Abstract
Objective
To study the relation of men’s meat intake and clinical outcomes in couples undergoing infertility treatment with the use of assisted reproductive technology (ART).
Design
Prospective cohort study.
Setting
Fertility center at an academic hospital.
Patient(s)
A total of 141 men whose female partners underwent 246 ART cycles from 2007 to 2014.
Intervention(s)
None. Total and specific types of meat intake were estimated from dietary questionnaires.
Main Outcome Measure(s)
Fertilization, implantation, clinical pregnancy, and live birth rates per initiated cycle. Mixed effect models account for multiple in vitro fertilization (IVF) cycles per woman.
Result(s)
There was a positive association between poultry intake and fertilization rate, with 13% higher fertilization rate among men in the highest quartile of poultry intake compared to those in the lowest quartile (78% vs. 65%). Processed meat intake was inversely related to fertilization rate in conventional IVF cycles, but not in IVF cycles using intracytoplasmic sperm injection (ICSI). The adjusted fertilization rates for men in increasing quartiles of processed meat intake were 82%, 67%, 70%, and 54% in conventional IVF cycles. Men’s total meat intake, including intake of specific types of meat, was not associated with implantation, clinical pregnancy, or live birth rates.
Conclusion(s)
Poultry intake was positively associated with fertilization rates, whereas processed meat intake was negatively associated with fertilization rates among couples undergoing conventional IVF. This, however, did not translate into associations with clinical pregnancy or live birth rates.
Keywords: Cohort studies, men, meat intake, infertility, assisted reproductive technology
INTRODUCTION
Infertility is a common problem for couples in the United States, with an estimated prevalence of 15 percent (1). Male factors, including azoospermia, oligospermia, and other semen analysis abnormalities contribute to roughly half of infertility cases (2). However, the impact that potentially modifiable risk factors may have on male infertility remains relatively unexplored. Increasing evidence suggests that diet may influence male reproductive function as evidenced by multiple reports of associations between dietary factors and conventional semen quality parameters (3-6).
One dietary factor that has received significant attention as a potential risk factor for male factor infertility is meat intake (7-10). Meats are a major source of saturated fat, which is related to lower sperm counts among men from a fertility clinic (11) and among young men from the general population (12). Furthermore, meats could serve as vehicles for environmental chemicals that may negatively impact spermatogenesis (13). We have previously reported that processed meat intake was associated with lower total sperm count among healthy young men (14) and with a lower percentage of morphologically normal sperm among men from subfertile couples presenting to a fertility clinic (7). However, given the poor ability of conventional semen parameters to predict fertility potential in natural and assisted conception (15, 16), it is not clear whether these associations necessarily translate into diminished fertility. To address this question, we evaluated the association of men’s meat intake with treatment outcomes of subfertile couples undergoing treatment using assisted reproductive technologies (ART).
MATERIALS AND METHODS
Study Population
Subfertile couples seeking evaluation and treatment at the Massachusetts General Hospital (MGH) Fertility Center were invited to participate in the Environment and Reproductive Health (EARTH) Study, an ongoing prospective cohort study focused on identifying how environmental factors impact human fertility (17). Men (aged 18 to 55) and women (aged 18 to 45) planning to use their own gametes during infertility treatment were eligible for the study. A food frequency questionnaire (FFQ) was introduced in 2007, and was completed by 241 of the 392 men (61%) recruited through June 2014. Of these 241 men, 107 were excluded: the female partners of 54 did not join the study, the female partners of 44 had not yet undergone any ART cycles, and the female partner of 9 men had already started an ART cycle before diet assessment. After these exclusions, there were 141 men whose female partners underwent at least one ART cycle (in vitro fertilization [IVF] with conventional insemination or intracytoplasmic sperm injection [ICSI]) and for whom pre-treatment diet data were collected during the study period. At the time of enrollment, trained research nurses measured height and weight of each subject, and completed a general health questionnaire including lifestyles, demographics, and reproductive history. This study was approved by the Human Subject Committees at the Harvard T.H. Chan School of Public Health and MGH. In addition, informed consent was obtained from all participants.
Dietary Assessment
Participants were asked to complete a previously validated FFQ and report how often, on average, they had consumed 131 foods and beverages during the past year (18). In a separately published validation study, the de-attenuated correlation coefficient ranged from 0.56 for chicken and turkey to 0.83 for processed red meats for meat intake assessed by FFQ and 1-year average of prospectively collected diet records (19). The FFQ had nine categories for intake frequency, from never to two or more servings/day. The nutritional content of each food and the specified portion size were obtained from a database of the United States Department of Agriculture (20). Total meat intake was defined as the sum of unprocessed red meat, processed red meat, poultry, fish, and organ meat intake. The definitions and serving size of each meat have been described elsewhere (7). Two dietary patterns were identified using principal components analysis: the Prudent pattern and the Western pattern, as previously described (21). A summary score for each pattern was calculated to reflect how closely each participant adhered to them (21). A higher score indicates higher adherence to the respective dietary pattern.
