Abstract
Nitrogenases are found in some microorganisms, and these enzymes convert atmospheric N2 to ammonia, thereby providing essential nitrogen atoms for higher organisms. Some nitrogenases reduce atmospheric N2 at the FeMoco, a sulfur-rich iron-molybdenum cluster1–5. The iron centers that are coordinated to sulfur and carbon atoms in FeMoco have been proposed as the substrate binding sites, based on kinetic and spectroscopic studies5,6. Studies on the enzyme indicate that iron atom Fe6 and possibly also adjacent belt iron sites are involved.5–8 In the resting state, the central Fe sites (including Fe6) have identical environments consisting of three sulfides and a carbide. Addition of electrons to the resting state causes the FeMoco to react with N2, but the geometry and bonding environment of N2-bound species remain unknown5. In this manuscript, we describe a synthetic complex with a sulfur-rich coordination sphere that, upon reduction, breaks an Fe-S bond and binds N2. The product is the first synthetic Fe–N2 complex in which iron has bonds to sulfur and carbon atoms, providing a model for N2 coordination in the FeMoco. Our results demonstrate that breaking an Fe-S bond is a chemically reasonable route to N2 binding in the FeMoco, and show structural and spectroscopic details for weakened N2 on a sulfur-rich iron site.
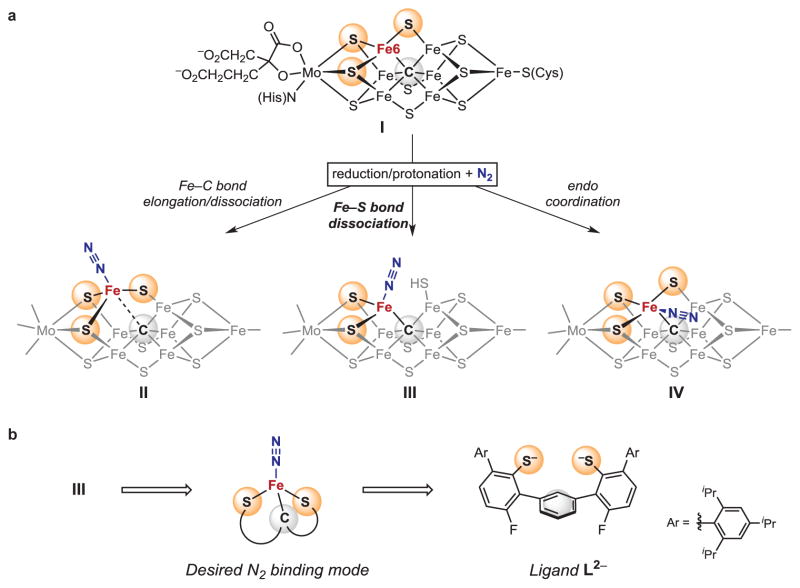
N2 binding to FeMoco is believed to take place at an iron center with three sulfur ligands following Fe-C bond elongation or dissociation (I to II, Fig. 1A)9–15. These sites could accommodate N2 binding by breaking an Fe-S or an Fe-C bond, but data on the enzyme do not yet distinguish between these possibilities. The likely enzymatic intermediates, iron-N2 species supported either solely by sulfur, or by sulfur and carbon ligands, are unprecedented in isolated coordination complexes. Here, we focus on an alternative hypothesis where one of the Fe-S bonds at the active site is broken upon reduction/protonation to expose the N2 binding site (I to III, Fig. 1A)16,17. N2 would thus bind at a pseudotetrahedral S,S,C-bound iron site. The feasibility of Fe-S bond cleavage in FeMoco is experimentally supported by the loss of this S atom in the structure of CO-inhibited nitrogenase7, and by the observation of Fe-S cleavage upon protonation in smaller FeS clusters18,19. Other N2 binding hypotheses include side-on binding, bridging, and endo coordination where N2 is positioned close to three additional iron atoms and opposite to a sulfur atom (IV, Fig. 1A)5,11,17.
Figure 1. N2 binding to iron in sulfur and carbon rich environments.
a, Schematic representations of FeMoco and three potential N2 binding modes. Potentially protonated sulfur ligands are not specified. b, Ligand design for a synthetic sulfur-carbon site.
