Abstract
Activation of invariant natural killer T (iNKT) cells with the model antigen α-galactosylceramide (αGalCer) induces rapid production of multiple cytokines, impacting a wide variety of different immune reactions. In contrast, following secondary activation with αGalCer, the behavior of iNKT cells is altered for months, with the production of most cytokines being strongly reduced. The requirements for the induction of this hypo-responsive state, however, remain poorly defined. Here, we show that Th1-biasing iNKT cell antigens could induce iNKT cell hypo-responsiveness, as long as a minimum antigenic affinity was reached. In contrast, the Th2-biasing antigen OCH did not induce a hypo-responsive state, nor did cytokine-driven iNKT cell activation by LPS or infections. Furthermore, while DCs and B cells have been reported to be essential for iNKT cell stimulation, neither DCs nor B cells were required to induce iNKT cell hypo-responsiveness. Therefore, our data indicate that while some bone marrow-derived cells could induce iNKT cell hypo-responsiveness, selective conditions, dependent on the structure and potency of the antigen, were required to induce hypo-responsiveness.
Keywords: natural killer T cells, T lymphocyte, lipid antigen, cytokine
Introduction
Invariant NKT (iNKT) cells are characterized by the expression of an identical TCRα rearrangement, Vα14-Jα18 in mice and Vα24-Jα18 in humans, and their recognition of lipid Ags presented by CD1d. iNKT cells rapidly produce copious amounts of various cytokines following activation with Ags or with cytokines, and they participate in innate immune responses, as well as bridging the innate and adaptive immune responses (1-5). Many studies of iNKT cells have used the model Ag α-galactosylceramide (αGalCer), which has an extraordinarily high affinity for the iNKT cell TCR when bound to CD1d. This response is characterized by the production of both Th1 cytokines such as IFNγ and Th2 cytokines such as IL-4 (1-5). However, it has been reported that iNKT cells become unresponsive to a secondary challenge following a primary activation with αGalCer in vivo, which has been compared to anergy in conventional T cells (6-8). We recently demonstrated that some αGalCer pre-treated iNKT cells secrete IL-10 when activated and display an IL-10-dependent regulatory function (9). Furthermore, smaller proportions of iNKT cells with a similar surface phenotype and the ability to produce IL-10 can be found in untreated mice and humans, indicating that IL-10+ iNKT cells are a new subset of iNKT cells that we termed NKT10 cells (9). Because not all the Ag-experienced iNKT cells produce IL-10, we will refer to ‘iNKT cell hypo-responsiveness’ here when discussing the function of populations of αGalCer pre-treated iNKT cells. The profound changes in iNKT cells that can be induced by αGalCer stimulation are long lasting, for at least 3-4 months (6-9). A better understanding of the underlying mechanisms, however, is essential to allow successful manipulation of iNKT cells for therapy. Here, we have explored the requirements, in terms of the type of stimulation and the relevant APC, for inducing this hypo-responsive iNKT cell phenotype.
Materials and Methods
Mice and bacteria
All mice were housed under SPF conditions at the vivarium of the La Jolla Institute for Allergy and Immunology (LJI, La Jolla, CA) in accordance with the Institutional Animal Care and Use Committee guidelines. C57BL/6J mice, 6.129S2-Igh-6tm1Cgn/J (μMT−/−) mice, B6.129S7-Ifngr1tm1Agt/J (Ifngr−/−), B6.129S1-Il12atm1Jm/J (Il12−/−), B6.129P2-Il18tm1Aki/J mice (Il18−/−) and B6.129S6-Cd1d1/Cd1d2tm1Spb/J (Cd1d−/−) on the C57BL/6 background were purchased from the Jackson Laboratories (Bar Harbor, ME). B6.129-Tcra-Jtm1Tgi (Ja18−/−) mice (10) and CD11c-DOG mice (11) on the C57BL/6 background were the kind gift of Dr. M. Taniguchi (RIKEN Institute, Yokohama, Japan) and Dr. Günter Hämmerling (DKFZ, Heidelberg, Germany), respectively. All mouse experiments were performed in an AAALAC-accredited facility with prior approval of the La Jolla Institute for Allergy and Immunology Animal Care Committee (IACUC) in accordance with the PHS Policy. Sphingobium yanoikuyae was purchased from American Type Culture Collection (Manassas, VA).
Reagents and monoclonal antibodies
The glycolipid Ags α-galactosylceramide (αGalCer) and OCH were obtained from Kyowa Hakko Kirin (Tokyo Research Park, Tokyo, Japan). C-glycoside and GalA-GSL (GSL-1’) were obtained from the NIH tetramer core facility (Emory University, Atlanta, GA). EF77 and SMC124 were prepared as described previously (12). LPS and diphtheria toxin (DTx) were purchased from Sigma-Aldrich (St. Louis, MO). Monoclonal antibodies against the following mouse Ags were used in this study: CD3ε (145.2C11, 17A2), CD4 (GK1.5, RM4-5), CD8α (53-6.7, 5H10), CD11b (M1/70), CD11c (HL3), CD19 (1D3, 6D5), CD25 (PC61.5), CD44 (IM7), CD45.1 (A20), CD45.2 (104), CD45R/B220 (RA3-6B2), CD69 (H1.2F3), CD279/PD-1 (J43, RMP1-30), FNγ (XMG1.2), IL-4 (11B11, BVD6-24G2), IL-10 (JES3-9D7), Ly6C/G (Gr1), NK1.1 (PK136), NRP1/CD304 (polyclonal), TCRβ (H57-597) and TNF (MP6-XT22). Antibodies were purchased from BD Biosciences (San Diego, CA), BioLegend (San Diego, CA), eBioscience (San Diego, CA), Invitrogen (Carlsbad, CA) or R&D Systems (Minneapolis, MN). Antibodies were biotinylated or conjugated to Pacific Blue, eFluor 450, V450, Brilliant Violet 421, Pacific Orange, V500, Brilliant Violet 570, Quantum Dot 605, Quantum Dot 655, eFluor 650, Brilliant Violet 650, Brilliant Violet 711, Brilliant Violet 785, Brilliant Violet 786, FITC, Alexa Fluor 488, PerCP, PerCP-Cy5.5, PerCP-eFluor 710, PE, PE-TexasRed, PE-CF594, PE-Cy5.5, PE-Cy7, APC, Alexa Fluor 647, eFluor 660, Alexa Fluor 700, APC-Cy7 or APC-eFluor 780. Anti-mouse CD16/32 antibody (2.4G2) used for Fc receptor blocking was purified in our laboratory. Unconjugated mouse and rat IgG antibodies were purchased from Jackson ImmunoResearch (West Grove, PA). Dead cells were labeled with Blue, Aqua or Yellow Dead Cell Stain Kit (Invitrogen). Preparation of fluorochrome-conjugated αGalCer loaded CD1d tetramers were performed as described previously (13).
ELISA and flow cytometry
IFNγ and IL-4 levels in plasma were determined by ELISA using BD Bioscience reagents (San Diego, CA), according to the manufacturer’s recommendations. Flow cytometry was performed as described previously (13). Vα14i NKT cells were defined throughout as live CD8α− CD19/CD45R− CD44+ TCR/CD3+ CD1d/αGalCer-tetramer+ cells. NK cells were defined as live TCR/CD3− NK1.1+ cells.
In vivo challenge
iNKT cells were αGalCer pretreated by injection of 4 μg αGalCer i.v. and analyzed 4-6 weeks later or as otherwise indicated. Acute activation in vivo was induced by injection of 1 μg αGalCer i.v. followed by analysis 90 min later or as otherwise indicated. For the depletion of NK cells mice were i.p. injected with 50μl/mouse of anti-asialo-GM1 antibody (rabbit IgG, IgM, IgA) (WakoPure Chemical Industries, Richmond, VA) 24 h in advance. For viral or bacterial infection 5 × 104 PFU of MCMV Smith strain (kindly provided by Chris Benedict, LJI, La Jolla, CA) or 1 × 108 Sphingobium yanoikuyae bacteria were injected i.p.. For depletion of DCs CD11c-DOG mice were i.p. injected with 8ng per gram body weight of DTx as described previously (11), resulting in a <95% loss of CD4+ and CD8+ CD11c+ DCs in the spleen within 24 h (Supplemental Fig. 4). One day after DTx treatment mice were challenged with αGalCer as indicated.
