Abstract
Background
Lambl’s excrescences (LEx) are detected by transesophageal echocardiography (TEE) and are characterized as thin, elongated, and hypermobile structures located at the leaflets’ coaptation point of the heart valves. The association of LEx with cerebrovascular disease (CVD) is still undefined and yet patients with LEx and suspected CVD receive unproven effective antiplatelet or anticoagulant therapy or even undergo valve surgery. Also, the association of LEx with aging and atherogenic, inflammatory, or thrombogenic parameters has not been reported.
Methods
77 patients with systemic lupus erythematosus (SLE) (71 women, age 37±12 years) and 26 age-and-sex matched healthy controls (22 women, age 34±11 years) prospectively underwent routine history and physical exam, transcranial Doppler, brain MRI, transesophageal echocardiography (TEE), carotid duplex, and clinical and laboratory evaluations of atherogenesis, inflammation, platelet activity, coagulation, and fibrinolysis. Subjects without stroke/TIA on enrollment (with and without LEx) had a median follow-up of 57 months.
Results
On enrollment, 33 (43%) of 77 patients had CVD manifested as acute stroke/TIA (23 patients), cerebromicroembolism by transcranial Doppler (17 patients), or cerebral infarcts by MRI (14 patients). Mitral or aortic valve LEx were equally frequent in healthy controls (46%) as in patients with and without any CVD (39% and 43%), stroke/TIA (35% and 43%), cerebromicroembolism (41% and 42%), or cerebral infarcts (36% and 43%) (all p≥0.72). Also, other mechanisms for CVD other than LEx such as Libman-Sacks vegetations, patent foramen ovale or interatrial septal aneurysm, aortic or carotid atherosclerosis, or thrombogenesis were found in ≥94% of patients with CVD. In addition, 36 subjects with and 44 without LEx had similar low incidence of stroke/TIA [1(1.3%) and 2(2.5%), respectively, p=1.0] during follow-up. Finally, LEx were not associated with aging, atherogenic risk factors, atherosclerosis, inflammation, or thrombogenesis.
Conclusions
In this study, LEx are similarly prevalent in healthy controls and SLE patients, are not associated with CVD, and are not associated with pathogenic risk factors. Therefore, the study findings suggest that LEx may not be cardioembolic substrates, may not represent pathologic valve structures, and may not require therapy.
Keywords: Lambl’s excrescences, cerebrovascular disease, magnetic resonance imaging, transcranial Doppler, transesophageal echocardiography
INTRODUCTION
Lambl’s excrescences (LEx) were first described in 1856, are currently detected by transesophageal echocardiography (TEE), and are characterized as thin, elongated, and hypermobile structures located at the coaptation point of valves’ leaflets [1–5]. An association of LEx with stroke/transient ischemic attacks (TIA) has been reported in retrospective or case-control studies, small case series, and isolated case reports [2, 3, 6–11]. However, patients >60 years old with risk factors for atherothrombosis or atheroembolism were included in those reports and other mechanisms of stroke/TIA and indicators of cerebroembolism such as cerebromicroembolism detected by transcranial Doppler or cerebral infarcts by magnetic resonance imaging (MRI) were not investigated. Therefore, the association of LEx with CVD is still undefined. Also, the association of LEx with aging and atherogenic, inflammatory, or thrombogenic markers has not been reported [2–11]. Patients with systemic lupus erythematosus (SLE) have a high prevalence and incidence of stroke/TIA, ischemic brain lesions on MRI, atherogenic risk factors, premature atherosclerosis, inflammation, and hypercoagulability [12–15]. Thus, patients with SLE are an ideal subset for studying the association of LEx with CVD and their potential pathogenic factors.
This 6-year, prospective, controlled, cross-sectional, and longitudinal study in SLE was designed with 2 objectives: 1) to determine if there is an association of LEx with CVD manifested as acute stroke/TIA, cerebromicroembolism by transcranial Doppler, or cerebral infarcts on MRI; and 2) to determine if there is an association of LEx with clinical and laboratory parameters of aging, atherogenesis, atherosclerosis, inflammation, platelet activity, coagulation, or fibrinolysis.
METHODS
Study Populations
This study is part of a protocol approved by the National Institutes of Health and Institutional Review Board for the study of cardiovascular and CVD in SLE [14]. All participants provided informed consent.
From December 2006 to December 2012, 77 (29%) of 266 patients with SLE [age 37±12 years (range, 18–60), 71 women)] actively followed at the rheumatology clinics of the University of New Mexico Health Sciences Center met inclusion criteria and agreed to participated in the study.
Exclusion criteria included: age <18 or >60 years, pregnancy, atrial fibrillation/flutter, cardiomyopathy (ejection fraction <50%), intracardiac thrombi, drug abuse, renal dysfunction, and heart or brain disease unrelated to SLE.
To provide a control reference and to validate blinded interpretation of studies, 26 age-and-sex matched volunteers [age 34±11 years (range, 18–57), 22 women] with a negative medical history and physical examination were studied.
All 103 participants prospectively underwent clinical and laboratory evaluations, brain MRI, transcranial Doppler, TEE, and carotid duplex within 1 week of enrollment. All studies were de-identified, coded, and interpreted by independent experienced observers unaware of subjects’ clinical and imaging data.
Clinical and laboratory evaluations
On enrollment, all participants were assessed for: 1) acute stroke/TIA (preceding 1–2 weeks); 2) general demographics; 3) parameters of atherogenesis; and 4) parameters of inflammation, platelet activity, coagulation, and fibrinolysis (Table 3). Also, SLE patients were characterized with regard to SLE duration, activity, and damage; standard inflammatory markers and autoantibodies; antiphospholipid antibodies (aPL); and corticosteroid, antimetabolite immunosuppressive, antimalarial, and antithrombotic therapy (Table 4).
Table 3.
