone via NOS inhibition (Ishikawa et al., 2005). CO signaling may constitute an important mechanism bridging information from levels 1 & 2 (Fig. 1) to the pial circulation (level 3, Fig 1) and NO/CO interactions may provide a negative feedback control for the upstream regulation of CBF (e.g. rapid CO-mediated vasodilation followed by constriction) (Leffler et al., 2011).
K+ signaling from astrocytes is a key mechanism contributing to NVC (Filosa et al., 2006). Astrocytic endfeet express, in a polarized manner, a number of ion channels including BK channels (Price et al., 2002, Higashimori et al., 2010). The polarized expression of these channels makes them ideally suited for participation in NVC signaling pathways given that K+ release at the gliovascular interface acts as a potent vasodilatory signal. The original hypothesis for K+-mediated vasodilation suggested that K+ released during neuronal activation was taken up by astrocyte processes at the synapse and released at endfeet processes surrounding blood vessels (Newman, 1986, Paulson and Newman, 1987). This mechanism was thought to be part of the K+ siphoning mechanism. However, it was later demonstrated that K+ siphoning does not contribute to NVC-mediated responses, at least in the retina (Metea et al., 2007). Yet, K+, at modest concentrations, acts as a powerful vasodilatory signal of both pial (Eckman and Nelson, 2001) and parenchymal arterioles (Dunn and Nelson, 2010). Modest extracellular K+ elevations lead to the activation of inward rectifier potassium channels (Kir) in VSMCs, subsequent VSMC hyperpolarization and vasodilation. Importantly, K+ concentration could have opposing effects on the vascular response. While mild to moderate (<20 mM) extracellular K+ levels elicit cerebrovascular dilation (Kuschinsky et al., 1972, McCarron and Halpern, 1990, Knot et al., 1996, Horiuchi et al., 2002, Filosa et al., 2006, Girouard et al., 2010), high extracellular K+ concentrations trigger vasoconstriction (Knot et al., 1996, Horiuchi et al., 2002). Girouard et al. (2010) furthered this concept showing that astrocytic intracellular Ca2+ levels >700 nM induced BK channel dependent cerebral arteriole vasoconstriction in cortical brain slices.
Overall considerations when studying NVC-mediated responses
The above studies clearly suggest that the conditions in which NVC is studied may significantly interfere with the polarity and magnitude of the vascular response. While much effort has been dedicated to maintaining the NVU in conditions similar to normal brain physiology during experimentation, the diversity of the observed vascular and astrocyte responses calls for attention to the inherent limitations of experimental methodologies. From a technical perspective, a number of non-physiological stimulation modalities have been used including: Ca2+ uncaging, electrical field stimulation and pharmacological stimulation (Zonta et al., 2003a, Filosa et al., 2004, Mulligan and MacVicar, 2004, Filosa et al., 2006, Straub et al., 2006, Blanco et al., 2008, Gordon et al., 2008, Girouard et al., 2010). Additionally, the use of genetic approaches, such as the IP3R2 knockout (KO) mouse, have added additional complexity to our elucidation of the role of astrocytes in the control of cerebrovascular tone. NVC clearly constitutes a mechanism involving a redundancy of signals and targets, as removal of a single signal/pathway does not completely abolish the vascular response. Further, stimulation-induced vascular responses of opposing polarities could be also accounted for by the species of animal used or its developmental stage. Sun et al., 2013 demonstrated that astrocytic expression of mGluR5 is developmentally regulated in mice (Sun et al., 2013). It must be also noted that many of the original in vitro brain slice studies were performed in neonatal rats with ages ranging between 9–21 days postnatal. Given that such young animals likely possess an underdeveloped NVU, developmental differences in astrocytic and vascular cell Ca2+ homeostasis may contribute to non-physiological vascular responses (e.g. vasoconstriction) upon neuronal stimulation. For in vitro studies, temperature is another important factor as it can affect the kinetics of transporters and various enzymes involved in the generation of vasoactive molecules as well as the ability of the microcirculation to develop tone and respond to vasoactive signals. Further, our group demonstrated that the basal level of vascular tone is an important factor determining the magnitude and polarity of astrocyte evoked-vascular responses (Blanco et al., 2008); the lesser the arteriole tone, the lesser the response. Moreover, vasoconstriction was favored in parenchymal arterioles with minimal or no tone (Blanco et al., 2008). Lastly, for in vivo studies, anesthesia has been shown to significantly alter astrocyte activity (Thrane et al., 2012) as well as cerebrovascular function (Ayata et al., 2004, Kuga et al., 2009, Wang et al., 2010). Thus, careful considerations of these two parameters must be made when interpreting in vivo observations.
It is generally accepted that IP3R2 activation is a major source of Ca2+ following astrocytic stimulation (Petravicz et al., 2008); this signaling pathway is also considered the putative mechanism contributing to the rise in Ca2+ during NVC. Thus, the role of astrocytes in FH was tested in IP3R2 KO mice in a series of recent in vivo studies (Nizar et al., 2013, Bonder and McCarthy, 2014, Jego et al., 2014). Intriguingly, results failed to elucidate a role for astrocytes in FH; thus, the notion that astrocytes participate in NVC is currently under debate. While these data clearly place astrocytes in a questionable position, a few considerations must be taken into account. Of note is the fact that while both wild type (WT) and IP3R2KO mice were tested, in essence, no differences were observed between these two groups. In the study by Nizar 2013, results showed normal hyperemic responses in IP3R2KO mice albeit no Ca2+ increases in astrocytes. In addition, the small percentage (~13%) of responding astrocytes from WT mice did so with a significant (>2sec) delay compared to the onset of the vascular response. Using the hM3Dq DREADD (designer receptor exclusively activated by designer drugs) (Armbruster et al., 2007) system to activate Gq-GPCR in vivo along with genetically encoded Ca2+ sensors [cyto-GCaMP3 (Chen et al., 2013) and Lck-GCaMP6 (Shigetomi et al., 2013a)], (Bonder and McCarthy, 2014) recently demonstrated that neither astrocytic Gq-GPRC nor IP3R2 signaling altered astrocytic Ca2+ in response to visual stimulation in lightly sedated mice (Bonder and McCarthy, 2014). These results again call in question the relevance of astrocyte-mediated vasodilation and their participation in NVC. However, contrary to these data, Winship et al. (2007) demonstrated rapid astrocytic Ca2+ responses to contralateral hindlimb stimulation. The authors reported that about 5% of responding sulforhodamine 101-labeled astrocytes reached a peak amplitude ~0.5 sec from the onset of the neuronal stimulation consistent with the idea that astrocytes initiate the hyperemic response (Winship et al., 2007). Further supporting a role for astrocytes in the initiation of FH, Lind et al. (2013) reported rapid astrocytic Ca2+ responses to increases in neuronal activity and correlation of astrocyte activation with the onset of the vasodilatory response. Lind et al. (2013) demonstrated stimulus-evoked Ca2+ increases in astrocytic soma, endfeet and processes using signal-enhancing analysis (Lind et al., 2013). Recently, Otsu et al., (2015) reported using a genetically encoded Ca2+ reporter (GCamP3) mouse that in vivo, odor stimulation evoked Ca2+ increases in astrocytic processes. These Ca2+changes preceded changes in blood flow by 1–2 seconds supporting a role for astrocytes in functional hyperemia (Otsu et al., 2015). Together, these studies confirm a role for astrocytes in FH and emphasize the need for improved image resolution techniques to detect Ca2+ signals in fine astrocytic processes.
Among important limitations arising from in vivo studies are that: 1) IP3R2 signaling stands as an important source of astrocytic Ca2+, yet other Ca2+ sources cannot be ruled out; 2) compensatory mechanisms in the IP3R2 KO mice may also account for a lack of response; 3) the temporal Ca2+ dynamics in astrocytic processes are independent of those in the soma, questioning the resolution of available tools and 4) it is possible that the delay in the astrocytic Ca2+ response may result from an earlier undetectable rise in Ca2+ which evokes the secondary “detectable” change, such as Ca2+-induced Ca2+ release (DiNuzzo, 2014). These questions remain, and the controversial issues raised by studies conducted in IP3R2 KO mice should be taken into full consideration. However, limited supporting evidence for astrocytes in FH was obtained from WT mice suggesting that, either as claimed by these investigators, normal FH and NVC can occur in the absence of detectable astrocytic Ca2+ changes or that, as mentioned earlier, alternative Ca2+ sources and better image resolution are needed to fully address the role of astrocytes in NVC in vivo. For now, we suggest that conscious effort must be dedicated towards the development of better approaches including genetically engineered animals (e.g. GCaMP) that would aid in resolving the issue of differential Ca2+ dynamics between the soma and fine astrocytic processes. Regardless of whether astrocytes directly control or are modulators of vascular tone, the complex interplay between neuro-glial signaling and vascular responses would likely be altered if astrocytes were to be completely removed from the working model. Thus, it is possible that a more accurate picture lies in the fact that astrocytes are indeed key participants of NVC by either: restoring tone, providing an additional modulatory pathway, prolonging the signals that regulate vascular responses and/or maintaining ionic homeostasis, to name a few.
