Abstract
Regional biomechanical and biochemical properties of bovine cartilaginous endplate (CEP) and its role in disc mechanics and nutrition were determined. The equilibrium aggregate modulus and hydraulic permeability between the central and lateral regions were examined by confined compression testing. Biochemical assays were conducted to quantify the amount of water, collagen, and glycosaminoglycan (GAG). The equilibrium aggregate modulus of the CEP in the central region (0.23±0.15 MPa) was significantly lower than for the lateral region (0.83±0.26 MPa). No significant regional difference was found for the permeability of the CEP (central region: 0.13±0.07×10−15 m4/Ns and lateral region: 0.09±0.03×10−15 m4/Ns). CEPs were an average of 75.6% water by wet weight, 41.1% collagen, and 20.4% GAG by dry weight in the central region, as well as an average of 70.2% water by wet weight, 73.8% collagen, and 11.7% GAG by dry weight in the lateral region. Regional differences observed for the equilibrium aggregate modulus were likely due to the regional variation in biochemical composition. The lateral bovine endplate is much stiffer and may share a greater portion of the load. Compared with the nucleus pulposus (NP) and annulus fibrosus (AF), a smaller hydraulic permeability was found for the CEP in both the central and lateral regions, which could be due to its lower water content and higher collagen content. Our results suggest that the CEP may block rapid fluid exchange and solute convection, allow pressurization of the interstitial fluid, and play a significant role in nutrient supply in response to loading.
Keywords: Intervertebral disc, Cartilaginous endplate, Aggregate modulus, Hydraulic permeability, Soft tissue mechanics
INTRODUCTION
The degenerative changes in the intervertebral disc (IVD) and disc herniation have been implicated as possible primary etiologic factors for low back pain (LBP) (Deyo, 1986; Luoma et al., 2000; Morinaga et al., 1996; Videman and Nurminen, 2004). A thin layer of hyaline cartilage endplate (CEP), which surrounds the cranial and caudal surfaces of the central regions of the disc, is critical for disc health by helping to resist disc herniation or tears (Harada and Nakahara, 1989; Lama et al., 2014; Rajasekaran et al., 2013). Abnormal loading may cause CEP damage and internal disc disruption, which then may initiate disc degeneration or the herniation processes (Adams and Hutton, 1982; Callaghan and McGill, 2001; Veres et al., 2010). CEP damage was also found to be strongly correlated with the onset of innervation in the bony endplate layer (interface between vertebral body and CEP) (Fields et al., 2014a). Mechanical and chemical stimuli may further sensitize the nerves under this pathological condition leading to the main cause of LBP (Cox, 1990; Koike et al., 2003; Lotz and Ulrich, 2006). Therefore, damage to the CEP may play an irreplaceable role in the progression of disc degeneration and LBP.
Due to the avascular nature of the disc, the nutrients that disc cells require for maintaining disc health are supplied by blood vessels at the margins of the disc. Two possible pathways for nutrient transport into the IVD include through the CEP as well as through the perianular region of the disc (Nachemson et al., 1970). Most in vivo studies (using animal models) and in vitro studies suggest that the endplate route is the main pathway for exchange of fluid and solutes between the nucleus pulposus (NP) [and inner annulus fibrosis (AF)] and surrounding blood vessels (Holm et al., 1981; Maroudas et al., 1975; Nachemson et al., 1970; Ogata and Whiteside, 1981; Urban et al., 1982). As a result of calcification, the water content/porosity of the CEP as well as its transport properties (lower hydraulic permeability and solute diffusivity) would be dramatically decreased (Gu and Yao, 2003; Gu et al., 2004; Roberts et al., 1993). The transport of nutrient solutes and metabolites such as glucose/oxygen inflow and lactate outflow may be hindered to a greater extent in a disc with a calcified CEP, than in a disc with a normal CEP (Roberts et al., 1996; Wu et al., 2013). By contrast, a degenerated or damaged CEP may have an inverse effect due to the loss of proteoglycan or small lesions in its extracellular matrix (ECM) (Johnstone and Bayliss, 1995; Rajasekaran et al., 2004; Urban and McMullin, 1988). It could “open up” the channels and accelerate the inflow of cytokines or enzymes, which have deleterious effects on the behavior of the disc cells (Koike et al., 2003; Roberts et al., 1996). Therefore, knowledge of the mechanical and transport properties of the CEP is crucial for understanding the mechanisms of disc mechanics, nutrition, and degeneration.
A previous study suggests that the average equilibrium tensile modulus of normal human CEP is similar to that of femoral articular cartilage (AC) in adults (Fields et al., 2014b). The compressive modulus of the baboon CEP is also found to be within the same range as that in bovine and human AC, while the hydraulic permeability of the baboon CEP is two orders of magnitude higher than that of human AC (Setton et al., 1993). By contrast, the permeability coefficient of human CEP is found to be about 1/3 and 1/10 of that in human AF and cartilage (Maroudas et al., 1975). Compared with NP and AF tissue, previous studies also indicated that CEP has a unique 3D morphology, inhomogeneous biochemical composition, and regional dependent solute diffusion rate (Fields et al., 2014b; Rajasekaran et al., 2004; Rajasekaran et al., 2008; Rajasekaran et al., 2010; Roberts et al., 1989; Roberts et al., 1996). Therefore, we hypothesized that the biphasic viscoelastic properties of the CEP may also be regional dependent. Due to the scarcity of normal human tissue as well as previous studies having shown that the bovine is an appropriate animal model to study human IVD biomechanics and biology, healthy bovine CEP was chosen for this study (Demers et al., 2004; Oshima et al., 1993). Specifically, we will determine the compressive aggregate modulus, swelling pressure, and hydraulic permeability of the bovine cartilage endplate in the central and lateral regions, and further characterize its related biochemical composition.
MATERIALS AND METHODS
Mechanical characterization
Bovine (2–3 years old; male) cartilaginous endplates were harvested at both the superior and inferior surfaces between C2-3 and C3-4 from bovine tails obtained from a local slaughterhouse within 4 hours of death. First, the discs were opened through the median plane with a scalpel, then using an 8 mm corneal trephine, cylindrical disc tissue plugs including the bone were obtained from both the central and lateral regions of the bovine disc (Figure 1A&B). The plugs were immediately wrapped in a plastic membrane and gauze soaked in a normal saline solution with protease inhibitors and stored at −80°C for less than one week.
Figure 1.
