Abstract
The causes of preeclampsia remain one of the great medical mysteries of our time. This syndrome is thought to occur in two stages with abnormal placentation leading to a maternal inflammatory response. Specific regions of the placenta have distinct pathological features. During normal pregnancy, cytotrophoblasts emigrate from the chorionic villi and invade the uterus, reaching the inner third of the myometrium. This unusual process is made even more exceptional by the fact that the placental cells are hemi-allogeneic, co-expressing maternal and paternal genomes. Within the uterine wall, cytotrophoblasts deeply invade the spiral arteries. Cytotrophoblasts migrate up these vessels and replace, in a retrograde fashion, the maternal endothelial lining. They also insert themselves amongst the smooth muscle cells that form the tunica media. As a result, the spiral arteries attain the physiological properties that are required to adequately perfuse the placenta. In comparison, invasion of the venous side of the uterine circulation is minimal, sufficient to enable venous return. In preeclampsia, cytotrophoblast invasion of the interstitial uterine compartment is frequently shallow, although not consistently so. In many locations, spiral artery invasion is incomplete. There are many fewer endovascular cytotrophoblasts and some vessels retain portions of their endothelial lining with relatively intact muscular coats while others are not modified. Work from our group showed that these defects mirror deficits in the differentiation program that enables cytotrophoblast invasion of the uterine wall. During normal pregnancy, invasion is accompanied by downregulation of epithelial-like molecules that are indicative of their ectodermal origin and upregulation of numerous receptors and ligands that are typically expressed by endothelial or vascular smooth muscle cells. For example, the expression of epithelial-cadherin, the cell-cell adhesion molecule that many ectodermal derivatives use to adhere to one another, becomes nearly undetectable, replaced by vascular-endothelial cadherin, which serves the same purpose in blood vessels. Invading cytotrophoblasts also modulate vascular endothelial growth factor ligands and receptors, at some point in the differentiation process expressing every (mammalian) family member. Molecules in this family play crucial roles in vascular and trophoblast biology, including prevention of apoptosis. In preeclampsia, this process of vascular mimicry is incomplete, which we theorize hinders the cells interactions with spiral arterioles. What causes these aberrations? Given what is known from animal models and human risk factors, reduced placental perfusion and/or certain disease states (metabolic, immune and cardiovascular) lie upstream. Recent evidence suggests the surprising conclusion that isolation and culture of cytotrophoblasts from the placentas of pregnancies complicated by preeclampsia enables normalization of their gene expression. The affected molecules include SEMA3B, which downregulates vascular endothelial growth factor signaling through the PI3K/AKT and GSK3 pathways. Thus, some aspects of the aberrant differentiation of cytotrophoblasts within the uterine wall that is observed in situ may be reversible. The next challenge is asking what the instigating causes are. There is added urgency to finding the answers, as these pathways could be valuable therapeutic targets for reversing abnormal placental function in patients.
Keywords: cytotrophoblast, dysgenesis, placenta, preeclampsia, neoplasm, pregnancy, natural killer cells, HLA-G, angiogenic factor, anti-angiogenic factors, placental growth factor, PlGF, soluble VEGFR-1, endoglin, spiral arteries, endothelial cells, inflammation
Given that many central aspects of normal pregnancy remain enigmatic (e.g., immune tolerance of the hemi-allogeneic fetus, initiation of labor), it is not surprising that explaining what goes wrong in preeclampsia is a challenging undertaking. The fact that this pregnancy complication has a placental component has been known for decades1. The defects involve some of the most interesting and unusual cell-cell interactions in all of human biology. That these cells are from two individuals—a mother and her offspring—and three genomes—maternal, paternal and fetal—adds even greater fascination.
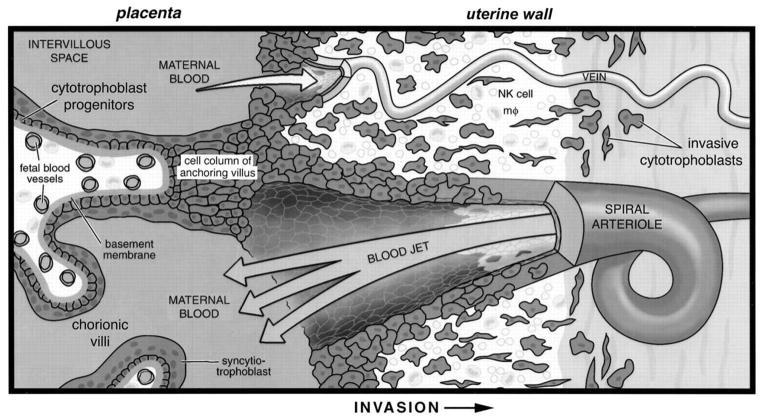
Formation of the affected area, the maternal-fetal interface, is a complex process (reviewed in2,3; diagrammed in Figure 1). In normal pregnancy, mononuclear cytotrophoblast (CTB) cells at the tips of placental anchoring villi depolarize, leaving the basement membrane to which they are attached in the rest of the placenta. The emigrating cells become strongly adherent to one another, forming aggregates that are attached at one end to the placenta and at the other end to the uterus. These so-called CTB columns must have sufficient structural integrity to bridge the gap between the placenta and the uterus. Additionally, these “highways” conduct the invasive cells that breach the uterine wall, migrating as far as the inner third of the myometrium where they stop for as yet unexplained reasons.
Figure 1.
Placental cytotrophoblasts invade the uterine wall where they breach veins and extensively remodel maternal spiral arterioles.
