Abstract
Aspirin exacerbated respiratory disease (AERD), a severe eosinophilic inflammatory disorder of the airways, involves overproduction of cysteinyl leukotrienes (cysLTs), activation of airway mast cells (MCs), and bronchoconstriction in response to nonselective cyclooxygenase inhibitors that deplete homeostatic prostaglandin (PG)E2. The mechanistic basis for MC activation in this disorder is unknown. We now demonstrate that patients with AERD have markedly increased epithelial expression of the alarmin-like cytokine IL-33 in nasal polyps, as compared to polyps from aspirin tolerant (AT) controls. The murine model of AERD, generated by dust mite priming of mice lacking microsomal PGE2 synthase (ptges−/− mice), shows a similar upregulation of IL-33 protein in the airway epithelium, along with marked eosinophilic bronchovascular inflammation. Deletion of LTC4 synthase (LTC4S), the terminal enzyme needed to generate cysLTs, eliminates the increased IL-33 content of the ptges−/− lungs and sharply reduces pulmonary eosinophilia and basal secretion of MC products. Challenges of dust mite-primed ptges−/− mice with lysine aspirin (Lys-ASA) induce IL-33-dependent MC activation and bronchoconstriction. Thus, IL-33 is a component of a cysLT-driven innate type 2 immune response that drives pathogenic MC activation and contributes substantially to AERD pathogenesis.
Introduction
Aspirin exacerbated respiratory disease (AERD) is a distinctive, idiopathic syndrome affecting 7–10% of all asthmatics and ~15% of severe asthmatics (1). It is characterized by severe eosinophilic respiratory mucosal inflammation, refractory nasal polyposis, and idiosyncratic respiratory reactions to aspirin and other nonselective cyclooxygenase (COX) inhibitors (2). Dysregulated activity of the 5-lipoxygenase (5-LO)/Leukotriene C4 synthase (LTC4S) pathway in AERD results in increased synthesis of leukotriene C4 (LTC4), the parent of the cysteinyl leukotrienes (cysLTs; LTC4, LTD4 and the stable metabolite LTE4) from arachidonic acid (2). As a consequence of this dysregulation, the levels of LTE4 in urine and respiratory secretions from subjects with AERD are elevated compared with those in aspirin tolerant asthmatic (ATA) controls, and increase further (by several fold) in response to provocative challenges with aspirin (3,4). Curiously, while subjects with AERD and ATA controls are equivalently sensitive to the bronchoconstricting effects of inhaled LTC4 (5) and LTD4 (6), both of which are powerful but short-lived contractile agonists, subjects with AERD develop airflow obstruction in response to challenges with LTE4, the weakest constrictor of the three cysLTs, at doses >1 log lower than those that induce bronchoconstriction in ATA controls (5,7). Thus, overproduction of cysLTs and hyperresponsiveness to LTE4 may each contribute to the persistent respiratory tract inflammation and to the pathognomonic clinical reactions associated with AERD. While high levels of LTC4S expression by tissue eosinophils (8) and aberrant transcellular cysLT generation by granulocyte-platelet complexes (9) contribute to cysLT overproduction, the basis for LTE4 hyperresponsiveness is unknown.
The surge in cysLT production characteristically elicited by challenges of AERD subjects with aspirin is accompanied by MC activation, as evidenced by increases in the levels of histamine, tryptase, and prostaglandin (PG)D2 metabolites in nasal lavage fluids, plasma, and urine (4,10). The administration of the MC-stabilizing drugs cromolyn or nedocromil prior to challenge prevents aspirin-induced bronchoconstriction (11,12), supporting the physiologic importance of MCs in the pathognomonic reactions. Although the cassette of mediators released by MCs in response to aspirin challenges in AERD is identical to that released in response to allergen challenges in sensitized individuals (13–15), there is no evidence that AERD involves classical allergen-driven immunopathology (16,17). The potency of COX-1 inhibition, rather than drug antigenicity or structure, is the major determinant of clinical reactivity to a given agent (18). Thus, the mechanism of MC activation in AERD likely involves disease-specific non-classical immune pathways that are “braked” by COX-1-derived PGs. Curiously, the administration of the 5-LO inhibitor zileuton to subjects with AERD not only prevents symptoms of reactions to intranasal challenge with lysine aspirin (Lys-ASA), but also blocks the release of MC products, suggesting that one or more endogenous 5-LO products (likely cysLTs) are necessary for MC activation in AERD (19). However, the precise identity of the products and the causative mechanism(s) by which they induce MC activation are unclear, and there is no explanation for why this mechanism is unique to AERD.
PGE2 stabilizes MCs (20) and prevents 5-LO activation (21) by acting at the E prostanoid (EP)2 receptor and activating protein kinase A (PKA). Deficient respiratory tract production of PGE2 (22), reduced expression of EP2 receptors by MCs and other tissue leukocytes (6,23), and diminished function of PKA (24) are all reported in association with AERD. Mice that are selectively PGE2-deficient due to targeted deletion of microsomal PGE2 synthase (ptges−/− mice) develop a phenotype strikingly similar to AERD when challenged with lysine aspirin (Lys-ASA) following a period of exposure to an extract (Df) from the house dust mite Dermatophagoides farinae to elicit airway inflammation (25). Lys-ASA elicits bronchoconstriction, pulmonary MC activation, and release of cysLTs from the lungs of ptges−/− mice, but not of PGE2-sufficient WT controls. All of these features are blocked by pre-treatment with zileuton or montelukast (an inhibitor of the type 1 receptor for cysLTs, CysLT1R) (25). We now demonstrate that ptges−/− mice also exhibit hyperresponsiveness to LTE4, and that exogenous LTE4, like aspirin, induces the release of MC-derived mediators. Remarkably, the cysLT-driven MC activation pathway hinges critically on the alarmin-like cytokine IL-33, which is markedly overexpressed in the lung epithelial cells of ptges−/− mice, as well as in nasal polyp epithelial cells from patients with AERD. Deletion of LTC4S, which eliminates cysLT synthesis in the ptges−/− mice, completely protects mice from pulmonary eosinophilic inflammation and markedly attenuates the overexpression of IL-33 in the lung. Thus, cysLT-amplified innate type 2 immunity contributes prominently to AERD, and IL-33 mediates a novel LTE4-induced pathway for MC activation.
Methods
Patient Characterization
Nonsmoking patients (18–70 years old) were recruited at the Brigham and Women’s Hospital (Boston, MA). Nasal polyp and sinus tissue were collected during surgical excision from subjects with AERD or from aspirin-tolerant (AT) controls with chronic hyperplastic sinusitis. AERD was suspected based on asthma, nasal polyposis, and a history of respiratory reaction upon ingestion of a COX inhibitor, and confirmed with a graded oral challenge to aspirin that resulted in characteristic sinonasal symptoms and/or a decrease in forced expiratory volume in 1 second of at least 15%. AT controls had taken aspirin or a nonsteroidal anti-inflammatory drug within the previous 6 months without an adverse reaction. All subjects had been treated with oral prednisone (20 mg daily) for the week leading up to their sinus surgery. The Institutional Review Board approved the study and all subjects provided written consent.
Polyp procurement and preparation
Portions of excised sinonasal tissue were transferred into CellLytic® MCell Lysis Reagent (Sigma-Aldrich, St. Louis, MO) with 2% protease inhibitor (Roche, Indianapolis, IN) for protein extraction. Tissue was homogenized using a gentleMACS® Dissociator (Miltenyi Biotec, San Diego, CA). The supernatants were stored at −80° C. Other portions were fixed in 4% paraformaldehyde, embedded in Tissue-Tek® O.C.T.™ Compound (Sakura Finetek), and kept at −80°C until sectioning. Sections of 10-μm thickness were freshly cut, thaw-mounted onto slides, and stored at −80°C until stained. For some patients a tissue segment was also placed in media containing 5% fetal bovine serum and chopped with a straight razor blade and then digested with 400 units/mL Type IV collagenase (Worthington Biochemical Corporation) and 200 μg/mL DNase (Sigma-Aldrich). The resulting suspension was passed through a 70 μM filter to retrieve a single cell suspension for flow cytometric sorting. These cells were stained with monoclonal antibodies against CD45, CD90, EpCAM, and CD31 (BD Biosciences) and were sorted into purified cell populations with a BD FACS Aria™ Fusion Cell-Sorter to collect tissue fibroblasts (CD45−/EpCAM−/CD31−/CD90+), epithelial cells (CD45−/EpCAM+/CD31−/CD90−), and endothelial cells (CD45−/EpCAM−/CD31+/CD90−) separately.
