Abstract
Much of our knowledge about Aβ aggregation and toxicity has been acquired using synthetic peptides and mouse models, whereas less attention has been paid to soluble proteins present in human brain. Here we report that aqueous extracts of AD brain can contain at least four different Aβ assembly forms which include: (i) monomers, (ii) a ~7kDa Aβ species, and larger species which ranged (iii) from ~30–150 kDa, and (iv) from ~160–700 kDa. The ~30–150 kDa species are by far the most prevalent and appear to be built from ~7 kDa Aβ species. The component ~7 kDa Aβ species resist denaturation by chaotropic agents and have a higher Aβ42/Aβ40 ratio than monomers, and are unreactive with antibodies to Asp1 of Aβ or APP residues N-terminal of Asp1. These results recommend further analysis of these brain-derived ~7 kDa Aβ species, the mechanism by which they assemble and the structures which they form.
Keywords: Alzheimer’s disease, amyloid β-protein, oligomers, SDS-stable dimer
Introduction
Strong genetic evidence indicates that the amyloid β-protein (Aβ) plays an initiating role in Alzheimer’s disease (AD) (Tanzi, 2012). In recent years much attention has focused on soluble non-fibrillar forms of Aβ loosely referred to as “oligomers” (Klein et al., 2001, Hardy and Selkoe, 2002, Benilova et al., 2012). Literally hundreds of studies have been conducted to investigate the formation, nature and activity of oligomers formed from synthetic Aβ, but much less effort has been directed at understanding soluble forms of Aβ present in human brain (Walsh and Teplow, 2012). We and others have shown that aqueous extracts of AD brain contain Aβ species which have disease-relevant bioactivity. In some experiments human brain-derived Aβ preparations were found to be orders of magnitude more potent than synthetic peptides (Shankar et al., 2008, Noguchi et al., 2009, Freir et al., 2011, Jin et al., 2011, Um et al., 2012) and were able to seed amyloidogenesis in amyloid precursor protein (APP) transgenic mice much more efficiently than synthetic assemblies (Langer et al., 2011, Stöhr et al., 2012). Consequently, understanding the assembly forms and composition of AD brain-derived water-soluble assemblies is of great importance.
A number of studies have investigated (usually by ELISA) the relationship between the levels of certain unspecified forms of water-soluble Aβ in brain samples from cases with AD, controls free of amyloid pathology and “controls” with high amyloid pathology. In some reports levels of water-soluble Aβ were selectively elevated in AD (Wang et al., 1999), whereas in others the levels in AD and high amyloid pathology controls were similar (Maarouf et al., 2009, Moore et al., 2012). Using immunoprecipitation (IP) coupled with denaturing SDS-PAGE and Western blotting (WB), we found that the levels of both Aβ monomers and what appeared to be SDS-stable dimers were higher in water-soluble extracts of AD brains than in brains from non-demented individuals or patients with other dementias (Mc Donald et al., 2010). In another widely cited paper, the levels of soluble Aβ in AD and control samples were analyzed by WB and found to correlate significantly with the number of plaques and tangles (McLean et al., 1999). In recent studies denaturing WB’ing revealed that ~4 and ~7 kDa Aβ species, but not slower migrating Aβ immunoreactive bands, increased in AD with increasing Braak stage (Lesné et al., 2013, Watt et al., 2013). Thus, in 6 out of 8 reports, at least some form of water-soluble Aβ was elevated in AD brain. In all such studies, “water-soluble Aβ” was defined operationally as Aβ that was extracted from brain in aqueous detergent-free buffer and remained in solution after high-speed centrifugation. Since water-soluble Aβ was described in this way and because it has been measured using a variety of techniques it is not clear that the species measured in each study were the same. For instance, it is known that certain ELISAs preferentially recognize Aβ monomer and under-detect aggregates of Aβ (Morishima-Kawashima et al., 1995, Enya et al., 1999, Stenh et al., 2005, Yang et al., 2013).
To avoid the confounding issue inherent with assays that cannot discriminate between Aβ monomer and higher assemblies, several groups developed immunoassays that appear not to detect Aβ monomer, and are said to be “oligomer-specific”. Using 2 distinct assay formats we found that levels of Aβ oligomers were significantly higher in aqueous brain extracts from patients with AD than in non-demented controls (Xia et al., 2009, Yang et al., 2013) and a recent study found significantly higher levels of Aβ oligomers in water-soluble extracts of AD than high amyloid pathology control brains (Esparza et al., 2013). Thus, there is consensus that Aβ oligomers are present at elevated levels in AD brain, but little is known about the nature of these oligomers.
Here we show that AD brain can contain four or more different sized Aβ species, the most prevalent of which range from ~30–150 kDa under native conditions. When treated with strong chaotropic agents these 30–150 kDa Aβ assemblies break down to yield two broad bands that migrate between: (i) 6.1 – 7.9 kDa, and (ii) 3.5 – 4.5 kDa. Quantitative WB’ing using a panel of 9 different anti-Aβ antibodies demonstrates that both the ~4 and ~7 kDa bands contain multiple different Aβ primary structures, and that there are significant primary structure differences between the ~4 and ~7 kDa species. Notably, the ~7 kDa material has a much higher Aβ42 content and more N-terminally truncated Aβ alloforms. Importantly, native sizing techniques confirm that the ~4 and ~7 kDa species have distinct physical properties consistent with the ~7 kDa species being a heterogeneous mixture of SDS-stable dimer formed from an array of different monomers. These results lead us to postulate that SDS-stable ~7 kDa Aβ species are a basic building block of intermediate molecular weight assemblies and that these assemblies play an important role in AD pathogenesis.
Materials and Methods
Reagents and chemicals
Blue dextran was purchased from GE Healthcare (San Francisco, CA) and unbranched dextrans of 5, 12, 25, 80, 150 and 270 kDa were purchased from Pharmacosmos (Holbaek, Denmark). Globular protein size exclusion chromatography (SEC) standards were from Bio-Rad (Hercules, CA). Aβ1-40 and Aβ1-42 were synthesised, purified and characterised by Dr. J. I. Elliott at Yale University (New Haven, CT). The mass and purity of peptides were determined by electrospray ionization/ion trap mass spectrometry and by reverse phase HPLC, respectively. Aβ40 beginning with pyroglutamic acid (AβpE3-40) was a gift from Probiodrug AG (Halle, Germany). A ~7 kDa APP fragment corresponding to Aβ1-40 plus the 31 residues of APP immediately N-terminal to Asp1 of Aβ (−31Aβ-40) was expressed and purified from E.coli (Walsh et al., 2009). Each synthetic peptide (Aβ1-40, Aβ1-42, AβpE3-40, −31Aβ-40) was dissolved in 50 mM Tris-HCl, pH 8.5 containing 7 M guanidium-HCl (GuHCl) and 5 mM EDTA at a concentration of 1 mg/ml and incubated at room temperature (RT) overnight to disaggregate pre-existing seeds. Samples were then centrifuged for 30 min at 16,000 g and chromatographed on a Superdex 75 10/300 column eluted at 0.5 ml/min with 50 mM ammonium bicarbonate, pH 8.5. The concentration of the peak fraction for each sample was determined by absorbance at 275 nm (O'Malley et al., 2014). The sample was then diluted to 5 ng/µl with 50 mM ammonium bicarbonate, pH 8.5, aliquoted and stored frozen at −80°C. When needed, an aliquot of a given peptide was thawed, used and any remaining sample was discarded. All other chemicals were of the highest purity available and unless indicated otherwise were obtained from Sigma-Aldrich (St. Louis, MO).
Antibodies
The antibodies used and their source are described in Table 1 and in Figure 1.
Table 1.
Antibodies used in this study and their sources.
| Antibody | Type | Recognises | Dilution for IP |
Western blot conc. |
ELISA conc. |
Source/Reference |
|---|---|---|---|---|---|---|
| 1G6 | Monoclonal | APP 573–576 | - | 1 µg/ml | - | Covance/ (Halford and Russell, 2009) |
| Z31 | Polyclonal | APP 577–596 | 1:500 | - | Selkoe Lab/ (Shoji et al., 2000) | |
| Pre-β | Polyclonal | APP 577–596 | 1:500 | - | Selkoe Lab/ (Podlisny et al., 1995) | |
| 3D6 | Monoclonal | Residue 1–5 of Aβ | - | 1 µg/ml | 1 µg/ml | Elan / (Johnson-Wood et al., 1997) |
| 07/1 | Monoclonal | Truncated Aβ that starts with pryoglutamate | - | 2 µg/ml | - | Probiodrug/ (Frost, 2013) |
| 6E10 | Monoclonal | Residues 6–10 of Aβ | - | 1 µg/ml | - | Signet/ (McLaurin et al., 2002) |
| 266 | Monoclonal | Residues 16–23 of Aβ | - | 1 µg/ml | 3 µg/ml | Elan/ (Seubert et al., 1992) |
| 4G8 | Monoclonal | Residues 17–24 of Aβ | - | 1 µg/ml | - | Signet /(Portelius et al., 2006) |
| 2G3 | Monoclonal | Aβ terminating at Val40 | - | 1 µg/ml | - | Elan /(Johnson-Wood et al., 1997) |
| HJ2 | Monoclonal | Aβ terminating at Val40 | - | 1 µg/ml | - | Holtzman D.M./ (Kim et al., 2012) |
| 21F12 | Monoclonal | Aβ terminating at Ile42 | - | 1 µg/ml | 1 µg/ml | Elan /(Johnson-Wood et al., 1997) |
| HJ7.4 | Monoclonal | Aβ terminating at Ile42 | - | 1 µg/ml | - | Holtzman D.M./ (Kim et al., 2012) |
| 1C22 | Monoclonal | Aβ aggregate specific | - | - | 3 µg/ml | Walsh lab/ (Yang et al., 2015) |
| AW7 | Polyclonal | Pan Aβ | 1:80 | - | - | Walsh Lab/ (Mc Donald et al., 2012) |
| 46-4 | Monoclonal | anti-HIV antibody, no Aβ reactivity (used as a negative control) | - | 1 µg/ml | - | ATCC/ (Reeves et al., 1995) |
Fig. 1. Epitopes of the anti-Aβ and anti-APP antibodies used in this study.
Line diagram of APP with the Aβ domain in red, and the single letter amino acid code of Aβ1-42. The reported binding epitopes for each antibody is indicated with a solid line under the corresponding amino acid sequence (see Table 1 for literature references). The location of the aspartate residue N-terminal of the Asp1 of Aβ is indicated by an arrow located at −31.
Preparation and characterization of aqueous extracts of human brain
Human cortical tissue from 36 donors (33 AD, 3 control) was obtained (Table 2) and used in agreement with local Ethics Committee guidelines. All AD cases met current diagnostic criteria based on tangle distribution and plaque burden. Post-mortem intervals were less than 48 hours. Frozen frontal or temporal cortical samples (~1 or ~2 g amounts) were allowed to thaw for ~30 sec at RT and diced into thin slices with a scalpel. Tissue was homogenised in 5 or 10 ml (for 1 or 2 g amounts respectively) of tris-buffered saline (TBS, 20 mM Tris-HCl, pH 7.4 containing 150 mM NaCl, 1 mg/ml pepstatin A, 1 mM pefabloc, 1 mg/ml aprotinin, 5 mM EGTA, sigma phosphatase inhibitor cocktail I and II, and 5 mM EDTA) with 25 strokes of a Dounce homogeniser (Fisher, Ottawa, Canada). Resulting 20% (w/v) homogenates were centrifuged at 150,000 g for 78 min and 4°C in a SW 55 Ti rotor (Beckman Coulter, Fullerton, CA). The upper 90% of the supernatant was removed from each sample, aliquoted and stored at −80°C prior to analysis.
Table 2.