Iron-N2 complexes supported solely by sulfur, or by sulfur and carbon supporting ligands, are likely N2-bound species in the nitrogenase catalytic cycle, but they are experimentally unprecedented. Though chemists have prepared complex iron-sulfur clusters inspired by the multimetallic structure of FeMoco, N2 does not bind to any known synthetic iron-sulfur cluster20. A number of well-defined iron complexes with B, N, and P supporting ligands are known to activate N2, and Peters has established P- and C-based systems capable of performing catalytic reduction of N2 to ammonia14,15,21–24. A few iron-N2 complexes have thioether/thiolate donors on the same iron center, and each is additionally supported by P- or N- donors25–27. To the best of our knowledge, there are no examples of terminal N2 complexes of any metal having immediate ligand environments similar to those in II-IV, which hinders scientists’ ability to predict the behavior of the FeMoco.
For this work, we designed bis(thiolate) ligand L2−, which offers only sulfur and carbon based coordination sites (indicated by yellow and grey spheres in Fig. 1B). Our approach was guided by the proposed binding mode III in Fig. 1A, which requires the presence of two coordinating sulfur atoms. These are provided by two chelating arylthiolate donors with bulky 2,4,6-triisopropylphenyl groups shielding the S sites. A central aromatic ring connects the two arylthiolate arms and additionally provides potential carbon based attachment sites28. Although carbide is electronically different than the arene ring in L2−, each could provide flexible bonding for stabilization of various intermediates during ammonia production14,15.
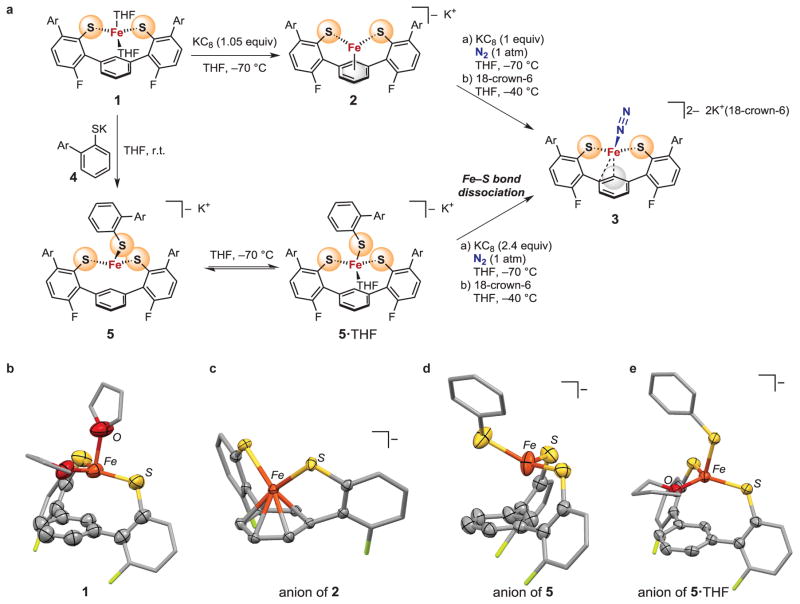
Iron(II) ions were installed in the ligand framework by treating LH2 with iron(II) bis(bis(trimethylsilyl)amide) in tetrahydrofuran (THF), which yielded the bright yellow, high spin iron(II) complex LFe(THF)2 (1, Fig. 2A). Its crystal structure reveals that it is four-coordinate, and that all Fe-C distances are at least 2.59 Å (Fig. 2B). Reduction of 1 to iron(I) with potassium graphite (KC8) results in the formation of brown-yellow 2, with close Fe-C distances (2.04 – 2.12 Å) indicating η6-binding of the central arene ring (Fig. 2A, 2C). Comparison of the molecular structures of 1 and 2 reveals that rotation of the arylthiolate arms enables the central aryl ring to move closer to the iron atom. Compound 2 has a rhombic EPR (electron paramagnetic resonance) spectrum with g = [2.180, 2.020, 1.989] and a solution magnetic moment of 2.1 μB, which indicate a low-spin (S = 1/2) iron(I) center.