Clinical Procedures and Assessment of Outcomes
Female partners underwent one of three stimulation protocols: (1) luteal phase GnRH-agonist protocol; (2) GnRH-antagonist protocol; or (3) follicular phase GnRH-agonist/flare protocol. Briefly, on day three of induced menses, treatment with gonadotropins was initiated, and the GnRH agonist or antagonist was continued or started after the usual ovarian stimulation protocols (22). Human chorionic gonadotropin (hCG) was administered 36 hours prior to oocyte retrieval to in order to trigger maturation. Oocyte retrieval was performed when transvaginal ultrasound showed at least three dominant follicles (≥16mm), and serum estradiol had reached at least 500pg/ml. Couples underwent IVF with conventional insemination or with ICSI, as clinically indicated. At our center, ICSI is typically recommended in cases of severe teratospermia (≤2% normal morphology), low total motile count (<1 M) after swim up or gradient separation, or prior failed fertilization with conventional insemination . Oocytes were classified by embryologists as germinal vesicle, metaphase I(MI),metaphase II(MII), or degenerated. Fertilized oocytes were classified as normally fertilized if they had two pronuclei. After an embryo was transferred, clinical outcomes were assessed. Successful implantation was defined as an elevation in plasma β-hCG levels above 6 IU/L measured two weeks after embryo transfer. Clinical pregnancy was defined as the presence of an intrauterine pregnancy confirmed by ultrasound at 6 weeks. Live birth was defined as the birth of a neonate on or after 24 weeks gestation.
Statistical Analysis
Men were categorized into quartiles according to total meat intake. To test for differences in demographic, reproductive, and dietary characteristics across quartiles, we used a Kruskal-Wallis test for continuous variables and an extended Fisher’s exact test for categorical variables. Multivariable generalized linear mixed models with random intercepts, binominal distribution, and logit link function were used to examine the association of meat intake with fertilization, implantation, clinical pregnancy, and live birth rates, while accounting for multiple treatment cycles per couple and adjusting for other covariates. Tests for linear trend were performed by modeling intake as a continuous variable where each man was assigned the median intake of his corresponding quartile category. Population marginal means were calculated (23) to allow presentation of results as probabilities adjusted for the covariates in the model. Four sets of models were used to account for potential confounding factors. The first model included terms for men’s age and total energy intake. The second model included men’s age, total energy intake, BMI, alcohol and caffeine consumption, and adherence to the Prudent and Western dietary patterns. The third model included all variables of the second model plus the couple’s primary infertility diagnosis and mode of insemination. The fourth model included all variables of the second model plus female meat intake. Because we have previously observed different effects of nutritional factors on ART outcomes according to mode of insemination which may reflect true biological differences, we evaluated whether the relations between meat intake and ART outcomes differed by mode of insemination (conventional vs. ICSI) by introducing a cross-product term between meat intake and type of cycle. Whenever this test of heterogeneity was statistically significant (p < 0.10), we presented separate results by cycle type. Statistical analyses were performed using Statistical Analysis Software (SAS) version 9.4 (SAS Institute Inc, Cary, NC).
RESULTS
The study population consisted of 141 men whose female partners underwent a total of 246 ART cycles. Men’s mean (SD) age and BMI were 37.0 (4.6) years and 27.0 (3.7) kg/m2. Most men were white (88.7%) and had never smoked (65.3%); 35.5% of the couples received a primary diagnosis of male factor infertility. Participant’s female partners had a mean (SD) age of 35.5 (3.9) years and BMI of 23.7 (4.0) kg/m2. Men who consumed more meat had a higher BMI and higher intake of alcohol, caffeine, protein, fat, total calories, and lower intake of carbohydrates. Meat intake was positively related to greater adherence scores for the Prudent and Western dietary patterns (Table 1). Men’s total meat intake was also positively associated with their female partner’s total meat intake (rspearman=0.35) and Western dietary pattern score (rspearman=0.27). Intake of poultry (31%) and processed meats (22%) accounted for more than half of the total meat intake. Three men reported not consuming any meat. Other baseline characteristics were not associated with men’s total meat intake.
TABLE 1.
Baseline demographic, nutritional, and reproductive characteristics of study participants by quartile of total meat intake