Demographic details of the cases used in this study.
| Case | Source | Age | Gender | Neuropathology diagnosis |
PMI (hr) | Clinical diagnosis |
|---|---|---|---|---|---|---|
| AD1 | Asterand UK Ltd. | 78 | F | AD | 5.33 | AD |
| AD2 | Washington University | 80 | M | AD | 16 | AD |
| AD3 | UCLA | 87 | F | AD | 75 | NA |
| AD4 | Beaumont Hospital, Dublin | 55 | F | AD | NA | AD |
| AD5 | Beaumont Hospital, Dublin | 66 | F | AD | <48 | AD |
| AD6 | Beaumont Hospital, Dublin | 73 | F | AD | <24 | AD |
| AD7 | University College London | 80 | F | AD | 32.3 | AD |
| AD8 | UCLA | 96 | F | AD | 9 | NA |
| AD9 | UCLA | 64 | M | AD | 34 | AD |
| AD10 | UCLA | 70 | M | AD | 6 | NA |
| AD11 | UCLA | 87 | M | AD | 5 | NA |
| AD12 | UCLA | 81 | M | AD | 15 | NA |
| AD13 | Massachusetts General Hospital | 83 | F | AD | NA | AD |
| AD14 | Massachusetts General Hospital | 69 | F | AD | 16 | AD |
| AD15 | UCLA | 84 | F | AD | 7 | NA |
| AD16 | UCLA | 80 | F | AD | 3 | NA |
| AD17 | Washington University | 79.6 | F | AD | 17.5 | AD |
| AD18 | Massachusetts General Hospital | 69 | F | AD | 4.5 | AD |
| AD19 | Massachusetts General Hospital | 74 | F | AD | 6 | AD |
| AD20 | University College London | 51 | F | AD | 32,3 | AD |
| AD21 | UCLA | 79 | F | AD | 5 | AD |
| AD22 | Massachusetts General Hospital | 83 | F | AD | <25 | AD |
| AD23 | Massachusetts General Hospital | 81 | F | AD | 24 | AD |
| AD24 | Massachusetts General Hospital | 80 | NA | AD | NA | NA |
| AD25 | Massachusetts General Hospital | 69 | F | AD | 3 | AD |
| AD26 | University of Minnesota | 84 | NA | AD/Vas dementia | NA | AD/LBD |
| AD27 | Washington University | 80 | F | AD | 5.15 | AD |
| AD28 | UCLA | 79 | M | AD | NA | NA |
| AD29 | Massachusetts General Hospital | 75 | F | AD | <48 | AD |
| AD30 | Massachusetts General Hospital | 92 | F | AD | 4 | AD |
| AD31 | Massachusetts General Hospital | 89 | F | AD | 4 | AD |
| AD32 | Massachusetts General Hospital | 79 | F | AD | NA | AD |
| AD33 | Massachusetts General Hospital | 74 | F | AD | 8 | AD |
| C1 | Massachusetts General Hospital | 93 | F | Control | 5 | Not demented |
| C2 | Massachusetts General Hospital | 44 | F | Control | 18 | Not demented |
| C3 | Massachusetts General Hospital | 87 | M | Control | 48 | NA |
M refers to male, F denotes female, NA = information not available. PMI = Post-mortem interval
Immunoprecipitations (IP)
In order to minimise detection of cross-reactive proteins that bind non-specifically to Protein A sepharose beads (PrA), samples (10 ml) were incubated with PrA (100 µl each) for one hour at 4°C with gentle shaking on a nutator. PrA was removed by centrifugation (3,500 g for 5 min) and the supernatant divided into 0.5 ml aliquots. A polyclonal antibody (AW7, Table 1) raised against aggregated synthetic Aβ1-42 and that recognises multiple assemblies and epitopes, was used for IP. Briefly, 20, 0.5 ml aliquots were incubated with AW7 plus PrA (15 µl each) on a nutator at 4°C overnight. Aβ-antibody-PrA complexes were collected and washed as previously described (Walsh et al., 2000). PrA pellets were pooled during the last wash step. Aβ was eluted by boiling in 300 µl of 1× sample buffer (50 mM Tris, 2% (w/v) SDS, 12% (v/v) glycerol with 0.01% (w/v) phenol red) and the supernatant collected. 15 µl of supernatant (equivalent to 0.5 ml of starting brain extract) was loaded per gel lane, so that the amount of IP’d material of a given sample was precisely the same on all gels. This approach removes variations in sample detection that could result from using individual IPs for each gel and therefore allows direct comparison between the same sample electrophoresed on separate gels used for WB’ing with different mAbs.
Denaturing sodium dodecyl sulphate polyacrylamide electrophoresis (SDS-PAGE) and Western blotting
Samples were electrophoresed on hand-poured, 15 well, 16% polyacrylamide tris-tricine gels or pre-cast 26 well, 16.5% polyacrylamide Criterion™ tris-tricine gels (Bio-Rad, Hercules, CA) at 75 V for 15 min and 125 V for an additional 3 hr. Gels were rinsed in transfer buffer (10% methanol, 0.192 M glycine and 25 mM Tris) and electroblotted onto 0.2 µM nitrocellulose membranes at 400 mA and 4°C for 2 hr. Synthetic Aβ standards of equal amounts were run at either ends of each gel to allow comparison of brain samples run on different gels and to ensure even transfer onto the nitrocellulose membrane. To improve sensitivity of detection, membranes were boiled in a microwave oven for 1.5 min at 800 W in 50 ml phosphate buffered saline (PBS) and allowed to stand for 3.5 min, turned, microwaved again and allowed to stand for an additional 3.5 min (Ida et al., 1996). To further ensure even transfer of protein, the nitrocellulose was stained with Ponceau S (Klein et al., 1995) and assessed by eye. Membranes were blocked with 50% Odyssey blocking bufferin PBS for 1 hr at RT to minimize non-specific binding of primary and secondary antibodies to the nitrocellulose. Blots were then incubated overnight at 4°C with an appropriate primary antibody (Figure 1 and Table 1) in PBS containing 0.05% (v/v) Tween 20 (PBST) and 1% BSA. Membranes were washed six times for 10 min with PBST and then incubated with goat anti-mouse infrared 800 antibody (Rockland, Gilbertville, PA) diluted 1:15,000 in PBS containing 0.02% (w/v) SDS for 1 hr at RT. Membranes were washed six times for 10 min with PBST and then a further two times with PBS, and immunoreactive bands were visualised using a Li-COR Odyssey infrared imaging system (Li-COR, Lincoln, NE).
Since glycosylation of APP at or near the 1G6, Z31 and Pre-β epitopes is possible (Halim et al., 2011) we were careful to test reactivity of APP antibodies on brain extracts treated +/− removal of O-linked sugar groups. For this AD TBS was IP’d with AW7, antigen was released with ammonium hydroxide, and the eluate lyophilized. Thereafter, the lyophilized sample was reconstituted in 45 µl of deglycosylation mix reaction buffer (Promega, Madison, WI) and incubated at 37°C for 18 hours in the presence or absence of deglycosylation enzyme mix (5 µl enzyme or 5 µl reaction buffer added). The deglycosylation mix is comprised of 5 enzymes: PNGase F, O-Glycosidase, Neuraminidase, β1–4 Galactosidase, β-N-Acetylglucosaminidase which allows for removal of both N and O-linked sugar groups. Thereafter 2× sample buffer was added, the sample boiled and analyzed by SDS-PAGE and WB.
Size exclusion chromatography (SEC)
For most experiments, 1 ml of TBS extract or immunoprecipitated TBS extract was chromatographed on a Superdex 75, 10/300 GL column eluted at 0.5 ml/min in 50 mM ammonium acetate, pH 8.5. One ml or 0.5 ml fractions were collected, lyophilized and used for direct immunoblot. For some experiments, 2 ml of TBS extract was injected on to a Superdex 75 10/300 GL column linked to a Superdex 200 10/300 GL column and eluted at 0.8 ml/min. To concentrate and denature Aβ from TBS extracts, samples were IP’d with AW7 and the concentrate treated with a strong denaturant. Briefly, Aβ-antibody complex was disrupted by vortexing in 150 mM ammonium hydroxide and the supernatant collected and lyophilized. This material was then re-suspended in a strong chaotrope, typically 50 mM Tris-HCl, pH 10.4 containing 6 M guanidium hydrochloride (GuHCl) and 5 mM EDTA. To assist dissociation of the high and intermediate molecular weight assemblies, samples were boiled for 10 min and incubated at RT for ~18 hr, centrifuged for 30 min at 16,000 g to remove insoluble debris and then chromatographed on a Superdex 75 10/300 GL column as described above.
To test if the SEC elution profile of Aβ species obtained using 50 mM ammonium acetate, pH 8.5 was comparable to that when a physiological relevant buffer was used, we chromatographed AD brain extracts on the same Superdex 75 column first in 50 mM ammonium acetate, pH 8.5 and then in artificial cerebrospinal fluid (aCSF), i.e. 1.25 mM sodium dihydrogen phosphate, 26 mM sodium bicarbonate containing 124 NaCl and 2.8 KCl, pH 7.5.
Aβ1-x and Aβx-42 Enzyme-linked immunosorbant assays (ELISA)
Assays utilised the Meso Scale Discovery (MSD) platform and reagents from Meso Scale (Rockville, MD). MULTI-ARRAY® 96 well small-spot black microplates were coated with 3 µg/ml of monoclonal antibody 266 in tris buffered saline (TBS) and incubated at RT for 18 hr. Monoclonal antibody 266 recognises the mid-region of Aβ (Fig.1), thus enabling detection of both N- and C-terminally heterogeneous Aβ species. Binding sites that were unoccupied were blocked in 150 µl of 5% Blocker A (MSD) in TBS containing 0.05% tween 20 (TBST) and agitated at 400 rpm for 1 hr and 22°C. Plates were washed 3 times with TBST and samples and standards were applied in duplicate, and agitated at 400 rpm for 2 hr and RT. After the capture phase, plates were washed 3 times with TBST and incubated with biotinylated antibody. To allow the detection of Aβ1-x or Aβx-42, we used biotinylated 3D6 (1 µg/ml) or 21F12 (1 µg/ml), respectively. Simultaneously, 1 µg/ml of the reporter reagent (SULFO-TAG Labelled Streptavidin) was added in ELISA diluent and incubated at RT with gentle agitation for 2 hr. Finally, plates were washed 3 times with PBST and 2× MSD read buffer (150 µl per well) was applied to allow for electrochemiluminesence detection. A SECTOR imager was used to measure the intensity of emitted light, thus allowing quantitative measurement of analytes present in the samples.
Oligomer-specific ELISA (oELISA)
We recently developed an MSD-based assay that reliably detects Aβ oligomers of a variety of different sizes (Yang et al., 2015). This assay was performed essentially as described above, but employed the novel aggregate-preferring mAb, 1C22, for capture and the Asp1-specific mAb, 3D6, for detection. This oELISA is >37,000-fold more sensitive for Aβ oligomers than Aβ monomer (Yang et al., 2015).
Preparation of dityrosine cross-linked Aβ (DiY) dimer
Aβ1-40 or Aβ1-42 monomer was oxidatively cross-linked using horseradish peroxidase (HRP) (Wiltfang et al., 1991, Moir et al., 2005, O'Malley et al., 2014). Briefly, Aβ monomer was isolated using a Superdex 75 10/300 column eluted in 50 mM ammonium bicarbonate, pH 8.5, diluted to 40 µM and incubated overnight at 37°C in the presence of 2.2 µM HRP and 250 µM H2O2 (O'Malley et al., 2014). The cross-linking reaction was quenched by addition of 0.02% (w/v) NaN3 and dimer was isolated from unreacted monomer and aggregates using a Superdex 75 16/600 column eluted in 50 mM ammonium bicarbonate, pH 8.5 (O'Malley et al., 2014).
Asymmetric flow field-flow fractionation (AF4)
Asymmetric field flow fractionation is a chromatography technique in which the separation of the sample is performed in a channel of several hundred µm height with a semipermeable membrane at the bottom. Because of the laminar flow of the carrier fluid, a parabolic flow profile is created. When a perpendicular (cross) flow is applied to the laminar stream, the analytes accumulate close to the semipermeable membrane. This concentration build-up causes particle diffusion away from the wall. Smaller particles have higher diffusion and thus have a higher mean elevation above the accumulation wall and elute faster than larger particles. The elution profile is thus the inverse order of SEC. AD-TBS samples were IP’d using AW7, and denatured in GuHCl (as above) and then injected onto an Eclipse DualTec® AF4 (Wyatt Technology, Santa Barbara, CA) and eluted with 50 mM ammonium acetate pH 8.5. The channel was 24.6 cm long and fitted with a 350 µm spacer and a 5 kDa MWCO polyethersulfone membrane. Samples were focused for 6 min during injection at 3 ml/min. The cross-flow was held for 10 min at 3 ml/min before linearly decreasing the flow to zero over 10 min. One ml fractions were collected, lyophilized and used for direct immunoblot.
Mass Spectrometry
MALDI TOF/TOF measurements were performed using a Bruker Daltonics UltraFleXtreme instrument (Bruker Daltonics, Bremen, Germany) operated in reflector or linear mode as described elsewhere (Pannee et al., 2014). Nanoflow LC-ESI-LQIT-FTICR measurements were conducted with an Ettan MDLC (GE Healthcare, Uppsala, Sweden), operated with a Zorbax 300 SB-C8 column (Agilent Technologies, Palo Alto, CA, USA), coupled to an LTQ FT Ultra (Thermo Fischer Scientific, Bremen, Germany) hybrid linear quadrupole ion trap–Fourier transform ion cyclotron resonance mass spectrometer as described previously (Brinkmalm et al., 2012). All spectra were acquired in FTICR mode.
Results
We prepared TBS extracts from a total of thirty six donor samples, using cortical tissue from thirty three AD and three control cases (Table 2). Initial analysis employed the pan anti-Aβ antibody, AW7, for IP and a combination of anti-Aβ40 and anti-Aβ42 monoclonal antibodies (mAbs) for WB. To assess the efficiency of the AW7 IP we used 2 different ELISAs; one specific for Aβ beginning at Asp1 (1-x) and another that detects Aβ termination at Ala42 (x-42), to measure Aβ in brain extracts before and after IP. In the eight AD samples, AW7 captured ~90% of all ELISA detectable Aβ (86.8% ± 26.9% of Asp1 Aβ, and 95.6% ± 2.7% of Aβ42). As in our previous work (Shankar et al., 2008, Mc Donald et al., 2010, Borlikova et al., 2013) IP/WB analysis of AD samples revealed two broad bands. By comparison to SeeBlue Plus prestained markers (MWs of 4 kDa, 7 kDa, 16 kDa, 34 kDa, 45 kDa, and 55 kDa), we estimated the apparent molecular weight of each band. Based on analysis of 8 different WBs, each of which contained at least one lane of standards and two lanes of IP’d AD brain TBS extract, we obtained average molecular weights of: (i) 3.5 – 4.5 kDa, and (ii) 6.1 – 7.9 kDa (Fig. 2). We refer to the ~4 kDa band as monomer and the ~7 kDa band as putative dimer or ~7 kDa Aβ. However, we are mindful that SDS-PAGE is not a reliable means to assess native assembly size (Bitan et al., 2005, Hepler et al., 2006) and that using mixtures of mAbs for WB provides no information about the relative abundance of primary structures present in these two bands. Therefore we applied a range of other approaches to investigate the molecular composition and native assembly size of these putative monomers and ~7 kDa Aβ species.