Functional significance for astrocyte-mediated vasodilation vs. vasoconstriction: relevance to activity-dependent and resting CBF regulation
Given the importance of astrocytes in modulating synaptic activity (the tripartite synapse (Araque et al., 1999)), one could envision an extension of this model in which vascular hemodynamics are incorporated. In such a paradigm, astrocytes would also monitor brain perfusion via mechanosensitive channels expressed preferentially on astrocytic endfeet and subsequently adjust synaptic activity and CBF accordingly. Under these conditions, astrocytes would not only constitute part of the now debated NVC response but also adjust baseline or resting CBF according to metabolic demands. Thus, we propose that astrocytes act as bidirectional signalers in the NVU, participating in the sensation of cerebral hemodynamics and modulation of synaptic activity (vessel-to-astrocyte-to-neuron signaling) as well as NVC (neuron-to-astrocyte-to-vessel signaling) thereby fine-tuning activity-dependent and resting CBF. Clearly, the field of glial physiology has been focused on the mechanisms underlying astrocyte-induced dilations whereas considerably less attention has been dedicated to the capability of astrocytes to elicit constrictions. This experimental emphasis on dilatory mechanisms is likely due to the fact that the physiological significance of astrocyte-induced dilations is clear - to facilitate FH – whereas the physiological relevance of astrocyte-induced constrictions remains elusive. Because astrocytes produce and release both vasodilatory and vasoconstrictive agents, the balance of these astrocyte-derived vasoactive compounds may contribute to the fine-tune regulation of cerebral vascular tone and CBF. We predict that under resting conditions, astrocytes tonically release vasoconstrictors, which ensure that cerebral artery tone is great enough to elicit optimal cerebral arteriole dilation upon exposure to vasodilatory agents. During NVC, physiological changes within the brain parenchyma may concurrently suppress vasoconstrictor production and increase vasodilator production thus favoring vasodilation at the site of increased neuronal activity (Fig. 2). At sites distant from neuronal stimulation (Devor et al., 2007), where vasodilatory signal release is diminished, tonic astrocytic release of vasoconstrictors in combination with myogenic constriction will be favored. This proposed spatial regulation of astrocyte-induced dilations and constrictions may contribute to the center-surround pattern of CBF observed during fMRI recordings of brain activity as suggested by Metea and Newman, (2006).
Cerebral resistance arterioles respond to hemodynamic changes (e.g. blood pressure and flow) in order to maintain constant CBF, thus protecting the brain from sudden changes in intracranial pressure and/or blood volume, which may induce edema. While numerous studies have addressed the mechanisms by which arterioles outside the brain parenchyma regulate CBF (Harder et al., 2011, Koller and Toth, 2012), little is known about whether parenchymal arterioles also contribute to the process of CA and to what extent the responses of these two vascular beds are integrated and coordinated to optimize resting CBF. However, like extracerebral arterioles, parenchymal arterioles constrict in response to increases in luminal pressure/flow (Bryan et al., 2001, Toth et al., 2011, Kim and Filosa, 2012) suggesting that this subpopulation of the cerebral vasculature (level 2, Fig. 1) also contributes to CA.
Given that parenchymal arterioles lose peripheral innervations past the Virchow Robin space (Iadecola, 2004, Hamel, 2006), parenchymal arteriole diameter is dependent upon intrinsic vascular signaling and signals originating from neurons and astrocytes. Although the pressure/flow-induced constrictions observed in isolated parenchymal arterioles are due to intrinsic vascular mechanisms (Bryan et al., 2001, Toth et al., 2011), the anatomical properties of perivascular astrocytes (specifically astrocytic endfeet encasement of blood vessels (Iadecola and Nedergaard, 2007)) along with the characteristic expression of specialized ion channels and receptors suggests that astrocytes may be key regulators/modulators of steady-state CBF and participants in CA (Iddings et al., 2015). Of note, astrocytes express mechanosensitive channels, including members of the transient receptor potential (TRP) family of ion channels, which may be capable of sensing arteriole strain/tension induced by hemodynamic stimuli.
Beginning with Pizzo et al. (2001), a number of studies have characterized the expression and function of multiple TRP channels in astrocytes. To date, members of the ankyrin (TRPA1 (Lee et al., 2012, Shigetomi et al., 2012, Shigetomi et al., 2013b)), canonical (TRPC1 (Golovina, 2005, Malarkey et al., 2008, Miyano et al., 2010, Shirakawa et al., 2010, Reyes et al., 2013), 2 (Miyano et al., 2010), 3 (Grimaldi et al., 2003, Miyano et al., 2010, Shirakawa et al., 2010), 4 (Golovina, 2005, Malarkey et al., 2008, Miyano et al., 2010), 5 (Malarkey et al., 2008), 6 (Beskina et al., 2007, Miyano et al., 2010, Shirakawa et al., 2010)), and vanilloid (TRPV4 (Benfenati et al., 2007, Benfenati et al., 2011, Dunn et al., 2013)) TRP subfamilies have been identified in astrocytes. Given that TRP channels can be activated by mechanical stimuli (Clapham, 2003) and are known to contribute to astrocytic intracellular Ca2+ regulation (Grimaldi et al., 2003, Ayata et al., 2004, Golovina, 2005, Benfenati et al., 2007, Beskina et al., 2007, Malarkey et al., 2008, Miyano et al., 2010, Shirakawa et al., 2010, Benfenati et al., 2011, Shigetomi et al., 2012, Dunn et al., 2013, Reyes et al., 2013, Shigetomi et al., 2013b), this family of non-selective cation channels may function to sense and subsequently modulate vascular tone. In addition, TRP channels may constitute an alternative route for astrocytic Ca2+ increases in addition to the proposed IP3R2 pathway and/or may be coupled to IP3Rs thus providing a mechanism for Ca2+ amplification as previously demonstrated (Fernandes et al., 2008, Garcia-Elias et al., 2008, Dunn et al., 2013). Modulation of intracellular Ca2+ levels by astrocytic TRP activation could facilitate astrocyte-induced vascular responses via a number of mechanisms including 20-HETE and EET production, ATP release and/or K+ efflux (>20 mM) all of which are Ca2+-dependent processes.
Of the TRP channels expressed in astrocytes, TRPV4 channels are the most attractive candidates for monitoring vascular tone given that they are expressed primarily in astrocytic endfeet (Benfenati et al., 2007, Benfenati et al., 2011). In support of this idea, a recent study by (Shibasaki et al., 2014) showed that a subpopulation of astrocytes release ATP in response to TRVP4 channel activation via a gap junction dependent mechanism. These findings are of interest as they go along with the idea that activation of TRPV4 channels expressed in astrocyte endfeet may trigger the release of vasoactive signals (e.g. ATP) thus contributing to the regulation of vascular tone. The anatomical properties of perivascular astrocytes along with the characteristic expression of specialized ion channels, particularly TRPV4 channels, calls for future studies addressing the ability of astrocytes to sense and transduce hemodynamic stimuli and participate in CA.
Evidence for pericytes in NVC-mediated signaling
In addition to the confounding data on the role of astrocytes in NVC-mediated responses, pericytes have recently taken center stage. Although pericytes are found along all vessel types (arteriole, venule and capillary), they are primarily associated with capillaries (Fig. 1). Compared to other vascular beds, the brain has the highest ratio of pericytes to endothelial cells - 3:1 (Shepro and Morel, 1993). In brain capillaries, pericytes are sandwiched between endothelial cells and astrocytes by a thin basal membrane (Peppiatt et al., 2006). While pericyte function has been well characterized with regards to its role in the establishment and maintenance of the BBB (Hellstrom et al., 2001, ArmulikAl Ahmad et al., 2009, Armulik et al., 2010, Daneman et al., 2010), an important role for pericytes in the regulation of capillary blood flow has also been proposed (Kawamura et al., 2003, Peppiatt et al., 2006, Hall et al., 2014). Pericytes express contractile proteins (Hirschi and D’Amore, 1996) and a number of ion channels and receptors which modulate intracellular Ca2+ changes and subsequently alter the contractile state of these cells. To this end, pericytes have been known to respond to vasoconstrictor signals such as ATP (Kawamura et al., 2003), dopamine (Wu et al., 2001), Ang II (Kawamura et al., 2004), noradrenaline (Peppiatt et al., 2006) and 20-HETE (Hall et al., 2014). Additionally, pericytes are known to relax to signals such as NO/cGMP (Sakagami et al., 2001), adenosine (Li and Puro, 2001) and PGE2 (Hall et al., 2014).
