Abstract
Hepatitis C virus (HCV) is the world’s most common blood-borne viral infection for which there is no vaccine. The rates of vertical transmission range between 3–6% with odds 90% higher in the presence of HIV co-infection. Prevention of vertical transmission is not possible due to lack of an approved therapy for use in pregnancy or an effective vaccine. Recently, HCV has been identified as an independent risk factor for pre-term delivery, perinatal mortality and other complications. In this study, we characterized the immune responses that contribute to the control of viral infection at the maternal-fetal interface (MFI) in the early gestational stages. Here we show that primary human trophoblast cells and an extravillous trophoblast cell line (HTR8), from first and second trimester of pregnancy, express receptors relevant for HCV binding/entry and are permissive for HCV-uptake. We found that HCV-RNA sensing by human trophoblast cells induces robust up-regulation of Type I/III IFNs and secretion of multiple chemokines that elicit recruitment and activation of decidual NK cells. Furthermore, we observed that HCV-RNA transfection induces a pro-apoptotic response within HTR8 that could affect the morphology of the placenta.
For the first time, we demonstrate that HCV-RNA sensing by human trophoblast cells elicits a strong antiviral response that alters the recruitment and activation of innate immune cells at the MFI. This work provides a paradigm shift in our understanding of HCV-specific immunity at the MFI, as well as novel insights into mechanisms that limit vertical transmission, but may paradoxically lead to virus-related pregnancy complications.
INTRODUCTION
Hepatitis C Virus (HCV) is the most common cause of chronic hepatitis in the Western world (1). Only a minority (~20%) of individuals exposed to HCV can spontaneously clear the infection, and most infected patients remain undiagnosed (2). The disease burden from HCV is staggering, with HCV-related liver failure as a leading cause of cirrhosis, liver cancer, and indication for liver transplantation (3).
Among pregnant women, the worldwide prevalence of HCV infection ranges from 1–8%; in the U.S. alone, over 40,000 births annually are affected (4). Infection with HCV is an independent risk factor for pre-term delivery, perinatal mortality, intrauterine growth restriction, and other complications of pregnancy (5, 6). Vertical transmission rates are between 3–6% in women without HIV co-infection; however, in presence of HIV co-infection (7), the odds of vertical transmission are ~90% higher (8). Thus, vertical transmission of HCV is an important public health concern. No perinatal management strategy has been shown to reduce the risk for HCV transmission (9). Mother-to-child transmission has become the major route of transmission in children and the leading cause of pediatric HCV cases (10). After several years, almost all children with chronic viremia develop hepatitis and decompensated HCV-related cirrhosis has been reported in children as young as 4 years (11). Despite the successful development of new therapies for HCV, many of the new drug combinations still include ribavirin, which is teratogenic and therefore incompatible with pregnancy. In the absence of an HCV vaccine or approved therapy during pregnancy, a greater understanding of HCV-host interactions is required to minimize viral transmission while maintaining pregnancy and allowing normal fetal development.
The placenta consists of specialized epithelium (the trophoblast) and blood vessels that, with their supportive connecting tissue, provide a potential barrier against maternal-fetal transmission. However, this placental barrier is not completely protective and most viruses (including HCV and hepatitis B virus) can be transmitted to the fetus through the placenta (12). The placenta mediates exchange of nutrients and waste between the maternal and fetal blood supplies via passage across the trophoblast and endothelial cell layers (13). The two primary areas where placental trophoblasts come in contact with the maternal blood and immune system are the villous syncytiotrophoblast, which lines the surface of the placenta, and the extravillous trophoblast cells (EVTs), which migrate out from the placenta and invade the endometrium of the pregnant uterus (decidua). The multinucleate syncytiotrophoblast layer originates from fusion of progenitor cytotrophoblast cells and is bathed by maternal blood delivered by the spiral arteries into the intervillous space. EVTs help form a physical anchor from the placenta to the uterus and are in direct contact with maternal immune and decidua cells as well as blood passing through the maternal spiral arteries (14).
Decidualization is the process in early pregnancy whereby the endometrium transforms into the decidua in preparation for development of the placenta (15). During decidualization, maternal leukocytes traffic to the uterus where the fetus-derived placenta has implanted (16), and they ultimately compose about 40% of total cells in the decidua (17). Early in pregnancy, approximately 70% of decidual immune cells consist of CD56brightCD16neg NK cells (18). The origin of these decidual NK cells is unknown, but it has been speculated that a subpopulation of peripheral NK cells with a similar phenotype traffic to the uterus and subsequently proliferate and differentiate (18, 19). The mechanism by which immune cells are recruited to the uterus is not well understood but chemokines are likely to be involved (20, 21), as described in other tissues. Chemokines are known to modulate cell trafficking to sites of inflammation (22) and secondary lymphoid organs (23). EVTs express multiple chemokine receptors and their cognate ligands including the one for CXCR3 and CXCR4 (IP-10, I-TAC and SDF-1). Peripheral CD56brightCD16dim NK cells are known to express CXCR3 and CXCR4; therefore, it has been postulated that EVTs might facilitate the migration of this maternal immune cell subset into decidual tissue (21, 24). The ability to cross the placental barrier is one key determinant of invasive viruses and pathogens. Little is known about the ability of dNK cells to provide protective immunity against these agents (25).
The aim of this study was to identify antiviral responses triggered by HCV infection in the human placenta at the time of early intrauterine infection. We found that primary trophoblast isolated from normal pregnancies and an EVT cell line (HTR8), expressed receptors important for HCV viral binding and entry. We also detected the non-structural protein NS5A, involved in HCV viral replication, within primary term syncytialized cytotrophoblast cells and HTR8 infected with full length HCV JFH-1 virus (Japanese Fulminant Hepatitis). Villous explants responded to various forms of HCV, including founder/transmitted virus RNA, sucrose-purified JFH-1 virus particles, full length JFH-1 RNA and the HCV pathogen-associated molecular pattern (PAMP), by marked up-regulation of Type I and III IFNs in villous explants and induced apoptosis in HTR8. In villous explants and HTR8, HCV-RNA transfection also elicited secretion of chemotactic chemokines involved in the recruitment of NK cells. Finally, we found that conditioned media from HCV-RNA-transfected HTR8 cells induced recruitment and activation of decidual NK cells. Collectively, these data suggest, to our knowledge for the first time that the placenta functions as a highly active immune organ that coordinates the responses to HCV at the maternal-fetal interface (MFI).
MATERIALS AND METHODS
Ethics Statement
These studies were approved by the University of Colorado at Denver (UCD) Institutional Review Board (Protocol #06-1098, #08-0653 and #08-0364). All adult patients signed the informed consent before samples were taken. A parent or guardian of any child participant signed the informed consent on their behalf.
Tissue collection
These studies were approved by the University of Colorado at Denver (UCD) Institutional Review Board (Protocol #06-1098 and 08-0653). Placental and decidual samples were collected from normal term deliveries and elective terminations between 10 and 20 weeks gestation. Tissue was washed extensively in cold 1X phosphate-buffered saline (PBS, Ambion, Life Technologies, Grand Island NY) and stored in cytowash [DMEM/HighGlucose (Thermo Fisher Scientific, Waltham MA) with 2.5% fetal bovine serum (FBS), 1% Penicillin/Streptomycin, 0.1% Gentamycin and 1% Glutamine (Gibco, Life Technologies)] for transport on ice. All the samples were processed within 1 hour.
Cytotrophoblast isolation
Cytotrophoblasts were isolated from first or second trimester (26) and term placentas (27) as described previously. Briefly, placental tissues were cut into small pieces (~2–3 mm), carefully removing any obvious blood vessels, membranes and decidual tissue. The fragments of chorionic villi were subjected to a series of enzymatic digestions, which removed the overlying syncytium and detached cytotrophoblast cells from the underlying stromal cores of the chorionic villi. Detached cytotrophoblasts were isolated on a Percoll gradient. The final purity of the cells was 90–95% positive for cytokeratin-7, with CD45+ cell contamination <10%, as assessed by flow cytometry.
Villous explant culture
Placental tissues, stored in cytowash, were cut into small pieces (~2–3 mm), carefully removing any obvious blood vessels, membranes, and decidual tissues. The anchoring chorionic villous ends were plated on 8.0 μm Polycarbonate Membrane 6.5 mm inserts (Fisher Scientific) coated with 150 μl of Matrigel (BD Biosciences, San Jose, CA) for 15 minutes at 37°C in 5% CO2. DMEM/F12 media [Corning Cellgro, Manassas VA with 10% FBS, 1% Penicillin/Streptomycin and 0.1% Fungizone (Gibco, Life Technologies)] was then added to the top (20 μl) and bottom of the inserts (500 μl). The explants were left in culture overnight before any further stimulation.