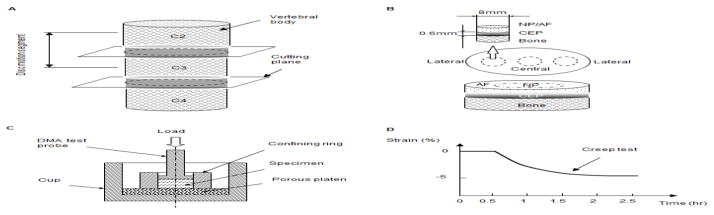
(A) Disc motion segments in the bovine tail used in this study. (B) Schematic of specimen preparation. The region and size of test specimens are shown. (C) Schematic of the confined compression test chamber. (D) Schematic of the mechanical testing protocol.
Before mechanical testing, the disc plugs were thawed at room temperature for 10 minutes and care was taken when separating the disc tissue from the bone under a dissecting microscope. A sledge microtome was used to carefully remove the NP or AF tissue (relatively more transparent than the CEP tissue) from the CEP to prepare disc shaped samples with an average height of 0.6 mm. The thickness of the CEP has been found to be approximately 0.6 mm in our histological study as well as in the literature (Roberts et al., 1989). The sample was then punched by a 5 mm corneal trephine and immediately used for mechanical testing to prevent swelling. In total, fourteen morphologically healthy bovine CEPs in the central region and eighteen bovine CEPs in the lateral region (total disc motion segments: n=7; tails: n=4) were sectioned and used for mechanical testing.
A Dynamic Mechanical Analyzer (DMA Q800, TA Instruments, New Castle, Delaware, USA) was used for the mechanical tests. The DMA displacement and force measurement precision was 0.1 μm and 0.1 mN, respectively. A test chamber was designed for confined compression testing (Figure 1C). A polished stainless steel confined ring was used to prevent radial deformation. The specimens were then compressed axially by the test probe (5 mm diameter) on top and a rigid porous permeable porous platen (20 μm average pore size) on the bottom (Kuo et al., 2010; Soltz and Ateshian, 2000; Yao et al., 2002). Figure 1D schematically shows the mechanical testing protocol.
Similar to the mechanical testing protocol in our previous studies on IVD tissues, hydrogels, and temporomandibular joint (TMJ) discs (Gu et al., 2003; Kuo et al., 2010; Yao et al., 2002), the specimens were first subjected to a minute compressive tare load (0.01 N) to measure initial height. Then, phosphate buffered saline (PBS) was carefully injected into the testing chamber and the equilibrium swelling pressure of the specimen (after ~40 minutes) was measured with the probe position held constant at the initial height. Secondly, a creep test (2 hours) was performed by applying a stress equal to 1.2 times the equilibrium stress at the initial height. The criterion for choosing the amplitude of load for the creep test was based on the fact that the resultant creep strain at the end of the test was on average 3–4%. This small creep strain was chosen to satisfy the assumption of the linear biphasic theory for the following curve fitting. By curve-fitting the creep data to the biphasic theory developed by Mow et al.(Mow et al., 1980), the equilibrium compressive aggregate modulus (HA) and hydraulic permeability coefficient (k) were determined for the CEP.
Histological study
Sagittal and frontal slices were taken from the disc plugs, which were harvested from the central and lateral regions in another four disc motion segments (Tails: n=2) for histological study. The segments were rapidly fixed in 10% neutral buffered formalin, decalcified, and paraffin wax embedded. Ehrlich’s hematoxylin and eosin (H&E) was used with the wax sections (7 μm) in order to study morphological characteristics of the CEP.
Biochemical analysis
During the preparation of CEP samples for mechanical testing, it was found that the CEP tissue may swell dramatically once immersed in PBS or even while thawing in air under room temperature. Accordingly, the CEP samples were directly transferred into the confined chamber for creep testing without any extra weight measurements. Therefore, four fresh disc motion segments from an additional two bovine tails were dissected for biochemical studies. CEPs in the central and lateral regions (n=8/each region) from both the superior and inferior surfaces of the disc, as well as adjacent NP and AF tissue (control groups; n=8/each region) were harvested and lyophilized (Figure 1A&B). The water content was determined using the difference between the wet and dry tissue weight, which was divided by the wet weight.
The lyophilized tissue was then assayed for total collagen and glycosaminoglycan (GAG) content. A modified chloramine-T hydroxyproline assay (Bergman and Loxley, 1970) was used to determine the total collagen content (μg collagen/μg dry tissue, unit: %). Collagen standards (Accurate Chemical and Scientific Corporation, Westbury, NY) were chosen for a more direct comparison instead of using hydroxyproline standards. The Blyscan Glycosaminoglycan Assay kit (Biocolor, Newtonabbey, Northern Ireland) was used to determine the total glycosaminoglycan (GAG) content based on 1,9-dimethylmethylene blue dye binding, with standards provided by the manufacturer.
Statistical analysis
Linear mixed effects models were fit to each of the mechanical and biochemical outcomes to assess for differences by disc region. The models allowed for error heterogeneity by disc region and three increasingly complex dependence structures, i.e. dependence among measures from the same animal, the same disc within animal, and the same surface within disc. Likelihood-based model selection supported the need to accommodate the error heterogeneity for all but swelling pressure and water content, but did not additionally support within-animal dependence. Thus all outcomes were modeled using ANOVA by region with heterogeneous variance fit by restricted maximum likelihood using the package nlme (Pinheiro et al., 2015) in R (R Core Team, 2015). Outcomes were summarized using model-based averages and standard deviations, and regional differences were assessed by model-based significance testing; adjusted p-values (Holm, 1979) are reported. Statistical differences were reported at p-values < 0.05.
RESULTS
General morphology and histological appearance
All of the bovine discs used in this study were dissected and morphologically examined. All discs were classified as Grade I or II (Thompson grading system) (Thompson et al., 1990). The lamellae structure in the AF region was visible and the NP was gelatinous. A thin hyaline cartilage layer called the CEP was found between the interface of the disc and bone. The CEP, as well as the NP and AF tissue, swelled immediately once exposed to the PBS solution. Based on our histology study, the cell density in the CEP layer was much higher than that in both the NP and AF regions (Figure 2). The average thickness of the CEP was approximately 0.6 mm. The collagen fibers in the CEP from both the central and lateral regions were more compacted than those in the NP and AF tissues, respectively. The collagen fibers from the AF also seemed to continue into the lateral endplate.
