Abstract
Human and animal studies show significant delays in neurobehavioral development in offspring after prolonged prenatal exposure to moderate and high ethanol doses resulting in high blood alcohol concentration (BECs). However, none have investigated the effects of lower ethanol doses given acutely during specific developmental time periods. Here, we sought to create a mouse model for modest and circumscribed human drinking during the 3rd and 4th weeks of pregnancy.
We acutely treated mice during embryo gastrulation on gestational day (GD) 7 or neurulation on GD8 with a low or moderate ethanol dose given via gavage that resulted in BECs of 107 and 177 mg/dl, respectively. We assessed neonatal physical development (pinnae unfolding, and eye opening); weight gain from postnatal day (PD) 3-65; and neurobehavioral maturation (pivoting, walking, cliff aversion, surface righting, vertical screen grasp, and rope balance) from PD3-17. We used a multiple linear regression model to determine the effects of dose, sex, day of treatment and birth in animals dosed during gastrulation or neurulation, relative to their vehicle controls.
We found that ethanol exposure during both time points (GD7 and GD8) resulted in some delays of physical development and significant sensorimotor delays of pivoting, walking, and thick rope balance, as well as additional significant delays in cliff aversion and surface righting after GD8 treatment. We also found that treatment with the low ethanol dose more frequently affected neurobehavioral development of the surviving pups than treatment with the moderate ethanol dose, possibly due to a loss of severely affected offspring. Finally, mice born prematurely were delayed in their physical and sensorimotor development.
Importantly, we showed that brief exposure to low dose ethanol, if administered during vulnerable periods of neuroanatomical development, results in significant neurobehavioral delays in neonatal mice. We thus expand concerns about alcohol consumption during the 3rd and 4th week of human pregnancy to include occasional light to moderate drinking.
Keywords: Mouse, Gastrulation, Neurulation, Low ethanol dose, Moderate ethanol dose, Sensorimotor development, Neurobehavioral delays, Premature birth
1. Introduction
Prenatal alcohol exposure of the developing human results in a continuum of structural anomalies and/or behavioral and neurocognitive disabilities that are termed fetal alcohol spectrum disorders [FASD] (Sokol et al., 2003; Hoyme et al., 2005; Riley et al., 2011). Fetal alcohol syndrome [FAS], first described 40 years ago (Jones et al., 1973) with diagnostic features of facial dysmorphology, growth restriction, and central nervous system/neurodevelopmental abnormalities, is now considered to lie at the extreme end of FASD (Sokol at al., 2003; Riley at al., 2011). In FASD, neurobehavioral deficits can include psychiatric disorders and impairments in intelligence, executive function, learning and memory, language, visual-spatial ability, motor function, attention, and activity levels (Mattson et al., 2011).
In the US, the estimated prevalence for FASD and FAS of 2–5% and 0.2–0.7% of live births, respectively, results in major public health issues and economic costs (May et al., 2009; Riley et al., 2011). Annual cost estimates for FAS alone were $4 billion in 1998 (Lupton et al., 2004) and could be expected to be much higher today due to inflation and when including FASD. Despite public health warnings, more than half of all nonpregnant women of childbearing age report alcohol use, and nearly 13% binge drink (Tsai et al., 2007). Annually, one in 8 pregnant women (500,000 pregnancies) report using alcohol during their pregnancy, and nearly 2% (80,000 pregnancies) report heavy drinking (Floyd and Sidhu, 2004). Nearly half of all pregnancies are unintended, are confirmed well into the course, and are often followed by belated or inadequate prenatal care – all of which could delay cessation of drinking (Finer and Zolna, 2011). Unfortunately, during the 3rd and 4th weeks of pregnancy, when gastrulation and neurulation respectively occur, the developing embryo is particularly vulnerable to ethanol insult, as documented in an autopsy study (Coulter et al., 1993). Additionally, such early ethanol exposure may increase the risk of spontaneous abortions or prematurity (Jaddoe et al., 2007; Sokol et al., 2007; Aliyu et al., 2008; O’Leary et al., 2009; Meyer-Leu et al., 2011; Andersen et al., 2012; Chiodo et al., 2012).
Prenatal rodent studies of heavy and acute ethanol exposure have clearly identified two susceptible times in brain and facial development: gastrulation, when the three germ layers are formed, and neurulation, when the neural tube closes (Webster et al., 1983; Sulik, 2005). In a mouse model for binge drinking during gastrulation, acute exposure to a high ethanol dose produces a subset of severely affected fetuses with ventral forebrain and facial defects, corresponding to those seen in human FAS (Sulik et al., 1981). Specifically, structural forebrain defects are seen in the olfactory bulbs, septal area, neostriatum, frontal cortex, corpus callosum, and hippocampus (Sulik et al., 1984; Schambra et al., 1990; Ashwell and Zhang, 1996; Sulik, 2005; Godin et al., 2010). Additionally, in a rat model, acute ethanol exposure during gastrulation results in a decreased number of neurons in two sensory trigeminal nuclei, and the trigeminal, facial and hypoglossal motor nuclei (Mooney and Miller, 2007). In contrast, acute ethanol exposure of mouse embryos during neurulation produces abnormalities in forebrain, cerebellum, cranial nerves and ganglia, and facial structures with features resembling the DiGeorge syndrome (Sulik et al., 1986; Kotch and Sulik, 1992a; Dunty et al., 2002; Sulik, 2005; Parnell et al., 2009).
Behavioral outcomes for the offspring of such heavy and acute prenatal ethanol exposures have been explored in a few studies with rats (Molina et al., 1984; 1987; Minetti et al., 1996) and mice (Dumas and Rabe, 1994; Enders et al., 2005). A decrease of successful deliveries and an increase of neonatal loss of pups have been reported in some of these high ethanol dose studies, and tests of sensorimotor maturation of the remaining ethanol exposed pups yielded few or no significant differences compared to unexposed pups (Molina et al., 1987; Dumas and Rabe, 1994; Enders et al., 2005). Prenatal studies found that the effects of ethanol within a litter are heterogeneous (Webster et al., 1983; Sulik et al., 1981; Schambra et al., 1990; Kotch and Sulik, 1992a). Thus, it is conceivable that in these postnatal studies the most severely affected pups died pre- or neonatally, whereas less affected pups survived but demonstrated few or no behavioral abnormalities. Computational modeling studies comparing the effects of prenatal ethanol exposure across species on neocortical neurogenesis indicate that a significantly higher BEC is required in rodents than in humans to produce similar neurodevelopmental defects. Thus, to predict similar deficits, a daily peak BEC of 20 mg/dl, reached by a woman drinking one drink within one hour on an empty stomach, would be comparable to a daily peak BEC of 100 mg/dl in a rat (Gohlke et al., 2008). The prenatal histological and the postnatal behavioral studies described above showed the adverse effects of high acute ethanol exposure (BECs well above 200 mg/dl), which are comparable to binge drinking (3–5 drinks per occasion) during gastrulation or neurulation. However, not all pregnant women who use alcohol may binge drink to this extent, but may rather consume 1 or 2 drinks before a meal on two consecutive days. This could result in BECs of 20 or 40 mg/dl, respectively, comparable to BECs of about 100 or 200 mg/dl in a rodent.
We chose a paradigm with two goals in mind: (1) to explore behavioral consequences for the offspring of such moderate or light social drinking during either of two vulnerable periods early in gestation, and (2) to avoid losing ethanol damaged offspring before or after birth. To mimic human drinking on an empty stomach, we delivered the alcohol doses to pregnant mice by gavage (intubation) before the mice began to feed. Pregnant mice were treated with ethanol doses that resulted in moderate or low BECs. Because gastrulation and neurulation take several days in humans and could be susceptible to a consecutive 2-day exposure, mice were given ethanol twice during either gastrulation on GD7 or neurulation on GD8. The offspring were evaluated for their physical and neurobehavioral development following published reports for mice (Fox, 1965; Schneiderman Fish et al., 1981) and rats (Molina et al., 1987).
We aimed to test several hypotheses: (1) relative to controls, animals treated with ethanol would be delayed in physical and neurobehavioral maturation, (2) treatment during gastrulation or neurulation would differentially affect physical and neurobehavioral maturation, and (3) relative to animals born on gestational day (GD) 20, animals born on GD19 would be delayed in physical and neurobehavioral maturation.
2. Materials and methods
2.1. Animals
Mice were offspring from C57BL/6J breeding pairs, obtained from Charles River (Raleigh, NC). Mice were housed in an AAALAC-accredited animal facility, with commercial lab chow and water available ad libitum. A 12/12 hour reversed-light cycle was maintained in the housing room, with lights off at 8 a.m. All procedures were conducted in strict compliance with the policies on animal welfare of the National Institutes of Health and East Tennessee State University. A single male was placed with a single female from 8 a.m. to 9 a.m., and successful copulation, determined by the presence of a mating plug, was designated gestational day zero, hour zero (GD0:0h). Pregnant females were weighed and given a single cage with nesting material.
2.2. Treatment
Pregnant dams were assigned to 1 of 7 groups, consisting of 3 control groups and 4 ethanol treated groups. The control groups were: (1) the untreated control group (UC), (2) a group treated with vehicle by gavage during gastrulation on GD7 (GC7) and (3) a group treated with vehicle by gavage during neurulation on GD8 (GC8). The 4 ethanol treated groups consisted of 2 each treated on GD7 or GD8 with either a low or moderate ethanol dose. Thus, the resultant ethanol groups were: (4) Low7, (5) Mod7, (6) Low8, and (7) Mod8.
The gavage ethanol dosing followed the established protocol for intraperitoneal (IP) dosing of 2 treatments, 4 hours apart (Sulik et al., 1981). The moderate dose 2.9 g ethanol and the low dose was 2.4 g ethanol/kg maternal body weight (BW), given in equivalent dosing volumes of 0.015 ml/g BW and prepared with solutions of 25% and 20% ethanol, respectively. We chose the gavage treatment to better replicate human drinking than the IP treatment commonly used, and to achieve lower BECs.
Dams were intubated with a #7910 curved stainless steel needle (Popper, New Hyde Park, NY) with either ethanol diluted in a rat infusion diet (#F1657, Bioserv, Frenchtown, NJ) for the ethanol treatment groups, or with vehicle only for the gavage controls (GC). To avoid intubation on a full stomach, we had accustomed the mice to a reversed light/dark cycle, so that the dark cycle and feeding period started at 8 a.m.; on day of treatment, we removed the chow until after treatment. We chose the rat infusion diet as the vehicle to minimize possibly detrimental short-term hypoglycemia of the embryos in the ethanol treatment groups (Smoak et al., 1990) by providing extra nourishment for these dams. Our gavage treatment times with either vehicle or ethanol solutions were based on prenatal mouse studies investigating ethanol exposure during gastrulation on GD7:0h at 8 a.m. and GD7:4h at 12:00 p.m. (Sulik et al., 1981), or neurulation on GD8:6h at 2 pm and GD8:10h at 6 p.m. (Kotch and Sulik, 1992b). Dams were weighed on GD0, the day of treatment (GD7 or GD8) and GD18 before parturition on GD19 or GD20. Dams were checked twice daily for parturition. Day of birth (DOB) was designated as postnatal day 0 (PD0).