Sample preparation
Single-cell suspensions from spleen were prepared as described previously (14). Heparinized whole blood was centrifuged at 2000 g for 10 min at room temperature to obtain plasma.
Bone marrow chimeras
Bone marrow transplantations were performed as described previously (15). Lethal irradiations were performed in a 137Cesium irradiator (600 rad twice, 3h apart) and C57BL/6J or Cd1d−/− mice were reconstituted with un-fractioned bone marrow from wild-type C57BL/6J mice as indicated. Mice were treated with trimethoprim-sulfomethoxazole in drinking water for two weeks after transplantation. Experiments were performed 3-4 months after bone marrow transplantation.
Statistical analysis
Results are expressed as mean ± standard error of the mean (SEM). Statistical comparisons were drawn using a two-tailed Student t-test (Excel, Microsoft Corporation, Redmond, WA; GraphPad Prism, GraphPad Software, San Diego, CA) for all paired samples or otherwise using an ANOVA test (GraphPad Prism). p-values <0.05 were considered statistically significant and are indicated with *p<0.05, **p≤0.01 and ***p≤0.001. Each experiment was repeated at least twice with 2-4 mice per group, and background values were subtracted. Graphs were generated with GraphPad Prism (GraphPad Software).
Results
iNKT cell hypo-responsiveness does not solely depend on strong TCR - mediated activation
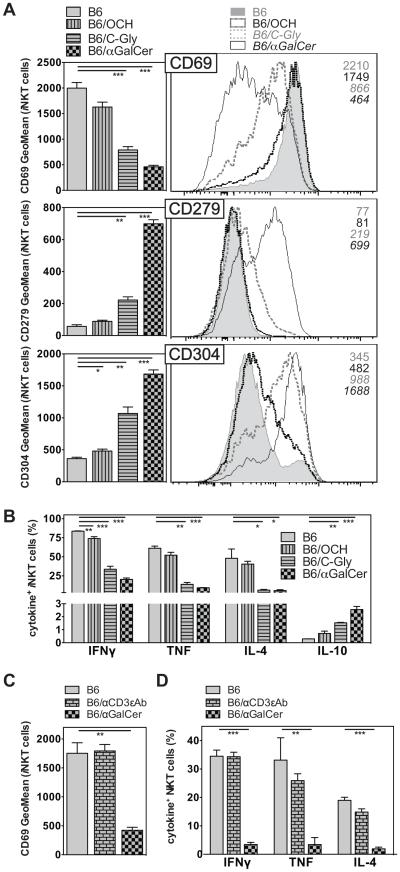
αGalCer is characterized by an exceptional antigenic potency, and therefore we addressed if other iNKT cell Ags, with differing degrees of antigenic strength, also would cause iNKT cell hypo-responsiveness. To this end, we compared the secondary iNKT cell response to αGalCer, so that each mouse received the same secondary stimulus, after an initial stimulation either with αGalCer or related compounds that differ with regard to relatively subtle chemical changes (Fig. 1). OCH is the prototypical Th2-biasing Ag. It has a sphingosine base reduced in length and exhibits a decreased antigenic potency and a weaker TCR affinity than αGalCer (16). C-glyoside (C-Gly) has a carbon-carbon bond substituting for the O-glycosidic linkage of the galactose sugar to the sphingosine (17). In terms of TCR affinity, C-Gly is weaker still compared to OCH (18-21), but induces a systemic Th1-response (17). The Th1-biasing effect of C-Gly is predominantly a consequence of increased IFNγ production by NK cells activated downstream of iNKT cell stimulation, as the ratio of IFNγ:IL-4 cytokines immediately produced by the iNKT cells themselves is comparable irrespective of the Ag injected (20). To investigate the long-term effects of stimulation with OCH or C-Gly on iNKT cells, we injected each compound once and measured the iNKT cell response one month later by re-challenge of the mice with αGalCer. We and others described previously that a single pre-treatment with αGalCer reduced the frequency of peripheral iNKT cells and led to wide range of phenotypic and functional changes in these cells (7-9). Markers such as CD25, CD69, CD122, CD127, CD154 (CD40L) and NK1.1 were expressed at lower levels, whereas markers associated with regulatory T cells, such as CD152 (CTLA4), CD279 (PD-1), CD304 (NRP1) and FR4 were strongly upregulated (9). Additionally, the expression of pro-inflammatory cytokines was reduced, whereas the production of IL-10 was increased in αGalCer pretreated iNKT cells (7-9). Similar changes were observed in this study one month after αGalCer injection (Fig. 2A, B), including decreased CD69 expression and intracellular cytokine staining for IFNγ, TNF and IL-4, together with increased expression of CD279 and CD304 and staining for IL-10 (Fig. 2A, B). Therefore these data demonstrate the expected iNKT cell hypo-responsiveness. In contrast, a single pre-treatment with OCH did not lead to significant alterations in the iNKT cell phenotype or effector function compared to control animals (Fig. 2A, B). Interestingly, a single pre-treatment with C-Gly resulted in a phenotype intermediate between the αGalCer and OCH treated mice (Fig. 2A, B). Similar changes were observed when IFNγ and IL-4 levels in plasma 90 min after αGalCer re-challenge were analyzed (Supplemental Fig. 2). To address the question if any type of strong TCR-triggering would lead to iNKT cell hypo-responsiveness, we injected αCD3ε mAbs i.v., which is known to activate iNKT cells and memory T cells preferentially (22). Despite the strong initial stimulation by the αCD3ε-antibody, the iNKT cell phenotype and response on re-challenge with αGalCer one month later was comparable to control animals (Fig. 2C, D and data not shown). Together, these data indicate that the potential of a TCR-mediated stimulus to induce iNKT cell hypo-responsiveness depends on antigen structure but does not directly correlate with its antigenic strength.
Figure 1. Chemical structures of the GSL Ags utilized.
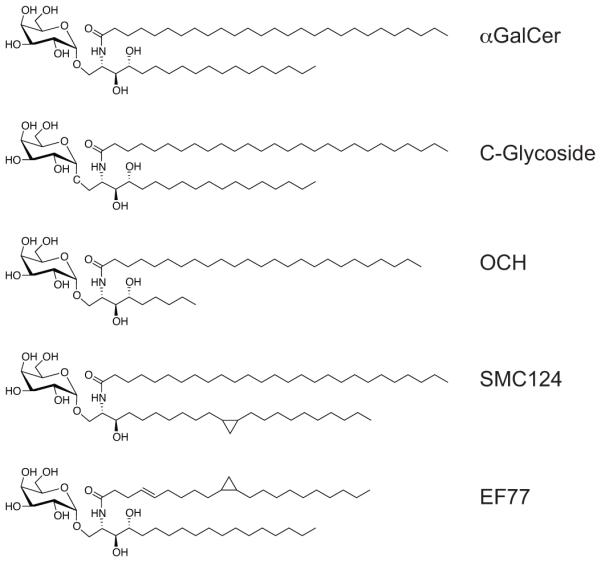
Figure 2. iNKT cell hypo-responsiveness does not solely depend on strong TCR - mediated activation.
(A, B) C57BL/6 (B6) mice were either left untreated or injected i.v. with 4μg of OCH, C-Gly or αGalCer as indicated. One month later mice were injected i.v. with 1μg αGalCer, and 90 min later expression surface markers (A) and the production of indicated cytokines (B) by splenic iNKT cells was analyzed. For (A) representative data (right panel) and summary graphs (left panel) are shown. The utilized gating strategy for iNKT cells is depicted in Supplemental Fig. 1. (C, D) C57BL/6 (B6) mice were either left untreated or i.v. injected with 4μg αGalCer or 1μg of αCD3ε (145.2C11) antibodies as indicated. One month later mice were injected i.v. with 1μg αGalCer, and 90 min later expression of CD69 (D) and of indicated cytokines (E) by splenic iNKT cells was analyzed. Statistically significant differences of treated groups versus the control group are indicated. Representative data from one of two independent experiments are shown.