Figure 2. N2 binding at an iron-sulfur-carbon site through Fe-S bond cleavage.
a, Reactions of synthetic iron-sulfur sites leading to N2-binding. The bottom pathway shows Fe-S cleavage with N2 binding. Ar = 2,4,6-triisopropylphenyl. b–e, Molecular structures of the synthetic mononuclear iron-sulfur sites presented here. Hydrogen atoms and Ar groups are omitted for clarity.
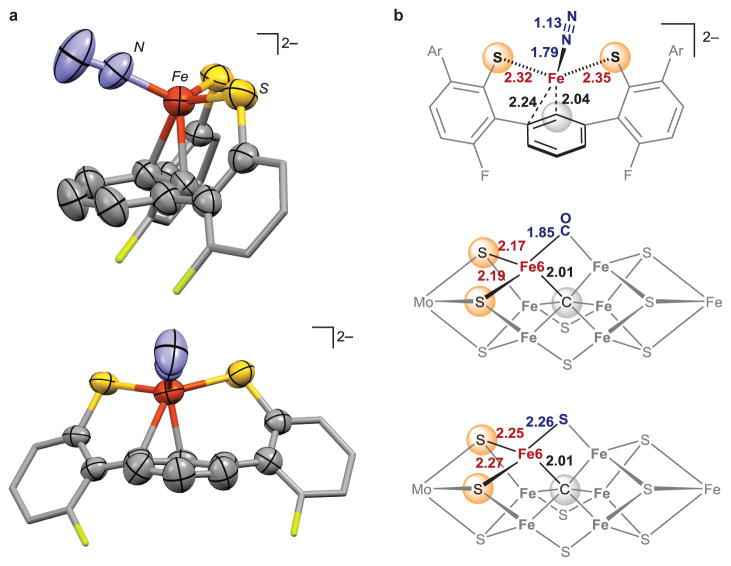
Encouraged by the ability of the ligand to stabilize low-valent iron sites, we further reduced the iron site to the iron(0) oxidation state. Reduction of a brown-yellow solution of 2 with one equivalent of KC8 under one atmosphere of N2 at −70 °C resulted in an immediate color change to deep red. After addition of 18-crown-6 to sequester potassium cations, dark red-brown crystals of 3 grew at −40 °C. X-ray diffraction analysis shows that 3 is [LFeN2][K(18-crown-6)(THF)2]2 (Fig. 2A and 3A). In 3, N2 is bound as a terminal ligand at a pseudotetrahedral iron(0) site, which is further bound to two S atoms and the arene of the supporting ligand. The closest Fe-C distance in 3 is 2.04 Å, and there is a second carbon atom within bonding distance (Fe-C = 2.24 Å), indicating asymmetric η2 coordination of the arene. The potassium cations do not bind to the N2 ligand.
Figure 3. Iron-N2 complex supported by sulfur and carbon ligands.
a, Two views of the molecular structure of the anionic part of 3. Hydrogen atoms and Ar groups are omitted. b, Comparison of geometric parameters with CO-inhibited FeMoco7 and resting state FeMoco2. All distances are reported in Ångstrøms.
The new N2 complex 3 provides a structural model of the pseudotetrahedral S,S,C supported N2 binding mode III proposed for FeMoco (Fig. 1A). It is compared to the experimental structures of resting state FeMoco and CO-inhibited FeMoco in Fig. 3B2,7. In the fourth coordination site that has labile S and CO ligands in nitrogenase structures7, 3 contains an N2 ligand. The Fe-S bond distances in 3 (2.32–2.35 Å) are somewhat longer than the Fe-S bonds in resting state FeMoco (2.25–2.27 Å), due to either the lesser negative charge of the thiolate or the greater steric hindrance. Remarkably, the Fe-C distance in 3 at 2.04 Å is very close to the Fe6-carbide distance of 2.01 Å in FeMoco structures. Overall, the relatively simple ligand L2− is capable of arranging appropriate atoms around iron and imparting a geometry that resembles the likely active iron site in FeMoco structures. However, the electronic structure of the iron(0) complex 3 may be different than the iron site in the N2-binding form of the FeMoco (for which the structure and iron oxidation state are unknown).
