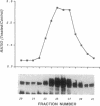
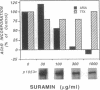
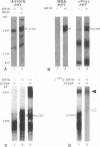
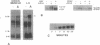Abstract
Motoneurons promote the accumulation of acetylcholine receptors (AChRs) at developing neuromuscular synapses. The AChR-inducing activity protein ARIA, which is purified from chicken brain and increases the synthesis of AChRs in chicken myotubes, may play a crucial role in this process. Here we show that ARIA induces the rapid tyrosine phosphorylation of a M(r) 185,000 protein (p185) in muscle cells. Phosphorylation of p185 correlates with AChR induction at each stage of ARIA purification. Moreover, medium conditioned by spinal cord motoneurons stimulates AChR synthesis and p185 phosphorylation. Studies with membrane-impermeant reagents and 125I-labeled ARIA indicate that p185 is a transmembrane ARIA-receptor tyrosine kinase. Our data suggests that muscle AChR synthesis can be regulated through tyrosine phosphorylation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987 Apr 25;262(12):5592–5595. [PubMed] [Google Scholar]
- Anderson M. J., Cohen M. W., Zorychta E. Effects of innervation on the distribution of acetylcholine receptors on cultured muscle cells. J Physiol. 1977 Jul;268(3):731–756. doi: 10.1113/jphysiol.1977.sp011879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg M. M., Sternberg D. W., Parada L. F., Chao M. V. K-252a inhibits nerve growth factor-induced trk proto-oncogene tyrosine phosphorylation and kinase activity. J Biol Chem. 1992 Jan 5;267(1):13–16. [PubMed] [Google Scholar]
- Birnbaum M., Reis M. A., Shainberg A. Role of calcium in the regulation of acetylcholine receptor synthese in cultured muscle cells*. Pflugers Arch. 1980 May;385(1):37–43. doi: 10.1007/BF00583913. [DOI] [PubMed] [Google Scholar]
- Burgess W. H., Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem. 1989;58:575–606. doi: 10.1146/annurev.bi.58.070189.003043. [DOI] [PubMed] [Google Scholar]
- Coffey R. J., Jr, Leof E. B., Shipley G. D., Moses H. L. Suramin inhibition of growth factor receptor binding and mitogenicity in AKR-2B cells. J Cell Physiol. 1987 Jul;132(1):143–148. doi: 10.1002/jcp.1041320120. [DOI] [PubMed] [Google Scholar]
- Cohen S. A., Fischbach G. D. Clusters of acetylcholine receptors located at identified nerve-muscle synapses in vitro. Dev Biol. 1977 Aug;59(1):24–38. doi: 10.1016/0012-1606(77)90237-8. [DOI] [PubMed] [Google Scholar]
- Cohen S. A., Fischbach G. D. Regulation of muscle acetylcholine sensitivity by muscle activity in cell culture. Science. 1973 Jul 6;181(4094):76–78. doi: 10.1126/science.181.4094.76. [DOI] [PubMed] [Google Scholar]
- Dohrmann U., Edgar D., Sendtner M., Thoenen H. Muscle-derived factors that support survival and promote fiber outgrowth from embryonic chick spinal motor neurons in culture. Dev Biol. 1986 Nov;118(1):209–221. doi: 10.1016/0012-1606(86)90089-8. [DOI] [PubMed] [Google Scholar]
- Falls D. L., Harris D. A., Johnson F. A., Morgan M. M., Corfas G., Fischbach G. D. Mr 42,000 ARIA: a protein that may regulate the accumulation of acetylcholine receptors at developing chick neuromuscular junctions. Cold Spring Harb Symp Quant Biol. 1990;55:397–406. doi: 10.1101/sqb.1990.055.01.040. [DOI] [PubMed] [Google Scholar]
- Fontaine B., Klarsfeld A., Changeux J. P. Calcitonin gene-related peptide and muscle activity regulate acetylcholine receptor alpha-subunit mRNA levels by distinct intracellular pathways. J Cell Biol. 1987 Sep;105(3):1337–1342. doi: 10.1083/jcb.105.3.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey E. W., Nitkin R. M., Wallace B. G., Rubin L. L., McMahan U. J. Components of Torpedo electric organ and muscle that cause aggregation of acetylcholine receptors on cultured muscle cells. J Cell Biol. 1984 Aug;99(2):615–627. doi: 10.1083/jcb.99.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D. A., Falls D. L., Dill-Devor R. M., Fischbach G. D. Acetylcholine receptor-inducing factor from chicken brain increases the level of mRNA encoding the receptor alpha subunit. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1983–1987. doi: 10.1073/pnas.85.6.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D. A., Falls D. L., Johnson F. A., Fischbach G. D. A prion-like protein from chicken brain copurifies with an acetylcholine receptor-inducing activity. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7664–7668. doi: 10.1073/pnas.88.17.7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfield J. F., Tank D. W., Greengard P., Huganir R. L. Functional modulation of the nicotinic acetylcholine receptor by tyrosine phosphorylation. Nature. 1988 Dec 15;336(6200):677–680. doi: 10.1038/336677a0. [DOI] [PubMed] [Google Scholar]
- Huang C. F., Tong J., Schmidt J. Protein kinase C couples membrane excitation to acetylcholine receptor gene inactivation in chick skeletal muscle. Neuron. 1992 Oct;9(4):671–678. doi: 10.1016/0896-6273(92)90030-h. [DOI] [PubMed] [Google Scholar]