Repetitive injection or increased dose can augment iNKT cell hypo-responsiveness
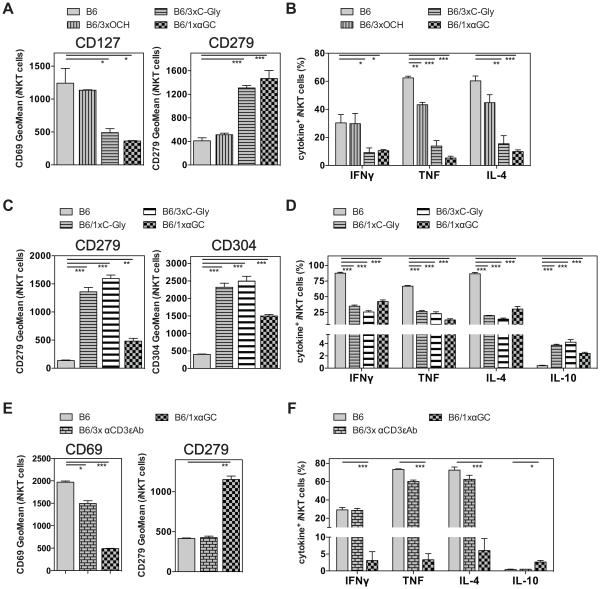
Repetitive antigenic stimulation has been shown to induce anergy in mainstream CD4 and CD8 T cells (23). Given the intermediate efficacy of C-Gly in the induction of iNKT cell hypo-responsiveness, we investigated whether repetitive challenge could augment hypo-responsiveness. Therefore, we injected either OCH or C-Gly three times and measured the iNKT cell response one month later. Similar to the results from a single injection (Fig. 2A, B), three injections of OCH did not significantly alter the phenotype or function of iNKT cells when re-stimulated and analyzed one month later (Fig. 3A and B). In contrast, three injections of C-Gly changed the phenotype and function of restimulated iNKT cells in a manner largely indistinguishable from the one following a single αGalCer injection (Fig. 3A and B). To discriminate if the observed effect of repetitive C-Gly challenge is due to the increased dose applied or due to the timing of the injections, we directly compared the injection of the same 12μg amount of C-Gly either given once or in a total of three separate aliquots of 4μg. Both treatments induced iNKT cell hypo-responsiveness to a similar degree that was comparable to a single αGalCer injection (Fig. 3C and D). We also tested three injections of αCD3ε mAbs and did not observe any changes in iNKT cells in regard to phenotype or cytokine production (Fig. 3E, F), confirming that the changes we observed with αGalCer and C-Gly were not simply the result of repeated strong TCR-triggering. Altogether, these data indicate that the efficiency of some Ags to induce iNKT cell hypo-responsiveness requires a minimum antigenic potency that can be achieved by repetitive challenge or by increasing the amount in a single dose.
Figure 3. Repetitive injection or increased dose can augment iNKT cell hypo-responsiveness.
(A, B) C57BL/6 (B6) mice were either left untreated or injected i.v. once with 4μg of αGalCer (1xαGC) or three times every other day with 4μg OCH (3xOCH) or C-Gly (3xC-Gly) as indicated. One month later mice were injected i.v. with 1μg αGalCer and 90 min later splenic iNKT cells were analyzed for the expression of surface makers (A) and of intracellular cytokines (B). (C, D) C57BL/6 (B6) mice were either left untreated or injected i.v. once with 4μg of αGalCer (αGC), once with 12μg of C-Gly (1xC-Gly) or three times every other day with 4μg C-Gly (3xC-Gly, i.e. 12μg in total) as indicated. One month later mice were injected i.v. with 1μg αGalCer and 90 min later splenic iNKT cells were analyzed for the expression of surface makers (C) and of intracellular cytokines (D). (E, F) C57BL/6 (B6) mice were either left untreated or injected i.v. once with 4μg of αGalCer (1xαGC) or three times every other day with 1μg αCD3ε (145.2C11) antibodies (3x αCD3εAb) as indicated. One month later mice were injected i.v. with 1μg αGalCer, and 90 min later splenic iNKT cells were analyzed for the expression of CD69 (D) and of intracellular cytokines (E). Differences in the amount of IFN□+ iNKT cells detectable in different experiments depended largely on the fluorochrome conjugated to the utilized antibody in the particular experiment (e.g. for IFNγ AF700 (3B, 3F) vs. PE-C594 (3D)). Regardless, within an experiment, consistent differences were observed between groups, and statistically significant differences are indicated. Representative data from one of at least two independent experiments are shown.
iNKT cell hypo-responsiveness is induced by Th1 - biasing compounds
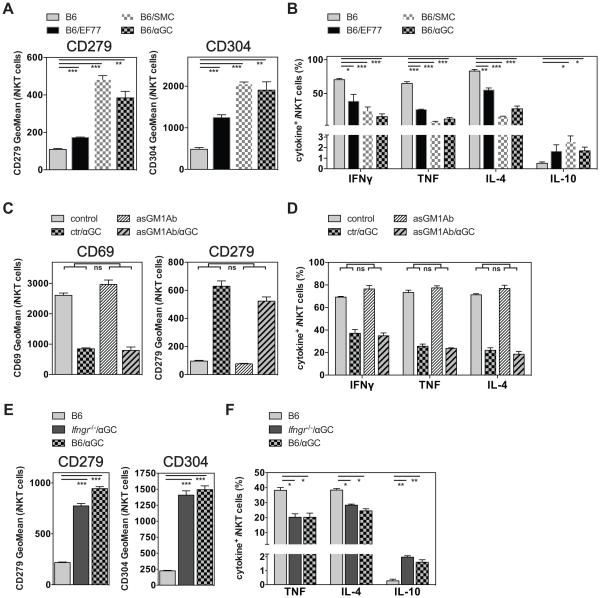
While αGalCer may be classified as a Th0 Ag, because of the large amounts of IFNγ and IL-4 it stimulates, C-Gly elicits a higher ratio of IFNγ to IL-4 (1-5, 17). Therefore we addressed if other Th1-biasing Ags also could induce iNKT cell hypo-responsiveness. To this end, we analyzed the responses to EF77 or SMC124 (Fig. 1), two glycosphingolipid (GSL) Ags based on the structure of the plakoside A GSL isolated from the marine sponge Plakortis simplex, which induce a Th1-biased pattern of cytokine secretion (1-5, 12). We injected these Ags i.v. and analyzed the iNKT cell response one month later after re-challenge with αGalCer. As shown in Figure 4A and B the phenotype and function of iNKT cells pre-treated with αGalCer or SMC124 were comparable. Furthermore, pre-treatment with EF77 also induced iNKT cell hypo-responsiveness, albeit to a lower degree (Fig. 4A, B). One of the key differences between the Th1 cytokine-vs Th2 cytokine-biasing iNKT cell Ags, is the ability of the Th1-biasing Ags to trans-activate NK cells, downstream of iNKT cell activation, to produce large amounts of IFNγ (6-8, 24, 25). To investigate if NK cells play a role in the induction of iNKT cell hypo-responsiveness we repeated the experiments after depletion of NK cells. However, NK cell depletion at the time of the initial αGalCer pretreatment did not reduce iNKT cell hypo-responsiveness upon re-challenge (Fig. 4C, D). Similarly, in mice deficient for the IFNγ-receptor (Ifngr−/−) the αGalCer induced iNKT cell hypo-responsiveness was unaffected (Fig. 4E, F). Therefore, the large amounts of IFNγ produced by NK cells, or any other function induced by these cells, is not required for the induction of iNKT cell hypo-responsiveness.
Figure 4. iNKT cell hypo-responsiveness is induced by Th1 - biasing compounds.
(A, B) C57BL/6 (B6) mice were either left untreated or injected i.v. with 4μg of EF77, SMC124 (SMC) or αGalCer (αGC) as indicated. One month later mice were injected i.v. with 1μg αGalCer, and 90 min later splenic iNKT cells were analyzed for the expression of surface makers (A) and intracellular cytokines (B). Statistically significant differences of treated groups versus the control group are indicated. (C, D) Control C57BL/6 (B6) mice or mice NK cell depleted one day earlier with α-asGM1 Ab (asGMAb) were either left untreated or injected i.v. with 4μg αGalCer (αGC). One month later 1μg αGalCer was injected i.v., and splenic iNKT cells were analyzed 90 min later for the expression of surface makers (C) and of intracellular cytokines (D). Statistically significant differences (Anova) of B6(control vs αGC) versus the NK-depleted asGM1(control vs αGC) groups are indicated. ns = not significant. (E, F) Control C57BL/6 (B6) mice or mice deficient for the IFNγ-receptor (Ifngr−/−) were either left untreated or injected i.v. with 4μg αGalCer (αGC). One month later 1μg αGalCer was injected i.v., and splenic iNKT cells were analyzed 90 min later for the expression of surface makers (E) and of intracellular cytokines (F). Statistically significant differences of treated groups versus the control group are indicated. Representative data from one of two independent experiments are shown.