Next, we designed a compound (5) intended to test the idea that Fe-S bond dissociation could provide a coordination site for N2 binding (I to III in Fig. 1A). The bis(thiolate) complex 1 reacted with thiolate 4 to give the iron tris(thiolate) complex 5 (Fig. 2A). This orange high-spin iron(II) complex contains three S ligands, like Fe6 in the FeMoco resting state (I in Fig. 1). The interaction of iron with the central arene ring is weak, with the closest Fe-C distance at 2.48 Å (Fig. 2D). Thus we view this site as three-coordinate and unsaturated, which is supported by the reversible binding of one THF molecule at low temperature (Fig. 2E and Supplementary Information show the X-ray crystal structure of 5·THF and temperature-dependent UV-vis spectra).
The tris(thiolate) iron(II) site in 5/5·THF was reduced to the iron(0) oxidation level with just over two equivalents of KC8, under conditions otherwise equivalent to those used for reduction of the iron(I) bis(thiolate) complex 2 (Fig. 2A). This yielded the same N2 complex 3 described above, and 1.0 equiv. of free thiolate was produced. Reduction thus causes an Fe-S bond to break concomitant with N2 binding, as in the proposed pathway for N2 binding to FeMoco in Fig. 1A (I to III). We note that tris(thiolate) 5 contains all the nearby atoms to support alternative binding modes II and IV in Fig. 1A, but Fe-S dissociation takes place instead.
We return to describe the further characterization of 3, which gives insight into potential properties of N2 after binding at FeMoco. Though complex 3 is very thermally sensitive, it was possible to isolate pure samples of 3 in >80% yield from reduction of 5 at low temperature and washing the crystals with cold butane at −70 °C. Analysis of these crystals by Mössbauer spectroscopy confirms the presence of a single iron species. Infrared spectroscopy (IR) analysis of single crystals of 3 revealed a strong N-N stretching band at 1880 cm−1. These frequencies are the lowest observed for any Fe-N2 complex with a terminal N2 ligand23, which shows that the thiolates are powerful electron donors that enable substantial backbonding into the N2 π* orbitals. The N2 ligand in 3 exchanges with free 15N2 (giving an 15N-15N stretching band at 1813 cm−1) at −70 °C in the solid state. Samples of 3 kept at room temperature for a few hours lack the N2 stretching vibration, further demonstrating the lability of N2. The lability suggests that the Fe-N2 interaction, though strong as judged by IR spectroscopy, may be compensated with tighter binding to the arene ring.
Compound 3 has a high spin (S = 1) electronic configuration, as determined by SQUID (superconducting quantum interference device) magnetometry on a crystalline sample. This experimental observation was confirmed with density functional theory calculations on a truncated model of 3. Optimization with S = 1 gave a model close to the experimental geometry, but optimization with S = 0 gave significantly different bond lengths and angles, and a Gibbs free energy (ΔG°) that was higher by 37 kJ/mol (see Supplementary Information). High spin iron(0) dinitrogen complexes are rare, and have been seen mainly in cases where high symmetry makes the frontier orbitals nearly degenerate29,30. To our knowledge, 3 is the first high spin iron complex that contains both S and N2 ligands25,26, and shows that high-spin iron (as expected in the weak-field sulfur-dominated environment of iron atoms in the FeMoco) can activate N2 substantially.
The preparation of an iron-N2 complex with a sulfur-rich environment provides structural and spectroscopic precedents for FeMoco-N2 binding, and also gives insight into the nitrogenase mechanism. Reduction of complex 5 breaks an Fe-S bond as in the hypothetical conversion of I to III in the FeMoco (Fig. 1), and binds N2 in a form where the N-N bond is greatly weakened. In this way, the results support the idea that the sulfur-rich iron site in the FeMoco is particularly well-suited for N2 activation, and that Fe-S bonds can be easily broken upon reduction to allow binding of N2.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (GM065313 to P.L.H.) and the Max Planck Society (E.B.). We thank Andreas Göbels for measurement of SQUID data and Gary Brudvig for use of an EPR spectrometer. Elemental analysis data were from the CENTC Elemental Analysis Facility at the University of Rochester, funded by the NSF (CHE-0650456), and we thank William Brennessel for collecting these data. This work was supported in part by the facilities and staff of the Yale High Performance Computing Center, which was partially funded by the NSF (CNS 08-21132). We thank Jim Mayer, Nilay Hazari, Simon Bonyhady, Nicholas Arnet, and Cory MacLeod for constructive criticism on the manuscript.