The bulk of the placenta is composed of numerous tree-like projections termed chorionic villi where maternal-fetal exchange occurs. These structures mediate the passage of nutrients, gases and wastes between fetal blood, which circulates through the villous core, and maternal blood, which circulates through the intervillous space. The uteroplacental circulation is established by cytotrophoblasts that acquire an invasive/endothelial phenotype as they leave the placenta and enter the uterine wall. Differentiation begins when cytotrophoblast progenitors that reside in a single layer surrounding the stromal core of anchoring villi emigrate, forming a cell column. These structures attach to the uterine wall and give rise to cells that invade the underlying decidual stroma. Invasive cytotrophoblasts breach uterine blood vessels connecting both the arterial and the venous circulation to the intervillous space. However, once this connection is made, remodeling of the venous side is halted. By contrast, cytotrophoblasts migrate up the lumina of spiral arterioles, eventually replacing the endothelial lining of the vessels and part of the muscular wall. This process encompasses the decidual and inner third of the myometrial segments of these vessels. NK, natural killer; mϕ, macrophage. Figure and legend reprinted with permission from Development80.
Interestingly, some early gestation placentas have large numbers of CTB aggregates, nascent columns that fail to make contact with the uterine wall. Termed cell islands, it is unclear why they are numerous in some pregnancies and not others4. They may be a sign of compensatory mechanisms in response to oxidative stress as we found that the appearance of these structures correlated with maternal smoking5 and cigarette use is protective against PE6. Islands have also been reported as increased in PE7. It would be interesting to know whether they are found more frequently during the early stages of placental development in pregnancies that are destined to be complicated by PE. If this is the case, they could be signs of failed compensation. At present it is technically impossible to visualize islands in vivo due to the low resolution of the available imaging techniques. With improved methods, it may eventually be possible to make this type of correlation, which would also lend insights into the origin of this syndrome.
Once CTBs enter the uterus they begin the process of radically altering its structure. In many areas, the uterine luminal epithelium is lost as CTBs track along its surface, in part, guided and fueled by the histiotrophe secretions of the glands8. Using cancer-like mechanisms, such as matrix metalloproteinases, CTBs invade the epithelial basement membrane that underlies these cells9. In response, stromal cells of the interstitium decidualize, a differentiation process that is driven by progesterone and cAMP10. Morphological and molecular changes ensue11. The formerly fibroblast-like cells take on a rounded appearance as they deposit a collection of basement membrane molecules and extracellular matrix components (laminin, type IV collagen, heparan sulfate proteoglycan, and fibronectin) in a pericellular location12. Their protein repertoire also changes. The products include hormones such as prolactin and many immune-type molecules. In culture, medium from isolated CTBs influences decidual stromal cell gene expression, including the induction of angiogenic factors, evidence of crosstalk13. CTBs also work cooperatively with decidual cells, for example, in production of a chemokine repertoire (e.g., CCL3) that retains the unusual population of immune cells (primarily NK cells with fewer numbers of macrophages and T-cells)14 that are recruited to the uterus during a nonpregnant cycle15.
CTB transformation of the uterine vasculature is a singular process, without direct parallels in biology or pathology. Some tumors coopt parts of this program, a haphazard process as compared to the precisely patterned structural changes that placental cells bring about during the first and second trimesters of pregnancy16. In the columns, they begin the process of vascular mimicry in which they downregulate the production of many epithelial-like molecules that are indicative of their ectodermal origin and upregulate numerous receptors and ligands that are typically expressed by endothelial or vascular smooth muscle cells (reviewed in17). For example, the expression of epithelial-cadherin, the cell-cell adhesion molecule that many ectodermal derivatives use to adhere to one another, becomes nearly undetectable, replaced by vascular-endothelial cadherin, which serves the same purpose in blood vessels. Invading CTBs also modulate vascular endothelial growth factor ligands and receptors, at some point in the differentiation process expressing every (mammalian) family member. The end result of this dramatic transformation is a placental cell with the molecular code of arterial endothelium. Likely, it is this switch that enables their extensive interactions with spiral arteries. CTBs migrate up these vessels and replace, in a retrograde fashion, the maternal endothelial lining. They also insert themselves amongst the smooth muscle cells that form the tunica media. This process, which encompasses the decidual portions of the arteries, extends into the inner myometrial segments. As a result, the spiral arteries attain the physiological and mechanical properties that are required to adequately perfuse the placenta. In comparison, invasion of the venous side of the uterine circulation is minimal, limited to the extent required to enable blood return. Somewhat paradoxically, immune cells are also involved in this transformation with NK cells cooperating in the process by secreting factors such as VEGF family members that play a role in the physiological transformation of the spiral arteries18.
In 1970, Brosens and colleagues described the defects in CTB patterning of the uterus that are frequently associated with PE1. CTB invasion of the interstitial uterine compartment is frequently shallow, although not consistently so. In many locations, spiral artery invasion is incomplete. Rather than the robust process that occurs in normal pregnancy, there are many fewer endovascular CTBs. Some vessels retain portions of their endothelial lining with relatively intact muscular coats, while others are not modified. As a result, they fail to enlarge to the extent that is required to deliver an adequate supply of maternal blood to the placenta. Several vessel abnormalities are evident—acute atherosis, fibrin deposits and the accumulation of lipid-laden macrophages19. In other settings, such as atherosclerosis, these so-called foam cell infiltrates are associated with inflammation20, a process with interesting relevance to formation of the maternal-fetal interface and aberrations thereof.
Pathological changes are also evident in the rest of the placenta, which is comprised of the floating chorionic villi. They include fibrin deposition, formation of syncytial knots and syncytiotrophoblast sloughing, either as cells or debris21. The intrinsic vasculature of the placenta may also be aberrant, and signs of vascular damage apparent. These changes are thought to be secondary to reduced perfusion of the maternal-fetal interface, which can be detected long before the signs of PE appear22. However, PE is also associated with reduction in the surface area of the villi23. The close ties between abnormalities in CTB patterning of maternal vessels, hypoperfusion of the placenta and reductions in surface area, presumed to occur in that order, are likely to set up a viscous cycle that culminates in PE. However, it is conceivable that any component of this circuit could be the driver rather than poor perfusion being the sole instigator.