Cytokine - driven stimulation does not induce iNKT cell hypo-responsiveness
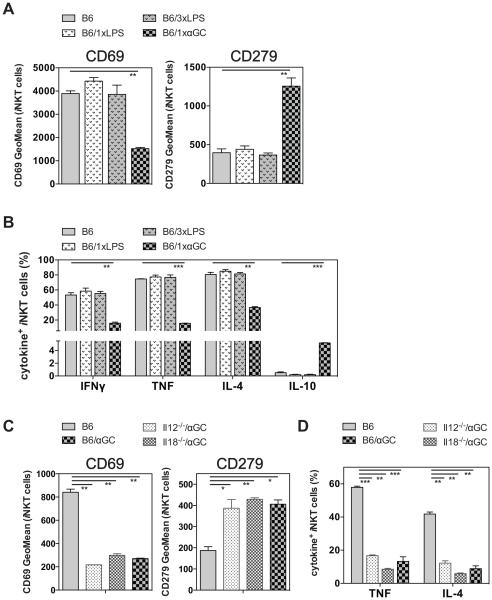
iNKT cells can be activated directly via the TCR or via cytokines, most prominently by IL-12 in concert with IL-18 for the majority population of Th1 cytokine biased iNKT cells (NKT1 cells) in C57BL/6 mice (9, 26, 27). Therefore, we addressed if such cytokine-driven activation would also lead to iNKT cell hypo-responsiveness. To this end, we injected 40 μg LPS either once or three times i.v. and analyzed the iNKT cell response one month later. As shown in Fig. 5A, B, LPS, even when given three times, did not induce iNKT cell hypo-responsiveness, as indicated by the unaltered phenotype and cytokine response compared to the control mice. To directly address the role of IL-12 and IL-18 in the induction of iNKT cell hypo-responsiveness we measured the long-term effects of αGalCer challenge in mice deficient for either cytokine. However, the lack of either the p35 subunit of IL-12 or IL-18 did not change the outcome of αGalCer induced iNKT cell hypo-responsiveness (Fig. 5C, D). We also tested the secondary response of iNKT cells following exposure to infectious agents. MCMV can stimulate iNKT cells via IL-12 and IL-18 (9, 27) or type I IFN (6-9, 28) and does not induce a TCR-mediated signal (10, 29, 30). In contrast, Sphingobium yanoikuyae bacteria provides both TCR and cytokine-dependent activation of iNKT cells (11, 31, 32). However, neither viral infection with MCMV nor bacterial infection with S. yanoikuyae induced any signs of hypo-responsiveness in the iNKT cells, based on the phenotype and cytokine response (Supplemental Fig. 3). Together, these data suggest that cytokine-driven activation of iNKT cells does not lead to hypo-responsiveness.
Figure 5. Cytokine - driven stimulation does not induce iNKT cell hypo-responsiveness.
(A, B) C57BL/6 (B6) mice were either left untreated or injected i.v. once with 4μg of αGalCer (1xαGC) or with 40μg LPS, either once (1xLPS) or three times every other day (3xLPS) as indicated. One month later mice were injected i.v. with 1μg αGalCer, and 90 min later splenic iNKT cells were analyzed for the expression of surface makers (A) and of intracellular cytokines (B). (C, D) Control C57BL/6 (B6) mice or mice deficient for the p35 chain of IL-12 (Il12−/−) or IL-18 (Il18−/−) were either left untreated or injected i.v. with 4μg αGalCer (αGC). One month later 1μg αGalCer was injected i.v., and splenic iNKT cells were analyzed 90 min later for the expression of surface makers (C) and of intracellular cytokines (D). Statistically significant differences of treated groups versus the control group are indicated. Representative data from one of at least two independent experiments are shown.
iNKT cell hypo-responsiveness can be induced by bone marrow-derived cells
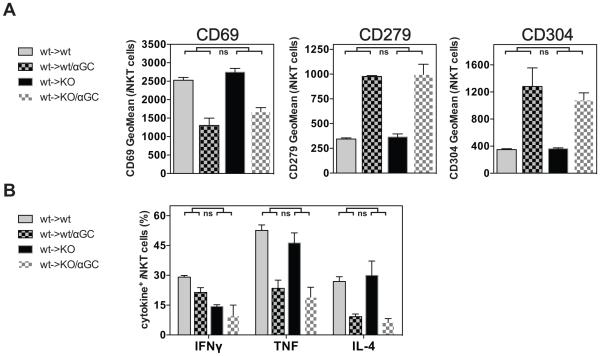
CD1d is widely expressed on hematopoietic, but also on non-hematopoietic cells (12, 33, 34). To investigate the cellular requirements for the αGalCer-induced iNKT cell hypo-responsiveness, we addressed the role of hematopoietic cells. To this end, we generated bone marrow chimeras by transferring C57BL/6 wild-type (wt) bone marrow into irradiated wild-type control (wt->wt) or CD1d-deficient hosts (wt->KO). As iNKT cells are selected in the thymus by double-positive thymocytes, they develop in wt->KO chimeras despite the absence of CD1d on non-hematopoietic cells (1, 4, 13, 35). After reconstitution, αGalCer was injected into wt->KO and control wt->wt chimeras and four weeks later the iNKT cell response was analyzed after re-challenge with αGalCer in vivo. The response of iNKT cells from wt->KO and control wt->wt chimeric mice was comparable, irrespective of the αGalCer pre-treatment, as indicated by the expression of surface markers and the production of cytokines (Fig. 6). These data indicate that presentation of αGalCer by CD1d on hematopoietic cells is sufficient to cause αGalCer-induced iNKT cell hypo-responsiveness. The reciprocal KO->wt chimeras could not be analyzed because these mice would not have iNKT cells, but our data do not role out a redundant role for expression of CD1d by non-hematopoietic cells in the induction of hypo-responsiveness.
Figure 6. iNKT cell hypo-responsiveness can be induced by bone marrow - derived cells.
(A, B) Lethally irradiated C57BL/6 (wt) or Cd1d−/− (KO) mice were reconstituted with C57BL/6 bone marrow (wt->wt or wt->KO), rested for 12 weeks and then either left untreated or injected i.v. with 4μg of αGalCer (αGC). One month later mice were injected i.v. with 1μg αGalCer, and 90 min later splenic iNKT cells were analyzed for the expression of surface markers (A) and intracellular cytokines (B). Statistically significant differences (Anova) of wt->wt(control vs αGC) versus the wt->KO(control vs αGC) groups are indicated. ns = not significant. Representative data from one of two independent experiments are shown.
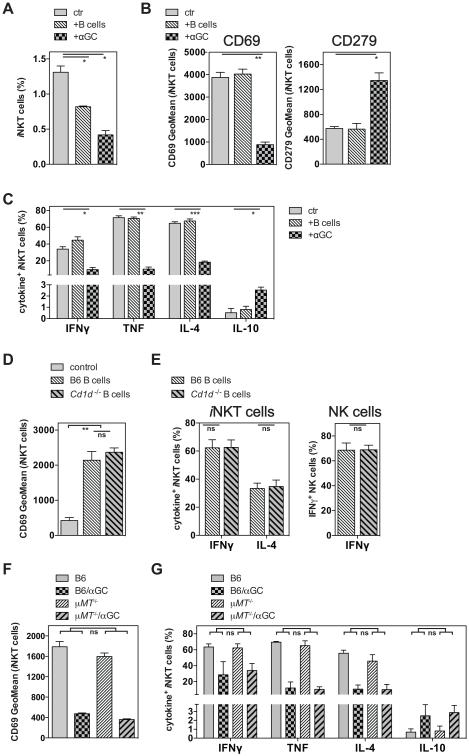
B cells are not required to induce iNKT cell hypo-responsiveness
It has been reported that injection of αGalCer-loaded B cells is sufficient to induce iNKT cell hypo-responsiveness in vivo (7, 13). This conclusion was based on the observation that a three day in vitro culture of splenocytes from such mice in the presence of αGalCer led to reduced proliferation, as measured by thymidine incorporation, and reduced levels of IFNγ in the culture supernatant (7, 11). However, we noticed a tendency for a reduced frequency of splenic iNKT cells in mice pre-treated with αGalCer ((9, 14) and data not shown), which in some, but not all experiments, was statistically significant. Nonetheless, this reduced number of responding cells could offer an alternative explanation for the previously reported in vitro findings (7, 16). To avoid this potential caveat, we restimulated iNKT cells with αGalCer in vivo and analyzed the iNKT cell response directly ex vivo on the single cell level. By this approach, the response of iNKT cells from control mice or mice injected one month earlier with αGalCer loaded B cells did not differ in the expression of surface markers or in the production of cytokines (Fig. 7A-C and data not shown). However, we observed that B cells loaded with αGalCer in vitro and injected i.v. led to an activation of iNKT cells in the host even when the transferred B cells were derived from Cd1d−/− mice (Fig. 7D, E). Furthermore, the trans-activation of NK cells was indistinguishable after the injection of B cells from either background (Fig. 7E). This indicated that αGalCer could efficiently be cross-presented by host cells in vivo after up-take of the injected B cells, and it reveals a cautionary note for defining the relevant APC type for iNKT cells in any experiment in which Ag-pulsed APCs are injected into recipients.