| Total meat intake (quartiles, Q) | |||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | P | |
| N | 35 | 35 | 36 | 35 | |
| Median, servings/day | 0.85 | 1.22 | 1.58 | 2.54 | |
| Range (min, max) | 0, 1.06 | 1.07, 1.40 | 1.41, 1.82 | 1.84, 4.97 | |
|
| |||||
| Median (IQR) or N (%) | |||||
| Men’s demographics | |||||
| Age , years | 37.0 (33.8, 39.2) | 35.5 (32.9, 37.7) | 37.6 (33.0, 41.6) | 38.2 (34.4, 41.4) | .11 |
| BMI, kg/m2 | 25.4 (23.0, 28.4) | 26.0 (23.2, 27.7) | 27.1 (24.5, 28.9) | 28.7 (27.4, 30.0) | .0002 |
| White, N (%) | 32 ( 91.4) | 29 (82.9) | 33 (91.7) | 31 (88.6) | .70 |
| Smoking status, N (%) | .46 | ||||
| Never smokers | 22 (62.9) | 21 (60.0) | 27 (75.0) | 22 (62.9) | |
| Past smokers | 10 (28.6) | 12 (34.3) | 9 (25.0) | 9 (25.7) | |
| Current smokers | 3 (8.5) | 2 (5.7) | 0 (0.0) | 4 (11.4) | |
| Moderate vigorous exercise, h/w | 3.0 (0.7, 7.2) | 3.5 (1.0, 6.5) | 3.7 (2.0, 9.5) | 4.0 (1.2, 6.5) | .71 |
| Total exercise, h/w | 6.7 (2.5, 10.5) | 6.0 (1.7, 10.0) | 8.5 (4.0, 12.0) | 5.5 (2.5, 9.0) | .30 |
| Diet | |||||
| Prudent pattern score | −0.67 (−1.16, −0.10) | −0.32 (−0.55, 0.41) | 0.04 (−0.57, 0.58) | 0.22 (−0.36, 1.00) | .001 |
| Western pattern score | −0.73 (−1.02, −0.13) | −0.26 (−0.71, 0.05) | −0.04 (−0.45, 0.46) | 1.26 (0.26, 1.98) | <.0001 |
| Folic acid, μg/d | 415.2 (357.6, 577.5) | 464.6(356.1, 527.4) | 397.8(337.1, 443.9) | 416.0(357.1, 468.0) | .24 |
| Vitamin B12, mg/d | 6.91 (4.16, 14.60) | 9.92 (5.17, 12.37) | 9.52 (6.44, 14.34) | 9.89 (6.50, 15.24) | .14 |
| Zn, mg/d | 12.49 (8.55, 19.20) | 11.93 (9.49, 21.93) | 15.06(11.45, 28.01) | 19.05(13.86, 28.54) | .0009 |
| Total carbohydrate, % energy | 51.7 (47.2, 57.9) | 49.8 (45.0, 53.5) | 46.9 (41.9, 51.3) | 42.1 (38.5, 46.4) | <.0001 |
| Total protein, % energy | 15.4 (13.6, 16.7) | 15.8 (14.4, 17.3) | 16.7 (15.2, 19.0) | 17.9 (14.8, 20.9) | .0002 |
| Total fat, % energy | 30.9 (26.0, 32.8) | 32.0 (28.0, 35.4) | 32.8 (27.5, 35.6) | 34.7 (30.0, 39.4) | .006 |
| Alcohol, g/d | 6.9 (1.0, 15.7) | 14.6 (6.7, 20.2) | 11.6 (3.0, 20.3) | 15.5 (8.0, 29,4) | .03 |
| Caffeine, g/d | 238.9 (93.1, 265.1) | 247.2(107.5, 292.4) | 116.8 (63.4, 240.5) | 243.1(111.3, 289.9) | .05 |
| Total energy intake, kcal/d | 1530 (1219, 2061) | 1913 (1592, 2228) | 2008 (1700, 2365) | 2529 (2105, 2877) | <.0001 |
| Reproductive History | |||||
| History of varicocele, N (%) | 3 (8.6) | 5 (14.3) | 3 (8.3) | 3 (8.6) | .88 |
| History of cryptorchidism,N (%) | 1 (2.9) | 2 (5.7) | 4 (11.1) | 1 (2.9) | .53 |
| Primary infertility Diagnosis, N (%) | .75 | ||||
| Male factor | 11 (31.4) | 12 (34.3) | 17 (47.2) | 10 (28.6) | |
| Female factor | 11 (31.4) | 10 (28.6) | 10 (27.8) | 11 (31.4) | |
| Diminished ovarian reserve | 4(11.4) | 3(8.6) | 3(8.3) | 2(5.6) | |
| Tubal disease | 4(11.4) | 0(0.0) | 2(5.6) | 3(8.6) | |
| Ovulatory | 2(5.7) | 3(8.6) | 5(13.9) | 3(8.6) | |
| Other disease | 1(2.9) | 4(11.4) | 0(0.0) | 3(8.6) | |
| Unexplained | 13 (37.2) | 13 (37.1) | 9 (25.0) | 14 (40.0) | |
| ICSI cycles, N(%) | 15 (42.9) | 14 (40.0) | 23 (63.9) | 8 (22.9) | .01 |
| Initial stimulation protocol, N (%) | .41 | ||||
| Antagonist | 7(20.0) | 1(2.9) | 4(11.1) | 4(11.4) | |
| Flare | 3(8.6) | 2(5.7) | 4(11.1) | 3(8.6) | |
| Luteal phase agonist | 25(71.4) | 32(91.4) | 28(77.8) | 28(80.0) | |
| No. of embryos transferred, N (%) | .63 | ||||
| None | 1(2.9) | 1(2.9) | 0(0.0) | 3(8.6) | |
| 1 | 7(20.0) | 7(20.0) | 6(16.7) | 2(5.7) | |
| 2 | 21(60.0) | 19(54.3) | 18(50.0) | 23(65.7) | |
| ≥3 | 4(11.4) | 5(14.2) | 10(27.7) | 4(11.3) | |
| Egg donor or cryo cycle | 2(5.7) | 3(8.6) | 2(5.6) | 3(8.6) | |
| Embryo transfer day, N (%) | .92 | ||||
| No embryos transferred | 1(2.9) | 1(2.9) | 0(0.0) | 3(8.6) | |
| Day2 | 1(2.9) | 1(2.9) | 1(2.8) | 3(8.6) | |
| Day3 | 17(48.6) | 18(51.4) | 19(52.8) | 15(42.9) | |
| Day5 | 14(40.0) | 12(34.3) | 14(38.9) | 11(31.4) | |
| Egg donor or cryo cycle | 2(5.7) | 3(8.6) | 2(5.6) | 3(8.6) | |
| Previous infertility exam, N (%) | 26 (74.3) | 31 (88.6) | 30 (83.3) | 25 (73.5) | .32 |
| Female Partner | |||||
| Age, years | 35.0 (32.0, 38.0) | 36.0 (33.0, 38.0) | 35.0 (32.0, 38.5) | 35.0 (33.0, 39.0) | .66 |
| BMI, kg/m2 | 22.8 (20.1, 26.1) | 23.0 (21.0, 24.9) | 22.9 (20.9, 25.8) | 23.4 (21.3, 27.0) | .86 |
| Total meat intake, sv/day | 0.94 (0.48, 1.27) | 1.02 (0.66, 1.25) | 1.37 (0.85, 1.73) | 1.35 (0.88, 1.68) | .003 |
| Prudent pattern score | −0.19 (−0.81, 0.24) | −0.46 (−0.84, 0.24) | −0.01 (−0.60, 0.65) | −0.22 (−0.70, 0.23) | .51 |
| Western pattern score | −0.23 (−0.87, 0.03) | −0.29 (−0.65, 0.24) | −0.16 (−0.43, 0.34) | 0.18 (−0.35, 0.89) | .02 |
P values from Kruskal-Wallis test for continuous variables and Fisher’s exact test for categorical variables. IQR=interquartile range, BMI=body mass index, sv=servings, h/w=hours/week. All variables use the measure for the first cycle.