Figure 2.
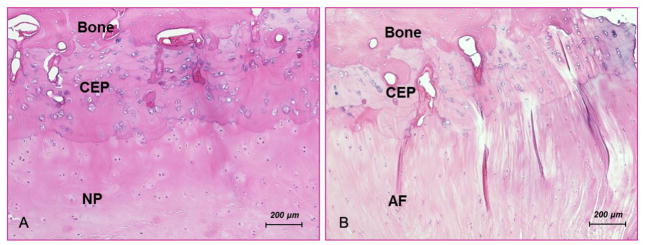
Histological section of bovine C2-3 (original magnification ×10), showing (A) the nucleus pulposus (NP), the cartilage endplate (CEP), and the vertebral body (VB) and (B) the annulus fibrosus (AF), the CEP, and the VB.
Creep compression behavior
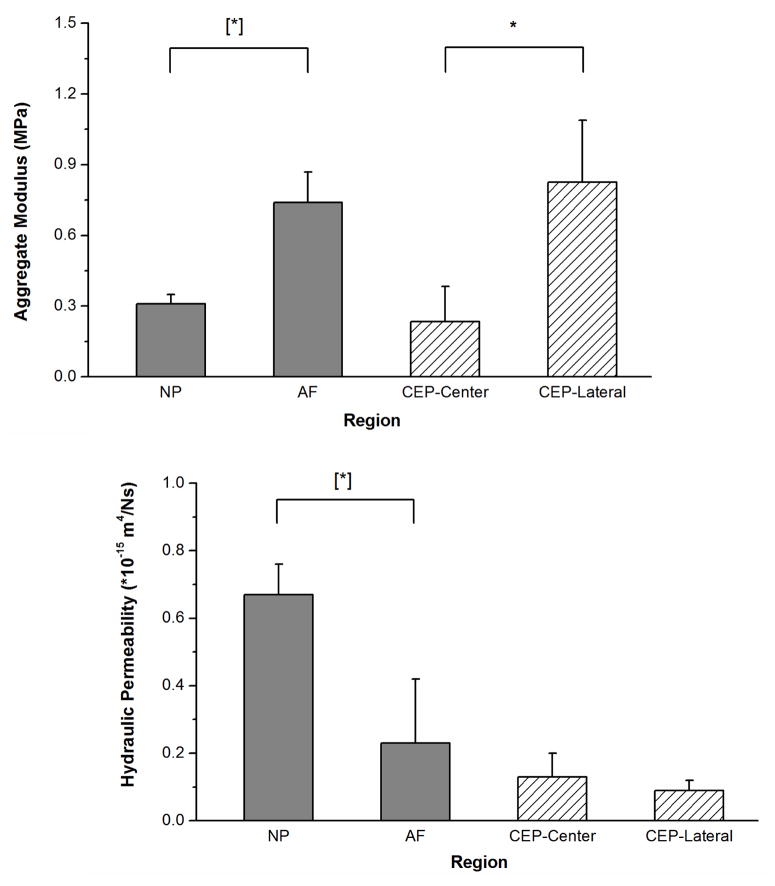
The initial heights of the CEPs were measured by the DMA before the creep test (central CEP: 0.60±0.07 mm, n=14; lateral CEP: 0.59±0.08 mm, n=18). The creep data were well fitted to the biphasic theory to determining the equilibrium compressive aggregate modulus and hydraulic permeability (Figure 3). A significant regional difference was found for the aggregate modulus in the CEP; 0.23±0.15 MPa in the central region was approximately 1/3 of that in the lateral region (0.83±0.26 MPa) (Figure 4A; p<0.0001). No significant regional differences were observed for the hydraulic permeability in the CEP (central region: 0.13±0.07×10−15 m4/Ns and lateral region: 0.09±0.03×10−15 m4/Ns; p=0.071) (Figure 4B). A significant regional difference was also found for the swelling pressure in the CEP (central region: 0.09±0.05 MPa and lateral region: 0.21±0.07 MPa; p<0.0001).
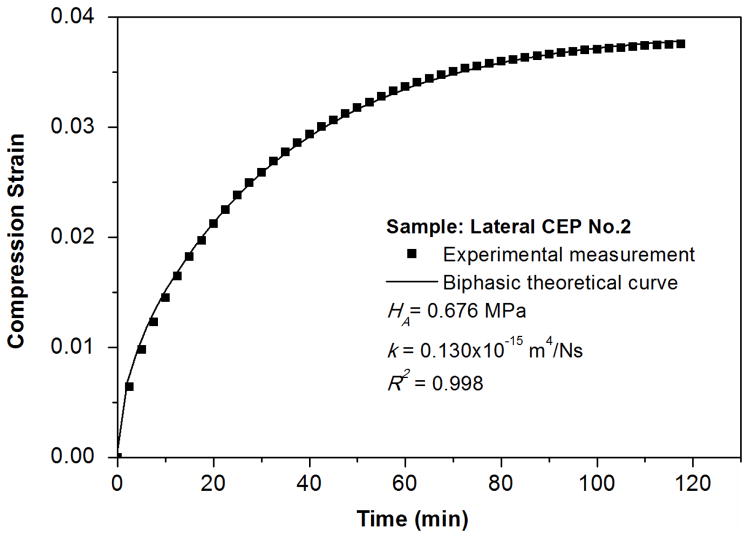
Figure 3.
Typical biphasic creep behavior of a bovine CEP. A good agreement is shown between the theoretical prediction and the experimental result.
Figure 4.