Figure 7. B cells are not required to induce iNKT cell hypo-responsiveness.
(A-C) C57BL/6 (B6) mice were either left untreated or injected i.v. with 4μg of αGalCer (+αGC) or with 5 × 106 αGalCer loaded (250ng/ml, 2h) B cells (+B cells) as indicated. One month later mice were injected i.v. with 1μg αGalCer, and 90 min later splenic iNKT cells were analyzed for frequency (A), the expression of surface makers (B) and of intracellular cytokines (C). Statistically significant differences of treated groups versus the control group are indicated. (D, E) C57BL/6 mice were either left untreated (control) or injected i.v. with 5 × 106 αGalCer loaded (250ng/ml, 2h) B cells derived from C57BL/6 (B6) or Cd1d−/− mice as indicated. 15 hours later purified splenocytes were incubated for 2h in vitro in the presence of protein transport inhibitors before iNKT cells (D, E) and NK cells (E) were analyzed for the expression of surface makers (D) and intracellular cytokines (E). (F, G) C57BL/6 (B6) or μMT−/− mice were either left untreated or injected i.v. with 4μg of αGalCer (αGC). One month later mice were injected i.v. with 1μg αGalCer, and 90 min later splenic iNKT cells were analyzed for the expression of CD69 (F) and of intracellular cytokines (G). Statistically significant differences (Anova) of B6(control vs αGC) versus the μMT−/−(control vs αGC) groups are indicated. ns = not significant. Representative data from one of at least two independent experiments are shown.
While these data indicate that cross-presentation of αGalCer does not lead to Ag-induced iNKT cell hypo-responsiveness, they do not settle the question as to whether or not B cells are required for this induction. To definitely address the requirement for B cells in the induction of iNKT cell hypo-responsiveness in vivo, we injected αGalCer into B cell deficient μMT−/− mice (17, 36) and analyzed the iNKT cells one month later. iNKT cells from both μMT−/− and wild-type control mice were similarly altered by the αGalCer pre-treatment and did not differ in the expression of surface markers or in the degree of reduction in the production of cytokines (Fig. 7F, G and data not shown). These data demonstrate that B cells are not necessary to induce αGalCer-induced iNKT cell hypo-responsiveness.
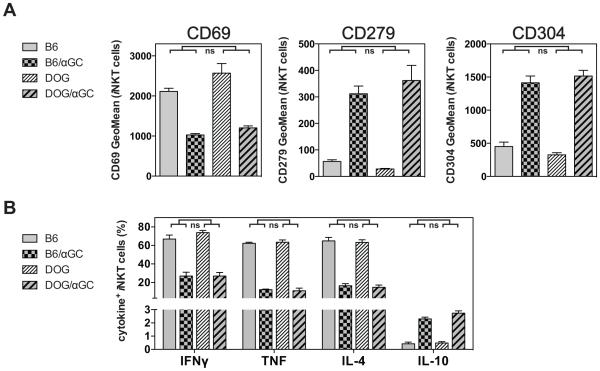
iNKT cell hypo-responsiveness does not require DCs
A recent study reported that CD8α+ DCs are the dominant APC type for activating iNKT cells with injected Ags (18-21, 37). Therefore, we addressed whether DCs also are necessary for the αGalCer induced iNKT cell hypo-responsiveness. To eliminate DCs in vivo we utilized transgenic mice expressing the DTx receptor under the control of the CD11c promoter (CD11c-DOG mice) (11, 17). Injection of DTx into CD11c-DOG mice led to depletion of <95% of CD4+ and CD8+ CD11c+ DCs in the spleen within 24 hours ((11, 20) and Supplemental Fig. 4). αGalCer was injected into control and DC-depleted CD11c-DOG mice and four weeks later the iNKT cell response was analyzed after re-challenge with αGalCer in vivo. However, iNKT cells from control and from DC-depleted CD11c-DOG mice were similarly altered by the αGalCer pre-treatment and did not differ in the expression of surface markers or in the production of cytokines (Fig. 8A, B and data not shown). These data demonstrate that CD11chigh DCs are not necessary to induce αGalCer-induced iNKT cell hypo-responsiveness.
Figure 8. iNKT cell hypo-responsiveness does not require DCs.
(A, B) CD11c-DOG mice were depleted <95% of CD4+ and CD8+ CD11c+ DCs in the spleen as described. C57BL/6 (B6) or DC-depleted CD11c-DOG (DOG) mice were either left untreated or injected i.v. with 4μg of αGalCer (αGC). One month later mice were injected i.v. with 1μg αGalCer, and 90 min later splenic iNKT cells were analyzed for the expression of surface markers (A) and of intracellular cytokines (B). Statistically significant differences (Anova) of B6(control vs αGC) versus the DC-depleted DOG(control vs αGC) groups are indicated. ns = not significant. Representative data from one of three independent experiments are shown.
Discussion
Initial activation of iNKT cells with αGalCer induces a rapid production of multiple cytokines; however, following secondary activation the production of most pro-inflammatory cytokines is blunted. Here, we report on two aspects of this iNKT cell hypo-responsiveness. First, our data demonstrate that Th0- and Th1-biasing GSL Ags can induce iNKT cell hypo-responsiveness, but not a Th2-biasing Ag or cytokine-driven iNKT cell activation due to TLR engagement as a result of LPS exposure or infections. Second, although presentation of αGalCer by hematopoietic cells can induce iNKT cell hypo-responsiveness, we did not find a nonredundant function either for B cells or DC for these changes.
Induction of iNKT cell hypo-responsiveness has previously largely been investigated with the Th0-Ag αGalCer (6-9). Here, we demonstrate that this feature is shared with several Th1-biasing Ags (Fig. 4A, B), in particular with C-glyoside (C-Gly) (9, 17) and the plakoside A analogs EF77 and SMC124 (7-9, 12). In contrast, the Th2-biasing compound OCH (16, 22) did not induce iNKT cell hypo-responsiveness (Fig. 2A, B and 3A, B). It has been suggested that the ability of an Ag to induce iNKT cell hypo-responsiveness correlates with its antigenic strength (23, 38). However, our data do not support this model. First, C-Gly was able induce long-term iNKT cell hypo-responsiveness, whereas OCH, which has more avid binding to the iTCR when complexed to CD1d than C-Gly, was not able to induce hypo-responsiveness (Fig. 2A, B and 3A, B). Second, a strong activation of iNKT cells with an agonistic αCD3ε-antibody, either once or repetitively, did not lead to iNKT cell hypo-responsiveness (Fig. 2C, D and 3E, F). Rather, our data indicated that the induction of long-term iNKT cell hypo-responsiveness is a particular feature of Th0- and Th1-biasing iNKT cell Ags, which is not shared with a Th2-biasing Ag. This interpretation is in line with some previous data suggesting that some Th1-biasing iNKT cell Ags (39-41), but not a Th2-biasing Ag (39) may induce long-term iNKT cell hypo-responsiveness. The reason for the opposite results described here and previously (38) is unknown. However, as Huang et al. (38) analyzed the secondary iNKT cell response only seven days after the initial challenge, the timing of the analysis could explain the differences between our studies. Indeed, it has been shown that the Th2-biasing iNKT cell Ag C20:2 can induce a short-lived hypo-responsiveness in iNKT cells that lasts for about one week; however, that is not sustained for the longer time frame of one month we investigated here (42).