In the isolated rat retinal preparation, bath applied ATP leads to pericyte-mediated capillary constriction (Kawamura et al., 2003). Such capillary responses were confirmed in the whole retina and cerebellar slices (Peppiatt et al., 2006), where pericyte-mediated capillary constriction induced by ATP or noradrenaline was observed. Consistent with in vitro observations, in vivo work further confirmed the role of pericytes as contractile cells (Fernández-Klett et al., 2010). However, a recent study by Hall et al. (2014) proposed pericytes as the initiators of the FH response. Using an in vitro and in vivo approach, the authors showed glutamate- and sensory-evoked pericyte-mediated capillary dilation (Hall et al., 2014). While the evidence confirmed the ability of pericytes to induce capillary dilation in response to neuronal stimulation, their relative contribution vs. VSMCs to CBF regulation remains unclear. Because both cell types can be stimulated by a number of overlapping vasoactive factors, the only current way to dissociate capillary vs. arteriole vascular responses is by measuring vessel caliber. Due to the thin properties of the capillary wall, which makes their membrane distensible and compressible, pericyte-evoked capillary dilation will be likely evoked earlier than the diameter change observed in arterioles. Thus, the increased flow and pressure within this site (level 1, Fig. 1) of the microcirculation will immediately result in wall distention. On the other hand, even if parenchymal arterioles VSMCs were to sense neuronal activity first or simultaneously with pericytes, their VSMCs may initially resist the increased flow/pressure (due to their intrinsic myogenic properties) resulting in a putative “delayed” response (i.e. change in vessel caliber). However, if pericytes were indeed the main drivers for the FH response, absence of pericyte-mediated vasodilatory signaling would prevent the pericyte-initiated capillary dilation, significantly delaying and/or compromising the kinetics of FH. Evidence for the latter is currently lacking. Thus, it is of critical importance to understand the exact sequence of events as well as the mechanisms underlying upstream vasodilation (e.g. from level 1 to level 3, Fig 1). For example, following sensory-evoked pericyte-initiated capillary dilation, does the endothelium conduct these events to the upstream circulation? If so, how is pericyte-to-endothelial cell signaling mediated? Are vasodilatory mechanisms (e.g. conduction of hyperpolarizing currents and/or amplification of vasodilatory signals) conducted to the upstream circulation via astrocytic gap junctions (Xu et al., 2008)? Do pericytes around parenchymal arterioles conduct information to parenchymal arteriole VSMCs? Future studies that incorporate pericytes are needed to better understand the hierarchy of cell-to-cell communication modalities underlying the orchestrated increase in CBF during the FH response.
Are microglia cells also components of the NVC response?
As the second most populous glial cell in the brain, it is important to consider the structural and functional role of microglia within the NVU and toward NVC. While microglial density varies by brain region, their presence is consistent throughout the brain. When compared to all macrophages (circulating, pulmonary, liver), microglia possess the largest surface area due to their highly ramified morphology (Lawson et al., 1990). The combination of this consentient distribution and ramified morphology allows for their continuous surveillance of vascular and cellular activity (Masuda et al., 2011). Unlike astrocytes, microglial processes are highly active under healthy conditions allowing for the complete and ongoing assessment of the brain parenchyma; this process becomes enhanced/focused and eventually blunted during microglial response to injury or pathogens (Petersen and Dailey, 2004, Davalos et al., 2005, Morrison and Filosa, 2013). It is well understood that microglia offer a diversity of responses that may or may not be attributed to a heterogeneous microglial population. However, it is difficult to elucidate whether diverse microglial responses arise from developmental programming (Rezaie et al., 2005, Nayak et al., 2012, Arnold and Betsholtz, 2013) or a dynamic environment of active astrocytes, neurons and vasculature present during health and disease states across diverse brain regions. Nevertheless, it remains that due to their dynamic and diverse capacity, microglia are prime candidates to sense and contribute to NVC mechanisms during health and disease.
Although a growing body of literature includes this notion of microglia as a vital component of the NVU, methodologies to assess microglias’ direct contribution toward NVC during physiologic and pathologic conditions have been limited. A small population of microglia—termed juxtavascular microglia—overlay the vasculature, a positioning that would facilitate immediate responses to vascular and blood born signaling (Grossmann et al., 2002, Nayak et al., 2012). Detailed cellular and molecular differences, if they exist, between microglial sub-populations remain understudied. However, in vivo imaging nicely illustrates that morphologic and locomotive responses differ between juxtavascular and non-juxtavascular microglia (Grossmann et al., 2002). Despite the presence of juxtavascular microglia and the ability of non-juxtavascular microglial processes to span greater than 30 μm to contact the vasculature or glia limitans (Lassmann et al., 1991), it is not yet clear whether these cells provide direct input to the vasculature and/or modify vascular responses during health and disease.
Independent of this small population of juxtavascular cells and different than astrocytes, microglia do not maintain prolonged physical contact with other components of the NVU nor do microglia form a syncytium. Thus, we suspect that their contribution toward NVC occurs via the release of small molecular messengers and ligand-receptor interactions. To support this notion, we will review microglial interactions with the other two primary constituents of the NVU—neurons and astrocytes. It is important to consider, then, that microglial function within the NVU and response to pathology is an integration of input and resulting cell signaling events from surrounding neurons, astrocytes and other microglia.
An emerging story surrounds microglia-neuron interactions in the development and maintenance of synaptic function. Microglia utilizes targeted phagocytosis for synapse organization during development and for re-organization after injury (Stevens et al., 2007, Wake et al., 2009, Tremblay et al., 2010). Microglia also modulate neuronal activity via small molecular messengers such as ATP, BDNF and CX3CL1 (Coull et al., 2005, Hayashi et al., 2006, Scianni et al., 2013, Siskova and Tremblay, 2013) thus placing microglia in the midst of an expanded paradigm—the “quad-partite” synapse (Schafer et al., 2013). In addition, microglia depend on continuous microglial-neuronal crosstalk which is mediated by neuronal ligand-microglia receptor interactions such as CX3CL1/CX3CR1, CD47/CD177, and CD22/CD45 to maintain their surveillance activities; the absence of this communication constitutes an initial “signal” to escalate/alter microglial function (Hanisch and Kettenmann, 2007, Saijo and Glass, 2011). However, not well understood is how microglia modulate, augment, or repress neuronal signaling during NVC; these data are confounded by astrocyte input as both cell types possess the capacity to monitor the synaptic cleft (Schafer et al., 2013).
A small but growing body of literature summarizes microglia-astrocyte communication. Methodologically, the assessment of astrocyte and microglia contributions to health and disease in independent cell populations and in situ remain challenging, as specific functional and morphologic assessment tools are limited (Wolf and Kirchhoff, 2008, Nimmerjahn, 2009, Sofroniew and Vinters, 2010). Current evidence proposes multiple mechanisms of microglia-astrocyte communication of which the mechanism may depend on contextual stimuli (Liu et al., 2011a). In an elegant set of experiments, (Pascual et al., 2012) support a paradigm where microglia immediately respond to lipopolysaccharide (LPS; within 5 minutes) to nudge astrocytes into a robust feed forward response via microglial ATP release binding to astrocytic P2Y1 receptors. Downstream responses of this microglia to astrocyte communication results in an excitatory neuronal network which may be applicable to an epileptic scenario (Pascual et al., 2012). Further evidence of microglia to astrocyte communication was reported by Abudara et al. (2015). In this experimental paradigm, lower dosage LPS incubations were extended to 3 hours and resulted in microglia TNF-α and IL-1β release. These microglial cytokines were necessary for astrocytic connexin43 hemichannel opening, thus increased astrocytic intracellular calcium concentrations facilitating glutamate release. However, in this paradigm, microglia to astrocyte communication resulted in decreased excitatory synaptic activity (Abudara et al., 2015). In addition, compelling evidence of astrocyte to microglia communication to quench microglia inflammatory responses after LPS induced activation is an important mechanism to provide checks and balances between these two inflammatory cells (Orellana et al., 2013). Unraveling the complexities of glial communication and these effects on NVC during health and disease is a story that holds great potential as both cells contribute to the dichotomous inflammatory response.