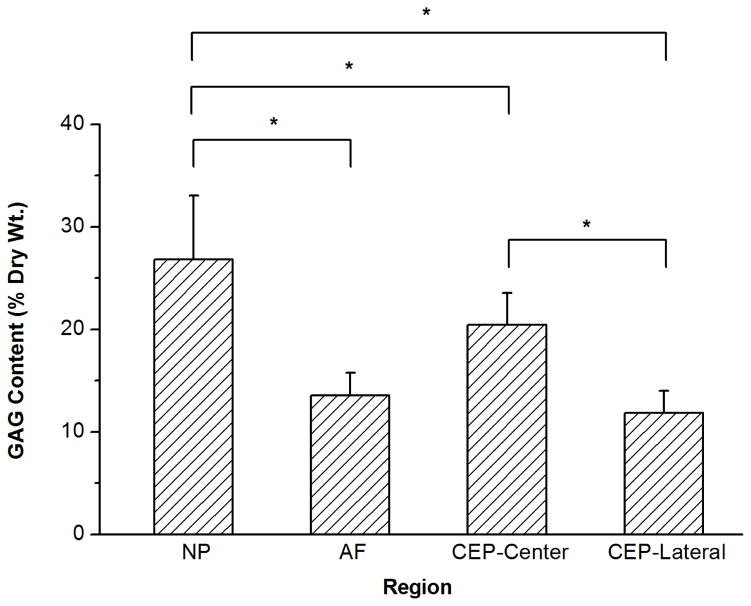
Comparison of aggregate modulus HA (A) and permeability k (B) of bovine CEP determined in this study (CEP-Central and CEP-Lateral) and bovine IVD tissue found in the literature (NP and AF) (Perie et al., 2005). For the aggregate modulus, a significant regional variation was detected in the CEP between the central and lateral regions (*p<0.0001; Central: n=14; Lateral: n=18). The aggregate modulus of the CEP in the central and lateral regions were comparable with that of the NP and AF, respectively ([*] p < 0.05 (Perie et al., 2005)). For hydraulic permeability, no significant regional variations were detected in the CEP between the central and lateral regions (p=0.071). Compared with that in the NP and AF (Perie et al., 2005), the permeability in the CEP is found to be much smaller.
Biochemical composition
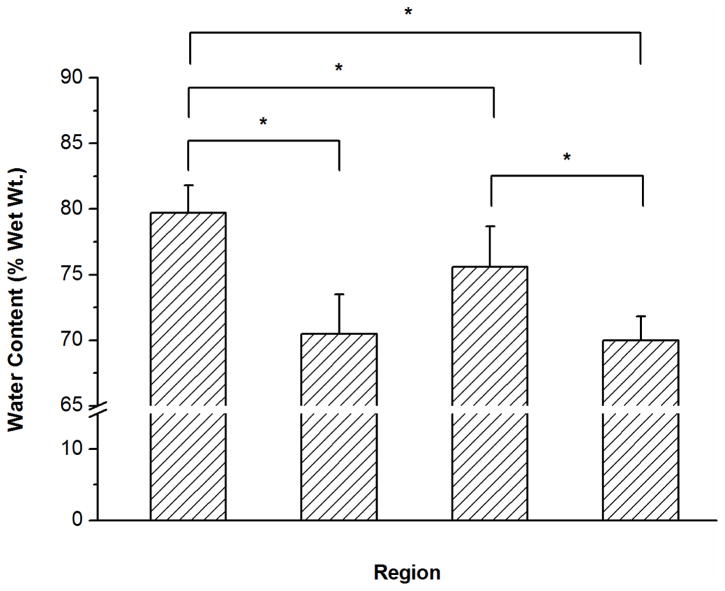
The water content (% wet weight), total collagen content (% dry weight), and total GAG content (% dry weight) of bovine CEP as well as NP and AF were shown in Figure 5. A significant regional difference was found for water content in the CEP between the central and lateral regions (central region: 75.6±3.1% and lateral region: 70.2±1.8%; p=0.004). Compared with that in the NP region (79.4±2.0%), the mean water content in the CEPs from both central and lateral regions (72.9±3.7%) was significantly lower (p<0.0001).
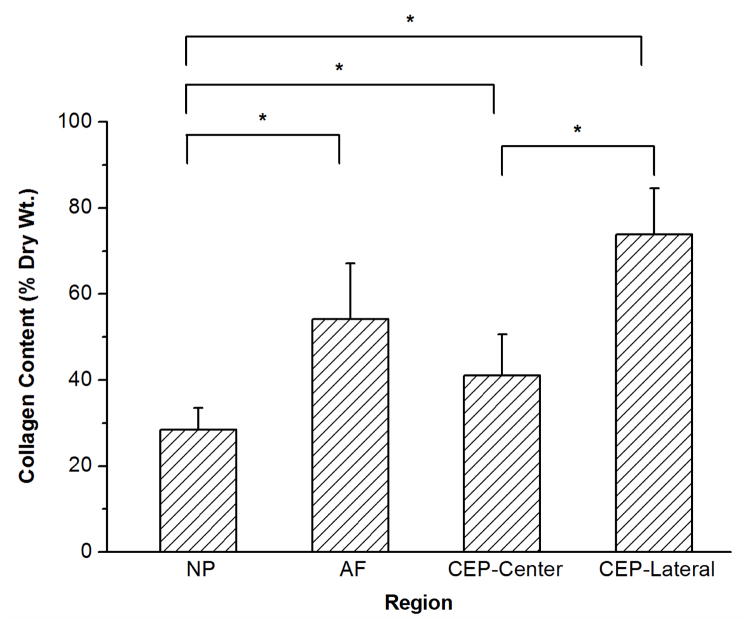
Figure 5.
Results of biochemical assays measuring water content (A), total collagen content (B), and total GAG content (C) for each region of the bovine IVD. Significant differences were detected for water, collagen, and GAG contents between the NP, AF, and CEP. Regional differences were also found for water, collagen, and GAG contents in the CEP between the central and lateral regions (*p values are lower than 0.05 as shown in the biochemical composition section; n=8 each group).
A significant regional difference was also found for GAG content in the CEP between the central and lateral regions (central region: 20.4±3.1% and lateral region: 11.7±2.1%; p<0.0001). Compared with that in the NP region (26.7±5.8%), the mean GAG content in the CEPs from both the central and lateral regions (16.1±5.1%) was significantly lower (p=0.0006). For collagen content, a significant regional difference was also found in the CEP between the central and lateral regions (central region: 41.1±9.5% and lateral region: 73.8±10.8%; p<0.0001). Compared with that in the NP region (28.5±4.6%), the mean collagen content in the CEPs from both central and lateral regions (57.5±19.2%) was significantly higher (p<0.0001).
DISCUSSION
The objective of this study was to investigate the regional viscoelastic properties of bovine cartilaginous endplate and determine its role in disc mechanics and nutrition. The linear biphasic model was used to curve fit the confined compression results with an average R2 value of 0.993 (Mow et al., 1980). It indicates that the biphasic theory can well model the creep behavior of bovine cartilaginous endplate. A regional variation was found for the aggregate modulus between the central and lateral regions in the CEP, indicating that the lateral region of the bovine endplate is much stiffer and might share a greater portion of the load in the disc.