2.3. Determination of Blood Ethanol Concentration (BEC)
We tested 1–3 mice per group to compare the BEC time courses resulting from IP treatment used in the prior prenatal studies (Sulik et al., 1981; Schambra et al., 1990) to the gavage ethanol treatment used in the present study. Both treatment doses of 2.4 g and 2.9 g ethanol/kg maternal BW were evaluated. These mice were not used as dams in the behavioral study.
Thirty minutes after ethanol treatment, the mouse was placed in a tailveiner mouse restrainer (Braintree Scientific, Braintree, MA), the tip of its tail clipped, and its blood collected into a 20μl heparinized micro-hematocrit capillary tube sealed at the bottom with Chao-seal (Fisher), capped with a double layer of Paraplast and refrigerated. Blood was collected every 30 min, up to 8.5 h, then analyzed in a Perkins-Elmer Sigma 2 Gas Chromatograph with a Hewlett Packard Model 3390A reporting integrator, using an ultra-micro method of direct sample injection and internal and ethanol standards (Manno and Manno, 1978).
2.4. Offspring experimental groups
Table 1 provides information on the number of litters and offspring for each of the seven groups, whether DOB was on GD19 or GD20, the neonatal survival data and the numbers of pups tested. Individual pups in each litter were uniquely identified up to PD7 by permanent red or black marker dots on their bellies. On PD8 the pup’s sex was confirmed when nipples on the females became prominent, and their tails additionally were marked with colored rings. On PD21 the mice were weaned, males and females were placed in separate cages, and identified with combinations of hole punches to their ears.
Table 1.
Group characteristics
| UC | GC7 | GC8 | Low7 | Mod7 | Low8 | Mod8 | |
|---|---|---|---|---|---|---|---|
| N litters | 13 | 7 | 8 | 8 | 12 | 7 | 7 |
| N pups born (alive and dead) | 95 | 48 | 62 | 66 | 89 | 52 | 61 |
| % pups dead at birth | 7.4 | 10.4 | 1.6 | 4.5 | 4.5 | 1.9 | 3.3 |
| % pups dead within 1st weekb | 9.5 | 20.8 | 8.1 | 22.7a | 21.3a | 13.5 | 9.8 |
| % pups born on GD19 | 33.7 | 41.7 | 22.6 | 10.6a | 70.8a | 15.4 | 27.9 |
| % pups born on GD19 survive | 78.1 | 80.0 | 85.7 | 14.3a | 74.6a | 87.5 | 94.1 |
| % pups born on GD20 survive | 92.0 | 78.6 | 93.7 | 84.7 | 88.5 | 85.7 | 88.6 |
| N GD19 pups | 25 | 16 | 12 | 1c | 47 | 7 | 16 |
| N GD20 pups | 58 | 22 | 45 | 50 | 23 | 36 | 39 |
| N Male pups | 40 | 24 | 26 | 34 | 34 | 19 | 30 |
| N pups/litter raised (mean ± SD) | 6.4 ± 2.0 | 5.4 ± 2.1 | 7.1 ± 1.5 | 6.4 ± 2.9 | 5.8 ± 2.2 | 6.1 ± 1.2 | 7.9 ± 1.1 |
suggestive differences (p-value < 0.1) in comparison with the UC group; comparison based on Wald test in a mixed model accounting for litter-level correlations.
inclusive of dead at birth.
This pup is the only one in the category and is excluded from further analyses.
UC = Untreated control
GC7 = Gavage control treated on GD7
GC8 = Gavage control treated on GD8
Low7 = Low dose ethanol treatment on GD7
Mod7 = Moderate dose ethanol treatment on GD7
Low8 = Low dose ethanol treatment on GD8
Mod8 = Moderate dose ethanol treatment on GD8
2.5 Outcomes
Unless otherwise noted, we recorded and analyzed the postnatal day at which a pup reached a specific set of characteristics or behaviors for a particular outcome, which we termed criterion. We also recorded the time to complete particular tests, which we termed latency.
2.5.1. Physical growth and maturation tests
2.5.1.1
Weights of all pups were recorded daily up to PD25 and on PD65 to the nearest 0.01 g on a DI electronic balance (Denver Instruments). Statistical analysis was performed for PD 3, 5, 10, 15, 20, 25 and 65.
2.5.1.2
Pinna unfolding was recorded from PD2 to PD5. Criterion was when both pinnae were unfolded.
2.5.1.3
Eye opening was recorded between PD11 and PD14, and criterion was when both eyes opened.
2.5.2. Neurobehavioral tests
We selected tests from previously established behavioral screens for neonatal rats (Altman and Sudarshan, 1975; Molina et al., 1987) and mice (Fox, 1965; Schneiderman Fish et al., 1981). All of the tests require the development of both motor and sensory function. Tests were conducted in the animal housing room to minimize stress on both dams and pups. Testing was done at the beginning of the dark cycle, and only the table area for testing was lit by a small lamp. Individual pups were given one test trial per day. Tests are described below with the postnatal testing days noted in parentheses.
2.5.2.1. Pivoting (PD3-13)
Each pup was placed on the table and seconds spent pivoting with its forelimbs around the planted hindlimbs within a given 60 s time span were recorded. Criterion was the last day of pivoting.
2.5.2.2. Walking (PD7-14)
Each pup was placed on the table and observed. Criterion was when the pup walked freely for ≥ 5 s on 2 consecutive days.
2.5.2.3. Cliff aversion (PD3-12)
Each pup was placed with the anterior half of the head and the front paws over the edge of the table and given a maximum of 30 s per trial. The latency to withdraw the head and entire body 1.5 cm from the cliff was recorded and criterion was reached when the pup withdrew 1.5 cm in ≤ 5 s on 2 consecutive days.
2.5.2.4. Surface righting (PD3-11)
Each pup was placed in a supine position on the table and immediately released. The latency to achieve prone position was recorded, or timed out at 30 s. Criterion was reached when the pup could right itself in ≤ 5 s on 2 consecutive days.
2.5.2.5. Vertical screen grasp (PD10-17)
Each pup was placed in the center of a vertically mounted wood-framed piece of wire mesh (20.5 × 23.5 cm with 0.5 cm2 holes) with all four paws touching the screen. Latency before falling onto a piece of foam rubber was recorded within a time frame of 30 s. Criterion was reached when the pup could hold onto the screen for 30 s or climb upward to the top of the screen on 2 consecutive days.
2.5.2.6. Rope balance tests (PD9-16) with a thick rope (10 mm diameter) and thin rope (5 mm diameter)
Ropes were tightly strung over a mouse cage, which was padded inside with foam rubber. Pups were placed at the center of each horizontal rope, starting with the thick rope and finishing with the thin rope, and latency (seconds balanced on a rope) and criterion (balancing for 30 s on 2 consecutive days) were recorded.
2.6. Statistical analyses
BEC time courses were visually inspected for differences, but the small sample size precluded formal statistical testing. Criterion and latency data were recorded for the above tests and analyzed offline. Latency data was used to determine the maturation span of sensorimotor behavior. We define maturation span as the time between the final day with the worst possible performance (timing out > 30 s for several variables, or immediately falling for the vertical screen and rope tests) and the day on which the pup reached criterion. We made the assumption that on the day prior to beginning observation, all pups would have had the worst possible performance of either 30 s or 0 s. We included this outcome in our analysis to evaluate for cases in which, despite a delayed onset of the behavior, there was a rapid “catching-up” of maturation, to result in relatively normal arrival at criterion.
Both the criterion and maturation span outcomes are continuous, and we modeled these responses using a multiple linear regression with day of treatment (GD7, GD8), DOB (GD19, GD20), dose (GC, Low, Mod), and sex (male, female) as categorical predictors. The regression framework that we used is an established statistical method that accounts for potential confounders when assessing the effect of a variable of interest. The key strength of this approach is the ability to isolate the effect of one variable while adjusting for the effects of remaining variables. Potential interactions between predictors of interest were considered, but were not found to be significant, and so are not included in the final models. Because pups are clustered within litters, we used a multilevel model to account for correlations across pups within litters; doing so allows the inclusion of all pups in our analysis rather than sampling a single pup from each litter. Specifically, we use random intercepts to model the litter effects by allowing litters to have higher or lower average response values. Comparisons between UC, GC7, and GC8 groups were made separately from analyses of other covariates because the UC group does not have a treatment day. Significance for each predictor was assessed using a Wald test with alpha = 0.05. All analyses were conducted using the R statistical computing platform (R Core Team, 2013).
3. Results
3.1. Determination of Blood Ethanol Concentration (BEC)
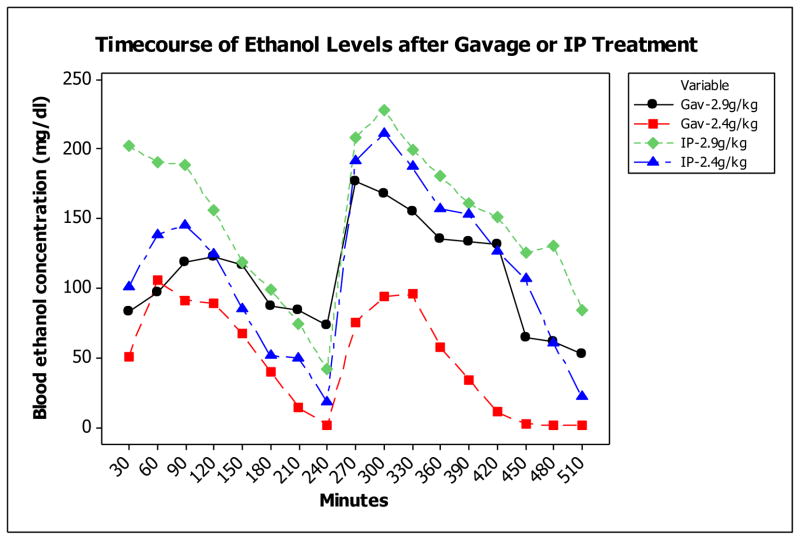
Intubation (gavage) ethanol treatment resulted in markedly lower BECs than intraperitoneal (IP) treatment after 2 doses of 2.9 or 2.4 g ethanol/kg BW, given 4 h apart (Fig. 1). By inspection, the highest peak BECs, achieved after the second ethanol dose, were considerably lower for both the 2.9 and 2.4 g/kg gavage doses (177 and 104 mg/dl, respectively) than the IP doses (228 and 211 mg/dl, respectively; Fig. 1). We refer to the two gavage doses here as a moderate dose (177mg/dl) and low dose (BEC 104 mg/dl)).
Fig. 1. Blood ethanol concentration (BEC).
after gavage (Gav) and intraperitoneal (IP) ethanol treatment on GD7 of either 2.9 or 2.4 g ethanol/kg maternal BW given twice, 4 h apart. N = 1 - 3 for each group.