Extraction of decidual mononuclear cells
Decidual tissue was separated from the placenta and placed in a tube filled with cytowash media. The tube was placed on ice and the sample was prepared within 1 hour. The decidua was washed with RPMI [Invitrogen, Carlsbad, CA with 10% FBS, 1% L-Glut, 10% Penicilin/Streptomycin (Gibco, Life Technologies), 10% Antibiotic-Antimycotic (Invitrogen) and 3.4 ml of IL-2 (Adesleukin, Novartis, Emeryville, CA)], placed on a large petri dish and cut into small pieces using two scalpels. Once the tissue was adequately minced into 3 mm pieces, it was placed in a 50ml conical tube containing digestion media [HBSS (Invitrogen) with 10% FBS, 250 mg collagenase, 100 mg DNAse (Sigma Aldrich, St. Louis, MO)] and placed on a plate rocker at 37°C for 1 hour. After the incubation, the tissue was passed through a 70μm filter and the cell suspension was centrifuged for 10 minutes at 335 × g. The supernatant was discarded and the pellet was re-suspended in 30ml of RPMI and spun again as described above. The cells were then frozen at 10 × 106/ml in freezing media [200 ml FBS, 10% RPMI with 1% Penicillin/Streptomycin (Gibco, Life Technologies) and 10% Human serum] until further analyses.
Cell lines
HTR-8/SVneo immortalized first trimester human trophoblast cells (28) (kindly provided by Dr. Virginia D Winn) were cultured in growth medium [RPMI 1640 medium supplemented with 1% Penicillin/Streptomycin (Gibco, Life Technologies) and 5% FBS,] at 37°C in 5% CO2.
Flow cytometric analysis of antigen expression
Multiparameter flow cytometry (FACS) was performed using a BD FACSCanto II instrument (BD Biosciences) compensated with single fluorochromes and analyzed using Diva™ software (BD Biosciences). Primary and immortalized trophoblast cells were identified by their characteristic forward scatter/side scatter (fsc/ssc) properties. Anti-CD45-FITC (clone HI30), anti-CD45-PE-Cy7 (clone HI30), anti-CD3-V500 (cloneUCHT1), anti-CD56-APC (clone B159), anti-CD56-V450 (clone B159), anti-TLR9-APC (clone eB72-1665) and anti IFNγ-V450 (clone B27), were purchased from BD Biosciences. Anti-TLR3-PE (clone TLR3.7) and anti-TLR4-APC (clone HTA125) were purchased from eBioscience (San Diego, CA). Anti-LDL-R-APC (clone 472418), anti-TLR7-PerCP (clone 533707) and anti-Claudin-1-APC (clone 421203) were obtained from R&D systems (Minneapolis, MN). Anti-CD107a-PerCP/Cy5.5 (clone H4A3) was purchased from BioLegend (San Diego, CA). Rabbit monoclonal antibody anti-Occludin (clone EPR8202, Abcam, Cambridge, MA) and polyclonal rabbit anti-human SR-BI (Novus Biologicals, Littleton, CO) were detected with anti-rabbit-Alexa Fluor 488 antibody (Invitrogen). Anti-CD45 and anti-Cytokeratin 7-FITC (clone LP5K, Millipore, Billerica MA) were used to identify primary cytotrophoblasts.
One million cells were stained for surface antigen expression at 4°C in the dark for 30 minutes and then washed in 2 ml PBS containing 1% bovine serum albumin (BSA) and 0.01% sodium azide (FACS Wash). The pellet was re-suspended in 100 μl of 2% paraformaldehyde (PFA, Sigma-Aldrich) and incubated for 20 minutes at 4°C. For intracellular antigens, one ml of BD PermBuffer III (BD Bioscience) was added overnight. Cells were then washed and incubated for 30 minutes in the presence of monoclonal Abs in the dark at 4°C and washed in 2 ml of FACS Wash. Isotype-matched control antibodies were used to determine background levels of staining.
Immunohistochemistry
First trimester (gestation age 5–8 weeks) were obtained from elective pregnancy termination (n=3–4 per antibody) and fixed in 4% PFA overnight, dehydrated through a series of increasing concentrations of ethanol, and embedded in paraffin. Sections (5 μm thick) were placed onto slides and subjected to antigen retrieval using a commercially available citric acid solution (BioCare Medical, Walnut Creek, CA). Alternatively, samples were fixed for 4 hours in 1% PFA, transferred to 18% sucrose, and embedded in OCT medium (Ted Pella Inc, Redding, CA). Then 10 μm thick sections were placed onto slides. All samples were blocked for nonspecific immunoglobulin binding in 10% normal goat, horse or rabbit serum. Primary antibodies against cytokeratin 7 (mouse clone CK-7, 5μg/ml, Abcam), CD81 (mouse clone B-11, 0.8 μg/ml, Santa Cruz Biotechnology, Dallas, TX), Claudin-1 (mouse clone 1C5-D9, 3 μg/ml, Abnova, Walnut, CA), LDL-R (rabbit polyclonal 3 μg/ml, Sigma), RIG-I (goat polyclonal, 5 μg/ml, LSBio, Seattle, WA), SR-B1 (rabbit polyclonal, 5 μg/ml, Thermo Fisher Scientific), TLR3 (rabbit polyclonal, 4 μg/ml, LSBio), TLR8 (rabbit polyclonal, 4 μg/ml, LSBio) or their controls were added to the tissues, which were then incubated overnight at 4°C, except in the case of TLR7 [rabbit clone EPR2088(2), 10 μg/ml, LSBio] antibody, which was added for 1 hour at room temperature. After addition of the appropriate secondary antibody, the samples were depleted of endogenous peroxidases by incubating in 0.5% H2O2/methanol. Antibody binding was detected using the streptavidin-peroxidase and aminoethyl carbazole reagent sets (Invitrogen), and tissues were lightly counterstained in Mayer hematoxylin. Positive staining was viewed microscopically as reddish-brown coloration, and images were captured on a Nikon Eclipse 80i microscope.
Immunofluorescence
Images were acquired on a Zeiss LSM 510 confocal microscope (Zeiss NLO 510 with META; Zeiss Plan-Apochromat 63/1.4NA oil; Thornwood, NY). Imaging settings were defined empirically to maximize the signal-to-noise ratio and to avoid saturation. In comparative imaging, all settings were kept constant between samples. The illumination was provided by 30 mW Argon (488 nm), 5 mW HeNe (633 nm) and 1 mW HeNe (543 nm) lasers. Image processing was performed using Zeiss ZEN 2009 software. Figures were mounted using Adobe Photoshop CS4 (Adobe System).
For the experimental setup, cells were seeded onto 35 mm glass bottom dishes (MatTek Corporation, Ashland, MA) and infected on day 1 and 7 with JFH1 at multiplicity of infection of 10 (MOI of 10). After 14 days of infection cells were fixed in 4% PFA in PBS. Cells were then blocked with 10% normal serum, labeled with primary polyclonal antibodies against HCV-NS5A (clone 9E10, 1:100, provided by C. Rice, Rockefeller University) and EEA1 (rabbit polyclonal 1:200, Abcam), and washed and labeled with the appropriate secondary antibody that was conjugated to Alexa-Fluor 633 or Alexa-Fluor 568 (Invitrogen). F-actin was concurrently stained with Alexa-Phalloidin 546 (Invitrogen). Images were acquired as described above.
HCV-PAMP preparation
pU/UC and X-region plasmids were kindly provided by Dr. Michael Gale (29). The plasmids were amplified using PCR (X-region Forward 5′-TAATACGACTCACTATAGGTGGCTCCATCTTAGCCCTA-3′; X-region Reverse 5′-ACTTGATCTGCAGAGAGGCCAGTATCA-3′; HCV pU/UC Forward 5′-TAATACGACTCACTATAGGCCATCCTGTTTTTTTCCC-3′; HCV pU/UC Reverse 5′-AAAGGAAAGAAAAGGAAAAAAAGAGG-3′) with a high fidelity polymerase (Invitrogen). The PCR products were separated by electrophoresis on an (1.5%) agarose gel. The bands of interest were extracted from the gel (Gel extraction kit, Qiagen, Valencia, CA) and transcribed in vitro (Applied Biosystems, SanFrancisco, CA). The final product was quantified using a Nanodrop microspectometer (Thermo Fisher Scientific).
HCV-PAMP stimulation
Villous explants were isolated from electively terminated in the first and second trimester and grown on Matrigel overnight at 37°C in 5% CO2 in RPMI (Invitrogen) supplemented with 10% FBS and 10 mM HEPES. HTR8 cells were plated in a 12 well low-adherence plate at a concentration of 1 × 106 cells in 1 ml per well. The next day, 1 μg of pU/UC RNA (PAMP) or X-region RNA (control) was transfected into the cells (co-incubation with Mirus 2250, Mirus Bio LLC, Madison, WI) for 6 or 24 hours at 37°C in 5% CO2. Cellular RNA was isolated using RNeasy Mini kit (Qiagen), quantified using a Nanodrop microspectometer and 1 μg of RNA was used to transcribe cDNA using Quantitect RT kit (Qiagen). qRT-PCR was performed using SYBR Green primers and master mix from Qiagen and run on a StepOnePlus qPCR machine (Applied Biosystems). Data was analyzed using the ΔΔCT method. All primers used in the qRT-PCR assays were purchased from Qiagen.