Conclusion and Perspectives
While the role of astrocytes in NVC is currently debated, in recent years a substantial amount of research has been published supporting the ability of astrocytes to modulate vascular tone. Astrocytes, via a Ca2+-dependent mechanism, engage numerous signaling pathways which lead to the release of vasoactive signals capable of both dilating and constricting arterioles. The kinetics and effectiveness of these signals is in turn determined by the metabolic state of the tissue, the level of basal arteriole tone and/or the magnitude and type of stimulus evoked. Future studies however are needed to better address current discrepancies and open questions in the field. To this end, improved image resolution is needed to better define dynamic Ca2+ events in astrocyte processes vs. somas. Moreover, while in vitro studies can provide important clues regarding the cellular signaling mechanisms controlling vascular tone, clear limitations (e.g. lack of flow and pressure in arterioles, temperature, O2 gradients) must be taken into consideration when interpreting in vitro results. Likewise, while in vivo studies provide meaningful information on the communication modalities contributing to activity-dependent changes in cerebral blood flow, the conditions of these studies (e.g. anesthesia, stimulus strength, acquisition parameters) must be also considered. The emerging availability of genetically encoded Ca2+ sensors along with improved two-photon imaging technology are key towards the re-evaluation of whether and how astrocytes participate in the regulation of vascular tone.
Highlights for review.
Constituents of the neurovascular unit
Role of astrocytes in the regulation of vascular tone
Functional significance for astrocyte-mediated vasodilation vs. vasoconstriction
Evidence for pericytes-evoked capillary dilation
Microglia a missing component of the neurovascular unit
Acknowledgments
We thank Adam Secrest for the illustration presented in Figure 1. This work was supported by the NIH National Heart, Lung and Blood Institute (NHLBI) R01 HL089067-02 to JAF, American Heart Association (AHA) 11PRE7400037 to JAI and (NINR) F32 NR013611 to HM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abudara V, Roux L, Dallerac G, Matias I, Dulong J, Mothet JP, Rouach N, Giaume C. Activated microglia impairs neuroglial interaction by opening Cx43 hemichannels in hippocampal astrocytes. Glia. 2015 doi: 10.1002/glia.22785. [DOI] [PubMed] [Google Scholar]
- Aguado F, Espinosa-Parrilla JF, Carmona MA, Soriano E. Neuronal activity regulates correlated network properties of spontaneous calcium transients in astrocytes in situ. J Neurosci. 2002;22:9430–9444. doi: 10.1523/JNEUROSCI.22-21-09430.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkayed NJ, Narayanan J, Gebremedhin D, Medhora M, Roman RJ, Harder DR. Molecular characterization of an arachidonic acid epoxygenase in rat brain astrocytes. Stroke. 1996;27:971–979. doi: 10.1161/01.str.27.5.971. [DOI] [PubMed] [Google Scholar]
- Alonso-Galicia M, Hudetz AG, Shen H, Harder DR, Roman RJ. Contribution of 20-HETE to vasodilator actions of nitric oxide in the cerebral microcirculation. Stroke. 1999;30:2727–2734. doi: 10.1161/01.str.30.12.2727. discussion 2734. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Bergher JP, Swanson RA. ATP-induced ATP release from astrocytes. J Neurochem. 2004;88:246–256. doi: 10.1111/j.1471-4159.2004.02204.x. [DOI] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- ArmulikAl Ahmad A, Gassmann M, Ogunshola OO. Maintaining blood-brain barrier integrity: pericytes perform better than astrocytes during prolonged oxygen deprivation. J Cell Physiol. 2009;218:612–622. doi: 10.1002/jcp.21638. [DOI] [PubMed] [Google Scholar]
- Arnold T, Betsholtz C. Correction: The importance of microglia in the development of the vasculature in the central nervous system. Vascular cell. 2013;5:12. doi: 10.1186/2045-824X-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayata C, Dunn AK, Gursoy OY, Huang Z, Boas DA, Moskowitz MA. Laser speckle flowmetry for the study of cerebrovascular physiology in normal and ischemic mouse cortex. J Cereb Blood Flow Metab. 2004;24:744–755. doi: 10.1097/01.WCB.0000122745.72175.D5. [DOI] [PubMed] [Google Scholar]
- Benfenati V, Amiry-Moghaddam M, Caprini M, Mylonakou MN, Rapisarda C, Ottersen OP, Ferroni S. Expression and functional characterization of transient receptor potential vanilloid-related channel 4 (TRPV4) in rat cortical astrocytes. Neuroscience. 2007;148:876–892. doi: 10.1016/j.neuroscience.2007.06.039. [DOI] [PubMed] [Google Scholar]
- Benfenati V, Caprini M, Dovizio M, Mylonakou MN, Ferroni S, Ottersen OP, Amiry-Moghaddam M. An aquaporin-4/transient receptor potential vanilloid 4 (AQP4/TRPV4) complex is essential for cell-volume control in astrocytes. Proc Natl Acad Sci U S A. 2011;108:2563–2568. doi: 10.1073/pnas.1012867108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beskina O, Miller A, Mazzocco-Spezzia A, Pulina MV, Golovina VA. Mechanisms of interleukin-1beta-induced Ca2+ signals in mouse cortical astrocytes: roles of store- and receptor-operated Ca2+ entry. Am J Physiol Cell Physiol. 2007;293:C1103–1111. doi: 10.1152/ajpcell.00249.2007. [DOI] [PubMed] [Google Scholar]
- Blanco VM, Stern JE, Filosa JA. Tone-dependent vascular responses to astrocyte-derived signals. Am J Physiol Heart Circ Physiol. 2008;294:H2855–2863. doi: 10.1152/ajpheart.91451.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleys RL, Cowen T. Innervation of cerebral blood vessels: morphology, plasticity, age-related, and Alzheimer’s disease-related neurodegeneration. Microsc Res Tech. 2001;53:106–118. doi: 10.1002/jemt.1075. [DOI] [PubMed] [Google Scholar]
- Bonder DE, McCarthy KD. Astrocytic Gq-GPCR-Linked IP3R-Dependent Ca2+ Signaling Does Not Mediate Neurovascular Coupling in Mouse Visual Cortex In Vivo. J Neurosci. 2014;34:13139–13150. doi: 10.1523/JNEUROSCI.2591-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayden JE, Earley S, Nelson MT, Reading S. Transient receptor potential (TRP) channels, vascular tone and autoregulation of cerebral blood flow. Clin Exp Pharmacol Physiol. 2008;35:1116–1120. doi: 10.1111/j.1440-1681.2007.04855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan RM, Jr, Marrelli SP, Steenberg ML, Schildmeyer LA, Johnson TD. Effects of luminal shear stress on cerebral arteries and arterioles. Am J Physiol Heart Circ Physiol. 2001;280:H2011–2022. doi: 10.1152/ajpheart.2001.280.5.H2011. [DOI] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci. 2002;22:183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmignoto G, Gomez-Gonzalo M. The contribution of astrocyte signalling to neurovascular coupling. Brain Res Rev. 2010;63:138–148. doi: 10.1016/j.brainresrev.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Cauli B, Hamel E. Revisiting the role of neurons in neurovascular coupling. Front Neuroenergetics. 2010;2:9. doi: 10.3389/fnene.2010.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan BS, Endo S, Kanai N, Schuster VL. Identification of lactate as a driving force for prostanoid transport by prostaglandin transporter PGT. Am J Physiol Renal Physiol. 2002;282:F1097–1102. doi: 10.1152/ajprenal.00151.2001. [DOI] [PubMed] [Google Scholar]
- Chen Y, Song X, Ye S, Miao L, Zhu Y, Zhang RG, Ji G. Structural insight into enhanced calcium indicator GCaMP3 and GCaMPJ to promote further improvement. Protein & cell. 2013;4:299–309. doi: 10.1007/s13238-013-2103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari M, Salomone S, Laureanti F, Copani A, Sortino MA. Modulation of cerebral vascular tone by activated glia: involvement of nitric oxide. J Neurochem. 2004;91:1171–1179. doi: 10.1111/j.1471-4159.2004.02782.x. [DOI] [PubMed] [Google Scholar]