Western blot analysis
Western blots were prepared as previously described (26) and probed with primary anti-IL-33 (R&D Systems, Minneapolis, MN), or anti-glyceraldehyde-3 dehydrogenase (GAPDH) (Cell Signaling Technology, Danvers, MA) antibodies, washed and then incubated with horseradish peroxidase-conjugated anti-rabbit IgG (Sigma-Aldrich) and visualized by enhanced chemiluminescence (GE HealthCare). The densities of the bands corresponding to full-length and proteolytically cleaved IL-33 (~30 and ~18 kDa, respectively) were measured in each lane using Quantity One 1-D Analysis Software (Bio-Rad, Hercules, California). The densities were divided by the densities of GAPDH for the same lane. Results were presented as the corrected expression for at least 10 mice/group.
ELISA
Lungs were homogenized 24 h after the final dose of PBS or Df. Total IL-33 content was measured with a commercial ELISA (R & D, Minneapolis, MN), and corrected for the protein content of each sample.
qPCR
RNA was extracted from the nasal tissue specimens or from the sorted cell populations with Tri Reagent® (Sigma-Aldrich, St. Louis, MO) and converted to cDNA using the RT2 First Strand Kit (Qiagen, Valencia, CA). The expression of IL-33 was examined using RT2 SybrGreen qPCR Master Mix (Qiagen). Expression levels of transcripts were normalized to the expression of glyceraldehyde-3-phosphate dehydrogenase (all primers from Qiagen).
Mice
C57BL/6 mice lacking mPGES-1 (ptges−/− mice) were from Dr. Shizuo Akira (Osaka University, Japan) (27). The mice were intercrossed with ltc4s−/− mice (28) to generate double knockouts. All of the mice and wild type C57BL/6 controls were housed at Charles River (Wilmington, MA). Six- to 8-wk-old male mice were used. All animal studies were approved by the Animal Care and Use Committee of the Dana-Farber Cancer Institute (Protocol 03-042). Airway inflammation was induced as described elsewhere (25). Mice were studied 24 h after the last of 6 intranasal treatments with Df (3 μg).
Reagents
Mice were treated with saline or Df obtained from Greer Laboratories (XPB81D3A25; Lenoir, NC). Montelukast was obtained from Brigham and Women’s Hospital Pharmacy. The mMCP-1 EIA kit was purchased from eBiosciences (San Diego, CA). Histamine, PGE2, TXB2, and cysLT EIA kits were from Cayman Chemical (Ann Arbor, MI).
Measurement of airway resistance
Airway resistance (RL) in response to Lys-ASA was assessed with an Invasive Pulmonary Function Device (Buxco, Sharon, CT). Briefly, mice were anesthetized 24 h after the last Df challenge, and a tracheotomy was performed. After allowing for RL to reach a stable baseline, Lys-ASA (12 μl of 100 mg/ml) was delivered to the lung via nebulizer, and RL was recorded for 45 min. In some experiments, mice were challenged with escalating doses of LTC4, LTD4, or LTE4. Since each cysLT elicited its peak response at 0.1 nmol/mouse, this dose was chosen for subsequent experiments. The results were expressed as percentage change of RL from baseline. Some mice were treated with montelukast (6.7 μg/ml in drinking water, p.o. 24 h before Lys-ASA), or SQ29.548 (50 μg/mouse, i.p. 24 h before Lys-ASA). Goat anti-mouse IL-33 antibody (3.6 μg/mouse, R&D Systems, Minneapolis, MN) or recombinant mouse ST2-Fc fusion protein (5 μg/mouse, R&D Systems) (29) were given intraperitoneally at 24 h before Lys-ASA or LTE4 challenge. The same amount of normal goat IgG (R&D Systems) and recombinant human IgG1 Fc (R&D Systems) were used as controls.
Immunohistologic analysis
Frozen sections of human nasal polyps were stained with rabbit IgG or rabbit anti-human IL-33 (Sigma HPA 024426) and developed with HRP-goat anti-rabbit IgG and Alexa Fluor 594 tyramide (Life Technologies T-20925). Other sections were incubated in Alexa Fluor 594 tyramide alone to control for nonspecific binding of tyramide. For mouse tissue, the left lungs were fixed in 10% neutral buffered formalin for 24 h and embedded in paraffin blocks. Tissue sections were deparaffinized and rehydrated. Endogenous peroxidase was inhibited by incubation with freshly prepared 3% H2O2 with 0.1% sodium azide. Rat anti-mouse IL-33 mAb (R&D Systems # MAB3626) was applied to the sections, and incubations were carried out for 1 h at room temperature. Ab binding was detected with rat-on-mouse HRP micro-polymer detection system (Biocare Medical) and visualized with DAB chromagen. Slides were counterstained with Gill’s No 2 hematoxylin, dehydrated, and mounted.
Statistical analysis
Data are expressed as ±SEM from at least 10 mice from at least two experiments, except where otherwise indicated. Analyses were performed with Prism software (Graphpad). Differences between two treatment groups were assessed using Student t test, and differences among multiple groups were assessed using one-way ANOVA and Bonferroni post hoc test. P<0.05 was considered statistically significant.
Results
IL-33 is strongly expressed in respiratory tissues from subjects with AERD and ptges−/− mice
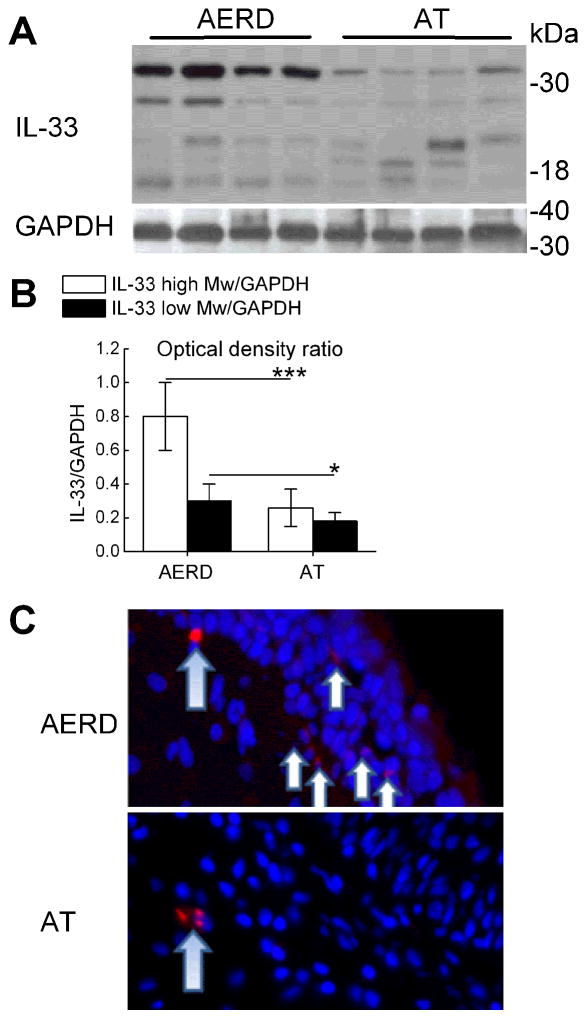
To determine whether IL-33 might contribute to tissue pathology in AERD, we examined surgically excised nasal polyps obtained from carefully phenotyped subjects with and without AERD for evidence of IL-33 expression. Western blots of whole polyp lysates from subjects with AERD displayed strong expression of IL-33 protein. Both the ~30 kDa unprocessed form and processed forms of 18–21 kDa and ~25 kDa were detected (Fig. 1A). Both the full-length form and the 18–21 kDa forms were more abundant in polyps from subjects with AERD than from controls (Fig. 1B). IL-33 protein was present in the basal layer of epithelial cells in the nasal polyps from patients with AERD, but not from ATA control subjects with nasal polyposis (Fig. 1C). The staining appeared primarily cytosolic. qPCR analysis of cells sorted from nasal polyps confirmed the presence of IL-33 transcript in the EPCAM+ epithelial cells from 5 of 5 samples tested, and in 4 of 5 samples of CD90+ fibroblasts and 4 of 5 samples of CD31+ endothelial cells sorted from the same polyps (not shown).