One difference between Th1- and Th2-biasing Ags is their differential ability to induce the trans-activation of NK cells in vivo (24, 25). However, this trans-activation of NK cells (Fig. 4C, D) or signaling by IFNγ (Fig. 4E, F) was not a requirement for the induction of iNKT cell hypo-responsiveness. Therefore, at this time the reason for the lack of iNKT cell hypo-responsiveness induced by the Th2-biasing Ag OCH is not known. It has been suggested that Th1-biasing Ags are characterized by prolonged iNKT cell stimulation in vivo, which could be due either to increased TCR affinity, stability of the Ag/CD1d-complexes or unknown pharmacokinetic properties of the Ags. For example, the synthesis and testing of C-Gly was stimulated by the supposition that the C-glycosidic bond would provide for a more stable compound resistant to catabolism (17). In line with this prolonged stimulation hypothesis, we previously reported that the CD1d complexes on the surface of APCs for several Th1-biasing Ags had an increased half-life in vivo (12, 20, 40). Furthermore, structural data suggest that some Th1-biasing compounds have increased molecular contacts with CD1d that may promote prolonged binding to CD1d in vivo, and therefore prolonged stimulation of iNKT cells (12, 40, 43). Together, our data support a model whereby only Th0/1-biasing Ags have the capability to induce long-term iNKT cell hypo-responsiveness, provided that they surpass a minimal antigenic strength. Once this threshold is reached, repetitive/chronic exposure or increased dose can amplify the functional changes in iNKT cells, as shown here for C-Gly.
Besides Ag-driven activation via the TCR, iNKT cells can also be activated by cytokines, most prominently IL-12 in concert with IL-18 or IFNα/β (26-28). Data presented here with Il12−/− and Il18−/− mice, LPS injection and MCMV infection indicate that cytokine-driven activation of iNKT cells does not lead to or influence hypo-responsiveness. Additionally, in preliminary experiments with Il15−/−, Il12rb−/− mice and with wild-type mice infected with E. coli we also did not observe any influence on iNKT cells hypo-responsiveness (data not shown). Together, these data support the conclusion that pro-inflammatory cytokines are not involved in the induction of αGalCer-induced iNKT cell hypo-responsiveness. In contrast, other reports suggested that some, but not all, bacterial infections could induce iNKT cell hypo-responsiveness (44). The reason for this discrepancy is not known. However, the timing could be important here as well, as following the i.v. injection of LPS a short-lived (2-3 days) lack of iNKT cell responsiveness toward TCR-triggering was reported that waned within one week (45).
It has been shown that αGalCer derived from αGalCer-loaded B16 melanoma cells can be cross-presented by DCs in vivo (46); however, this has not been shown for hematopoietic cells. Here, we demonstrate that αGalCer associated with Cd1d−/− B cells is efficiently cross-presented after i.v. injection, leading to an iNKT cell activation that is indistinguishable from the stimulation achieved with Ag-loaded wild-type B cells (Fig. 7D, E). Similar preliminary results were obtained after injection of αGalCer-loaded Cd1d−/− bone marrow - derived DCs (data not shown). Our finding that αGalCer is efficiently cross-presented in vivo provides a cautionary note for the interpretation of experiments involving transfer of αGalCer loaded cells. Such experiments cannot discriminate between stimulation of iNKT cells by αGalCer presented by the injected cells and cross-presented by host cells.
Ag presentation by different APC populations has been suggested to be critical for iNKT cell stimulation with particular Ags and in particular organs (37, 47-49). For example, it was reported that the presentation of Th1-biasing Ags is largely dependent on presentation by DCs/macrophages, whereas Th2-biasing compounds are more promiscuous with regard to the APC type (48). In contrast, there is evidence indicating that CD8α+ DCs are the critical APC for the initial presentation of all Ags in vivo, irrespective of their Th1- or Th2-biasing properties (37). Nonetheless, the requirements for the immediate iNKT cell activation are not necessarily identical with the requirements for long-term effects leading to of iNKT cell hypo-responsiveness. Therefore, we investigated here the role of two APC populations, B cells and DCs. Based on in vitro data generated after the transfer of αGalCer-loaded B cells it had been suggested that B cells could induce the hypo-responsive state in iNKT cells (7). In contrast, when we analyzed the iNKT cell response from similarly treated mice on a single cell level rather than on a population level, we could not detect any long-term changes in the iNKT cell phenotype and cytokine response (Fig. 7B, C). However, we noticed a tendency for a reduced iNKT cell frequency in splenocytes from mice pre-treated with αGalCer-loaded B cells (Fig. 7A), which could potentially explain the previous in vitro findings (7). Importantly, our data with B cell deficient μMT−/− mice directly demonstrated that B cells are not required for the induction of iNKT cell hypo-responsiveness in vivo (Fig. 7F, G). We cannot exclude the possibility that the few B-1 cells remaining in μMT−/− mice (50, 51) could be responsible for the observed induction of iNKT cell hypo-responsiveness. However, we consider this unlikely, in light of the systemic nature of anergy induction (7, 9), the fact that iNKT cells in many organs do not circulate extensively (52, 53), and the paucity of B-1 B cells in some sites (54).
It has been reported that i.v. injection of αGalCer-loaded bone marrow - derived DCs (BM-DCs) (6) or primary splenic DCs (7) does not induce iNKT cell hypo-responsiveness, and we could reproduce this finding with BM-DCs (data not shown). However, as noted above, because of extensive Ag cross-presentation, no conclusion could be drawn about the role of DCs in the induction of hypo-responsiveness (Fig. 7D, E). Importantly, our data with CD11c-DOG mice (11) indicated that CD11chigh DCs are not required to induce αGalCer-induced iNKT cell hypo-responsiveness in vivo (Fig. 8). Altogether, our data demonstrate that although presentation of αGalCer by hematopoietic cells is sufficient to cause iNKT cell hypo-responsiveness (Fig. 6), neither presentation by B cells nor DCs is required. It has been reported that the depletion of macrophages via clodronate liposome treatment also does not reduce iNKT cell hypo-responsiveness (55). Together, these data suggest that none of the classical bone marrow - derived APCs, DCs, B cells and macrophages, are essential for the presentation of αGalCer in the induction of iNKT cell hypo-responsiveness in vivo. It is likely, however, that hypo-responsiveness by iNKT cells requires specific properties of the cell presenting αGalCer. It has been suggested that Th1-biasing Ags preferentially load onto CD1d in lysosomes and localize on the cell surface in lipid rafts (56, 57). It also was reported that Th1-biasing Ags also cause changes in DCs, such as increased CD70 and CD86 expression, that support Th1 responses (37). How these changes might be correlated with a long-term decrease in the ability of iNKT cells to respond to Ag stimulation remains unknown.
In summary, we demonstrate here that Th0- and Th1-biasing, but not a Th2-biasing Ags can induce long-term iNKT cell hypo-responsiveness once a minimal threshold of antigenic strength is reached. This can be achieved by a sufficient Ag dose or repetitive/chronic exposure. Furthermore, although hematopoietic cells can induce iNKT cell hypo-responsiveness, neither B cells nor DC are essential for these changes.
Supplementary Material
Acknowledgments
The authors wish to thank Archana Khurana, the Flow Cytometry Core Facility as well as the Department of Laboratory Animal Care at the La Jolla Institute for Allergy and Immunology for excellent technical assistance. We thank Günter Hämmerling and Chris Benedict for kindly providing CD11c-DOG mice and MCMV, respectively. We are grateful to Barbara Sullivan, Bo Pei, Aaron Tyznik, Jose Luis Véla, Norihito Kawasaki, James C. Paulson, Tri Giang Phan and Arens Ramon for their scientific contributions.
Funding: This work was funded by NIH grants RO1 AI45053 and R37 AI71922 (M.K.), RO1 GM087136 (A.R.H.) and an Outgoing International Fellowship by the Marie Curie Actions (G.W.).
Abbreviations
- αGalCer
α-galactosylceramide
- BM-DCs
bone marrow - derived dendritic cells
- C-Gly
C-Glycoside
- DC
dendritic cell
- DTx
diphtheria toxin
- GSL
glycosphingolipid
- i
invariant
- NKT
Natural Killer T
- SPF
specific pathogen free
- TLR-L
toll like receptor ligand
- Vα14i
invariant Vα14 to Jα18 TCR rearrangement
- Vα24i
invariant Vα24 to Jα18 TCR rearrangement
Footnotes
Competing Interest: The authors have no competing interests regarding this work.