Clinical and Laboratory Parameters in SLE Patients and Healthy Controls with and without Lambl’s Excrescences.
| SLE Patients (N = 77) | Healthy Controls (N = 26) | |||||
|---|---|---|---|---|---|---|
| Parameter | LEx (N = 32) |
No LEx (N = 45) |
P value |
LEx (N = 12) |
No LEx (N = 14) |
P value |
| Demographic Parameters | ||||||
| Age (years) | 36 ± 13 | 37 ± 12 | 0.88 | 36 ± 11 | 32 ± 10 | 0.38 |
| Women | 31 (97%) | 40 (89%) | 0.39 | 8 (67%) | 14 (100%) | 0.03‡ |
| Body mass index | 28 ± 5 | 27 ± 6 | 0.79 | 27 ± 5 | 27 ± 6 | 0.91 |
| Parameters of Atherogenesis and Atherosclerosis | ||||||
| HTN (>140/90 mmHg) | 3/27 (11%) | 5 (11%) | 1.0 | 0 | 0 | |
| Cholesterol (mg/dl) | 188 ± 48 | 174 ± 44 | 0.21 | 194 ± 48 | 192 ± 39 | 0.88 |
| HDL (mg/dL) | 52 ± 17 | 49 ± 15 | 0.38 | 43 ± 14 | 58 ± 20 | 0.10 |
| LDL (mg/dL) | 111 ± 43 | 107 ± 38 | 0.69 | 111 ± 41 | 112 ± 23 | 0.98 |
| Triglicerides (mg/dL) | 160 ± 89 | 149 ± 74 | 0.59 | 170 ± 99 | 150 ± 97 | 0.61 |
| Dyslipidemia | 13/28 (46%) | 21 (47%) | 1.00 | 6 (50%) | 3/13 (23%) | 0.23 |
| Diabetes mellitus | 0/29 (0%) | 3 (7%) | 0.28 | 0 | 0 | |
| Smoking (currently) | 7/31 (23%) | 17 (38%) | 0.21 | 2 (17%) | 4 (29%) | 0.65 |
| Any risk factor | 16/31 (52%) | 26 (58%) | 0.64 | 6 (50%) | 3 (21%) | 0.22 |
| Aortic or carotid atherosclerosis | 13 (41%) | 19 (42%) | 1.00 | 4 (33%) | 1 (7%) | 0.15 |
| Parameters of Inflammation, Platelet Activity, Coagulation, and Fibrinolysis | ||||||
| White blood cell count (×10^3/mm3) | 6.5 ± 3.1 | 5.9 ± 2.2 | 0.41 | 6.4 ± 1.8 | 6.6 ± 1.5 | 0.75 |
| C3a (pg/mL) | 1443 (967–1999) | 1350 (1084–2034) | 0.71* | 1440 (1197–1839) | 1335 (1206–1601) | 0.78* |
| C5a (pg/mL) | 26 ± 10 | 29 ± 11 | 0.28 | 26 ± 11 | 28 ± 9 | 0.55 |
| Total microparticles (U/mL) | 3206 (2321–5265) | 2750 (1857–3820) | 0.11* | 2150 (1512–2799) | 2987 (2777–4158) | 0.06* |
| Monocyte-derived microparticles (U/uL) | 182 (82 – 719) | 256 (55 – 676) | 0.90* | 195 (81–280) | 664 (228–1080) | 0.03*‡ |
| Platelets (×10^3/mm3) | 252 ± 91 | 234 ± 83 | 0.37 | 269 ± 36 | 279 ± 46 | 0.52 |
| P-selectin (ng/dL) | 35 ± 17 | 42 ± 25 | 0.16 | 40 ± 20 | 36 ± 12 | 0.48 |
| Platelet-derived microparticles (U/uL) | 268 (67–579) | 453 (111–820) | 0.11* | 298 (179 – 404) | 405 (225–936) | 0.27* |
| Endothelium-derived microparticles (U/uL) | 43 (20–63) | 86 (20–162) | 0.15* | 61 (31–93) | 152 (55–319) | 0.09* |
| Peak thrombin generation (nmol/L) | 336 ± 165 | 306 ± 165 | 0.47 | 382 ± 132 | 481 ± 107 | 0.05*‡ |
| Thrombin-antithrombin complexes (ng/mL) | 4.6 (3.1–8.8) | 2.7 (1.6–5.1) | 0.02*† | 3.0 (2.5–4.0) | 2.8 (2.6–6.2) | 0.51* |
| D-dimer (mg/dL) | 0.4 (0.5–0.9) | 0.40 (0.1–0.6) | 0.41* | 0.11 (0.1–0.3) | 0.35 (0.1–0.5) | 0.04*‡ |
| Tissue plasminogen antigen (ng/mL) | 10.1 ± 4.2 | 9.8 ± 6.3 | 0.79 | 9.6 ± 4.2 | 6.4 ± 3.6 | 0.06 |
| Plasminogen activator inhibitor-1 (U/mL) | 4.3 (2.2–8.9) | 5.4 (2.0–10.3) | 0.56* | 8.5 (3.9–14.9) | 3.5 (1.7–11.3) | 0.24* |
Values are n (%), mean ± SD, or median (interquartile range).
By Wilcoxon Rank Sum test.
p value non-significant after Bonferoni’s correction.
p value non-significant after Bonferoni’s correction and association occurred in the opposite direction.
Abbreviations: HTN = hypertension, HDL = high density lipoproteins, LDL = low density lipoproteins Other abbreviations as in previous Tables.
Table 4.
Clinical, Laboratory, and Therapy Data in SLE Patients with and without Lambl’s Excrescences
| Variable | Excrescences (N = 32) |
No Excrescences (N = 45) |
P Value |
|---|---|---|---|
| Disease Duration, Activity, and Damage | |||
| Duration of SLE (years) | 7.3 ± 6.9 | 8.3 ± 6.6 | 0.55 |
| Age of onset of SLE | 28 ± 12 | 29 ± 13 | 0.80 |
| Total SLE-Disease Activity Index (SLEDAI) (U) | 14 ± 13 | 11 ± 8 | 0.33 |
| Non-Neurologic SLEDAI (U) | 9.2 ± 7.2 | 7.0 ± 4.8 | 0.15 |
| Neurologic SLEDAI (U) | 0 (0 – 8) | 0 (0 – 8) | 0.99* |
| Total SLE Damage Index (U) | 2.9 ± 1.6 | 3.2 ± 2.6 | 0.51 |
| Non-Neurologic SLE Damage Index (U) | 2.4 ± 1.5 | 2.4 ± 1.8 | 0.95 |
| Neurologic SLE Damage index (U) | 0 (0 – 1) | 0 (0 – 1) | 0.66 |
| Parameters of Inflammation | |||
| C-reactive protein (mg/dl) | 0.6 (0.4 – 1.3) | 0.6 (0.5 – 0.9) | 0.90* |
| Erythrocyte sedimentation rate (mm/hr) | 33 ± 23 | 26 ± 25 | 0.23 |
| C3 (mg/dl) | 91 ± 40 | 99 ± 32 | 0.35 |
| C4 (mg/dl) | 14 (7 – 21) | 16 (11 – 23) | 0.15 |
| CH50 (mg/dl) | 71 ± 44 | 82 ± 38 | 0.23 |
| Standard Autoantibodies | |||
| dsDNA positive | 17/31 (55%) | 21/43 (49%) | 0.64 |
| dsDNA titer | 15 (0 – 122) | 10 (0 – 45) | 0.35 |
| Ribonucleoprotein titer | 0 (0 – 1) | 0 (0 – 1) | 0.06* |
| Smith antibody positive | 18/31 (58%) | 12 (27%) | 0.01† |
| SSA antibody positive | 14/31 (45%) | 17 (38%) | 0.64 |
| SSB antibody positive | 9/31 (29%) | 6 (15%) | 0.14 |
| Antiphospholipid Antibodies | |||
| Any antiphospholipid antibody positive | 14/31 (45%) | 31 (69%) | 0.06 |
| Beta-2 glycoprotein I antibody positive | 2/29 (7%) | 16/43 (37%) | 0.005‡ |
| Lupus-like inhibitor positive | 7/31 (23%) | 18 (40%) | 0.14 |
| IgG, IgM, or IgA anticardiolipin positive | 10/31 (32%) | 23 (51%) | 0.16 |
| IgM anticardiolipin antibody (IU) | 4.6 (1.8 – 12) | 8.8 (2 – 21) | 0.19* |
| IgG anticardiolipin antibody (IU) | 6.1 (2.2 – 12) | 8 (3 – 25) | 0.19* |
| IgA anticardiolipin antibody (IU) | 2.9 (0.8 – 5.5) | 2.9 (1 – 9) | 0.49* |
| Anti-inflammatory and Anti-thrombotic Therapy | |||