Figure 1. Expression of IL-33 protein in nasal polyps.
A. Western blotting of nasal polyp proteins from 4 subjects with AERD and 4 CHES controls showing full-length (30 kDa) and processed (25, 18–21 kDa) forms of IL-33. B. Quantitative densitometry of full-length and 18–21 kDa IL-33 signals, corrected for GAPDH. The densitometry results are from 8 samples per clinical group, including the 4 from each group depicted in A. C. Tyramide-amplified anti-IL-33 immunofluorescent staining of nasal polyps from subjects with AERD and with AT chronic hyperplastic eosinophilic sinusitis. Specific immunofluorescence is shown in cells localizing to the basal layer of the epithelium (short arrows). Nonspecific staining with tyramide (larger arrows) appears in both specimens. * p<0.05, *** p<0.001.
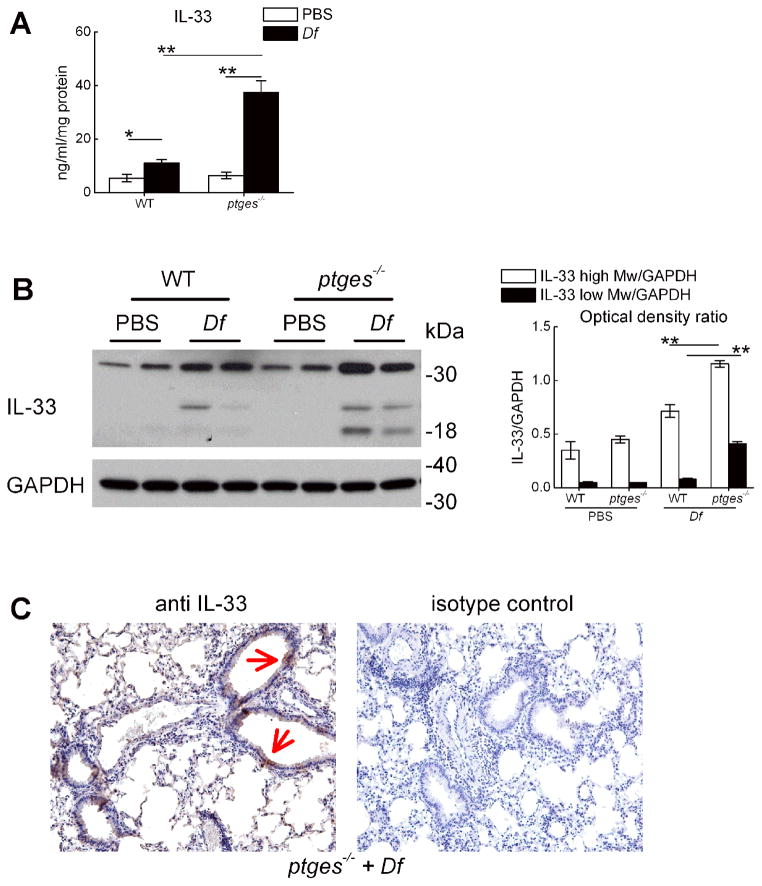
To determine whether IL-33 was also overexpressed in the lungs of AERD-like ptges−/− mice, we performed ELISA for total IL-33 protein on lysates of whole lungs of naïve and Df-treated WT and ptges−/− mice. We also performed western blots to detect the presence of the full-length and cleaved IL-33 isoforms. Low levels of IL-33 protein were detected in the lungs of the naïve mice. These levels increased significantly in response to Df, and were markedly greater in the lungs of Df-treated ptges−/− mice than WT controls (Fig. 2A). Both the full-length protein and proteolytically processed fragments (including the active 18 kDa fragment) were detected by western blot, and were more abundant in the lungs of the Df-treated ptges−/− mice than controls (Fig. 2B, 2C). Immunohistochemistry revealed prominent cytosolic staining of bronchial epithelial cells for IL-33 protein in the lungs of ptges−/− mice (Fig. 2D, arrows).
Figure 2. Expression of IL-33 protein in the lungs of AERD-like mice.
A. ELISA measurement of IL-33 in lysates of whole lung from the indicated groups. B. Western blot showing full-length and cleaved IL-33 protein in the lungs collected from WT and ptges−/− mice 24 h after the last of 6 intranasal doses of either PBS or Df. C. Immunohistochemical staining for IL-33 protein (left) or isotype control (right) in the lungs of Df-treated ptges−/− mice. Epithelial staining is indicated by arrows. Results in A–C are representative of at least 10 mice in two separate experiments. * p<0.05, ** p<0.01,
CysLT-deficient mice are protected from upregulation of IL-33 and basal MC activation
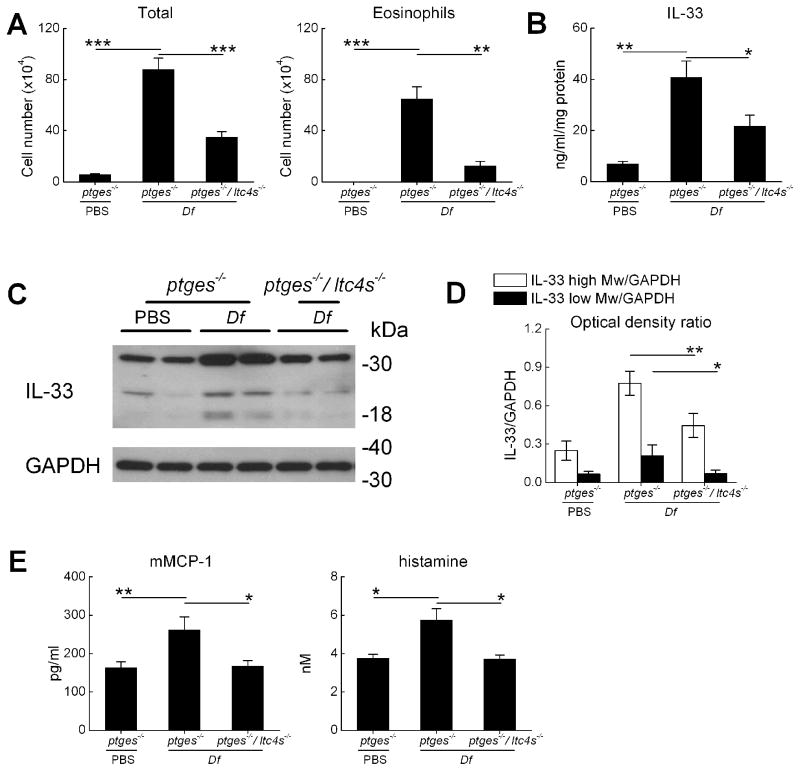
Persistent high level cysLT generation is a hallmark of AERD that distinguishes it from aspirin tolerant asthma (3). This feature is recapitulated by Df-treated ptges−/− mice (25). To determine whether endogenous cysLTs might drive IL-33 overexpression, ptges−/− mice were intercrossed with mice lacking LTC4S (ltc4s−/− mice) (28) to generate double knockout ptges−/−/ltc4s−/− mice. The resultant ptges−/−/ltc4s−/− mice and ptges−/− controls were treated with Df on 6 occasions. Compared with the lungs of the Df-treated ptges−/− mice, the ptges−/−/ltc4s−/− mice displayed reduced levels of BAL fluid total cells and eosinophils twenty four hours after the final exposure to Df (Fig. 3A). The lungs of the ptges−/−/ltc4s−/− mice also contained substantially less IL-33 protein than did the lungs of the ptges−/− controls (Fig. 3B), with reductions in both the 30 kDa and 18 kDa bands (Fig. 3C, 3D). Df treatment of the ptges−/− mice modestly increased the BAL fluid levels of histamine and mouse mast cell protease 1 (mMCP-1) compared with PBS-treated controls. These increases were absent in the BAL fluids of Df-treated ptges−/−/ltc4s−/− mice (Fig. 3E).