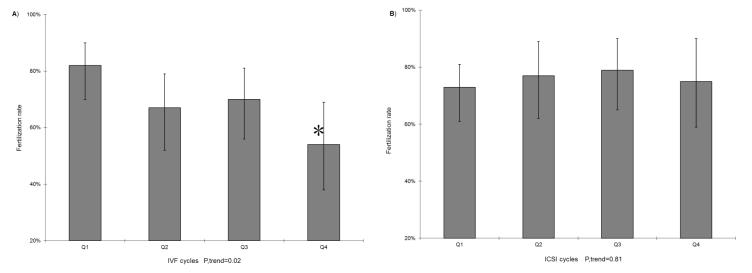
Men’s total meat intake was not associated with fertilization rate (Table 2). However, when specific types of meat were examined separately, there was a positive association between poultry intake and fertilization rate. Specifically, fertilization rate among men in the highest quartile of poultry intake was 13% higher than that of men in the lowest quartile of intake (78% vs. 65%; p=0.03). This relation did not differ between conventional insemination and ICSI cycles (p, heterogeneity=0.53). In addition, processed meat intake was inversely related to fertilization rate in conventional insemination cycles, but not in ICSI cycles (p, heterogeneity=0.08). The adjusted fertilization rates for men in increasing quartiles of processed meat intake were 82%, 67%, 70%, and 54% in conventional insemination cycles (p, trend = 0.02) and 73%, 77%, 79%, and 75% in ICSI cycles (p, trend = 0.81) (Figure 1). Intakes of unprocessed red meat, fish, and organ meat were not associated with fertilization rate. These relations were unchanged after adjustment for the female partner’s meat intake or her overall dietary patterns.
TABLE 2.
Men’s meat intake and fertilization rate
| Adjusted mean fertilization rate (95% Confidence Interval) | ||||
|---|---|---|---|---|
| MODEL | Model 1 | Model 2 | Model 3 | Model 4 |
| Total number of cycles | 206 | 206 | 206 | 183 |
| Quartile intake of total meat [Range] (Number of Men) | ||||
| Q1 [0.00-1.06] (N=52) | 0.72 (0.65 -0.78) | 0.73 (0.65 -0.79) | 0.72 (0.64- 0.79) | 0.74 (0.66-0.81) |
| Q2 [1.07-1.40] (N=45) | 0.69 (0.62-0.76) | 0.70 (0.62 -0.77) | 0.69 (0.61- 0.77) | 0.69 (0.61 -0.76) |
| Q3 [1.41-1.82] (N=44) | 0.74 (0.67 -0.79) | 0.74 (0.67 -0.80) | 0.72 (0.64 -0.79) | 0.76 (0.68- 0.82) |
| Q4 [ 1.84-4.97] (N=65) | 0.73 (0.66-0.79) | 0.72 (0.65-0.79) | 0.71 (0.63 -0.78) | 0.72 (0.63 -0.79) |
| P trend | .64 | .94 | .95 | .85 |
| Quartile intake of unprocessed red meat | ||||
| Q1 [0.00-0.12] (N=53) | 0.75 (0.68-0.81) | 0.76 (0.68- 0.81) | 0.75 (0.68 -0.81) | 0.78 (0.70 -0.83) |
| Q2 [0.16-0.24] (N=42) | 0.68 (0.61- 0.74) | 0.68 (0.61 -0.74) | 0.67 (0.58- 0.75) | 0.68 (0.60 -0.75) |
| Q3 [0.24-0.30] (N=46) | 0.75 (0.68- 0.81) | 0.75 (0.68- 0.81) | 0.74 (0.65 -0.80) | 0.76 (0.68 -0.82) |
| Q4 [0.36-1.29] (N=65) | 0.71 (0.64-0.76) | 0.71 (0.64-0.76) | 0.70 (0.62 -0.76) | 0.70 (0.62 -0.77) |
| P trend | .65 | .52 | .54 | .39 |
| Quartile intake of processed meat | ||||
| Q1 [0.00-0.22] (N=52) | 0.73 (0.67- 0.79) | 0.76 (0.69-0.82) | 0.75 (0.67- 0.81) | 0.77 (0.70- 0.83) |
| Q2 [0.24-0.38] (N=46) | 0.71 (0.64- 0.77) | 0.71 (0.64 -0.78) | 0.71 (0.63- 0.78) | 0.73 (0.64- 0.79) |
| Q3 [0.38-0.59] (N=49) | 0.74 (0.68 -0.80) | 0.74 (0.67- 0.80) | 0.73 (0.65- 0.79) | 0.75 (0.67- 0.81) |
| Q4 [0.62-2.79] (N=59) | 0.70 (0.62- 0.76) | 0.68 (0.60-0.75) | 0.67 (0.58 -0.75) | 0.66 (0.57- 0.74) |
| P trend | .54 | .17 | .18 | .09 |
| Quartile intake of poultry | ||||
| Q1 [0.00-0.18] (N=54) | 0.66 (0.58 -0.72) | 0.65 (0.57 -0.72) | 0.65 (0.56- 0.72) | 0.65 (0.56 -0.73) |
| Q2 [0.18-0.42] (N=45) | 0.72 (0.65 -0.79) | 0.72 (0.65- 0.78) | 0.71 (0.62- 0.78) | 0.73 (0.64- 0.80) |