Footnotes
Supplementary Information is available in the online version of the paper.
Author Contributions I.Č. designed the iron-sulfur-carbon system for N2 binding, performed the laboratory experiments, and analyzed data. B.Q.M. collected and interpreted crystallographic data. E.B. interpreted solid-state (SQUID) magnetic data. D.J.V. collected and fit EPR data. P.L.H. supervised the research, and I.Č. and P.L.H. wrote the manuscript.
X-ray crystallographic data have been deposited in the Cambridge Crystallographic Data Centre (http://www.ccdc.cam.ac.uk/) with deposition numbers CCDC1402555-1402559.
The authors declare no competing financial interests.
Readers are welcome to comment on the online version of this article at www.nature.com/nature.
References
- 1.Einsle O, et al. Nitrogenase MoFe-Protein at 1.16 Å Resolution: A Central Ligand in the FeMo-Cofactor. Science. 2002;297:1696–1700. doi: 10.1126/science.1073877. [DOI] [PubMed] [Google Scholar]
- 2.Spatzal T, et al. Evidence for Interstitial Carbon in Nitrogenase FeMo Cofactor. Science. 2011;334:940. doi: 10.1126/science.1214025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lancaster KM, et al. X-ray Emission Spectroscopy Evidences a Central Carbon in the Nitrogenase Iron-Molybdenum Cofactor. Science. 2011;334:974–977. doi: 10.1126/science.1206445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiig JA, Hu Y, Lee CC, Ribbe MW. Radical SAM-Dependent Carbon Insertion into the Nitrogenase M-Cluster. Science. 2012;337:1672–1675. doi: 10.1126/science.1224603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffman BM, Lukoyanov D, Yang ZY, Dean DR, Seefeldt LC. Mechanism of Nitrogen Fixation by Nitrogenase: The Next Stage. Chem Rev. 2014;114:4041–4062. doi: 10.1021/cr400641x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seefeldt LC, Hoffman BM, Dean DR. Mechanism of Mo-Dependent Nitrogenase. Annu Rev Biochem. 2009;78:701–722. doi: 10.1146/annurev.biochem.78.070907.103812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spatzal T, Perez KA, Einsle O, Howard JB, Rees DC. Ligand binding to the FeMo-cofactor: Structures of CO-bound and reactivated nitrogenase. Science. 2014;345:1620–1623. doi: 10.1126/science.1256679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yandulov DV, Schrock RR. Catalytic Reduction of Dinitrogen to Ammonia at a Single Molybdenum Center. Science. 2003;301:76–78. doi: 10.1126/science.1085326. [DOI] [PubMed] [Google Scholar]
- 9.Holland PL. Low-coordinate iron complexes as synthetic models of nitrogenase. Can J Chem. 2005;83:296–301. [Google Scholar]
- 10.MacBeth CE, Harkins SB, Peters JC. Synthesis and characterization of cationic iron complexes supported by the neutral ligands NPi-Pr3, NArPi-Pr3, and NSt-Bu3. Can J Chem. 2005;83:332–340. [Google Scholar]
- 11.Dance I. Ramifications of C-centering rather than N-centering of the active site FeMo-co of the enzyme nitrogenase. Dalton Trans. 2012;41:4859–4865. doi: 10.1039/c2dt00049k. [DOI] [PubMed] [Google Scholar]
- 12.Hinnemann B, Nørskov JK. Chemical Activity of the Nitrogenase FeMo Cofactor with a Central Nitrogen Ligand: Density Functional Study. J Am Chem Soc. 2004;126:3920–3927. doi: 10.1021/ja037792s. [DOI] [PubMed] [Google Scholar]