| Prednisone therapy | 17/31 (55%) | 14 (31%) | 0.06 |
| Average prednisone (mg/day) | 8.0 ± 7.7 | 6.0 ± 5.3 | 0.21 |
| Prednisone current dose (mg/d) | 5 (0 – 10) | 0 (0 – 10) | 0.07* |
| Years of prednisone | 4 (1 – 9) | 6 (1 – 10) | 0.46* |
| Cyclophosphamide therapy | 11/31 (35%) | 16 (36%) | 1.00 |
| Years of cyclophosphamide | 0 (0 – 1) | 0 (0 – 1) | 0.55* |
| Mycophenalate, methotrexate, or rituximab | 6/31 (19%) | 15 (33%) | 0.20 |
| Hydroxycloroquine or cloroquine therapy | 24/31 (77%) | 27 (60%) | 0.14 |
| Aspirin, warfarin, or either | 12/31 (39%) | 20 (44%) | 0.64 |
Values are n (%), mean ± SD, or median (interquartile range).
By Wilcoxon Rank Sum test.
p value non-significant after Bonferoni’s correction.
Association occurred in the opposite direction.
Abbreviations: SLE = systemic lupus erythematosus, DNA = double stranded nuclear antibody, ANA = antinuclear antibody, SSA = Ro antibody, SSB = La antibody, U = units, IU = international units.
Brain MRI
Standard volume-acquired T1-weighted, T2-weighted fluid attenuated inversion recovery (T2-FLAIR), and diffusion-weighted images were obtained using a 1.5-T Siemens Sonata scanner with an 8-channel head coil. Brain lesions were classified as recent or old cerebral infarcts using standard criteria [15]. Inter-observer agreement for detection of cerebral infarcts in 72 studies was 93% (Kappa 0.72).
Transcranial Doppler
Both middle cerebral arteries were interrogated for 90 minutes for detection of microembolism defined as audible, high intensity, and short lasting unidirectional signals within the Doppler blood flow velocity and vessel lumen [16]. Intraobserver agreement for detection of microembolism was 96% (Kappa 0.83).
Transesophageal echocardiography
Participants underwent TEE with IE-33 Philips systems (Andover, Massachusetts, USA) and images were digitally acquired for off-line interpretation. Heart valves were carefully imaged in multiple planes at depths of 4–8 cm with a narrow sector scan to improve image resolution.
Criteria for interpretation
LEx were defined as thin (≤2 mm) and elongated (≥6 mm) structures with independent and undulating hypermobility seen at the leaflet’s coaptation point, on the atrial side of the mitral leaflets and ventricular side of the aortic cusps [2–5]. In 53 randomly selected TEE studies (27 patients, 26 controls), inter-observer agreement for the detection of mitral and aortic valve LEx were 96% (Kappa 0.92) and 91% (Kappa 0.81), respectively. To further validate the presence and characteristics of LEx, a third observer measured and averaged the length and width of LEx during 3 cardiac cycles. Finally, the length and width of LEx were measured by a fourth observer in 20 randomly selected studies (13 patients, 7 controls) with 25 LEx. The length and width of LEx were similar between the third and fourth observers (all p≥0.20) (Supplemental Table 1).
Other cardiogenic mechanisms for CVD were carefully assessed: 1) Libman-Sacks vegetations defined as abnormal localized, sessile, oval shape, and protruding echodensities of ≥3 mm in diameter with well-defined borders either as part of or adjacent to valve leaflets, subvalvular apparatus, or great vessels [14]. Inter-observer agreement for detection of vegetations in 30 randomly selected TEE studies (22 patients, 8 controls) was 93% (kappa 0.87). 2) Intracardiac thrombus and spontaneous echocardiographic contrast on the left atrium and ventricle. 3) Patent foramen ovale or interatrial septal aneurysms by two-dimensional, color-Doppler, and saline contrast imaging. And 4) atherosclerosis of the ascending aorta, arch, and descending thoracic aorta by two-dimensional and M-mode imaging and defined as intima-media thickening (≥2SD above the mean of healthy controls) or plaques (focal thickening of the intima-media exceeding 50% of the surrounding vessel wall) [14, 17]. Inter-observer variability for measurement of aortic intima-media thickness in 10 randomly selected studies (7 patients, 3 controls) demonstrated a mean percent error of 4.9%.
Carotid duplex
From longitudinal B-mode images of both common carotid arteries, 6 measurements of intima-media thickness of the far and near walls were performed [18]. Inter-observer variability for measurement of carotid intima-media thickness in 10 randomly selected studies (6 patients, 4 controls) revealed a mean percent error of 3.8%. Carotid atherosclerosis was defined with the same criteria described for the aorta.
Cerebrovascular disease
After completion of evaluations, the presence of CVD was defined as acute stroke/TIA, cerebromicroembolism, or cerebral infarcts.
Follow-up
Subjects without stroke/TIA on enrollment had a median follow-up of 57 months (IQR 25–64 months) to assess an association of LEx with incident stroke/TIA.
Statistical analysis
Descriptive statistics were mean±SD, median (IQR) for asymmetrically distributed data, or frequencies (%). Comparisons between 3 study groups were performed by analysis of variance (ANOVA) for continuous measures and verified by Kruskal-Wallis tests. Fisher’s exact tests were used for comparisons of binary measures among 3 groups. Student’s t-tests (Wilcoxon’s rank sum test for asymmetrically distributed data) and Fisher’s exact tests were used to compare continuous and binary measures between 2 groups, respectively. A two-tailed p value <0.05 was considered significant. Analyses were performed using SAS 9.3.