| Q3 [0.45-0.71] (N=52) | 0.74 (0.68- 0.80) | 0.75 (0.68- 0.80) | 0.74 (0.66- 0.80) | 0.75 (0.68- 0.81) |
| Q4 [0.71-2.82] (N=55) | 0.76 (0.70- 0.81)* | 0.77 (0.70- 0.82)* | 0.76 (0.69 -0.82)* | 0.78 (0.71 -0.84)* |
| P trend | .05 | .03 | .03 | .04 |
| Quartile intake of dark fish | ||||
| Q1 [0.00-0.02] (N=47) | 0.69 (0.62 -0.76) | 0.70 (0.62 -0.76) | 0.69 (0.61-0.76) | 0.72 (0.64-0.79) |
| Q2 [0.04-0.08 (N=50) | 0.78 (0.72 -0.83) | 0.78 (0.71- 0.83) | 0.77 (0.70-0.83) | 0.79 (0.72 -0.85) |
| Q3 [0.10-0.14] (N=50) | 0.71 (0.64-0.77) | 0.71 (0.63 -0.77) | 0.70 (0.61-0.77) | 0.72 (0.64 -0.78) |
| Q4 [0.16-0.80] (N=59) | 0.71 (0.64 -0.76) | 0.71 (0.64- 0.77) | 0.70 (0.62-0.77) | 0.70 (0.62-0.76) |
| P trend | .99 | .92 | .95 | .48 |
| Quartile intake of white fish | ||||
| Q1 [0.00-0.02] (N=67) | 0.77 (0.71 -0.82) | 0.77 (0.71-0.82) | 0.76 (0.70-0.81) | 0.79 (0.73-0.84) |
| Q2 [0.04-0.04] (N=15) | 0.70 (0.57- 0.81) | 0.70 (0.56 -0.81) | 0.67 (0.52 -0.80) | 0.71 (0.56 -0.83) |
| Q3 [0.08-0.10] (N=85) | 0.70 (0.64-0.74) | 0.70 (0.64-0.75) | 0.69 (0.63 -0.75) | 0.70 (0.64 -0.75) |
| Q4 [0.14-0.51] (N=39) | 0.70 (0.62-0.77) | 0.71 (0.62 -0.78) | 0.69 (0.60 -0.77) | 0.70 (0.60-0.78) |
| P trend | .11 | .18 | .16 | .09 |
| Category of shellfish intake | ||||
| Q1 [0.00- −0.02] (N=77) | 0.72 (0.67 -0.77) | 0.72 (0.67 -0.77) | 0.71 (0.65 -0.77) | 0.74 (0.68 -0.80) |
| Q2 [0.08- −0.43] (N=129) | 0.72 (0.68 -0.76) | 0.72 (0.68-0.76) | 0.71 (0.66 -0.76) | 0.72 (0.67 -0.76) |
| P trend | .87 | .91 | .99 | .53 |
| Category of organ meat | ||||
| Q1 [0.00] (N=163) | 0.73 (0.69 -0.76) | 0.73 (0.70- 0.77) | 0.72 (0.68 -0.77) | 0.74 (0.70 -0.78) |
| Q2[0.02--0.94] (N=43) | 0.68 (0.60 -0.75) | 0.68 (0.59- 0.75) | 0.67 (0.58- 0.75) | 0.67 (0.58 -0.76) |
| P trend | .25 | .20 | .21 | .16 |
Note: Model 1: Adjusted for age and total energy intake; Model 2: adjusted for total energy intake, age, BMI, alcohol, caffeine, Prudent dietary pattern, and Western dietary pattern; Model 3: model 2+ infertility diagnoses, mode of insemination; Model 4: model 2+ female meat intake.
P<0.05 compared to men in the lowest category of intake.
Tests for trend across quartiles using the median activity level in each quartile as a continuous variable.
FIGURE 1. Men’s processed meat intake and fertilization rate in A) conventional IVF and B) ICSI cycles.
(85 conventional IVF cycles vs. 98 ICSI cycles) Results are adjusted for total energy intake, age, BMI, alcohol, caffeine, female meat intake, and data-derived dietary patterns (Prudent and Western patterns). IVF=in vitro fertilization; ICSI=intracytoplasmic sperm injection; BMI=body mass index.* p<0.05 compared to Q1.
We then examined the relationship of men’s meat intake with implantation, clinical pregnancy, and live birth rates (Table 3). Men’s total meat intake, as well as intake of specific meat types, was not associated with these outcomes (Table 3). There was no evidence of difference in these associations between ICSI and conventional IVF cycles. Further adjustment for infertility diagnosis, cycle type (IVF vs. ICSI) and female meat intake did not change the results (data not shown).
TABLE 3.