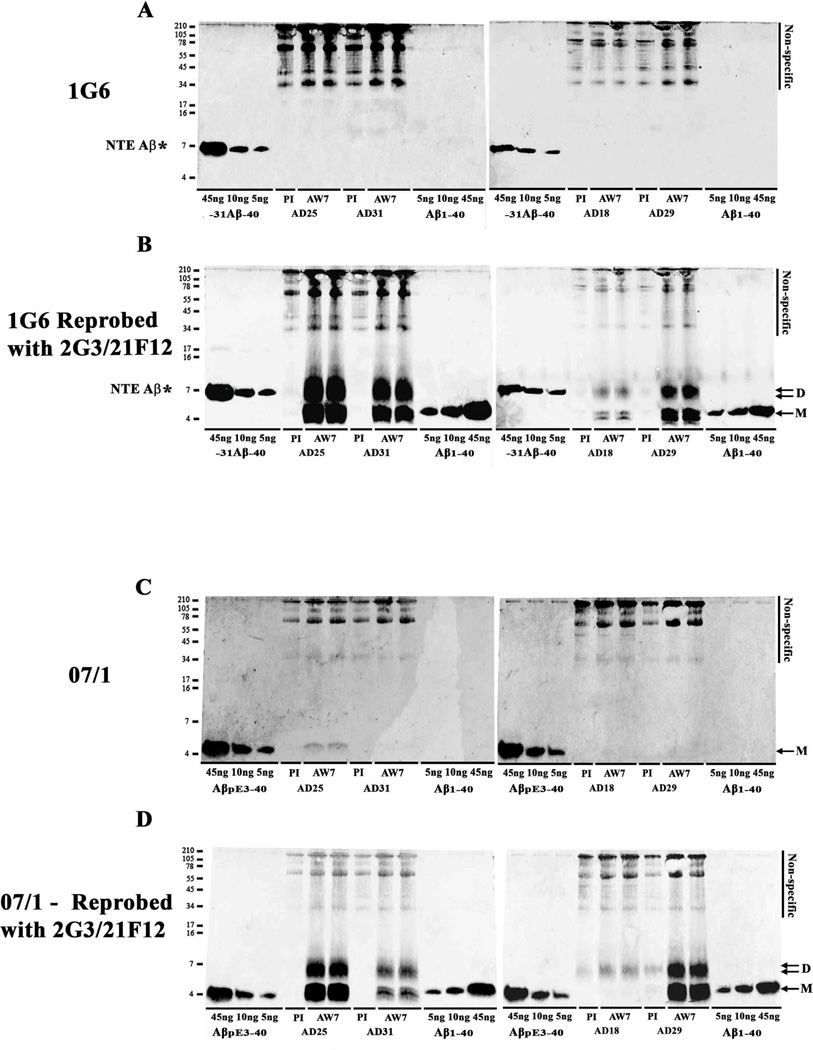
Fig. 2. Immunoprecipitation/Western blotting reveals the presence of ~4 and ~7 kDa Aβ species in the aqueous phase of AD brain.
TBS extracts from four AD brains were IP’d with AW7 or pre-immune serum (PI) and WB’d with a combination of the Aβ40 and Aβ42 specific mAbs, 2G3 and 21F12. AD brain numbers and whether PI or AW7 antiserum was used for IP is indicated below each lane. Molecular weight markers are shown on the left. M (single arrow) denotes monomer and D (double arrow) refers to dimer. Non-specific indicates bands detected when PI was used are indicated by a solid black line. To allow comparison between blots, Aβ1-40 (5, 10 and 45 ng) and Aβ1-42 (5, 10 and 45 ng) were electrophoresed on each gel. The pattern shown is representative of more than 100 AD brains analyzed by this method, the sensitive of which is sufficient to detect Aβ at concentrations as a low as ~10 ng/g wet weight tissue.
IP/WB demonstrates that AD TBS ~7 kDa Aβ contain a higher Aβ42/Aβ40 ratio than Aβ monomer
To gain insight into the primary structures and relative abundance of Aβ species present in AD TBS we used a series of sequence-specific antibodies in a quantitative WB approach. Large batches of samples were IP’d with AW7, and equal volumes of IP eluate electrophoresed on SDS-PAGE alongside synthetic Aβ of known concentration, and WB’d with one of 13 different mAbs (Fig. 3 – 4, Supplementary Fig. 1 – 4). In total, TBS extracts from eight different AD brains and three control brains were analyzed. In order to facilitate comparisons between samples within gels and between samples on different gels we were careful to: (i) include synthetic standards at each end of each gel, and (ii) to ponceau S stain blots to ensure even transfer of protein across blots. No Aβ was detected in two out of three control brains and in the one extract in which Aβ was detected (C1) the amount of Aβ was less than that detected in any of the extracts from AD brains (Supplementary Fig. 2). When probed with mAbs to the unmodified Aβ sequence one or two specific immunoreactive bands were detected in all of the AD samples (Fig. 3 and Supplementary Fig. 1–3). The most commonly encountered species migrated on SDS-PAGE as a broad band centered at ~4 kDa (Fig. 2) and contained at least 3 electrophoretically distinct species (Supplementary Fig. 1C and D). A broad multi-component putative dimer band centered ~7 kDa was also detected in all AD samples probed with 4G8, 266 and 21F12 (Fig. 3, Supplementary Fig. 2 and 3), and a faint diffuse smear between ~9–30 kDa was seen with mid-region and C-terminal mAbs.
Fig. 3. The primary structure composition of the ~4 and ~7 kDa species are different.
TBS extracts from four AD brains were IP’d with AW7 antiserum or pre-immune serum (PI) and WB’d with 3D6 (A), 6E10 (B), 4G8 (C), 2G3 (D) or 21F12 (E). To allow comparison between blots, Aβ1-40 (5, 10 and 45 ng) and Aβ1-42 (5, 10 and 45 ng) were electrophoresed on each gel. The epitope recognized by each mAb is indicated in Figure 1. AD brain numbers and use of PI or AW7 for IP is indicated below each lane. Molecular weight markers are shown on the left; M denotes monomer and D, dimer. Non-specific bands detected when PI was used for IP are indicated by a solid black line on the right of each blot.
Fig. 4. Aβ in AD-TBS extracts contains little pE3 Aβ and no detectible N-terminally extended Aβ species.
TBS extracts from the same four AD brains shown in Figures 2 and 3 were IP’d with AW7 or pre-immune serum (PI) and WB’d with 1G6 (A) or mAb 07/1 (C). Recombinantly produced −31Aβ-40 (5, 10 and 45 ng) included on gels in panel A were readily detected, whereas no 1G6 immunoreactive species were detected in AD-TBS samples. Importantly, when the same blots were reprobed with a combination of 2G3 and 21F12 (B), abundant ~4 and ~7 kDa species were detected. AβpE3-40 (5, 10 and 45 ng) standards were readily detected with 07/1. A mAb 07/1-immunoreactive band that co-migrated with synthetic AβpE3-40 was detected in one of the 4 AD brains examined. When the same blots were reprobed with a combination of 2G3 and 21F12 (D), abundant ~4 and ~7 kDa species were detected. AD brain numbers and the use of PI or AW7 for IP is indicated below each lane. Molecular weight markers are shown on the left. M denotes monomer and D, dimer. * indicates N-terminally extended (NTE) −31Aβ-40 standard.
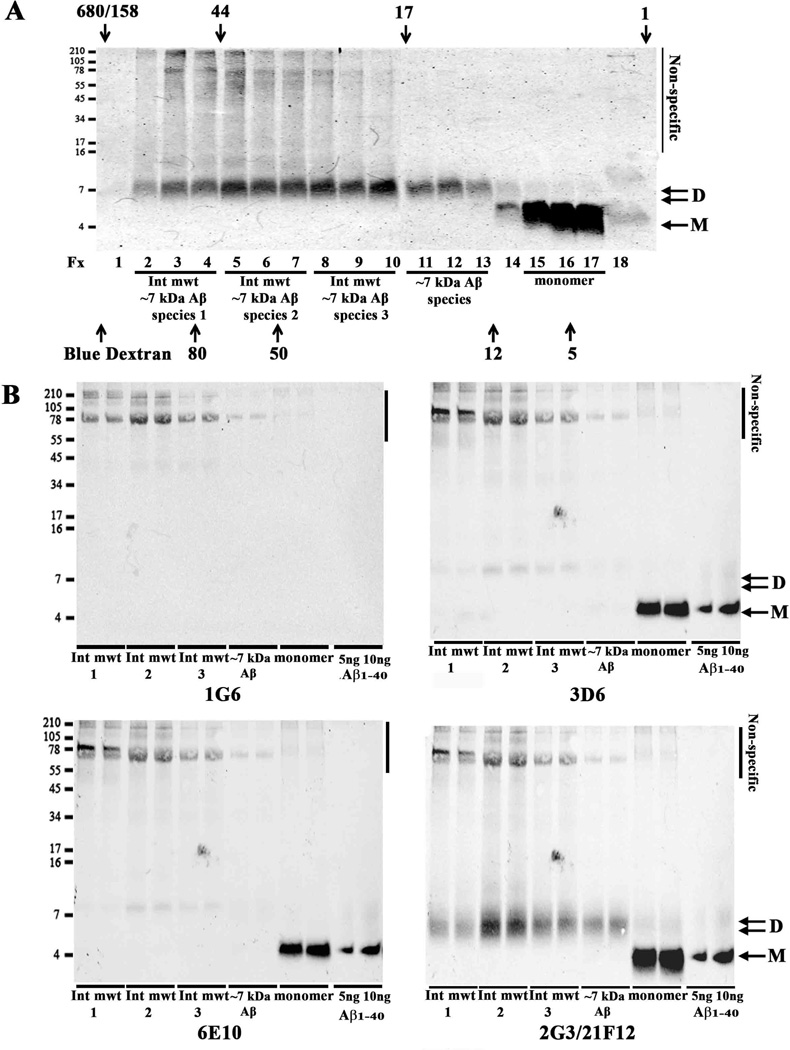
The amount of monomer varied from case to case and depended on the mAb used for detection. The ~4 kDa band frequently resolved to yield three distinct bands (Supplementary Fig. 1C and D, Table 3), and within a sample, N-terminal, mid-region and 40/42-specific mAbs detected varying levels of monomer (Fig. 3 and Supplementary Fig. 1–3). 3D6 detected a single band that co-migrated with Aβ1-40 and Aβ1-42 standards (Fig. 3A, Supplementary Figs. 1D and 2A, and Table 3). 6E10 and 4G8 detected the same band as 3D6 plus a lower migrating band (Fig. 3B and C, Supplementary Figs. 1D, 2B and 2C, and Table 3), and C-terminal specific mAbs detected the same bands as 6E10 and 4G8 plus a third faster migrating band (Fig. 3D and E, Supplementary Fig. 1, Supplementary Fig. 2D and E, Table 3). These results indicate that the upper monomer band contains Aβ species that begin at Asp1, the middle band is composed of N-terminally truncated species, and the bottom band is likely p3 (17–40/2). The amount of Aβ detected by the Asp1-specific mAb, 3D6, varied widely between samples with Asp1 monomer present in 7/8 AD brains (Fig. 3A, Supplementary Fig. 2A and Table 3). 2G3-reactive Aβ40 monomer was present in 7/8 samples, but tended to be at lower levels than monomer detected by 6E10, 4G8 or 21F12. When comparing the amount of Aβ detected in each band by different mAbs it is clear that the monomer and dimer bands have distinct compositions (Table 3). Specifically, Aβ40 was detected in monomer from most (7/8) AD brains and was a dominant C-terminal species in 5 of these (Fig. 3, Supplementary Fig. 1 and 2), and although Aβ40 was present in dimer bands in 7 of 8 cases it was never a major dimer species (Fig. 3, Supplementary Fig. 1 and 2, and Table 3). In contrast, Aβ42 was present in all monomer and dimer bands, with the amount of Aβ42 in the dimer exceeding that in the monomer in 7/8 cases (Fig. 3E, and Supplementary Fig. 1B and Supplementary Fig. 2E, and Table 3). Thus in all 8 cases the amount of Aβ42 relative to Aβ40 was always higher in ~7 kDa Aβ species than monomers. Reactivity with the mid-region mAb, 4G8, and lack of detection with (i) an mAb, 46-4, that does not detect Aβ; and (ii) AD brain extract IP’d with pre-immune serum, confirm that both the ~4 kDa and ~7 kDa are authentic Aβ species (Fig. 3C, Supplementary Fig. 2C, Supplementary Fig. 3 and Table 3). It is also noteworthy that a significant portion of Aβ monomer in all 8 samples was reactive with the N-terminally directed mAb, 6E10 (Fig. 3B and Supplementary Fig. 2B, and Table 3), and that at least some of the monomer in 7 of 8 cases was also detected with the Asp1-specific mAb, 3D6 (Fig. 3A and Supplementary Fig. 2A, and Table 3). As was the case with their C-termini, the composition of Aβ monomers differed from those of ~7 kDa Aβ species with regard to N-termini. For instance, a significant portion of monomer in all cases was faintly detected with 6E10, whereas just 3 of the same samples had dimer that was detected by 6E10, and in no case was the amount of 6E10-reactive-dimer higher than the amount of 6E10-reactive-monomer (Fig. 3B and Supplementary Fig. 2B, and Table 3). It is interesting to note that 6E10 does not react with Aβ phosphorylated at Ser8 (Kumar et al., 2011) and is weakly reactive to Aβ cross-linked at Tyr10 (T. O’Malley & D. Walsh, unpublished), raising the possibility that at least some of the ~7 kDa dimer could be post-translationally modified.