RESULTS
On enrollment, 33 (43%) of 77 SLE patients had CVD manifested as acute stroke/TIA in 23 patients (stroke in 7, TIA in 16), cerebromicroembolism in 17 patients with 28 microembolic events, or cerebral infarcts in 14 patients with 47 infarcts (11 recent, 36 old). No CVD was detected in healthy controls.
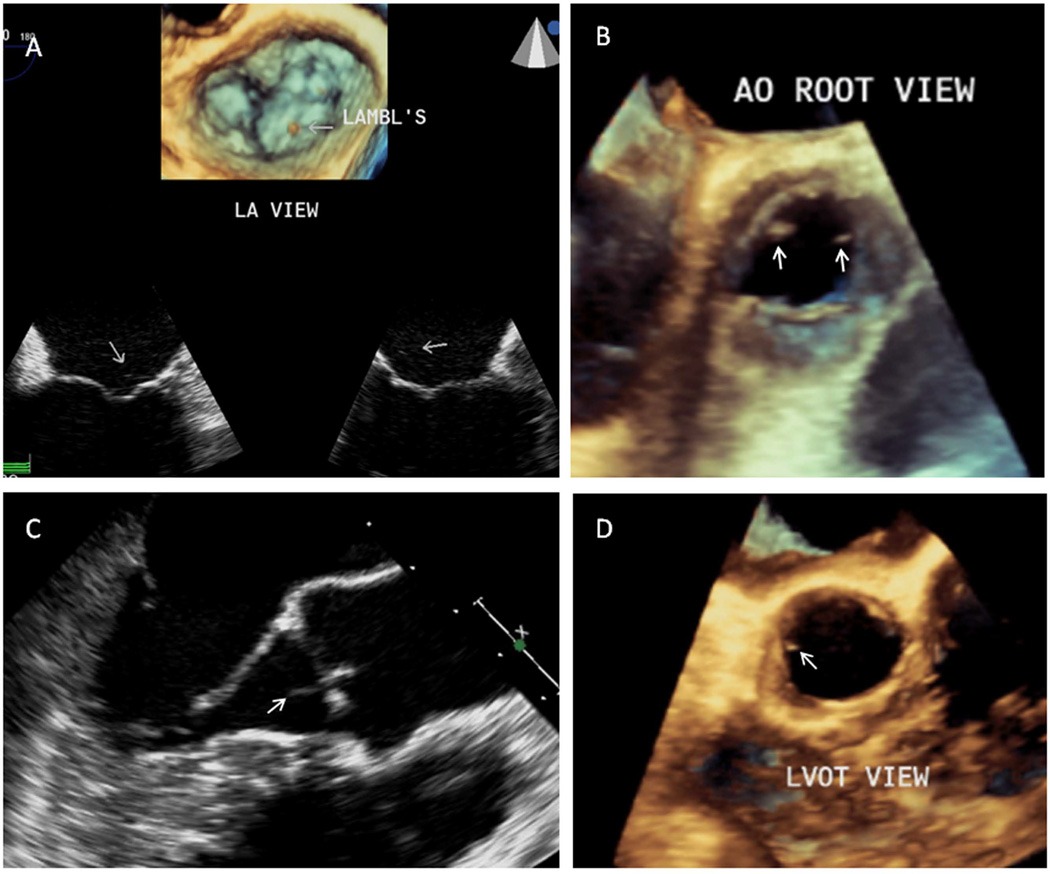
The frequency of LEx on the mitral, aortic, or either valve were similar in patients and controls [21 (27%), 20 (26%), or 32 (42%) versus 7 (27%), 9 (35%), or 12 (46%), respectively, all p≥0.45]. Of most importance, the frequency of LEx was similar in patients with and without CVD as well as in healthy controls (all p≥0.55) (Table 1, Figure 1 with video clips).
Table 1.
Frequency and Distribution of Lambl’s Excrescences in 77 SLE Patients with and without Cerebrovascular Disease and in 26 Healthy Controls
| Stroke/TIA (N = 23) |
No Stroke/TIA (N = 54) |
Controls (N = 26) |
P value |
|
|---|---|---|---|---|
| MV LEx | 4 (17%) | 16 (30%) | 7 (27%) | 0.58 |
| AoV LEx | 7 (30%) | 13 (24%) | 9 (35%) | 0.59 |
| Either | 8 (35%) | 23 (43%) | 12 (46%) | 0.72 |
|
Cerebromicroembolism (N = 17) |
No Cerebromicroembolism (N = 60) |
Controls | ||
| MV LEx | 4 (24%) | 17 (28%) | 7 (27%) | 0.95 |
| AoV LEx | 5 (29%) | 15 (25%) | 9 (35%) | 0.65 |
| Either | 7 (41%) | 25 (42%) | 12 (46%) | 0.96 |
| Cerebral Infarcts (N = 14) | No Cerebral Infarcts* (N =61) | Controls | ||
| MV LEx | 3 (21%) | 17 (28%) | 7 (27%) | 0.95 |
| AoV LEx | 5 (36%) | 15 (25%) | 9 (35%) | 0.57 |
| Either | 5 (36%) | 26 (43%) | 12 (46%) | 0.81 |
| †Overall CVD (N = 33) | No CVD (N = 44) | Controls | ||
| MV LEx | 8 (24%) | 13 (30%) | 7 (27%) | 0.96 |
| AoV LEx | 10 (30%) | 10 (23%) | 9 (35% | 0.55 |
| Either | 13 (39%) | 19 (43%) | 12 (46%) | 0.91 |
One patient refused MRI and a second patient had poor quality MRI.
Overall CVD = stroke/TIA, cerebromicroembolism, or cerebral infarcts.
Abbreviations: LEx=Lambl’s excrescences, MV=mitral valve, AoV=aortic valve, TIA=transient ischemic attack.
Also, the length and width of LEx were similar in patients with (11.9 ± 4.9 mm and 0.77 ± 0.25 mm) and without CVD (11.7 ± 3.5 mm and 0.90 ± 0.20 mm) and in controls (9.1 ± 3.4 mm and 0.85 ± 0.18 mm, respectively) (all p≥0.13 for length and p≥0.24 for width) (Supplemental Table 2).
In addition, sources of cardioembolism other than LEx, aortic or carotid atherosclerosis, or thrombogenesis were found in ≥94% of patients with CVD (Table 2).
Table 2.