Figure 3. Effect of LTC4S deletion on IL-33 expression and attendant physiologic effects.
A. BAL fluid total cell counts and eosinophil counts in the lungs of ptges−/− and ptges−/−/ltc4s−/− mice measured 24 h after the last of 6 doses of PBS or Df. B. ELISA of total IL-33 protein levels in lung lysates from the indicated strains. C. Western blotting of lung proteins from the indicated strains showing full-length and cleaved forms of IL-33. D. Quantitative densitometry showing effect of ltc4s deletion from ptges−/− mice on lung IL-33 protein levels. E. BAL fluid levels of MC granule-derived mediators from the indicated groups. Results in A, B, and D and E are mean ± SEM from at least 10 mice/group in two separate experiments. * p<0.05, ** p<0.01, *** p<0.001.
IL-33 is responsible for MC activation and changes in lung function
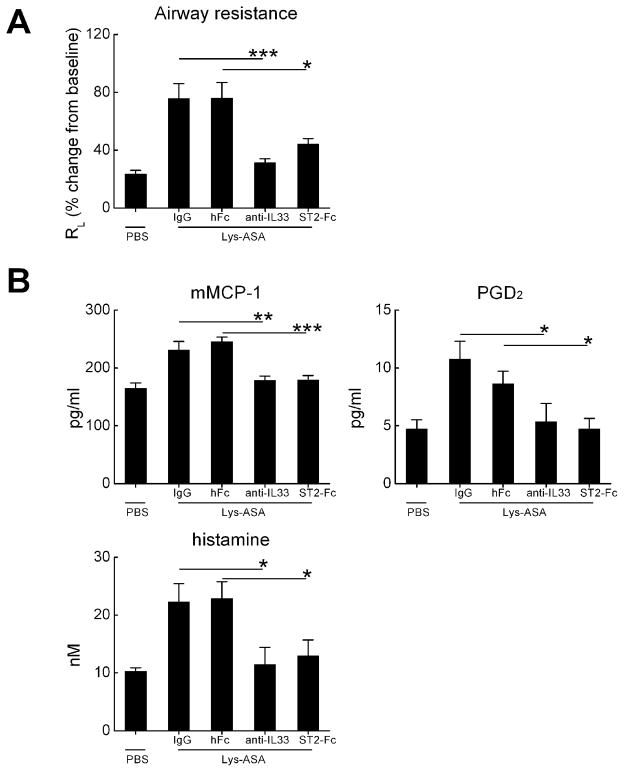
IL-33 can directly activate human MCs to generate cytokines in vitro (30), and elicits systemic MC-dependent anaphylaxis and IgE synthesis in vivo when injected into naïve mice (31). To determine whether IL-33 was directly involved in the airway physiology changes and MC activation occurring in response to Lys-ASA, we administered a blocking Ab against IL-33, a soluble ST-Fc fusion protein, a control IgG, or a control Fc protein to separate cohorts of Df-treated ptges−/− mice prior to inhalation challenges with inhaled Lys-ASA. Both anti-IL-33 and anti-ST2 completely blocked the changes in RL occurring in response to Lys-ASA (Fig. 4A). Both blockers also prevented the increases in BAL fluid mMCP-1, histamine, and PGD2 (Fig. 4B).
Figure 4. Role of IL-33 in Lys-ASA-induced changes in lung function and MC activation.
A. Effect of blocking IL-33 or ST2 on pulmonary responses to Lys-ASA challenge. Ptges−/− mice were challenged with Lys-ASA by inhalation 24 h after the last of 6 intranasal doses of Df. RL was monitored continuously for 45 min and the peak change from baseline was recorded for each mouse. The indicated groups received single intraperitoneal doses of rat anti-mouse IL-33, IgG isotype control, recombinant ST2-Fc fusion protein, or Fc control. B. Levels of BAL fluid mMCP-1, histamine, and PGD2 in BAL fluids from the same mice. Results are from 10 mice/group in two separate experiments. * p<0.05, ** p<0.01, *** p<0.001.
LTE4 is responsible for IL-33-dependent MC activation and airflow obstruction
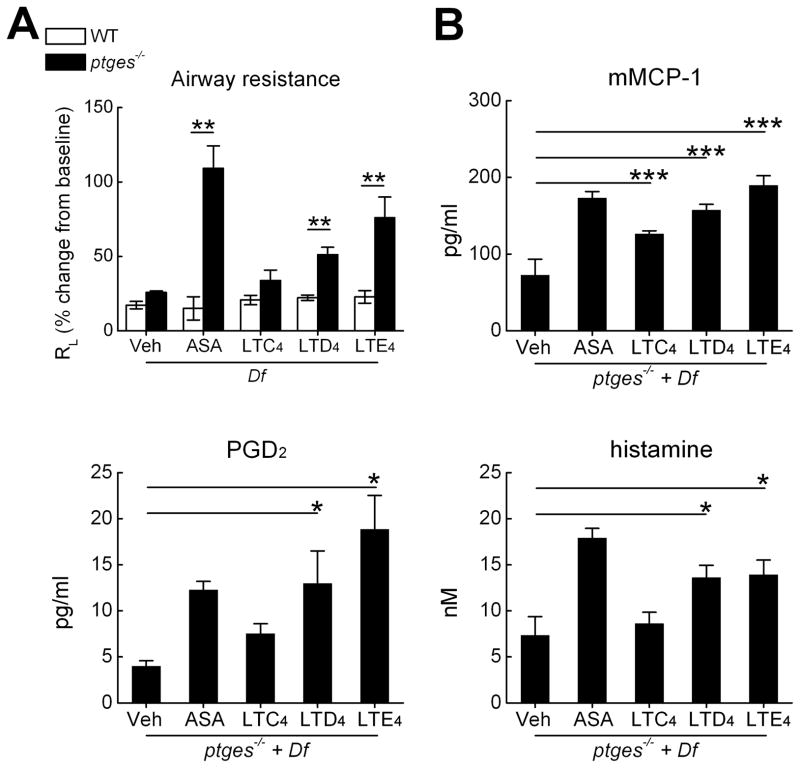
The administration of either zileuton or montelukast to ptges−/− mice blocked Lys-ASA-induced changes in RL in our previous studies (25), indicating that their airways were sensitive to contractile effects of endogenous cysLTs. These drugs also blocked the release of MC products, implying that the endogenous cysLTs in this model were required for MC activation. To determine which cysLT was most responsible for MC activation and consequent physiologic effects, we directly administered LTC4, LTD4, and LTE4 to anaesthetized, sedated, and mechanically ventilated WT and ptges−/− mice, with or without prior exposure to 6 intranasal doses of Df to elicit airway inflammation. Lung resistance (RL) was monitored directly for 45 minutes. Df-treated ptges−/− mice displayed significant increases in RL following inhalation challenges with LTE4 and LTD4, but not with LTC4, when compared with WT controls (Fig. 5A). The increases in RL in response to LTE4 tended to exceed those to LTD4, and approached ~75% of the magnitude of the response to Lys-ASA, administered as a positive control. Compared with vehicle-challenged controls, BAL fluids from LTD4- and LTE4-challenged ptges−/− mice displayed increased quantities of mMCP-1, PGD2, and histamine (Fig. 5B).In contrast, cysLT challenges failed to elicit increases in MC products in the BAL fluids of WT control mice (not shown).
Figure 5. Effect of exogenous cysLTs on airway physiology and intrapulmonary MC activation.