Table 3.
Qualitative scoring of Aβ band intensities visualized by Western blotting.
| 3D6 | 6E10 | 4G8 | 2G3 | 21F12 | ||
|---|---|---|---|---|---|---|
| AD25 | Mon1 | ++ | + | ++ | + | ++ |
| Mon2 | − | ++ | ++ | + | ++ | |
| Mon3 | − | − | − | − | ++ | |
| Dimer | + | + | +++ | + | +++ | |
| AD31 | Mon1 | + | + | + | − | ++ |
| Mon2 | − | + | + | − | ++ | |
| Mon3 | − | − | − | − | + | |
| Dimer | − | − | ++ | − | +++ | |
| AD18 | Mon1 | + | + | + | + | + |
| Mon2 | − | ++ | + | + | + | |
| Mon3 | − | − | − | − | + | |
| Dimer | − | − | ++ | + | ++ | |
| AD29 | Mon1 | ++ | ++ | ++ | + | ++ |
| Mon2 | − | ++ | ++ | ++ | ++ | |
| Mon3 | − | − | − | − | ++ | |
| Dimer | + | + | +++ | + | +++ | |
| AD30 | Mon1 | + | + | ++ | ++ | ++ |
| Mon2 | − | +++ | ++ | ++ | ++ | |
| Mon3 | − | − | − | − | + | |
| Dimer | − | + | +++ | ++ | ++ | |
| AD33 | Mon1 | ++ | ++ | ++ | ++ | ++ |
| Mon2 | − | ++ | ++ | ++ | ++ | |
| Mon3 | − | − | − | − | ++ | |
| Dimer | − | + | +++ | ++ | +++ | |
| AD32 | Mon1 | + | + | +++ | + | ++ |
| Mon2 | − | ++ | +++ | ++ | ++ | |
| Mon3 | − | − | − | − | + | |
| Dimer | − | + | +++ | ++ | +++ | |
| AD19 | Mon1 | + | + | + | + | + |
| Mon2 | − | ++ | + | + | ++ | |
| Mon3 | − | − | − | − | + | |
| Dimer | − | − | ++ | ++ | ++ |
For an example of all three monomer bands see Supplementary Fig. 1. Estimates of abundance were made by visual inspection of WBs shown in Fig. 3 and Supplementary Fig. 2 and are the average of determinations from 3 independent observers.
Not detected = −
Detected, < 5 ng = +
Detected, < 10 ng = ++
Detected between 10 ng and 45 ng = +++
In a small number of cases certain monomer and dimer bands were detected by all 5 mAbs and although the amount of each epitope varied considerably this may indicate that at least some of the monomers and ~7 kDa Aβ species contained full length Aβ1-40 and/or Aβ1-42 (Fig. 3 and Supplementary Figs. 1 and 2, and Table 3). However, only a fraction of all monomers and ~7 kDa Aβ species contained N-termini detected by 3D6, thus indicating that most AD TBS Aβ: (i) extends N-terminal from Asp1 (Kaneko et al., 2014, Welzel et al., 2014), (ii) begins after Asp1, or (iii) that the N-terminus is otherwise modified. To address some of these possibilities we used antibodies specific for the presence of pyroGlu3 (pE3) and for APP sequences N-terminal of Asp1 (Fig. 4). We employed 3 antibodies each of which recognize different epitopes within the 30 residues stretch of the APP sequence immediately N-terminal to the Aβ domain. Since glycosylation of APP at or near these sites could influence antibody recognition we tested reactivity plus and minus treatment to remove N- and O-linked sugars under conditions that readily degycosylated full length APP (not shown). No N-terminally extended Aβ (NTE) species were evident in any of the AD TBS extracts examined, whereas the recombinantly produced NTE (−31Aβ40) was readily detected by the anti-APP antibodies 1G6 (Fig. 4A and B), Z31 and pre-β (Supplementary Fig. 4A and B). Synthetic pE3 was readily and specifically detected, but pE3 was detected in only one of four brain extracts, and even in that case only a very small amount of pE3 Aβ was detected (Fig. 4A and C). Importantly, re-probing of blots with 2G3 and 21F12 demonstrated that there was abundant monomer and dimer in samples which did not react with antibodies to NTE or pE3 (Fig. 4 and Supplementary Fig. 4).
Taken together, our quantitative immunoprofiling study indicates that AD TBS contains a heterogeneous mixture of Aβ sequences and that the relative abundance of these species varies between individual AD cases. Moreover, it is clear that within cases the composition of primary structures of Aβ differ significantly for Aβ monomers versus ~7 kDa Aβ species. In particular, two disease linked changes (Nilsberth et al., 2001, Tomiyama et al., 2008) are increased in ~7 kDa Aβ species relative to monomers. The ~7 kDa Aβ species contains higher Aβ42/Aβ40 ratios, and more N-terminally modified Aβ.
Non-denaturing size exclusion chromatography (SEC) of AD TBS reveals the presence of Aβ in at least 4 different molecular weight fractions, the most prevalent of which ranged from ~30–150 kDa
SDS-PAGE is highly denaturing and can provide little information about native assemblies, and IP could potentially induce formation of the ~7 kDa species, and/or higher aggregates, thus we used non-denaturing SEC to estimate the size of Aβ assemblies. Aqueous extracts of AD brain were chromatographed in a volatile buffer, fractions collected and concentrated to dryness. Thereafter fractions were electrophoresed on SDS-PAGE and used for WB. Thirty samples were analyzed using SEC (for an example see Fig. 5A), and Aβ was detected in at least one fraction from each sample (Table 4). In all but two cases Aβ immunoreactive species were detected in fxs 1, 2 or 3. As fxs 1–3 occur just after the void volume and before elution of ovalbumin (44 kDa) we refer to Aβ that eluted in these fractions as intermediate molecular weight (Int mwt) Aβ (Fig. 5A). Roughly one third of AD TBS extracts also contained Aβ which eluted in fractions with predicted sizes consistent for putative dimers (7/30) and monomers (8/28), and we refer to these as ~7 kDa Aβ and “true” monomers (Table 4). A portion (9/30) of extracts contained Aβ that eluted in the void volume, and we refer to this material as high molecular weight (High mwt) Aβ (Table 4).
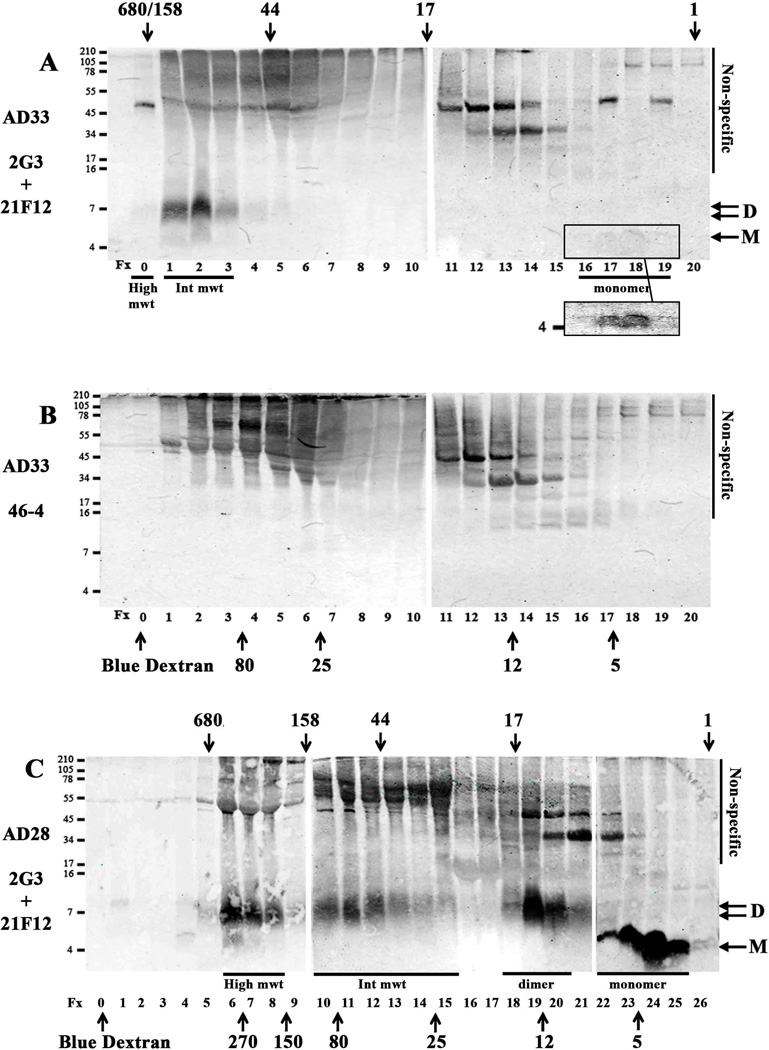
Fig. 5. Aqueous extracts of human brain contain up to 4 different sized Aβ assemblies separable by SEC.
TBS extract (1 ml) from one AD brain (AD33) was chromatographed on a Superdex 75 10/30 HR column (A and B). Half ml fractions were collected, a 0.25 ml aliquot was lyophilized and analyzed by WB using a combination of 2G3 and 21F12 (A) or a monoclonal antibody, 46-4, which does not recognize Aβ (B). Bands specifically reactive with anti-Aβ mAbs were detected in fxs 1 and 2, (High mwt), 3, and 4 (Int mwt) 17 and 18 (monomer). A darker exposure (A inset) is shown to highlight bands in fxs 17 and 18. Two ml of AD-TBS (AD28) was chromatographed on a Superdex 75 10/30 HR column linked in tandem with a Superdex 200 10/30 HR column (C). Fractions (1 ml) were lyophilized and analyzed by WB using 2G3/21F12. Because the fractions from a single chromatographic run couldn’t be accommodated on a single gel, samples were electrophoresed on 2 (A and B) or 3 (C) gels alongside synthetic Aβ1-42 standards (not shown) which were used to normalise the exposure of each blot. Species that migrated on SDS-PAGE as monomer and dimer are indicated by arrows and molecular weight markers shown on the left, and fraction numbers are provided below each lane. Four peaks of Aβ immunoreactivity were detected in the SEC fractions, and based on their elution relative to SEC standards are designated as (i) high molecular weight (high mwt), (ii) intermediate molecular weight (Int mwt), (iii) ~7 kDa Aβ species and (iv) true monomer (monomer) - these species are highlighted with an underline. The elution of linear dextran standards is indicated by up-pointing arrows and labelled 5, 12, 25, 80, 150, 270 kDa, while the elution of globular standards, is indicated by down-pointing arrows labelled 1, 17, 44, 158 and 670 kDa. Fraction 0 indicates the peak fraction in which Blue dextran eluted. Non-specific indicates bands detected when 46-4 was used.
Table 4.
SEC analysis of AD brains.
| Case # | High mwt | Intermediate mwt | ~7 kDa Aβ species | True Monomer | Column |
|---|---|---|---|---|---|
| AD1 | − | + M +D | + | − | S75 |
| AD2 | + M +D | − | − | + | S75 |
| AD3 | + M +D | + M +D | + | + | S75 |
| AD4 | − | + D | + | + | S75 |
| AD5 | + D | + D | + | − | S75 |
| AD6 | + D | + D | − | + | S75 |
| AD7 | − | + M +D | + | + | S75 |
| AD8 | − | + M +D | − | − | S75 |
| AD9 | + M +D | + M +D | − | − | S75 |
| AD10 | − | + M +D | − | − | S75 |
| AD11 | − | + M +D | − | − | S75 |
| AD12 | − | + M +D | − | − | S75 |
| AD13 | − | + M +D | − | − | S75 |
| AD14 | − | + M +D | − | − | S75 |
| AD15 | − | + M +D | − | − | S75 |
| AD16 | − | + M +D | − | − | S75 |
| AD17 | − | + M +D | − | − | S75 |
| AD18 | + M +D | + D | + | + | S75 |
| AD19 | − | + M +D | − | − | S75 |
| AD20 | − | + D | + | + | S75 |
| AD21 | + M +D | + M +D | − | − | S75 |
| AD22 | − | + M +D | − | − | S75 |
| AD23 | − | + M +D | − | − | S75 |
| AD24 | − | + M +D | − | − | S75 |
| AD25 | + M +D | − | − | − | S75 |
| AD26 | + D | + M +D | + | − | S75/S200 |
| AD27 | + M +D | + D | + | + | S75/S200 |
| AD28 | + D | + D | + | + | S75/S200 |
| AD29 | − | + M +D | − | − | S75 |
| AD30 | +M | +M +D | − | −* | S75 |
| AD31 | − | + M +D | − | − | S75 |
| AD32 | − | + M +D | − | − | S75 |
| AD33 | − | + M +D | − | + * | S75 |
The presence or absence of a species is indicated by + or −, respectively. M and D refers to the migration of Aβ on SDS-PAGE, were M=monomer, D=dimer. +* = a very weak signal by WB, but readily detected by ELISA, and −* not detectable by WB, but detectable by ELISA.