Mechanisms of Cerebrovascular Disease in SLE Patients
| Abnormality | Stroke/TIA (N = 23) |
Cerebro- Microembolism (N = 17) |
Cerebral Infarcts (N = 14) |
Overall Cerebrovascular Disease (N = 33) |
|---|---|---|---|---|
| Libman-Sacks vegetations | 22 (96%) | 12 (71%) | 14 (100%) | 27 (82%) |
| PFO/IASA | 1 (4%) | 3 (18%) | 0 | 4 (12%) |
| Aortic atherosclerosis | 9 (39%) | 6 (35%) | 7 (50%) | 13 (39%) |
| Carotid atherosclerosis | 6 (26%) | 2 (12%) | 5 (36%) | 8 (24%) |
| Positive aPL | 16 (70%) | 10 (59%) | 9 (64%) | 19 (58%) |
| Any abnormality | 23 (100%) | 16 (94%) | 14 (100%) | 32 (97%) |
Abbreviations: PFO=patent foramen ovale, IASA=interatrial septal aneurysm, Positive aPL= abnormal levels of IgG, IgM, or IgA anticardiolipin antibodies or positive lupus-like antibody or β2-glycoprotein antibody.
Furthermore, the incidence of stroke/TIA during follow-up was similarly low in 36 subjects with LEx and 44 without LEx [1(1.3%) versus 2(2.5%), respectively, p=1.0]. The patient with LEx and stroke/TIA also had Libman-Sacks vegetations and aPL.
Finally, in SLE patients and controls, LEx were not associated with aging [age <30 (43%), 30–39 (45%), 40–49 (35%), and 50–60 (47%); Jonckheere-Terpstra trend test p=0.93], atherogenic risk factors, atherosclerosis, inflammation, or thrombogenesis (Table 3). Also in SLE patients, LEx were not associated with disease duration, activity, and damage; standard parameters of inflammation; serology and autoantibodies including aPL antibodies; and anti-inflammatory or antithrombotic therapy (Table 4).
DISCUSSION
Major findings
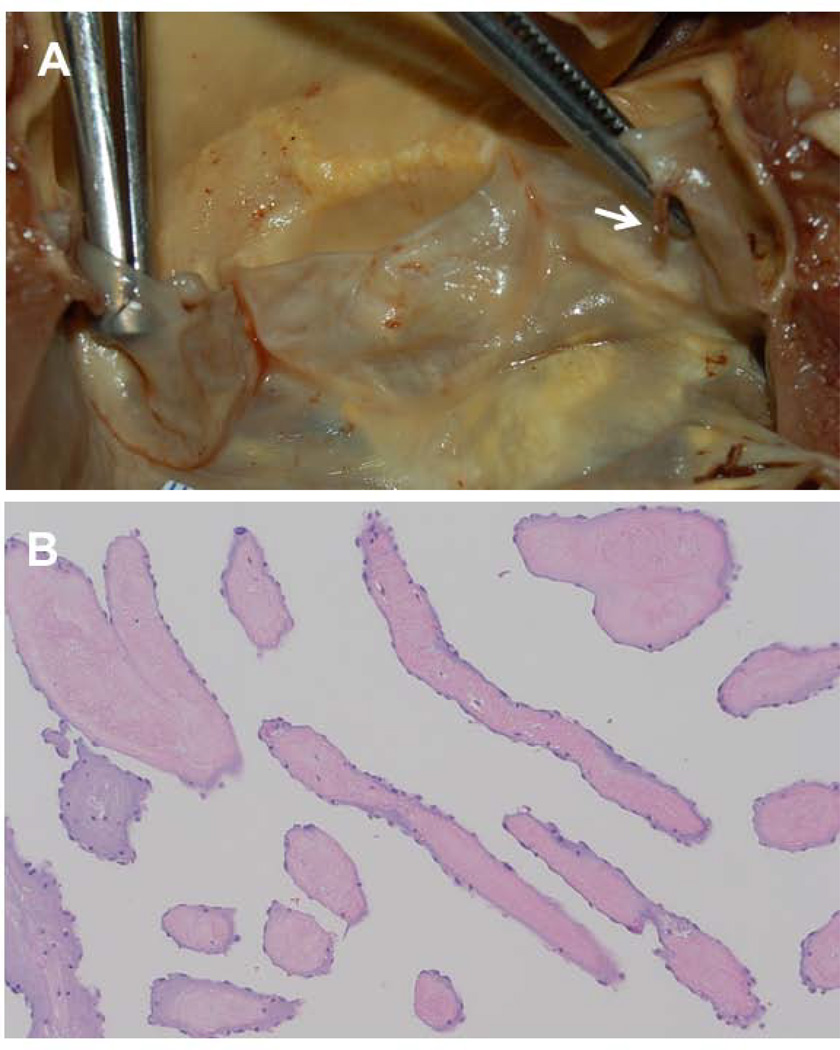
This study demonstrated 4 major findings: 1) LEx had similar frequency, distribution, and characteristics in healthy controls as in SLE patients with and without CVD; 2) most patients with CVD had other proven sources of cardioembolism, atherosclerosis, or increased thrombogenesis; 3) LEx were not associated with incident stroke/TIA; and 4) LEx were not associated with aging, atherogenic risk factors, atherosclerosis, inflammation, or thrombogenesis. To our knowledge, this is the first highly integrated, multidisciplinary, controlled, cross-sectional and longitudinal study to support that LEx may not be pathologic valve structures, may not be cardioembolic substrates, and therefore may not warrant therapy. LEx may be normal variants with a protective blunting effect on the leaflets from the impact of valve closure or from shear forces of blood flow jets or are normal variants resulting from constant bending and buckling of leaflets leading to transient tearing of endothelial cells and subendothelial collagen and elastic fibers with subsequent normal reparative endothelialization [5, 19, 20]. The predominance of LEx on the left heart valves where high ventricular and aortic pressure gradients impact valve closure may support such pathogeneses. In fact, pathology studies of normal valves report similar frequency of LEx during different decades of age with equal sex distribution, and with similar frequency, distribution, and characteristics to those described by TEE in healthy subjects [4, 5, 19–22]. Finally, the histology of LEx consisting of a core of connective tissue with collagen and elastic fibrils or of an acellular hyaline material covered by normal endothelium does not indicate an aging, atherogenic, inflammatory, or thrombogenic process [1, 19–22] (Figure 2).
Figure 2. Histology of Lambl’s Excrescences.
A. Gross anatomic view of a mildly sclerotic aortic valve with a thin (1 mm) and elongated (6 mm) Lambl’s excrescence (arrow) at the coaptation point and ventricular side of the left coronary cusp. B. Histology of longitudinal, cross-sectional, and oblique cuts of the Lambl’s excrescence demonstrate a core of fibroelastic, hypocellular, and avascular connective tissue covered by a single layer of endothelial cells.