A. Peak changes in RL in Df-treated mice from the indicated genotypes challenged by inhalation with Lys-ASA or with LTC4, LTD4, or LTE4. B. Levels of mediators in BAL fluids collected 45 min after challenges with Lys-ASA or the indicated cysLT. Results in A and B are from 10 mice/group in two separate experiments. * p<0.05, ** p<0.01, *** p<0.001.
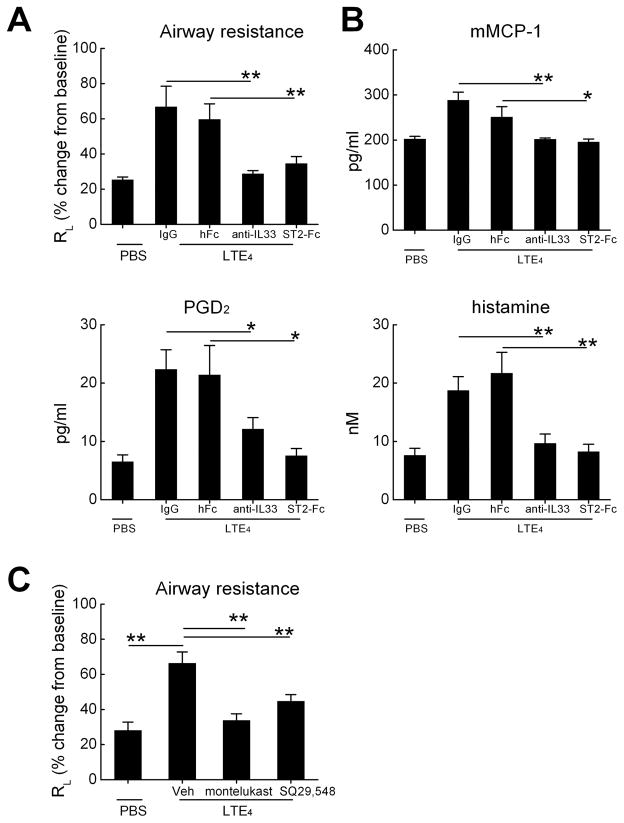
To determine whether LTE4, like Lys-ASA, caused MC activation and bronchoconstriction by inducing the release of IL-33, we treated mice with anti-IL-33 or ST2-Fc and corresponding controls 24 hours before inhalation challenges with LTE4. Treatment of the ptges−/− mice with either anti-IL-33 or with ST2-Fc blocked the increases in RL (Fig. 6A), and eliminated the increases in MC-derived products in the BAL fluid (Fig. 6B).
Figure 6.
Effect of anti-IL-33 and ST2 blockade on pulmonary responses to direct challenges with LTE4. The indicated groups were treated single intraperitoneal doses of rat anti-mouse IL-33, IgG isotype control, recombinant ST2-Fc fusion protein, or Fc control 24 h before the challenge with LTE4. A. Peak percent changes in RL from baseline are displayed. B. BAL fluid content of histamine, mMCP-1, and PGD2. C. Effects of CysLT1R blockade or TP receptor antagonism on LTE4-induced changes in RL The indicated groups received Montelukast P.O. in drinking water beginning 24 h before challenge, or received a single intraperitoneal dose of the selective TP receptor antagonist SQ29,548. * p<0.05, ** p<0.01, *** p<0.001.
LTE4-induced changes in RL and cell activation are montelukast-sensitive and depend partially on MC-derived prostaglandins
We used a pharmacologic approach to identify the receptors required for LTE4-mediated changes in RL, and to determine whether these responses were direct, or whether they involved the contractile effects of LTE4-elicited prostaglandins as reported in humans (32,33). Prior to inhalation of LTE4, Df-treated ptges−/− mice were given the CysLT1R antagonist montelukast overnight in their drinking water. Because LTE4 elicited the release of PGD2, which requires T prostanoid (TP) receptor signaling to elicit smooth muscle constriction (34), we treated additional mice with a single intraperitoneal dose of the TP receptor antagonist SQ29,548. Montelukast treatment and SQ29,548 both blocked the LTE4-mediated increase in RL (Fig. 6C). Montelukast also tended to block the modest increase in RL induced by LTD4, although the narrow window in these experiments precluded statistical significance (Supplemental Fig. 1).
Discussion
Chronic severe eosinophilic respiratory tract inflammation, baseline overproduction of cysLTs, and hyperresponsiveness to LTE4 are all features of AERD. The administration of nonselective COX inhibitors elicits explosive cysLT generation and MC activation that are both blocked by the administration of the 5-LO inhibitor zileuton (19). The fact that AERD often occurs in nonatopic hosts (16), some of whom nevertheless show significantly increased total serum IgE (17) and all of whom display marked eosinophilic tissue inflammation, suggests that the disease involves immune mechanisms that are distinct from allergen-specific adaptive type 2 immune responses. Whereas IL-33 is released as an alarmin from necrotic cells (35), it is also released in a controlled manner from epithelial cells in response to danger signals from viruses (36), fungi (37), and helminths (38). IL-33 acts at the IL-1 receptor-like protein ST2 to activate both myeloid and lymphoid innate effector cells, resulting in the production of cytokines (IL-5, IL-13, IL-9) that drive eosinophil-rich pathology, either independently of adaptive immunity (39,40), or in concert with conventional allergen-driven Th2 responses (41). MCs express ST2 and can be activated by IL-33 in vivo, either in response to direct systemic administration (31) or in models of fungal protease challenge (37) and cellular damage (42). Although IL-33 is strongly expressed in both refractory nasal polyposis (43) and severe asthma (44), no previous study had addressed the potential role of IL-33 in the pathogenesis of AERD. In this study, we used complementary approaches in humans and mice to determine the potential role of IL-33 in disease pathophysiology, particularly in the enigmatic, 5-LO-dependent MC activation that occurs characteristically in response to challenges with aspirin in AERD.
We first surveyed surgically excised nasal polyps for IL-33 expression. Whole nasal polyp lysates from subjects with AERD contained substantially more IL-33 full-length and cleaved IL-33 protein than did AT controls (Fig. 1A, 1B). The lower molecular weight species identified on the western blot (~25 and 18–21 kDa, respectively) are consistent with the sizes of the fragments generated by cleavage of full-length IL-33 by MC-and neutrophil-derived proteases (45,46). These fragments are ~30-fold more active than the full length form in vitro and in vivo. Total IL-33 protein was markedly increased in the lungs of the Df-treated ptges−/− mice relative to controls (Fig. 2A). Both full-length and the 18 kDa cleaved IL-33 protein were detected by western blots (Fig. 2B), and both were more abundant in the lungs of AERD-like ptges−/− mice than in WT controls (Fig. 2C). In both AERD nasal polyps (Fig. 1C) and in the lungs of Df-treated ptges−/− mice, (Fig. 2D), IL-33 protein localized primarily to epithelial cells, and qPCR analysis of sorted nasal polyp cells suggested additional expression by both fibroblasts and endothelial cells. The robust expression of IL-33 (and the presence of bioactive forms) is consistent with a potential role for IL-33 in particular (and innate type 2 immunity in general) in driving the eosinophil-rich pathology that is typical of AERD.
In mice, cysLTs contribute to the induction and amplification of eosinophilic pulmonary inflammation through several mechanisms, including CysLT1R-mediated priming of DCs for Th2 responses (47), and CysLT2R-mediated upregulation of endothelial adhesion receptor expression via platelets (48). Accordingly, mice lacking LTC4S, the terminal enzyme necessary to generate cysLTs, are protected from Th2-type sensitization and pulmonary eosinophilia induced by Df (47). LTC4S in AERD is highly expressed by eosinophils (8) and by platelets that adhere to granulocytes with high frequency in both the blood and respiratory tissue (9). Remarkably, the deletion of LTC4S from ptges−/− mice not only abrogated cysLT generation in the lung as expected (not shown), but attenuated Df-induced pulmonary eosinophilia (Fig. 3A), IL-33 expression (Fig. 3B–D), and the increases in basal BAL fluid levels of MC activation markers (mMCP-1, histamine) (Fig. 3E). Thus, cysLTs act upstream of IL-33 in this model to drive sustained eosinophilic inflammation and associated basal activation of MCs. The ability to amplify IL-33 expression may be an additional mechanism by which cysLTs may contribute to type 2 immunity and inflammation.
