Abstract
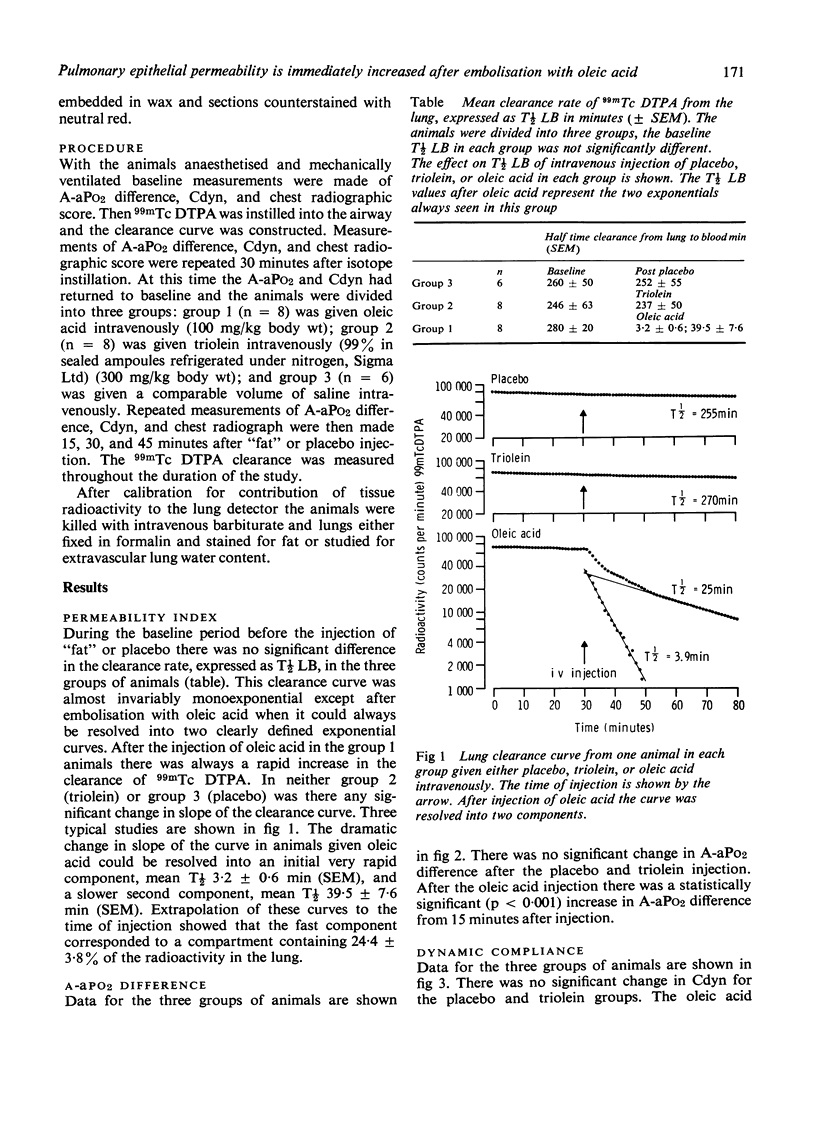
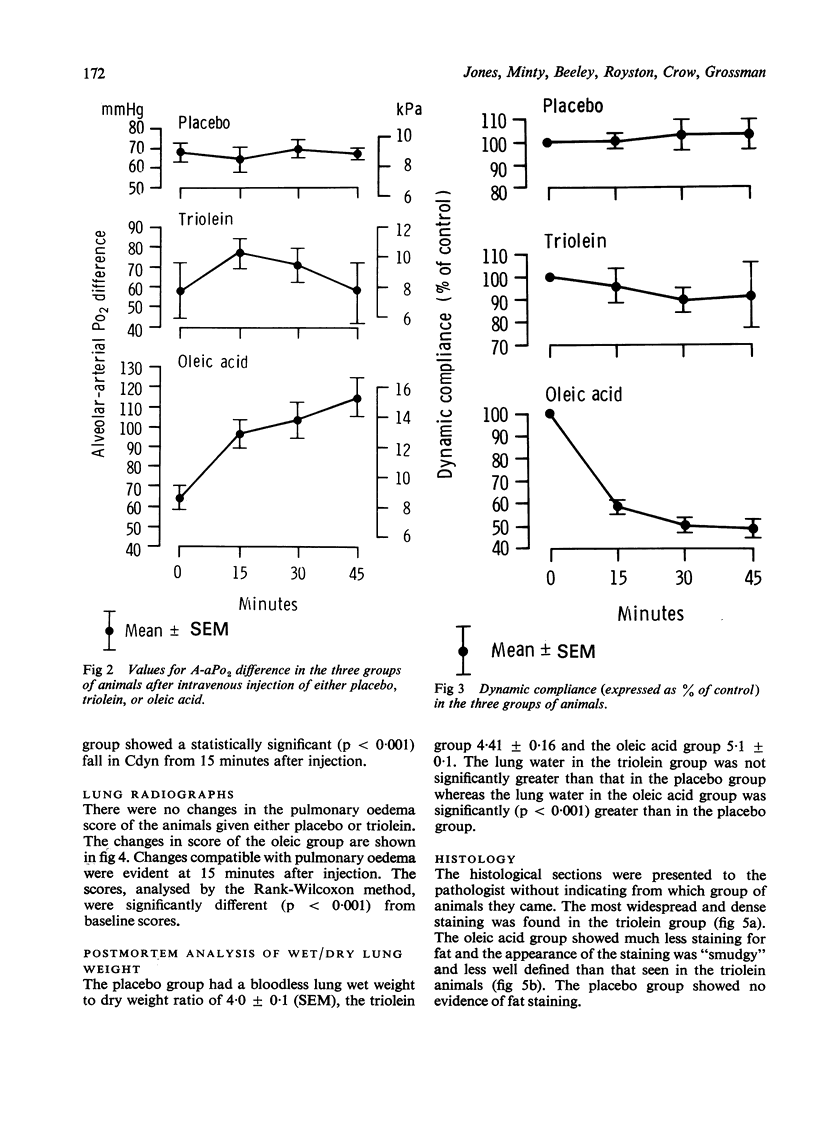
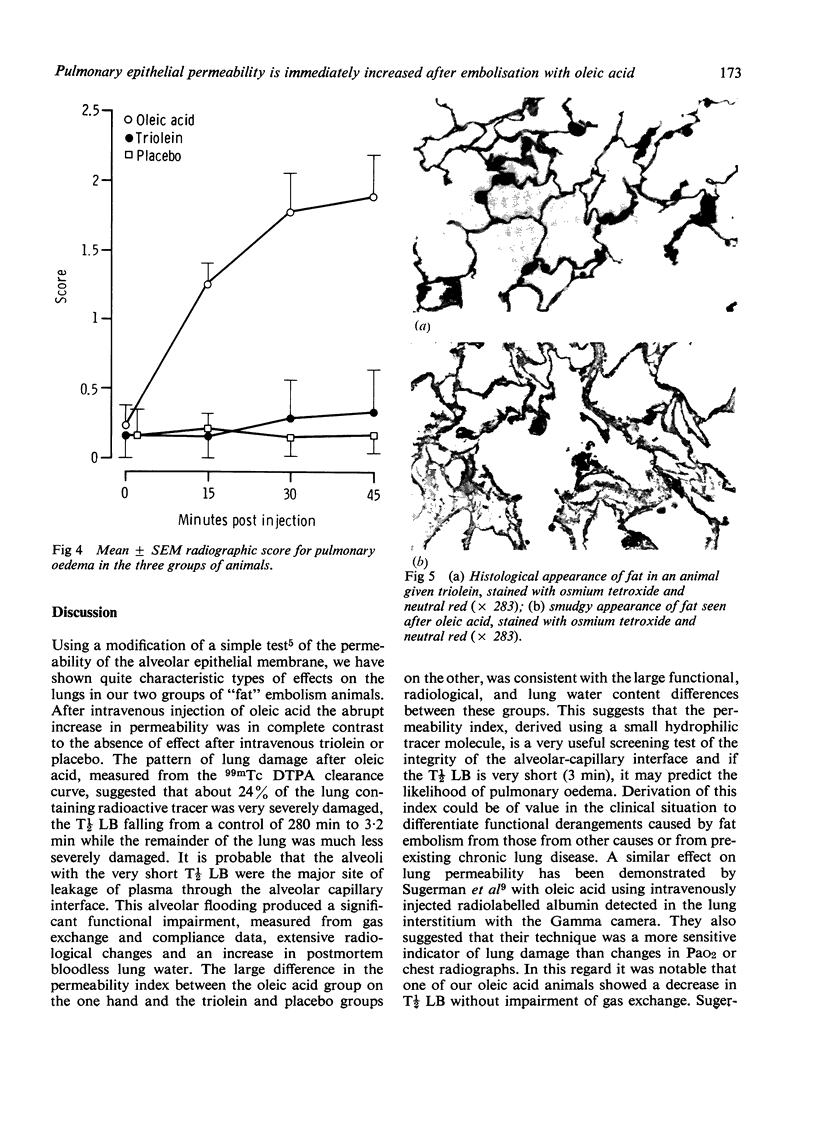
Pulmonary fat embolism occurs frequently after trauma but its functional significance is often unclear. To obtain direct evidence of lung damage caused by fat embolism we have measured changes in permeability of the alveolar-capillary interface. A permeability index was derived from the half time clearance from lung to blood (T1/2LB) of 99mTcDTPA introduced into the lung in a 1 ml bolus. Three groups of rabbits were studied. Baseline T1/2LB. did not differ significantly between groups. After intravenous injection of saline placebo in one group and of 300 mg/kg triolein in another group there was no change in permeability index. After intravenous injection of 100 mg/kg oleic acid in the third group there was an immediate change in T1/2LB from a monoexponential baseline 280 +/- 20 min (SEM) to a multiexponential curve which was resolved into two components, one with a T1/2LB of 3.2 +/- 0.6 min (SEM) and the other 39.5 +/- 7.6 min (SEM). Statistically significant changes in alveolar-arterial PO2 difference, dynamic compliance, chest radiography, and postmortem lung water accompanied the changes in T1/2LB in this group. There were no significant changes in these variables in the placebo or triolein group. Histological studies of the lung tissue of these animals using the osmic acid stain for fat showed no fat in the placebo group, extensive fat embolisation which was densely stained in the triolein group and much less densely stained fat in the oleic acid group. Measurement of the permeability