Comparison with previous studies
Four previous studies support our findings. Cohen et al [23] in a case-control study of 284 patients with stroke (all >60 years old) and 276 controls reported mitral valve LEx in 22.5% of cases and 12.1% in controls (p=0.005). However, during 2–4 years of follow-up, incident stroke was similarly low in patients with and without LEx (6.0 and 4.2 per 100 person-years, respectively), LEx were not independent predictors of recurrent stroke (relative risk 1.3, 95% CI 0.5–3.1, p=0.54), and incident stroke in patients with LEx was unrelated to antiplatelet, anticoagulant, or no therapy (7.1, 6.9, and 0 per 100 person-year, respectively). Our group [5] in 1997 in a different population and in part retrospective study reported similar frequency of LEx in 38% of 90 healthy volunteers, in 47% of 88 patients undergoing TEE for reasons other than cardioembolism, and in 41% of 49 patients undergoing TEE for suspected cardioembolism, of whom 85% had other cardiovascular mechanisms for CVD. Also, incident stroke/TIA in subjects with and without LEx was similarly low (≤2%) during 5-years of follow-up. Nighoghossian et al [24] reported LEx in 30 of 160 (18.8%) patients with stroke. In that study, all 30 patients had atherogenic risk factors, atrial septal aneurysms and patent foramen ovale were the predominant abnormalities in patients with presumed cardioembolic stroke, 40% had large or small vessel disease as cause of stroke, one patient with recurrent stroke had pathologic confirmation of thrombotic vegetations, and 85% of LEx persisted on follow-up TEE despite antithrombotic therapy. Of most importance, Homma et al [4], in a large, prospective, cross-sectional and longitudinal study of 619 patients with carefully categorized stroke types, meticulously assessed TEE studies for LEx, and randomly and double blindly assigned patients with and without LEx to warfarin or aspirin therapy reported LEx in 244 (39%) of 619 patients with stroke (in 38.6% with cryptogenic stroke and in 40.1% with known cause of stroke). During 2-year follow-up and independent of stroke type or of warfarin or aspirin therapy, the incidence of stroke or death was similar in those with and without LEx (16.4% versus15.5%, respectively).
Other studies suggesting LEx as cardioembolic substrates have design and methodologic characteristics that preclude establishing a causal association. Recently, Leitman, et al (6) in a 5-year retrospective case-controlled echocardiographic database study reported valve strands in 150 of 21,000 studies (0.7%, the lowest ever reported prevalence of LEx). They report a 27% frequency of stroke/TIA or peripheral ischemia in 40 (27%) of those 150 patients with as compared with 20 (13%) of 150 case-controls without strands. However, an association of valve strands with cerebral or peripheral ischemia cannot be established for several reasons: 1) study design, 2) ascertainment bias given that records of patients in whom LEx were reported upon were retrospectively reviewed aware that echocardiographic studies were frequently performed for suspected cardioembolism, 3) 43% of patients had transthoracic echocardiography with an expected low sensitivity and low specificity for valve strands, and 4) study population with high risk for atherothrombosis, atheroembolism, or proven sources of cardioembolism as follows: a) elderly population [mean age, 61±17 years (range 18 to >80)], b) 76% hospitalized patients, c) 63% with atherogenic risk factors, d) 37% with coronary artery disease; e) ≥68% with thickened or calcified heart valves; f) 23% with atrial fibrillation, and g) an undefined proportion with prosthetic valves. Lee et al [2] in a non-controlled study reported mitral valve LEx in 11 (22%) of 50 patients undergoing TEE for suspected cardioembolism. However, patients’ mean age was 63 years, 9 had valve thickening or calcification, and 7 had history of arrhythmias, myocardial infarction, cardiomyopathy, or hypertension. Freedberg et al [3], in a retrospective analysis of 1,559 patients with a mean age of 66 years identified LEx by TEE in 63 (10.6%) of 597 patients with suspected cardioembolism as compared to 23 (2.3%) of 962 without. However, their study population was old and also had a 10% prevalence of prosthetic or native valve disease. Roberts et al [7] in a retrospective case-control study reported LEx in 34 (47%) of 73 patients with stroke as compared to 12 (16%) of 73 stroke-free age-matched controls (OR=4.4; 95% CI=2.0–9.6). However, the cases mean age was 63 with a high prevalence (≥55%) of atherogenic risk factors and paradoxically lower frequency (27%) of LEx in patients with presumed cardioembolic stroke than in those with atherothrombotic (42%) or lacunar (62%) strokes. Isolated case reports of LEx and stroke have either alternative mechanisms of stroke or incomplete stroke work-up [8–10, 25–27]. Finally, 4 cases of stroke associated with “giant LEx” described as long (>20 mm) and multiple strands forming a complex are suggestive of papillary fibroelastomas on echocardiography and histology [9, 11, 28–30]. In this study, one subject had a LEx of >20 mm.
Study limitations
The discrete control group may have led to overestimation of the frequency of LEx as compared to that of a general healthy population. However, similar high frequency of LEx were reported in a study with larger number of healthy controls [5] and this limitation does not affect the main finding of the lack of an association of LEx with prevalent and incident CVD in SLE patients. Although lack of histologic confirmation limits conclusions about the nature of LEx seen on TEE, autopsy studies report similar frequency, distribution, and characteristics of LEx as those described by TEE [21, 22]. Concomitant Libman-Sacks vegetations may decrease the specificity of TEE for LEx, yet such vegetations are structurally distinct from LEx [14] (Figure 1B). Although the study included predominantly females as is typical of SLE, similar frequency of LEx has been reported in males [2–5, 21, 22].
Figure 1. Lambl’s excrescences of the mitral and aortic valves.
A. Two-dimensional (2D) TEE 4 and 2-chamber views of the mitral valve (bottom images) in a 21 year-old healthy female demonstrate a long, thin, and hypermobile Lambl’s excrescence located at the coaptation point and atrial side of normal mitral leaflets (Figure 1A video clip). Corresponding three-dimensional (3D) TEE atrial view of the mitral valve (top image) shows a Lambl’s excrescence at the coaptation point of the middle anterior and posterior mitral scallops (Figure 1A video clip). B. This aortic root 3D-TEE view of the aortic valve in a 44 year-old female with SLE demonstrates a Lambl’s excrescence (right arrow) on the tip and ventricular side of the left coronary cusp and a Libman-Sacks vegetation (left arrow) located on the tip and ventricular side of a thickened non-coronary cusp (Figure 1B video clip). C,D. This longitudinal 2-D TEE view (C) of the aortic valve in a 34 year-old female with SLE demonstrates a thin, elongated, and mobile Lambl’s excrescence at the coaptation point of the left and right coronary cusps prolapsing into the ventricular outflow tract during diastole (arrow) (Figure 1C video clip). Corresponding left ventricular outflow tract (LVOT) 3D-TEE systolic view (D) demonstrates a Lambl’s excrescence located on the ventricular side and tip of the left coronary cusp (Figure 1D video clip).