- Cipolla MJ, Smith J, Kohlmeyer MM, Godfrey JA. SKCa and IKCa Channels, myogenic tone, and vasodilator responses in middle cerebral arteries and parenchymal arterioles: effect of ischemia and reperfusion. Stroke. 2009;40:1451–1457. doi: 10.1161/STROKEAHA.108.535435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990;247:470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- Dabertrand F, Hannah RM, Pearson JM, Hill-Eubanks DC, Brayden JE, Nelson MT. Prostaglandin E2, a postulated astrocyte-derived neurovascular coupling agent, constricts rather than dilates parenchymal arterioles. J Cereb Blood Flow Metab. 2013;33:479–482. doi: 10.1038/jcbfm.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. Nat Neurosci. Vol. 8. United States: 2005. ATP mediates rapid microglial response to local brain injury in vivo; pp. 752–758. [DOI] [PubMed] [Google Scholar]
- Davis RJ, Murdoch CE, Ali M, Purbrick S, Ravid R, Baxter GS, Tilford N, Sheldrick RL, Clark KL, Coleman RA. EP4 prostanoid receptor-mediated vasodilatation of human middle cerebral arteries. Br J Pharmacol. 2004;141:580–585. doi: 10.1038/sj.bjp.0705645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Labra C, Rivadulla C, Espinosa N, Dasilva M, Cao R, Cudeiro J. Different sources of nitric oxide mediate neurovascular coupling in the lateral geniculate nucleus of the cat. Frontiers in systems neuroscience. 2009;3:9. doi: 10.3389/neuro.06.009.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Zoppo GJ, Milner R. Integrin-matrix interactions in the cerebral microvasculature. Arterioscler Thromb Vasc Biol. 2006;26:1966–1975. doi: 10.1161/01.ATV.0000232525.65682.a2. [DOI] [PubMed] [Google Scholar]
- Devor A, Tian P, Nishimura N, Teng IC, Hillman EM, Narayanan SN, Ulbert I, Boas DA, Kleinfeld D, Dale AM. Suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative blood oxygenation level-dependent signal. J Neurosci. 2007;27:4452–4459. doi: 10.1523/JNEUROSCI.0134-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNuzzo M. Isn’t functional neuroimaging all about Ca2+ signaling in astrocytes? J Neurophysiol jn 00826. 2014:02014. doi: 10.1152/jn.00826.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CT, Iadecola C. The role of neuronal signaling in controlling cerebral blood flow. Brain Lang. 2007;102:141–152. doi: 10.1016/j.bandl.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Dunn KM, Hill-Eubanks DC, Liedtke WB, Nelson MT. TRPV4 channels stimulate Ca2+-induced Ca2+ release in astrocytic endfeet and amplify neurovascular coupling responses. Proc Natl Acad Sci U S A. 2013;110:6157–6162. doi: 10.1073/pnas.1216514110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KM, Nelson MT. Potassium channels and neurovascular coupling. Circ J. 2010;74:608–616. doi: 10.1253/circj.cj-10-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckman DM, Nelson MT. Potassium ions as vasodilators: role of inward rectifier potassium channels. Circ Res. 2001;88:132–133. doi: 10.1161/01.res.88.2.132. [DOI] [PubMed] [Google Scholar]
- Edvinsson LM, MacKenzie ET. General and comparative anatomy of the cerebral circulation. New York: Lippincott Williams & Wilkins; 2002. [Google Scholar]
- Faraci FM, Sobey CG. Role of soluble guanylate cyclase in dilator responses of the cerebral microcirculation. Brain Res. 1999;821:368–373. doi: 10.1016/s0006-8993(99)01110-5. [DOI] [PubMed] [Google Scholar]
- Fellin T, Carmignoto G. Neurone-to-astrocyte signalling in the brain represents a distinct multifunctional unit. J Physiol. 2004;559:3–15. doi: 10.1113/jphysiol.2004.063214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergus A, Jin Y, Thai QA, Kassell NF, Lee KS. Tonic protein kinase C-mediated vasoconstriction is unmasked when nitric oxide synthase is inhibited in cerebral microvessels. Neuroscience. 1996;74:927–934. doi: 10.1016/0306-4522(96)00158-3. [DOI] [PubMed] [Google Scholar]
- Fergus A, Lee KS. Regulation of cerebral microvessels by glutamatergic mechanisms. Brain Res. 1997;754:35–45. doi: 10.1016/s0006-8993(97)00040-1. [DOI] [PubMed] [Google Scholar]
- Fernandes J, Lorenzo IM, Andrade YN, Garcia-Elias A, Serra SA, Fernandez-Fernandez JM, Valverde MA. IP3 sensitizes TRPV4 channel to the mechano- and osmotransducing messenger 5′-6′-epoxyeicosatrienoic acid. J Gen Physiol. 2008;131:i2. doi: 10.1085/JGP1315OIA2. [DOI] [PubMed] [Google Scholar]
- Fernández-Klett F, Offenhauser N, Dirnagl U, Priller J, Lindauer U. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc Natl Acad Sci U S A. 2010;107:22290–22295. doi: 10.1073/pnas.1011321108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa XF, Duling BR. Gap junctions in the control of vascular function. Antioxid Redox Signal. 2009;11:251–266. doi: 10.1089/ars.2008.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosa JA. Vascular tone and neurovascular coupling: considerations toward an improved in vitro model. Front Neuroenergetics. 2010;2 doi: 10.3389/fnene.2010.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosa JA, Bonev AD, Nelson MT. Calcium dynamics in cortical astrocytes and arterioles during neurovascular coupling. Circ Res. 2004;95:e73–81. doi: 10.1161/01.RES.0000148636.60732.2e. [DOI] [PubMed] [Google Scholar]
- Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, Nelson MT. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci. 2006;9:1397–1403. doi: 10.1038/nn1779. [DOI] [PubMed] [Google Scholar]
- Fiumana E, Parfenova H, Jaggar JH, Leffler CW. Carbon monoxide mediates vasodilator effects of glutamate in isolated pressurized cerebral arterioles of newborn pigs. Am J Physiol Heart Circ Physiol. 2003;284:H1073–1079. doi: 10.1152/ajpheart.00881.2002. [DOI] [PubMed] [Google Scholar]
- Garcia-Elias A, Lorenzo IM, Vicente R, Valverde MA. IP3 receptor binds to and sensitizes TRPV4 channel to osmotic stimuli via a calmodulin-binding site. J Biol Chem. 2008;283:31284–31288. doi: 10.1074/jbc.C800184200. [DOI] [PubMed] [Google Scholar]
- Giaume C, Koulakoff A, Roux L, Holcman D, Rouach N. Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat Rev Neurosci. 2010;11:87–99. doi: 10.1038/nrn2757. [DOI] [PubMed] [Google Scholar]
- Giaume C, Liu X. From a glial syncytium to a more restricted and specific glial networking. J Physiol Paris. 2012;106:34–39. doi: 10.1016/j.jphysparis.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Girouard H, Bonev AD, Hannah RM, Meredith A, Aldrich RW, Nelson MT. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc Natl Acad Sci U S A. 2010;107:3811–3816. doi: 10.1073/pnas.0914722107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goadsby PJ, Edvinson L. Neurovascular control of the cerebral circulation. New York: Lippincott Williams & Wilkins; 2002. [Google Scholar]
- Golovina VA. Visualization of localized store-operated calcium entry in mouse astrocytes. Close proximity to the endoplasmic reticulum. J Physiol. 2005;564:737–749. doi: 10.1113/jphysiol.2005.085035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon GR, Choi HB, Rungta RL, Ellis-Davies GC, MacVicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456:745–749. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon GR, Mulligan SJ, MacVicar BA. Astrocyte control of the cerebrovasculature. Glia. 2007;55:1214–1221. doi: 10.1002/glia.20543. [DOI] [PubMed] [Google Scholar]
- Grimaldi M, Maratos M, Verma A. Transient receptor potential channel activation causes a novel form of [Ca 2+]I oscillations and is not involved in capacitative Ca 2+ entry in glial cells. J Neurosci. 2003;23:4737–4745. doi: 10.1523/JNEUROSCI.23-11-04737.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann R, Stence N, Carr J, Fuller L, Waite M, Dailey ME. Juxtavascular microglia migrate along brain microvessels following activation during early postnatal development. Glia. 2002;37:229–240. [PubMed] [Google Scholar]
- Gulbenkian S, Uddman R, Edvinsson L. Neuronal messengers in the human cerebral circulation. Peptides. 2001;22:995–1007. doi: 10.1016/s0196-9781(01)00408-9. [DOI] [PubMed] [Google Scholar]
- Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O’Farrell FM, Buchan AM, Lauritzen M, Attwell D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508:55–60. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel E. Cholinergic modulation of the cortical microvascular bed. Prog Brain Res. 2004;145:171–178. doi: 10.1016/S0079-6123(03)45012-7. [DOI] [PubMed] [Google Scholar]
- Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol. 2006;100:1059–1064. doi: 10.1152/japplphysiol.00954.2005. [DOI] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Harder DR, Narayanan J, Gebremedhin D. Pressure-induced myogenic tone and role of 20-HETE in mediating autoregulation of cerebral blood flow. Am J Physiol Heart Circ Physiol. 2011;300:H1557–1565. doi: 10.1152/ajpheart.01097.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder DR, Zhang C, Gebremedhin D. Astrocytes function in matching blood flow to metabolic activity. News in physiological sciences: an international journal of physiology produced jointly by the International Union of Physiological Sciences and the American Physiological Society. 2002;17:27–31. doi: 10.1152/physiologyonline.2002.17.1.27. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Ishibashi H, Hashimoto K, Nakanishi H. Potentiation of the NMDA receptor-mediated responses through the activation of the glycine site by microglia secreting soluble factors. Glia. 2006;53:660–668. doi: 10.1002/glia.20322. [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, Betsholtz C. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153:543–553. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashimori H, Blanco VM, Tuniki VR, Falck JR, Filosa JA. Role of epoxyeicosatrienoic acids as autocrine metabolites in glutamate-mediated K+ signaling in perivascular astrocytes. Am J Physiol Cell Physiol. 2010;299:C1068–1078. doi: 10.1152/ajpcell.00225.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KK, D’Amore PA. Pericytes in the microvasculature. Cardiovasc Res. 1996;32:687–698. [PubMed] [Google Scholar]
- Horiuchi T, Dietrich HH, Hongo K, Dacey RG., Jr Mechanism of extracellular K+-induced local and conducted responses in cerebral penetrating arterioles. Stroke. 2002;33:2692–2699. doi: 10.1161/01.str.0000034791.52151.6b. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Neurogenic control of the cerebral microcirculation: is dopamine minding the store? Nat Neurosci. 1998;1:263–265. doi: 10.1038/1074. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- Iddings JA, Kim KJ, Zhou Y, Higashimori H, Filosa JA. Enhanced parenchymal arteriole tone and astrocyte signaling protect neurovascular coupling mediated parenchymal arteriole vasodilation in the spontaneously hypertensive rat. J Cereb Blood Flow Metab. 2015 doi: 10.1038/jcbfm.2015.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M, Kajimura M, Adachi T, Maruyama K, Makino N, Goda N, Yamaguchi T, Sekizuka E, Suematsu M. Carbon monoxide from heme oxygenase-2 Is a tonic regulator against NO-dependent vasodilatation in the adult rat cerebral microcirculation. Circ Res. 2005;97:e104–114. doi: 10.1161/01.RES.0000196681.34485.ec. [DOI] [PubMed] [Google Scholar]
- Jaggar JH, Leffler CW, Cheranov SY, Tcheranova D, ES, Cheng X. Carbon monoxide dilates cerebral arterioles by enhancing the coupling of Ca2+ sparks to Ca2+-activated K+ channels. Circ Res. 2002;91:610–617. doi: 10.1161/01.res.0000036900.76780.95. [DOI] [PubMed] [Google Scholar]
- Jakovcevic D, Harder DR. Role of astrocytes in matching blood flow to neuronal activity. Curr Top Dev Biol. 2007;79:75–97. doi: 10.1016/S0070-2153(06)79004-4. [DOI] [PubMed] [Google Scholar]
- Jego P, Pacheco-Torres J, Araque A, Canals S. Functional MRI in mice lacking IP3-dependent calcium signaling in astrocytes. J Cereb Blood Flow Metab. 2014;34:1599–1603. doi: 10.1038/jcbfm.2014.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacem K, Lacombe P, Seylaz J, Bonvento G. Structural organization of the perivascular astrocyte endfeet and their relationship with the endothelial glucose transporter: a confocal microscopy study. Glia. 1998;23:1–10. [PubMed] [Google Scholar]
- Kawamura H, Kobayashi M, Li Q, Yamanishi S, Katsumura K, Minami M, Wu DM, Puro DG. Effects of angiotensin II on the pericyte-containing microvasculature of the rat retina. J Physiol. 2004;561:671–683. doi: 10.1113/jphysiol.2004.073098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura H, Sugiyama T, Wu DM, Kobayashi M, Yamanishi S, Katsumura K, Puro DG. ATP: a vasoactive signal in the pericyte-containing microvasculature of the rat retina. J Physiol. 2003;551:787–799. doi: 10.1113/jphysiol.2003.047977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KJ, Filosa JA. Advanced in vitro approach to study neurovascular coupling mechanisms in the brain microcirculation. J Physiol. 2012;590:1757–1770. doi: 10.1113/jphysiol.2011.222778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knot HJ, Zimmermann PA, Nelson MT. Extracellular K(+)-induced hyperpolarizations and dilatations of rat coronary and cerebral arteries involve inward rectifier K(+) channels. J Physiol. 1996;492 (Pt 2):419–430. doi: 10.1113/jphysiol.1996.sp021318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler RC, Roman RJ, Harder DR. Astrocytes and the regulation of cerebral blood flow. Trends Neurosci. 2009;32:160–169. doi: 10.1016/j.tins.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Koller A, Toth P. Contribution of flow-dependent vasomotor mechanisms to the autoregulation of cerebral blood flow. J Vasc Res. 2012;49:375–389. doi: 10.1159/000338747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuga N, Hirata T, Sakai I, Tanikawa Y, Chiou HY, Kitanishi T, Matsuki N, Ikegaya Y. Rapid and local autoregulation of cerebrovascular blood flow: a deep-brain imaging study in the mouse. J Physiol. 2009;587:745–752. doi: 10.1113/jphysiol.2008.163253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano Y, Echeverry G, Miekisiak G, Kulik TB, Aronhime SN, Chen JF, Winn HR. Role of adenosine A2 receptors in regulation of cerebral blood flow during induced hypotension. J Cereb Blood Flow Metab. 2010;30:808–815. doi: 10.1038/jcbfm.2009.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuschinsky W, Wahl M, Bosse O, Thurau K. Perivascular potassium and pH as determinants of local pial arterial diameter in cats. A microapplication study. Circ Res. 1972;31:240–247. doi: 10.1161/01.res.31.2.240. [DOI] [PubMed] [Google Scholar]
- Laranjinha J, Santos RM, Lourenco CF, Ledo A, Barbosa RM. Nitric oxide signaling in the brain: translation of dynamics into respiration control and neurovascular coupling. Ann N Y Acad Sci. 2012;1259:10–18. doi: 10.1111/j.1749-6632.2012.06582.x. [DOI] [PubMed] [Google Scholar]
- Lassmann H, Zimprich F, Vass K, Hickey WF. Microglial cells are a component of the perivascular glia limitans. J Neurosci Res. 1991;28:236–243. doi: 10.1002/jnr.490280211. [DOI] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- Lee SM, Cho YS, Kim TH, Jin MU, Ahn DK, Noguchi K, Bae YC. An ultrastructural evidence for the expression of transient receptor potential ankyrin 1 (TRPA1) in astrocytes in the rat trigeminal caudal nucleus. Journal of chemical neuroanatomy. 2012;45:45–49. doi: 10.1016/j.jchemneu.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Leffler CW, Balabanova L, Fedinec AL, Waters CM, Parfenova H. Mechanism of glutamate stimulation of CO production in cerebral microvessels. Am J Physiol Heart Circ Physiol. 2003;285:H74–80. doi: 10.1152/ajpheart.01081.2002. [DOI] [PubMed] [Google Scholar]
- Leffler CW, Parfenova H, Jaggar JH. Carbon monoxide as an endogenous vascular modulator. Am J Physiol Heart Circ Physiol. 2011;301:H1–H11. doi: 10.1152/ajpheart.00230.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffler CW, Parfenova H, Jaggar JH, Wang R. Carbon monoxide and hydrogen sulfide: gaseous messengers in cerebrovascular circulation. J Appl Physiol. 2006;100:1065–1076. doi: 10.1152/japplphysiol.00793.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Sul JY, Haydon PG. A calcium-induced calcium influx factor, nitric oxide, modulates the refilling of calcium stores in astrocytes. J Neurosci. 2003;23:10302–10310. doi: 10.1523/JNEUROSCI.23-32-10302.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Puro DG. Adenosine activates ATP-sensitive K(+) currents in pericytes of rat retinal microvessels: role of A1 and A2a receptors. Brain Res. 2001;907:93–99. doi: 10.1016/s0006-8993(01)02607-5. [DOI] [PubMed] [Google Scholar]