None of these pathologies are unique features of PE24–26. The constellation is most frequently associated with the severe early onset form of the condition rather than the appearance of this pregnancy complication at term. However, this conclusion is tempered by the fact that the precisely patterned interactions between CTBs and maternal cells that are observed earlier in gestation degrade toward term. Presumably placental connections to the uterus and its vasculature weaken in preparation for birth. For example, CTBs occupancy of the uterine arteries appears to regress27. Thus, it is difficult to determine whether the defects that are the hallmark of severe PE were once present in the late onset form of this syndrome or never occurred. Regardless, it is clear that some features of PE placentas overlap other conditions. Deficient invasion of spiral arteries has long been understood to also occur in IUGR22, 28. Likewise, more recent work has shown that some cases of preterm birth are also associated with deficient remodeling of the spiral arterioles29. Additionally, PE-associated lesions of the spiral arteries are also not specific to PE, but rather shared among many pregnancy complications. The aberrations of the floating chorionic villi are also nonspecific. Why poor placentation leads to PE in some women and other similarly affected pregnancies end in seemingly very different complications (or with a normal birth) is a key unanswered question.
Ultimately, the answer probably lies in the different molecular pathways that are involved, which drive divergence of the phenotypes, with fetal placental and maternal factors being key players. Some of the placental defects that are associated with the severe forms of PE that occur in the late second trimester/early third trimester interval, necessitating delivery, are understood. The stereotypical process by which CTBs invade the uterine wall is an elaborate differentiation process. Every major transition is marked by dramatic changes in the molecules that the cells express. These stage-specific antigens have enabled researchers to ask whether faulty CTB invasion in PE mirrors failures in portions of the differentiation program that lead to uterine invasion. In fact, this is the case (reviewed in30). In this syndrome, CTBs within the uterine wall retain portions of their original epithelial-like phenotype and fail to fully transform into vascular-like cells. We theorize that this mixed identity may hinder their interactions with uterine vessels.
Molecular defects involving other types of molecules are also apparent. Given the “two-stage” theory of PE, immune regulators are particularly relevant. Specifically, abnormal placentation, the first stage, is proposed to lead to immune activation, the second stage31, 32. In this family of molecules, HLA-G is an interesting example. Among all the cells of the body, the expression of this non-classical major histocompatibility complex class 1 molecule is limited to invasive CTBs33. As with the vasculogenic factors, HLA-G expression, which is normally upregulated to the full extent in the columns, is substantially diminished34. Whether or how HLA-G functions in preventing maternal immune responses is not yet clear. NK cell KIR2DL4 has been implicated and refuted as the HLA-G receptor35–37 as well as LILRB1 and 238, 39. A detailed understanding of HLA-G functions in normal pregnancy would, in turn, enable analysis of the predicted shifts in PE, thereby providing important new insights into the immunological component of this syndrome. Of note, myriad other immune alterations have been documented in PE (reviewed in40).
Dysregulated expression of vasculogenic and/or angiogenic factors has also been implicated in the maternal signs of PE. Several lines of evidence led investigators in this direction. For example, it has been known for some time that factors in the blood of PE patients damage cultured endothelial cells41. Given that many of the maternal signs of PE—hypertension, proteinuria and edema—can be explained by vascular damage, there has been intensive interest in identifying the factors that are involved. During the last ten years, the concept of an angiogenic imbalance has emerged (reviewed in26,42). For example, in PE, a soluble VEGF receptor (sFLT1), which sequesters this ligand, and endoglin, a TGFβ family member, circulate at higher levels. Conversely, PLGF levels dramatically decrease. Recreating this milieu in pregnant rodents leads to a PE-like syndrome, including hypertension, proteinuria and the kidney lesions that are typically observed in this syndrome as well as cerebral vascular changes, which may be related to the seizure component. What causes this imbalance? In some cases, the placenta appears to be the source. For example, in severe PE, isolated CTBs from the affected placentas produce higher levels of sFLT1 than control cells43. Microarray analyses also show that sFLT1 and endoglin production are upregulated at the mRNA level in this syndrome44 together with the dysregulation of other angiogenic factors45. However, it is likely that the maternal vasculature also contributes to observed shifts in the circulating levels of these molecules.
This body of work is an interesting example of how basic research into the causes of PE can lead to changes in clinical care. With regard to prediction and diagnosis of PE, sFLT1, endoglin and PLGF levels in maternal blood have been proposed as biomarkers46. More recently, PLGF measurements in combination with certain clinical risk factors were shown to have the highest positive predictive value at 14–16 wks of gestation. Adding measurements of sFLT, endoglin or other proposed biomarkers did not increase performance47. However, the authors cautioned that improvements, such as the addition of other molecular measurements to the test, were needed to achieve clinical utility. With regard to treatment, there is interest in whether reducing sFLT1 levels in women with the severe early onset form of PE could safely prolong their pregnancies, leading to better outcomes.
Although less well studied in comparison to the placental defects, the uterine lining may also be abnormal in PE. The vasculature and/or any of the cells that reside in this location could be involved. For instance, PE is associated with increased maternal risk of cardiovascular disease later in life48. Whether CTB failure to properly remodel uterine arteries is due to a damaged substrate is an interesting question. Are these faulty interactions a proxy for more widespread vessel disease that is not yet evident? The more frequent occurrence of PE in women with hypertension prior to pregnancy could be indicative of such a relationship49. In this regard, a recent study found evidence of impaired decidual angiogenesis (and lymphangiogenesis) in these patients50. With regard to specific cell types, molecular defects in the decidua have been described early in pregnancy before for the signs of PE appear51. Additionally, overexpression of VEGF in the decidua leads to a PE-like syndrome in mice52. With regard to the immune cells that occupy the uterus during pregnancy, PE-associated failure of Treg induction has been reported53. Given the important role of decidual NK cells in remolding of the spiral arteries, it is not surprising that their aberrant activation has been proposed in PE54, 55.