While promoting tissue eosinophilia by inducing cytokine generation from lymphoid cells (49,50), IL-33 can also induce full activation of MCs (37), including systemic anaphylaxis (31), when released in response to environmental danger signals. The fact that endogenous cysLTs were involved in the basal expression, release, and processing of IL-33 in the lungs of ptges−/− mice (Fig. 3) led us to postulate that the increases in cysLT production might result in incremental IL-33-driven MC activation and airflow obstruction during provocative challenges with Lys-ASA. Indeed, short-term blockade of either IL-33 (with a monoclonal Ab) or ST2 (with a fusion protein of soluble ST2 with Fc) markedly attenuated the Lys-ASA-induced change in RL (Fig. 4A), and prevented the increases in MC-derived products, including PGD2, histamine, and mMCP-1 (Fig. 4B). We verified that IL-33 was the end-effector of the cysLTs released during the reaction by challenging Df-primed WT and ptges−/− mice by inhalation of cysLTs. LTE4 (the most biologically stable and abundant of three cysLTs), and to a lesser extent LTD4, replicated the changes in RL (Fig. 5A) and MC activation (Fig. 5B) elicited by Lys-ASA. Remarkably, the effects of LTE4 on RL and mediator release were also abrogated by blockade of IL-33 or ST2 (Fig. 6A, 6B). Thus, the cysLT-driven mechanism of airflow obstruction and MC activation in AERD (19) and ptges−/− mice (25) may be due to incremental release and actions of IL-33 in response to endogenous LTE4. The deficiency in PGE2 is permissive for both cysLT overproduction and IL-33-driven hyperresponsiveness to LTE4.
Although LTE4 is the weakest bronchoconstrictor among the three cysLTs, it is disproportionately potent as a constrictor in the airways of subjects with asthma (51), and even more so in AERD (5). LTE4 is also the only cysLT that can induce the accumulation of eosinophils and basophils in the bronchial mucosa when inhaled by mild asthmatics (52). It is tempting to speculate that these findings reflect the actions of LTE4-elicited IL-33 on MCs and other components of the innate type 2 immune system. Although LTE4 is a weak agonist at CysLT1R in transfected cells, its effects in our study were blocked completely by the selective CysLT1R antagonist montelukast (Fig. 6C). The administration of zafirlukast (another CysLT1R antagonist) blocked LTE4-induced bronchoconstriction and induction of eosinophilia in AT asthmatic subjects in a previous study (53). It is possible that the magnitude of the LTE4 effect (relative to the negligible LTC4 effect and the modest LTD4 effect) reflects the comparative inability of LTE4 to elicit signaling through CysLT2R, which inhibits CysLT1R signaling in some contexts (54,55). Early studies had suggested that some actions of LTE4 on human bronchi depended on endogenous COX products that signal through TP receptors (32). The blocking effect of the TP receptor antagonist SQ29,548 in our model (Fig. 6C) suggests that the airflow obstruction consequent to MC activation is at least partly attributable to PGD2, which requires TP receptors to elicit smooth muscle contraction (56).
Our study suggests that IL-33 is a prominent effector of AERD that bridges the upstream production of the cysLTs to the idiosyncratic MC activation typical of the disorder. CysLTs may drive persistent tissue eosinophilia and MC activation in response to COX-1 inhibition by driving the expression and release of IL-33. High-dose aspirin, which produces substantial symptomatic improvements and reduces recurrence rates for nasal polyps in AERD, does not decrease systemic levels of cysLTs (57), but eliminates the selective hyperresponsiveness to LTE4 (7). The latter effect could reflect the capacity of high-dose aspirin to deplete TP-active PGs (58). Our study also suggests that AERD, in which eosinophilic inflammation and MC activation are prominent despite a clear association with atopy, is largely driven by innate type 2 immunity. Drugs that block IL-33 functions may have therapeutic applications in AERD.
Supplementary Material
Footnotes
This work was supported by NIH grants AI078908, AI095219, AT002782, AI082369, HL111113, HL117945, and by the Vinik and Kaye Families.
Nonstandard abbreviations
AERD, aspirin exacerbated respiratory disease; cysLTs, cysteinyl leukotrienes; CysLT1R, type 1 cysteinyl leukotriene receptor; CysLT2R, type 2 cysteinyl leukotriene receptor; LT, leukotriene; LTC4S, leukotriene C4 synthase; MC, mast cell; mMCP-1, mouse MC protease 1; RL, lung resistance; TP, thromboxane prostanoid
References
- 1.Rajan JP, Wineinger NE, Stevenson DD, White AA. Prevalence of aspirin-exacerbated respiratory disease among asthmatic patients: A meta-analysis of the literature. J Allergy Clin Immunol. 2015;135:676–681. doi: 10.1016/j.jaci.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 2.Laidlaw TM, Boyce JA. Pathogenesis of aspirin-exacerbated respiratory disease and reactions. Immunol Allergy Clin North Am. 2013;33:195–210. doi: 10.1016/j.iac.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christie PE, Tagari P, Ford-Hutchinson AW, Charlesson S, Chee P, Arm JP, Lee TH. Urinary leukotriene E4 concentrations increase after aspirin challenge in aspirin-sensitive asthmatic subjects. Am Rev Respir Dis. 1991;143:1025–1029. doi: 10.1164/ajrccm/143.5_Pt_1.1025. [DOI] [PubMed] [Google Scholar]
- 4.Sladek K, Szczeklik A. Cysteinyl leukotrienes overproduction and mast cell activation in aspirin-provoked bronchospasm in asthma. Eur Respir J. 1993;6:391–399. [PubMed] [Google Scholar]
- 5.Christie PE, Schmitz-Schumann M, Spur BW, Lee TH. Airway responsiveness to leukotriene C4 (LTC4), leukotriene E4 (LTE4) and histamine in aspirin-sensitive asthmatic subjects. Eur Respir J. 1993;6:1468–1473. [PubMed] [Google Scholar]
- 6.Corrigan CJ, Napoli RL, Meng Q, Fang C, Wu H, Tochiki K, Reay V, Lee TH, Ying S. Reduced expression of the prostaglandin E2 receptor E-prostanoid 2 on bronchial mucosal leukocytes in patients with aspirin-sensitive asthma. J Allergy Clin Immunol. 2012;129:1636–1646. doi: 10.1016/j.jaci.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Arm JP, O’Hickey SP, Spur BW, Lee TH. Airway responsiveness to histamine and leukotriene E4 in subjects with aspirin-induced asthma. Am Rev Respir Dis. 1989;140:148–153. doi: 10.1164/ajrccm/140.1.148. [DOI] [PubMed] [Google Scholar]
- 8.Cowburn AS, Sladek K, Soja J, Adamek L, Nizankowska E, Szczeklik A, Lam BK, Penrose JF, Austen FK, Holgate ST, Sampson AP. Overexpression of leukotriene C4 synthase in bronchial biopsies from patients with aspirin-intolerant asthma. J Clin Invest. 1998;101:834–846. doi: 10.1172/JCI620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laidlaw TM, Kidder MS, Bhattacharyya N, Xing W, Shen S, Milne GL, Castells MC, Chhay H, Boyce JA. Cysteinyl leukotriene overproduction in aspirin-exacerbated respiratory disease is driven by platelet-adherent leukocytes. Blood. 2012;119:3790–3798. doi: 10.1182/blood-2011-10-384826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bochenek G, Nagraba K, Nizankowska E, Szczeklik A. A controlled study of 9alpha,11beta-PGF2 (a prostaglandin D2 metabolite) in plasma and urine of patients with bronchial asthma and healthy controls after aspirin challenge. J Allergy Clin Immunol. 2003;111:743–749. doi: 10.1067/mai.2003.1387. [DOI] [PubMed] [Google Scholar]