References
- 1.Bendelac A, Savage PB, Teyton L. The Biology of NKT Cells. Annu. Rev. Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 2.Salio M, Silk JD, Yvonne Jones E, Cerundolo V. Biology of CD1- and MR1-Restricted T Cells. Annu. Rev. Immunol. 2014;32:323–366. doi: 10.1146/annurev-immunol-032713-120243. [DOI] [PubMed] [Google Scholar]
- 3.Rossjohn J, Pellicci DG, Patel O, Gapin L, Godfrey DI. Recognition of CD1d-restricted antigens by natural killer T cells. Nature reviews. 2012;12:845–857. doi: 10.1038/nri3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kronenberg M. Towards an Understanding of NKT Cell Biology: Progress and Paradoxes. Annu. Rev. Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 5.Wingender G, Kronenberg M. Role of NKT cells in the digestive system. IV. The role of canonical natural killer T cells in mucosal immunity and inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;294:G1–8. doi: 10.1152/ajpgi.00437.2007. [DOI] [PubMed] [Google Scholar]
- 6.Fujii S, Shimizu K, Kronenberg M, Steinman RM. Prolonged IFN-gamma-producing NKT response induced with alpha-galactosylceramide-loaded DCs. Nat Immunol. 2002;3:867–874. doi: 10.1038/ni827. [DOI] [PubMed] [Google Scholar]
- 7.Parekh VV, Wilson MT, Olivares-Villagómez D, Singh AK, Wu L, Wang C-R, Joyce S, Van Kaer L. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin. Invest. 2005;115:2572–2583. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uldrich AP, Crowe NY, Kyparissoudis K, Pellicci DG, Zhan Y, Lew AM, Bouillet P, Strasser A, Smyth MJ, Godfrey DI. NKT cell stimulation with glycolipid antigen in vivo: costimulation-dependent expansion, Bim-dependent contraction, and hyporesponsiveness to further antigenic challenge. J. Immunol. 2005;175:3092–3101. doi: 10.4049/jimmunol.175.5.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sag D, Krause P, Hedrick CC, Kronenberg M, Wingender G. IL-10–producing NKT10 cells are a distinct regulatory invariant NKT cell subset. J Clin. Invest. 2014;124:3725–3740. doi: 10.1172/JCI72308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, Kaneko Y, Koseki H, Kanno M, Taniguchi M. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 11.Hochweller K, Striegler J, Hammerling GJ, Garbi N. A novel CD11c.DTR transgenic mouse for depletion of dendritic cells reveals their requirement for homeostatic proliferation of natural killer cells. Eur. J. Immunol. 2008;38:2776–2783. doi: 10.1002/eji.200838659. [DOI] [PubMed] [Google Scholar]
- 12.Tyznik AJ, Farber E, Girardi E, Birkholz A, Li Y, Chitale S, So R, Arora P, Khurana A, Wang J, Porcelli SA, Zajonc DM, Kronenberg M, Howell AR. Glycolipids that Elicit IFN-γ-Biased Responses from Natural Killer T Cells. Chemistry & Biology. 2011;18:1620–1630. doi: 10.1016/j.chembiol.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wingender G, Rogers P, Batzer G, Lee MS, Bai D, Pei B, Khurana A, Kronenberg M, Horner AA. Invariant NKT cells are required for airway inflammation induced by environmental antigens. J. Exp. Med. 2011;208:1151–1162. doi: 10.1084/jem.20102229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wingender G, Krebs P, Beutler B, Kronenberg M. Antigen-specific cytotoxicity by invariant NKT cells in vivo is CD95/CD178-dependent and is correlated with antigenic potency. J. Immunol. 2010;185:2721–2729. doi: 10.4049/jimmunol.1001018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wingender G, Hiss M, Engel I, Peukert K, Ley K, Haller H, Kronenberg M, von Vietinghoff S. Neutrophilic granulocytes modulate invariant NKT cell function in mice and humans. J. Immunol. 2012;188:3000–3008. doi: 10.4049/jimmunol.1101273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413:531–534. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 17.Schmieg J, Yang G, Franck RW, Tsuji M. Superior protection against malaria and melanoma metastases by a C-glycoside analogue of the natural killer T cell ligand alpha-Galactosylceramide. J. Exp. Med. 2003;198:1631–1641. doi: 10.1084/jem.20031192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aspeslagh S, Li Y, Yu ED, Pauwels N, Trappeniers M, Girardi E, Decruy T, Van Beneden K, Venken K, Drennan M, Leybaert L, Wang J, Franck RW, Van Calenbergh S, Zajonc DM, Elewaut D. Galactose-modified iNKT cell agonists stabilized by an induced fit of CD1d prevent tumour metastasis. The EMBO Journal. 2011;30:2294–2305. doi: 10.1038/emboj.2011.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel O, Cameron G, Pellicci DG, Liu Z, Byun H-S, Beddoe T, McCluskey J, Franck RW, Castaño AR, Harrak Y, Llebaria A, Bittman R, Porcelli SA, Godfrey DI, Rossjohn J. NKT TCR recognition of CD1d-α-C-galactosylceramide. J. Immunol. 2011;187:4705–4713. doi: 10.4049/jimmunol.1100794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sullivan BA, Nagarajan NA, Wingender G, Wang J, Scott I, Tsuji M, Franck RW, Porcelli SA, Zajonc DM, Kronenberg M. Mechanisms for glycolipid antigen-driven cytokine polarization by Valpha14i NKT cells. J. Immunol. 2010;184:141–153. doi: 10.4049/jimmunol.0902880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wun KS, Cameron G, Patel O, Pang SS, Pellicci DG, Sullivan LC, Keshipeddy S, Young MH, Uldrich AP, Thakur MS, Richardson SK, Howell AR, Illarionov PA, Brooks AG, Besra GS, McCluskey J, Gapin L, Porcelli SA, Godfrey DI, Rossjohn J. A Molecular Basis for the Exquisite CD1d-Restricted Antigen Specificity and Functional Responses of Natural Killer T Cells. Immunity. 2011:1–13. doi: 10.1016/j.immuni.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wingender G, Schumak B, Schurich A, Gessner JE, Endl E, Limmer A, Knolle PA. Rapid and preferential distribution of blood-borne alphaCD3epsilonAb to the liver is followed by local stimulation of T cells and natural killer T cells. Immunology. 2006;117:117–126. doi: 10.1111/j.1365-2567.2005.02272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz RH. T Cell Anergy. Annu. Rev. Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 24.Carnaud C, Lee D, Donnars O, Park SH, Beavis A, Koezuka Y, Bendelac A. Cutting edge: Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J. Immunol. 1999;163:4647–4650. [PubMed] [Google Scholar]
- 25.Eberl G, Macdonald HR. Selective induction of NK cell proliferation and cytotoxicity by activated NKT cells. Eur. J. Immunol. 2000;30:985–992. doi: 10.1002/(SICI)1521-4141(200004)30:4<985::AID-IMMU985>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 26.Nagarajan NA, Kronenberg M. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J. Immunol. 2007;178:2706–2713. doi: 10.4049/jimmunol.178.5.2706. [DOI] [PubMed] [Google Scholar]
- 27.Tyznik AJ, Tupin E, Nagarajan NA, Her MJ, Benedict CA, Kronenberg M. Cutting edge: the mechanism of invariant NKT cell responses to viral danger signals. J. Immunol. 2008;181:4452–4456. doi: 10.4049/jimmunol.181.7.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wesley JD, Tessmer MS, Chaukos D, Brossay L. NK Cell–Like Behavior of Vα14i NK T Cells during MCMV Infection. PLoS Pathog. 2008;4:e1000106. doi: 10.1371/journal.ppat.1000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tyznik AJ, Verma S, Wang Q, Kronenberg M, Benedict CA. Distinct Requirements for Activation of NKT and NK Cells during Viral Infection. J. Immunol. 2014;192:3676–3685. doi: 10.4049/jimmunol.1300837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holzapfel KL, Tyznik AJ, Kronenberg M, Hogquist KA. Antigen-Dependent versus -Independent Activation of Invariant NKT Cells during Infection. J. Immunol. 2014;192:5490–5498. doi: 10.4049/jimmunol.1400722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, Tsuji M, Kawahara K, Wong CH, Kronenberg M. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 32.Mattner J, Debord KL, Ismail N, Goff RD, Cantu C, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, Hoebe K, Schneewind O, Walker D, Beutler B, Teyton L, Savage PB, Bendelac A. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 33.Porcelli SA, Modlin RL. The CD1 system: antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu. Rev. Immunol. 1999;17:297–329. doi: 10.1146/annurev.immunol.17.1.297. [DOI] [PubMed] [Google Scholar]