The burning question remains as to the causes of the morphological and molecular defects summarized above. Animal models are one source of answers (reviewed in56,57). There are downsides to studying PE, which is specific to human pregnancy, in animals that do not naturally develop this syndrome. Also, the placental component of PE, deficient endovascular invasion, is rarely observed in these models, probably due to the many unique aspects of the human placenta—from its cellular composition to formation of the maternal-fetal interface. However, there are also significant upsides to employing animal models in terms of drawing causal relationships.
In this context, transgenic mice are often the model system of choice, enabling functional confirmation of a molecule’s role in the human disease or the discovery of new pathways. For example, when transgenic female mice expressing angiotensinogen were mated with transgenic males expressing renin, the mothers developed hypertension and proteinuria that resolved after delivery. There was histological evidence of placental damage, glomerular nephritis and myocardial hypertrophy58. Pregnant mice that lack the expression of catechol-O-methyltransferase develop a PE-like syndrome attributable to the absence of 2-methoxyoestridiaol, which inhibits angiogenesis59. Transgenic mice were used to show the importance of balancing fetal and maternal adrenomedullin levels to correct formation of the maternal-fetal interface60, 61. Atrial natriuretic peptide-converting enzyme (e.g., corin), which activates this peptide, promotes trophoblast invasion and spiral artery remodeling. In mice, lack of corin results in PE-like signs during pregnancy62. Pregnant MMP-9 null mice develop features of PE, including growth restriction, possibly due to deficient trophoblast invasion63. Beside transgenic approaches, rodent models have been exploited in other ways. Davisson et al. (2002)64 showed that mice with borderline high blood pressure, the BPH/5 strain, develop hypertension, proteinuria and glomerular nephritis during pregnancy, ultimately delivering smaller litters. Overexpression, via viral vectors, of angiogenic factors (e.g., sFlt1, endoglin) in pregnant rats produces features of PE65.
Reductions in uterine perfusion are used to model one of the causes of PE. In the 1940s, Ogden and Page sought to demonstrate direct ties between placental hypoxia and the signs of PE. Using pregnant dogs as the model, they showed that constricting the abdominal aorta between the origins of the renal and uterine arteries increased blood pressure (1940). Since then many investigators have followed in their footsteps, reproducing these results in several animal models. Some of the most convincing evidence has come from work with non-human primates. Aortic banding via the same strategy that was used in dogs results in many features of PE: hypertension, proteinuria, glomerular nephritis and increased levels of circulating sFLT1, which were attributable to increased mRNA expression by the placenta and peripheral blood mononuclear cells66. However, The placental effects are diametrically opposite to those observed in PE. CTB invasion of the uterus is dramatically increased, reaching the periphery of the myometrium67. Currently, the reduced uterine perfusion pressure model, developed by Granger and colleagues, is most widely used due to its high degree of reproducibility and low cost as compared to nonhuman primates68, 69. Uterine perfusion is reduced ~40% by restricting blood flow at several points—the aorta above the iliac bifurcation and the right/left uterine arcades61. The sequelae include many PE features such as hypertension, proteinuria and aspects of the immunological dysregulation observed in this syndrome (e.g., increases in TNF-α, IL-6, CD4+ T cells). Additionally, the production of agonistic autoantibodies to the angiotensin AT1 receptor is observed, with subsequent activation contributing to the hypertension that is a hallmark of PE70. However, this model also fails to reproduce the placental component of PE. Why? The answer may lie in the fact that the ability of human CTBs to proliferate in response to hypoxia is largely lost after the uteroplacental circulation is established around the end of the first trimester71. This appears not to be the case in the aforementioned animal models.
Important insights into the causes of PE can also be gained from the risk factors that predispose a pregnant woman to develop this syndrome. They include a family history of this disorder, nulliparity, multiple pregnancy, previous PE, African American race, egg donation and overweight/obesity72, 73. The contribution of a positive family history and PE in a prior pregnancy is taken as evidence of a genetic component as does the influence of African American race. In the latter instance, it is interesting to note the unique features of PE in this population, which may include more severe signs74. In this context, different KIR B regions appear to protect sub-Saharan Africans and Europeans from PE75. The risk conferred by nulliparity may reflect the beneficial effects of uterine remodeling during an initial pregnancy on subsequent placentation. Egg donation entails maternal exposure to third party antigens with unknown consequences. Being overweight or obese, which is associated with low-grade inflammation, could also play a role at the level of metabolic dysregulation, likely an important factor in placental health76. Of note, BMI is the most rapidly growing modifiable risk factor, having a does-response relationship with PE and the severe forms of this syndrome. Individual disease states are also informative. Diabetes is an important risk factor reinforcing the contributions of vascular and immune alterations as well as a metabolic component to the etiology of PE. Anti-phospholipid syndromes are associated with the increased risk of PE and in its most severe forms77. In some pregnant patients with systemic lupis erythematosus, IFNα induces an anti-angiogenic milieu, increasing the sensitivity of endothelial cells to soluble FLT-1, and contributing to the pathogenesis78.
Where should future efforts to understand the role of placental dysgenesis in PE be directed? Work from our group revealed the surprising conclusion that CTBs isolated from the placentas of affected pregnancies normalized their gene expression over 48 h in vitro79. Although caveats abound, this unexpected finding suggests that some aspects of the aberrant differentiation of CTBs within the uterine wall that is observed in situ may be reversible. This unexpected result points to the importance of environment factors in driving the phenotype. The next challenge is identifying what they are. It is clear that this will be no easy task as the mechanisms are doubtlessly complicated and intertwined. Given what is known from animal models and human risk factors, reduced perfusion and/or certain disease states (metabolic, immune and cardiovascular) lie upstream of the eventual derangement of the maternal-fetal interface. There are new clues to the longstanding mystery of why placentation is abnormal in PE and added urgency to finding the answers, as these pathways could be valuable therapeutic targets for reversing abnormal placental function in patients.