To assess apoptosis, HTR8 were plated overnight, and the next day HCV-PAMP (or X-region control) transfection was performed as described above. Cells were harvested after 24 hours of culture and stained, as described above with Anti-Active Caspase-2-AF647, Annexin V (BD Biosciences) following manufacturer’s instruction.
Chemotaxis Assay
NK cells were isolated using CD56 magnetic-labeled beads (Miltenyi Biotec, San Diego CA) from peripheral blood mononuclear cells (PBMCs) of healthy donors (patients were recruited and consented using UCD Institutional Review Board approved protocol #08-0364) and decidual mononuclear cells (DMNCs) of elective terminations (10 and 20 weeks gestation). Purity of isolated NK cells was assessed by flow cytometry (>80%). Cells were loaded (100 μl) onto Transwell filters (5-μm pore size, 24-well cell clusters; Corning) and were incubated in wells containing 600 μl of conditioned media of PAMP-transfected, X-region-transfected, JFH-1-transfected, mock-transfected or mock control media for 3 hours at 37°C and 5% CO2. The upper chambers were then removed and cells in the bottom chamber were collected and counted by flow cytometry. The migration index (MI) was calculated using the formula: MI=Mt/Mc where Mt is the migration area with PAMP and X-region-transfected or JFH-1 and mock-transfected conditioned media, and Mc is the mean migration area of control cultures.
ELISAs
IFNA (VeriKine Human Interferon Alpha, PBL Interferon Source, Piscataway, NJ], IFNL ELISA [VeriKine-DIY Human Interferon Lambda (IL-28B/29/28A)] and the Multi-Analyte ELISArray (Qiagen) were performed according to the manufacturer’s instructions. All supernatants were harvested at 24 or 48 hours after PAMP or X-region stimulation. Conditioned media of JFH-1 and mock transfected villous explants were harvest after 48 hours and analyzed by Multi Analyte ELISArray (Qiagen) according to manufacturer’s instructions.
Statistical analysis
Results are expressed as mean (± SEM). The Wilcoxon signed-rank test was used to compare fold increases of stimulated conditions with control conditions. The Mann-Whitney test and t-test were used to compare differences between groups. Calculations were performed using Graphpad Prism software. Statistical significance was defined as p < 0.05.
RESULTS
Human Trophoblast Cells Express Cellular Receptors Relevant for HCV Uptake and Sensing
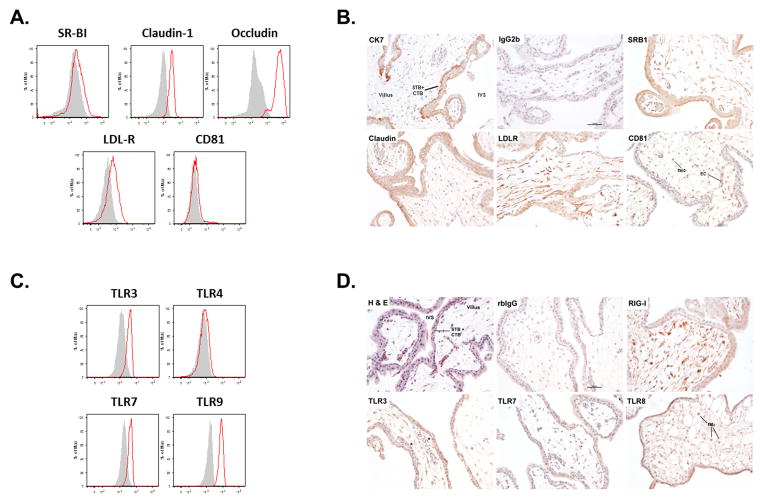
To investigate the expression of cell surface receptors important for HCV binding and entry, we stained primary human villous cytotrophoblast cells, isolated from first or second trimester elective pregnancy terminations (30, 31), for the scavenger receptor class B type I (SR-BI), the tight junction components Claudin-1 and Occludin, the lipoprotein receptor (LDL-R) and the tetraspanin CD81. Primary trophoblast cells expressed high levels of Occludin, Claudin-1 and LDL-R, which are necessary for HCV binding and entry (Figure 1A). Immunohistochemical staining for HCV binding and entry receptors confirmed in situ expression of these proteins in trophoblast cells (Figure 1B). First trimester syncytiotrophoblast and villous cytotrophoblast cells, both of which can be identified by cytokeratin 7 immunoreactivity, strongly and uniformly expressed SR-BI, Claudin-1 and LDL-R. Notably, syncytiotrophoblast expressed SR-BI most strongly at the apical brush border, which directly interfaces with maternal blood. Fetal fibroblasts and macrophages were also positive for SR-BI, Claudin-1, LDL-R, and CD81. Extravillous trophoblast cells (EVTs) that are associated with cell columns also strongly expressed SR-BI, Claudin-1 and LDL-R; again, these cells were negative for CD81 (data not shown). Together flow cytometric analysis and immunohistochemical staining unequivocally show that human villous cytotrophoblast cells and syncytiotrophoblast express multiple receptors necessary for HCV entry.
Figure 1. Characterization of primary villous cytotrophoblast and syncytiotrophoblast.
FACS analysis demonstrates that primary villous cytotrophoblast (between 10 and 20 week gestation) cells express (A) several receptors involved in HCV binding and entry and (C) are positive for Toll-like receptors (TLRs) 3, 7 and 9 (n=3). Shaded histograms represent isotype controls. (B) Immunohistochemical staining confirmed in situ expression of HCV receptors in trophoblast cells. (D) Syncytiotrophoblast and cytotrophoblast revealed positivity for the expression of RIG-I, TLR3, TLR7 and TLR8 (n=3 or 4). Photomicrographs were captured using a 20X objective; scale bars in the lower right corners of the (B) IgG2b and (D) rbIgG represent 100 μm. Intervillous space (IVS), the endothelial cell (EC), the syncytiotrophoblast (STB), the cytotrophoblasts (CTB) and the fetal macrophages (fMΦ) are shown.
We next asked whether trophoblast cells express the pattern recognition receptors (PRRs) necessary for conferring responsiveness to infection by HCV. Primary cytotrophoblast cells and villous explants express multiple PRRs implicated in innate antiviral immunity including Toll-like receptors (32, 33). Flow cytometry analyses of primary villous cytotrophoblast cells (isolated from first or second trimester placentas) showed expression of TLR3, TLR7 and TLR9 (Figure 1C). Immunohistochemical staining of first trimester placentas revealed strong immunoreactivity of cytotrophoblast, syncytiotrophoblast and EVTs for the retinoic acid inducible gene-I (RIG-I), the cytosolic receptor for HCV (34), as well as TLR3; weaker, but positive immunoreactivity was observed for TLR7 (Figure 1D). Syncytiotrophoblast and EVTs were also positive for TLR8; weaker and more variable staining of cytotrophoblast was observed. Further, fetal macrophages were strongly positive for all four pattern recognition receptors. The immortalized first trimester EVT cell line (HTR8) was also phenotyped by flow cytometry (Supplemental Figure 1), showing a profile similar to the primary cytotrophoblasts.
Human Trophoblasts are Permissive for HCV Uptake
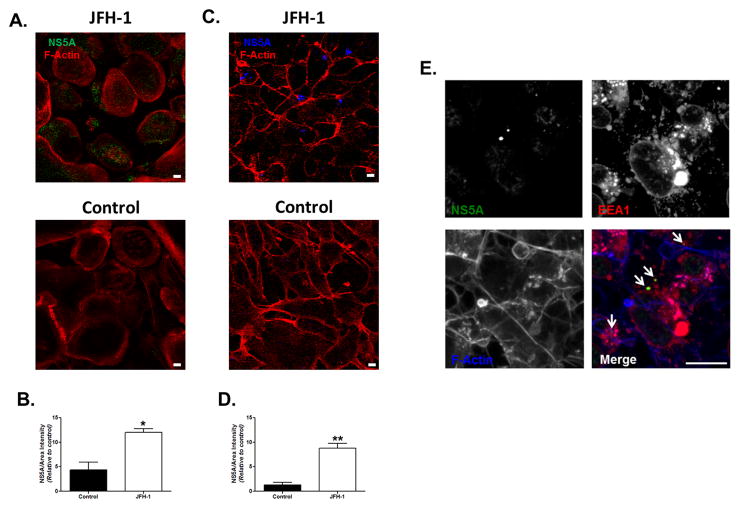
In order to determine whether HCV is taken up by human trophoblasts, we cultured primary term cytotrophoblast (Figure 2A–B) and HTR8 cells (Figure 2C–D) with full-length Japanese Fulminant Hepatitis-1 virus (JFH-1). After 14 days of culture with JFH-1 infected conditioned media, primary syncytialized cytotrophoblasts and HTR8 cells demonstrated HCV viral protein, in keeping with and extending a recent report (35). Co-localization of NS5A with the early endosomal antigen 1 (EEA1) suggests that endocytosis is involved in HCV uptake (Figure 2E) by human trophoblast cells.
Figure 2. HCV is taken up by human trophoblasts.