- 11.Robuschi M, Gambaro G, Sestini P, Pieroni MG, Refini RM, Vaghi A, Bianco S. Attenuation of aspirin-induced bronchoconstriction by sodium cromoglycate and nedocromil sodium. Am J Respir Crit Care Med. 1997;155:1461–1464. doi: 10.1164/ajrccm.155.4.9105094. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida S, Amayasu H, Sakamoto H, Onuma K, Shoji T, Nakagawa H, Tajima T. Cromolyn sodium prevents bronchoconstriction and urinary LTE4 excretion in aspirin-induced asthma. Ann Allergy Asthma Immunol. 1998;80:171–176. doi: 10.1016/S1081-1206(10)62951-1. [DOI] [PubMed] [Google Scholar]
- 13.Wenzel SE, Westcott JY, Larsen GL. Bronchoalveolar lavage fluid mediator levels 5 minutes after allergen challenge in atopic subjects with asthma: relationship to the development of late asthmatic responses. J Allergy Clin Immunol. 1991;87:540–548. doi: 10.1016/0091-6749(91)90013-e. [DOI] [PubMed] [Google Scholar]
- 14.Wenzel SE, Westcott JY, Smith HR, Larsen GL. Spectrum of prostanoid release after bronchoalveolar allergen challenge in atopic asthmatics and in control groups. An alteration in the ratio of bronchoconstrictive to bronchoprotective mediators. Am Rev Respir Dis. 1989;139:450–457. doi: 10.1164/ajrccm/139.2.450. [DOI] [PubMed] [Google Scholar]
- 15.Wenzel SE, Fowler AA, III, Schwartz LB. Activation of pulmonary mast cells by bronchoalveolar allergen challenge. In vivo release of histamine and tryptase in atopic subjects with and without asthma. Am Rev Respir Dis. 1988;137:1002–1008. doi: 10.1164/ajrccm/137.5.1002. [DOI] [PubMed] [Google Scholar]
- 16.Szczeklik A, Nizankowska E, Duplaga M. Natural history of aspirin-induced asthma. AIANE Investigators. European Network on Aspirin-Induced Asthma. Eur Respir J. 2000;16:432–436. doi: 10.1034/j.1399-3003.2000.016003432.x. [DOI] [PubMed] [Google Scholar]
- 17.Johns CB, Laidlaw TM. Elevated total serum IgE in nonatopic patients with aspirin-exacerbated respiratory disease. Am J Rhinol Allergy. 2014;28:287–289. doi: 10.2500/ajra.2014.28.4054. [DOI] [PubMed] [Google Scholar]
- 18.Szczeklik A, Gryglewski RJ, Czerniawska-Mysik G, Zmuda A. Aspirin-induced asthma. Hypersensitivity to fenoprofen and ibuprofen in relation to their inhibitory action on prostaglandin generation by different microsomal enzymic preparations. J Allergy Clin Immunol. 1976;58:10–18. doi: 10.1016/0091-6749(76)90102-0. [DOI] [PubMed] [Google Scholar]
- 19.Fischer AR, Rosenberg MA, Lilly CM, Callery JC, Rubin P, Cohn J, White MV, Igarashi Y, Kaliner MA, Drazen JM. Direct evidence for a role of the mast cell in the nasal response to aspirin in aspirin-sensitive asthma. J Allergy Clin Immunol. 1994;94:1046–1056. doi: 10.1016/0091-6749(94)90123-6. [DOI] [PubMed] [Google Scholar]
- 20.Kay LJ, Yeo WW, Peachell PT. Prostaglandin E2 activates EP2 receptors to inhibit human lung mast cell degranulation. Br J Pharmacol. 2006;147:707–713. doi: 10.1038/sj.bjp.0706664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flamand N, Surette ME, Picard S, Bourgoin S, Borgeat P. Cyclic AMP-mediated inhibition of 5-lipoxygenase translocation and leukotriene biosynthesis in human neutrophils. Mol Pharmacol. 2002;62:250–256. doi: 10.1124/mol.62.2.250. [DOI] [PubMed] [Google Scholar]
- 22.Yoshimura T, Yoshikawa M, Otori N, Haruna S, Moriyama H. Correlation between the prostaglandin D(2)/E(2) ratio in nasal polyps and the recalcitrant pathophysiology of chronic rhinosinusitis associated with bronchial asthma. Allergol Int. 2008;57:429–436. doi: 10.2332/allergolint.o-08-545. [DOI] [PubMed] [Google Scholar]
- 23.Ying S, Meng Q, Scadding G, Parikh A, Corrigan CJ, Lee TH. Aspirin-sensitive rhinosinusitis is associated with reduced E-prostanoid 2 receptor expression on nasal mucosal inflammatory cells. J Allergy Clin Immunol. 2006;117:312–318. doi: 10.1016/j.jaci.2005.10.037. [DOI] [PubMed] [Google Scholar]
- 24.Laidlaw TM, Cutler AJ, Kidder MS, Liu T, Cardet JC, Chhay H, Feng C, Boyce JA. Prostaglandin E resistance in granulocytes from patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2014 doi: 10.1016/j.jaci.2013.12.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu T, Laidlaw TM, Katz HR, Boyce JA. Prostaglandin E2 deficiency causes a phenotype of aspirin sensitivity that depends on platelets and cysteinyl leukotrienes. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1313185110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cummings HE, Liu T, Feng C, Laidlaw TM, Conley PB, Kanaoka Y, Boyce JA. Cutting edge: Leukotriene C4 activates mouse platelets in plasma exclusively through the type 2 cysteinyl leukotriene receptor. J Immunol. 2013;191:5807–5810. doi: 10.4049/jimmunol.1302187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uematsu S, Matsumoto M, Takeda K, Akira S. Lipopolysaccharide-dependent prostaglandin E(2) production is regulated by the glutathione-dependent prostaglandin E(2) synthase gene induced by the Toll-like receptor 4/MyD88/NF-IL6 pathway. J Immunol. 2002;168:5811–5816. doi: 10.4049/jimmunol.168.11.5811. [DOI] [PubMed] [Google Scholar]
- 28.Kanaoka Y, Maekawa A, Penrose JF, Austen KF, Lam BK. Attenuated zymosan-induced peritoneal vascular permeability and IgE-dependent passive cutaneous anaphylaxis in mice lacking leukotriene C4 synthase. J Biol Chem. 2001;276:22608–22613. doi: 10.1074/jbc.M103562200. [DOI] [PubMed] [Google Scholar]
- 29.Lee HY, Rhee CK, Kang JY, Byun JH, Choi JY, Kim SJ, Kim YK, Kwon SS, Lee SY. Blockade of IL-33/ST2 ameliorates airway inflammation in a murine model of allergic asthma. Exp Lung Res. 2014;40:66–76. doi: 10.3109/01902148.2013.870261. [DOI] [PubMed] [Google Scholar]
- 30.Allakhverdi Z, Smith DE, Comeau MR, Delespesse G. Cutting edge: The ST2 ligand IL-33 potently activates and drives maturation of human mast cells. J Immunol. 2007;179:2051–2054. doi: 10.4049/jimmunol.179.4.2051. [DOI] [PubMed] [Google Scholar]
- 31.Komai-Koma M, Brombacher F, Pushparaj PN, Arendse B, McSharry C, Alexander J, Chaudhuri R, Thomson NC, McKenzie AN, McInnes I, Liew FY, Xu D. Interleukin-33 amplifies IgE synthesis and triggers mast cell degranulation via interleukin-4 in naive mice. Allergy. 2012;67:1118–1126. doi: 10.1111/j.1398-9995.2012.02859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacques CA, Spur BW, Johnson M, Lee TH. The mechanism of LTE4-induced histamine hyperresponsiveness in guinea-pig tracheal and human bronchial smooth muscle, in vitro. Br J Pharmacol. 1991;104:859–866. doi: 10.1111/j.1476-5381.1991.tb12518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christie PE, Hawksworth R, Spur BW, Lee TH. Effect of indomethacin on leukotriene4-induced histamine hyperresponsiveness in asthmatic subjects. Am Rev Respir Dis. 1992;146:1506–1510. doi: 10.1164/ajrccm/146.6.1506. [DOI] [PubMed] [Google Scholar]