Notably, the fractions that contained true monomer migrated on SDS-PAGE exclusively at ~4 kDa, and the fractions that contained putative dimer migrated on SDS-PAGE at ~7 kDa. SEC-isolated High mwt or Int mwt species appeared as low molecular weight species on SDS-PAGE (Fig. 5A and C, Table 4) and no other specific bands were detected (Supplementary Fig. 5). Taken together these results demonstrate that aqueous extracts of AD brain contain at least 4 different sized fractions: true monomer and ~7 kDa Aβ species which migrate appropriately on SDS-PAGE; Int mwt and High mwt Aβ which are SDS labile and break down to Aβ monomers and ~7 kDa Aβ species.
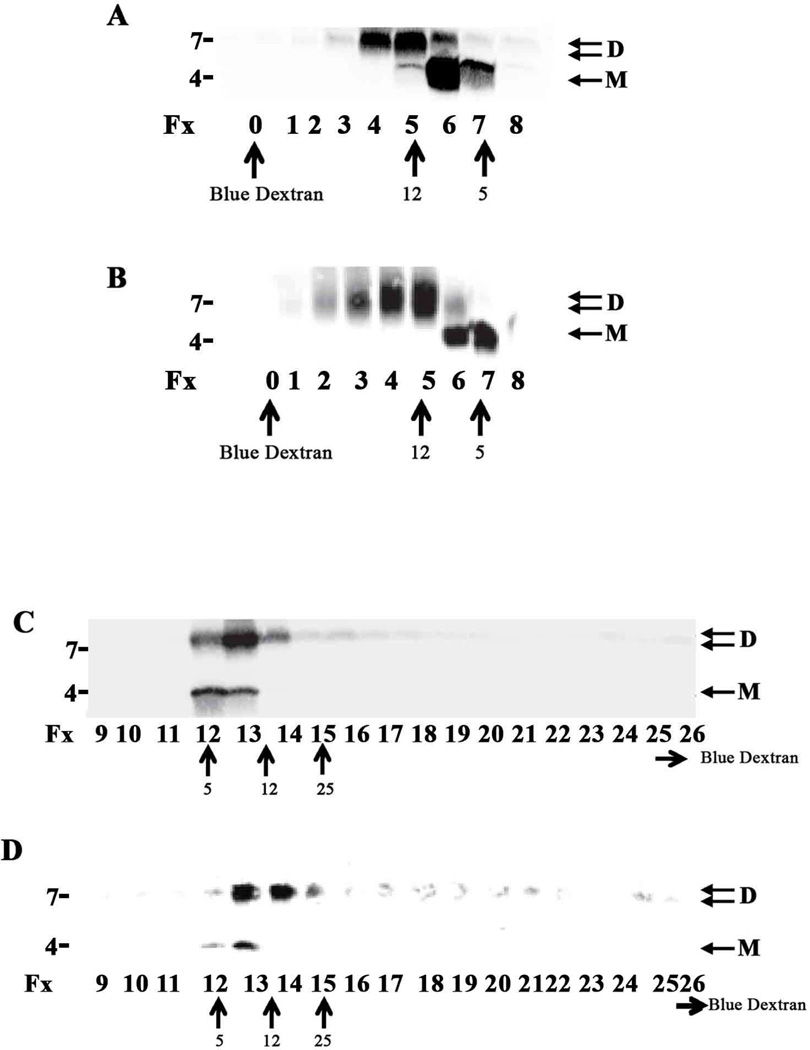
In a pilot experiment extract from AD30 was chromatographed on a Superdex 75 column eluted in aCSF or in ammonium acetate, pH 8.5. Since aCSF is not volatile and fractions eluted in this buffer are not amenable to lyophilization and subsequent WB we detected Aβ using ELISAs. We employed the Aβx-42 ELISA, which preferentially recognizes Aβ monomers, and an ELISA specific for oligomers that contain at least one 3D6-reactive component (Yang et al., 2015). The oELISA signal was strongest in fxs 1–5 (Fig. 6A and B), whereas the Aβx-42 ELISA detected a weak signal in fxs 1–4 and a strong signal in fxs 14–18 (Fig. 6C and D). The pattern was the same in both elution buffers. Two prominent Aβ species were detected: the major component eluted as Int mwt Aβ and the minor component as Aβ monomer. Moreover, when ammonium acetate fractions were lyophilized and used for WB the bulk of the Aβ was found in fxs 1–3 as bands that migrated on SDS-PAGE at ~4 and ~7 kDa (Fig. 7A).
Fig.6. High and Intermediate mwt oligomers are detected by ELISA in aqueous extracts of AD brain fractionated on SEC in physiological buffer.
TBS extract (1 ml) from AD brain 30 was chromatographed on a Superdex 75 10/30 HR column eluted in 50 mM ammonium acetate (A and C) or artificial CSF (aCSF) (B and D). Half ml fractions were collected and analyzed using the oligomer-specific ELISA (oELISA) (A and B) or the Aβx-42 ELISA (C and D). High/intermediate mwt oligomers were detected in fxs 1–4, whereas the majority of Aβx-42 was found in fxs 14–18. Oligomer concentrations were calculated using an ADDL standard with units of equivalent weight of Aβ1-42 monomer (see Yang et al. 2015) and Aβx-42 concentration were calculated based on the molar content of SEC-isolated Aβ1-42 monomer. Fraction “0” was not examined by ELISA, and ND indicates that the concentration was not determined.
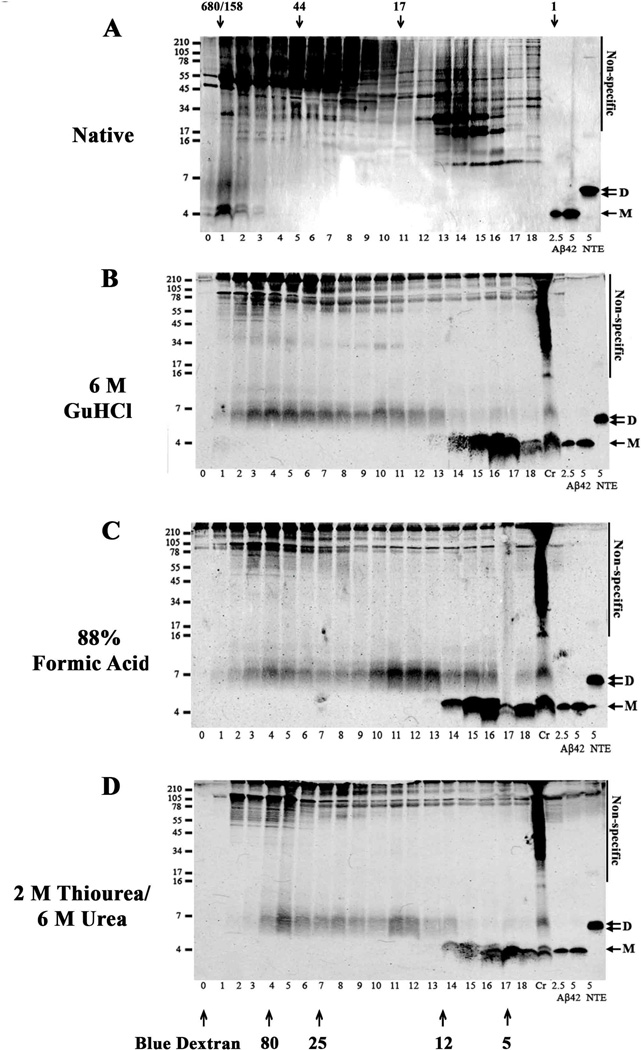
Fig. 7. AD-TBS extract contains high molecular weight Aβ complexes which chaotropes break down to yield mostly SDS-stable dimer.
An AD-TBS extract (AD31) was chromatographed on a Superdex 75 10/30 HR column (A). One ml fractions were collected, lyophilized and analyzed by WB using 2G3 and 21F12. The same AD-TBS (1 ml × 3) was IP’d with AW7, and the antigen-antibody complex eluted from the protein A sepharose beads by vortexing in 150 mM AmOH. The AmOH eluate was lyophilized, and then incubated in 6 M GuHCl (B), 88 % formic acid (C) or 2 M thiourea/6M urea (D) and chromatographed on a Superdex 75 10/30 HR column. One ml fractions were lyophilized and analyzed by WB using 2G3 and 21F12. Molecular weight markers are shown on the left and fraction numbers are indicated below each lane. Exposures of the blots were adjusted so that the intensity of the synthetic Aβ1-42 standards was comparable. Non-specific bands are indicated. Fraction 0 refers to the elution of Blue dextran. Bands that migrate with molecular weights consistent for Aβ monomer (M) and dimer (D) are indicated with arrows. The patterns shown are representative of that seen with aqueous extracts from 4 other AD brains.
In an effort to improve resolution of larger molecular weight assemblies we used a Superdex 75 column linked in series to a Superdex 200 column (Supplementary Fig. 6), and analyzed three AD extracts, each which we knew to contain at least 3 different sized Aβ species. We used linear dextran standards to predict the molecular weight of small Aβ species, since it is known that Aβ monomer has an extended conformation, the size of which is best estimated by reference to structurally extended linear dextrans (Paivio et al., 2004). This approach confirmed that true monomers and putative ~7 kDa dimers eluted as expected with peak fractions having masses of ~4 – 5 kDa and ~8 – 12 kDa (estimated by reference to linear dextrans), respectively (Fig. 5C). The High mwt species which eluted in the void of a single superdex 75 column was now detected in the included volume of the combined columns and eluted in a range consistent with globular proteins of ~160–700 kDa and had a molecular weight of 150–300 kDa in comparison to linear dextrans (Fig. 5C). In accord with our initial SEC analysis (Table 4, Fig. 5A), the Int mwt species in the three samples analyzed on the tandem system spanned a mass range of ~25–150 kDa based on linear dextrans and 30–150 kDa based on globular proteins (Fig. 5C, Table 4).
SDS-stable ~7 kDa Aβ species are an important component of intermediate molecular weight Aβ assemblies
Our SEC studies indicate that Int mwt Aβ is by far the most commonly encountered Aβ species in aqueous extracts of AD brain (Table 4). On denaturing SDS-PAGE this material breaks down to yield species that migrate with molecular weights expected for Aβ monomers and ~7 kDa Aβ species (Fig. 5 and Table 4). However, it is not clear if the SDS-PAGE ~7 kDa band represents a true Aβ dimer, or an aberrantly migrating Aβ monomer. Indeed, several studies have found that a fraction of synthetic Aβ migrates anomalously on SDS-PAGE (Bitan et al., 2005, Hepler et al., 2006, Watt et al., 2013). Bearing in mind that our quantitative WB experiments revealed that ~7 kDa Aβ species contained more Aβ42 than did monomers (Fig. 3 and Supplementary Fig. 1 and 2) it is conceivable that the ~7 kDa band was not actually a dimer, but an aberrantly migrating N-terminally truncated Aβ42 monomer. Since we know that natively isolated High mwt/ Int mwt Aβ breaks down on SDS-PAGE to yield ~7 kDa species, we reasoned that if we first denatured High mwt/Int mwt Aβ we could then use native SEC to estimate the size of the Aβ species produced by breaking up native High mwt/Int mwt Aβ assemblies. Thus we undertook a series of experiments that combined the use of strong chaotropes and SEC. GuHCl, formic acid and urea were used to dissociate High mwt/Int mwt Aβ and then non-denaturing SEC was employed to size the breakdown products (Fig. 7). When the TBS extract of AD brain 30 was chromatographed on a Superdex 75 column only High/Int mwt Aβ was detected (Fig. 7A). These species migrated on SDS-PAGE at ~4 kDa and ~7 kDa, (fxs 1–2). Whenever the same extract was IP’d with AW7, treated with strong chaotropes and then chromatographed, little or no High mwt Aβ was detected and there was a substantial increase in the amount of ~4 and 7 kDa Aβ species (Compare Fig. 7A with Fig. 7B–D). Importantly, similar results were obtained with 5 different brains (not shown) and when SEC fractions of AD brain 30 were analyzed by ELISA (Supplementary Fig. 7). In AD brain 30 extract not treated with denaturant Aβ oligomers were readily detected in fxs 1–4, whereas prior incubation of the brain extract with GuHCl abolished the High mwt signal and produced a greatly enhanced monomer signal (fxs 14–18), and a new signal appeared in spanning fxs 2–13 (Supplementary Figure 7C and G). These ELISA results nicely complement our WB experiments (Fig. 7A–D) from which it is apparent that the major species in AD brain are relatively High mwt oligomers, but that upon treatment with denaturants these break down to yield: (1) low mwt species which behave on SDS-PAGE and SEC as Aβ monomer and SDS-stable dimers; and (2) Int mwt species which migrate on SDS-PAGE as putative dimers.