- 34.Bleicher PA, Balk SP, Hagen SJ, Blumberg RS, Flotte TJ, Terhorst C. Expression of murine CD1 on gastrointestinal epithelium. Science. 1990;250:679–682. doi: 10.1126/science.1700477. [DOI] [PubMed] [Google Scholar]
- 35.Wei DG, Lee H, Park S-H, Beaudoin L, Teyton L, Lehuen A, Bendelac A. Expansion and long-range differentiation of the NKT cell lineage in mice expressing CD1d exclusively on cortical thymocytes. J. Exp. Med. 2005;202:239–248. doi: 10.1084/jem.20050413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 37.Arora P, Baena A, Yu KOA, Saini NK, Kharkwal SS, Goldberg MF, Kunnath-Velayudhan S, Carreño LJ, Venkataswamy MM, Kim J, Lazar-Molnar E, Lauvau G, Chang Y-T, Liu Z, Bittman R, Al-Shamkhani A, Cox LR, Jervis PJ, Veerapen N, Besra GS, Porcelli SA. A Single Subset of Dendritic Cells Controls the Cytokine Bias of Natural Killer T Cell Responses to Diverse Glycolipid Antigens. Immunity. 2014;40:105–116. doi: 10.1016/j.immuni.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang J-R, Tsai Y-C, Chang Y-J, Wu J-C, Hung J-T, Lin K-H, Wong C-H, Yu AL. alpha-Galactosylceramide but not phenyl-glycolipids induced NKT cell anergy and IL-33-mediated myeloid-derived suppressor cell accumulation via upregulation of egr2/3. J. Immunol. 2014;192:1972–1981. doi: 10.4049/jimmunol.1302623. [DOI] [PubMed] [Google Scholar]
- 39.Blumenfeld HJ, Tohn R, Haeryfar SMM, Liu Y, Savage PB, Delovitch TL. Structure-guided design of an invariant natural killer T cell agonist for optimum protection from type 1 diabetes in non-obese diabetic mice. Clinical and experimental immunology. 2011;166:121–133. doi: 10.1111/j.1365-2249.2011.04454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Birkholz AM, Girardi E, Wingender G, Khurana A, Wang J, Zhao M, Zahner S, Illarionov PA, Wen X, Li M, Yuan W, Porcelli SA, Besra GS, Zajonc DM, Kronenberg M. A Novel Glycolipid Antigen for NKT Cells That Preferentially Induces IFN-Production. J. Immunol. 2015;195:924–933. doi: 10.4049/jimmunol.1500070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silk JD, Salio M, Reddy BG, Shepherd D, Gileadi U, Brown J, Masri SH, Polzella P, Ritter G, Besra GS, Jones EY, Schmidt RR, Cerundolo V. Cutting edge: nonglycosidic CD1d lipid ligands activate human and murine invariant NKT cells. J. Immunol. 2008;180:6452–6456. doi: 10.4049/jimmunol.180.10.6452. [DOI] [PubMed] [Google Scholar]
- 42.Tohn R, Blumenfeld H, Haeryfar SMM, Veerapen N, Besra GS, Porcelli SA, Delovitch TL. Stimulation of a shorter duration in the state of anergy by an invariant natural killer T cell agonist enhances its efficiency of protection from type 1 diabetes. Clinical and experimental immunology. 2011;164:26–41. doi: 10.1111/j.1365-2249.2011.04323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aspeslagh S, Nemcovic M, Pauwels N, Venken K, Wang J, Van Calenbergh S, Zajonc DM, Elewaut D. Enhanced TCR footprint by a novel glycolipid increases NKT-dependent tumor protection. J. Immunol. 2013;191:2916–2925. doi: 10.4049/jimmunol.1203134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim S, Lalani S, Parekh VV, Vincent TL, Wu L, Van Kaer L. Impact of bacteria on the phenotype, functions, and therapeutic activities of invariant NKT cells in mice. J Clin. Invest. 2008;118:2301–2315. doi: 10.1172/JCI33071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chiba A, Dascher CC, Besra GS, Brenner MB. Rapid NKT cell responses are self-terminating during the course of microbial infection. J. Immunol. 2008;181:2292–2302. doi: 10.4049/jimmunol.181.4.2292. [DOI] [PubMed] [Google Scholar]
- 46.Shimizu K, Kurosawa Y, Taniguchi M, Steinman RM, Fujii S. Cross-presentation of glycolipid from tumor cells loaded with alpha-galactosylceramide leads to potent and long-lived T cell mediated immunity via dendritic cells. J. Exp. Med. 2007;204:2641–2653. doi: 10.1084/jem.20070458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmieg J, Yang G, Franck RW, van Rooijen N, Tsuji M. Glycolipid presentation to natural killer T cells differs in an organ-dependent fashion. Proc. Natl. Acad. Sci. U.S.A. 2005;102:1127–1132. doi: 10.1073/pnas.0408288102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bai L, Constantinides MG, Thomas SY, Reboulet R, Meng F, Koentgen F, Teyton L, Savage PB, Bendelac A. Distinct APCs Explain the Cytokine Bias of alpha-Galactosylceramide Variants In Vivo. J. Immunol. 2012;188:3053–3061. doi: 10.4049/jimmunol.1102414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bezbradica JS, Stanic AK, Matsuki N, Bour-Jordan H, Bluestone JA, Thomas JW, Unutmaz D, Van Kaer L, Joyce S. Distinct roles of dendritic cells and B cells in Va14Ja18 natural T cell activation in vivo. J. Immunol. 2005;174:4696–4705. doi: 10.4049/jimmunol.174.8.4696. [DOI] [PubMed] [Google Scholar]
- 50.Ghosh S, Hoselton SA, Schuh JM. m-Chain-Deficient Mice Possess B-1 Cells and Produce IgG and IgE, but Not IgA, following Systemic Sensitization and Inhalational Challenge in a Fungal Asthma Model. J. Immunol. 2012;189:1322–1329. doi: 10.4049/jimmunol.1200138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Macpherson AJ, Lamarre A, McCoy K, Harriman GR, Odermatt B, Dougan G, Hengartner H, Zinkernagel RM. IgA production without mu or delta chain expression in developing B cells. Nat Immunol. 2001;2:625–631. doi: 10.1038/89775. [DOI] [PubMed] [Google Scholar]
- 52.Lynch L, Michelet X, Zhang S, Brennan PJ, Moseman A, Lester C, Besra G, Vomhof-Dekrey EE, Tighe M, Koay H-F, Godfrey DI, Leadbetter EA, Sant’Angelo DB, von Andrian U, Brenner MB. Regulatory iNKT cells lack expression of the transcription factor PLZF and control the homeostasis of Treg cells and macrophages in adipose tissue. Nat Immunol. 2015;16:85–95. doi: 10.1038/ni.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomas SY, Scanlon ST, Griewank KG, Constantinides MG, Savage AK, Barr KA, Meng F, Luster AD, Bendelac A. PLZF induces an intravascular surveillance program mediated by long-lived LFA-1-ICAM-1 interactions. J. Exp. Med. 2011;208:1179–1188. doi: 10.1084/jem.20102630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nature Reviews Immunology. 2010;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 55.Biburger M, Tiegs G. Activation-induced NKT cell hyporesponsiveness protects from alpha-galactosylceramide hepatitis and is independent of active transregulatory factors. Journal of leukocyte biology. 2008;84:264–279. doi: 10.1189/jlb.0607352. [DOI] [PubMed] [Google Scholar]
- 56.Arora P, Venkataswamy MM, Baena A, Bricard G, Li Q, Veerapen N, Ndonye R, Park J-J, Lee JH, Seo K-C, Howell AR, Chang Y-T, Illarionov PA, Besra GS, Chung S-K, Porcelli SA. A Rapid Fluorescence-Based Assay for Classification of iNKT Cell Activating Glycolipids. J. Am. Chem. Soc. 2011;133:5198–5201. doi: 10.1021/ja200070u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bai L, Sagiv Y, Liu Y, Freigang S, O. Yu K, Teyton L, Porcelli SA, Savage PB, Bendelac A. Lysosomal recycling terminates CD1d-mediated presentation of short and polyunsaturated variants of the NKT cell lipid antigen alphaGalCer. Proc. Natl. Acad. Sci. U.S.A. 2009;106:10254–10259. doi: 10.1073/pnas.0901228106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.