Primary cytotrophoblast cells from (A) term placentas and (C) HTR8 cells were plated for 14 days with supernatant of JFH-1 infected Huh7.5.1 at an MOI=10. Cells were stained for F-actin (red) and NS5A [green (A), blue (C)] (magnification 40X). Cells were visualized using confocal microscopy as described in the Methods. Bar represents 10 μm. Densitometry analysis confirmed significantly higher expression of NS5A protein in JFH-1-infected (B) primary syncytialized cytotrophoblasts and (D) HTR8 cells compared to non-infected control cells. Bars represent mean plus SEM, t test (n=3), * p<0.05, ** p<0.01. (E) HTR8 infected for 14 days with supernatant of JFH-1 infected Huh7.5.1 were stained for NS5A (green), early endosomal antigen 1 (EEA1, red) and actin (blue). Co-localization of NS5A with EEA1 suggests that endocytosis is involved in HCV uptake by human trophoblast cells.
Innate Responses Induced by HCV Sensing in Human Trophoblasts
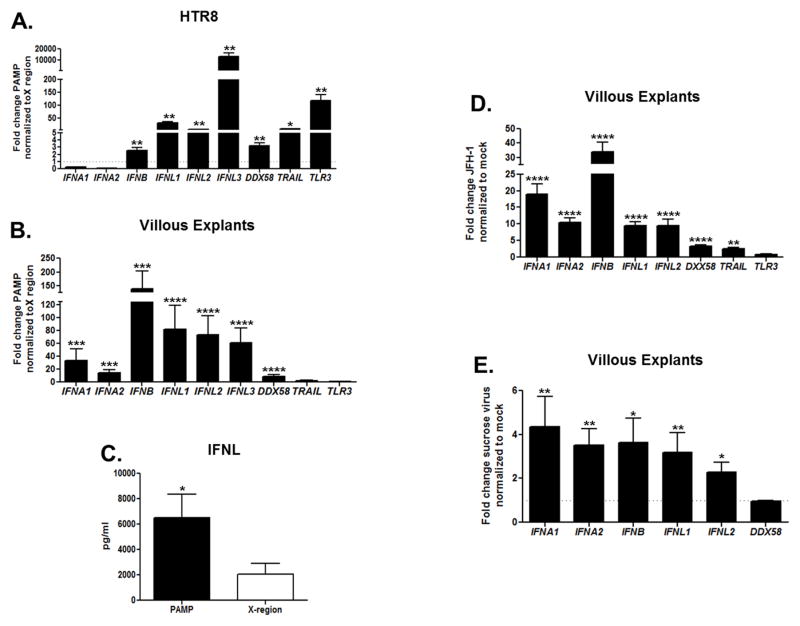
To elucidate the immune responses generated by HCV, we transfected human trophoblasts with a pathogen-associated molecular pattern (PAMP) specifically expressed by HCV (polyuridine motif of the HCV genome 3′ non-translated region), previously shown to function as the substrate of retinoic acid-inducible gene (RIG-I), the cytosolic PRR for HCV (29, 36, 37). Extensive analysis and characterization of the HCV genomic RNA has identified that the pU/UC tract in the 3′ UTR has the greatest capacity to stimulate interferon beta (IFNB) production in hepatocytes (29). As a negative control, trophoblast cells were transfected with the adjacent highly-conserved X-region (3′-UTR), which is non-immunogenic in hepatocytes (29). HCV-PAMP transfection of HTR8 cells (Figure 3A) and also of villous explants (Figure 3B) for 6 hours induced robust up-regulation of several key antiviral genes. Of particular interest is the induction of Type III IFN mRNAs [IFNL1 (IL29), IFNL2 (IL28A), and IFNL3 (IL28B)], recently identified as critical for the host defense against HCV (1, 38, 39). Moreover, supernatants from HCV-PAMP-stimulated placental explants showed extremely high levels of interferon lambda (IFNL) (>5000 pg/ml) at 24 hours (Figure 3C) compared control (29, 40). Interferon alpha (IFNA) production was also 2.3 fold higher (58.9 pg/ml,) in PAMP-transfected villous explants compared to our specificity control (data not shown). Next, we transfected villous explants for 6 hours with full JFH-1 RNA. Also, in this case we detected significant up-regulation of Type I and Type III IFN genes, similar to the immune response we observed after HCV-PAMP RNA transfection (Figure 3D). Moreover, when villous explants were cultured with supernatant containing sucrose-purified JFH-1 viral particles (41) (Figure 3E) and viral-RNA of a full-length infectious molecular clone of HCV known to efficiently transmit infection [transmitted founder (T/F) virus (42, 43)] (Supplemental Figure 2) for 48 hours, we found significant transcription of multiple Type I and Type III IFN genes. These data indicate that HCV is taken up directly by human trophoblasts and induces innate immune responses.
Figure 3. HCV-RNA sensing by human trophoblasts induces strong Type I and III IFN responses.
(A) HTR8 (n=3) and (B) villous explants (n=8) were transfected with the HCV-PAMP for 6 hours (normalized to X-region control, 3′-UTR). Gene up-regulation was assessed by real-time RT-PCR. Bars represent mean plus SEM, Wilcoxon signed-rank test. (C) IFNL protein secretion was higher in supernatants of 24 hours HCV-PAMP-transfected villous explants compared to control as assessed by ELISA. Bars represent mean plus SEM, t test (n=3). (D) Villous explants were transfected with JFH-1 RNA for 6 hours (normalized to mock transfection). Gene up-regulation was assessed by real-time PCR. Bars represent mean plus SEM, Wilcoxon signed-rank test (n=8). (E) Villous explants were cultured for 48 hours with sucrose purified JFH-1 virus. Gene up-regulation was assessed by real-time RT-PCR. Villous explants were dissected from early terminated pregnancy between 10 and 20 week gestation. Bars represent mean plus SEM, Wilcoxon signed-rank test (n=8), * p<0.05, ** p<0.01, ***p< 0.001, ****p< 0.0001.
HCV Sensing Induces Apoptosis in Human EVTs
Next, we determined the impact of HCV sensing on the viability of human EVT cell line HTR8 to define if HCV infection could alter normal placental development during the early gestational stages. HCV-PAMP transfection for 24 hours induced a pro-apoptotic response within HTR8 cells, as evidenced by a significant increase in Annexin-V staining (Figure 4A–B) and caspase-3 activation (Figure 4C–D) compared to HCV X-region stimulated control. Induction of apoptosis was also investigated after JFH-1 transfection. HTR8 transfected for 24 hours with JFH-1 RNA induced significant increase of capsase-3 activation compared to mock transfected cells (Supplemental Figure 3).
Figure 4. HCV-PAMP sensing induces apoptosis in EVTs.
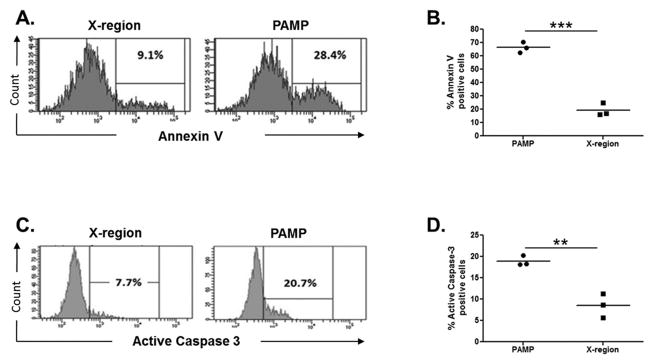
HTR8 were transfected with HCV-PAMP (or the control X-region) for 24 hours. (A–B) Annexin V staining showed significant higher induction of apoptosis over control. (C–D) Concurrent staining is shown for active caspase 3. Lines represent mean, t test, **p<0.01, ***p< 0.001.
HCV Sensing by Human Trophoblasts Induces Secretion of Chemotactic Molecules
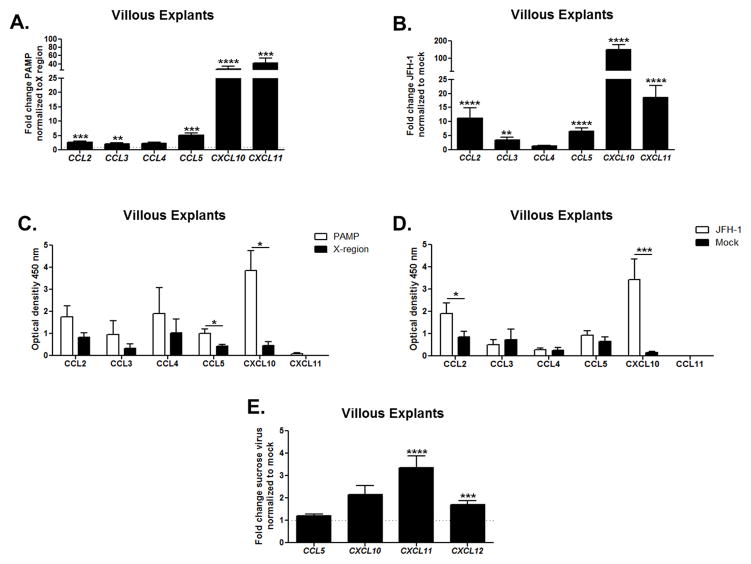
To elucidate whether HCV sensing by trophoblast cells induces up-regulation of chemokines involved in the recruitment of immune cells, we transfected villous explants from first and second trimester of pregnancy with HCV-PAMP versus X-region control for 24 hours and examined chemokine gene up-regulation. PAMP-transfected villous explants induced strong up-regulation of several chemokine genes that are known to play a central role in NK cell recruitment (Figure 5A). Specifically, CCL5 (RANTES), CXCL10 (IP-10), and CXCL11 (I-TAC) were up-regulated. A similar response was observed after transfection of villous explants with JFH-1 RNA (Figure 5B). Chemokines (e.g., CXCL10) that are known to play key roles in the recruitment of dNK cells (44) were also confirmed at the protein level by ELISA after 48 hours of transfection with PAMP and JFH-1 RNA (Figure 5C–D). Interestingly CXCL11 gene up-regulation observed in both experimental settings was not confirmed at the protein level. When villous explants were cultured with sucrose-purified viral particles for 48 hours, we detected significant up-regulation of the CXCL11 (I-TAC) and CXCL12 (SDF-1) genes (Figure 5E). Exposure of villous explants to T/F viral-RNA also induced significant CXCL10 (IP-10) and CXCL11 (I-TAC) gene up-regulation (Supplemental Figure 2). These results indicate that HCV sensing by human trophoblasts induces release of chemotactic mediators that might elicit recruitment of dNK cells.