- 34.Larsson AK, Hagfjard A, Dahlen SE, Adner M. Prostaglandin D(2) induces contractions through activation of TP receptors in peripheral lung tissue from the guinea pig. Eur J Pharmacol. 2011;669:136–142. doi: 10.1016/j.ejphar.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 35.Miller AM. Role of IL-33 in inflammation and disease. J Inflamm (Lond) 2011;8:22. doi: 10.1186/1476-9255-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackson DJ, Makrinioti H, Rana BM, Shamji BW, Trujillo-Torralbo MB, Footitt J, Jerico D, Telcian AG, Nikonova A, Zhu J, Aniscenko J, Gogsadze L, Bakhsoliani E, Traub S, Dhariwal J, Porter J, Hunt D, Hunt T, Hunt T, Stanciu LA, Khaitov M, Bartlett NW, Edwards MR, Kon OM, Mallia P, Papadopoulos NG, Akdis CA, Westwick J, Edwards MJ, Cousins DJ, Walton RP, Johnston SL. IL-33-dependent type 2 inflammation during rhinovirus-induced asthma exacerbations in vivo. Am J Respir Crit Care Med. 2014;190:1373–1382. doi: 10.1164/rccm.201406-1039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snelgrove RJ, Gregory LG, Peiro T, Akthar S, Campbell GA, Walker SA, Lloyd CM. Alternaria-derived serine protease activity drives IL-33-mediated asthma exacerbations. J Allergy Clin Immunol. 2014;134:583–592. doi: 10.1016/j.jaci.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hung LY, I, Lewkowich P, Dawson LA, Downey J, Yang Y, Smith DE, Herbert DR. IL-33 drives biphasic IL-13 production for noncanonical Type 2 immunity against hookworms. Proc Natl Acad Sci U S A. 2013;110:282–287. doi: 10.1073/pnas.1206587110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL-33-responsive lineage- CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol. 2012;188:1503–1513. doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim HY, Chang YJ, Subramanian S, Lee HH, Albacker LA, Matangkasombut P, Savage PB, McKenzie AN, Smith DE, Rottman JB, Dekruyff RH, Umetsu DT. Innate lymphoid cells responding to IL-33 mediate airway hyperreactivity independently of adaptive immunity. J Allergy Clin Immunol. 2012;129:216–227. doi: 10.1016/j.jaci.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oliphant CJ, Barlow JL, McKenzie AN. Insights into the initiation of type 2 immune responses. Immunology. 2011;134:378–385. doi: 10.1111/j.1365-2567.2011.03499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Enoksson M, Lyberg K, Moller-Westerberg C, Fallon PG, Nilsson G, Lunderius-Andersson C. Mast cells as sensors of cell injury through IL-33 recognition. J Immunol. 2011;186:2523–2528. doi: 10.4049/jimmunol.1003383. [DOI] [PubMed] [Google Scholar]
- 43.Reh DD, Wang Y, Ramanathan M, Jr, Lane AP. Treatment-recalcitrant chronic rhinosinusitis with polyps is associated with altered epithelial cell expression of interleukin-33. Am J Rhinol Allergy. 2010;24:105–109. doi: 10.2500/ajra.2010.24.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo Z, Wu J, Zhao J, Liu F, Chen Y, Bi L, Dong L, Liu S. IL-33 promotes airway remodeling and is a marker of asthma disease severity. J Asthma. 2014 doi: 10.3109/02770903.2014.921196. [DOI] [PubMed] [Google Scholar]
- 45.Lefrancais E, Roga S, Gautier V, Gonzalez-de-Peredo A, Monsarrat B, Girard JP, Cayrol C. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc Natl Acad Sci U S A. 2012;109:1673–1678. doi: 10.1073/pnas.1115884109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lefrancais E, Duval A, Mirey E, Roga S, Espinosa E, Cayrol C, Girard PJ. Central domain of IL-33 is cleaved by mast cell proteases for potent activation of group-2 innate lymphoid cells. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1410700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barrett NA, Rahman OM, Fernandez JM, Parsons MW, Xing W, Austen KF, Kanaoka Y. Dectin-2 mediates Th2 immunity through the generation of cysteinyl leukotrienes. J Exp Med. 2011 doi: 10.1084/jem.20100793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cummings HE, Liu T, Feng C, Laidlaw TM, Conley PB, Kanaoka Y, Boyce JA. Cutting Edge: Leukotriene C4 Activates Mouse Platelets in Plasma Exclusively through the Type 2 Cysteinyl Leukotriene Receptor. J Immunol. 2013 doi: 10.4049/jimmunol.1302187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dyer KD, Percopo CM, Rosenberg HF. IL-33 promotes eosinophilia in vivo and antagonizes IL-5-dependent eosinophil hematopoiesis ex vivo. Immunol Lett. 2013;150:41–47. doi: 10.1016/j.imlet.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bessa J, Meyer CA, de Vera Mudry MC, Schlicht S, Smith SH, Iglesias A, Cote-Sierra J. Altered subcellular localization of IL-33 leads to non-resolving lethal inflammation. J Autoimmun. 2014;55:33–41. doi: 10.1016/j.jaut.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 51.Arm JP, O’Hickey SP, Hawksworth RJ, Fong CY, Crea AE, Spur BW, Lee TH. Asthmatic airways have a disproportionate hyperresponsiveness to LTE4, as compared with normal airways, but not to LTC4, LTD4, methacholine, and histamine. Am Rev Respir Dis. 1990;142:1112–1118. doi: 10.1164/ajrccm/142.5.1112. [DOI] [PubMed] [Google Scholar]
- 52.Laitinen A, Lindqvist A, Halme M, Altraja A, Laitinen LA. Leukotriene E(4)-induced persistent eosinophilia and airway obstruction are reversed by zafirlukast in patients with asthma. J Allergy Clin Immunol. 2005;115:259–265. doi: 10.1016/j.jaci.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 53.Laitinen A, Lindqvist A, Halme M, Altraja A, Laitinen LA. Leukotriene E(4)-induced persistent eosinophilia and airway obstruction are reversed by zafirlukast in patients with asthma. J Allergy Clin Immunol. 2005;115:259–265. doi: 10.1016/j.jaci.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 54.Jiang Y, Borrelli LA, Kanaoka Y, Bacskai BJ, Boyce JA. CysLT2 receptors interact with CysLT1 receptors and down-modulate cysteinyl leukotriene dependent mitogenic responses of mast cells. Blood. 2007;110:3263–3270. doi: 10.1182/blood-2007-07-100453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barrett NA, Fernandez JM, Maekawa A, Xing W, Li L, Parsons MW, Austen KF, Kanaoka Y. Cysteinyl leukotriene 2 receptor on dendritic cells negatively regulates ligand-dependent allergic pulmonary inflammation. J Immunol. 2012;189:4556–4565. doi: 10.4049/jimmunol.1201865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Safholm J, Manson ML, Bood J, Delin I, Orre AC, Bergman P, Al-Ameri M, Dahlen SE, Adner M. Prostaglandin E inhibits mast cell-dependent bronchoconstriction in human small airways through the E prostanoid subtype 2 receptor. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2015.04.002. in press. [DOI] [PubMed] [Google Scholar]
- 57.Nasser SM, Patel M, Bell GS, Lee TH. The effect of aspirin desensitization on urinary leukotriene E4 concentrations in aspirin-sensitive asthma. Am J Respir Crit Care Med. 1995;151:1326–1330. doi: 10.1164/ajrccm.151.5.7735581. [DOI] [PubMed] [Google Scholar]
- 58.Cahill KN, Bensko JC, Boyce JA, Laidlaw TM. Prostaglandin D2: A dominant mediator of aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2015;135:245–252. doi: 10.1016/j.jaci.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.