3.2. Group characteristics
Table 1 summarizes litter, pup and group characteristics. Treatment on GD7, but not on GD8, lowered the pup survival rate due to peri- and neonatal death of pups, indicating that gastrulation may be a stage particularly sensitive to any disturbance. There was an uneven distribution of pups born on GD19 vs. GD20 across groups. For example, 70.8% Mod7 pups were born on GD19, whereas 27.9% Mod8 pups were born on GD19. Because only one pup born on GD19 survived in the Low7 group, the Low7–19 group was removed from analyses due to small sample size. Since DOB occurred either on GD19 or GD20, and because earlier DOB could potentially be related to prematurity and delay of developmental milestones, we evaluated our data using DOB as one of the predictors.
3.3. Effects of predictors on physical and behavioral outcomes
3.3.1. Body weight
We analyzed pup weights on PD 3, 5, 10, 15, 20, 25, and 65 using a linear mixed model with fixed effects for PD, Tx (day of treatment; GD7, GD8), Sex (M, F), Dose (GC, Low, Mod), and DOB (GD19, GD20). We included random intercepts and slopes at the litter- and pup-level to allow random fluctuations in birth weight and growth rate. Our model indicates that PD is the most important predictor of pup weight, which is to be expected, with a roughly 0.3 g increase in weight per day for female pups and 0.4 g per day increase in weight for male pups, keeping all other variables fixed (p-value for difference in slopes <0.00001). Pups born on GD19 were, on average, lighter than pups born on GD20 by 0.45 g (p-value <0.0001); while not significant, it is also possible that GD19 pups grew at a slower rate than GD20 pups (p-value for interaction between GD19 and PD = 0.08). Pups treated on GD7 were smaller than pups treated on GD8 by 0.25 g (p = 0.002) on PD3 and this significant difference continued to PD10. The effects of dose level were not significant, nor were interactions with PD or sex.
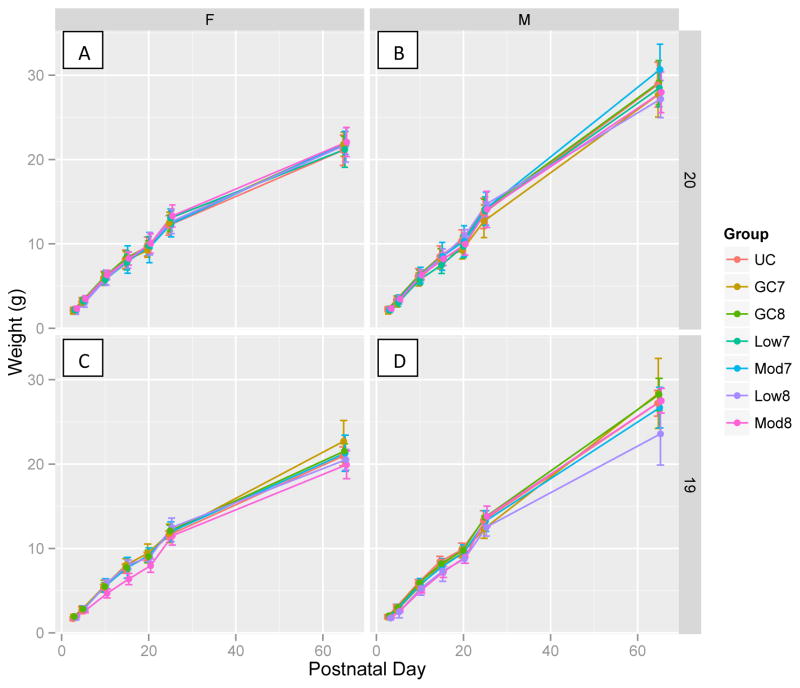
Figure 2 shows the increase in weight over PD for all groups (UC, GC7, GC8, Low7, Low8, Mod7, Mod8), separated by sex and DOB. This figure illustrates the effects found in the regression analysis, specifically that PD is the main predictor of weight and that the effect of PD differs by sex. From PD20 on, males were statistically heavier than females, i.e. on PD20 by 0.31 g (p = 0.006) due to a faster rate of growth rather than to an interaction with dose. We probed the apparent weight difference on PD65 between Low8 and GC8 males seen in Fig. 2D using a t-test for the comparison and found a significant difference (p = 0.043). However, since the sample size was small and this result is not corrected for multiple comparisons, we hesitate to over-interpret this result and suggest that future studies may explore this further.
Fig. 2. Weight gain for PD 3-65.
shown in g (± SD). Groups are split by female (A, C) and male (B, D), and birth on GD20 (A, B), and GD19 (C, D).
3.3.2. PD of reaching criterion
In Tables 2A–C, the mean PD (± SD) of reaching physical and behavioral criterion is given for each group, split by DOB. When sex was a significant predictor for outcome, as for eye opening, surface righting and thick rope balance, sex-specific data are also shown.
Table 2A. Criterion.
Postnatal day (mean ± SD) of reaching criterion for pinnae unfolding, eyes opened (females and males), pivoting and walking.
| Pinnae unfolded | Eyes opened, females | Eyes opened, males | Pivoting | Walking | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GD | 19 | 20 | 19 | 20 | 19 | 20 | 19 | 20 | 19 | 20 |
| UC | 4.0 ± 0.6 | 3.5 ± 0.5 | 13.2 ± 0.4 | 13.0 ± 0.6 | 13.1 ± 0.3 | 12.8 ± 0.6 | 6.9 ± 1.2 | 6.6 ± 1.2 | 9.1 ± 0.9 | 9.1 ± 0.8 |
| GC7 | 4.8 ± 0.4 | 4.0 ± 0.0 | 13.4 ± 0.5 | 13.0 ± 0.0 | 13.5 ± 0.5 | 13.0 ± 0.0 | 7.1 ± 0.9 | 6.9 ± 0.7 | 9.8 ± 0.4 | 9.4 ± 1.1 |
| GC8 | 4.1 ± 0.3 | 3.5 ± 0.5 | 13.9 ± 0.4 | 12.9 ± 0.4 | 13.0 ± 0.0 | 12.7 ± 0.5 | 7.0 ± 0.9 | 6.0 ± 1.2 | 9.5 ± 0.9 | 8.5 ± 0.8 |
| Low7 | N/A | 3.6 ± 0.5 | N/A | 12.9 ± 0.3 | N/A | 12.8 ± 0.5 | N/A | 7.9 ± 1.7 | N/A | 10.6 ± 1.2 |
| Mod7 | 3.9 ± 0.5 | 3.6 ± 0.5 | 13.5 ± 0.7 | 13.0 ± 0.0 | 13.0 ± 0.7 | 12.9 ± 0.7 | 7.6 ± 1.9 | 6.0 ± 1.2 | 9.9 ± 1.4 | 8.4 ± 0.9 |
| Low8 | 4.0 ± 0.0 | 3.3 ± 0.5 | 14.0 ± 0.0 | 12.8 ± 0.4 | 13.5 ± 0.7 | 12.6 ± 0.6 | 9.4 ± 2.1 | 7.4 ± 1.6 | 11.7 ± 2.0 | 9.8 ± 1.5 |
| Mod8 | 3.9 ± 0.7 | 3.3 ± 0.5 | 13.9 ± 0.4 | 12.6 ± 0.5 | 13.7 ± 0.5 | 12.5 ± 0.5 | 7.3 ± 0.6 | 6.7 ± 0.6 | 9.4 ± 0.5 | 8.8 ± 0.5 |
Table 2C. Criterion.
Postnatal day (mean ± SD) of reaching criterion for thick rope (females and males) and thin rope balance.
| Thick rope balance, females | Thick rope balance, males | Thin rope balance | ||||
|---|---|---|---|---|---|---|
| GD | 19 | 20 | 19 | 20 | 19 | 20 |
| UC | 12.2 ± 1.6 | 11.4 ± 0.9 | 12.6 ± 0.7 | 11.4 ± 0.9 | 14.2 ± 0.6 | 13.5 ± 0.8 |
| GC7 | 11.8 ± 1.3 | 11.6 ± 0.8 | 11.5 ± 1.0 | 12.1 ± 1.2 | 14.4 ± 0.6 | 14.2 ± 0.9 |
| GC8 | 12.3 ± 1.0 | 11.0 ± 0.7 | 12.4 ± 1.5 | 11.4 ± 0.7 | 14.4 ± 1.1 | 13.6 ± 1.0 |
| Low7 | N/A | 12.9 ± 0.8 | N/A | 12.2 ± 1.2 | N/A | 14.1 ± 0.7 |
| Mod7 | 12.2 ± 0.7 | 11.2 ± 0.6 | 12.4 ± 0.8 | 11.3 ± 1.0 | 14.1 ± 0.7 | 13.7 ± 0.8 |
| Low8 | 12.6 ± 1.5 | 11.6 ± 1.0 | 12.5 ± 2.1 | 11.8 ± 0.9 | 14.6 ± 1.0 | 13.7 ± 0.7 |
| Mod8 | 11.3 ± 0.8 | 11.0 ± 1.0 | 11.4 ± 1.1 | 11.8 ± 1.2 | 13.9 ± 0.8 | 13.0 ± 0.9 |
3.3.3. Maturation span
Table 3 represents the mean number of days (± SD) that each group, split by DOB, took from first consistent manifestation of the examined behavior to arrival at criterion. Of note, pinnae unfolding, eye opening, and walking are not included in the maturation span analyses because the outcomes are dichotomous.
Table 3. Maturation span.
Days to maturation of neurobehavioral outcomes (mean ± SD). Note: No significant sex differences.