Figure 5. HCV sensing by human trophoblasts induces up-regulation of chemokines at the gene and protein level.
Villous explants were transfected for 24 hours with (A) HCV-PAMP (or the X-region control) and (B) JFH-1 RNA (normalized to mock). Gene up-regulation was assessed by real-time RT-PCR. Bars represent mean plus SEM, Wilcoxon signed-rank test (n=8). Supernatant analysis of (C) villous explants PAMP (or X-region-transfected, n=4) and (D) JFH-1 (or mock transfected, n=8) for 48 hours with Multi-Analyte ELISArray Kit (QIAGEN) confirmed that HCV-PAMP induced statistically higher protein secretion of CCL5 (RANTES) and CXCL10 (IP-10) and that JFH-1 elicited significant higher secretion of CCL2 (MCP1) and CXCL10 (IP-10). Bars represent mean plus SEM, Mann-Whitney test. (E) Villous explants were cultured for 48 hours with sucrose purified JFH-1 virus. Villous explants were dissected from early terminated pregnancy between 10 and 20 week gestation. Gene up-regulation was assessed by real-time RT-PCR. Bars represent mean plus SEM, Wilcoxon signed-rank test (n=8), *<p0.05, ** p<0.01, ***p< 0.001, ****p<0.0001.
We also addressed whether the EVTs, which invade the maternal decidua and are in direct contact with maternal immune leukocytes, release chemokines after sensing HCV-RNA. Considering the technical difficulty in isolating purified EVTs for in vitro studies (18), we performed the experiments using the first trimester immortalized cell line HTR8. We transfected HTR8 cells with HCV-PAMP for 48 hours and examined gene up-regulation and protein secretion of chemotactic chemokines involved in NK cell recruitment. PAMP transfection of HTR8 induced significant up-regulation of multiple chemokines at the gene (Figure 6A) and protein level (Figure 6B), including CCL3 (MIP-1α), CCL5 (RANTES), CXCL8 (IL-8). These chemokines have established roles in the recruitment of NK cells to the decidua (20, 45).
Figure 6. EVTs sensing HCV secrete chemokines.
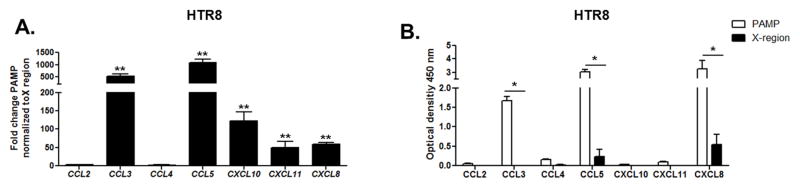
(A) HTR8 cells were transfected for 48 hours with HCV-PAMP or X-region control. Gene up-regulation was assessed by real-time RT-PCR. Bars represent mean plus SEM, Wilcoxon signed-rank test (n=3). (B) Supernatants from PAMP-transfected HTR8 has a significantly higher CCL3 (MIP-1α), CCL5 (RANTES) and CXCL10 (IP-10) protein concentration compared to the control (X-region-transfected cells) as assessed by ELISA. Bars represent mean plus SEM, Mann Whitney test (n=3), *<p0.05, **p<0.01, ***p< 0.001.
HCV Sensing by Human Trophoblasts Elicits Recruitment and Activation of Decidual NK Cells
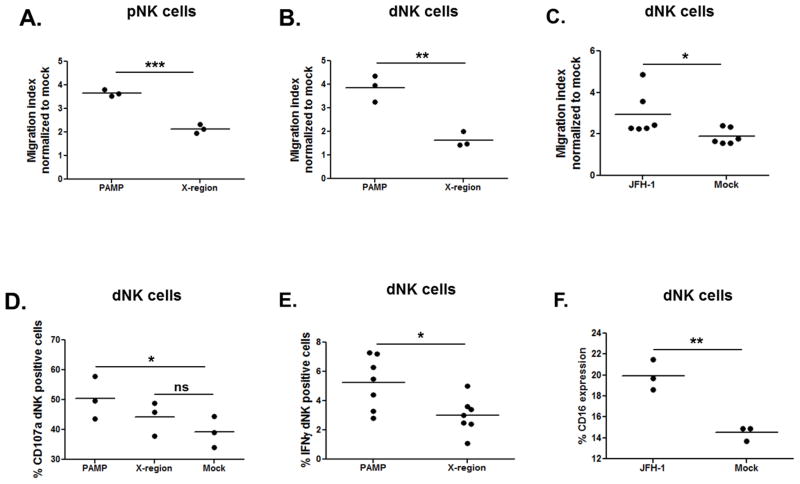
To assess whether HCV sensing by HTR8 could induce recruitment of peripheral NK cells (pNK), we performed a chemotaxis assay. We cultured CD56 bead-purified pNK cells from 3 healthy individuals on transwell filters plated in wells containing conditioned media from PAMP and X-region-transfected HTR8 (Figure 7A). After 3 hours of culture, we detected a significantly increased cell migration index (MI) of pNK cultured with PAMP-transfected conditioned media. To assess whether migration of decidual NK (dNK) cells was altered in a similar way by HCV-PAMP conditioned media we repeated the experiment using dNK cells from 3 early termination pregnancies (first and second trimester). Similarly, dNK cells cultured with PAMP-transfected conditioned media showed significantly higher migration index compared to dNK cultured with X-region-transfected media (Figure 7B). When dNK cells were cultured with supernatant of JFH-1-transfected HTR8, we observed significantly higher migration index compared to dNK cells cultured with mock-transfected media (Figure 7C). These results suggest that HCV sensing by EVTs elicits release of chemotactic molecules able to recruit both pNK and dNK cells to the maternal-fetal interface (MFI).
Figure 7. EVTs sensing HCV elicit recruitment and activation of NK cells.
Supernatant of PAMP-transfected HTR8 cultured with CD56 bead-purified (A) peripheral NK (pNK) and (B) decidual NK (dNK) cells for 3 hours induced a significantly higher migration index of pNK and dNK compared to the control. (C) CD56 bead-purified dNK cells showed significantly higher migration index also when cultured with supernatant of JFH-1-transfected HTR8. (D) The percentage of degranulating (CD107a+) CD56 bead-purified dNK cells cultured with PAMP-transfected, X-region-transfected or control media is displayed. Compared to control media, PAMP-transfected media enhanced cytotoxicity of dNK. (E) Decidual mononuclear cells (DMNCs) were cultured for 24 hours with supernatant of PAMP or X-region-transfected HTR8. dNK cells (CD3neg, CD56high) showed significant higher IFNγ secretion when cultured with PAMP-conditioned media. (F) The same experimental setting was performed using conditioned media of JFH-1-transfected HTR8. In this case, dNK cells showed significant higher CD16 expression when cultured with JFH-1-transfected supernatant. DMNCs and dNK cells were isolated from decidua of early terminated pregnancy between 10 and 20 week gestation. Lines represent mean, t-test, * p<0.05, **<0.01.
Next, we wanted to define whether dNK cells cultured with PAMP-transfected conditioned media for 24 hours were more cytotoxic compared to cells cultured with X-region-transfected conditioned media and media control. It is known that dNK cells contain high levels of perforin and granzyme (comparable to CD56dim pNK cells); however, they display a poor ability to kill classical NK targets (18, 46). Correspondingly, CD56 bead-purified dNK cells cultured for 24 hours with PAMP-transfected media showed a significantly higher degranulation rate compared to dNK cells cultured with control media (Figure 7D). Cells cultured with X-region-transfected conditioned media did not show a significant higher activation state compared to cells cultured with control media. The same experiment was performed using decidual mononuclear cells (DMNCs), and again, in this case we observed higher degranulation rate (data not shown) and significantly higher IFNγ secretion (Figure 7E) in dNK cells cultured with PAMP-transfected HTR8. When DMNCs were cultured with supernatant of JFH-1 transfected HTR8, we observed significantly higher percentage of dNK cells CD16 positive cells compared to control cells (Figure 7F). Taken together, our data suggest that HCV-transfected conditioned media contains soluble components that activate dNK cells through mechanisms that might play a role in limiting the spread of HCV to fetal tissue.