Clinical implications
The detection of LEx in patients with CVD poses a diagnostic and therapeutic challenge. The detection of LEx is of particular importance in a relatively large proportion (~25%) of young patients with presumed cryptogenic stroke/TIA [31) in whom TEE is recommended. The detection of LEx in these patients, which will increase further with the use of three-dimensional TEE [32], may lead to misclassification of a stroke/TIA as cardioembolic, underdiagnosis of the true etiology of stroke/TIA, and potentially to unnecessary antiplatelet or anticoagulant therapy or even valve surgery. Thus, detection of LEx in a patient with suspected cardioembolism should not be considered a de facto cause of cerebroembolism. In such a patient other known cardiovascular sources of embolism and mechanisms of CVD should be thoroughly investigated before initiating a specific therapy or considering valve surgery [4, 5, 14, 23,24, 31]. Patients with suspected cardioembolism and a normal transthoracic echocardiogram may not need to undergo TEE for detection of LEx. In these patients, clinical, laboratory, and transthoracic echocardiography predict absence on TEE of established cardioembolic substrates in ≥95% of patients [34]. The incidental finding of LEx in patients undergoing TEE for reasons other than suspected cardioembolism may not warrant prophylactic antiplatelet therapy. Finally, awareness of the high frequency, distinctive echocardiographic characteristics, lack of association with pathogenic factors, and lack of response to anti-inflammatory and antithrombotic therapy of LEx should improve the diagnostic accuracy of TEE for detection of echocardiographically distinctive non-infective and infective vegetations and papillary fibroelastomas [5, 14, 35, 36].
Conclusions
In this study, LEx are similarly prevalent in healthy controls and SLE patients, are not associated with CVD, and are not associated with pathogenic risk factors. Therefore, the study findings suggest that LEx may not be cardioembolic substrates, may not represent pathologic valve structures, and may not require therapy.
Supplementary Material
ACKNOWLEDGEMENTS
To Janeen Sharrar, RN, and Julia Middendorf, RN, for outstanding coordination of the study, and to Ryan Berry, M.D., for the expert histopatologic illustration of Lambl’s excrescences.
GRANT SUPPORT
Grant RO1-HL04722-01-A6 by the National Institutes of Health/National Heart Lung and Blood Institute and in part by grant 8UL1-TR000041 by the National Center for Research Resources and National Center for Advancing Translational Sciences.
Footnotes
CONFLICTS OF INTEREST
None of the authors has conflicts of interest to disclose.
REFERENCES
- 1.Lambl VA. Papillare excrescenzen und der semilunar-klappe der aorta. Wien Med Wochenschr. 1856;16:244–250. [Google Scholar]
- 2.Lee RJ, Bartzokis T, Yeoh T, Grogin HR, Choi D, Schnittger I. Enhanced detection of intracardiac sources of cerebral emboli by transesophageal echocardiography. Stroke. 1991;22:734–739. doi: 10.1161/01.str.22.6.734. [DOI] [PubMed] [Google Scholar]
- 3.Freedberg RS, Goodkin GM, Perez JL, Tunick PA, Kronzon I. Valve strands are strongly associated with systemic embolization: a transesophageal echocardiographic study. J Am Coll Cardiol. 1995;26:1709–1712. doi: 10.1016/0735-1097(95)00394-0. [DOI] [PubMed] [Google Scholar]
- 4.Homma S, Di Tullio MR, Sciacca RR, Sacco RL, Mohr JP. Effect of aspirin and warfarin therapy in stroke patients with valvular strands. PICSS Investigators. Stroke. 2004;35:1436–1442. doi: 10.1161/01.STR.0000126100.53682.a0. [DOI] [PubMed] [Google Scholar]
- 5.Roldan CA, Shively BK, Crawford MH. Valve Excrescences: Prevalence, evolution and risk for cardioembolism. J Am Coll Cardiol. 1998;30:1308–1314. doi: 10.1016/s0735-1097(97)00315-x. [DOI] [PubMed] [Google Scholar]
- 6.Leitman M, Tyomkin V, Peleg E, Shmueli R, Krakover R, Vered Z. Clinical significance and prevalence of valvular strands during routine echo examinations. Eur Heart J Cardiovasc Imaging. 2014;15:1226–1230. doi: 10.1093/ehjci/jeu110. [DOI] [PubMed] [Google Scholar]
- 7.Roberts JK, Omarali I, Di Tullio MR, Sciacca RR, Sacco RL, Homma S. Valvular strands and cerebral ischemia. Effect of demographics and strand characteristics. Stroke. 1997;28:2185–2188. doi: 10.1161/01.str.28.11.2185. [DOI] [PubMed] [Google Scholar]
- 8.Kalavakunta JK, Peddi P, Bantu V, Tokala H, Kodenchery M. Lambl's excrescences: a rare cause of stroke. J Heart Valve Dis. 2010;19:669–670. [PubMed] [Google Scholar]
- 9.Wolf RC, Spiess J, Vasic N, Huber R. Valvular strands and ischemic stroke. Eur Neurol. 2007;57:227–31. doi: 10.1159/000100016. Review. [DOI] [PubMed] [Google Scholar]
- 10.Aziz F, Baciewicz FA., Jr Lambl's excrescences: review and recommendations. Tex Heart Inst J. 2007;34:366–368. Review. [PMC free article] [PubMed] [Google Scholar]
- 11.Aggarwal A, Leavitt BJ. Images in clinical medicine. Giant Lambl's excrescences. N Engl J Med. 2003;349:e24. doi: 10.1056/ENEJMicm010900. [DOI] [PubMed] [Google Scholar]
- 12.Mok CC, Ho LY, To CH. Annual incidence and standardized incidence ratio of cerebrovascular accidents in patients with systemic lupus erythematosus. Scand J Rheumatol. 2009;38:362–368. doi: 10.1080/03009740902776927. [DOI] [PubMed] [Google Scholar]
- 13.Chiu CC, 1, Huang CC, Chan WL, Chung CM, Huang PH, Lin SJ, Chen JW, Leu HB. Increased risk of ischemic stroke in patients with systemic lupus erythematosus: a nationwide population-based study. Intern Med. 2012;51:17–21. doi: 10.2169/internalmedicine.51.6154. [DOI] [PubMed] [Google Scholar]