| Pivoting | Cliff aversion | Surface righting | Vertical screen grasp | Thick rope balance | Thin rope balance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GD | 19 | 20 | 19 | 20 | 19 | 19 | 19 | 20 | 19 | 20 | 19 | 20 |
| UC | 4.8± 1.2 | 4.4 ± 1.2 | 2.2 ± 0.5 | 2.5 ± 0.9 | 2.5 ± 0.7 | 3.4 ± 1.4 | 3.1 ± 1.3 | 3.1 ± 1.3 | 3.4 ± 1.4 | 3.2 ± 0.9 | 2.4 ± 0.6 | 3.1 ± 1.1 |
| GC7 | 5.1 ± 0.9 | 4.9 ± 0.7 | 2.6 ± 0.8 | 2.3 ± 0.6 | 2.9 ± 1.0 | 3.2 ± 0.9 | 3.6 ± 1.4 | 3.2 ± 0.9 | 3.2 ± 0.9 | 3.6 ± 1.1 | 3.4 ± 0.8 | 3.7 ± 0.9 |
| GC8 | 5.0 ± 0.9 | 4.0 ± 1.2 | 2.8 ± 0.9 | 2.3 ± 0.5 | 3.1 ± 0.8 | 3.7 ± 0.9 | 2.6 ± 0.8 | 3.2 ± 1.2 | 3.7 ± 0.9 | 3.2 ± 0.8 | 3.2 ± 1.3 | 3.6 ± 1.1 |
| Low7 | N/A | 5.6 ± 1.8 | N/A | 2.3 ± 0.9 | N/A | N/A | N/A | 3.1 ± 1.2 | N/A | 3.6 ± 1.0 | N/A | 3.1 ± 0.9 |
| Mod7 | 4.9 ± 1.9 | 3.9 ± 1.5 | 2.5 ± 0.8 | 2.5 ± 1.1 | 3.2 ± 0.9 | 3.9 ± 0.9 | 2.8 ± 1.6 | 3.2 ± 1.1 | 3.9 ± 0.9 | 3.2 ± 0.8 | 3.3 ± 1.0 | 3.7 ± 1.0 |
| Low8 | 6.4 ± 3.2 | 5.4 ± 1.6 | 3.4 ± 0.8 | 2.8 ± 1.2 | 2.1 ± 0.4 | 3.9 ± 1.3 | 3.1 ± 1.3 | 3.0 ± 1.5 | 3.9 ± 1.3 | 3.3 ± 0.8 | 2.9 ± 0.9 | 3.1 ± 0.9 |
| Mod8 | 5.3 ± 0.6 | 4.7 ± 0.7 | 2.4 ± 0.8 | 2.3 ± 0.8 | 2.8 ± 0.7 | 3.2 ± 1.1 | 3.1 ± 1.3 | 2.9 ± 1.1 | 3.2 ± 1.1 | 3.1 ± 0.9 | 3.2 ± 1.1 | 2.9 ± 0.8 |
3.4. Statistical analyses of criterion and maturation span
3.4.1. Comparison of untreated and gavage controls
To identify whether the treatment delivery method alone is sufficient to alter neonate behavior, we compared outcomes of GC7 and GC8 pups to UC pups (Table 4). We used a linear regression framework with random effects at the litter level to account for correlations across pups, and accounted for DOB, sex and control group as covariates. The outcome of interest was the PD of reaching criterion and estimated differences between groups; p-values indicate the significance of those differences. As an example for the interpretation of the results, holding DOB and sex constant, GC7 pups were significantly (p < 0.0005) delayed by 0.62 days in pinnae unfolding relative to UC pups. Overall, we found significant delays in reaching criterion for GC7 pups relative to UC on several outcomes (pinnae unfolding, vertical screen grasp, and thin rope balance) with a suggestive delay in one (walking). GC8 pups reached criterion significantly faster than UC on several outcomes (pivot, walk, and surface righting), but were significantly delayed in one (vertical screen grasp). There were significant delays in reaching criterion for all tasks except cliff aversion and surface righting for pups born on GD19 relative to GD20. Males were only significantly faster than females for eye opening. Thus, given the potential confound of treatment delivery on a particular GD, we compared the ethanol treated groups to their respective gavage controls only. We found no significant differences in maturation span outcomes between the gavage and untreated controls, and the results are not shown.
Table 4.
Criterion outcomes, control groups, effect sizes (days and p-values). Reference group is UC-20 females (intercept).
| Pinnae unfolded | Eyes opened | Pivoting | Walking | Cliff aversion | Surface righting | Vertical screen grasp | Thick rope balance | Thin rope balance | |
|---|---|---|---|---|---|---|---|---|---|
| Intercept | 3.50 | 12.95 | 6.48 | 8.88 | 5.14 | 6.80 | 13.10 | 11.51 | 13.37 |
| Treatment: GC7 | 0.62 (<.0005) | 0.21 (0.068) | 0.23 (0.309) | 0.35 (0.054) | 0.20 (0.421) | −0.27 (0.435) | 0.79 (<.0005) | −0.05 (0.801) | 0.52 (0.002) |
| Treatment: GC8 | −0.02 0.769) | 0.01 (0.863) | −0.41 (0.033) | −0.38 (0.014) | 0.11 (0.599) | −0.99 (0.001) | 0.44 (0.007) | −0.21 (0.221) | 0.13 (0.391) |
| DOB: GD19 | 0.57 (<.0005) | 0.41 (<.0005) | 0.47 (0.010) | 0.39 (0.010) | −0.16 (0.458) | 0.08 (0.770) | 0.67 (<.0005) | 0.73 (<.0005) | 0.58 (<.0005) |
| Sex: Male | −0.10 (0.148) | −0.22 (0.002) | 0.15 (0.364) | 0.15 (0.250) | −0.08 (0.631) | −0.29 (0.268) | 0.22 (0.107) | 0.14 (0.328) | 0.09 (0.470) |
3.4.2. Criterion outcome for developmental milestones and neurobehavioral development
We assessed sources of delay in developmental milestones and sensorimotor behaviors across groups treated on the same day (i.e. GD7 or GD8). We modeled the effects of DOB, Dose, and Sex on mean PDs to reach criterion. We fit a random intercept model for all criterion outcomes. Interactions between predictors were explored but found to not be significant, and were therefore not included in the model. The resulting estimated regression coefficients and p-values for GD7- and GD8-treated groups are shown in Tables 5A and 5B, respectively.
Table 5A.
Criterion outcomes, GD7 treated groups, effect sizes (days and p-values). Reference group is GC7-20 females (intercept).
| Pinnae unfolded | Eyes opened | Pivoting | Walking | Cliff aversion | Surface Righting | Vertical screen grasp | Thick rope balance | Thin rope balance | |
|---|---|---|---|---|---|---|---|---|---|
| Intercept | 4.19 | 13.19 | 6.90 | 9.36 | 5.03 | 6.48 | 13.98 | 11.38 | 14.06 |
| DOB: GD19 | 0.49 (<.0005) | 0.34 (0.005) | 0.92 (0.003) | 1.12 (<.0005) | 0.22 (0.555) | 0.48 (0.205) | 0.37 (0.096) | 0.61 (0.003) | 0.30 (0.054) |
| Dose: Low | −0.55 (<.0005) | −0.23 (0.141) | 1.36 (<.0005) | 1.47 (<.0005) | −0.07 (0.866) | 0.64 (0.136) | −0.43 (0.095) | 0.58 (0.010) | −0.09 (0.611) |
| Dose: Mod | −0.67 (<.0005) | −0.16 (0.203) | −0.50 (0.121) | −0.54 (0.029) | 1.76 (<.0005) | 0.55 (0.158) | −0.48 (0.037) | 0.03 (0.890) | −0.38 (0.017) |
| Sex: Male | −0.18 (0.015) | −0.20 (0.030) | −0.24 (0.270) | −0.06 (0.717) | −0.06 (0.829) | −0.67 (0.024) | 0.17 (0.309) | 0.18 (0.237) | 0.16 (0.191) |
Table 5B.
Criterion outcomes, GD8 treated groups, effect sizes (days and p-values). Reference group is GC8-20 females (intercept).
| Pinnae unfolded | Eyes opened | Pivoting | Walking | Cliff aversion | Surface Righting | Vertical screen grasp | Thick rope balance | Thin rope balance | |
|---|---|---|---|---|---|---|---|---|---|
| Intercept | 3.42 | 12.85 | 6.02 | 8.45 | 5.21 | 5.76 | 13.65 | 10.99 | 13.55 |
| DOB: GD19 | 0.61 (<.0005) | 1.01 (<.0005) | 1.08 (<.0005) | 1.14 (<.0005) | 0.33 (0.372) | 0.76 (0.010) | 0.96 (<.0005) | 0.61 (0.002) | 0.90 (<.0005) |
| Dose: Low | −0.16 (0.142) | −0.01 (0.893) | 1.55 (<.0005) | 1.46 (<.0005) | 1.11 (0.004) | 1.79 (<.0005) | −0.34 (0.093) | 0.53 (0.010) | 0.12 (0.506) |
| Dose: Mod | −0.16 (0.078) | −0.15 (0.091) | 0.55 (0.015) | 0.12 (0.538) | 0.10 (0.779) | 0.68 (0.019) | −0.89 (<.0005) | −0.08 (0.667) | −0.66 (<.0005) |
| Sex: Male | 0.11 (0.203) | −0.21 (0.006) | 0.05 (0.786) | −0.01 (0.969) | −0.33 (0.249) | −0.47 (0.054) | 0.001 (0.996) | 0.36 (0.019) | −0.003 (0.983) |
As an example for the interpretation of these results, consider the PD when pinnae unfolded for GD7-treated groups. For a pup j from litter i, the fitted model for the outcome yij is:
where bi is the litter-level random intercept and eij is the pup-level residual. There were no significant interactions between predictors. The reference group is GC7-20 females, whose pinnae unfolded on PD 4.19 (intercept). Coefficients are interpreted individually by “keeping other predictors fixed”, i.e. by not varying dose or sex when examining the effect of DOB. With this interpretation, GC7 pups born on GD19 showed pinnae unfolding 0.49 days later than pups born on GD20 on average; pups exposed to low dose ethanol showed pinnae unfolding 0.55 days earlier than pups in the control group; pups exposed to moderate dose ethanol showed pinnae unfolding 0.67 days earlier than pups in the control group; and male pups showed pinnae unfolding 0.18 days earlier than female pups.
From the aggregated criterion outcomes in Table 5A for GD7-treated mice, keeping all other predictors fixed and using GC7-20 females as the reference, we see that: (1) relative to GD20, pups born on GD19 were very significantly delayed in all outcomes, except cliff aversion, surface righting and vertical screen grasp; (2) relative to GC, pups exposed to a low ethanol dose were significantly delayed in pivoting, walking, and thick rope balance, but significantly hastened in pinnae unfolding; (3) relative to GC, pups exposed to a moderate ethanol dose were significantly delayed in cliff aversion but hastened in pinnae unfolding, walking, and vertical screen grasp; and (4) relative to females, male pups were significantly hastened in pinnae unfolding, eye opening and surface righting.
From the aggregated criterion outcomes in Table 5B for GD8-treated mice, keeping all predictors fixed and using the GC8-20 females as the reference, we see that: (1) relative to GD20, pups born on GD19 were very significantly delayed in all outcomes, except cliff aversion; (2) relative to GC, pups exposed to the low ethanol dose were significantly delayed in pivoting, walking, cliff aversion, surface righting and thick rope balance; (3) relative to GC, pups exposed to the moderate ethanol dose were significantly delayed in pivoting and surface righting, and hastened in vertical screen grasp, and thin rope balance; (4) relative to females, male pups were significantly delayed in thick rope balance but hastened in eye opening and surface righting.
Overall, across GD7- and GD8-treated groups, birth on GD19 was associated with significant delays in reaching nearly all criterion outcomes (Tables 5A and B). Effects of low ethanol dose were more treatment day-specific (i.e. GD7 or GD8), with overlapping significant delays in pivoting, walking and thick rope balance, but specific with significant delays for pinnae unfolding in Low7 mice, and for cliff aversion and surface righting in Low8 mice. Effects of moderate ethanol dose were likewise treatment day-specific, with overlapping hastening of reaching criterion in vertical screen grasp, but GD-specific significant delays for cliff aversion of Mod7 mice, and for pivoting of Mod8 mice, as well as significant hastening for Mod 7 mice (pinnae unfolding, walking, thin rope balance) and for Mod8 mice (thin rope balance). Effect of gender showed males with hastened eye opening and surface righting in both GD7- and GD8-treated groups, and in pinnae unfolding in GD7-treated groups.
3.4.3. Maturation span outcomes of sensorimotor behavior
We next evaluated the time a sensorimotor behavior took to mature from the day of its first consistent manifestation until reaching criterion. As before, we analyzed outcomes within groups treated on the same day (i.e. GD7 or GD8). To assess sources of delay, we modeled the effects of DOB, Dose, and Sex on influencing maturation span. We fit a random intercept model for sensorimotor outcomes for GD7- or GD8-treated groups; the respective estimated effect sizes (in days and p-values) are shown in Tables 6A–B. Models are interpreted the same as for the criterion outcomes. For example, for the pivoting outcome in GD7-treated groups, the fitted model is:
where bi is the litter-level random intercept and eij is the pup-level residual.