- Lind BL, Brazhe AR, Jessen SB, Tan FC, Lauritzen MJ. Rapid stimulus-evoked astrocyte Ca2+ elevations and hemodynamic responses in mouse somatosensory cortex in vivo. Proc Natl Acad Sci U S A. 2013;110:E4678–4687. doi: 10.1073/pnas.1310065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindauer U, Leithner C, Kaasch H, Rohrer B, Foddis M, Fuchtemeier M, Offenhauser N, Steinbrink J, Royl G, Kohl-Bareis M, Dirnagl U. Neurovascular coupling in rat brain operates independent of hemoglobin deoxygenation. J Cereb Blood Flow Metab. 2010;30:757–768. doi: 10.1038/jcbfm.2009.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindauer U, Megow D, Matsuda H, Dirnagl U. Nitric oxide: a modulator, but not a mediator, of neurovascular coupling in rat somatosensory cortex. Am J Physiol. 1999;277:H799–811. doi: 10.1152/ajpheart.1999.277.2.H799. [DOI] [PubMed] [Google Scholar]
- Liu W, Tang Y, Feng J. Life Sci: 2011. Elsevier Inc; 2011a. Cross talk between activation of microglia and astrocytes in pathological conditions in the central nervous system. [DOI] [PubMed] [Google Scholar]
- Liu X, Li C, Gebremedhin D, Hwang SH, Hammock BD, Falck JR, Roman RJ, Harder DR, Koehler RC. Epoxyeicosatrienoic acid-dependent cerebral vasodilation evoked by metabotropic glutamate receptor activation in vivo. Am J Physiol Heart Circ Physiol. 2011b;301:H373–381. doi: 10.1152/ajpheart.00745.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malarkey EB, Ni Y, Parpura V. Ca2+ entry through TRPC1 channels contributes to intracellular Ca2+ dynamics and consequent glutamate release from rat astrocytes. Glia. 2008;56:821–835. doi: 10.1002/glia.20656. [DOI] [PubMed] [Google Scholar]
- Masuda T, Croom D, Hida H, Kirov SA. Capillary blood flow around microglial somata determines dynamics of microglial processes in ischemic conditions. Glia. 2011;59:1744–1753. doi: 10.1002/glia.21220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia. 2010;58:1094–1103. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- McCarron JG, Halpern W. Potassium dilates rat cerebral arteries by two independent mechanisms. Am J Physiol. 1990;259:H902–908. doi: 10.1152/ajpheart.1990.259.3.H902. [DOI] [PubMed] [Google Scholar]
- McCaslin AF, Chen BR, Radosevich AJ, Cauli B, Hillman EM. In vivo 3D morphology of astrocyte-vasculature interactions in the somatosensory cortex: implications for neurovascular coupling. J Cereb Blood Flow Metab. 2011;31:795–806. doi: 10.1038/jcbfm.2010.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metea MR, Kofuji P, Newman EA. Neurovascular coupling is not mediated by potassium siphoning from glial cells. J Neurosci. 2007;27:2468–2471. doi: 10.1523/JNEUROSCI.3204-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metea MR, Newman EA. Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J Neurosci. 2006;26:2862–2870. doi: 10.1523/JNEUROSCI.4048-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A, Hamid A, Newman EA. Oxygen modulation of neurovascular coupling in the retina. Proc Natl Acad Sci U S A. 2011;108:17827–17831. doi: 10.1073/pnas.1110533108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyano K, Morioka N, Sugimoto T, Shiraishi S, Uezono Y, Nakata Y. Activation of the neurokinin-1 receptor in rat spinal astrocytes induces Ca2+ release from IP3-sensitive Ca2+ stores and extracellular Ca2+ influx through TRPC3. Neurochem Int. 2010;57:923–934. doi: 10.1016/j.neuint.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Morrison HW, Filosa JA. A quantitative spatiotemporal analysis of microglia morphology during ischemic stroke and reperfusion. J Neuroinflammation. 2013;10:4. doi: 10.1186/1742-2094-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- Nayak D, Zinselmeyer BH, Corps KN, McGavern DB. In vivo dynamics of innate immune sentinels in the CNS. Intravital (Print) 2012;1:95–106. doi: 10.4161/intv.22823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA. High potassium conductance in astrocyte endfeet. Science. 1986;233:453–454. doi: 10.1126/science.3726539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A. Astrocytes going live: advances and challenges. J Physiol. 2009;587:1639–1647. doi: 10.1113/jphysiol.2008.167171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizar K, Uhlirova H, Tian P, Saisan PA, Cheng Q, Reznichenko L, Weldy KL, Steed TC, Sridhar VB, MacDonald CL, Cui J, Gratiy SL, Sakadzic S, Boas DA, Beka TI, Einevoll GT, Chen J, Masliah E, Dale AM, Silva GA, Devor A. In vivo stimulus-induced vasodilation occurs without IP3 receptor activation and may precede astrocytic calcium increase. J Neurosci. 2013;33:8411–8422. doi: 10.1523/JNEUROSCI.3285-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata K, Kosaka T. Structural and quantitative analysis of astrocytes in the mouse hippocampus. Neuroscience. 2002;113:221–233. doi: 10.1016/s0306-4522(02)00041-6. [DOI] [PubMed] [Google Scholar]
- Orellana JA, Montero TD, von Bernhardi R. Astrocytes inhibit nitric oxide-dependent Ca(2+) dynamics in activated microglia: involvement of ATP released via pannexin 1 channels. Glia. 2013;61:2023–2037. doi: 10.1002/glia.22573. [DOI] [PubMed] [Google Scholar]
- Parfenova H, Tcheranova D, Basuroy S, Fedinec AL, Liu J, Leffler CW. Functional role of astrocyte glutamate receptors and carbon monoxide in cerebral vasodilation response to glutamate. Am J Physiol Heart Circ Physiol. 2012;302:H2257–2266. doi: 10.1152/ajpheart.01011.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual O, Ben Achour S, Rostaing P, Triller A, Bessis A. Proc Natl Acad Sci U S A. Vol. 109. United States: 2012. Microglia activation triggers astrocyte-mediated modulation of excitatory neurotransmission; pp. E197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson OB, Newman EA. Does the release of potassium from astrocyte endfeet regulate cerebral blood flow? Science. 1987;237:896–898. doi: 10.1126/science.3616619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Carhuapoma JR, Bhardwaj A, Alkayed NJ, Falck JR, Harder DR, Traystman RJ, Koehler RC. Suppression of cortical functional hyperemia to vibrissal stimulation in the rat by epoxygenase inhibitors. Am J Physiol Heart Circ Physiol. 2002;283:H2029–2037. doi: 10.1152/ajpheart.01130.2000. [DOI] [PubMed] [Google Scholar]
- Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen MA, Dailey ME. Diverse microglial motility behaviors during clearance of dead cells in hippocampal slices. Glia. 2004;46:195–206. doi: 10.1002/glia.10362. [DOI] [PubMed] [Google Scholar]
- Petravicz J, Fiacco TA, McCarthy KD. Loss of IP3 receptor-dependent Ca2+ increases in hippocampal astrocytes does not affect baseline CA1 pyramidal neuron synaptic activity. J Neurosci. 2008;28:4967–4973. doi: 10.1523/JNEUROSCI.5572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DL, Ludwig JW, Mi H, Schwarz TL, Ellisman MH. Distribution of rSlo Ca2+-activated K+ channels in rat astrocyte perivascular endfeet. Brain Res. 2002;956:183–193. doi: 10.1016/s0006-8993(02)03266-3. [DOI] [PubMed] [Google Scholar]
- Rancillac A, Rossier J, Guille M, Tong XK, Geoffroy H, Amatore C, Arbault S, Hamel E, Cauli B. Glutamatergic Control of Microvascular Tone by Distinct GABA Neurons in the Cerebellum. J Neurosci. 2006;26:6997–7006. doi: 10.1523/JNEUROSCI.5515-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes RC, Verkhratsky A, Parpura V. TRPC1-mediated Ca2+ and Na+ signalling in astroglia: differential filtering of extracellular cations. Cell Calcium. 2013;54:120–125. doi: 10.1016/j.ceca.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaie P, Dean A, Male D, Ulfig N. Microglia in the cerebral wall of the human telencephalon at second trimester. Cereb Cortex. 2005;15:938–949. doi: 10.1093/cercor/bhh194. [DOI] [PubMed] [Google Scholar]
- Saijo K, Glass CK. Microglial cell origin and phenotypes in health and disease. Nature reviews Immunology. 2011;11:775–787. doi: 10.1038/nri3086. [DOI] [PubMed] [Google Scholar]
- Sakagami K, Kawamura H, Wu DM, Puro DG. Nitric oxide/cGMP-induced inhibition of calcium and chloride currents in retinal pericytes. Microvasc Res. 2001;62:196–203. doi: 10.1006/mvre.2001.2343. [DOI] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Stevens B. The “quad-partite” synapse: microglia-synapse interactions in the developing and mature CNS. Glia. 2013;61:24–36. doi: 10.1002/glia.22389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlageter KE, Molnar P, Lapin GD, Groothuis DR. Microvessel organization and structure in experimental brain tumors: microvessel populations with distinctive structural and functional properties. Microvasc Res. 1999;58:312–328. doi: 10.1006/mvre.1999.2188. [DOI] [PubMed] [Google Scholar]