Men’s meat intake in relation to adjusted* rates of clinical outcomes per initiated ART cycle
| Implantation rate |
Clinical pregnancy
rate |
Live birth rate | |
|---|---|---|---|
|
Number of
cycles |
246 | 246 | 246 |
| Total meat intake [Range] | |||
| Q1 [0.00-1.06] | 0.54 (0.39- 0.68) | 0.48 (0.34- 0.62) | 0.34 (0.21- 0.49) |
| Q2 [1.07-1.40] | 0.66 (0.50- 0.79) | 0.61 (0.46- 0.74) | 0.46 (0.31- 0.61) |
| Q3 [1.41-1.82] | 0.58 (0.43- 0.72) | 0.52 (0.38- 0.66) | 0.38 (0.25- 0.53) |
| Q4 [1.84-4.97] | 0.52 (0.37- 0.67) | 0.45 (0.32- 0.59) | 0.35 (0.22- 0.50) |
| P trend | .67 | .56 | .82 |
| Unprocessed red meat intake | |||
| Q1 [0.00-0.12] | 0.56 (0.42- 0.70) | 0.49 (0.35- 0.63) | 0.36 (0.24- 0.51) |
| Q2 [0.16-0.24] | 0.61 (0.46- 0.75) | 0.58 (0.43- 0.71) | 0.44 (0.30- 0.59) |
| Q3 [0.24-0.30] | 0.56 (0.40- 0.70) | 0.52 (0.38- 0.67) | 0.36 (0.23- 0.51) |
| Q4 [0.36-1.29] | 0.56 (0.42- 0.69) | 0.47 (0.35- 0.60) | 0.36 (0.25- 0.49) |
| P trend | .81 | .59 | .73 |
| Processed meat intake | |||
| Q1 [0.00-0.22] | 0.54 (0.39 -0.69) | 0.50 (0.36- 0.65) | 0.38 (0.24- 0.53) |
| Q2 [0.24-0.38] | 0.58 (0.43- 0.71) | 0.51 (0.37- 0.65) | 0.31 (0.19- 0.46) |
| Q3 [0.38-0.59] | 0.58 (0.43- 0.71) | 0.51 (0.37- 0.65) | 0.40 (0.27- 0.55) |
| Q4 [0.62-2.79] | 0.58 (0.43- 0.72) | 0.52 (0.37- 0.66) | 0.43 (0.29- 0.58) |
| P trend | .79 | .88 | .45 |
| Poultry intake | |||
| Q1 [0.00-0.18] | 0.59 (0.43- 0.73) | 0.51 (0.36- 0.65) | 0.36 (0.23- 0.51) |
| Q2 [0.18-0.42] | 0.50 (0.36- 0.65) | 0.46 (0.32- 0.60) | 0.35 (0.22- 0.50) |
| Q3 [0.45-0.71] | 0.64 (0.49- 0.76) | 0.58 (0.44- 0.71) | 0.48 (0.34- 0.62) |
| Q4 [0.71-2.82] | 0.54 (0.39- 0.68) | 0.49 (0.35- 0.64) | 0.33 (0.21- 0.48) |
| P trend | .99 | .86 | .96 |
| Dark meat fish intake | |||
| Q1 [0.00-0.02] | 0.59 (0.44- 0.73) | 0.52 (0.37- 0.66) | 0.41 (0.27- 0.57) |
| Q2 [0.04-0.08] | 0.51 (0.36- 0.65) | 0.45 (0.31- 0.59) | 0.32 (0.20- 0.47) |
| Q3 [0.10-0.14] | 0.60 (0.44- 0.73) | 0.57 (0.43- 0.71) | 0.42 (0.28- 0.57) |
| Q4 [0.16-0.80] | 0.59 (0.46- 0.71) | 0.51 (0.38- 0.63) | 0.37 (0.26- 0.50) |
| P trend | .87 | .95 | .80 |
| White meat fish intake | |||
| Q1 [0.00-0.02] | 0.56 (0.43- 0.68) | 0.49 (0.37- 0.61) | 0.39 (0.28- 0.52) |
| Q2 [0.04-0.04] | 0.63 (0.35- 0.84) | 0.54 (0.29- 0.78) | 0.34 (0.15- 0.61) |
| Q3 [0.08-0.10] | 0.54 (0.42- 0.65) | 0.49 (0.38- 0.60) | 0.38 (0.28- 0.50) |
| Q4 [0.14-0.51] | 0.63 (0.47- 0.77) | 0.57 (0.41- 0.71) | 0.37 (0.23- 0.54) |
| P trend | .57 | .53 | .88 |
| Shellfish intake | |||
| Q1 [0.00- 0.02] | 0.55 (0.44- 0.66) | 0.49 (0.38- 0.60) | 0.38 (0.28- 0.50) |
| Q2 [0.08- 0.43] | 0.58 (0.49- 0.67) | 0.52 (0.43- 0.61) | 0.37 (0.29- 0.46) |
| P trend | .70 | .67 | .86 |
| Organ meat intake | |||
| Q1 [0.00-0.00] | 0.59 (0.51- 0.67) | 0.53 (0.45- 0.60) | 0.40 (0.33- 0.48) |
| Q2 [0.02-0.94] | 0.48 (0.33- 0.64) | 0.44 (0.29- 0.59) | 0.29 (0.17- 0.44) |
| P trend | .23. | .28 | .18 |
Note: Adjusted for total energy intake, age, BMI, alcohol, caffeine, Prudent dietary pattern, and Western dietary pattern.
DISCUSSION
We prospectively evaluated the relation between men’s meat intake and treatment outcomes of their partners undergoing ART. We found that poultry intake was related to a higher fertilization rate. In addition, men’s processed meat intake was associated with a lower fertilization rate in couples undergoing IVF with conventional insemination, but not in couples undergoing ICSI. These differences in fertilization rate, however, did not translate into differences in clinical pregnancy or live birth rates.
Although we and others have previously reported on the relation between meat intake and semen quality parameters as a proxy for male fertility (7-10), the literature on the relation between men’s meat intake with more direct measures of fertility outcome is scarce. To date, only one previous study has addressed this question. Contrary to our findings, Braga et al. found that in couples undergoing ICSI, the consumption of red meat was inversely related to implantation and clinical pregnancy rates (24). Differences in analytical approaches and study characteristics may account for the discrepancies between studies. For example, in keeping with the nutritional epidemiology literature on chronic disease risk, we separated red meats into unprocessed meat and processed meat whereas Braga et al. considered all red meat as a single construct. On the other hand, Braga’s study had a larger sample size (250 men) raising the possibility that the differences in findings could be due to differences in statistical power between the studies. Further research is necessary to clarify these issues.