Table 6A.
Maturation span outcome, GD7 treated groups, effect size (days and p-values). Reference group is GC7-20 females (intercept).
| Pivoting | Cliff aversion | Surface righting | Vertical screen grasp | Thick rope balance | Thin rope balance | |
|---|---|---|---|---|---|---|
| Intercept | 4.74 | 2.31 | 3.67 | 3.15 | 3.13 | 3.62 |
| DOB: GD19 | 0.69 (0.039) | 0.10 (0.598) | −0.08 (0.766) | −0.03 (0.945) | 0.27 (0.179) | −0.40 (0.031) |
| Dose: Low | 0.98 (0.010) | −0.01 (0.969) | −0.38 (0.200) | 0.63 (0.268) | 0.31 (0.175) | −0.59 (0.006) |
| Dose: Mod | −0.76 (0.030) | 0.10 (0.597) | −0.46 (0.089) | 0.67 (0.168) | 0.49 (0.621) | −0.83 (0.370) |
| Sex: Male | 0.17 (0.481) | 0.07 (0.612) | −0.40 (0.056) | 0.15 (0.692) | 0.13 (0.525) | 0.001 (0.995) |
Table 6b.
Maturation span outcome, GD8 treated groups, effect size (days and p-values). Reference group is GC8-20 females (intercept).
| Pivoting | Cliff aversion | Surface righting | Vertical screen grasp | Thick rope balance | Thin rope balance | |
|---|---|---|---|---|---|---|
| Intercept | 3.96 | 2.30 | 3.03 | 3.77 | 3.14 | 3.56 |
| DOB: GD19 | 0.88 (<.0005) | 0.36 (0.030) | −0.10 (0.681) | −0.02 (0.970) | 0.35 (0.049) | −0.04 (0.824) |
| Dose: Low | 1.43 (<.0005) | 0.56 (0.001) | −0.02 (0.950) | 0.46 (0.361) | 0.14 (0.449) | −0.51 (0.011) |
| Dose: Mod | 0.60 (0.013) | −0.05 (0.759) | −0.09 (0.714) | 0.11 (0.830) | −0.25 (0.147) | −0.48 (0.011) |
| Sex: Male | 0.11 (0.582) | −0.02 (0.895) | 0.06 (0.780) | −0.18 (0.603) | 0.18 (0.214) | −0.06 (0.711) |
From the aggregated maturation span outcomes in Table 6A for GD7-treated mice, keeping all predictors fixed and using GC7-20 females as the reference, we see that: (1) relative to GD20, pups born on GD19 took significantly longer to mature pivoting, but took significantly less time to mature thin rope balance; (2) relative to GC7, pups exposed to the low ethanol dose took significantly longer to mature pivoting, but significantly less time to mature thin rope balance; (3) relative to GC7, pups exposed to the moderate ethanol dose took significantly less time to mature pivoting; and (4) gender differences were nonsignificant for all tests, except that surface righting approached significance for the faster males.
From the aggregated maturation span outcomes in Table 6B for GD8-treated mice, keeping all predictors fixed and using GC8-20 females as the reference, we see that: (1) relative to GD20, pups born on GD19 took significantly longer to mature pivoting, cliff aversion, and thick rope balance; (2) relative to GC8, pups exposed to the low ethanol dose took significantly longer to mature pivoting and cliff aversion, but significantly less time to mature thin rope balance; (3) relative to GC8, pups exposed to the moderate ethanol dose took significantly longer to mature pivoting but took significantly less time to mature thin rope balance; and (4) gender differences were nonsignificant.
Overall, across GD7- and GD8-treated groups, birth on GD19 was associated with delayed maturation of pivoting only, but with delayed cliff aversion for GD8-treated pups and hastened thin rope balance for GD7-treated pups. Low ethanol dose was associated with overlapping delayed maturation of pivoting and hastened maturation of thin rope balance. Moderate ethanol dose was associated with a specific delay of pivoting maturation for Mod8 mice and hastening of pivoting for Mod7 mice, as well as hastened thin rope maturation for Mod8 mice.
4. Discussion
This behavioral study was based on prenatal mouse models for human binge drinking, which investigated the histological effects on specific brain structures from heavy IP ethanol exposure. These studies focused on the earliest vulnerable processes in brain development: gastrulation (GD7; Sulik et al., 1981; 1984; Schambra et al., 1990) and neurulation (GD8; Sulik et al., 1986; Kotch and Sulik, 1992b; Dunty et al., 2001; 2002) and defined the time, pattern, and dose of ethanol treatment to mimic heavy human drinking of 3–5 drinks in one session on 2 consecutive days. In this study, we aimed to model a common pattern of alcohol consumption, namely social weekend drinking, characterized by 1 or 2 drinks on an empty stomach on 2 consecutive days. As expected, the gavage treatment resulted in lower BECs than IP treatment. This approach allowed us to assess the physical and neurobehavioral characteristics of mice acutely exposed on GD7 or on GD8 to a low or moderate dose of ethanol. Our main findings were: (1) treatment during gastrulation or neurulation had several overlapping and some treatment day-specific effects on neurobehavioral maturation, but no effects on physical maturation; (2) low dose more frequently affected neurobehavioral development than moderate dose ethanol; and (3) animals born prematurely were developmentally delayed.
BECs and dose nomenclature
We characterized the differences in BECs resulting from IP and gavage treatment, and found BECs for IP administration were greater than those for an equal gavage dose. Differences in BECs can be explained by differences in ethanol absorption and biotransformation with the different routes of administration, i.e., ethanol is directly absorbed into systemic circulation when given IP, while it is first metabolized in the stomach and liver when given by gavage, resulting in a lower BEC.
For many prenatal rodent studies, BECs vary widely. Using a similar IP treatment paradigm for a 2.9 g/kg dose, others have observed peak BECs of 215 mg/dl (Sulik et al., 1981), 500 mg/dl (Webster et al., 1983), and 440 mg/dl (Godin et al., 2010). No BEC comparison is available for the lower dose of 2.4 g/kg given by IP or gavage. Because of these discrepancies, we performed our own precise and accurate gas chromatography analysis with appropriate quality controls, and found that our IP dose of 2.9 g/kg resulted in a peak BEC of 228 mg/dl, comparable to the lower BECs previously reported. It is conceivable that higher BECs may have been found in other studies due to differences in BEC analysis technique.
BECs can be influenced by rate of ethanol administration (bolus, split doses, or 24 h access to ethanol in a liquid diet or drinking water), route of administration (IP, gavage, drinking), and context (on empty or full stomach). Given the myriad factors affecting BEC, it is most appropriate to use peak BECs rather than total ethanol dose (g/kg/day) to delineate ethanol effects on offspring survival, development, and behavior. One glass wine drunk within one hour by a 130-pound person results in a BEC of 19 mg/dl, and 2 glasses of wine drunk within 2 h in a BEC of 39 mg/dl (http://my.clevelandclinic.org/health/tools-quizzes/Blood_Alcohol_Calculator). In a comparison of ethanol-induced toxicity in the developing neocortex across several species, similar deficits in neocortical neurogenesis were observed in humans and monkeys after daily peak BECs 20mg/dl and in rodents after daily peak BECs of 100 mg/dl (Gohlke et al., 2008). This indicates that primates are 5 times more susceptible than rodents to ethanol-induced cell loss during neocortical neurogenesis. Thus, a rodent BEC of ~100 mg/dl and a human BEC of ~20 mg/dl may result in comparable sequelae; a rodent BEC of ~170 mg/dl and a human BEC of ~39 mg/dl (arising from ~ 2 drinks) may likewise result in comparable ill effects. We therefore reference IP doses of 2.9 and 2.4 g/kg (with peak BECs over 200 mg/dl) as high ethanol doses, the gavage dose of 2.9 g/kg (with peak BEC 177 mg/dl) as a moderate dose, and the gavage dose of 2.4 g/kg (peak BEC of 104 mg/dl) as a low dose.
Effect of gavage treatment on development
In comparing the controls, we observed both significant delays (GC7 and GC8) and hastening (GC8) of time to reach criterion by gavage controls relative to untreated controls (Table 4); maturation spans were not significantly different across groups. The observation of significant delays in several criterion outcomes raises the possibility that maternal stress by gavage treatment on GD7 or GD8 may alone influence neonatal neurobehavioral development. In rats, repeated gavage dosing with lipid vehicles induced a long-lasting activation of the stress response (increased adrenal output of corticosterone) in a volume-dependent fashion; the authors suggest that dose volumes should not exceed 0.010 ml/g (Brown et al., 2000). Although a dosing volume of 0.015 ml/g BW is standard for IP treatment of mice, it is conceivable that this volume is too large for gavage treatment of heavier mice and could cause a prolonged stress response. While most animal studies of long-term behavioral consequences of prenatal stress are based on extended stress during late gestation (see review by Weinstock, 2008), pregnant rhesus monkeys in one study were exposed to daily stress during early gestation, and their offspring presented with reduced neuromotor functioning (Schneider et al., 2002). In GC8 animals, some criterion outcomes were reached more quickly than in UC mice. Although there is no clear physiologic explanation for this, it is possible that the liquid rat diet used as vehicle had a mixed beneficial effect during neurulation. To avoid these issues in the future, behavioral studies of acute and low dose ethanol treatment may consider using a saline vehicle and a dosing volume of 0.01 ml/kg BW. Given the potential confound of treatment delivery method on neurobehavioral outcomes, we thus compared the ethanol treated groups to their respective gavage controls.
The effect of ethanol dose on postnatal outcome
The majority of human and animal behavioral studies focus on chronic moderate-to-high dose ethanol exposure during part or all of gestation, but a minority of rodent studies are based on acute ethanol administration (see reviews by Driscoll et al., 1990; Becker et al., 1996; Eckardt et al., 1998; Mattson and Riley, 1998; Guerri et al., 2009; Mattson et al., 2011; Schneider et al., 2011; Valenzuela et al., 2012). Three of the acute studies are relevant to the present investigation (Vigliecca et al., 1986; Molina et al., 1987; Enders et al., 2005). These studies followed the ethanol dosing and timing of treatment during gastrulation or neurulation used in earlier teratological studies (Sulik et al., 1981; Webster et al., 1983). We used a similar protocol as Sulik and colleagues, but treated by gavage instead of IP and added a lower ethanol dose group (2.4 g/kg). Both our gavage doses resulted in lower BECs than observed with IP. The higher BECs of the prenatal studies resulted in a spectrum of effects on fetuses; the ones with the most severe malformations were chosen to typify defects specific to ethanol treatment during gastrulation or neurulation (Webster et al., 1983; Sulik et al., 2005). These severely affected offspring are generally not viable, and thus are not available for behavioral studies. Acute high dose ethanol treatment in rodents during gastrulation results in significantly increased perinatal pup mortality (Webster et al., 1983; Cook et al., 1987; Molina et al., 1987; Dumas and Rabe, 1994) and during neurulation, increased prenatal mortality (Webster et al., 1983; Kotch and Sulik, 1986a; Enders et al., 2005).