Moreover, ~7 kDa Aβ species and true monomer from both native and chaotrope-treated AD brain extracts eluted in the same fractions as synthetic dimer (fxs 3–5), and monomer (fxs 6 and 7) (Fig. 8A and B). Thus, given that the major chaotrope-induced breakdown products of Int mwt Aβ co-eluted from SEC with synthetic cross-linked dimer (Fig. 8) it is reasonable to assume that these brain-derived species are authentic Aβ dimers.
Fig. 8. AD-TBS Aβ treated with GuHCl elutes from SEC and AF4 predominantly as monomer and dimer.
A mixture of Aβ1-42 monomer and DiYAβ1-40 dimer was resuspended in 6 M GuHCl, boiled for 10 min and chromatographed on a Superdex 75 10/30 HR column. One ml fractions were collected, lyophilized and analyzed by WB using 2G3 and 21F12 (A). In a similar manner, AD-TBS (AD 21) was IP’d with AW7 and the antigen-antibody complex eluted from Protein A sepharose beads by boiling in 6M GuHCl and chromatographed (B). One ml fractions were collected and analyzed by WB using 2G3 and 21F12 (B). The same samples as used in (A) and (B) were also analyzed using AF4. Samples were injected onto a 24.6 cm long channel and 1 ml fractions were collected, lyophilized and analyzed by WB. Results for the synthetic Aβ mixture and the AD TBS extract are shown in (C) and (D), respectively. SDS-PAGE molecular weight markers are shown on the left, while elution of linear dextrans from SEC or AF4 are indicated by upward facing arrows. The elution of blue dextran is shown by an upward facing blue arrow for SEC (fx 0), but for AF4 blue dextran eluted outside the range shown (fx 29), a horizontal arrow denotes the later elution of blue dextran. No specific immunoreactive bands were detected above 10 kDa so blots were cropped accordingly. M refers to monomer and D to dimer.
In an orthogonal approach, we then used quantitative WB’ing to compare the primary structure of monomer and dimer isolated by SEC of denatured AD TBS. Five ml of AD TBS (AD33) was immunoprecipitated using AW7, eluted in 150 mM ammonium hydroxide (AmOH), lyophilized and dissolved in 6M GuHCl. The resulting sample was chromatographed, 0.5 ml fractions collected (to maximize species resolution on a single Superdex 75 column) and a portion of each fraction (100 µl) lyophilized and analyzed by WB. 2G3/21F12-reactive material that migrated on SDS-PAGE at ~7 kDa was detected in fxs 2–13, and ~4 kDa monomer was detected in fxs 14–17 (Fig. 9A). The presence of SDS-stable dimers across such a wide molecular weight range indicates incomplete disassembly of the High/ Int mwt species present in this particular brain extract (compare SEC of native AD33 extract in Fig. 5A vs. denatured AD33 extract, Fig. 9A). Nonetheless this profile allowed us to compare the primary structure of Aβ species present in different sized assemblies built from SDS-stable dimers. To this end we pooled fractions from 5 distinct molecular weight ranges (intermediate mwt dimer 1, fxs 2–4; intermediate mwt dimer 2, fxs 5–7; intermediate mwt dimer 3, fxs 8–10; dimer, fxs 11–13; monomer, fxs 15–17) (Fig. 9A). The N-terminal anti-Aβ mAbs 3D6 and 6E10 readily detected Aβ monomer, but none of the SDS-stable dimers (Fig. 9B). In contrast, a combination of 2G3 and 21F12 readily detected both Aβ monomer and the 4 different pools of dimer (Fig. 9B). The results obtained with SEC-isolated monomers and dimers from denatured AD brain extract (Fig. 9B) are entirely consistent with our IP/WB results (Fig. 3 and Supplementary Fig. 2). These results also jibe with the finding that High mwt and Int mwt aggregates built of Aβ monomers and ~7 kDa Aβ are detected by the 3D6-based oELISA, whereas oligomers composed solely of ~7 kDa Aβ (which we know to be N-terminally truncated) are not detected by the oELISA (Supplementary Fig. 7). Specifically, when a TBS extract from AD brain 33 was IP’d with AW7; monomer, but not dimer was detected when 3D6 and 6E10 were used for western blotting, whereas both monomer and dimer were detected with 4G8, 2G3 and 21F12 (Supplementary Fig. 2).
Fig. 9. SEC-isolated Aβ monomer and dimers have distinct primary structure compositions.
Five ml of AD TBS extract (AD43) was IP’d with AW7 (10 × 0.5 ml), and the antigen-antibody complex eluted from the protein A sepharose beads by vortexing in 150 mM ammonium hydroxide (AmOH). The AmOH eluate was lyophilized, and the lyophilizate incubated in 6 M GuHCl and then chromatographed on a Superdex 75 10/30 HR column. Half ml fractions were collected and 100 µl of each fraction was lyophilized and analyzed by WB using 2G3 and 21F12 (A). The remaining fractions were pooled in groups of 3 as indicated on the figure. These pools were lyophilized, dissolved in sample buffer and a sample of each pool (in duplicate) used for western blotting with 1G6 to detect N-terminally extended peptides and the same blot then serially reprobed with anti-Aβ mAbs in the following order: 3D6, 6E10, and a combination of 2G3 and 21F12 (B). Molecular weight markers are shown on the left and the SEC origins of the samples are indicated below each lane. Exposures of the blots were adjusted so that the intensity of the synthetic Aβ1-40 standards are comparable. Non-specific indicates bands detected when 46-4 was used (not shown). Bands that migrate with molecular weights consistent for Aβ monomer (M) and dimer (D) are indicated with arrows.
Since interpretation of molecular weight by SEC can be confounded by non-ideal interactions with it’s stationary phase (Irvine, 1997, Benilova et al., 2012) we employed a second native separation method, asymmetric flow field-flow fractionation (AF4). AF4 is a flow-based method that does not require a stationary phase (Wahlund and Giddings, 1987) and therefore avoids some of the issues that may complicate interpretation of SEC. In AF4, separation takes place in a channel where sample retention is caused by the action of a cross-flow that is generated by a second independent stream that runs across the channel at right angles to the primary channel flow (Wahlund and Giddings, 1987, Wahlund and Litzen, 1989). The utility of AF4 was tested using the same synthetic Aβ monomer and dimer mixture as described above (Fig. 8A). Partial separation of monomer and dimer was achieved using AF4, with, the lower molecular weight monomer clearly eluting ahead of the heavier cross-linked dimer (Fig. 8C)*. Importantly, when GuHCl-treated AD TBS was analyzed by AF4, the brain-derived species fractionated in a manner similar to that seen with synthetic Aβ. Most monomer eluted in fx12 and fx13; and the majority of dimer eluted in fxs 13–14 (Compare Fig. 8C vs. Fig. 8D). These AF4 experiments agree with our SEC studies and provide additional evidence that authentic Aβ dimers are important constituents of Int mwt and High mwt Aβ.
In sum our results indicate that both authentic Aβ monomers and 7 kDa Aβ are present in high molecular weight assemblies and that the primary structure composition of 7kDa Aβ is distinct from that of monomers, with the former containing more N-terminal modifications and relevantly higher levels of Aβ42.
Discussion
We previously reported that water-soluble extracts of AD brain potently disrupt hippocampal long term potentiation (LTP), alter synaptic form and number, and impair memory consolidation (Shankar et al., 2008, Barry et al., 2011, Freir et al., 2011, Borlikova et al., 2013). In all cases these effects were shown to be dependent on Aβ and were reversed when Aβ was specifically removed (Shankar et al., 2008, Barry et al., 2011, Freir et al., 2011, Borlikova et al., 2013). The Aβ present in these samples migrated on SDS-PAGE with molecular weights consistent for monomers and dimers – a pattern we have seen in aqueous extracts of 33 AD brains used in this study and in more than 100 AD brains analyzed in our lab (Shankar et al., 2008, Mc Donald et al., 2010, Mc Donald, 2012) (J. Mc Donald, T. O’Malley, W. Liu, A. Mably and D. Walsh, unpublished). However, since the gels used to resolve these species are highly denaturing, the size estimates they provide do not necessarily reflect native Aβ assembly size. Rather, they simply indicate the presence of at least two different species.
Because fibrils are removed by centrifugation the Aβ species detected on SDS-PAGE are unlikely to be SDS-induced breakdown products of fibrils. Thus, the ~4 kDa species could be true monomers, and/or monomers derived from pre-fibrillar assemblies that are unstable when electrophoresed in SDS. Interpretation of the ~7 kDa band is more complicated. The most straightforward explanation for these species relies on SDS-PAGE providing an accurate estimate of molecular weight. In this case, the ~7 kDa material could be attributed to four different entities: (i) native dimers of Aβ formed from two Aβ monomers tightly bound together or covalently cross-linked, (ii) Aβ dimers that are generated from the denaturation of pre-fibrillar assemblies (O'Nuallain et al., 2010), (iii) small ~7 kDa fragments of APP that contain all or part of the Aβ sequence (Portelius et al., 2013, Kaneko et al., 2014, Welzel et al., 2014), or (iv) Aβ monomer bound to another biomolecule such that it alters it’s migration on SDS-PAGE, SEC and AF4. If the molecular weight predicted by denaturing gel electrophoresis is unreliable yet other explanations would be possible. For instance, certain N-terminal fragments of Aβ (e.g. Aβ1-20) are known to migrate aberrantly on SDS-PAGE and produce bands with apparent molecular weights of ~6–7 kDa (Chesneau et al., 2000). Similarly, it is well-recognized that monomeric Aβ1-42 can give rise to small amounts of anomalously migrating species (Hepler et al., 2006) which have been erroneously designated as “trimers” and “tetramers” (Chromy et al., 2003).
In this study we set out to shed light on the native assembly size of Aβ found in aqueous extracts of AD brain and to clarify the nature and involvement of the material that migrates on SDS-PAGE at ~7 kDa. Previously, we used native SEC to analyze Aβ in an aqueous extract from a single AD brain and we found that Aβ eluted in fractions consistent with monomer, dimer and high molecular weight species of undefined size (Shankar et al., 2008). Detection of a ~7 kDa species by SEC provided the first support that SDS-PAGE accurately reflected the molecular weight of at least some of the ~7 kDa species. Moreover, when each SEC fraction was tested for its ability to block LTP, only the dimer fraction had significant activity. Together these results demonstrate that the SEC-isolated ~7 kDa species is physically and functionally distinct from the ~4 kDa monomer. In the current study we used SEC to analyze water-soluble extracts from 33 AD brains. We found ~7 kDa Aβ species in a third of the samples, thus confirming that some of the ~7 kDa SDS-PAGE species were indeed native dimers and could be detected in the absence of monomer. Intermediate (~30–150 kDa) or high (~160–700 kDa) molecular weight species which broke down on SDS-PAGE to yield ~7 kDa Aβ were detected in all the brain extracts studied (Fig. 5, Supplementary Fig. 5, Table 4). Although we did not assess the activity of different SEC fractions, the fact that the majority of AD brains contain no ~7 kDa Aβ species, but had abundant Int mwt/High mwt species that broke down on SDS-PAGE to yield dimers, suggests that synaptotoxicity can be mediated by multiple species, of which dimers are likely to be important building blocks. Moreover, in future studies it will be important to determine how the different profiles of High mwt, Int mwt, ~7 kDa Aβ species and true monomer relate to clinicopathological stage, postmortem interval (PMI) and ApoE genotype.
To determine if the breakdown products of High mwt and Int mwt Aβ are real dimers or artefacts of SDS-PAGE, we first treated aqueous extracts from AD brains with strong denaturants and then analyzed them using SEC (Fig. 7). In each brain extract tested there was a significant shift in the elution pattern of the component Aβ species, with denaturants causing a loss of High mwt and Int mwt material and an increase in the amount of Aβ eluting as ~7 kDa Aβ species and true monomer. Importantly, when covalently cross-linked Aβ dimers were chromatographed on SEC these eluted in exactly the same fractions as the chaotrope-treated, brain-derived Aβ dimers. Furthermore, using a separate chromatographic technique, AF4, we also found that the SDS-PAGE ~7 kDa species eluted differently from Aβ monomer. Thus multiple lines of evidence indicate that the aqueous phase of AD brain contains 2 major forms of Aβ, a 6.1 – 7.9 kDa heterogeneous species centered at ~7 kDa, which most likely represents a mixture of highly stable N-terminally truncated Aβ dimers and a range of species from 3.5 – 4.5 kDa and centered at ~4 kDa, i.e. Aβ monomer beginning at Asp1, N-terminally truncated Aβ and p3. Since dimers are particularly abundant breakdown products of aggregates, it seems likely that dimers are either a fundamental building block of aggregates, or that monomer-monomer interactions are stabilized in aggregates such that when aggregates breakdown they yield SDS-stable dimers.