- 13.George SJ, et al. EXAFS and NRVS Reveal a Conformational Distortion of the FeMo-cofactor in the MoFe Nitrogenase Propargyl Alcohol Complex. J Inorg Biochem. 2012;112:85–92. doi: 10.1016/j.jinorgbio.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Creutz SE, Peters JC. Catalytic Reduction of N2 to NH3 by an Fe–N2 Complex Featuring a C-Atom Anchor. J Am Chem Soc. 2014;136:1105–1115. doi: 10.1021/ja4114962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson JS, Rittle J, Peters JC. Catalytic conversion of nitrogen to ammonia by an iron model complex. Nature. 2013;501:84–87. doi: 10.1038/nature12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kästner J, Blöchl PE. Ammonia Production at the FeMo Cofactor of Nitrogenase: Results from Density Functional Theory. J Am Chem Soc. 2007;129:2998–3006. doi: 10.1021/ja068618h. [DOI] [PubMed] [Google Scholar]
- 17.Schimpl J, Petrilli HM, Blöchl PE. Nitrogen Binding to the FeMo-Cofactor of Nitrogenase. J Am Chem Soc. 2003;125:15772–15778. doi: 10.1021/ja0367997. [DOI] [PubMed] [Google Scholar]
- 18.Alwaaly A, Dance I, Henderson RA. Unexpected explanation for the enigmatic acid-catalysed reactivity of [Fe4S4X4]2− clusters. Chem Commun. 2014;50:4799–4802. doi: 10.1039/c4cc00922c. [DOI] [PubMed] [Google Scholar]
- 19.Saouma CT, Morris WD, Darcy JW, Mayer JM. Protonation and Proton-Coupled Electron Transfer at S-Ligated [4Fe-4S] Clusters. Chem Eur J. 2015;21:9256–9260. doi: 10.1002/chem.201500152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee SC, Lo W, Holm RH. Developments in the Biomimetic Chemistry of Cubane-Type and Higher Nuclearity Iron–Sulfur Clusters. Chem Rev. 2014;114:3579–3600. doi: 10.1021/cr4004067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ung G, Peters JC. Low-Temperature N2 Binding to Two-Coordinate L2Fe0 Enables Reductive Trapping of L2FeN2− and NH3 Generation. Angew Chem Int Ed. 2015;54:532–535. doi: 10.1002/anie.201409454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez MM, Bill E, Brennessel WW, Holland PL. N2 Reduction and Hydrogenation to Ammonia by a Molecular Iron-Potassium Complex. Science. 2011;334:780–783. doi: 10.1126/science.1211906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hazari N. Homogeneous iron complexes for the conversion of dinitrogen into ammonia and hydrazine. Chem Soc Rev. 2010;39:4044–4056. doi: 10.1039/b919680n. [DOI] [PubMed] [Google Scholar]
- 24.Danopoulos AA, Wright JA, Motherwell WB. Molecular N2 complexes of iron stabilised by N-heterocyclic ‘pincer’ dicarbene ligands. Chem Commun. 2005:784–786. doi: 10.1039/b415562a. [DOI] [PubMed] [Google Scholar]
- 25.Takaoka A, Mankad NP, Peters JC. Dinitrogen Complexes of Sulfur-Ligated Iron. J Am Chem Soc. 2011;133:8440–8443. doi: 10.1021/ja2020907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bart SC, Lobkovsky E, Bill E, Wieghardt K, Chirik PJ. Neutral-Ligand Complexes of Bis(imino)pyridine Iron: Synthesis, Structure, and Spectroscopy. Inorg Chem. 2007;46:7055–7063. doi: 10.1021/ic700869h. [DOI] [PubMed] [Google Scholar]
- 27.Creutz SE, Peters JC. Diiron Bridged-Thiolate Complexes That Bind N2 at the FeIIFeII, FeIIFeI, and FeIFeI Redox States. J Am Chem Soc. 2015;137:7310–7313. doi: 10.1021/jacs.5b04738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ellison JJ, Ruhlandt-Senge K, Power PP. Synthesis and Characterization of Thiolato Complexes with Two-Coordinate Iron(II) Angew Chem Int Ed Engl. 1994;33:1178–1180. [Google Scholar]
- 29.Suess DLM, Peters JC. H–H and Si–H Bond Addition to Fe≡NNR2 Intermediates Derived from N2. J Am Chem Soc. 2013;135:4938–4941. doi: 10.1021/ja400836u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moret ME, Peters JC. Terminal Iron Dinitrogen and Iron Imide Complexes Supported by a Tris(phosphino)borane Ligand. Angew Chem Int Ed. 2011;50:2063–2067. doi: 10.1002/anie.201006918. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.