Viability is possible with lower acute ethanol doses, where a dose-dependent reduction of hemispheric and cerebellar length, width and weight; a decrease of body weight on PD1; and a dose-dependent increase in abnormal hyperactivity have been reported (Molina et al., 1984). Thus, we anticipated that a lower acute ethanol dose would result in milder deficits compatible with life, and that pups could be assessed with neurobehavioral testing. To our knowledge, our study is the first to acutely treat mice with a low ethanol dose and determine several milestones of neurobehavioral development.
However, we did not find a similar dose-related increase of neurobehavioral and physical abnormalities with increasing doses (Molina, 1984). We found more significant neurobehavioral delays in our low dose groups than in our moderate dose groups. Although these results may appear counterintuitive, it has been observed in prenatal studies that ethanol effects are heterogeneous; that is, within a litter, one pup may be severely affected while others are grossly unscathed (Webster et al., 1983; Sulik et al., 1984; Schambra et al., 1990; Godin et al., 2010). The mechanism for this phenomenon may be related to slightly different developmental stages of the embryos at the time of ethanol exposure (Dunty et al., 2001). The critical dose threshold, above which an animal will die rather than survive with testable deficits, has not been determined. It is conceivable that the moderate ethanol dose was above this threshold, resulting in a greater loss of the most affected offspring and a disproportionately larger number of surviving pups with minimal physical and neurobehavioral deficits. While there was an increase in pups dead within the first week in the mice treated on GD7, there was no apparent increase in neonatal deaths in the moderate ethanol groups treated on GD8 (Table 1). It is possible that intrauterine loss and resorptions may have underestimated the death rate for this group, but we cannot definitely conclude this because we did not assess dams for resorptions.
Interestingly, we also observed that for some outcomes, the moderate dose groups were expedited in reaching criterion relative to their GCs (for GD7 treatment: pinnae unfolding, walking, vertical screen grasp, thin rope balance; for GD8 treatment: pinnae unfolding, vertical screen grasp). These findings require consideration of two alternative explanations. First, ethanol could directly improve neurobehavioral outcomes. To date, no studies have reported beneficial behavioral effects of prenatal ethanol treatment, making this explanation unlikely. Second, ethanol could be protective against possible stress effects of gavage administration, as discussed above. Thus, moderate dose groups may appear expedited relative to the delayed gavage controls. In these cases, apparent accelerations in development should be interpreted with caution.
Effect of gender
Gender differences played a minor role in outcomes and are shown when they occurred. We observed delays for females in eye opening, pinnae unfolding, and surface righting. These findings are largely consistent with previous studies. While significantly increased hyperactive behaviors and deficits in spatial learning have been noted in female rats exposed to ethanol during gastrulation, no significant gender differences in any measures of sensorimotor maturation have been previously documented (Molina et al., 1984; 1987; Minetti et al., 1996).
Effect of day of birth on physical and neurobehavioral development
Relative to mice born on GD20, mice born on GD19 were significantly delayed in reaching criterion for most of the physical and neurobehavioral tests. Across controls, delays in premature pups ranged from 0.38 days to 0.73 days relative to GD20 pups, supporting the decision to use DOB as a factor in the statistical analyses. Similar delays were found in premature GD7 animals (in pivoting, walking, and rope balance), and GD8 animals (in eye opening, surface righting, and vertical screen grasp). Overall we conclude that prematurity leads to delay.
In human studies, late or moderate preterm infants (born at 32–36 weeks) represent >80% of premature births. These children experience increased mortality and morbidity when compared to full-term born infants (Gouyon et al., 2012), and are at risk for school problems, lower cognitive functioning, behavior problems, and psychiatric disorders (de Jong et al., 2012). Premature infants show significantly lower scores in neurobehavioral and development tests relative to term infants when tested at term, 3 and 6 months of corrected age (Wolf et al., 2002), and corrected ages of 18–24 months (Guerra et al., 2014).
Alcohol is associated with both mild prematurity (< 37 weeks) and extreme prematurity (< 32 weeks), the latter accounting for the greatest proportion of perinatal morbidity and long-term disability (Sokol et al.; 2007). Jaddoe et al. (2007) reported a dose-response related tendency between drinking one or more drinks during early pregnancy and low birth weight, small size for gestational age, and preterm birth. Moderate (2–4 glasses/week) and higher levels of alcohol consumption during the first trimester were associated with preterm birth (O’Leary et al., 2009; Meyer-Leu et al., 2011). In our sample, we did not detect a clear propensity for prematurity associated with ethanol; for example, we saw relative to GC7, both a significantly higher percentage of premature births for Mod7 pups, but a significantly lower percentage of premature birth for Low7 pups.
The effect of ethanol timing on neurobehavioral development
We found that, relative to their gavage controls, ethanol treatment on both GD7 and GD8 resulted in delays of reaching criterion for pivoting, walking, cliff aversion and thick rope balance. Surface righting was delayed for treatment on GD8 only. Our results confirm some of the findings of two studies investigating the effects of specific timing of prenatal high dose ethanol IP treatment of rats during gastrulation (Vigliecca et al., 1986; Molina et al., 1987). Similar to us, they found treated animals were significantly delayed in cliff aversion, but only when compared to untreated controls and not to vehicle controls, as we found. They also found no delays in eye opening or the vertical screen test. Unlike us, their treated animals showed delays in surface righting, and had significantly lower body weights on PD1. We found significantly lower weights from PD3 to PD10 of mice treated on GD7 compared to those treated on GD8. The investigators also found significant delays in the horizontal screen test and opening of external auditory canals, which we did not test.
One study treated mice on GD8 and, consistent with our results, found a significant delay in cliff aversion and no delay in vertical screen grasp (Enders et al., 2005). Unlike our results, they found delayed eye opening but no significant delay in surface righting. None of these studies tested for pivoting, walking or rope balance, three outcomes that were significantly delayed in our GD7 and GD8 exposed mice relative to their gavage controls.
Some of the discrepancies noted in the comparisons above may be due to inter-species differences in sensorimotor development, which occur earlier in mice than rats, thus possibly shifting the time of greatest vulnerability (Allam and Abo-Eleneen, 2012); differences in treatment time during neurulation (ours was on GD8:6h and GD8:10h, Enders et al. treated at an unspecified time on GD8); and higher BECs due to higher ethanol dose and intraperitoneal delivery. Overall, these findings collectively confirm that ethanol treatment during the earliest stages of brain development results in delayed sensorimotor and physical development.
Possible neuroanatomical loci associated with delays
The purpose of the present study was to expand on the aforementioned behavioral studies by adding a lower ethanol dose. We found widespread delays in alcohol treated animals in multiple neurobehavioral outcomes. Because arrival at criterion performance for each test requires sufficiently functioning motor and sensory systems, it is impossible to infer from abnormal behavior if one or both were affected by treatment. Several prenatal histological studies using IP ethanol administration point to brain structures vulnerable to timing-specific ethanol exposure, which may underlie the abnormal development we observed. These studies document motor system-specific neuronal loss after ethanol treatment during gastrulation in the septal area, striatum (caudate/putamen), and frontal cortex (Sulik et al., 1984; Schambra et al., 1990; Ashwell and Zhang, 1996; Godin et al., 2010). Hypoplasia in these structures after treatment of mice on GD7 is related to increased programmed cell death in the embryonic ectoderm, specifically the anterior neural ridge, from which cortical and striatal progenitors arise (Dunty et al., 2001; Sulik, 2005; Kilburn et al., 2006). Additionally, ethanol treatment during gastrulation results in decreased numbers of neurons of trigeminal sensory nuclei in rats (Mooney and Miller, 2007), and in lower numbers of neurons in the trigeminal sensory nuclei and somatosensory cortex in macaques (Miller 2007).
In contrast, ethanol exposure of mice during neurulation causes excessive cell death in the developing trigeminal ganglion, in the apical portion of the alar plate of the hindbrain from which progenitors for the vestibular nuclei and cerebellum arise, and at a later developmental stage in a hypoplastic cerebellum (Kotch and Sulik, 1992b; Dunty et al., 2001; 2002; Parnell et al., 2009).
To our knowledge, no histological studies have investigated the effects of low dose acute ethanol exposure on the developing motor or sensory systems. Studies are needed to correlate behavioral changes with histological abnormalities in the same animal. Human studies also point to neuroanatomical abnormalities and associated behavioral delays in ethanol-exposed children. Various degrees of gross and fine motor dysfunction and delays have been seen at different developmental stages of ethanol exposed children (see reviews by Mattson and Riley, 1998; Riley and Mc Gee, 2005; Connor et al., 2006; Bay and Kesmodel, 2011; Mattson et al., 2011). Children with FAS were significantly delayed in their motor development and had significantly lower fine motor scores relative to unexposed controls (Kalberg et al., 2006). FAS children were also found to have significant delays in response programming and movement times (Simmons et al., 2010). Alcohol exposed children without FAS features, considered to be within fetal alcohol spectrum disorder (FASD), are also delayed in their motor development compared to unexposed children, but less affected than FAS children. Specific neuroanatomical insults in the motor system have been found in both children with FAS and FASD. When regional patterns of brain hypoplasia were studied with structural MRI in juveniles with FAS and FASD, significant microcephaly and volume reductions in the caudate nucleus, parietal cortex, and cerebral white matter were demonstrated in the FAS cohort, and volume reductions of basal ganglia and parietal lobe approached significance in the FASD children relative to nonexposed controls (Archibald et al., 2001). Additionally, children with FASD have sensory processing deficits that correlated with problem behaviors such as being anxious/depressed, having somatic complaints, social and attention problems, and aggressive behaviors (Franklin et al., 2008). Although these human studies point to neuroanatomical insults that could underpin abnormal behavior, they are confounded by unclear dosing and timing of ethanol exposure. It is our hope that future histopathological-behavioral correlations in humans and/or animal models, controlling for timing and dose of ethanol exposure, may better illustrate the temporal vulnerability of specific neuroanatomical areas and behavioral ramifications.
Maturation span
Criterion outcome provided information on the mean time a group needs to reach the set goal from the first day of recording a particular test. However, we anecdotally noticed that some groups seemed to start with a delay but then reached criterion at an accelerated or a slower rate. Thus, we used the latency data to analyze the maturation span of sensorimotor behavior development--the number of days a sensorimotor behavior took to mature, from first consistent manifestation to arrival at criterion. In this analysis, we were interested in determining whether delayed pups “caught up” once sufficient neural resources were in place to develop the behavior. We found as many cases of delayed animals with delayed maturation (particularly for pivoting), as there were of delayed animals with accelerated maturation (particularly for thin rope). In general, we did not find a clear picture of accelerated maturation after a delayed start, resulting in a normal criterion outcome time.