Our finding of an inverse relation between processed meat intake and fertilization rate in conventional insemination cycles is consistent with our previous report of an inverse relation between intake of these meats and sperm morphology among subfertile men presenting to a fertility clinic (7). Since sperm morphology is related to fertilization rate (25), an inverse relation between processed meat intake and fertilization rate mediated through the effects of processed meats on morphology was to be expected. Also expected is the fact that this association was observed in conventional insemination cycles, but not in ICSI cycles where the combined effects of sperm selection and direct injection of sperm into the oocyte could negate any effects of environmental exposures on conventional semen quality parameters. We also found an unexpected positive association between poultry intake and fertilization rate. Eslamian et al. reported an association for poultry intake and lower risk of asthenospermia (10). However, others (8, 9) have failed to identify a relation between poultry intake and markers of male fertility, including our previous work in this and other populations (7, 14). This unexpected finding raises the possibility of at least two competing hypotheses. On one hand, this could represent a true association that is not mediated via conventional semen quality parameters. It is well known that semen quality parameters are not robust predictors of fertility in natural or assisted conception (15, 16). Thus, it is not surprising that environmental exposures affecting the sperm micro-environment, membrane composition, mitochondrial function, DNA integrity, epigenome, or transcriptome (without altering numbers, morphology, or motility) could have an impact on men’s reproductive potential. We are unaware, however, of any known biological mechanism that could explain these results. The other likely hypothesis is that a positive association between poultry intake and fertilization rate represents a chance finding. Therefore, the impact that meat intake may have on men’s contributions to couples’ fertility deserves further investigation.
The present research has some limitations. First, as is true for any observational study, we cannot eliminate the possibility of unmeasured confounding. However, our results were adjusted for a number of potential confounding factors identified based on previous findings reported in the literature. Second, dietary assessment using FFQs is not free of errors. However, because diet was assessed prior to treatment, the most likely effect of dietary mismeasurement is attenuation of the results towards the null. Third, there were very few men who consumed no meat. These men usually would be an ideal reference group. As a result our findings cannot provide insight into the potential effects that avoiding meat may have but rather provide estimated impacts that increasing meat consumption may have. In addition, it may not be possible to extrapolate the findings to a general population who wants to conceive without medical intervention. However, couples in our study underwent ARTs, and findings may thus be generalizable to couples undergoing infertility treatment.
The strengths of our study include the prospective design and the use of a previously validated FFQ. Furthermore, using more direct and objective measures of male fertility potential, including fertilization rate and live birth rate, is a novel approach that improves on the traditional approach of using semen quality parameters as a proxy for male fertility. In addition, meat intake in this study was comparable to intake among the general U.S. population (26), further supporting the relevance of the findings.
In summary, in this prospective study among men from couples undergoing infertility treatment with ART, we found that poultry intake was positively associated with fertilization rate, whereas processed meat intake was negatively associated with fertilization rate among couples undergoing IVF with conventional insemination only. These associations with fertilization rate, however, did not translate into differences in implantation, clinical pregnancy, or live birth rates. Our study expands the growing literature regarding the relationship between diet and markers of male fertility. However, due to the scarcity of data on how men’s diets in general and meat intake in particular influence infertility treatment outcomes, further research is needed to clarify these relations in order to allow the formulation of clinically relevant recommendations in the future.
Acknowledgments
The authors acknowledge all members of the EARTH study team, specifically the Harvard School of Public Health research nurses Jennifer B. Ford and Myra G. Keller, research staff Ramace Dadd and Patricia Morey, physicians and staff at Massachusetts General Hospital fertility center and a special thanks to all the study participants.
Supported by NIH grants R01ES009718, R01ES022955, P30ES000002, P30DK46200 and T32DK007703.Dr. Xia was supported by the China Scholarship Council.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
W.X. has nothing to disclose. Y.H.C. has nothing to disclose. P.L.W. has nothing to disclose. A.J.G. has nothing to disclose. T.L.T. has nothing to disclose. C.T. has nothing to disclose. R.H. has nothing to disclose. J.E.C. has nothing to disclose.