Even though maternal HCV infection is an independent risk factor for pregnancy complications, its effect on pregnancy is not devastating. Therefore, we investigated whether a compensatory mechanism limiting dNK activation at the MFI could be induced by HCV sensing. There is evidence that increased pNK cells and activated NK cells are associated with increased risk of miscarriage and failed in vitro fertilization treatment (47, 48). The healthy trophoblast does not express classical HLA-A and HLA-B products; however, trophoblasts do express the non-classical MHC class I molecule HLA-E, the ligand for the inhibitory CD94/NKG2A and activating CD94/NKG2C receptors (49). Increased expression of HLA-E within the livers of HCV-infected patients has been implicated as an immune evasion strategy because it suppresses innate immune cell function (50). Therefore, we explored whether HCV sensing by trophoblasts affected HLA-E expression. FACS phenotyping reveals that HLA-E was constitutively expressed on trophoblasts, and it was further up-regulated by HCV-PAMP and JFH-1 transfection compared to control transfection (Figure 8A–B). In order to investigate the effect that HLA-E up-regulation induced by HCV-sensing might have on dNK, we transfected HTR8 with JFH-1 for 24 hours and co-cultured them with DMNCs additionally for 24 hours. dNK cells co-cultured with JFH-1-trnasfected HTR8 showed significantly higher IFNγ and TNFα secretion but, no significant reduction in CD107a (Figure 8C–D–E). No differences were observed in NKG2A and NKG2C expression levels (data not shown). Collectively, our results suggest that HCV sensing by EVTs activates dNK cells that might contribute to viral control but also be responsible for immune-mediated pathology triggered by HCV.
Figure 8. HCV sensing by EVTs induces HLA-E up-regulation and cytokine production by dNK cells.
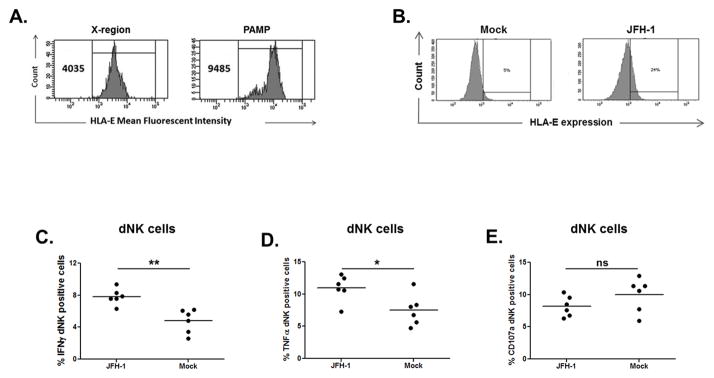
(A) HLA-E expression was up-regulated in HTR8 transfected with HCV-PAMP (compared to the control X-region) for 24 hours. (B) The same up-regulation was observed in HTR8 transfected with JFH-1 RNA compared to mock transfected cells. One representative experiment of n=3 is shown. (C–D–E) DMNCs were added to JFH-1-transfected (or mock-transfected) HTR8 after 24 hours culture. dNK cells (CD3neg CD56high) co-cultured for 24 hours with JFH-1-transfected HTR8 showed significant higher IFNγ and TNFα secretion but a non-significant reduction in CD107a expression. DMNCs were isolated from decidua of early terminated pregnancy between 10 and 20 week gestation. Lines represent mean, t-test, * p<0.05, **<0.01, ns=non-significant.
DISCUSSION
The pregnant state represents a paradoxical challenge whereby tolerance to the allogeneic fetus is balanced with host defense against pathogens (51). Immune responses against microorganisms at the maternal-fetal interface (MFI) may have a significant impact on pregnancy outcomes. Multiple components of the immune system can survey, recognize, and eliminate invading microorganisms with variable success rates (32, 52–54). For example, whereas more than 70% of children born to mothers positive for hepatitis B e antigen become chronically infected (55), the rate of mother-to-child transmission of HCV is significantly lower (9, 56). Here, we tested the hypothesis that fetal trophoblasts recognize and respond to HCV infection as a mechanism to explain decreased transmission.
Both human villous and extravillous trophoblast cells (EVTs) express many of the receptors involved in HCV entry and innate signaling. Accordingly, by immunofluorescence, we detected HCV NS5A protein after a two week culture period co-localized with the early endosomal marker EEA1, suggesting endocytosis is likely involved in the uptake of HCV by human trophoblast cells. These results expand an electron microscopic study demonstrating HCV-like particles within cytotrophoblasts near the rough endoplasmic reticulum (35). Further work is warranted to examine different steps in the viral life cycle within trophoblast cells. Using sucrose-purified HCV without potential contaminants from infectious cell-culture-derived HCV (HCVcc) (57) and the exogenous HCV-specific T/F viral-RNA, we demonstrated that HCV directly triggers Type I and III IFN responses from primary villous trophoblasts. Furthermore, HCV-PAMP and JFH-1 transfection of placental explants consistently up-regulated mRNA. Gene up-regulation of IFNA and IFNL was also confirmed at the protein level in PAMP-transfected explants. Of interest, a recent study shows a greater spontaneous recovery rate among infants infected with HCV who have the IFNL3 (IL-28B) CC rs12979860 polymorphism (58).
Another important antiviral mechanism in tissue involves the migration of innate immune cells. The first trimester of pregnancy is characterized by high numbers of dNK cells within the decidua, perhaps accounting for the low rate of congenital cytomegalovirus (CMV) infection (25). It has been shown that NK cells that infiltrate during the first trimester can switch their phenotype after sensing infection and that dNK cells can be found in the vicinity of infected cells within floating chorionic villi (59). Thus, modulation of their migration phenotype might be a response to invading pathogens. For the first time, we present data that HCV-RNA sensing by villous explants and EVTs induced remarkably broad and robust expression of chemokines, including CXCL10 (IP-10), CCL3 (MIP-1α) and CCL5 (RANTES) (20, 44, 45), that are known to elicit recruitment of NK cells. Hepatitis C viral sensing by EVTs, which invade deep into the uterus wall (60) and are in direct contact with maternal immune cells (14), leads to recruitment of peripheral and decidual NK cells. Further, dNK cells showed significantly higher expression of CD16, IFNγ secretion and increased degranulation when cultured with conditioned media of HCV-transfected EVTs. Interestingly, we also detected a higher HLA-E expression in EVTs transfected with HCV-PAMP and JFH-1 RNA. HLA-E is the ligand for the inhibitory receptor CD94/NKG2A expressed at high levels in all dNK cells and for the activating receptor CD94/NKG2C (18). DMNCs co-cultured with JFH-1-transfected HTR8 showed a significant higher IFNγ and TNFα secretion but a non-significant reduction in CD107a. These results suggest that HCV viral sensing by EVTs not only elicits recruitment of NK cells but also activates them, and enhances antiviral cytokine production, which might be important in limiting HCV transmission but also may mediate some of the observed complications noted in HCV-positive pregnancies, e.g., intrauterine growth restriction. Moreover, the fact that HCV-PAMP-transfected EVTs demonstrated increased apoptosis, in keeping with prior studies which demonstrated that viral ssRNA induces apoptosis in first trimester trophoblasts through the activation of an inflammatory mechanism (33), point to an additional regulatory mechanism. These results underscore the delicate balance between immune response that controls the virus and an overzealous response that damages the MFI (32, 52–54, 61). An excessive immune response may account for the higher complication rate in HCV-infected mothers. Collectively, these data provide conceptual insights and a novel paradigm for innate immune signaling by fetal-derived trophoblasts (Figure 9), which contributes to local control of HCV and attenuated transmission. These results have broader implications for understanding protection against vertical transmission of infections.
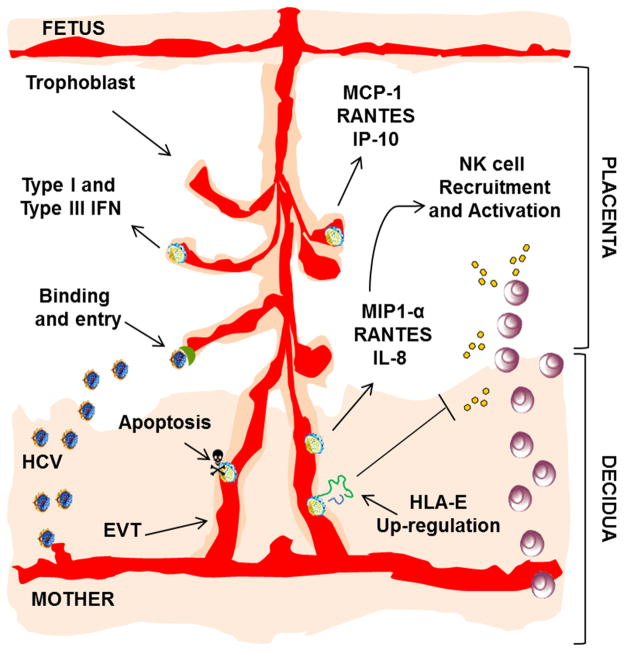
Figure 9. Working model of the role of the placenta in the context of HCV infection.