of the alveolar-capillary interface provides direct evidence of lung damage after oleic acid embolisation. There were no functional changes in animals with extensive embolisation with triolein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMIN J., GRANT R. T. Observations on gross pulmonary fat embolism in man and the rabbit. Clin Sci. 1951 Nov;10(4):441–469. [PubMed] [Google Scholar]
- Delaunois L., Sergysels R., Martin R. R. Acute effects on airways mechanics of pulmonary edema induced by intravenous oleic acid in dogs. Bull Eur Physiopathol Respir. 1980 Jan-Feb;16(1):47–55. [PubMed] [Google Scholar]
- Derks C. M., Jacobovitz-Derks D. Embolic pneumopathy induced by oleic acid. A systematic morphologic study. Am J Pathol. 1977 Apr;87(1):143–158. [PMC free article] [PubMed] [Google Scholar]
- Ellison L. T., McPherson J. C., Jr, Anabtawi I. N., Ellison R. G. Incidence of free fat in the lung during open-heart surgery. Ann Thorac Surg. 1969 Jun;7(6):509–517. doi: 10.1016/s0003-4975(10)66390-4. [DOI] [PubMed] [Google Scholar]
- Jacobs R. R., Wheeler E. J., Jelenko C., 3rd, McDonald T. F., Bliven F. E. Fat embolism: a microscopic and ultrastructure evaluation of two animal models. J Trauma. 1973 Nov;13(11):980–993. [PubMed] [Google Scholar]
- Jones J. G., Berry M., Hulands G. H., Crawley J. C. The time course and degree of change in alveolar-capillary membrane permeability induced by aspiration of hydrochloric acid and hypotonic saline. Am Rev Respir Dis. 1978 Dec;118(6):1007–1013. doi: 10.1164/arrd.1978.118.6.1007. [DOI] [PubMed] [Google Scholar]