REFERENCES
- 1.Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, Buck Louis GM. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril. 2013;99:1324–31. doi: 10.1016/j.fertnstert.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thonneau P, Marchand S, Tallec A, Ferial ML, Ducot B, Lansac J, Lopes P, Tabaste JM, Spira A. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988–1989) Hum Reprod. 1991;6:811–6. doi: 10.1093/oxfordjournals.humrep.a137433. [DOI] [PubMed] [Google Scholar]
- 3.Minquez LA, Mendiola J, Lopez EJ, Sarabia CL, Vivero SG, Vioque J, Navarrete EM, Torres AC. Dietary intake of antioxidant nutrients is associated with semen quality in young university students. Hum Reprod. 2012;27:2807–14. doi: 10.1093/humrep/des247. [DOI] [PubMed] [Google Scholar]
- 4.Jensen TK, Swan SH, Skakkebaek NE, Rasmussen S, Jorgensen N. Caffeine intake and semen quality in a population of 2,554 young Danish men. Am J Epidemiol. 2010;171:883–91. doi: 10.1093/aje/kwq007. [DOI] [PubMed] [Google Scholar]
- 5.Chavarro JE, Toth TL, Sadio SM, Hauser R. Soy food and isoflavone intake in relation to semen quality indicators among men from an infertility clinic. Hum Reprod. 2008;23:2584–90. doi: 10.1093/humrep/den243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attaman JA, Toth TL, Furtado J, Campos H, Hauser R, Chavarro JE. Dietary fat and semen quality among men attending a fertility clinic. Hum Reprod. 2012;27:1466–74. doi: 10.1093/humrep/des065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Afeiche MC, Gaskins AJ, Willianms PL, Toth TL, Wright DL, Tanrikut C, Hauser R, Chavarro JE. Processed meat intake is unfavorably and fish intake favorably associated with semen quality indicators among men attending a fertility clinic. Journal Nutrition. 2014;144:1091–8. doi: 10.3945/jn.113.190173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mendiola J, Torres-Cantero AM, Moreno-Grau JM, Ten J, Roca M, Moreno-Grau S, Bernabeu R. Food intake and its relationship with semen quality: a case-control study. Fertil Steril. 2009;91:812–8. doi: 10.1016/j.fertnstert.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 9.Twigt JM, Bolhuis ME, Steegers EA, Hammiche F, van Inzen WG, Laven JS, Steegers-Theunissen RP. The preconception diet is associated with the chance of ongoing pregnancy in women undergoing IVF/ICSI treatment. Hum Reprod. 2012;27:2526–31. doi: 10.1093/humrep/des157. [DOI] [PubMed] [Google Scholar]
- 10.Eslamian G, Amirjannati N, Rashidkhani B, Sadeghi MR, Hekmatdoost A. Intake of food groups and idiopathic asthenozoospermia: a case-control study. Hum Reprod. 2012;27:3328–36. doi: 10.1093/humrep/des311. [DOI] [PubMed] [Google Scholar]
- 11.Attaman JA, Toth TL, Furtado J, Campos H, Hauser R, Chavarro JE. Dietary fat and semen quality among men attending a fertility clinic. Hum Reprod. 2012;27:1466–74. doi: 10.1093/humrep/des065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen TK, Heitmann BL, Blomberg Jensen M, Halldorsson TI, Andersson AM, Skakkebæk NE, Joensen UN, Lauritsen MP, Christiansen P, Dalgård C, Lassen TH, Jørgensen N. High dietary intake of saturated fat is associated with reduced semen quality among 701 young Danish men from the general population. Am J Clin Nutr. 2013;97:411–8. doi: 10.3945/ajcn.112.042432. [DOI] [PubMed] [Google Scholar]
- 13.Swan SH, Liu F, Overstreet JW, Brazil C, Skakkebaek NE. Semen quality of fertile US males in relation to their mothers’ beef consumption during pregnancy. Hum Reprod. 2007;22:1497–1502. doi: 10.1093/humrep/dem068. [DOI] [PubMed] [Google Scholar]
- 14.Afeiche MC, Williams PL, Mendiola J, Gaskins AJ, JR, Chavarro JE. Dietary fat and semen intake and reproductive parameters among physically active young men. Epidemiology. 2014;25:323–30. doi: 10.1097/EDE.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jedrzejczak P, Taszarek-Hauke G, Hauke J, Pawelczyk L, Duleba AJ. Prediction of spontaneous conception based on semen quality indicators. Int J Androl. 2008;31:499–507. doi: 10.1111/j.1365-2605.2007.00799.x. [DOI] [PubMed] [Google Scholar]
- 16.Buck Louis GM, Sundaram R, Schisterman EF, Sweeney A, Lynch CD, Kim S, Maisog JM, Gore-Langton R, Eisenberg ML, Chen Z. Semen quality and time to pregnancy: the Longitudinal Investigation of Fertility and the Environment Study. Fertil Steril. 2014;101:453–62. doi: 10.1016/j.fertnstert.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chavarro JE, Toth TL, Wright DL, Meeker JD, Hauser R. Body mass index in relation to semen quality, sperm DNA integrity, and serum reproductive hormone levels among men attending an infertility clinic. Fertil Steril. 2010;93:2222–31. doi: 10.1016/j.fertnstert.2009.01.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–26. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 19.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93:790–6. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 20.USDA/Agricultural Research Service [cited 2012];USDA National Nutrient Database for Standard Reference, Release 25. Nutrient data laboratory home page. Available from: http://www.ars.usda.gov/ba/bhnrc/ndl.
- 21.Gaskins AJ, Colaci DS, Mendiola J, Swan SH, Chavarro JE. Dietary patterns and semen quality in young men. Hum Reprod. 2012;27:2899–907. doi: 10.1093/humrep/des298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colaci DS, Afeiche MC, Gaskins AJ, Wright DL, Toth TL, Tanrikut C, Hauser R, Chavarro JE. Men’s body mass index in relation to embryo quality and clinical outcomes in couples undergoing in vitro fertilization. Fertil Steril. 2012;98:1193–9 e1. doi: 10.1016/j.fertnstert.2012.07.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Searle SR, Speed FM, Milliken GA. Population marginal means in the linear model: an alternative to least square means. Am Stat. 1980;4:216–221. [Google Scholar]
- 24.Braga DP, Halpern G, Figueira RC, Setti AS, Laconelli AJ, Borges EJ. Food intake and social habits in male patients and its relationship to intracytoplasmic sperm injection outcomes. Fertil Steril. 2012;97:53–9. doi: 10.1016/j.fertnstert.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Zhang K, Zhu W, Fan L, Gong F. Human normal sperm morphology rate and in vitro fertilization outcome. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2010;35:738–42. doi: 10.3969/j.issn.1672-7347.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 26.Daniel RC, Cross JA, Koebnick C, Sinha R. Trends in meat consumption in the United States. Public Health Nutr. 2011;14:575–583. doi: 10.1017/S1368980010002077. [DOI] [PMC free article] [PubMed] [Google Scholar]