Figure 2.
VE-cadherin is not detected on cytotrophoblasts (CTBs) in pre-eclamptic placentas. Sections of age-matched 26-wk control (CON, A–D) and HELLP pre-eclamptic (PE, E–H) tissue double stained for cytokeratin (CK; A, C, E, and G) and VE-cadherin (B, D, F, and H). (A, B) Control anchoring villus and placental bed. VE-cadherin staining is not detected on villous CTBs but is present on CTBs within columns and the uterine wall. (C, D) Section of a maternal spiral artery in control placental bed tissue. VE-cadherin staining is especially strong on intravascular CTBs and CTBs that are associated with the vessel wall. (E, F) Section of preeclamptic anchoring villus and placental bed. VE-cadherin staining is not detected. (G, H) Section through a small blood vessel surrounded by CTBs. VE-cadherin is not detected on vessel-associated CTBs or CTBs within the surrounding tissue. However, the endothelial cells (EC) that line the vessel did stain (arrows). Figure and legend reprinted with permission from Journal of Clinical Investigation81.
Figure 3.
Severe PE-associated aberrations in cytotrophoblast (CTB) gene expression return to control values after 48 hours of culture. RNA was collected immediately after the cells were isolated (0 hour) and after 12, 24, and 48 hours in culture. The relative gene expression levels for CTBs isolated from gestational age matched placentas of patients who delivered due to severe PE (n = 5) relative to noninfected preterm labor (nPTL) are shown as a heat map. The average fold changes for each time point (severe PE vs. nPTL) are shown. ns, no significant difference (LIMMA). Figure and legend reprinted with permission from Journal of Clinical Investigation79. The data are a truncated form of the original heatmap that was published.
Acknowledgments
Portions of this work were supported by grants from the National Institutes of Child Health and Human Development (P50 HD055764 and R37 HD076253).
Footnotes
The author declares no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of pre-eclampsia. The Journal of pathology. 1970;101(4):Pvi. [PubMed] [Google Scholar]
- 2.Maltepe E, Bakardjiev AI, Fisher SJ. The placenta: transcriptional, epigenetic, and physiological integration during development. J Clin Invest. 2010;120(4):1016–25. doi: 10.1172/JCI41211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carter AM, Enders AC, Pijnenborg R. The role of invasive trophoblast in implantation and placentation of primates. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2015;370(1663):20140070. doi: 10.1098/rstb.2014.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Genbacev O, Miller RK. Post-implantation differentiation and proliferation of cytotrophoblast cells: in vitro models--a review. Placenta. 2000;21(Suppl A):S45–9. doi: 10.1053/plac.1999.0523. [DOI] [PubMed] [Google Scholar]
- 5.Zdravkovic T, Genbacev O, Prakobphol A, Cvetkovic M, Schanz A, McMaster M, et al. Nicotine downregulates the l-selectin system that mediates cytotrophoblast emigration from cell columns and attachment to the uterine wall. Reprod Toxicol. 2006;22(1):69–76. doi: 10.1016/j.reprotox.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Conde-Agudelo A, Althabe F, Belizan JM, Kafury-Goeta AC. Cigarette smoking during pregnancy and risk of preeclampsia: a systematic review. American journal of obstetrics and gynecology. 1999;181(4):1026–35. doi: 10.1016/s0002-9378(99)70341-8. [DOI] [PubMed] [Google Scholar]
- 7.Redline RW, Patterson P. Pre-eclampsia is associated with an excess of proliferative immature intermediate trophoblast. Human pathology. 1995;26(6):594–600. doi: 10.1016/0046-8177(95)90162-0. [DOI] [PubMed] [Google Scholar]
- 8.Jones CJ, Aplin JD, Burton GJ. First trimester histiotrophe shows altered sialylation compared with secretory phase glycoconjugates in human endometrium. Placenta. 2010;31(7):576–80. doi: 10.1016/j.placenta.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Librach CL, Werb Z, Fitzgerald ML, Chiu K, Corwin NM, Esteves RA, et al. 92-kD type IV collagenase mediates invasion of human cytotrophoblasts. J Cell Biol. 1991;113(2):437–49. doi: 10.1083/jcb.113.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gellersen B, Brosens J. Cyclic AMP and progesterone receptor cross-talk in human endometrium: a decidualizing affair. The Journal of endocrinology. 2003;178(3):357–72. doi: 10.1677/joe.0.1780357. [DOI] [PubMed] [Google Scholar]
- 11.Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med. 2012;18(12):1754–67. doi: 10.1038/nm.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wewer UM, Faber M, Liotta LA, Albrechtsen R. Immunochemical and ultrastructural assessment of the nature of the pericellular basement membrane of human decidual cells. Laboratory investigation; a journal of technical methods and pathology. 1985;53(6):624–33. [PubMed] [Google Scholar]
- 13.Hess AP, Hamilton AE, Talbi S, Dosiou C, Nyegaard M, Nayak N, et al. Decidual stromal cell response to paracrine signals from the trophoblast: amplification of immune and angiogenic modulators. Biology of reproduction. 2007;76(1):102–17. doi: 10.1095/biolreprod.106.054791. [DOI] [PubMed] [Google Scholar]
- 14.Drake PM, Gunn MD, Charo IF, Tsou CL, Zhou Y, Huang L, et al. Human placental cytotrophoblasts attract monocytes and CD56(bright) natural killer cells via the actions of monocyte inflammatory protein 1alpha. J Exp Med. 2001;193(10):1199–212. doi: 10.1084/jem.193.10.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gellersen B, Brosens JJ. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocrine reviews. 2014;35(6):851–905. doi: 10.1210/er.2014-1045. [DOI] [PubMed] [Google Scholar]