- 14.Roldan CA, Sibbitt WL, Jr, Qualls CR, Jung RE, Greene ER, Gasparovic CM, Hayek RA, Charlton GA, Crookston K. Libman-Sacks endocarditis and embolic cerebrovascular disease. JACC:Cardiovascular Imaging. 2013;6:973–983. doi: 10.1016/j.jcmg.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sibbitt WL, Jr, Sibbitt RR, Brooks WM. Neuroimaging in neuropsychiatric systemic lupus erythematosus. Arthritis Rheum. 1999;42:2026–2038. doi: 10.1002/1529-0131(199910)42:10<2026::AID-ANR2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 16.Choi Y, Saqqur M, Asil T, Jin A, Stewart E, Stephenson C. A combined power M-mode and single gate transcranial Doppler ultrasound microemboli signal criteria for improving emboli detection and reliability. J Neuroimaging. 2009;32:1–9. doi: 10.1111/j.1552-6569.2009.00446.x. [DOI] [PubMed] [Google Scholar]
- 17.Roldan CA, Joson J, Sharrar J, Qualls CR, Sibbitt WL., Jr Premature aortic atherosclerosis in systemic lupus erythematosus: a controlled transesophageal echocardiographic study. J Rheumatol. 2010;37:71–78. doi: 10.3899/jrheum.090665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roman MJ, Naqvi TZ, Gardin JM, Gerhard-Herman M, Jaff M, Mohler E. Clinical application of noninvasive vascular ultrasound in cardiovascular risk stratification: A report from the American Society of Echocardiography and the Society of Vascular Medicine and Biology. J Am Soc Echocardiogr. 2006;19:943–954. doi: 10.1016/j.echo.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 19.Hurle JM, Garcia-Martinez V, Sanchez-Quintana D. Morphologic characteristics and structure of surface excrescences (Lambl’s excrescences) in the normal aortic valve. Am J Cardiol. 1986;58:1223–1227. doi: 10.1016/0002-9149(86)90386-3. [DOI] [PubMed] [Google Scholar]
- 20.Riddle JM, Wang CH, Magilligan DJ, Stein PD. Scanning electron microscopy of surgically excised human mitral valves in patients over 45 years of age. Am J Cardiol. 1989;63:471–477. doi: 10.1016/0002-9149(89)90322-6. [DOI] [PubMed] [Google Scholar]
- 21.Nistal JF, Garcia-Martinez V, Fernandez MD, Hurle A, Hurle JM, Revuelta JM. Age-dependent dystrophic calcification of the aortic valve leaflets in normal subjects. J Heart Valve Dis. 1994;3:37–40. [PubMed] [Google Scholar]
- 22.Magarey FR. On the mode of formation of Lambl’s excrescences and their relation to chronic thickening of the mitral valve. J Pathol Bacteriol. 1949;61:203–208. doi: 10.1002/path.1700610207. [DOI] [PubMed] [Google Scholar]
- 23.Cohen A, Tzourio C, Chauvel C, Bertrand B, Crassard I, Bernard Y. Mitral valve strands and the risk of ischemic stroke in elderly patients. The French Study of Aortic Plaques in Stroke (FAPS) Investigators. Stroke. 1997;28:1574–1578. doi: 10.1161/01.str.28.8.1574. [DOI] [PubMed] [Google Scholar]
- 24.Nighoghossian N, Derex L, Perinetti M, Honnorat J, Barthelet M, Loire R. Course of valvular strands in patients with stroke: cooperative study with transesophageal echocardiography. Am Heart J. 1998;136:1065–1069. doi: 10.1016/s0002-8703(98)70164-4. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez-Juanatey C, Garcia-Porrua C, Testa A, Gonzalez-Gay MA. Potential role of mitral valve strands on stroke recurrence in rheumatoid arthritis. Arthritis Rheum. 2003;49:866–867. doi: 10.1002/art.11469. [DOI] [PubMed] [Google Scholar]
- 26.Jaffe W, Figueredo VM. An example of Lambl's excrescences by transesophageal echocardiogram: a commonly misinterpreted lesion. Echocardiography. 2007;24:1086–1089. doi: 10.1111/j.1540-8175.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- 27.Rhee HY, Choi HY, Kim SB, Shin WC, Kim SH. Acute ischemic stroke in a patient with a native valvular strand. Case Rep Neurol. 2010;2:91–95. doi: 10.1159/000317117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu TY, Gerber IL, Roxburgh RH. Thromboembolic cerebral infarction secondary to giant Lambl’s excrescence. J Clin Neurosci. 2013;20:1632–1634. doi: 10.1016/j.jocn.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 29.Nighossian N, Trouillas P, Perinetti M, Barthelet M, Ninet J, Loire R. Lambl’s excrescence: an uncommon cause of cerebral embolimsm. Rev Neurol. 1995;151:583–585. [PubMed] [Google Scholar]
- 30.Mariscalco G, Bruno VD, Borsani P, Dominici C, Sala A. Papillary fibroelastoma: insight to a primary cardiac valve tumor. J Card Surg. 2010;25:198–205. doi: 10.1111/j.1540-8191.2009.00993.x. [DOI] [PubMed] [Google Scholar]
- 31.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2015 update: a report from the American heart association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 32.Dumaswala B, Dumaswala K, Hsiung MC, Quiroz LD, Sungur A, Escanuela MG, Mehta K, Oz TK, Bhagatwala K, Karia NM, Nanda NC. Incremental value of three-dimensional transesophageal echocardiography over two-dimensional transesophageal echocardiography in the assessment of Lambl's excrescences and nodules of Arantius on the aortic valve. Echocardiography. 2013;30:967–975. doi: 10.1111/echo.12310. [DOI] [PubMed] [Google Scholar]
- 33.Armstrong WF. Valve excrescences: harmless and common or strokes-in-waiting? J Am Coll Cardiol. 1997;30:1315–1316. doi: 10.1016/s0735-1097(97)00322-7. [DOI] [PubMed] [Google Scholar]
- 34.Leung DY1, Black IW, Cranney GB, Walsh WF, Grimm RA, Stewart WJ, Thomas JD. Selection of patients for transesophageal echocardiography after stroke and systemic embolic events: role of transthoracic echocardiography. Stroke. 1995;26:1820–1824. doi: 10.1161/01.str.26.10.1820. [DOI] [PubMed] [Google Scholar]
- 35.Blanchard DG, Ross RS, Dittrich HC. Nonbacterial thrombotic endocarditis: assessment by transesophageal echocardiography. Chest. 1992;102:954–956. doi: 10.1378/chest.102.3.954. [DOI] [PubMed] [Google Scholar]
- 36.Job FP, Franke S, Lethen H, Flachskampf Hanrath P. Incremental value of multiplane transesophageal echocardiography for the assessment of active infective endocarditis. Am J Cardiol. 1995;75:1033–1037. doi: 10.1016/s0002-9149(99)80719-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.