The average hydraulic permeability of bovine CEP in this study is substantially different from that observed in the NP and AF (Figure 4B), although no significant regional variation was found for the hydraulic permeability of bovine CEP. The average hydraulic permeability of bovine CEP is 0.11×10−15 m4/Ns, which is about 1/6 of that in bovine NP, 1/2 of that in bovine and human AF, 1/20 of that in bovine AC, and 1/10 of that in human AC (Table 1). It suggests that, due to the small hydraulic permeability, the cartilaginous endplate blocks rapid fluid exchange and allows pressurization of the interstitial fluid in response to loading. Furthermore, as shown in the literature, the cartilage endplate also plays a significant role in preventing the disc from swelling with its intrinsic tensile resistance (high stiffness of collagen fibrils and entangled GAGs) (Fields et al., 2014b; Veres et al., 2010; Vernon-Roberts et al., 2007). The nucleus pulposus contains a high density of fixed negative charges which are bonded with proteoglycans in the ECM. It could cause a high swelling pressure which helps to sustain the compressive load in the spine (Gu et al., 2003; Urban and McMullin, 1985). The AF is formed by a series of concentric encircling lamellae, which could provide a strong resistant tensile force to prevent the whole disc from swelling (Inoue, 1981; Yu et al., 2005). As shown in this study, the cartilage endplates above and below the disc play a significant role in IVD load supporting mechanisms by allowing pressurization of the interstitial fluid and maintaining swelling pressure in the disc.
Table 1.
Biphasic viscoelastic properties (aggregate modulus and hydraulic permeability: mean ± SD) of CEP, other disc tissues (NP and AF), and articular cartilage (AC) tissues.
| Aggregate modulus (MPa) | Permeability (10−15 m4/Ns) | Method | Species | Reference |
|---|---|---|---|---|
| 0.23±0.15 | 0.13±0.07 | Confined compression | Central CEP | This study |
| 0.83±0.26 | 0.09±0.03 | Confined compression | Lateral CEP | This study |
| 0.31±0.04 | 0.67±0.09 | Confined compression | Bovine NP | (Perie et al., 2005) |
| 0.74±0.13 | 0.23±0.19 | Confined compression | Bovine AF | (Perie et al., 2005) |
| 0.56±0.21 | 0.21±0.10 | Confined compression | Human AF | (Iatridis et al., 1998) |
| 0.40±0.14 | 2.70±1.50 | Confined compression | Bovine AC | (Ateshian et al., 1997) |
| ~0.60 | ~1.48 | Indentation | Human AC | (Froimson et al., 1997) |
| 1.15±0.50 | 0.71±0.36 | Indentation | Human AC | (Athanasiou et al., 1994) |
The significantly lower hydraulic permeability found in the CEP could block the nutrient solutes or the proteoglycan fragment outflow caused by the rapid convection under mechanical loading. Together with the low solute diffusivities of the CEP found in the literature, this study suggested that the CEP could act as a gateway for solute transport into the disc and help to maintain a constant physiological nutrient and ECM ionic environment inside the IVD (Maroudas et al., 1975; Moon et al., 2013; Nachemson et al., 1970; Roberts et al., 1996; Urban et al., 2004). Furthermore, the CEP could play an important role in preventing angiogenesis progression into the human IVD by blocking the rapid diffusion of angiogenic molecules such as vascular endothelial growth factor (VEGF) into the disc (Fields et al., 2014a; Karamouzian et al., 2010; Koike et al., 2003; Lama et al., 2014; Lotz and Ulrich, 2006).
The central region of the bovine CEP had a lower aggregate modulus and swelling pressure than that in the lateral region, which could be correlated with their different biochemical compositions (higher water content in the central region of the CEP). These results are consistent with the inverse relationship between aggregate modulus and the water content found in the literature (Perie et al., 2006). This observation supported the hypothesis that water content is generally a stronger determinant of material properties than the GAG content (Perie et al., 2006). In this study, hydraulic permeability in the central CEP is higher than that in the lateral CEP, although it is not statistically significant (p=0.071). Correspondingly, the water content is higher in the central CEP than in the lateral CEP. Similarly, the correlation between hydraulic permeability and water content was found in bovine NP and AF, and porcine AF in the literature (Gu and Yao, 2003; Maroudas, 1975; Perie et al., 2006).
Due to the scarcity of human disc tissue, bovine IVD has previously been used as an alternative source for the study of disc metabolism, function, and biomechanics (Aguiar et al., 1999; Horner et al., 2002; Horner and Urban, 2001; Ohshima et al., 1995; Race et al., 2000). Although some structural differences have been found in the annular wall as well as the curvature of the vertebral body, the overall structure of the bovine disc is similar to the young and healthy human IVD (Demers et al., 2004; Race et al., 2000; Simunic et al., 2001). The biochemical composition of bovine discs has also been found to be similar to the human disc (Urban and McMullin, 1988; Urban and Roberts, 2003). Furthermore, it is reported that the biphasic mechanical properties as well as the resting stress of bovine AF are in the same range of that in the human AF (Drost et al., 1995; Iatridis et al., 1998; Perie et al., 2005). In this study, the biochemical composition (average water, GAG, and collagen contents) in the control groups (bovine NP and AF) was found to be consistent with that in the literature (Demers et al., 2004; Perie et al., 2006). Therefore, the bovine disc could be considered as a good animal model (Demers et al., 2004) for the study of biphasic mechanical properties of human cartilage endplate while normal human CEP tissue is too rare to collect.
In this study, the confined swelling condition, instead of the free swelling condition, was chosen to represent the physiological water content in the CEP. In addition, during the sample preparation, the CEP swelled dramatically once immersed in the PBS or even while being thawed in the air under room temperature. Therefore, the sample was immediately moved into the confined chamber following the microtome preparation. There may have been a small amount of GAG leakage from the tissue into the PBS solution after the confined compression test. This may have introduced some error to the measured mechanical parameters, however this effect was expected to be small (Perie et al., 2006). In our histological study, the thickness of the CEP was found to be approximately 0.6 mm, which is consistent with that in the literature (the human CEP: 0.62±0.29 mm and thickness of baboon CEP: 0.81±0.26 mm) (Roberts et al., 1989; Setton et al., 1993). Therefore, attention was taken while preparing the CEP samples using the microtome to maintain the initial thickness of the sample as close to 0.6 mm.