- 16.Wagenblast E, Soto M, Gutierrez-Angel S, Hartl CA, Gable AL, Maceli AR, et al. A model of breast cancer heterogeneity reveals vascular mimicry as a driver of metastasis. Nature. 2015;520(7547):358–62. doi: 10.1038/nature14403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Y, Genbacev O, Fisher SJ. The human placenta remodels the uterus by using a combination of molecules that govern vasculogenesis or leukocyte extravasation. Annals of the New York Academy of Sciences. 2003;995:73–83. doi: 10.1111/j.1749-6632.2003.tb03211.x. [DOI] [PubMed] [Google Scholar]
- 18.Ratsep MT, Felker AM, Kay VR, Tolusso L, Hofmann AP, Croy BA. Uterine natural killer cells: supervisors of vasculature construction in early decidua basalis. Reproduction. 2015;149(2):R91–102. doi: 10.1530/REP-14-0271. [DOI] [PubMed] [Google Scholar]
- 19.Sheppard BL, Bonnar J. The ultrastructure of the arterial supply of the human placenta in pregnancy complicated by fetal growth retardation. British journal of obstetrics and gynaecology. 1976;83(12):948–59. doi: 10.1111/j.1471-0528.1976.tb00781.x. [DOI] [PubMed] [Google Scholar]
- 20.Chaabane C, Coen M, Bochaton-Piallat ML. Smooth muscle cell phenotypic switch: implications for foam cell formation. Current opinion in lipidology. 2014;25(5):374–9. doi: 10.1097/MOL.0000000000000113. [DOI] [PubMed] [Google Scholar]
- 21.Redman CW, Tannetta DS, Dragovic RA, Gardiner C, Southcombe JH, Collett GP, et al. Review: Does size matter? Placental debris and the pathophysiology of pre-eclampsia. Placenta. 2012;33(Suppl):S48–54. doi: 10.1016/j.placenta.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Khong TY. Placental vascular development and neonatal outcome. Seminars in neonatology : SN. 2004;9(4):255–63. doi: 10.1016/j.siny.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 23.Boyd PA, Scott A. Quantitative structural studies on human placentas associated with pre-eclampsia, essential hypertension and intrauterine growth retardation. British journal of obstetrics and gynaecology. 1985;92(7):714–21. doi: 10.1111/j.1471-0528.1985.tb01454.x. [DOI] [PubMed] [Google Scholar]
- 24.Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. American journal of obstetrics and gynecology. 2011;204(3):193–201. doi: 10.1016/j.ajog.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brosens I, Pijnenborg R, Benagiano G. Defective myometrial spiral artery remodelling as a cause of major obstetrical syndromes in endometriosis and adenomyosis. Placenta. 2013;34(2):100–5. doi: 10.1016/j.placenta.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 26.Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R. Pre-eclampsia part 1: current understanding of its pathophysiology. Nature reviews Nephrology. 2014;10(8):466–80. doi: 10.1038/nrneph.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Enders AC, Blankenship TN. Modification of endometrial arteries during invasion by cytotrophoblast cells in the pregnant macaque. Acta anatomica. 1997;159(4):169–93. doi: 10.1159/000147983. [DOI] [PubMed] [Google Scholar]
- 28.Lyall F, Robson SC, Bulmer JN. Spiral artery remodeling and trophoblast invasion in preeclampsia and fetal growth restriction: relationship to clinical outcome. Hypertension. 2013;62(6):1046–54. doi: 10.1161/HYPERTENSIONAHA.113.01892. [DOI] [PubMed] [Google Scholar]
- 29.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345(6198):760–5. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McMaster MT, Zhou Y, Fisher SJ. Abnormal placentation and the syndrome of preeclampsia. Seminars in nephrology. 2004;24(6):540–7. doi: 10.1016/s0270-9295(04)00124-x. [DOI] [PubMed] [Google Scholar]
- 31.Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. American journal of obstetrics and gynecology. 1998;179(1):80–6. doi: 10.1016/s0002-9378(98)70254-6. [DOI] [PubMed] [Google Scholar]
- 32.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308(5728):1592–4. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 33.McMaster MT, Librach CL, Zhou Y, Lim KH, Janatpour MJ, DeMars R, et al. Human placental HLA-G expression is restricted to differentiated cytotrophoblasts. J Immunol. 1995;154(8):3771–8. [PubMed] [Google Scholar]
- 34.Lim KH, Zhou Y, Janatpour M, McMaster M, Bass K, Chun SH, et al. Human cytotrophoblast differentiation/invasion is abnormal in pre-eclampsia. The American journal of pathology. 1997;151(6):1809–18. [PMC free article] [PubMed] [Google Scholar]
- 35.Rajagopalan S, Long EO. A human histocompatibility leukocyte antigen (HLA)-G-specific receptor expressed on all natural killer cells. J Exp Med. 1999;189(7):1093–100. doi: 10.1084/jem.189.7.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li C, Houser BL, Nicotra ML, Strominger JL. HLA-G homodimer-induced cytokine secretion through HLA-G receptors on human decidual macrophages and natural killer cells. Proc Natl Acad Sci U S A. 2009;106(14):5767–72. doi: 10.1073/pnas.0901173106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le Page ME, Goodridge JP, John E, Christiansen FT, Witt CS. Killer Ig-like receptor 2DL4 does not mediate NK cell IFN-gamma responses to soluble HLA-G preparations. J Immunol. 2014;192(2):732–40. doi: 10.4049/jimmunol.1301748. [DOI] [PubMed] [Google Scholar]