Despite extensive efforts the precise primary structure of these putative dimers remains enigmatic. Using mass spectrometry we have identified more than 10 different primary structures in SEC-isolated monomer fractions, but as yet we have been unable to obtain useful spectra from SEC-isolated dimers (Supplementary Fig. 8). Although these efforts provide no information about the primary structure of the ~7 kDa species, they again demonstrate a physical difference between SEC-isolated monomers and dimers. Given that dimers have been refractory to mass spectrometric analysis we used a quantitative WB approach to gain insight into the molecular composition of dimers. For this we employed nine monoclonal antibodies that specifically recognize different epitopes within Aβ and 3 antibodies to a region of APP just N-terminal to the Aβ domain. In extracts from eight AD brains, the majority of the material present in the ~7 kDa bands was recognized by mAbs specific for the Aβ42 C-terminus (21F12, HJ7.4) and the mid-region of Aβ (4G8, 266), and to a lesser extent the Aβ40 C-terminus (2G3, HJ2). The anti-APP mAb 1G6 recognises an epitope just N-terminal of Aβ and readily detected a recombinant NTE-Aβ, but importantly 1G6 did not detect the ~7 kDa band found in aqueous extracts of AD brain. These findings indicate that the ~7 kDa band contains neither N-terminally extended, nor C-terminally truncated Aβ species.
Given our demonstration that the ~4 kDa band contains at least three different species, (two of which are N-terminally truncated) it is evident that many different types of dimers could be formed (Roberts et al., 2012). In this regard it is important to note that the ~7 kDa band seen on SDS-PAGE is more broad and fuzzy than the ~4 kDa monomer. Under native SEC conditions this ~7 kDa Aβ dimer species is a predominant component of both Int mwt and High mwt assemblies. Although, it is unclear whether the Int mwt and High mwt Aβ species are homo-oligomers of Aβ, or are complexes of Aβ bound to other proteins e.g. ApoE (Hashimoto et al., 2012, Takeda, 2013), our data suggest that preventing the formation and further aggregation of Aβ dimers may offer a novel target for the treatment of AD.
Supplementary Material
Acknowledgements
This work was supported by grants to DMW from the National Institutes of Health (AG027443 and AG046275), BrightFocus Foundation, the Medical Research Council, UK (MRC CFAS, grant number G0900582), and to the Massachusetts Alzheimer’s Disease Research Center (AG05134). We thank Drs. H. Vinters, T. Revesz, M. Farrell, N. Cairns and J. Cleary for providing human brain tissue. Drs. D. Schenk, P. Seubert, and G. Basi kindly provided 3D6, 266, 2G3 and 21F12. Dr. D. Holtzman provided HJ2 and HJ7.4 and Dr. D. Selkoe provided pre-β and Z31. Probiodrug provided antibody 07/1 and synthetic AβpE3-40. We are grateful to K. Fitch for technical assistance and to Dr. J. Champagne for use of the Eclipse DualTec AF4.
Abbreviations
- aCSF
artificial cerebrospinal fliud
- AD
Alzheimer's disease
- Aβ
amyloid β-protein
- APP
amyloid precursor protein
- BSA
bovine serum albumin
- EDTA
Ethylenediaminetetraacetic acid
- EGTA
ethyleneglycoltetraacetic acid
- ELISA
enzyme linked immunosorbant assay
- fx
fraction
- GuHCl
guanidinium hydrochloride
- HPLC
high performance liquid chromatography
- HRP
horseradish peroxidase
- IP
immunoprecipitation
- LTP
long term potentiation
- mAb
monoclonal antibody
- mwt
molecular weight
- NTE
N-terminally extended Aβ
- oELISA
Aβ oligomer-specific ELISA
- PAGE
polyacrylamide gel electrophoresis
- PBS(T)
phosphate buffer saline (0.05% (v/v) Tween 20)
- PrA
Protein A-sephrose
- SDS
sodium dodecylsulfate
- SEC
size exclusion chromatography
- TBS(T)
tris buffer saline (0.05% (v/v) Tween 20)
- WB
Western blot
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Footnote: on AF4, low molecular weight species elute first.
Author Contributions
DMW conceived the project, designed experiments, directed research and wrote the paper. JMMcD and TTOM designed and performed experiments, and wrote the paper. WL, AJM and WMW performed experiments. MPF performed histological examination of brain tissue. GB and EP did mass spec analysis. All authors provided critical assessment of the text.
References
- Barry A, Klyubin I, Mc Donald JM, Mably AJ, Farrell MA, Scott M, et al. Alzheimer's Disease brain-derived amyloid-β-mediated inhibiton of LTP in vivo is prevented by immunotargetiong cellular prion protein. J Neurosci. 2011;31(20):7259–7263. doi: 10.1523/JNEUROSCI.6500-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benilova I, Karran E, De Strooper B. The toxic Aβ oligomer and Alzheimer's disease: an emperor in need of clothes. Nat neurosci. 2012;15(3):349–357. doi: 10.1038/nn.3028. [DOI] [PubMed] [Google Scholar]
- Bitan G, Fradinger E, Spring SM, Teplow DB. Neurotoxic protein oligomers-what you see is not always what you get. Amyloid. 2005;12:88–95. doi: 10.1080/13506120500106958. [DOI] [PubMed] [Google Scholar]
- Borlikova GG, Trejo M, Mably AJ, Mc Donald JM, Sala Frigerio C, Regan CM, Murphy KJ, Masliah W, Walsh DM. Alzheimer brain-derived amyloid beta-protein impairs synaptic remodeling and memory consolidation. Neurobiol Aging. 2013;34(5):1315–1327. doi: 10.1016/j.neurobiolaging.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmalm G, Portelius E, Ohrfelt A, Mattsson N, Persson R, Gustavsson MK, Vite CH, Gobom J, Mansson JE, Nilsson J, Halim A, Larson G, Ruetschi U, Zetterberg H, Blennow K, Brinkmalm A. An online nano-LC-ESI-FTICR-MS method for comprehensive characterization of endogenous fragments from amyloid beta and amyloid precursor protein in human and cat cerebrospinal fluid. J Mass Spectrom. 2012;47(5):591–603. doi: 10.1002/jms.2987. [DOI] [PubMed] [Google Scholar]
- Chesneau V, Vekrellis K, Rosner MR, Selkoe DJ. Purified recombinant insulin-degrading enzyme degrades amyloid beta-protein but does not promote its oligomerization. Biochem J. 2000;351(2):509–516. [PMC free article] [PubMed] [Google Scholar]
- Chromy BA, Nowak RJ, Lambert MP, Viola KL, Chang L, Velasco PL, Jones BW, Fernandez SJ, Lacor PN, Horowitz P, Finch CE, Krafft GA, Klein WL. Self-assembly of Abeta1-42 into Globular Neurotoxins. Biochemistry. 2003;42:12749–12760. doi: 10.1021/bi030029q. [DOI] [PubMed] [Google Scholar]
- Enya M, Morishima-Kawashima M, Yoshimura M, Shinkai Y, Kusui K, Khan K, Games D, Schenk D, Sugihara S, Yamaguchi H, Ihara Y. Appearance of sodium dodecyl sulfate-stable amyloid beta-protein (Abeta) dimer in the cortex during aging. Am J Pathol. 1999;154(1):271–279. doi: 10.1016/s0002-9440(10)65273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esparza TJ, Zhao H, Cirrito JR, Cairns NJ, Bateman RJ, Holtzman DM, Brody DL. Amyloid-β oligomerization in Alzheimer dementia versus high-pathology controls. Ann Neurol. 2013;73(1):104–119. doi: 10.1002/ana.23748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freir D, Nicoll AJ, Klyubin I, Panico S, Mc Donald JM, Risse E, Asante EA, Farrow MA, Sessions RB, Saibil HR, Clarke AR, Rowan MJ, Walsh DM, Collinge J. Interaction between prion protein and toxic amyloid β assemblies can be therapeutically targeted at multiple sites. Nat Commun. 2011;2(336) doi: 10.1038/ncomms1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost J, Le KX, Cynis H, Ekpo E, Kleinschmidt M, Palmur RM, Ervin FR, Snigdha S, Cotman CW, Saido TC, Vassar RJ, St George-Hyslop P, Ikezu T, Schilling S, Demuth HU, Lemere CA. Pyroglutamate-3 amyloid-β deposition in the brains of humans, non-human primates, canines, and Alzheimer disease-like transgenic mouse models. Am J Pathol. 2013;183(2):369–381. doi: 10.1016/j.ajpath.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford RW, Russell DW. Reduction of cholesterol synthesis in the mouse brain does not affect amyloid formation in Alzheimer's disease, but does extend lifespan. Proc Natl Acad Sci. 2009;106(9):3502–3506. doi: 10.1073/pnas.0813349106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halim A, Brinkmalm G, Ruetschi U, Westman-Brinkmalm A, Portelius E, Zetterberg H, Blennow K, Larson G, Nilsson J. Site-specific characterization of threonine, serine, and tyrosine glycosylations of amyloid precursor protein/amyloid beta-peptides in human cerebrospinal fluid. Proc Natl Acad Sci. 2011;108(29):11848–11853. doi: 10.1073/pnas.1102664108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Serrano-Pozo A, Hori Y, Adams KW, Takeda S, Olaf Banerji A, Mitani A, Joyner D, Thyssen DH, Bacskai BJ, Frosch MP, Spires-Jones TL, Finn MB, Holtzman DM, Hyman BT, Apolipoprotein E. Especially Apolipoprotein E4, Increases the Oligomerization of Amyloid beta Peptide. Neurobiol Dis. 2012;32(43):15181–15192. doi: 10.1523/JNEUROSCI.1542-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler RW, Grimm KM, Nahas DD, Breese R, Dodson EC, Acton P, Keller PM, Yeager M, Wang H, Shughrue P, Kinney G, Joyce JG. Solution state characterization of amyloid beta-derived diffusible ligands. Biochemistry. 2006;45(51):15157–15167. doi: 10.1021/bi061850f. [DOI] [PubMed] [Google Scholar]
- Ida N, Hartmann T, Pantel J, Schroder J, Zerfass R, Forstl H, Sandbrink R, Masters CL, Beyreuther K. Analysis of heterogeneous A4 peptides in human cerebrospinal fluid and blood by a newly developed sensitive Western blot assay. J Biol Chem. 1996;271(37):22908–22914. doi: 10.1074/jbc.271.37.22908. [DOI] [PubMed] [Google Scholar]
- Irvine GB. Size-exclusion high-performance liquid chromatography of peptides. Anal Chim Acta. 1997;352:387–397. [Google Scholar]
- Jin M, Shepardson N, Yang T, Chen G, Walsh D, Selkoe DJ. Soluble amyloid beta-protein dimers isolated from Alzheimer cortex induce tau hyperphospohrylation and neuritic degeneration. Proc Natl Acad Sci. 2011;108(14):5819–5824. doi: 10.1073/pnas.1017033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Wood K, Lee M, Motter R, Hu K, Gordon G, Barbour R, Khan K, Tan H, Games D, Leiberburg I, Schenk D, Seubert P, McConlogue L. Amyloid precursor protein processing and Aβ42 deposition in a transgenic mouse model of Alzheimer disease. Proc Natl Acad Sci. 1997;94(4):1550–1555. doi: 10.1073/pnas.94.4.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko N, Yamamoto R, Sato TA, Tanaka K. Identification and quantification of amyloid beta-related peptides in human plasma using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Proc Jap Acad Ser B, Phys Biol Sci. 2014;90(3):104–117. doi: 10.2183/pjab.90.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Eltorai AE, Jiang H, Liao F, Verghese PB, Stewart FR, Basak JM, Holtzman DM. Anti-apoE immunotherapy inhibits amyloid accumulation in a transgenic mouse model of Abeta amyloidosis. Journal Exp Med. 2012;209(12):2149–2156. doi: 10.1084/jem.20121274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein D, Kern RM, Sokol RZ. A method for quantification and correction of proteins after transfer to immobilization membranes. Biochem Mol Biol Int. 1995;36(1):59–66. [PubMed] [Google Scholar]
- Klein WL, Krafft GA, Finch CE. Targeting small Abeta oligomers: the solution to an Alzheimer's disease conundrum? Trends Neurosci. 2001;24(4):219–224. doi: 10.1016/s0166-2236(00)01749-5. [DOI] [PubMed] [Google Scholar]