In summary, the regional biomechanical and biochemical characterizations of the bovine CEP were investigated to address the lack of data on CEP tissues currently available in the literature. Based on our initial gross observation, the histological, biochemical, and mechanical characterizations confirmed that a layered structure of hyaline cartilage exists on the top and bottom of the bovine disc. Second, due to the higher aggregate modulus and swelling pressure found in the lateral region of the CEP, the lateral bovine endplate may be much stiffer than the central endplate and may share a greater portion of the load. This characteristic could be linked to the lower water content determined in the lateral region of the CEP. This observation confirmed a previous hypothesis that the water content is generally a stronger determinant of tissue material properties than GAG content (Perie et al., 2006). Third, compared with the NP and AF, a smaller hydraulic permeability was found in the CEP, which indicated that the CEP could block rapid solute convection and allow pressurization of the interstitial fluid in response to loading. In conclusion, our results indicate that the CEP may act as a mechanical barrier to help sustaining mechanical loading as well as a gateway for nutrition supply and preventing angiogenesis.
Acknowledgments
This project was supported by NIH grants AR055775, DE018741 and DE021134, a grant (SCIRF0307) from the South Carolina Spinal Cord Injury Research Fund, and a NSF Graduate Research Fellowship to SEC.
Footnotes
Conflict of Interest:
None of the authors of this paper have a conflict of interest that might be construed as affecting the conduct or reporting of the work presented.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams MA, Hutton WC. Prolapsed intervertebral disc. A hyperflexion injury. Spine. 1982;7:184–191. [PubMed] [Google Scholar]
- Aguiar DJ, Johnson SL, Oegema TR. Notochordal cells interact with nucleus pulposus cells: regulation of proteoglycan synthesis. Experimental Cell Research. 1999;246:129–137. doi: 10.1006/excr.1998.4287. [DOI] [PubMed] [Google Scholar]
- Ateshian GA, Warden WH, Kim JJ, Grelsamer RP, Mow VC. Finite deformation biphasic material properties of bovine articular cartilage from confined compression experiments. Journal of Biomechanics. 1997;30:1157–1164. doi: 10.1016/s0021-9290(97)85606-0. [DOI] [PubMed] [Google Scholar]
- Athanasiou KA, Agarwal A, Dzida FJ. Comparative study of the intrinsic mechanical properties of the human acetabular and femoral head cartilage. Journal of Orthopaedic Research. 1994;12:340–349. doi: 10.1002/jor.1100120306. [DOI] [PubMed] [Google Scholar]
- Callaghan JP, McGill SM. Intervertebral disc herniation: studies on a porcine model exposed to highly repetitive flexion/extension motion with compressive force. Clinical Biomechanics. 2001;16:28–37. doi: 10.1016/s0268-0033(00)00063-2. [DOI] [PubMed] [Google Scholar]
- Cox JM. Low Back Pain: Mechanism, Diagnosis and Treatment. Williams & Wilkins, Maryland; 1990. [Google Scholar]
- Demers CN, Antoniou J, Mwale F. Value and limitations of using the bovine tail as a model for the human lumbar spine. Spine. 2004;29:2793–2799. doi: 10.1097/01.brs.0000147744.74215.b0. [DOI] [PubMed] [Google Scholar]
- Deyo RA. Early diagnostic evaluation of low back pain. Journal of General Internal Medicine. 1986;1:328–338. doi: 10.1007/BF02596214. [DOI] [PubMed] [Google Scholar]
- Drost MR, Willems P, Snijders H, Huyghe JM, Janssen JD, Huson A. Confined compression of canine annulus fibrosus under chemical and mechanical loading. Journal of Biomechanical Engineering. 1995;117:390–396. doi: 10.1115/1.2794197. [DOI] [PubMed] [Google Scholar]
- Fields AJ, Liebenberg EC, Lotz JC. Innervation of pathologies in the lumbar vertebral end plate and intervertebral disc. The Spine Journal. 2014;14:513–521. doi: 10.1016/j.spinee.2013.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields AJ, Rodriguez D, Gary KN, Liebenberg EC, Lotz JC. Influence of biochemical composition on endplate cartilage tensile properties in the human lumbar spine. Journal of Orthopaedic Research. 2014;32:245–252. doi: 10.1002/jor.22516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froimson MI, Ratcliffe A, Gardner TR, Mow VC. Differences in patellofemoral joint cartilage material properties and their significance to the etiology of cartilage surface fibrillation. Osteoarthritis and Cartilage. 1997;5:377–386. doi: 10.1016/s1063-4584(97)80042-8. [DOI] [PubMed] [Google Scholar]
- Gu WY, Yao H. Effects of hydration and fixed charge density on fluid transport in charged hydrated soft tissues. Annals of Biomedical Engineering. 2003;31:1162–1170. doi: 10.1114/1.1615576. [DOI] [PubMed] [Google Scholar]
- Gu WY, Yao H, Huang CY, Cheung HS. New insight into deformation-dependent hydraulic permeability of gels and cartilage, and dynamic behavior of agarose gels in confined compression. Journal of Biomechanics. 2003;36:593–598. doi: 10.1016/s0021-9290(02)00437-2. [DOI] [PubMed] [Google Scholar]
- Gu WY, Yao H, Vega AL, Flagler D. Diffusivity of ions in agarose gels and intervertebral disc: effect of porosity. Annals of Biomedical Engineering. 2004;32:1710–1717. doi: 10.1007/s10439-004-7823-4. [DOI] [PubMed] [Google Scholar]
- Harada Y, Nakahara S. A pathologic study of lumbar disc herniation in the elderly. Spine. 1989;14:1020–1024. doi: 10.1097/00007632-198909000-00017. [DOI] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- Holm S, Maroudas A, Urban JP, Selstam G, Nachemson A. Nutrition of the intervertebral disc: solute transport and metabolism. Connective Tissue Research. 1981;8:101–119. doi: 10.3109/03008208109152130. [DOI] [PubMed] [Google Scholar]