- 38.Gonen-Gross T, Achdout H, Gazit R, Hanna J, Mizrahi S, Markel G, et al. Complexes of HLA-G protein on the cell surface are important for leukocyte Ig-like receptor-1 function. J Immunol. 2003;171(3):1343–51. doi: 10.4049/jimmunol.171.3.1343. [DOI] [PubMed] [Google Scholar]
- 39.Shiroishi M, Kuroki K, Rasubala L, Tsumoto K, Kumagai I, Kurimoto E, et al. Structural basis for recognition of the nonclassical MHC molecule HLA-G by the leukocyte Ig-like receptor B2 (LILRB2/LIR2/ILT4/CD85d) Proc Natl Acad Sci U S A. 2006;103(44):16412–7. doi: 10.1073/pnas.0605228103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Staff AC, Johnsen GM, Dechend R, Redman CW. Preeclampsia and uteroplacental acute atherosis: immune and inflammatory factors. Journal of reproductive immunology. 2014;101–102:120–6. doi: 10.1016/j.jri.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 41.Rodgers GM, Taylor RN, Roberts JM. Preeclampsia is associated with a serum factor cytotoxic to human endothelial cells. American journal of obstetrics and gynecology. 1988;159(4):908–14. doi: 10.1016/s0002-9378(88)80169-8. [DOI] [PubMed] [Google Scholar]
- 42.Maynard S, Epstein FH, Karumanchi SA. Preeclampsia and angiogenic imbalance. Annual review of medicine. 2008;59:61–78. doi: 10.1146/annurev.med.59.110106.214058. [DOI] [PubMed] [Google Scholar]
- 43.Zhou Y, McMaster M, Woo K, Janatpour M, Perry J, Karpanen T, et al. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. The American journal of pathology. 2002;160(4):1405–23. doi: 10.1016/S0002-9440(10)62567-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cox B, Sharma P, Evangelou AI, Whiteley K, Ignatchenko V, Ignatchenko A, et al. Translational analysis of mouse and human placental protein and mRNA reveals distinct molecular pathologies in human preeclampsia. Molecular & cellular proteomics : MCP. 2011;10(12):M111 012526. doi: 10.1074/mcp.M111.012526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winn VD, Gormley M, Fisher SJ. The Impact of Preeclampsia on Gene Expression at the Maternal-Fetal Interface. Pregnancy hypertension. 2011;1(1):100–8. doi: 10.1016/j.preghy.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang A, Holston AM, Yu KF, Zhang J, Toporsian M, Karumanchi SA, et al. Circulating anti-angiogenic factors during hypertensive pregnancy and increased risk of respiratory distress syndrome in preterm neonates. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2012;25(8):1447–52. doi: 10.3109/14767058.2011.640368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Myers JE, Kenny LC, McCowan LM, Chan EH, Dekker GA, Poston L, et al. Angiogenic factors combined with clinical risk factors to predict preterm pre-eclampsia in nulliparous women: a predictive test accuracy study. BJOG : an international journal of obstetrics and gynaecology. 2013;120(10):1215–23. doi: 10.1111/1471-0528.12195. [DOI] [PubMed] [Google Scholar]
- 48.Chen CW, Jaffe IZ, Karumanchi SA. Pre-eclampsia and cardiovascular disease. Cardiovascular research. 2014;101(4):579–86. doi: 10.1093/cvr/cvu018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roberts JM, Cooper DW. Pathogenesis and genetics of pre-eclampsia. Lancet. 2001;357(9249):53–6. doi: 10.1016/s0140-6736(00)03577-7. [DOI] [PubMed] [Google Scholar]
- 50.Liu H, Li Y, Zhang J, Rao M, Liang H, Liu G. The defect of both angiogenesis and lymphangiogenesis is involved in preeclampsia. Placenta. 2015;36(3):279–86. doi: 10.1016/j.placenta.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 51.Rabaglino MB, Post Uiterweer ED, Jeyabalan A, Hogge WA, Conrad KP. Bioinformatics approach reveals evidence for impaired endometrial maturation before and during early pregnancy in women who developed preeclampsia. Hypertension. 2015;65(2):421–9. doi: 10.1161/HYPERTENSIONAHA.114.04481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fan X, Rai A, Kambham N, Sung JF, Singh N, Petitt M, et al. Endometrial VEGF induces placental sFLT1 and leads to pregnancy complications. J Clin Invest. 2014;124(11):4941–52. doi: 10.1172/JCI76864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hsu P, Santner-Nanan B, Dahlstrom JE, Fadia M, Chandra A, Peek M, et al. Altered decidual DC-SIGN+ antigen-presenting cells and impaired regulatory T-cell induction in preeclampsia. The American journal of pathology. 2012;181(6):2149–60. doi: 10.1016/j.ajpath.2012.08.032. [DOI] [PubMed] [Google Scholar]
- 54.Sargent IL, Borzychowski AM, Redman CW. NK cells and pre-eclampsia. Journal of reproductive immunology. 2007;76(1–2):40–4. doi: 10.1016/j.jri.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 55.Erlebacher A. Immunology of the maternal-fetal interface. Annu Rev Immunol. 2013;31:387–411. doi: 10.1146/annurev-immunol-032712-100003. [DOI] [PubMed] [Google Scholar]
- 56.McCarthy FP, Kingdom JC, Kenny LC, Walsh SK. Animal models of preeclampsia; uses and limitations. Placenta. 2011;32(6):413–9. doi: 10.1016/j.placenta.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 57.Pennington KA, Schlitt JM, Jackson DL, Schulz LC, Schust DJ. Preeclampsia: multiple approaches for a multifactorial disease. Disease models & mechanisms. 2012;5(1):9–18. doi: 10.1242/dmm.008516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takimoto E, Ishida J, Sugiyama F, Horiguchi H, Murakami K, Fukamizu A. Hypertension induced in pregnant mice by placental renin and maternal angiotensinogen. Science. 1996;274(5289):995–8. doi: 10.1126/science.274.5289.995. [DOI] [PubMed] [Google Scholar]
- 59.Kanasaki K, Palmsten K, Sugimoto H, Ahmad S, Hamano Y, Xie L, et al. Deficiency in catechol-O-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature. 2008;453(7198):1117–21. doi: 10.1038/nature06951. [DOI] [PubMed] [Google Scholar]