- Scianni M, Antonilli L, Chece G, Cristalli G, Di Castro MA, Limatola C, Maggi L. Fractalkine (CX3CL1) enhances hippocampal N-methyl-D-aspartate receptor (NMDAR) function via D-serine and adenosine receptor type A2 (A2AR) activity. J Neuroinflammation. 2013;10:108. doi: 10.1186/1742-2094-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepro D, Morel NM. Pericyte physiology. FASEB J. 1993;7:1031–1038. doi: 10.1096/fasebj.7.11.8370472. [DOI] [PubMed] [Google Scholar]
- Shi Y, Liu X, Gebremedhin D, Falck JR, Harder DR, Koehler RC. Interaction of mechanisms involving epoxyeicosatrienoic acids, adenosine receptors, and metabotropic glutamate receptors in neurovascular coupling in rat whisker barrel cortex. J Cereb Blood Flow Metab. 2008;28:111–125. doi: 10.1038/sj.jcbfm.9600511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki K, Ikenaka K, Tamalu F, Tominaga M, Ishizaki Y. A novel subtype of astrocytes expressing TRPV4 (transient receptor potential vanilloid 4) regulates neuronal excitability via release of gliotransmitters. J Biol Chem. 2014;289:14470–14480. doi: 10.1074/jbc.M114.557132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Bushong EA, Haustein MD, Tong X, Jackson-Weaver O, Kracun S, Xu J, Sofroniew MV, Ellisman MH, Khakh BS. Imaging calcium microdomains within entire astrocyte territories and endfeet with GCaMPs expressed using adeno-associated viruses. J Gen Physiol. 2013a;141:633–647. doi: 10.1085/jgp.201210949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Jackson-Weaver O, Huckstepp RT, O’Dell TJ, Khakh BS. TRPA1 channels are regulators of astrocyte basal calcium levels and long-term potentiation via constitutive D-serine release. J Neurosci. 2013b;33:10143–10153. doi: 10.1523/JNEUROSCI.5779-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Tong X, Kwan KY, Corey DP, Khakh BS. TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat Neurosci. 2012;15:70–80. doi: 10.1038/nn.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa H, Sakimoto S, Nakao K, Sugishita A, Konno M, Iida S, Kusano A, Hashimoto E, Nakagawa T, Kaneko S. Transient receptor potential canonical 3 (TRPC3) mediates thrombin-induced astrocyte activation and upregulates its own expression in cortical astrocytes. J Neurosci. 2010;30:13116–13129. doi: 10.1523/JNEUROSCI.1890-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard M, Arcuino G, Takano T, Liu QS, Nedergaard M. Signaling at the gliovascular interface. J Neurosci. 2003;23:9254–9262. doi: 10.1523/JNEUROSCI.23-27-09254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard M, Nedergaard M. The neurobiology of glia in the context of water and ion homeostasis. Neuroscience. 2004;129:877–896. doi: 10.1016/j.neuroscience.2004.09.053. [DOI] [PubMed] [Google Scholar]
- Siskova Z, Tremblay ME. Microglia and synapse: interactions in health and neurodegeneration. Neural plasticity. 2013;2013:425845. doi: 10.1155/2013/425845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobey CG, Faraci FM. Effect of nitric oxide and potassium channel agonists and inhibitors on basilar artery diameter. Am J Physiol. 1997;272:H256–262. doi: 10.1152/ajpheart.1997.272.1.H256. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SW, Barres BA. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Straub SV, Bonev AD, Wilkerson MK, Nelson MT. Dynamic inositol trisphosphate-mediated calcium signals within astrocytic endfeet underlie vasodilation of cerebral arterioles. J Gen Physiol. 2006;128:659–669. doi: 10.1085/jgp.200609650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun CW, Falck JR, Okamoto H, Harder DR, Roman RJ. Role of cGMP versus 20-HETE in the vasodilator response to nitric oxide in rat cerebral arteries. Am J Physiol Heart Circ Physiol. 2000;279:H339–350. doi: 10.1152/ajpheart.2000.279.1.H339. [DOI] [PubMed] [Google Scholar]
- Sun W, McConnell E, Pare JF, Xu Q, Chen M, Peng W, Lovatt D, Han X, Smith Y, Nedergaard M. Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science. 2013;339:197–200. doi: 10.1126/science.1226740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- Theis M, Sohl G, Eiberger J, Willecke K. Emerging complexities in identity and function of glial connexins. Trends Neurosci. 2005;28:188–195. doi: 10.1016/j.tins.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Thrane AS, Rangroo Thrane V, Zeppenfeld D, Lou N, Xu Q, Nagelhus EA, Nedergaard M. General anesthesia selectively disrupts astrocyte calcium signaling in the awake mouse cortex. Proc Natl Acad Sci U S A. 2012;109:18974–18979. doi: 10.1073/pnas.1209448109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Rozsa B, Springo Z, Doczi T, Koller A. Isolated human and rat cerebral arteries constrict to increases in flow: role of 20-HETE and TP receptors. J Cereb Blood Flow Metab. 2011;31:2096–2105. doi: 10.1038/jcbfm.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS biology. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udosen IT, Jiang H, Hercule HC, Oyekan AO. Nitric oxide-epoxygenase interactions and arachidonate-induced dilation of rat renal microvessels. Am J Physiol Heart Circ Physiol. 2003;285:H2054–2063. doi: 10.1152/ajpheart.00075.2003. [DOI] [PubMed] [Google Scholar]
- Vetri F, Xu H, Mao L, Paisansathan C, Pelligrino DA. ATP hydrolysis pathways and their contributions to pial arteriolar dilation in rats. Am J Physiol Heart Circ Physiol. 2011;301:H1369–1377. doi: 10.1152/ajpheart.00556.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Schuler B, Vogel O, Arras M, Vogel J. What is the optimal anesthetic protocol for measurements of cerebral autoregulation in spontaneously breathing mice? Exp Brain Res. 2010;207:249–258. doi: 10.1007/s00221-010-2447-4. [DOI] [PubMed] [Google Scholar]
- Wiencken AE, Casagrande VA. Endothelial nitric oxide synthetase (eNOS) in astrocytes: another source of nitric oxide in neocortex. Glia. 1999;26:280–290. [PubMed] [Google Scholar]
- Winship IR, Plaa N, Murphy TH. Rapid astrocyte calcium signals correlate with neuronal activity and onset of the hemodynamic response in vivo. J Neurosci. 2007;27:6268–6272. doi: 10.1523/JNEUROSCI.4801-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf F, Kirchhoff F. Neuroscience. Imaging astrocyte activity. Science. 2008;320:1597–1599. doi: 10.1126/science.1160122. [DOI] [PubMed] [Google Scholar]
- Wu DM, Kawamura H, Li Q, Puro DG. Dopamine activates ATP-sensitive K+ currents in rat retinal pericytes. Vis Neurosci. 2001;18:935–940. [PubMed] [Google Scholar]
- Xi Q, Tcheranova D, Basuroy S, Parfenova H, Jaggar JH, Leffler CW. Glutamate-induced calcium signals stimulate CO production in piglet astrocytes. Am J Physiol Heart Circ Physiol. 2011;301:H428–433. doi: 10.1152/ajpheart.01277.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HL, Mao L, Ye S, Paisansathan C, Vetri F, Pelligrino DA. Astrocytes are a key conduit for upstream signaling of vasodilation during cerebral cortical neuronal activation in vivo. Am J Physiol Heart Circ Physiol. 2008;294:H622–632. doi: 10.1152/ajpheart.00530.2007. [DOI] [PubMed] [Google Scholar]
- Yang G, Chen G, Ebner TJ, Iadecola C. Nitric oxide is the predominant mediator of cerebellar hyperemia during somatosensory activation in rats. Am J Physiol. 1999;277:R1760–1770. doi: 10.1152/ajpregu.1999.277.6.R1760. [DOI] [PubMed] [Google Scholar]
- Yang G, Huard JM, Beitz AJ, Ross ME, Iadecola C. Stellate neurons mediate functional hyperemia in the cerebellar molecular layer. J Neurosci. 2000;20:6968–6973. doi: 10.1523/JNEUROSCI.20-18-06968.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003a;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- Zonta M, Sebelin A, Gobbo S, Fellin T, Pozzan T, Carmignoto G. Glutamate-mediated cytosolic calcium oscillations regulate a pulsatile prostaglandin release from cultured rat astrocytes. J Physiol. 2003b;553:407–414. doi: 10.1113/jphysiol.2003.046706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorec R, Araque A, Carmignoto G, Haydon PG, Verkhratsky A, Parpura V. Astroglial excitability and gliotransmission: an appraisal of Ca2+ as a signalling route. ASN Neuro. 2012;4 doi: 10.1042/AN20110061. [DOI] [PMC free article] [PubMed] [Google Scholar]