- Horner HA, Roberts S, Bielby RC, Menage J, Evans H, Urban JP. Cells from different regions of the intervertebral disc: effect of culture system on matrix expression and cell phenotype. Spine. 2002;27:1018–1028. doi: 10.1097/00007632-200205150-00004. [DOI] [PubMed] [Google Scholar]
- Horner HA, Urban JP. Effect of nutrient supply on the viability of cells from the nucleus pulposus of the intervertebral disc. Spine. 2001;26:2543–2549. doi: 10.1097/00007632-200112010-00006. [DOI] [PubMed] [Google Scholar]
- Iatridis JC, Setton LA, Foster RJ, Rawlins BA, Weidenbaum M, Mow VC. Degeneration affects the anisotropic and nonlinear behaviors of human anulus fibrosus in compression. Journal of Biomechanics. 1998;31:535–544. doi: 10.1016/s0021-9290(98)00046-3. [DOI] [PubMed] [Google Scholar]
- Inoue H. Three-dimensional architecture of lumbar intervertebral discs. Spine. 1981;6:139–146. doi: 10.1097/00007632-198103000-00006. [DOI] [PubMed] [Google Scholar]
- Johnstone B, Bayliss MT. The large proteoglycans of the human intervertebral disc. Changes in their biosynthesis and structure with age, topography, and pathology. Spine. 1995;20:674–684. doi: 10.1097/00007632-199503150-00008. [DOI] [PubMed] [Google Scholar]
- Karamouzian S, Eskandary H, Faramarzee M, Saba M, Safizade H, Ghadipasha M, Malekpoor AR, Ohadi A. Frequency of lumbar intervertebral disc calcification and angiogenesis, and their correlation with clinical, surgical, and magnetic resonance imaging findings. Spine. 2010;35:881–886. doi: 10.1097/BRS.0b013e3181b9c986. [DOI] [PubMed] [Google Scholar]
- Koike Y, Uzuki M, Kokubun S, Sawai T. Angiogenesis and inflammatory cell infiltration in lumbar disc herniation. Spine. 2003;28:1928–1933. doi: 10.1097/01.BRS.0000083324.65405.AE. [DOI] [PubMed] [Google Scholar]
- Kuo J, Zhang L, Bacro T, Yao H. The region-dependent biphasic viscoelastic properties of human temporomandibular joint discs under confined compression. Journal of Biomechanics. 2010;43:1316–1321. doi: 10.1016/j.jbiomech.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lama P, Zehra U, Balkovec C, Claireaux HA, Flower L, Harding IJ, Dolan P, Adams MA. Significance of cartilage endplate within herniated disc tissue. European Spine Journal. 2014;23:1869–1877. doi: 10.1007/s00586-014-3399-3. [DOI] [PubMed] [Google Scholar]
- Lotz JC, Ulrich JA. Innervation, inflammation, and hypermobility may characterize pathologic disc degeneration: review of animal model data. Journal of Bone and Joint Surgery Am. 2006;88(Suppl 2):76–82. doi: 10.2106/JBJS.E.01448. [DOI] [PubMed] [Google Scholar]
- Luoma K, Riihimaki H, Luukkonen R, Raininko R, Viikari-Juntura E, Lamminen A. Low back pain in relation to lumbar disc degeneration. Spine. 2000;25:487–492. doi: 10.1097/00007632-200002150-00016. [DOI] [PubMed] [Google Scholar]
- Maroudas A. Biophysical chemistry of cartilaginous tissues with special reference to solute and fluid transport. Biorheology. 1975;12:233–248. doi: 10.3233/bir-1975-123-416. [DOI] [PubMed] [Google Scholar]
- Maroudas A, Stockwell RA, Nachemson A, Urban J. Factors involved in the nutrition of the human lumbar intervertebral disc: cellularity and diffusion of glucose in vitro. Journal of Anatomy. 1975;120:113–130. [PMC free article] [PubMed] [Google Scholar]
- Moon SM, Yoder JH, Wright AC, Smith LJ, Vresilovic EJ, Elliott DM. Evaluation of intervertebral disc cartilaginous endplate structure using magnetic resonance imaging. European Spine Journal. 2013;22:1820–1828. doi: 10.1007/s00586-013-2798-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinaga T, Takahashi K, Yamagata M, Chiba T, Tanaka K, Takahashi Y, Nakamura S, Suseki K, Moriya H. Sensory innervation to the anterior portion of lumbar intervertebral disc. Spine. 1996;21:1848–1851. doi: 10.1097/00007632-199608150-00002. [DOI] [PubMed] [Google Scholar]
- Mow VC, Kuei SC, Lai WM, Armstrong CG. Biphasic creep and stress relaxation of articular cartilage in compression: Theory and experiments. Journal of Biomechanical Engineering. 1980;102:73–84. doi: 10.1115/1.3138202. [DOI] [PubMed] [Google Scholar]
- Nachemson A, Lewin T, Maroudas A, Freeman MA. In vitro diffusion of dye through the end-plates and the annulus fibrosus of human lumbar inter-vertebral discs. Acta Orthopaedica Scandinavica. 1970;41:589–607. doi: 10.3109/17453677008991550. [DOI] [PubMed] [Google Scholar]
- Ogata K, Whiteside LA. Nutritional pathways of the intervertebral disc. An experimental study using hydrogen washout technique. Spine. 1981;6:211–216. [PubMed] [Google Scholar]
- Ohshima H, Urban JP, Bergel DH. Effect of static load on matrix synthesis rates in the intervertebral disc measured in vitro by a new perfusion technique. Journal of Orthopaedic Research. 1995;13:22–29. doi: 10.1002/jor.1100130106. [DOI] [PubMed] [Google Scholar]
- Oshima H, Ishihara H, Urban JP, Tsuji H. The use of coccygeal discs to study intervertebral disc metabolism. Journal of Orthopaedic Research. 1993;11:332–338. doi: 10.1002/jor.1100110304. [DOI] [PubMed] [Google Scholar]
- Perie D, Korda D, Iatridis JC. Confined compression experiments on bovine nucleus pulposus and annulus fibrosus: sensitivity of the experiment in the determination of compressive modulus and hydraulic permeability. Journal of Biomechanics. 2005;38:2164–2171. doi: 10.1016/j.jbiomech.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Perie DS, Maclean JJ, Owen JP, Iatridis JC. Correlating material properties with tissue composition in enzymatically digested bovine annulus fibrosus and nucleus pulposus tissue. Annals of Biomedical Engineering. 2006;34:769–777. doi: 10.1007/s10439-006-9091-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1–120. 2015 Retrieved from http://CRAN.R-project.org/package=nlme.