Primary trophoblasts express receptors involved in HCV binding and entry. Trophoblasts internalize HCV and respond to HCV-RNA sensing with production of IFNs and induction of chemokines responses that recruit and activate dNK cells at the MFI. Furthermore, HCV sensing up-regulates HLA-E expression and triggers an apoptotic response in human EVTs that may contribute to pregnancy-related complications.
Supplementary Material
Acknowledgments
The authors thank Takaji Wakita for the use of JFH-1, Charles M. Rice for the HCV-NS5A antibody, and George Shaw for the founder/transmitted HCV.
Support: This work was supported by NIH R01-HD075549 and R56AI100991 (to H.R.R.).
Abbreviations
- HCV
hepatitis C virus
- MFI
maternal-fetal interface
- EVT
extravillous trophoblast cell
- dNK
decidual NK
- JFH-1
Japanese Fulminant Hepatitis
References
- 1.Rosen HR. Clinical practice. Chronic hepatitis C infection. N Engl J Med. 2011;364:2429–2438. doi: 10.1056/NEJMcp1006613. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell AE, Colvin HM, Palmer Beasley R. Institute of Medicine recommendations for the prevention and control of hepatitis B and C. Hepatology. 2010;51:729–733. doi: 10.1002/hep.23561. [DOI] [PubMed] [Google Scholar]
- 3.Davis GL, Albright JE, Cook SF, Rosenberg DM. Projecting future complications of chronic hepatitis C in the United States. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2003;9:331–338. doi: 10.1053/jlts.2003.50073. [DOI] [PubMed] [Google Scholar]
- 4.Arshad M, El-Kamary SS, Jhaveri R. Hepatitis C virus infection during pregnancy and the newborn period--are they opportunities for treatment? J Viral Hepat. 2011;18:229–236. doi: 10.1111/j.1365-2893.2010.01413.x. [DOI] [PubMed] [Google Scholar]
- 5.Pergam SA, Wang CC, Gardella CM, Sandison TG, Phipps WT, Hawes SE. Pregnancy complications associated with hepatitis C: data from a 2003–2005 Washington state birth cohort. Am J Obstet Gynecol. 2008;199:38.e31–39. doi: 10.1016/j.ajog.2008.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zanetti AR, Tanzi E, Newell ML. Mother-to-infant transmission of hepatitis C virus. J Hepatol. 1999;31(Suppl 1):96–100. doi: 10.1016/s0168-8278(99)80383-3. [DOI] [PubMed] [Google Scholar]
- 7.Hayashida A, Inaba N, Oshima K, Nishikawa M, Shoda A, Hayashida S, Negishi M, Inaba F, Inaba M. Re-evaluation of the true rate of hepatitis c virus mother-to-child transmission and its novel risk factors based on our two prospective studies. J Obstet Gynaecol Res. 2007;33:417–422. doi: 10.1111/j.1447-0756.2007.00582.x. [DOI] [PubMed] [Google Scholar]
- 8.Polis CB, Shah SN, Johnson KE, Gupta A. Impact of maternal HIV coinfection on the vertical transmission of hepatitis C virus: a meta-analysis. Clin Infect Dis. 2007;44:1123–1131. doi: 10.1086/512815. [DOI] [PubMed] [Google Scholar]
- 9.Cottrell EB, Chou R, Wasson N, Rahman B, Guise JM. Reducing risk for mother-to-infant transmission of hepatitis C virus: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013;158:109–113. doi: 10.7326/0003-4819-158-2-201301150-00575. [DOI] [PubMed] [Google Scholar]
- 10.Babik JM, Cohan D, Monto A, Hartigan-O’Connor DJ, McCune JM. The human fetal immune response to hepatitis C virus exposure in utero. J Infect Dis. 2011;203:196–206. doi: 10.1093/infdis/jiq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birnbaum AH, Shneider BL, Moy L. Hepatitis C in children. N Engl J Med. 2000;342:290–291. doi: 10.1056/NEJM200001273420414. [DOI] [PubMed] [Google Scholar]
- 12.Yeung LT, Roberts EA. Current issues in the management of paediatric viral hepatitis. Liver Int. 2010;30:5–18. doi: 10.1111/j.1478-3231.2009.02145.x. [DOI] [PubMed] [Google Scholar]
- 13.Huppertz B. The anatomy of the normal placenta. Journal of clinical pathology. 2008;61:1296–1302. doi: 10.1136/jcp.2008.055277. [DOI] [PubMed] [Google Scholar]
- 14.Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol. 2006;6:584–594. doi: 10.1038/nri1897. [DOI] [PubMed] [Google Scholar]
- 15.Brosens JJ, Pijnenborg R, Brosens IA. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: a review of the literature. Am J Obstet Gynecol. 2002;187:1416–1423. doi: 10.1067/mob.2002.127305. [DOI] [PubMed] [Google Scholar]
- 16.Cross JC, Werb Z, Fisher SJ. Implantation and the placenta: key pieces of the development puzzle. Science. 1994;266:1508–1518. doi: 10.1126/science.7985020. [DOI] [PubMed] [Google Scholar]
- 17.Lash GE, Robson SC, Bulmer JN. Review: Functional role of uterine natural killer (uNK) cells in human early pregnancy decidua. Placenta. 2010;31(Suppl):S87–92. doi: 10.1016/j.placenta.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 18.Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol. 2002;2:656–663. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- 19.van den Heuvel MJ, Chantakru S, Xuemei X, Evans SS, Tekpetey F, Mote PA, Clarke CL, Croy BA. Trafficking of circulating pro-NK cells to the decidualizing uterus: regulatory mechanisms in the mouse and human. Immunol Invest. 2005;34:273–293. doi: 10.1081/imm-200064488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drake PM, Gunn MD, Charo IF, Tsou CL, Zhou Y, Huang L, Fisher SJ. Human placental cytotrophoblasts attract monocytes and CD56(bright) natural killer cells via the actions of monocyte inflammatory protein 1alpha. J Exp Med. 2001;193:1199–1212. doi: 10.1084/jem.193.10.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanna J, Wald O, Goldman-Wohl D, Prus D, Markel G, Gazit R, Katz G, Haimov-Kochman R, Fujii N, Yagel S, Peled A, Mandelboim O. CXCL12 expression by invasive trophoblasts induces the specific migration of CD16- human natural killer cells. Blood. 2003;102:1569–1577. doi: 10.1182/blood-2003-02-0517. [DOI] [PubMed] [Google Scholar]
- 22.Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol. 2001;2:123–128. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- 23.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 24.Wu X, Jin LP, Yuan MM, Zhu Y, Wang MY, Li DJ. Human first-trimester trophoblast cells recruit CD56brightCD16- NK cells into decidua by way of expressing and secreting of CXCL12/stromal cell-derived factor 1. J Immunol. 2005;175:61–68. doi: 10.4049/jimmunol.175.1.61. [DOI] [PubMed] [Google Scholar]
- 25.Jabrane-Ferrat N, Siewiera J. The up side of decidual natural killer cells: new developments in immunology of pregnancy. Immunology. 2014;141:490–497. doi: 10.1111/imm.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fisher SJ, Cui TY, Zhang L, Hartman L, Grahl K, Zhang GY, Tarpey J, Damsky CH. Adhesive and degradative properties of human placental cytotrophoblast cells in vitro. J Cell Biol. 1989;109:891–902. doi: 10.1083/jcb.109.2.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petroff MG, Phillips TA, Ka H, Pace JL, Hunt JS. Isolation and culture of term human trophoblast cells. Methods in molecular medicine. 2006;121:203–217. doi: 10.1385/1-59259-983-4:201. [DOI] [PubMed] [Google Scholar]
- 28.Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, Lala PK. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res. 1993;206:204–211. doi: 10.1006/excr.1993.1139. [DOI] [PubMed] [Google Scholar]
- 29.Saito T, Owen DM, Jiang F, Marcotrigiano J, Gale M., Jr Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature. 2008;454:523–527. doi: 10.1038/nature07106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF. Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986;118:1567–1582. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- 31.Librach CL, Werb Z, Fitzgerald ML, Chiu K, Corwin NM, Esteves RA, Grobelny D, Galardy R, Damsky CH, Fisher SJ. 92-kD type IV collagenase mediates invasion of human cytotrophoblasts. J Cell Biol. 1991;113:437–449. doi: 10.1083/jcb.113.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abrahams VM. Pattern recognition at the maternal-fetal interface. Immunol Invest. 2008;37:427–447. doi: 10.1080/08820130802191599. [DOI] [PubMed] [Google Scholar]