- Ingalls H. M., Goodloe-Holland C. M., Luna E. J. Junctional plasma membrane domains isolated from aggregating Dictyostelium discoideum amebae. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4779–4783. doi: 10.1073/pnas.83.13.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell T. M., Siegel R. E., Fischbach G. D. Induction of acetylcholine receptors on cultured skeletal muscle by a factor extracted from brain and spinal cord. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5397–5401. doi: 10.1073/pnas.76.10.5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirilovsky J., Duclert A., Fontaine B., Devillers-Thiery A., Osterlund M., Changeux J. P. Acetylcholine receptor expression in primary cultures of embryonic chick myotubes--II. Comparison between the effects of spinal cord cells and calcitonin gene-related peptide. Neuroscience. 1989;32(2):289–296. doi: 10.1016/0306-4522(89)90079-1. [DOI] [PubMed] [Google Scholar]
- Klarsfeld A., Laufer R., Fontaine B., Devillers-Thiéry A., Dubreuil C., Changeux J. P. Regulation of muscle AChR alpha subunit gene expression by electrical activity: involvement of protein kinase C and Ca2+. Neuron. 1989 Mar;2(3):1229–1236. doi: 10.1016/0896-6273(89)90307-3. [DOI] [PubMed] [Google Scholar]
- Lyall R. M., Zilberstein A., Gazit A., Gilon C., Levitzki A., Schlessinger J. Tyrphostins inhibit epidermal growth factor (EGF)-receptor tyrosine kinase activity in living cells and EGF-stimulated cell proliferation. J Biol Chem. 1989 Aug 25;264(24):14503–14509. [PubMed] [Google Scholar]
- Martinou J. C., Falls D. L., Fischbach G. D., Merlie J. P. Acetylcholine receptor-inducing activity stimulates expression of the epsilon-subunit gene of the muscle acetylcholine receptor. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7669–7673. doi: 10.1073/pnas.88.17.7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahan U. J. The agrin hypothesis. Cold Spring Harb Symp Quant Biol. 1990;55:407–418. doi: 10.1101/sqb.1990.055.01.041. [DOI] [PubMed] [Google Scholar]
- Nicola N. A., Metcalf D. Subunit promiscuity among hemopoietic growth factor receptors. Cell. 1991 Oct 4;67(1):1–4. doi: 10.1016/0092-8674(91)90564-f. [DOI] [PubMed] [Google Scholar]
- Onoda T., Iinuma H., Sasaki Y., Hamada M., Isshiki K., Naganawa H., Takeuchi T., Tatsuta K., Umezawa K. Isolation of a novel tyrosine kinase inhibitor, lavendustin A, from Streptomyces griseolavendus. J Nat Prod. 1989 Nov-Dec;52(6):1252–1257. doi: 10.1021/np50066a009. [DOI] [PubMed] [Google Scholar]
- Role L. W., Matossian V. R., O'Brien R. J., Fischbach G. D. On the mechanism of acetylcholine receptor accumulation at newly formed synapses on chick myotubes. J Neurosci. 1985 Aug;5(8):2197–2204. doi: 10.1523/JNEUROSCI.05-08-02197.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Yamaguchi Y. Proteoglycans as modulators of growth factor activities. Cell. 1991 Mar 8;64(5):867–869. doi: 10.1016/0092-8674(91)90308-l. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Ullrich A. Growth factor signaling by receptor tyrosine kinases. Neuron. 1992 Sep;9(3):383–391. doi: 10.1016/0896-6273(92)90177-f. [DOI] [PubMed] [Google Scholar]
- Shainberg A., Burstein M. Decrease of acetylcholine receptor synthesis in muscle cultures by electrical stimulation. Nature. 1976 Nov 25;264(5584):368–369. doi: 10.1038/264368a0. [DOI] [PubMed] [Google Scholar]
- Shing Y., Folkman J., Sullivan R., Butterfield C., Murray J., Klagsbrun M. Heparin affinity: purification of a tumor-derived capillary endothelial cell growth factor. Science. 1984 Mar 23;223(4642):1296–1299. doi: 10.1126/science.6199844. [DOI] [PubMed] [Google Scholar]
- Staros J. V. N-hydroxysulfosuccinimide active esters: bis(N-hydroxysulfosuccinimide) esters of two dicarboxylic acids are hydrophilic, membrane-impermeant, protein cross-linkers. Biochemistry. 1982 Aug 17;21(17):3950–3955. doi: 10.1021/bi00260a008. [DOI] [PubMed] [Google Scholar]
- Uehara Y., Fukazawa H. Use and selectivity of herbimycin A as inhibitor of protein-tyrosine kinases. Methods Enzymol. 1991;201:370–379. doi: 10.1016/0076-6879(91)01033-x. [DOI] [PubMed] [Google Scholar]
- Umezawa K., Hori T., Tajima H., Imoto M., Isshiki K., Takeuchi T. Inhibition of epidermal growth factor-induced DNA synthesis by tyrosine kinase inhibitors. FEBS Lett. 1990 Jan 29;260(2):198–200. doi: 10.1016/0014-5793(90)80102-o. [DOI] [PubMed] [Google Scholar]
- Usdin T. B., Fischbach G. D. Purification and characterization of a polypeptide from chick brain that promotes the accumulation of acetylcholine receptors in chick myotubes. J Cell Biol. 1986 Aug;103(2):493–507. doi: 10.1083/jcb.103.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace B. G., Qu Z., Huganir R. L. Agrin induces phosphorylation of the nicotinic acetylcholine receptor. Neuron. 1991 Jun;6(6):869–878. doi: 10.1016/0896-6273(91)90227-q. [DOI] [PubMed] [Google Scholar]