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2015. version 3.2.0. Retrieved from http://www.R-project.org/ [Google Scholar]
- Race A, Broom ND, Robertson P. Effect of loading rate and hydration on the mechanical properties of the disc. Spine. 2000;25:662–669. doi: 10.1097/00007632-200003150-00003. [DOI] [PubMed] [Google Scholar]
- Rajasekaran S, Babu JN, Arun R, Armstrong BR, Shetty AP, Murugan S. A study of diffusion in human lumbar discs: a serial magnetic resonance imaging study documenting the influence of the endplate on diffusion in normal and degenerate discs. Spine. 2004;29:2654–2667. doi: 10.1097/01.brs.0000148014.15210.64. [DOI] [PubMed] [Google Scholar]
- Rajasekaran S, Bajaj N, Tubaki V, Kanna RM, Shetty AP. The anatomy of failure in lumbar disc herniation: an in vivo, multimodal, prospective study of 181 subjects. Spine. 2013;38:1491–1500. doi: 10.1097/BRS.0b013e31829a6fa6. [DOI] [PubMed] [Google Scholar]
- Rajasekaran S, Venkatadass K, Naresh Babu J, Ganesh K, Shetty AP. Pharmacological enhancement of disc diffusion and differentiation of healthy, ageing and degenerated discs: Results from in-vivo serial post-contrast MRI studies in 365 human lumbar discs. European Spine Journal. 2008;17:626–643. doi: 10.1007/s00586-008-0645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekaran S, Vidyadhara S, Subbiah M, Kamath V, Karunanithi R, Shetty AP, Venkateswaran K, Babu M, Meenakshi J. A study of effects of in vivo mechanical forces on human lumbar discs with scoliotic disc as a biological model: results from serial postcontrast diffusion studies, histopathology and biochemical analysis of twenty-one human lumbar scoliotic discs. Spine. 2010;35:1930–1943. doi: 10.1097/BRS.0b013e3181e9a156. [DOI] [PubMed] [Google Scholar]
- Roberts S, Menage J, Eisenstein SM. The cartilage end-plate and intervertebral disc in scoliosis: calcification and other sequelae. Journal of Orthopaedic Research. 1993;11:747–757. doi: 10.1002/jor.1100110517. [DOI] [PubMed] [Google Scholar]
- Roberts S, Menage J, Urban JP. Biochemical and structural properties of the cartilage end-plate and its relation to the intervertebral disc. Spine. 1989;14:166–174. doi: 10.1097/00007632-198902000-00005. [DOI] [PubMed] [Google Scholar]
- Roberts S, Urban JP, Evans H, Eisenstein SM. Transport properties of the human cartilage endplate in relation to its composition and calcification. Spine. 1996;21:415–420. doi: 10.1097/00007632-199602150-00003. [DOI] [PubMed] [Google Scholar]
- Setton LA, Zhu W, Weidenbaum M, Ratcliffe A, Mow VC. Compressive properties of the cartilaginous end-plate of the baboon lumbar spine. Journal of Orthopaedic Research. 1993;11:228–239. doi: 10.1002/jor.1100110210. [DOI] [PubMed] [Google Scholar]
- Simunic DI, Broom ND, Robertson PA. Biomechanical factors influencing nuclear disruption of the intervertebral disc. Spine. 2001;26:1223–1230. doi: 10.1097/00007632-200106010-00010. [DOI] [PubMed] [Google Scholar]
- Soltz MA, Ateshian GA. Interstitial fluid pressurization during confined compression cyclical loading of articular cartilage. Annals of Biomedical Engineering. 2000;28:150–159. doi: 10.1114/1.239. [DOI] [PubMed] [Google Scholar]
- Thompson JP, Pearce RH, Schechter MT, Adams ME, Tsang IK, Bishop PB. Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine. 1990;15:411–415. doi: 10.1097/00007632-199005000-00012. [DOI] [PubMed] [Google Scholar]
- Urban JP, Holm S, Maroudas A, Nachemson A. Nutrition of the intervertebral disc: effect of fluid flow on solute transport. Clinical Orthopaedics and Related Research. 1982:296–302. [PubMed] [Google Scholar]
- Urban JP, McMullin JF. Swelling pressure of the inervertebral disc: influence of proteoglycan and collagen contents. Biorheology. 1985;22:145–157. doi: 10.3233/bir-1985-22205. [DOI] [PubMed] [Google Scholar]
- Urban JP, McMullin JF. Swelling pressure of the lumbar intervertebral discs: influence of age, spinal level, composition, and degeneration. Spine. 1988;13:179–187. doi: 10.1097/00007632-198802000-00009. [DOI] [PubMed] [Google Scholar]
- Urban JP, Roberts S. Degeneration of the intervertebral disc. Arthritis Research & Therapy. 2003;5:120–130. doi: 10.1186/ar629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban JP, Smith S, Fairbank JC. Nutrition of the intervertebral disc. Spine. 2004;29:2700–2709. doi: 10.1097/01.brs.0000146499.97948.52. [DOI] [PubMed] [Google Scholar]
- Veres SP, Robertson PA, Broom ND. How loading rate influences disc failure mechanics: a microstructural assessment of internal disruption. Spine. 2010;35:1897–1908. doi: 10.1097/BRS.0b013e3181d9b69e. [DOI] [PubMed] [Google Scholar]
- Vernon-Roberts B, Moore RJ, Fraser RD. The natural history of age-related disc degeneration: the pathology and sequelae of tears. Spine. 2007;32:2797–2804. doi: 10.1097/BRS.0b013e31815b64d2. [DOI] [PubMed] [Google Scholar]
- Videman T, Nurminen M. The occurrence of anular tears and their relation to lifetime back pain history: a cadaveric study using barium sulfate discography. Spine. 2004;29:2668–2676. doi: 10.1097/01.brs.0000146461.27105.2b. [DOI] [PubMed] [Google Scholar]
- Wu Y, Cisewski S, Sachs BL, Yao H. Effect of cartilage endplate on cell based disc regeneration: a finite element analysis. Molecular & Cellular Biomechanics. 2013;10:159–182. [PMC free article] [PubMed] [Google Scholar]
- Yao H, Justiz MA, Flagler D, Gu WY. Effects of swelling pressure and hydraulic permeability on dynamic compressive behavior of lumbar annulus fibrosus. Annals of Biomedical Engineering. 2002;30:1234–1241. doi: 10.1114/1.1523920. [DOI] [PubMed] [Google Scholar]
- Yu J, Fairbank JC, Roberts S, Urban JP. The elastic fiber network of the anulus fibrosus of the normal and scoliotic human intervertebral disc. Spine. 2005;30:1815–1820. doi: 10.1097/01.brs.0000173899.97415.5b. [DOI] [PubMed] [Google Scholar]