Clinical meaning of delays
This study found significant delays in several tests, but are these delays clinically meaningful? Because this study, to our best knowledge, is the first study to document these norms, we extrapolated to human time scale. The Jackson Laboratory gives a maturation rate for mice during their first month as 150 times faster than humans (Flurkey et al., 2007). As an example, Low7 and Low8 mice were delayed by about 1.5 days relative to their respective controls in walking outcome. Since the median age for human walking is 12.1 months, and failure to walk by 18 months is a sign of developmental delay (WHO Multicentre Growth Reference Study Group, 2006), the delay of 1.5 days observed in treated mice would translate to 225 human days, or a median delayed walking onset of around 19.6 months. Premature birth in the mice treated on GD7 or GD8 resulted in a delay of 1.1 day, which would translate to a delay of walking onset of 5.5 months, at 17.6 months. Thus, even apparent minor delays in mice would translate to weeks-months differences on the human time scale. Despite these abnormalities, we cannot assume that neonatal mice expressing developmental delays will go on to show long-lasting abnormalities into adulthood. Future studies will need to evaluate the long-term behavioral implications of developmental delays in similarly exposed mice.
Table 2B. Criterion.
Postnatal day (mean ± SD) of reaching criterion for cliff aversion, surface righting (females and males), and vertical screen grasp
| Cliff aversion | Surface righting, females | Surface righting, males | Vertical screen grasp | |||||
|---|---|---|---|---|---|---|---|---|
| GD | 19 | 20 | 19 | 20 | 19 | 20 | 19 | 20 |
| UC | 4.6 ± 1.0 | 5.0 ± 1.3 | 7.2 ± 2.1 | 6.5 ± 1.9 | 5.8 ± 1.5 | 6.8 ± 2.0 | 13.6 ± 1.0 | 13.4 ± 0.9 |
| GC7 | 5.5 ± 1.4 | 4.8 ± 1.0 | 6.6 ± 1.3 | 6.8 ± 2.1 | 6.3 ± 1.6 | 6.1 ± 1.7 | 14.9 ± 0.6 | 13.9 ± 0.9 |
| GC8 | 4.8 ± 0.9 | 5.0 ± 1.3 | 6.1 ± 1.8 | 5.7 ± 1.5 | 6.2 ± 1.3 | 5.4 ± 0.9 | 14.8 ± 0.7 | 13.5 ± 1.0 |
| Low7 | N/A | 4.9 ± 1.6 | N/A | 7.2 ± 1.8 | N/A | 6.7 ± 2.0 | N/A | 13.7 ± 1.2 |
| Mod7 | 6.9 ± 2.4 | 7.0 ± 1.7 | 7.7 ± 2.2 | 7.3 ± 1.1 | 7.1 ± 2.0 | 6.1 ± 1.6 | 13.9 ± 1.3 | 13.9 ±0.7 |
| Low8 | 6.3 ± 0.5 | 6.1 ± 3.0 | 9.2 ± 2.9 | 8.1 ± 1.7 | 6.5 ± 0.7 | 6.4 ± 1.3 | 14.3 ± 1.1 | 13.3 ± 1.3 |
| Mod8 | 5.6 ± 2.3 | 4.8 ± 1.2 | 6.3 ± 1.3 | 6.2 ± 1.5 | 7.4 ± 1.6 | 6.1 ± 1.3 | 13.3 ± 0.9 | 12.9 ± 0.7 |
Highlights.
Acute low dose ethanol exposure during gastrulation or neurulation results in significant neurobehavioral delays
Acute moderate dose ethanol exposure results in increased perinatal death and few neurobehavioral delays in the surviving pups.
Premature birth results in significantly neurobehavioral delays
Acknowledgments
The first author thanks with great appreciation Dr. Michael Woodruff for his help in the design of this study. Heartfelt thanks also to Dr. Ken Ferslew for the BEC analysis, Dr. Sheryl Moy for preliminary statistical analyses, Dr. Edith Seier, for statistical advice, and Dr. Jean Lauder for critical reading of the manuscript. This work was supported by a Research and Development Grant, the former Department of Anatomy and Cell Biology, and the Department of Biomedical Sciences, East Tennessee State University.
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allam AA, Abo-Eleneen RE. The development of sensorimotor reflexes in albino mice; albino rats and black-hooded rats. Int J Devl Neurosci. 2012;30:545–53. doi: 10.1016/j.ijdevneu.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Altman J, Sudarshan K. Postnatal development of locomotion in the laboratory rat. Anim Behav. 1975;23:896–920. doi: 10.1016/0003-3472(75)90114-1. [DOI] [PubMed] [Google Scholar]
- Aliyu MH, Wilson RE, Zoorob R, Chakrabarty S, Alio AP, Kirby RS, et al. Alcohol consumption during pregnancy and the risk of early stillbirth among singletons. Alcohol. 2008;42:369–374. doi: 10.1016/j.alcohol.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Andersen AM, Andersen PK, Olsen J, Grønbæk M, Strandberg-Larsen K. Moderate alcohol intake during pregnancy and risk of fetal death. Int J Epidemiol. 2012;41:405–13. doi: 10.1093/ije/dyr189. [DOI] [PubMed] [Google Scholar]
- Archibald SL, Fennema-Notestine C, Gamst A, Riley EP, Mattson SN, Jernigan TL. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Developmental Medicine and Child Neurology. 2001;43:148–154. [PubMed] [Google Scholar]
- Ashwell KWS, Zhang L. Forebrain hypoplasia following acute prenatal ethanol exposure: Quantitative analysis of effects on specific forebrain nuclei. Pathology. 1996;28:161–6. doi: 10.1080/00313029600169803. [DOI] [PubMed] [Google Scholar]
- Bay B, Kesmodel US. Prenatal alcohol exposure – a systematic review of the effects on child motor function. Acta Obstet Gynecol Scand. 2011;90:210–26. doi: 10.1111/j.1600-0412.2010.01039.x. [DOI] [PubMed] [Google Scholar]
- Becker HC, Diaz-Granados JL, Randall CL. Teratogenic actions of ethanol in the mouse: A minireview. Pharmacol Biochem Behav. 1996;55:501–13. doi: 10.1016/s0091-3057(96)00255-9. [DOI] [PubMed] [Google Scholar]
- Brown AP, Dinger N, Levine BS. Stress produced by gavage administration in the rat. Contemporary Topics. 2000;39:17–21. [PubMed] [Google Scholar]
- Chiodo LM, Bailey BA, Sokol RJ, Janisse J, Delaney-Black V, Hannigan JH. Recognized spontaneous abortions in mid-pregnancy and patterns of pregnancy alcohol use. Alcohol. 2012;46:261–7. doi: 10.1016/j.alcohol.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor PD, Sampson PD, Streissguth AP, Bookstein FL, Barr HM. Effects of prenatal alcohol exposure on fine motor coordination and balance: A study of two adult samples. Neuropsychologia. 2006;44:744–51. doi: 10.1016/j.neuropsychologia.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Cook CS, Nowotny AZ, Sulik KK. Fetal alcohol syndrome. Eye malformations in a mouse model. Arch Phthalmol. 1987;105:1791–8. doi: 10.1001/archopht.1987.01060110122045. [DOI] [PubMed] [Google Scholar]
- Coulter CL, Leech RW, Schaefer GB, Scheithauer BW, Brumback RA. Midline cerebral dysgenesis, dysfunction of the hypothalamic-pituitary axis, and fetal alcohol effects. Arch Neurol. 1993;50:771–5. doi: 10.1001/archneur.1993.00540070083022. [DOI] [PubMed] [Google Scholar]
- de Jong M, Verhoeven M, van Baar A. School outcome, cognitive functioning, and behaviour problems in moderate and late preterm children and adults: a review. Seminars in Fetal & Neonatal Medicine. 2012;17:163–9. doi: 10.1016/j.siny.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Driscoll CD, Streissguth AP, Riley EP. Prenatal alcohol exposure: comparability of effects in humans and animal models. Neurotox Teratol. 1990;12:231–7. doi: 10.1016/0892-0362(90)90094-s. [DOI] [PubMed] [Google Scholar]
- Dumas RM, Rabe A. Augmented memory loss in aging mice after one embryonic exposure to alcohol. Neurotox Teratol. 1994;16:605–12. doi: 10.1016/0892-0362(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Dunty WC, Jr, Chen S, Zucker RM, Dehart DB, Sulik KK. Selective vulnerability of embryonic cell populations to ethanol-induced apoptosis: Implications for alcohol-related birth defects and neurodevelopmental disorder. Alcohol Clin Exp Res. 2001;25:1523–35. [PubMed] [Google Scholar]
- Dunty WC, Jr, Zucker RM, Sulik KK. Hindbrain and cranial nerve dysmorphogenesis result from acute maternal ethanol administration. Dev Neurosci. 2002;24:328–42. doi: 10.1159/000066748. [DOI] [PubMed] [Google Scholar]
- Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, Hoffman PL, et al. Effects of moderate alcohol consumption on the central nervous system. Alcohol Clin Exp Res. 1998;22:998–1040. doi: 10.1111/j.1530-0277.1998.tb03695.x. [DOI] [PubMed] [Google Scholar]
- Endres M, Toso L, Roberson R, Park J, Abebe D, Poggi S, Spong CY. Prevention of alcohol-induced developmental delays and learning abnormalities in a model of fetal alcohol syndrome. Am J Obstetr Gynecol. 2005;193:1028–34. doi: 10.1016/j.ajog.2005.05.052. [DOI] [PubMed] [Google Scholar]
- Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception. 2011;84:478–85. doi: 10.1016/j.contraception.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd RL, Sidhu JS. Monitoring prenatal alcohol exposure. Am J Med Genet C (Semin Med Genet) 2004;127C:3–9. doi: 10.1002/ajmg.c.30010. [DOI] [PubMed] [Google Scholar]
- Flurkey K, Currer JM, Harrison DE. The Mouse in Aging Research. In: Fox JG, et al., editors. The Mouse in Biomedical Research. 2. American College Laboratory Animal Medicine (Elsevier); Burlington, MA: 2007. pp. 637–672. [Google Scholar]
- Fox WM. Reflex-ontogeny and behavioural development of the mouse. Animal Behav. 1965;13:234–241. doi: 10.1016/0003-3472(65)90041-2. [DOI] [PubMed] [Google Scholar]
- Franklin L, Deitz J, Jirikowic T, Astley S. Children with fetal alcohol spectrum disorders: Problem behaviors and sensory processing. Am J Occup Ther. 2008;62:265–73. doi: 10.5014/ajot.62.3.265. [DOI] [PubMed] [Google Scholar]
- Godin EA, O’Leary-Moore SK, Khan AA, Parnell SE, Amet JJ, Dehart DB, et al. Magnetic resonance microscopy defines ethanol-induced brain abnormalities in prenatal mice: Effects of acute insult on gestational day 7. Alcohol Clin Exp Res. 2010;34:98–111. doi: 10.1111/j.1530-0277.2009.01071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohlke JM, Griffith WC, Faustman EM. Computational models of ethanol-induced neurodevelopmental toxicity across species: Implications for risk assessment. Birth Defects Res B Dev Reprod Toxicol. 2008;83:1–11. doi: 10.1002/bdrb.20137. [DOI] [PubMed] [Google Scholar]
- Gouyon J-B, Iacobelli S, Ferdynus C, Bonsante F. Neonatal problems of late and moderate preterm infants. Sminars in Fetal & Neonatal Medicine. 2012;17:146–52. doi: 10.1016/j.siny.2012.01.015. [DOI] [PubMed] [Google Scholar]