- 33.Aldo PB, Mulla MJ, Romero R, Mor G, Abrahams VM. Viral ssRNA induces first trimester trophoblast apoptosis through an inflammatory mechanism. Am J Reprod Immunol. 2010;64:27–37. doi: 10.1111/j.1600-0897.2010.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosen HR. Emerging concepts in immunity to hepatitis C virus infection. J Clin Invest. 2013;123:4121–4130. doi: 10.1172/JCI67714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nie QH, Gao LH, Cheng YQ, Huang XF, Zhang YF, Luo XD, Wang JQ, Wang YY. Hepatitis C virus infection of human cytotrophoblasts cultured in vitro. J Med Virol. 2012;84:1586–1592. doi: 10.1002/jmv.23380. [DOI] [PubMed] [Google Scholar]
- 36.Horner SM, Gale M. Intracellular innate immune cascades and interferon defenses that control hepatitis C virus. J Interferon Cytokine Res. 2009;29:489–498. doi: 10.1089/jir.2009.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giugliano S, Kriss M, Golden-Mason L, Dobrinskikh E, Stone AE, Soto-Gutierrez A, Mitchell A, Khetani SR, Yamane D, Stoddard M, Li H, Shaw GM, Edwards MG, Lemon SM, Gale M, Jr, Shah VH, Rosen HR. Hepatitis C virus infection induces autocrine interferon signaling by human liver endothelial cells and release of exosomes, which inhibits viral replication. Gastroenterology. 2015;148:392–402 e313. doi: 10.1053/j.gastro.2014.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balagopal A, Thomas DL, Thio CL. IL28B and the Control of Hepatitis C Virus Infection. Gastroenterology. 2010;139:1865–1876. doi: 10.1053/j.gastro.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas E, V, Gonzalez D, Li Q, Modi AA, Chen W, Noureddin M, Rotman Y, Liang TJ. HCV infection induces a unique hepatic innate immune response associated with robust production of type III interferons. Gastroenterology. 2012;142:978–988. doi: 10.1053/j.gastro.2011.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hornung V, Ellegast J, Kim S, Brzozka K, Jung A, Kato H, Poeck H, Akira S, Conzelmann KK, Schlee M, Endres S, Hartmann G. 5′-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314:994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- 41.Vieyres G, Thomas X, Descamps V, Duverlie G, Patel AH, Dubuisson J. Characterization of the envelope glycoproteins associated with infectious hepatitis C virus. J Virol. 2010;84:10159–10168. doi: 10.1128/JVI.01180-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parrish NF, Gao F, Li H, Giorgi EE, Barbian HJ, Parrish EH, Zajic L, Iyer SS, Decker JM, Kumar A, Hora B, Berg A, Cai F, Hopper J, Denny TN, Ding H, Ochsenbauer C, Kappes JC, Galimidi RP, West AP, Jr, Bjorkman PJ, Wilen CB, Doms RW, O’Brien M, Bhardwaj N, Borrow P, Haynes BF, Muldoon M, Theiler JP, Korber B, Shaw GM, Hahn BH. Phenotypic properties of transmitted founder HIV-1. Proc Natl Acad Sci U S A. 2013;110:6626–6633. doi: 10.1073/pnas.1304288110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitchell AM, Stone AE, Cheng L, Ballinger K, Edwards MG, Stoddard M, Li H, Golden-Mason L, Shaw GM, Khetani S, Rosen HR. Transmitted/founder hepatitis C viruses induce cell-type- and genotype-specific differences in innate signaling within the liver. MBio. 2015;6:e02510. doi: 10.1128/mBio.02510-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lockwood CJ, Huang SJ, Chen CP, Huang Y, Xu J, Faramarzi S, Kayisli O, Kayisli U, Koopman L, Smedts D, Buchwalder LF, Schatz F. Decidual cell regulation of natural killer cell-recruiting chemokines: implications for the pathogenesis and prediction of preeclampsia. Am J Pathol. 2013;183:841–856. doi: 10.1016/j.ajpath.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chantakru S, Kuziel WA, Maeda N, Croy BA. A study on the density and distribution of uterine Natural Killer cells at mid pregnancy in mice genetically-ablated for CCR2, CCR 5 and the CCR5 receptor ligand, MIP-1 alpha. J Reprod Immunol. 2001;49:33–47. doi: 10.1016/s0165-0378(00)00076-0. [DOI] [PubMed] [Google Scholar]
- 46.Le Bouteiller P, Tabiasco J. Killers become builders during pregnancy. Nat Med. 2006;12:991–992. doi: 10.1038/nm0906-991. [DOI] [PubMed] [Google Scholar]
- 47.Karami N, Boroujerdnia MG, Nikbakht R, Khodadadi A. Enhancement of peripheral blood CD56(dim) cell and NK cell cytotoxicity in women with recurrent spontaneous abortion or in vitro fertilization failure. J Reprod Immunol. 2012;95:87–92. doi: 10.1016/j.jri.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 48.Ozturk OG, Sahin G, Karacor ED, Kucukgoz U. Evaluation of KIR genes in recurrent miscarriage. Journal of assisted reproduction and genetics. 2012;29:933–938. doi: 10.1007/s10815-012-9811-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Apps R, Murphy SP, Fernando R, Gardner L, Ahad T, Moffett A. Human leucocyte antigen (HLA) expression of primary trophoblast cells and placental cell lines, determined using single antigen beads to characterize allotype specificities of anti-HLA antibodies. Immunology. 2009;127:26–39. doi: 10.1111/j.1365-2567.2008.03019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nattermann J, Nischalke HD, Hofmeister V, Ahlenstiel G, Zimmermann H, Leifeld L, Weiss EH, Sauerbruch T, Spengler U. The HLA-A2 restricted T cell epitope HCV core 35–44 stabilizes HLA-E expression and inhibits cytolysis mediated by natural killer cells. Am J Pathol. 2005;166:443–453. doi: 10.1016/S0002-9440(10)62267-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeldovich VB, Bakardjiev AI. Host defense and tolerance: unique challenges in the placenta. PLoS Pathog. 2012;8:e1002804. doi: 10.1371/journal.ppat.1002804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abrahams VM. The role of the Nod-like receptor family in trophoblast innate immune responses. J Reprod Immunol. 2011;88:112–117. doi: 10.1016/j.jri.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 53.Koga K, Cardenas I, Aldo P, Abrahams VM, Peng B, Fill S, Romero R, Mor G. Activation of TLR3 in the trophoblast is associated with preterm delivery. Am J Reprod Immunol. 2009;61:196–212. doi: 10.1111/j.1600-0897.2008.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bere A, Denny L, Burgers WA, Passmore JA. Polyclonal expansion of cervical cytobrush-derived T cells to investigate HIV-specific responses in the female genital tract. Immunology. 2010;130:23–33. doi: 10.1111/j.1365-2567.2009.03172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee C, Gong Y, Brok J, Boxall EH, Gluud C. Effect of hepatitis B immunisation in newborn infants of mothers positive for hepatitis B surface antigen: systematic review and meta-analysis. BMJ. 2006;332:328–336. doi: 10.1136/bmj.38719.435833.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Network, E. P. H. C. V. Three broad modalities in the natural history of vertically acquired hepatitis C virus infection. Clin Infect Dis. 2005;41:45–51. doi: 10.1086/430601. [DOI] [PubMed] [Google Scholar]
- 57.Boonstra A, van der Laan LJ, Vanwolleghem T, Janssen HL. Experimental models for hepatitis C viral infection. Hepatology. 2009;50:1646–1655. doi: 10.1002/hep.23138. [DOI] [PubMed] [Google Scholar]
- 58.Ruiz-Extremera A, Muñoz-Gámez JA, Salmerón-Ruiz MA, de Rueda PM, Quiles-Pérez R, Gila-Medina A, Casado J, Belén Martín A, Sanjuan-Nuñez L, Carazo A, Pavón EJ, Ocete-Hita E, León J, Salmerón J. Genetic variation in interleukin 28B with respect to vertical transmission of hepatitis C virus and spontaneous clearance in HCV-infected children. Hepatology. 2011;53:1830–1838. doi: 10.1002/hep.24298. [DOI] [PubMed] [Google Scholar]
- 59.Siewiera J, El Costa H, Tabiasco J, Berrebi A, Cartron G, Le Bouteiller P, Bouteiller P, Jabrane-Ferrat N. Human cytomegalovirus infection elicits new decidual natural killer cell effector functions. PLoS Pathog. 2013;9:e1003257. doi: 10.1371/journal.ppat.1003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tarrade A, Lai Kuen R, Malassine A, Tricottet V, Blain P, Vidaud M, Evain-Brion D. Characterization of human villous and extravillous trophoblasts isolated from first trimester placenta. Lab Invest. 2001;81:1199–1211. doi: 10.1038/labinvest.3780334. [DOI] [PubMed] [Google Scholar]
- 61.Koga K, Izumi G, Mor G, Fujii T, Osuga Y. Toll-like receptors at the maternal-fetal interface in normal pregnancy and pregnancy complications. Am J Reprod Immunol. 2014;72:192–205. doi: 10.1111/aji.12258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.