- 60.Li M, Yee D, Magnuson TR, Smithies O, Caron KM. Reduced maternal expression of adrenomedullin disrupts fertility, placentation, and fetal growth in mice. J Clin Invest. 2006;116(10):2653–62. doi: 10.1172/JCI28462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li M, Schwerbrock NM, Lenhart PM, Fritz-Six KL, Kadmiel M, Christine KS, et al. Fetal-derived adrenomedullin mediates the innate immune milieu of the placenta. J Clin Invest. 2013;123(6):2408–20. doi: 10.1172/JCI67039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cui Y, Wang W, Dong N, Lou J, Srinivasan DK, Cheng W, et al. Role of corin in trophoblast invasion and uterine spiral artery remodelling in pregnancy. Nature. 2012;484(7393):246–50. doi: 10.1038/nature10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Plaks V, Rinkenberger J, Dai J, Flannery M, Sund M, Kanasaki K, et al. Matrix metalloproteinase-9 deficiency phenocopies features of preeclampsia and intrauterine growth restriction. Proc Natl Acad Sci U S A. 2013;110(27):11109–14. doi: 10.1073/pnas.1309561110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Davisson RL, Hoffmann DS, Butz GM, Aldape G, Schlager G, Merrill DC, et al. Discovery of a spontaneous genetic mouse model of preeclampsia. Hypertension. 2002;39(2 Pt 2):337–42. doi: 10.1161/hy02t2.102904. [DOI] [PubMed] [Google Scholar]
- 65.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12(6):642–9. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 66.Makris A, Thornton C, Thompson J, Thomson S, Martin R, Ogle R, et al. Uteroplacental ischemia results in proteinuric hypertension and elevated sFLT-1. Kidney international. 2007;71(10):977–84. doi: 10.1038/sj.ki.5002175. [DOI] [PubMed] [Google Scholar]
- 67.Zhou Y, Chiu K, Brescia RJ, Combs CA, Katz MA, Kitzmiller JL, et al. Increased depth of trophoblast invasion after chronic constriction of the lower aorta in rhesus monkeys. American journal of obstetrics and gynecology. 1993;169(1):224–9. doi: 10.1016/0002-9378(93)90172-f. [DOI] [PubMed] [Google Scholar]
- 68.Crews JK, Herrington JN, Granger JP, Khalil RA. Decreased endothelium-dependent vascular relaxation during reduction of uterine perfusion pressure in pregnant rat. Hypertension. 2000;35(1 Pt 2):367–72. doi: 10.1161/01.hyp.35.1.367. [DOI] [PubMed] [Google Scholar]
- 69.Granger JP, LaMarca BB, Cockrell K, Sedeek M, Balzi C, Chandler D, et al. Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Methods in molecular medicine. 2006;122:383–92. doi: 10.1385/1-59259-989-3:381. [DOI] [PubMed] [Google Scholar]
- 70.Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A, et al. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103(7):945–52. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997;277(5332):1669–72. doi: 10.1126/science.277.5332.1669. [DOI] [PubMed] [Google Scholar]
- 72.Pare E, Parry S, McElrath TF, Pucci D, Newton A, Lim KH. Clinical risk factors for preeclampsia in the 21st century. Obstetrics and gynecology. 2014;124(4):763–70. doi: 10.1097/AOG.0000000000000451. [DOI] [PubMed] [Google Scholar]
- 73.English FA, Kenny LC, McCarthy FP. Risk factors and effective management of preeclampsia. Integrated blood pressure control. 2015;8:7–12. doi: 10.2147/IBPC.S50641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nakimuli A, Chazara O, Byamugisha J, Elliott AM, Kaleebu P, Mirembe F, et al. Pregnancy, parturition and preeclampsia in women of African ancestry. American journal of obstetrics and gynecology. 2014;210(6):510–20. e1. doi: 10.1016/j.ajog.2013.10.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nakimuli A, Chazara O, Hiby SE, Farrell L, Tukwasibwe S, Jayaraman J, et al. A KIR B centromeric region present in Africans but not Europeans protects pregnant women from pre-eclampsia. Proc Natl Acad Sci U S A. 2015;112(3):845–50. doi: 10.1073/pnas.1413453112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mission JF, Marshall NE, Caughey AB. Pregnancy Risks Associated with Obesity. Obstetrics and gynecology clinics of North America. 2015;42(2):335–53. doi: 10.1016/j.ogc.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 77.Haddad T, Hakem D, Berrah A. PP144. Profile of lupus pregnancy in internal medecine practice. Pregnancy hypertension. 2012;2(3):317–8. doi: 10.1016/j.preghy.2012.04.255. [DOI] [PubMed] [Google Scholar]
- 78.Andrade D, Kim M, Blanco LP, Karumanchi SA, Koo GC, Redecha P, et al. Interferon-alpha and angiogenic dysregulation in pregnant lupus patients who develop preeclampsia. Arthritis Rheumatol. 2015;67(4):977–87. doi: 10.1002/art.39029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou Y, Gormley MJ, Hunkapiller NM, Kapidzic M, Stolyarov Y, Feng V, et al. Reversal of gene dysregulation in cultured cytotrophoblasts reveals possible causes of preeclampsia. J Clin Invest. 2013;123(7):2862–72. doi: 10.1172/JCI66966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Red-Horse K, Kapidzic M, Zhou Y, Feng KT, Singh H, Fisher SJ. EPHB4 regulates chemokine-evoked trophoblast responses: a mechanism for incorporating the human placenta into the maternal circulation. Development. 2005;132(18):4097–106. doi: 10.1242/dev.01971. [DOI] [PubMed] [Google Scholar]
- 81.Zhou Y, Damsky CH, Fisher SJ. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. One cause of defective endovascular invasion in this syndrome? J Clin Invest. 1997;99(9):2152–64. doi: 10.1172/JCI119388. [DOI] [PMC free article] [PubMed] [Google Scholar]