- Guerra CC, de Moraes Barros MC, Goulart AL, Fernandes LV, Kopelman BI. Premature infants with birth weights of 1500–1999 g exhibit considerable delays in several developmental areas. Acta Paediatrica. 2014;103:e1–e6. doi: 10.1111/apa.12430. [DOI] [PubMed] [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, et al. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: Clarification of the 1996 Institute of Medicine criteria. Pediatrics. 2005;115:39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaddoe VWV, Bakker R, Hofman A, Mackenbach JP, Moll HA, Steegers EAP, et al. Moderate alcohol consumption during pregnancy and the risk of low birth weight and preterm birth. The Generaton R Study. Ann Epidemiol. 2007;17:834–40. doi: 10.1016/j.annepidem.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW, Ulleland CN, Streissguth P. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet. 1973 Jun 9;1(7815):1267–71. doi: 10.1016/s0140-6736(73)91291-9. [DOI] [PubMed] [Google Scholar]
- Kalberg WO, Provost B, Tollison SJ, Tabachnik BG, Robinson LK, Hoyme HE, Trulillo PM, Buckley D, Aragon AS, May PA. Comparison of motor delays in young children with fetal alcohol syndrome to those with prenatal alcohol exposure and with no prenatal alcohol exposure. Alcohol Clin Exp Res. 2006;30:2037–45. doi: 10.1111/j.1530-0277.2006.00250.x. [DOI] [PubMed] [Google Scholar]
- Kilburn BA, Chiang PJ, Wang J, Flentke GR, Smith SM, Armant DR. Rapid induction of apoptosis in gastrulating mouse embryos by ethanol and its prevention by HB-EGF. Alcohol Clin Exp Res. 2006;30:127–34. doi: 10.1111/j.1530-0277.2006.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotch LE, Sulik KK. Experimental fetal alcohol syndrome: proposed pathogenic basis for a variety of associated facial and brain anomalies. Am J Med Genet. 1992a;44:168–76. doi: 10.1002/ajmg.1320440210. [DOI] [PubMed] [Google Scholar]
- Kotch LE, Sulik KK. Patterns of ethanol-induced cell death in the developing nervous system of mice; neural fold states through the time of anterior neural tube closure. Int J Devl Neurosci. 1992b;10:273–9. doi: 10.1016/0736-5748(92)90016-s. [DOI] [PubMed] [Google Scholar]
- Lupton C, Burd L, Harwood R. Cost of fetal alcohol spectrum disorders. Am J Med Genet Part C (Semin Med Genet) 2004;127C:42–50. doi: 10.1002/ajmg.c.30015. [DOI] [PubMed] [Google Scholar]
- Manno BR, Manno JE. A simple approach to gas chromatographic microanalysis of alcohols in blood and urine by direct-injection technique. J Analyt Tox. 1978;2:257–61. [Google Scholar]
- Mattson SN, Riley EP. A review of neurobehavioral deficits in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcohol Clin Exp Res. 1998;22:279–94. doi: 10.1111/j.1530-0277.1998.tb03651.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Crocker N, Nguyen TT. Fetal alcohol spectrum disorders: neuropsychological and behavioral features. Neuropsychol Rev. 2011;21(2):81–101. doi: 10.1007/s11065-011-9167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, et al. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Disabil Res Rev. 2009;15:176–92. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- Meyer-Leu Y, Lemola S, Daeppen J-B, Deriaz O, Gerber S. Association of moderate alcohol use and binge drinking during pregnancy with neonatal health. Alcohol Clin Exp Res. 2011;35:1669–77. doi: 10.1111/j.1530-0277.2011.01513.x. [DOI] [PubMed] [Google Scholar]
- Miller MW. Exposure to ethanol during gastrulation alters somatosensory-motor cortices and the underlying white matter in the macaque. Cerebral Cortex. 2007;17:2961–71. doi: 10.1093/cercor/bhm024. [DOI] [PubMed] [Google Scholar]
- Minetti A, Arolfo MP, Virgolini MB, Brioni JD, Fulginiti S. Spatial learning in rats exposed to acute ethanol intoxication on gestational day 8. Pharmacol Biochem Behav. 1996;53:361–7. doi: 10.1016/0091-3057(95)02035-7. [DOI] [PubMed] [Google Scholar]
- Molina JC, Moyano HF, Spear LP, Spear NE. Acute alcohol exposure during gestational day 8 in the rat: Effects upon physical and behavioral parameters. Alcohol. 1984;1:459–64. doi: 10.1016/0741-8329(84)90022-3. [DOI] [PubMed] [Google Scholar]
- Molina JC, Hoffmann H, Spear LP, Spear NE. Sensorimotor maturation and alcohol responsiveness in rats prenatally exposed to alcohol during gestational day 8. Neurotox Teratol. 1987;9:121–8. doi: 10.1016/0892-0362(87)90088-2. [DOI] [PubMed] [Google Scholar]
- Mooney SM, Miller MW. Time-specific effects of ethanol exposure on cranial nerve nuclei: Gastrulation and neurogenesis. Exp Neurol. 2007;205:56–63. doi: 10.1016/j.expneurol.2007.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary CM, Nassar N, Kurinczuk JJ, Bower C. The effect of maternal alcohol consumption on fetal growth and preterm birth. BJOG. 2009;116:390–400. doi: 10.1111/j.1471-0528.2008.02058.x. [DOI] [PubMed] [Google Scholar]
- Parnell SE, O’Leary-Moore SK, Godin EA, Dehart DB, Johnson BW, Johnson GA, et al. Magnetic resonance microscopy defines ethanol-induced brain abnormalities in prenatal mice: Effects of acute insult on gestational day 8. Alcohol Clin Exp Res. 2009;33:1001–11. doi: 10.1111/j.1530-0277.2009.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. Foundation for Statistical Computing; Vienna, Austria: 2013. [Google Scholar]
- Riley EP, McGee CL. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med. 2005;230:357–65. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- Riley EP, Infante MA, Warren KR. Fetal alcohol spectrum disorders: an overview. Neuropsychol Rev. 2011;21:73–80. doi: 10.1007/s11065-011-9166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schambra UB, Lauder JM, Petrusz P, Sulik KK. Development of neurotransmitter systems in the mouse embryo following acute ethanol exposure: A histological and immunocytochemical study. Int J Devl Neurosci. 1990;8:507–22. doi: 10.1016/0736-5748(90)90043-2. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Kraemer GW, Roberts AD, DeJesus OT. The impact of prenatal stress, fetal alcohol exposure, or both on development: perspectives from a primate model. Psychoneuroendocrinology. 2002;27:285–98. doi: 10.1016/s0306-4530(01)00050-6. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Adkins MM. The effects of prenatal alcohol exposure on behavior: rodent and primate studies. Neuropsychol Rev. 2011;21:186–203. doi: 10.1007/s11065-011-9168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiderman Fish BS, Rank SA, Wilson JR, Collins AC. Viability and sensorimotor development of mice exposed to prenatal short-term ethanol. Pharmacol Biochem Behav. 1981;14:57–65. doi: 10.1016/0091-3057(81)90103-9. [DOI] [PubMed] [Google Scholar]
- Simmons RW, Thomas JD, Levy SS, Riley EP. Motor response programming and movement time in children with heavy prenatal alcohol exposure. Alcohol. 2010;44:371–78. doi: 10.1016/j.alcohol.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoak IW, Sadler TW. Embryopathic effects of short-term exposure to hypoglycemia in mouse embryos in vitro. Am J Obstet Gynecol. 1990;163:619–24. doi: 10.1016/0002-9378(90)91213-v. [DOI] [PubMed] [Google Scholar]
- Sokol RJ, Delaney-Black V, Nordstrom B. Fetal alcohol spectrum disorder. JAMA. 2003;290:2996–9. doi: 10.1001/jama.290.22.2996. [DOI] [PubMed] [Google Scholar]
- Sokol RJ, Janisse JJ, Louis JM, Bailey BN, Ager J, Jacobson SW, et al. Extreme prematurity: an alcohol-related birth defect. Alcohol Clin Exp Res. 2007;31:1031–7. doi: 10.1111/j.1530-0277.2007.00384.x. [DOI] [PubMed] [Google Scholar]
- Sulik KK, Johnston MC, Webb MA. Fetal alcohol syndrome: Embryogenesis in a mouse model. Science. 1981;214:936–38. doi: 10.1126/science.6795717. [DOI] [PubMed] [Google Scholar]
- Sulik KK, Lauder JM, Dehart DB. Brain malformations in prenatal mice following acute maternal ethanol administration. Int J Devl Neurosci. 1984;2:203–14. doi: 10.1016/0736-5748(84)90014-5. [DOI] [PubMed] [Google Scholar]
- Sulik KK, Johnston MC, Daft PA, Russell WE, Dehart DB. Fetal alcohol syndrome and DiGeorge anomaly: Critical ethanol exposure periods for craniofacial malformations as illustrated in an animal model. Am J Med Gen Suppl. 1986;2:97–112. doi: 10.1002/ajmg.1320250614. [DOI] [PubMed] [Google Scholar]
- Sulik KK. Genesis of alcohol-induced craniofacial dysmorphism. Exp Biol Med. 2005;230:366–75. doi: 10.1177/15353702-0323006-04. [DOI] [PubMed] [Google Scholar]
- Tsai J, Floyd RL, Green PP, Boyle CA. Patterns and average volume of alcohol use among women of childbearing age. Matern Child Health J. 2007;11:437–45. doi: 10.1007/s10995-007-0185-4. [DOI] [PubMed] [Google Scholar]
- Valenzuela CF, Morton RA, Diaz MR, Topper L. Does moderate drinking harm the fetal brain? Insights from animal models. Trends Neurosci. 2012;35:284–92. doi: 10.1016/j.tins.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigliecca NS, Moyano HF, Molina JC. Acute prenatal alcohol exposure in rats: a behavioral study. Acta Physiol Pharmacol Latinoam. 1986;36:463–72. [PubMed] [Google Scholar]
- Webster WS, Walsh DA, McEwen SE, Lipson AH. Some teratogenic properties of ethanol and acetaldehyde in C57BL/6J mice: implications for the study of the fetal alcohol syndrome. Teratology. 1983;27:231–43. doi: 10.1002/tera.1420270211. [DOI] [PubMed] [Google Scholar]
- Weinstock M. The long-term behavioural consequences of prenatal stress. Neuroscience and Behavioral Review. 2008;32:1073–86. doi: 10.1016/j.neubiorev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- WHO Multicenter Growth Reference Study Group. WHO Motor Development Study: Windows of achievement for six gross motor development milestones. Acta Paediatrica. 2006;(Suppl 450):86–95. doi: 10.1111/j.1651-2227.2006.tb02379.x. [DOI] [PubMed] [Google Scholar]
- Wolf M-J, Kodewijn K, Beelen A, Smit B, Hedlund R, de Groot IJM. Neurobehavioral and developmental profile of very low birthweight preterm infants in early infancy. Acta Paediatr. 2002;91:930–8. doi: 10.1080/080352502760148667. [DOI] [PubMed] [Google Scholar]