- Kumar S, Rezaei-Ghaleh N, Terwel D, Thal DR, Richard M, Hoch M, Mc Donald JM, Wullner U, Glebov K, Heneka MT, Walsh DM, Zweckstetter M, Walter J. Extracellular phosphorylation of the amyloid β-peptide promotes formation of toxic aggregates during the pathogenesis of Alzheimer's disease. EMBO J. 2011;30(11):2255–2265. doi: 10.1038/emboj.2011.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer F, Eisele YS, Fritschi SK, Staufenbiel M, Walker LC, Jucker M. Soluble Aβ seeds are potent inducers of cerebral β-amyloid deposition. J Neurosci. 2011;31(41):14488–14495. doi: 10.1523/JNEUROSCI.3088-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesné SE, Sherman MA, Grant M, Kuskowski M, Schneider JA, Bennett DA, Ashe KH. Brain amyloid-β oligomers in ageing and Alzheimer's disease. Brain. 2013;136(5):1383–1398. doi: 10.1093/brain/awt062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maarouf CL, Andacht TM, Kokjohn TA, Castano EM, Sue LI, Beach TG, Roher AE. Proteomic analysis of Alzheimer's disease cerebrospinal fluid from neuropathologically diagnosed subjects. Curr Alzheimer Res. 2009;6(4):399–406. doi: 10.2174/156720509788929318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mc Donald JM, Savva G, Brayne C, Welzel AT, Forster G, Shankar GM, Selkoe DJ, Ince PG, Walsh DM. The presence of sodium dodecyl sulphate-stable Aβ dimers is strongly associated with Alzheimer-type dementia. Brain. 2010;133(5):1297–1299. doi: 10.1093/brain/awq065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mc Donald JM, Cairns NJ, Taylor-Reinwald L, Holtzman D, Walsh DM. The levels of water-soluble and triton-soluble Aβ are increased in Alzheimer's disease brain. Brain Res. 2012;1450:138–147. doi: 10.1016/j.brainres.2012.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaurin J, Cecal R, Kierstead ME, Tian X, Phinney AL, Manea M, French JE, Lambermon MH, Darabie AA, Brown ME, Janus C, Chishti MA, Horne P, Westaway D, Fraser PE, Mount HT, Przybylski M, St George-Hyslop P. Therapeutically effective antibodies against amyloid-beta peptide target amyloid-beta residues 4–10 and inhibit cytotoxicity and fibrillogenesis. Nat Med. 2002;8(11):1263–1269. doi: 10.1038/nm790. [DOI] [PubMed] [Google Scholar]
- McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, Bush AI, Masters CL. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer's disease. Ann Neurol. 1999;46(6):860–866. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Moir RDTK, Soscia S, Hyman BT, Irizarry MC, Tanzi RE. Autoantibodies to redox-modified oligomeric Abeta are attenuated in the plasma of Alzheimer's disease patients. J Biol Chem. 2005;280(17):17458–17463. doi: 10.1074/jbc.M414176200. [DOI] [PubMed] [Google Scholar]
- Moore BD, Chakrabarty P, Levites Y, Kukar TL, Baine AM, Moroni T, Ladd TB, Das P, Dickson DW, Golde TE. Overlapping profiles of Aβ peptides in the Alzheimer's disease and pathological aging brains. Alzheimers Res Ther. 2012;4(3):18. doi: 10.1186/alzrt121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima-Kawashima M, Hasegawa M, Takoo K, Suzuki M, Yoshida H, Titani K, et al. Proline-directed and non-proline-directed phosphorylation of PHF-tau. J Biol Chem. 1995;270(2):823–829. doi: 10.1074/jbc.270.2.823. [DOI] [PubMed] [Google Scholar]
- Nilsberth C, Westlind-Danielsson A, Eckman CB, Condron MM, Axelman K, Forsell C, Stenh C, Luthman J, Teplow DB, Younkin SG, Naslund J, Lannfelt L. The 'Arctic' APP mutation (E693G) causes Alzheimer's disease by enhanced Abeta protofibril formation. Nat Neurosci. 2001;4(9):887–893. doi: 10.1038/nn0901-887. [DOI] [PubMed] [Google Scholar]
- Noguchi A, Matsumura S, Dewzawa M, Tada M, Yanazawa M, Ito A, Akioka M, Kikuchi S, Sato M, Ideno S, Noda M, Fukunari A, Muramatsu S, Itokazu Y, Sato K, Takashi H, Teplow D, Nabeshima Y, Kakita A, Imahori K, Hoshi M. Isolation and characterisation of patient-derived, toxic, high mass amyloid beta-protein assembly from Alzheimer's Disease brains. J Biol Chem. 2009;284(47):32895–32905. doi: 10.1074/jbc.M109.000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley TT, Oktaviani NA, Zhang D, Lomakin A, O'Nuallain B, Linse S, Benedek GB, Rowan MJ, Mulder FAA, Walsh DM. Aβ dimers differ from monomers in structural propensity, aggregation paths, and population of synaptotoxic assemblies. Biochem J. 2014;461(3):413–426. doi: 10.1042/BJ20140219. [DOI] [PubMed] [Google Scholar]
- O'Nuallain B, Freir D, Nicoll A, Risse E, Ferguson N, Herron CE, Colllinge J, Walsh DM. Amyloid β-protein dimers rapidly form stable synaptotoxic protofibrils. J Neurosci. 2010;30(43):14411–14419. doi: 10.1523/JNEUROSCI.3537-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paivio A, Jarvet J, Graslund A, Lannfelt L, Westlind-Danielsson A. Unique physicochemical profile of beta-amyloid peptide variant Abeta1-40E22G protofibrils: conceivable neuropathogen in arctic mutant carriers. J Mol Biol. 2004;339(1):145–159. doi: 10.1016/j.jmb.2004.03.028. [DOI] [PubMed] [Google Scholar]
- Pannee J, Tornqvist U, Westerlund A, Ingelsson M, Lannfelt L, Brinkmalm G, Persson R, Gobom J, Svensson J, Johansson P, Zetterberg H, Blennow K, Portelius E. The amyloid-beta degradation pattern in plasma--a possible tool for clinical trials in Alzheimer's disease. Neurosci Lett. 2014;573:7–12. doi: 10.1016/j.neulet.2014.04.041. [DOI] [PubMed] [Google Scholar]
- Podlisny MB, Ostaszewski BL, Squazzo SL, Koo EH, Rydell RE, Teplow DB, Selkow DJ. Aggregation of secreted amyloid beta-protein into sodium dodecyl sulfate-stable oligomers in cell culture. J Biol Chem. 1995;270(16):9564–9570. doi: 10.1074/jbc.270.16.9564. [DOI] [PubMed] [Google Scholar]
- Portelius E, Olsson M, Brinkmalm G, Rüetschi U, Mattsson N, Andreasson U, Gobom J, Brinkmalm A, Hölttä M, Blennow K, Zetterberg H. Mass spectrometric characterization of amyloid-β species in the 7PA2 cell model of Alzheimer's disease. J Alzheimer's Dis. 2013;33(1):85–93. doi: 10.3233/JAD-2012-120994. [DOI] [PubMed] [Google Scholar]
- Portelius E, Westman-Brinkmalm A, Zetterberg H, Blennow K. Determination of beta-amyloid peptide signatures in cerebrospinal fluid using immunoprecipitation-mass spectrometry. J Proteome Res. 2006;5(4):1010–1016. doi: 10.1021/pr050475v. [DOI] [PubMed] [Google Scholar]
- Reeves JP, Lo CY, Klinman DM, Epstein SL. Mouse monoclonal antibodies to human immunodeficiency virus glycoprotein 120 generated by repeated immunization with glycoprotein 120 from a single isolate, or by sequential immunization with glycoprotein 120 from three isolates. Hybridoma. 1995;14(3):235–242. doi: 10.1089/hyb.1995.14.235. [DOI] [PubMed] [Google Scholar]
- Roberts BR, Ryan TM, Bush AI, Masters CL, Duce JA. The role of metallobiology and amyloid-beta peptides in Alzheimer's disease. J Neurochem. 2012;120(Suppl 1):149–166. doi: 10.1111/j.1471-4159.2011.07500.x. [DOI] [PubMed] [Google Scholar]
- Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, Davis D, et al. Isolation and quantification of soluble Alzheimer's beta-peptide from biological fluids. Nature. 1992;359(6393):325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-β protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14(8):837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji M, Kawarabayashi T, Matsubara E, Ikeda M, Ishiguro K, Harigaya Y, Okamoto K. Distribution of amyloid beta protein precursor in the Alzheimer's disease brain. Psy Clin Neurosci. 2000;54(1):45–54. doi: 10.1046/j.1440-1819.2000.00636.x. [DOI] [PubMed] [Google Scholar]
- Stenh C, Englund H, Lord A, Johansson AS, Almeida CG, Gellerfors P, Greengard P, Gouras GK, Lannfelt L, Milsson LN. Amyloid-beta oligomers are inefficiently measured by enzyme-linked immunosorbent assay. Ann Neurol. 2005;58(1):147–150. doi: 10.1002/ana.20524. [DOI] [PubMed] [Google Scholar]
- Stöhr J, Watts JC, Mensinger ZL, Oehler A, Grillo SK, DeArmond SJ, Prusiner SB, Giles K. Purified and synthetic Alzheimer's amyloid beta (Aβ) prions. Proc Natl Acad Sci. 2012;109(27):11025–11030. doi: 10.1073/pnas.1206555109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S, Hashimoto T, Roe AD, Hori Y, Spires-Jones TL, Hyman BT. Brain interstitial oligomeric amyloid β increases with age and is resistant to clearance from brain in a mouse model of Alzheimer's disease. FASEB J. 2013;27(8):3239–3248. doi: 10.1096/fj.13-229666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi RE. The genetics of Alzheimer disease. Cold Spring Harb Persp Med. 2012;1(2):10. doi: 10.1101/cshperspect.a006296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiyama T, Nagata T, Shimada H, Teraoka R, Fukushima A, Kanemitsu H, Takuma H, Kuwano R, Imagawa M, Ataka S, Wada Y, Yoshioka E, Nishizaki T, Watanabe Y, Mori H. A new amyloid beta variant favoring oligomerization in Alzheimer's-type dementia. Annal Neurol. 2008;63(3):377–387. doi: 10.1002/ana.21321. [DOI] [PubMed] [Google Scholar]
- Um JW, Nygaard HB, Heiss JK, Kostylev MA, Stagi M, Vortmeyer A, Wisniewski T, Gunther EC, Strittmatter SM. Alzheimer amyloid-β oligomer bound to postsynaptic prion protein activates Fyn to impair neurons. Nature neurosci. 2012;15(9):1227–1235. doi: 10.1038/nn.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlund KG, Giddings JC. Properties of an asymmetrical flow field-flow fractionation channel having one permeable wall. Anal Chem. 1987;59(9):1332–1339. doi: 10.1021/ac00136a016. [DOI] [PubMed] [Google Scholar]
- Wahlund KG, Litzen A. Application of an asymmetrical flow field-flow fractionation channel to the separation and characterization of proteins, plasmids, plasmid fragments, polysaccharides and unicellular algae. J Chrom. 1989;461:73–87. doi: 10.1016/s0021-9673(00)94276-6. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Thulin E, Minogue AM, Gustavsson N, Pang E, Teplow DB, Linse S. A facile method for expression and purification of the Alzheimer's disease-associated amyloid β-peptide. FEBS J. 2009;276(5):1266–1281. doi: 10.1111/j.1742-4658.2008.06862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Tseng BP, Rydel RE, Podlisny MB, Selkoe DJ. Detection of intracellular oligomers of amyloid β-protein in cells derived from human brain. Biochemistry. 2000;39:10831–10839. doi: 10.1021/bi001048s. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Teplopw DB. Alzheimer's disease and the amyloid β-protein. Prog Mol Biol Transl Sci. 2012;107:101–124. doi: 10.1016/B978-0-12-385883-2.00012-6. [DOI] [PubMed] [Google Scholar]
- Wang J, Dickson DW, Trojanowski JQ, Lee VM. The levels of soluble versus insoluble brain Abeta distinguish Alzheimer's disease from normal and pathologic aging. Exp Neurol. 1999;158(2):328–337. doi: 10.1006/exnr.1999.7085. [DOI] [PubMed] [Google Scholar]
- Watt AD, Perez KA, Rembach A, Sherrat NA, Hung LW, Johanssen T, McLean CA, Kok WM, Hutton CA, Fodero-Tavoletti M, Masters CL, Villemagne VL, Barnham KJ. Oligomers, fact or artefact? SDS-PAGE induces dimerization of β-amyloid in human brain samples. Acta Neuropathol. 2013;125(4):549–564. doi: 10.1007/s00401-013-1083-z. [DOI] [PubMed] [Google Scholar]
- Welzel AT, Maggio JE, Shankar GM, Walker DE, Ostaszewski BL, Li S, Klyubin I, Rowan MJ, Seubert P, Walsh DM, Selkoe DJ. Secreted amyloid β-proteins in a cell culture model include N-terminally-extended prptides that impair synaptic plasticity. Biochemistry. 2014;53(24):3908–3921. doi: 10.1021/bi5003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltfang J, Arold N, Neuhoff V. A new multiphasic buffer system for sodium dodecyl sulfate-polyacrylamide gel electrophoresis of proteins and peptides with molecular masses 100,000-1000, and their detection with picomolar sensitivity. Electrophoresis. 1991;12(5):352–366. doi: 10.1002/elps.1150120507. [DOI] [PubMed] [Google Scholar]
- Xia W, Yang T, Shankar G, Smith IM, Shen Y, Walsh DM, Selkoe DJ. A specific enzyme-linked immunosorbent assay for measuring beta-amyloid protein oligomers in human plasma and brain tissue of patients with Alzheimer disease. Arch Neurol. 2009;2009(66):2. doi: 10.1001/archneurol.2008.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Hong S, O'Malley T, Sperling RA, Walsh DM, Selkoe DJ. New ELISAs with high specificity for soluble oligomers of amyloid β-protein detect natural Aβ oligomers in human brain but not CSF. Alz Dement. 2013;9(2):99–112. doi: 10.1016/j.jalz.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, O'Malley T, Kanmert D, Jerecic J, Zieske L, Zetterberg H, Hyman B, Walsh DM, Selkoe DJ. A highly sensitive novel immunoassay specifically detects low levels of soluble Aβ oligomers in human CSF. Alz Res Therapy. 2015 doi: 10.1186/s13195-015-0100-y. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.