- Jones J. G., Minty B. D., Lawler P., Hulands G., Crawley J. C., Veall N. Increased alveolar epithelial permeability in cigarette smokers. Lancet. 1980 Jan 12;1(8159):66–68. doi: 10.1016/s0140-6736(80)90493-6. [DOI] [PubMed] [Google Scholar]
- Minty B. D., Jordan C., Jones J. G. Rapid improvement in abnormal pulmonary epithelial permeability after stopping cigarettes. Br Med J (Clin Res Ed) 1981 Apr 11;282(6271):1183–1186. doi: 10.1136/bmj.282.6271.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEARCE M. L., YAMASHITA J., BEAZELL J. MEASUREMENT OF PULMONARY EDEMA. Circ Res. 1965 May;16:482–488. doi: 10.1161/01.res.16.5.482. [DOI] [PubMed] [Google Scholar]
- PELTIER L. F. Fat embolism. III. The toxic properties of neutral fat and free fatty acids. Surgery. 1956 Oct;40(4):665–670. [PubMed] [Google Scholar]
- Pietra G. G., Rüttner J. R., Wüst W., Glinz W. The lung after trauma and shock--fine structure of the alveolar-capillary barrier in 23 autopsies. J Trauma. 1981 Jun;21(6):454–462. [PubMed] [Google Scholar]
- Reidbord H. E. Pulmonary fat embolism. An ultrastructural study. Arch Pathol. 1974 Aug;98(2):122–125. [PubMed] [Google Scholar]
- Shaffer J. W., Sealy W. C., Seaber A. V., Goldner J. L. Etiology of fat embolism syndrome: early morphologic lung changes of respiratory distress syndrome produced by triolein. Surg Forum. 1976;27(62):516–518. [PubMed] [Google Scholar]
- Sugerman H. J., Strash A. M., Hirsch J. I., Glauser F. L., Shirazi K. K., Sharp D. E., Greenfield L. J. Sensitivity of scintigraphy for detection of pulmonary capillary albumin leak in canine oleic acid ARDS. J Trauma. 1981 Jul;21(7):520–527. doi: 10.1097/00005373-198107000-00003. [DOI] [PubMed] [Google Scholar]




